Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the pathogen responsible for coronavirus disease 2019 (COVID-19) has been a major cause of morbidity and mortality globally. Older age, and the presence of certain components of metabolic syndrome, including hypertension have been associated with increased risk for severe disease and death in COVID-19 patients. The role of antihypertensive agents in the pathogenesis of COVID-19 has been extensively studied since the onset of the pandemic. This review discusses the potential pathophysiologic interactions between hypertension and COVID-19 and provides an up-to-date information on the implications of newly emerging SARS-CoV-2 variants, and vaccines on patients with hypertension.
Keywords: Hypertension, COVID-19, ACE inhibitors, Angiotensin receptor blockers, Calcium channel blockers, Catecholamines, Vaccines, Delta variant, SARS-CoV-2, Alpha blockers, Beta blockers
Introduction
Coronavirus disease 2019 (COVID-19), caused by the SARS-CoV-2 virus, swept across the world beginning in late 2019, and was officially declared a pandemic by the World Health Organization (WHO) in March of 2020. With the persistent spread of the virus across the globe, particularly in unvaccinated communities, new and more transmissible variants have emerged, and the disease remains a critical threat. Therefore, continued understanding of the pathophysiology, risk factors, prevention and treatment remains of utmost importance. Prior studies have identified risk factors associated with severe disease, including diabetes and old age [1], [2]. Hypertension has also been linked to worse outcomes [3], [4], [5], [6], [7], [8], [9]. Globally, 1.13 billion people carry a diagnosis of hypertension and in the United States alone, this number reaches upwards of 108 million individuals [10], [11], [12], [13]. Several studies from around the globe have demonstrated associations between hypertension and risk for severe COVID-19, as well as increased rates of hospitalization and need for mechanical ventilation [14], [15], [16], [17]. Further studies have evaluated the role of various antihypertensive agents in SARS-CoV-2 infection and have found differing outcomes. Distinct focus has been laid on the drugs affecting the renin-angiotensin-aldosterone system (RAAS) pathway, especially the angiotensin converting enzyme inhibitors (ACEis) and angiotensin receptor blockers (ARBs), due to the well-established data on the requirement of ACE2 receptor for the entry of SARS-CoV virion into the host cell [18]. This review aims to discuss the potential pathophysiological links between hypertension and COVID-19, updated literature on hypertension as a risk factor in COVID-19, the effects of anti-hypertensives on COVID-19 outcomes, and the emerging data on relation among vaccines, novel SARS-CoV-2 variants, and hypertension.
Pathophysiological interactions between SARS-CoV-2 virus and hypertension, and their potential implications on anti-hypertensive therapy
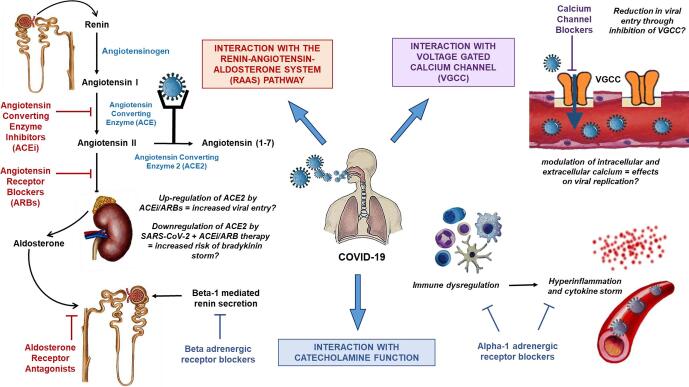
The various points of interaction among the SARS-CoV-2 virion, the pathways involved in development of hypertension, and the targets for anti-hypertensive agents are described in Fig. 1. Studies on the SARS-CoV, the virus responsible for SARS, as well as more recent studies on SARS-CoV-2 have demonstrated that the spike (S) protein facilitates viral entry by binding to angiotensin-converting enzyme 2 (ACE2) receptors on target cells, and subsequently downregulates ACE2 enzyme via proteolytic degradation [18], [19]. ACE2 is expressed broadly in over 15 organ systems including the heart, kidneys, lung alveolar epithelial cells, and type I and type II pneumocytes [20]. Of note, ACE2 receptors have been found to have higher expression in adipocytes, even in relation to lung tissue and could help explain the increased rates of SARS-CoV-2 infection in obese patients [21], [22]. In the RAAS pathway, the renal juxtaglomerular cells release renin which cleaves angiotensinogen to angiotensin I, which through the action of the angiotensin-converting enzyme (ACE) generates angiotensin II. Angiotensin II is a powerful vasoconstrictor and the main effector molecule of the RAAS [23]. ACE2 serves as a counter-regulatory enzyme in the RAAS pathway, degrading angiotensin II to angiotensin (1–7), a vasodilator with anti-inflammatory effects [24], [25], [26], [27], tempering the effects of angiotensin II on vasoconstriction, sodium retention, cellular growth, and fibrosis. It was initially hypothesized that use of RAAS pathway modulators (such as ACEi and ARBs) which inhibit the production/activity of angiotensin II, may potentiate an upregulation of ACE2 receptors, and thereby increase risk of COVID-19 disease due to enhanced viral entry via the increased number of ACE2 receptors. As well, another hypothesis has been put forward that may be a “bradykinin storm” is responsible for some of the manifestations attributed to the cytokine storm, which could be aggravated by the use of both ACEis and ARBs [27], [28]. As SARS-CoV-2 virus binds to ACE2, the number of ACE2 moieties on the cells reduce, thereby resulting in a downregulation of ACE2 receptors. This may lead to increased levels of ACE2 substrates, including des-Arg(9)-bradykinin [27], [29]. ACEi use may potentially aggravate this effect by facilitating further production of bradykinin. On the contrary, SARS-CoV-2-mediated ACE2 downregulation also leads to increased angiotensin II, and the use of ACEi/ARB may in fact be beneficial in alleviating the deleterious effects of angiotensin II.
Fig. 1.
Molecular interactions in the setting of co-existing COVID-19 and hypertension, and the potential effects of antihypertensive agents.
Beta blockers (beta adrenergic receptor blockers) also modulate the RAAS pathway by inhibiting sympathetic activation of the juxtaglomerular cells decreasing renin secretion. With a reduction in renin release, there is downregulation in the downstream pathways, including the activation and expression of ACE2, and potentially decreasing SARS-CoV-2 viral entry [30]. It has previously been demonstrated that established beta-blocker therapy is associated with decreased mortality in patients admitted to the Intensive Care Unit (ICU) with acute respiratory failure and sepsis [31], [32]. Sepsis is an inflammatory response to infection with high levels of cytokine production, and beta-blockers have the potential to modulate these pathways [32]. COVID-19 may potentiate a cytokine storm, and previous studies have shown that inflammatory markers seen in severe COVID-19 disease are similarly elevated in patients with other critical illness including sepsis and acute respiratory distress syndrome (ARDS) [33]. Therefore, it can be postulated that beta blockers can be a treatment modality for COVID-19 disease by downregulating the pro-inflammatory cytokines that lead to a cytokine storm.
One of the main mechanisms by which SARS-CoV-2 affects various organ systems is through hyper-inflammation. This has been correlated with high inflammatory markers including C-reactive protein (CRP), ferritin and D-dimer in COVID-19 patients [34]. Rapid viral replication and localized cellular destruction causes release of cytokines and chemokines, which attract immune cells to the region. In some patients, an inappropriate immune response can occur triggering a cytokine release storm [35]. Several of these effects are likely mediated by circulating catecholamines [36], [37], [38], [39]. The immune modulatory effects of catecholamines, such as the inhibition of antigen presenting cells, and downregulation of T lymphocytes may also facilitate viral replication [12]. Therefore, alpha blockers were initially suggested as potential agents to utilize in COVID-19, not only for control of hypertension but also for mitigating the hyper-inflammatory response, the cytokine storm, and viral replication [12], [40].
Calcium is an important substrate in viral infection and replication. Voltage-gated calcium channels (VGCC) are found on the surface of various excitable cells, and activation of these channels results in an influx of calcium ions, allowing for downstream physiologic effects including synaptic transmission and muscle activation and relaxation [41]. Interaction between viral surface proteins and host VGCC allows for viral entry [42]. Reduction in calcium can therefore lead to decreased viral entry, and prior studies have demonstrated this to hold true for SARS-CoV [43]. Viruses are also capable of overtaking host calcium modulation to support their own replication and survival [44]. Calcium serves an integral role in many aspects of viral pathogenesis and could play a role in the fight against SARS-CoV-2 virus. There is a possibility that calcium channel blockers may have an effect on one or more of these aspects of calcium physiology.
Anti-Hypertensive agents and COVID-19: What is known so far?
Acei and ARBs
ACEis and ARBs are among the most commonly used prescription medications to treat hypertension in the United States. Due to the known interaction between SARS-CoV viruses and the ACE2 receptor, numerous studies have evaluated the association between ACEi/ARB use and COVID-19. This has led to varying recommendations regarding the continuation of ACEis and ARBs in patients hospitalized with COVID-19 [45].
Some studies have identified a lack of association between ACE2 protein expression and the use of ACEis and ARBs [46]. In a meta-analysis of 27 studies by Caldeira et al, the association between prior exposure to ACEi and/or ARBs and the risk of acquiring COVID-19 infection was evaluated. ACEi and ARB group did not have an increased risk of a positive test for COVID-19 infection (OR 0.99, 95%CI 0.91–1.11) nor were ACEi use alone (OR 0.94, 95% CI 0.87 to 1.02) or ARB use alone (OR 1.01, 95%CI 0.93 to 1.10) associated with increased risk of a positive test [47]. In another meta-analysis of 10 studies by Flacco et al, the association between the use of ACEi/ARB use and risk of severe or lethal COVID-19 was explored. The study found that risk of severe or lethal COVID-19 was comparable between patients who were treated with ACEi and the untreated group (OR 0.90; 95% CI 0.65 to 1.26). Similarly, the risk between ARB use and developing severe or lethal COVID-19 compared to non-users was non-significant (OR = 0.92; 95% CI 0.75 to 1.12). The results of the study did not change when adjusted for three outlier studies which reported either a higher or lower risk of severe illness among the treated group [48]. A recent multicenter prospective study identified no increased risk for severe COVID-19, ICU admission, intubation, or mortality with ACEi/ARB use [49]. In fact, continued maintenance of ACEi/ARB use decreased the risk of death from COVID-19 [49]. In a meta-analysis by Wang et al, the results indicate that treatment with ACEi/ARB was significantly associated with a lower risk of mortality in COVID-19 infected hypertensive patients (OR = 0.624, 95% CI = 0.457 to 0.852, p = .003), and also reduced the odds of requiring ventilator support (OR = 0.682, 95% CI 0.475 to 1.978, p = .037) [50].
In a large population-based study by Hippisley-Cox et al, ACEi and ARBs were noted to have a decreased risk for COVID-19 disease (adjusted HR = 0.71, 95% CI 0.67 to 0.74). However, there was no association with ICU admission, even when controlled for possible confounding variables. Interestingly, the study also noted some significant differences among ethnic groups. There was increased risk of COVID-19 among Caribbean ACEi users (adjusted HR 1.05, 95% CI 0.87 to1.28) and also among Black Africans with ACEi use (adjusted HR 1.31, 95% CI 1.08 to 1.59) and ARB use (adjusted HR 1.24, 95% CI 0.99 to 1.58) [51].
Calcium channel blockers
Calcium channel blockers are widely prescribed medications with utility in management of blood pressure, arrhythmias, angina, and migraines. In a retrospective study of 77 patients who were hospitalized with COVID-19 and were treated calcium channel blockers (nifedipine or amlodipine) for hypertension, that patients treated with a calcium channel blocker were significantly less likely to have undergone intubation and mechanical ventilation. Additionally, in the group treated with the calcium channel blockers, 50% survived the hospitalizations, while only 14.6% of the untreated group survived (p = 0.0036) [52]. This was further supported by a meta-analysis conducted by Alsagaff et.al where 31 studies were analyzed to identify the association between calcium channel blocker use, and COVID-19 outcomes. Pooled analysis indicated that CCB use was not associated with decreased mortality (OR of 1.21, 95% CI 0.98 to 1.49). However, when subgroup analysis was done in just hypertensive patients, there was significantly reduced mortality (OR of 0.69, 95% CI 0.52 to 0.91) [53]. In a meta-analysis of 53 studies by Ren et al., there was no association noted between the use of calcium channel blockers with incidence of COVID-19 (OR of 1.15, 95% CI 0.87 to 1.53, I2 = 93.61%), or between use of calcium channel blockers and severity of disease (OR of 0.94, 95% CI 0.80-1.10) [54].
Beta blockers and diuretics
There is a paucity of data regarding the association between the use of beta blockers and diuretics, and the role in COVID-19 susceptibility, morbidity, and mortality. In a retrospective observational study conducted in China by Gao et al, the association of hypertensive treatment class with mortality among hospitalized patients with COVID-19 was explored. Patients with a history of hypertension and not on hypertensive therapy had a significantly higher rate of mortality compared to those on antihypertensive treatment (7.3 vs. 3.2%, HR 2.54, 95% CI). This study also compared rates of mortality between COVID-19 hypertensive patients on RAAS inhibitors vs non-RAAS inhibitors (including beta blockers, diuretics, and calcium channel blockers), and the results were similar for symptom onset, severity of disease, and percent requiring ventilator support [55]. However, 15 patients on spironolactone (an anti-hypertensive modulating RAAS pathway) were counted as being on a ‘non-RAAS inhibitor’. Ren et al. also described the association between the use of beta-blockers and diuretics with COVID-19 disease incidence and mortality. There was no association between the use of beta-blockers with incidence (OR 1.03, 95% CI 0.78 to 1.35) or severity (OR 1.23, 95% CI 0.74 to 2.04), or with prior diuretic use with incidence (OR 0.86, 95% CI 0.54 to 1.38) or severity (OR 0.96, 95%CI 0.81 to 1.15) [54].
In a large multicenter observational study conducted in patients hospitalized with COVID-19 with pre-existing diabetes, clinical characteristics prior to admission were compared. Among them was use of various anti-hypertensive agents with the primary outcomes of intubation and/or death within the first 7 days of hospitalization. Prior use of loop diuretics, thiazide diuretics, and beta-blockers were noted in 19.1%, 20.3%, and 33.6% of patients, respectively. Use of beta-blockers and loop diuretics were associated with increased odds of death on day 7. (OR 1.03, 95% CI 0.80 to 1.32) (OR 1.10, 95% CI0.81 to1.48) (OR 1.08, 95% CI 0.81 to 1.45) [56].
Alpha blockers
Alpha blockers (alpha adrenergic receptor blockers) are gaining traction in the fight against viral illness due to the potential impact on cytokine storms. Prior animal studies have demonstrated that alpha blockade can prevent cytokine storms and their deleterious effects [36]. Prior exposure to any alpha blocker was associated with a relative risk reduction of 34% in patients with ARDS (OR 0.70, 95% CI 0.49 to 0.99), and in patients with pneumonia, there was a relative risk reduction of 8% (OR 0.86, 95% CI 0.82 to 0.91) [57]. In COVID-19 specifically, outpatient use of any alpha blocker is not associated with COVID-19 outcomes in hospitalized patients (OR 0.749, 95% CI 0.527 to 1.064) [58]. However, inpatient use of alpha blockers was associated with improved outcomes, specifically, in-hospital mortality (OR 0.633, 95% CI 0.434 to 0.921) [58].
Vaccinations, Variants, and COVID-19
Despite the use and promotion of contact tracing methods, social distancing, and hand hygiene, SARS-CoV-2 has continued to have a drastic spread with limited treatment options. Vaccinations offer a means of preventing illness, and thereby diminishing the risk of viral spread and mutation. Numerous vaccines have been approved and are in use globally. In the United States, the three most common vaccines include two mRNA-based vaccines; mRNA-1273 vaccine (Moderna®) and BNT162b2 (Pfizer®), and an adenovirus-vector based vaccine (Johnson and Johnson®). Two doses of the Moderna vaccine offers a 94.1% efficacy at preventing viral infection, and severe disease [59]. Similarly, a two dose regimen of the Pfizer vaccine provides 95% protection against COVID-19 [60]. The Johnson and Johnson vaccine is a single-injection vaccine that confers 64% efficacy against disease, and 81.7% against critical illness [61]. Findings on vaccine efficacy were replicated in various countries’ vaccination efforts and suggest that vaccines are highly effective in preventing illness and decrease risk for severe disease [62], [63]. Additionally, vaccines are likely effective in populations with pre-existing conditions like hypertension. Data for the BNT162b2 vaccine indicated that the vaccine is highly efficacious in patients with hypertension at 94.6% (95% CI 68.7 to 99.9) [60]. Data for the Johnson and Johnson vaccine indicated that vaccine efficacy in patients older than 60 with at least one comorbid condition was 82%. Hypertension was identified as one of the comorbid conditions, being prevalent in 10.3% of the study population [61]. The Moderna vaccine study did not include hypertension as one of the specific pre-existing conditions, and there is therefore no data specific to that population. While vaccine efficacy remains robust in populations with pre-existing conditions, it is important to note that studies have demonstrated lower antibody titers in patients with certain comorbidities including hypertension and central obesity [64]. This highlights the need for additional investigations into determining the best vaccine schedule and need for earlier boosters in these special populations.
Gam-COVID-Vac (Sputnik®) is an adenovirus-based vaccine mainly utilized in Russia. This vaccine boasts an efficacy of 91.6% (95% CI 85.6 to 95.2) and of 100% (95% CI 94.4 to 100) against severe COVID-19. The vaccine trial included patients with hypertension as a comorbid condition, along with diabetes mellitus, ischemic heart disease, and obesity. Seven participants in the placebo group with polymerase chain reaction-confirmed COVID-19 and no comorbid conditions, while 25 patients in the vaccine group were confirmed to have COVID-19 after vaccination, with 3 of the 25 also having underlying comorbidities [65]. BBIBP COVID-19 vaccine (Sinopharm®) manufactured and first approved for use in China is noted to have an overall efficacy of 78.1% (95% CI 64.9 to 86.3). While the vaccine efficacy in hypertensive sub-group was not estimated, of the 374 patients in the vaccine group who had hypertension, none were noted to have COVID-19. In the control group, 367 patients had an underlying diagnosis of hypertension, with 4 patients noted to have COVID-19 infections [66]. BBV152 (Bharat Biotech®) vaccine used mostly in India is an inactivated SARS-CoV-2 vaccine that confers 77.8% efficacy (95% CI 65.2 to 86.4). 20% of the study population was composed of “high-risk” populations; either being over 60 years of age, or having a coexisting comorbidity, but the data does not specifically indicate the efficacy rate among patients with pre-existing hypertension [67]. Another prominent vaccine candidate is the ChAdOx1nCoV-19 (Covishield), an adenovirus-based vaccine. In a study comparing two standard doses to one low dose with a subsequent standard dose, vaccine efficacy across both groups was noted to be 70.4% (95% CI 54.8 to 80.6) [68]. While the study describes efficacy in groups with co-morbid conditions, hypertension is not specifically detailed.
In this context, the concept of heterologous immunity and cross-reactivity of adaptive immune lymphocytes in support of protection from infection should be considered. Heterologous effects of vaccines include cross-reactivity between shared epitopes of unrelated pathogens, trained immunity in innate cells such as natural killer T cells and monocytes, modulation of type 1 and type 17, regulatory and memory T cells, cytokine responses, and modulation of mean concentration of antibodies and cross-reactive antibodies [69]. SARS-CoV-2-reactive CD4 T cells have been detected in unexposed individuals and T cell cross-reactivity between SARS-CoV-2 and other circulating coronaviruses which is expected as they share high sequence similarity [70], [71]. In individuals who were vaccinated with Measles-Mumps-Rubella (MMR) or Tetanus-diphtheria-pertussis (Tdap) vaccine, a propensity-weighted analysis of 73,582 COVID-19 patients showed that severe disease outcomes were reduced by 32–38% and 20–23%, respectively, suggesting that SARS-CoV-2 reactivates memory T cells generated by MMR and Tdap vaccines [72]. Better delineation of the risk for future SARS-CoV infections may be accomplished by long-term assessment of SARS-CoV-2 memory B and T cell-mediated immune responses in patients recovering from an infection or those with cross-reactive immunological memory [73]. Such immunological heterologous reactivity phenomena may play a role in the outcomes among patients with underlying co-morbidities such as hypertension who receive vaccination against COVID-19, which warrants further investigation.
RNA viruses like SARS-CoV-2 are prone to mutations as long as there is continued transmission, and recent variants of interest include B.1.1.7 (Alpha), B.1.351 (Beta), P.1 (Gamma), and B.1.617.2 (Delta) [74]. These variants have been shown to have more transmissibility with possibility for increased severity of disease [75]. The Delta variant is currently the most prevalent variant in the United States [74]. However, vaccines such as Pfizer, AstraZeneca, and Moderna are still able to generate adequate levels of neutralizing antibodies in recipients [76]. Hypertension continues to remain an important risk factor for the Delta variant. Factors associated with increased risk of COVID-19-related hospitalization, even in fully vaccinated adults include age greater than or equal to 65 years (75%), and hypertension seen in 70.5% of fully vaccinated adults, compared to 59.9% in unvaccinated adults [77]. In a hospital based cohort-study, 36.5% of unvaccinated and 51.7% of vaccinated individuals (with Covaxin® or Covishield®) who acquired the Delta variant had hypertension/diabetes [78]. Additionally, hypertensive patients with other spike protein mutations still had a significant risk for hospitalization (OR 17, 95% CI 2–144) [79].
Treatment of hypertension during COVID-19: Telemedicine and addressing inequities are crucial
Hypertension is responsible for millions of deaths globally and has been shown to be a strong risk factor in the development of severe COVID-19. In addition to social distancing, hand hygiene, and vaccinations, effective management and treatment of elevated blood pressure will be key in the fight against COVID-19. Regular blood pressure monitoring will allow for early detection and management. Racial and ethnic minorities in the United States are more likely to experience hospitalization and mortality from COVID-19 [80]. They are also more likely to have co-morbid conditions like hypertension and obesity that puts them at risk for more severe disease [81], [82]. Additionally, the risk of disease exposure is also higher for ethnic minorities as they are overrepresented in several frontline industries [83]. In the United States, African Americans and Hispanics also make up a lower percentage of the vaccinated population, especially when compared to their disproportionate case rate [84].
During the pandemic, telemedicine has emerged as an important means of healthcare delivery. Telemedicine offers the opportunity for management of chronic illness, including hypertension, from the safety of ones’ home [85], [86]. Additionally, appropriate pharmacotherapy with anti-hypertensive agents, and monitoring for adverse medication adverse effects such as electrolyte abnormalities, dehydration, and kidney injury will allow for optimal control of hypertension. Finally, healthy dietary habits coupled with an active lifestyle with regular physical activity can lead to overall well-being in addition to tighter blood pressure control. Amendment of local and federal governmental policies are crucial to address issues of socioeconomic and other regional inequities, and improve access to affordable healthcare, telemedicine, and ancillary services.
Future directions
Tremendous progress has been made in understanding the SARS-CoV-2 pathogen and COVID-19 disease since the onset of the pandemic. Despite the many advances in knowledge, treatment, and prevention, the global devastation continues to be unparalleled. Managing risk factors such as hypertension will be key in the fight against COVID-19. As Table 1 highlights, there are currently many clinical trials underway to evaluate the role of various anti-hypertensive agents, both in the form of observational studies, and randomized controlled trials. The pathophysiologic interactions between COVID-19 and anti-hypertensive therapy may vary among different races. For instance, in a study on over 19,000 COVID-19 patients in the United Kingdom, increased risk for COVID-19 was observed with the use of ACEis and ARBs among Caribbeans and Black Africans [51]. Glucocorticoids, especially dexamethasone, whose beneficial effects in severe COVID-19 has been well-established [87], cause sodium retention thereby increasing the risk for hypertension [88], [89]. However, information on glucocorticoid-induced hypertension in COVID-19 is lacking in current literature, which therefore warrants further research.
Table 1.
List of current clinical trials for the treatment and management of COVID-19 (registered under ClinicalTrials.gov).
| Trial Name | Study phase | Study status | Location | NCT ID Number |
|---|---|---|---|---|
| I. Anti-Hypertensives | ||||
| Association Between Hypertension, Renin-Angiotensin-Aldosterone System Inhibitors and COVID-19 | Not Yet Recruiting | France | NCT04374695 | |
| Phase IV Observational Study to Associate Hypertension and Hypertension Treatment to COVID-19 | Phase IV | Active, Recruiting | Italy | NCT04331574 |
| COVID-19 Blood Pressure Endothelium Interaction Study | Active, Recruiting | United Kingdom | NCT04409847 | |
| Prospective Monitoring of Drug Safety and the Occurrence of Complications During Hospitalization in Patients with Cardiovascular Diseases with COVID-19. | – | Active, Recruiting | Poland | NCT04374110 |
| Effects of Discontinuing Renin-angiotensin System Inhibitors in Patients With and Without COVID-19 | – | Active, Recruiting | Denmark | NCT04351581 |
| Switch or Maintenance of Renin-Angiotensin System Inhibitors in Patients with COVID-19: A Randomized Proof of Concept Trial | Phase 3 | Active, Recruiting | Brazil | NCT04493359 |
| Efficacy of Hydroxychloroquine, Telmisartan and Azithromycin on the Survival of Hospitalized Elderly Patients with COVID-19: a Randomized, Multicenter, Adaptive Study | Phase 3 | Active, Recruiting | France | NCT04359953 |
| Randomized, Double-Blind, Placebo-Controlled Pilot Clinical Trial of the Safety and Efficacy of Telmisartan for the Treatment of COVID-19 in Hospitalized Patients | Phase 2 | Active, Recruiting | United States | NCT04715763 |
| II. Vaccines | ||||
| Study to Evaluate the Effectiveness of BCG Vaccines in Reducing Morbidity and Mortality in Elderly Individuals in COVID-19 Hotspots in India | Phase 3 | Active, Recruiting | India | NCT04475302 |
| III. Innovations | ||||
| Personalized Electronic Record Supported Optimization When Alone for Patients with Hypertension- Pilot Study for Remote Medical Management of Hypertension During the COVID-19 Pandemic | Phase 4 | Recruiting | United Kingdom | NCT04559074 |
| Comparison of Physical Activity Levels and Disease Attitudes of Hypertensive And Healthy Individuals Under Social Isolation During the COVID-19 pandemic | – | Active, Recruiting | Turkey | NCT04583345 |
| Integrated Distance Management Strategy for Patients with Cardiovascualr Disease (Ischaemic Coronary Rtery Disease, High Blood Pressure, Heart Failure) in the context of the COVID-19 Pandemic | – | Active, Recruiting | Romania | NCT04325867 |
| Optimization of the Management of COVID-19 Through Tailored Recommendations to the Citizens | – | Active, Recruiting | Canada | NCT04699851 |
| Evaluating the Comparative Effectiveness of Telemedicine in Primary Care: Learning from the COVID-19 Pandemic | – | Active, Recruiting | United States | NCT04684836 |
| The Measurement of Vital Signs by Lifelight Data Collect Software in comparison to the Standard of Care in Acutely Unwell Patients – The VISION-Acute Study | – | Active, Recruiting | United Kingdom | NCT04589923 |
As COVID-19 pandemic continues to change the traditional model of healthcare, studies that look into the efficacy and feasibility of delivering tele-medicine will be of significant importance. Tele-medicine can also potentially play a role in the management of COVID-19 via the use of remote home monitoring models. In previous studies done utilizing this method, patients who were considered at high-risk for clinical deterioration were monitored remotely at home using pulse oximetry and with frequent, regular communication between patients and healthcare workers [90]. Such tele-medicine models, along with country-specific guidance [91] to tailor remote monitoring of hypertension and other such co-morbidities can allow for patients to be monitored more closely and for care to be escalated appropriately to prevent worsening of COVID-19 severity. Finally, as the pandemic continues with the potential for emergence of novel SARS-CoV-2 variants, the efficacy of vaccinations, particularly in at-risk populations like those with hypertension will need to be assessed.
Funding
This study was funded in part by the intramural program of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Wolff D, Nee S, Hickey NS, Marschollek M. Risk factors for Covid-19 severity and fatality: a structured literature review. Infection 2020 49:2020;49(1):15-28. [DOI] [PMC free article] [PubMed]
- 2.Del Sole F., Farcomeni A., Loffredo L., Carnevale R., Menichelli D., Vicario T., et al. Features of severe COVID-19: A systematic review and meta-analysis. Eur J Clin Invest. 2020;50(10) doi: 10.1111/eci.v50.1010.1111/eci.13378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu L, Chen S, Fu Y, Gao Z, Long H, Ren H-w, et al. Risk Factors Associated With Clinical Outcomes in 323 Coronavirus Disease 2019 (COVID-19) Hospitalized Patients in Wuhan, China. Clinical Infectious Diseases. 2020;71(16):2089-98. [DOI] [PMC free article] [PubMed]
- 4.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W., et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323(20):2052. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guan W-j, Ni Z-y, Hu Y, Liang W-h, Ou C-q, He J-x, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. https://doiorg/101056/NEJMoa2002032. 2020;382(18):1708-20. [DOI] [PMC free article] [PubMed]
- 6.Zhang J.-J., Dong X., Cao Y.-Y., Yuan Y.-D., Yang Y.-B., Yan Y.-Q., et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan. China. Allergy. 2020;75(7):1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 7.Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368. [DOI] [PMC free article] [PubMed]
- 8.Du Y., Zhou N., Zha W., Lv Y. Hypertension is a clinically important risk factor for critical illness and mortality in COVID-19: A meta-analysis. Nutrition, Metabolism and Cardiovascular Diseases. 2021;31(3):745–755. doi: 10.1016/j.numecd.2020.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barrera FJ, Shekhar S, Wurth R, Moreno-Pena PJ, Ponce OJ, Hajdenberg M, et al. Prevalence of diabetes and hypertension and their associated risks for poor outcomes in Covid-19 patients. Journal of the Endocrine Society. 2020;4(9):bvaa102. [DOI] [PMC free article] [PubMed]
- 10.World Health Organization 'Hypertension' https://www.who.int/news-room/fact-sheets/detail/hypertension. 2021.
- 11.Whelton P.K., Carey R.M., Aronow W.S., Casey D.E., Collins K.J., Himmelfarb C.D., et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults a report of the American College of Cardiology/American Heart Association Task Force on Clinical practice guidelines. Hypertension. 2018;71(6):E13–E115. doi: 10.1161/HYP.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 12.Gubbi S., Nazari M.A., Taieb D., Klubo-Gwiezdzinska J., Pacak K. Catecholamine physiology and its implications in patients with COVID-19. Lancet Diabetes Endocrinol. 2020;8(12):978–986. doi: 10.1016/S2213-8587(20)30342-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hannah-Shmouni F., Gubbi S., Spence J.D., Stratakis C.A., Koch C.A. Resistant hypertension: a clinical perspective. Endocrinol Metab Clin North Am. 2019;48(4):811–828. doi: 10.1016/j.ecl.2019.08.010. [DOI] [PubMed] [Google Scholar]
- 14.Wang Z., Deng H., Ou C., Liang J., Wang Y., Jiang M., et al. Clinical symptoms, comorbidities and complications in severe and non-severe patients with COVID-19: A systematic review and meta-analysis without cases duplication. Medicine. 2020;99(48):e23327. doi: 10.1097/MD.0000000000023327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iqbal F, Soliman A, Sanctis VD, Mushtaq K, Nair AP, Masalamani MAA, et al. Prevalence, Clinical Manifestations, and Biochemical Data of Hypertensive versus Normotensive Symptomatic Patients with COVID-19: A Comparative Study. Acta Bio Medica : Atenei Parmensis. 2020;91(4):e2020164-e. [DOI] [PMC free article] [PubMed]
- 16.Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A., et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region. Italy. JAMA. 2020;323(16):1574. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Almeida-Pititto B., Dualib P.M., Zajdenverg L., Dantas J.R., de Souza F.D., Rodacki M., et al. Severity and mortality of COVID 19 in patients with diabetes, hypertension and cardiovascular disease: a meta-analysis. Diabetol Metab Syndr. 2020;12(1) doi: 10.1186/s13098-020-00586-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181(2):271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamming I., Timens W., Bulthuis MLC, Lely A.T., Navis G.J., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanchis-Gomar F., Lavie C.J., Mehra M.R., Henry B.M., Lippi G. Obesity and Outcomes in COVID-19: When an Epidemic and Pandemic Collide. Mayo Clin Proc. 2020;95(7):1445–1453. doi: 10.1016/j.mayocp.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma A., Garg A., Rout A., Lavie C.J. Association of Obesity With More Critical Illness in COVID-19. Mayo Clin Proc. 2020;95(9):2040–2042. doi: 10.1016/j.mayocp.2020.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamming I., Cooper M.E., Haagmans B.L., Hooper N.M., Korstanje R., Osterhaus ADME, et al. The emerging role of ACE2 in physiology and disease. J Pathol. 2007;212(1):1–11. doi: 10.1002/path.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vaduganathan M., Vardeny O., Michel T., McMurray J.J.V., Pfeffer M.A., Solomon S.D. Renin–Angiotensin–Aldosterone System Inhibitors in Patients with Covid-19. N Engl J Med. 2020;382(17):1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang K., Gheblawi M., Oudit G.Y. Angiotensin Converting Enzyme 2. Circulation. 2020;142(5):426–428. doi: 10.1161/CIRCULATIONAHA.120.047049. [DOI] [PubMed] [Google Scholar]
- 26.Muniyappa R., Gubbi S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am J Physiol Endocrinol Metab. 2020;318(5):E736–E741. doi: 10.1152/ajpendo.00124.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilczynski S.A., Wenceslau C.F., McCarthy C.G., Webb R.C. A cytokine/bradykinin storm comparison: what is the relationship between hypertension and COVID-19? Am J Hypertens. 2021;34(4):304–306. doi: 10.1093/ajh/hpaa217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Oliveira A.A., Priviero F., Lima V.V., Webb R.C., Nunes K.P. COVID-19 and ROS Storm: what is the forecast for hypertension. Am J Hypertens. 2021;34(8):779–782. doi: 10.1093/ajh/hpab085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roche J.A., Roche R. A hypothesized role for dysregulated bradykinin signaling in COVID-19 respiratory complications. Faseb j. 2020;34(6):7265–7269. doi: 10.1096/fj.202000967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vasanthakumar N. Can beta-adrenergic blockers be used in the treatment of COVID-19? Med Hypotheses. 2020;142:109809. doi: 10.1016/j.mehy.2020.109809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Noveanu M., Breidthardt T., Reichlin T., Gayat E., Potocki M., Pargger H., et al. Effect of oral beta-blocker on short and long-term mortality in patients with acute respiratory failure: results from the BASEL-II-ICU study. Crit Care. 2010;14(6):R198. doi: 10.1186/cc9317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tan K., Harazim M., Tang B., McLean A., Nalos M. The association between premorbid beta blocker exposure and mortality in sepsis—a systematic review. Crit Care. 2019 23:1. 2019,;23(1):1–12. doi: 10.1186/s13054-019-2562-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson J.G., Simpson L.J., Ferreira A.-M., Rustagi A., Roque J., Asuni A., et al. Cytokine profile in plasma of severe COVID-19 does not differ from ARDS and sepsis. JCI Insight. 2020;5(17) doi: 10.1172/jci.insight.14028910.1172/jci.insight.140289DS110.1172/jci.insight.140289DS2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeng F., Huang Y., Guo Y., Yin M., Chen X., Xiao L., et al. Association of inflammatory markers with the severity of COVID-19: A meta-analysis. Int J Infect Dis. 2020;96:467–474. doi: 10.1016/j.ijid.2020.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.V S, Ry B, K K, M D, Ml D, Gj R, et al. Disruption of a self-amplifying catecholamine loop reduces cytokine release syndrome. Nature. 2018;564(7735):273–277. doi: 10.1038/s41586-018-0774-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oberbeck R. Catecholamines: Physiological Immunomodulators During Health and Illness. Curr Med Chem. 2006;13(17):1979–1989. doi: 10.2174/092986706777584997. [DOI] [PubMed] [Google Scholar]
- 38.Seiffert K., Hosoi J., Torii H., Ozawa H., Ding W., Campton K., et al. Catecholamines Inhibit the Antigen-Presenting Capability of Epidermal Langerhans Cells. J Immunol. 2002;168(12):6128–6135. doi: 10.4049/jimmunol.168.12.6128. [DOI] [PubMed] [Google Scholar]
- 39.Bao J.-Y., Huang Y., Wang F., Peng Y.-P., Qiu Y.-H. Expression of α-AR Subtypes in T Lymphocytes and Role of the α-ARs in Mediating Modulation of T Cell Function. NeuroImmunoModulation. 2007;14(6):344–353. doi: 10.1159/000129670. [DOI] [PubMed] [Google Scholar]
- 40.Konig MF, Powell M, Staedtke V, Bai R-Y, Thomas DL, Fischer N, et al. Preventing cytokine storm syndrome in COVID-19 using α-1 adrenergic receptor antagonists. The Journal of Clinical Investigation. 2020;130(7):3345-7. [DOI] [PMC free article] [PubMed]
- 41.Atlas D. Voltage-gated calcium channels function as Ca2+-activated signaling receptors. Trends Biochem Sci. 2014;39(2):45–52. doi: 10.1016/j.tibs.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 42.Chen X, Cao R, Zhong W. Host Calcium Channels and Pumps in Viral Infections. Cells 2020, Vol 9, Page 94. 2019;9(1):94-. [DOI] [PMC free article] [PubMed]
- 43.Straus M.R., Tang T., Lai A.L., Flegel A., Bidon M., Freed J.H., et al. Ca 2+ Ions Promote Fusion of Middle East Respiratory Syndrome Coronavirus with Host Cells and Increase Infectivity. J Virol. 2020;94(13) doi: 10.1128/JVI.00426-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Casciano JC, Duchemin NJ, Lamontagne RJ, Steel LF, Bouchard MJ. Hepatitis B virus modulates store-operated calcium entry to enhance viral replication in primary hepatocytes. PLOS ONE. 2017;12(2):e0168328-e. [DOI] [PMC free article] [PubMed]
- 45.Sanchis-Gomar F., Lavie C.J., Perez-Quilis C., Henry B.M., Lippi G. Angiotensin-Converting Enzyme 2 and Antihypertensives (Angiotensin Receptor Blockers and Angiotensin-Converting Enzyme Inhibitors) in Coronavirus Disease 2019. Mayo Clin Proc. 2020;95(6):1222–1230. doi: 10.1016/j.mayocp.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sriram K., Insel P.A. Risks of ACE Inhibitor and ARB Usage in COVID-19: Evaluating the Evidence. Clin Pharmacol Ther. 2020;108(2):236–241. doi: 10.1002/cpt.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Caldeira D., Alves M., Gouveia e Melo R., Silvério António P., Cunha N., Nunes-Ferreira A., et al. Angiotensin-converting enzyme inhibitors and angiotensin-receptor blockers and the risk of COVID-19 infection or severe disease: Systematic review and meta-analysis. IJC Heart & Vasculature. 2020;31:100627. doi: 10.1016/j.ijcha.2020.100627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Flacco M.E., Acuti Martellucci C., Bravi F., Parruti G., Cappadona R., Mascitelli A., et al. Treatment with ACE inhibitors or ARBs and risk of severe/lethal COVID-19: a meta-analysis. Heart. 2020;106(19):1519–1524. doi: 10.1136/heartjnl-2020-317336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hakeam H.A., Alsemari M., Duhailib Z.A., Ghonem L., Alharbi S.A., Almutairy E., et al. Association of angiotensin-converting enzyme inhibitors and angiotensin II blockers with severity of COVID-19: a multicenter, prospective study. Cardiovasc Pharmacol Ther. 2021;26(3):244–252. doi: 10.1177/1074248420976279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Y., Chen B., Li Y., Zhang L., Wang Y., Yang S., et al. The use of renin–angiotensin–aldosterone system (RAAS) inhibitors is associated with a lower risk of mortality in hypertensive COVID-19 patients: A systematic review and meta-analysis. J Med Virol. 2021;93(3):1370–1377. doi: 10.1002/jmv.26625. [DOI] [PubMed] [Google Scholar]
- 51.Hippisley-Cox J., Young D., Coupland C., Channon K.M., Tan P.S., Harrison D.A., et al. Risk of severe COVID-19 disease with ACE inhibitors and angiotensin receptor blockers: cohort study including 8.3 million people Special populations. Heart. 2020;106(19):1503–1511. doi: 10.1136/heartjnl-2020-317393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Solaimanzadeh I. Nifedipine and Amlodipine Are Associated With Improved Mortality and Decreased Risk for Intubation and Mechanical Ventilation in Elderly Patients Hospitalized for COVID-19. Cureus. 2020;12(5) doi: 10.7759/cureus.8069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alsagaff M.Y., Mulia E.P.B., Maghfirah I., Luke K., Nugraha D., Rachmi D.A., et al. Association of calcium channel blocker use with clinical outcome of COVID-19: A meta-analysis. Diabetes Metab Syndr: Clin. Res Rev. 2021;15(5):102210. doi: 10.1016/j.dsx.2021.102210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ren L.u., Yu S., Xu W., Overton J.L., Chiamvimonvat N., Thai P.N. Lack of association of antihypertensive drugs with the risk and severity of COVID-19: A meta-analysis. J Cardiol. 2021;77(5):482–491. doi: 10.1016/j.jjcc.2020.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gao C, Cai Y, Zhang K, Zhou L, Zhang Y, Zhang X, et al. Association of hypertension and antihypertensive treatment with COVID-19 mortality: a retrospective observational study. European Heart Journal. 2020;41(22):2058-66. [DOI] [PMC free article] [PubMed]
- 56.Cariou B, Hadjadj S, Wargny M, Pichelin M, Al-Salameh A, Allix I, et al. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: the CORONADO study. Diabetologia 2020 63:8. 2020;63(8):1500-15. [DOI] [PMC free article] [PubMed]
- 57.Koenecke A, Powell M, Xiong R, Shen Z, Fischer N, Huq S, et al. Alpha-1 adrenergic receptor antagonists to prevent hyperinflammation and death from lower respiratory tract infection. eLife. 2021;10. [DOI] [PMC free article] [PubMed]
- 58.Li S, Jun T, Wang Z, Kao Y-H, Schadt E, Konig MF, et al. COVID-19 outcomes among hospitalized men with or without exposure to alpha-1-adrenergic receptor blocking agents. medRxiv. 2021:2021.04.08.21255148-2021.04.08.
- 59.Baden LR, Sahly HME, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. https://doiorg/101056/NEJMoa2035389. 2020;384(5):403-16. [DOI] [PMC free article] [PubMed]
- 60.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B, et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. https://doiorg/101056/NEJMoa2101544. 2021;384(23):2187-201. [DOI] [PMC free article] [PubMed]
- 62.Dagan N., Barda N., Kepten E., Miron O., Perchik S., Katz M.A., et al. BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. N Engl J Med. 2021;384(15):1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bernal J.L., Andrews N., Gower C., Robertson C., Stowe J., Tessier E., et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. 2021;373 doi: 10.1136/bmj.n1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Watanabe M, Balena A, Tuccinardi D, Tozzi R, Risi R, Masi D, et al. Central obesity, smoking habit, and hypertension are associated with lower antibody titres in response to COVID-19 mRNA vaccine. Diabetes Metab Res Rev. 2021:e3465. [DOI] [PMC free article] [PubMed]
- 65.Logunov D.Y., Dolzhikova I.V., Shcheblyakov D.V., Tukhvatulin A.I., Zubkova O.V., Dzharullaeva A.S., et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. The Lancet. 2021;397(10275):671–681. doi: 10.1016/S0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Evidence Assessment: Sinopharm/BBIBP COVID-19 vaccine FOR RECOMMENDATION BY THE STRATEGIC ADVISORY GROUP OF EXPERTS (SAGE) ON IMMUNIZATION Prepared by the SAGE Working Group on COVID-19 vaccines 2 EVIDENCE ASSESSMENT: BBIBP-CorV Key evidence to inform policy recommendations on the use of BBIBP-CorV.
- 67.Ella R, Reddy S, Blackwelder W, Potdar V, Yadav P, Sarangi V, et al. Efficacy, safety, and lot to lot immunogenicity of an inactivated SARS-CoV-2 vaccine (BBV152): a, double-blind, randomised, controlled phase 3 trial. medRxiv. 2021:2021.06.30.21259439-2021.06.30.
- 68.Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. The Lancet. 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Agrawal B. Heterologous Immunity: Role in Natural and Vaccine-Induced Resistance to Infections. Front Immunol. 2019;10:2631. doi: 10.3389/fimmu.2019.02631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grifoni A., Weiskopf D., Ramirez S.I., Mateus J., Dan J.M., Moderbacher C.R., et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell. 2020;181(7):1489–1501.e15. doi: 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reche P.A. Potential cross-reactive immunity to SARS-CoV-2 from common human pathogens and vaccines. Front Immunol. 2020;11:586984. doi: 10.3389/fimmu.2020.586984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mysore V., Cullere X., Settles M.L., Ji X., Kattan M.W., Desjardins M., et al. Protective heterologous T cell immunity in COVID-19 induced by the trivalent MMR and Tdap vaccine antigens. Med (N Y). 2021;2(9):1050–1071.e7. doi: 10.1016/j.medj.2021.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jordan S.C. Innate and adaptive immune responses to SARS-CoV-2 in humans: relevance to acquired immunity and vaccine responses. Clin Exp Immunol. 2021;204(3):310–320. doi: 10.1111/cei.13582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lauring A.S., Malani P.N. Variants of SARS-CoV-2. JAMA. 2021;326(9):880. doi: 10.1001/jama.2021.14181. [DOI] [PubMed] [Google Scholar]
- 75.Rio Cd, Malani PN, Omer SB. Confronting the Delta Variant of SARS-CoV-2, Summer 2021. JAMA. 2021. [DOI] [PubMed]
- 76.Baraniuk C. Covid-19: How effective are vaccines against the delta variant? BMJ. 2021;374 doi: 10.1136/bmj.n1960. n1960-n. [DOI] [PubMed] [Google Scholar]
- 77.Havers FP, Pham H, Taylor CA, Whitaker M, Patel K, Anglin O, et al. COVID-19-associated hospitalizations among vaccinated and unvaccinated adults ≥18 years – COVID-NET, 13 states, January 1 – July 24, 2021. medRxiv. 2021:2021.08.27.21262356-2021.08.27. [DOI] [PMC free article] [PubMed]
- 78.Kumar VJ, Sowpati DT, Munigela A, Banu S, Siva AB, Sasikala M, et al. Clinical outcomes in vaccinated individuals hospitalized with Delta variant of SARS-CoV-2. medRxiv. 2021:2021.07.13.21260417-2021.07.13.
- 79.Gadjah Mada U, Hospital Mohamad Saifudin Hakim Universitas Gadjah Mada Hendra Wibawa S, El Khair Universitas Gadjah Mada R, Hospital Kristy Iskandar S, Balai Besar Teknik Kesehatan Lingkungan dan Pengendalian Penyakit Nungki Anggorowati Universitas Gadjah Mada I, Hospital Edwin Widyanto Daniwijaya S, et al. The Impact of SARS-CoV-2 Multiple Spike Protein Mutations on COVID-19 Outcomes. 2021.
- 80.CDC. Risk for COVID-19 Infection, Hospitalization, and Death By Race/Ethnicity https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-race-ethnicity.html (accessed August 27, 2021) 2021.
- 81.Brancati F.L., Kao W.H., Folsom A.R., Watson R.L., Szklo M. Incident type 2 diabetes mellitus in African American and white adults: the Atherosclerosis Risk in Communities Study. JAMA. 2000;283(17):2253–2259. doi: 10.1001/jama.283.17.2253. [DOI] [PubMed] [Google Scholar]
- 82.Petersen R., Pan L., Blanck H.M. Racial and Ethnic Disparities in Adult Obesity in the United States: CDC's Tracking to Inform State and Local Action. Prev Chronic Dis. 2019;16:E46. doi: 10.5888/pcd16.180579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hye Jin Rho HB, Shawn Fremstad. A Basic Demographic Profile of Workers in Frontline Industries https://cepr.net/a-basic-demographic-profile-of-workers-in-frontline-industries/ (accessed on September 16th, 2021) 2020.
- 84.CDC. COVID-19 Vaccine Equity for Racial and Ethnic Minority Groups n.d. https://www.cdc.gov/coronavirus/2019-ncov/community/health-equity/vaccine-equity.html#13 (accessed August 27, 2021). 2021.
- 85.Omboni S., McManus R.J., Bosworth H.B., Chappell L.C., Green B.B., Kario K., et al. Evidence and Recommendations on the Use of Telemedicine for the Management of Arterial Hypertension: An International Expert Position Paper. Hypertension. 2020;76(5):1368–1383. doi: 10.1161/HYPERTENSIONAHA.120.15873. [DOI] [PubMed] [Google Scholar]
- 86.Bansal R, Gubbi S, Muniyappa R. Metabolic syndrome and COVID 19: endocrine-immune-vascular interactions shapes clinical course. Endocrinology. 2020;161(10):bqaa112. [DOI] [PMC free article] [PubMed]
- 87.Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L., et al. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Braz-de-Melo H.A., Faria S.S., Pasquarelli-do-Nascimento G., Santos I.O., Kobinger G.P., Magalhães K.G. The Use of the Anticoagulant Heparin and Corticosteroid Dexamethasone as Prominent Treatments for COVID-19. Front Med (Lausanne). 2021;8 doi: 10.3389/fmed.2021.615333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Goodwin J.E., Geller D.S. Glucocorticoid-induced hypertension. Pediatr Nephrol. 2012;27(7):1059–1066. doi: 10.1007/s00467-011-1928-4. [DOI] [PubMed] [Google Scholar]
- 90.Vindrola-Padros C., Sidhu M.S., Georghiou T., Sherlaw-Johnson C., Singh K.E., Tomini S.M., et al. The implementation of remote home monitoring models during the COVID-19 pandemic in England. EClinicalMedicine. 2021;34:100799. doi: 10.1016/j.eclinm.2021.100799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.NHS. Pulse oximetry to detect early deterioration of patients with COVID-19 in primary and community care settings https://www.england.nhs.uk/coronavirus/publication/pulse-oximetry-to-detect-early-deterioration-of-patients-with-covid-19-in-primary-and-community-care-settings/ (accessed November 17th, 2021) 2020 [.