Abstract
Objective:
Cardiorespiratory fitness (as measured by peak oxygen consumption [VO2peak]) is an independent predictor of cardiovascular disease and all-cause mortality. Limited data exist on VO2peak following repair for an acute type A aortic dissection (ATAAD) or proximal thoracic aortic aneurysm (pTAA). This study prospectively evaluated VO2peak, functional capacity, and health-related quality of life (HR-QOL) following open repair.
Methods:
Participants with a history of an ATAAD (n=21) or pTAA (n=43) performed cardiopulmonary exercise testing (CPX), six-minute walk testing, and HR-QOL at 3 (early) and 15 (late) months following open repair.
Results:
The median age at time of surgery was 55-years-old and 60-years-old in the ATAAD and pTAA groups, respectively. Body mass index significantly increased between early and late timepoints for both ATAAD (p=0.0245, 56% obese) and pTAA groups (p=0.0045, 54% obese). VO2peak modestly increased by 0.8 mLO2·kg−1·min−1 within the ATAAD group (P=0.2312) while VO2peak significantly increased by 2.2 mLO2·kg−1·min−1 within the pTAA group (P=0.0003). Anxiety significantly decreased in the ATAAD group whereas functional capacity and HR-QOL metrics (social roles and activities, physical function) significantly improved in the pTAA group (p values<0.05). There were no serious adverse events during CPX.
Conclusion:
Cardiorespiratory fitness among the ATAAD group remained 36% below predicted normative values >1 year after repair. CPX should be considered post-operatively to evaluate exercise tolerance and blood pressure response to determine whether mild-to-moderate aerobic exercise should be recommended to reduce future risk of morbidity and mortality.
Keywords: exercise testing, aortic dissection, cardiorespiratory fitness, thoracic aortic aneurysm
Graphical Abstract
INTRODUCTION
Cardiac rehabilitation is a cornerstone of postoperative care for most cardiac surgery populations as there is long-standing evidence to support its efficacy at lowering cardiovascular-related mortality through improvements in cardiorespiratory fitness, reductions in hospitalizations, and improvements in quality of life1. In chronic cardiac conditions, cardiorespiratory fitness is considered a clinical vital sign with assessment recommended across all phases of clinical decision making2,3. At present, cardiorespiratory fitness is not clinically evaluated following open aortic repair for thoracic aortic disease, especially acute type A aortic dissection (ATAAD)4. The clinical importance of evaluating cardiorespiratory fitness in this setting is warranted as preliminary data from cross-sectional studies highlight that cardiorespiratory fitness (as measured by peak oxygen consumption, VO2peak) is 34–40% below normative values5,6.
This study prospectively evaluated cardiorespiratory fitness and health-related quality of life outcomes after open proximal aortic repair for an ATAAD or proximal thoracic aortic aneurysm (pTAA) at two postoperative timepoints (3- and 15-months) that align with clinical follow-up. We hypothesized that cardiorespiratory fitness would remain impaired greater than a year after repair, especially among ATAAD, given scant guidance on the need to evaluate cardiorespiratory fitness and on the value of implementing exercise training in this setting.
METHODS
Trial Design and Study Population
Eligible participants underwent open proximal aortic repair through sternotomy for an emergent ATAAD or elective pTAA. Society of Thoracic Surgery data elements from the University of Michigan Department of Cardiac Surgery Data Warehouse were utilized to determine pre-, intra-, and post-operative (defined as in-hospital postoperative events) outcomes as previously reported 7. ATAAD and pTAA participants were evaluated at approximately 3 months (early timepoint) and 15 months (late timepoint) following open repair between March 2016 and July 2018 at the University of Michigan, Michigan Medicine (Supplemental Figure 1). Early and late timepoints were selected as they align with clinical guidelines for follow-up8. Eligible participants were >18 years of age, cleared by their aortic surgeon to perform cardiopulmonary exercise testing (CPX), and without a known syndromic diagnosis or underlying genetic predisposition (this phenotype confers an increased risk of dissection)9. All study procedures were reviewed and approved by the Michigan Medicine institutional review board (IRBMED, HUM00110749) and all participants provided written informed consent. Data supporting the findings of this study are available from the corresponding authors upon reasonable request.
The primary endpoint was change in VO2peak which was evaluated using an electronic/motorized treadmill test performed by two advanced cardiovascular life support and American College of Sports Medicine–certified clinical exercise physiologists under the supervision of the interpreting cardiologist10. Expired gases were analyzed continuously by an Ultima CPX metabolic stress test system (MCG Diagnostics). After stable resting values had been achieved, participants were tested according to the Cornell protocol until patient request to stop due to fatigue, maximal effort, or clinical decision to terminate10. The Cornell protocol increases the treadmill speed and grade every two minutes with standardized metabolic equivalent increases per stage. VO2peak was defined as the greatest VO2 value for a given 30-sec interval; anaerobic threshold was calculated using standard methods11. Percent-predicted VO2peak was calculated according to the Fitness Registry and the Importance of Exercise National Database (FRIEND) normative values for VO2 (referred to as normative values from this point onward)12. A peak CPX was defined as a respiratory exchange ratio (RER) ≥1.05 5,13–15. Heart rate (HR) response was evaluated utilizing continuous 12-lead Mac 5000 ECG Monitoring system (GE Healthcare) and blood pressure response was measured manually by an auscultatory sphygmomanometer every two minutes during CPX procedures. Blood pressure termination criterion during CPX included a decrease in systolic pressure > 20mm Hg16 or an increase ≥180 mm Hg systolic or ≥90 mm Hg diastolic pressure4. It is possible that blood pressure may have exceeded the upper-limit thresholds because of the time interval between blood pressure measurements. EKG termination criterion during CPX included ischemic EKG changes, complex ectopy, or second or third degree heart blocks16. Participants were not asked to discontinue medications prior to CPX. The criterion utilized to determine an abnormal HR recovery was ≤12 bpm (max HR – 1-min HR recovery) following a walking protocol17. Exercise breathing reserve indicates how closely ventilation approaches maximal ventilatory volume during exercise, and is calculated (1-[peak ventilation/maximal ventilatory volume])16. Echocardiography and computed tomography angiography (CTA) outcomes including left ventricular ejection fraction (LVEF), presence of moderate-to-severe aortic valve insufficiency, aortic diameters, and evidence of residual aneurysmal disease or distal dissection, particularly for ATAAD participants, were assessed18.
Secondary endpoints were safety, functional capacity, and patient-reported outcomes (PROs). Safety was evaluated by the type and prevalence of serious (e.g., requiring hospitalization, life-threatening) and non-serious (e.g., musculoskeletal pain) adverse events during CPX5. Functional capacity was assessed by the six-minute walk test and measured by the six-minute walk distance (6MWD)19. Health-related quality of life (Patient Reported Outcomes Measurement Information System, PROMIS)20, anxiety (Generalized Anxiety Disorder 7-Item, GAD-7 Scale)21, pain (20-item Brief Pain Inventory)22, sleep quality (Pittsburgh Sleep Inventory)23, and physical activity (Godin-Leisure Time Exercise Questionnaire)24 were also evaluated.
Statistical Analysis
The analysis provided descriptive information on demographic, clinical, and surgical outcomes in the overall population and amongst the ATAAD and pTAA groups. Results are presented as median (interquartile range (IQR): 25% and 75%) for continuous data and n (%) for categorical data. Means were utilized for percent of predicted and percent change. For repeated measurements on surgical outcomes, paired Wilcoxon signed-rank tests and McNemar’s test were conducted to assess the difference for continuous and categorical variables, respectively, between early and late timepoints. The difference in anxiety level (ordinal variable) over time was evaluated by cumulative link mixed effects model with patient ID as random effect. Additionally, group by time interactions were evaluated by linear mixed model with group, timepoint, and group by timepoint as fixed effects and participant ID as random effect. Longitudinal analysis was performed for participants with complete data. Correlations between health-related quality of life measurements and the change in VO2peak and 6MWD over time were evaluated using both Pearson correlation and Spearman correlation tests. All statistical calculations used SAS 9.4 (SAS Institute, Cary, NC) and R 3.4.2. Statistical significance was considered at p<0.05.
RESULTS
The analytic cohort consisted of 64 participants that underwent open proximal aortic repair through sternotomy for an emergent ATAAD (n=21) and elective pTAA (n=43). Median time from open repair to the postoperative CPX was 3.4 months (3.1, 3.6) for the ATAAD group and 3.2 months (3.0, 3.5) for the pTAA group. The postoperative CPX occurred a median of 15.9 months (13.0, 17.3) for the ATAAD group and 15.2 months (12.6, 15.7) for pTAA group following open repair. Fifty-one participants completed a CPX at both timepoints (ATAAD, n=16 and pTAA, n=35), with 13 participants lost to follow-up due to death (one death per group, n=2), contraindications to CPX at time of follow-up (e.g., hip arthroplasty, stroke, vertigo, type B intramural hematoma, n=4), and inability to re-contact (n=7). One participant in the ATAAD group and two participants in the pTAA group participated in cardiac rehabilitation between study timepoints.
Study Outcomes for ATAAD
Clinical Characteristics and Operative Outcomes
The ATAAD group was predominately male (86%) with a median age of 55 years. At the time of open repair, 24% of participants had moderate-to-severe aortic insufficiency and 14% had moderate-to-severe aortic stenosis. Regarding cardiovascular disease risk, 81% of participants had hypertension, 43% had peripheral vascular disease, and 9.5% had previous cardiac surgery (Table 1). Thirty-eight percent underwent aortic root replacement and 29% underwent a zone 1/2/3 arch replacement. The median cardiopulmonary bypass time was 216 minutes and hypothermic circulatory arrest was utilized in 100% of participants with predominantly (86%) antegrade cerebral perfusion. Nine and half percent of participants suffered a postoperative stroke and 9.5% had renal failure requiring dialysis; the median postoperative length of stay was nine days (Table 2).
Table 1.
Preoperative Characteristics and Comorbidities
| All Participants n=64 | ATAAD n=21 | pTAA n=43 | |
|---|---|---|---|
|
| |||
| Age (years) | 57 (49, 64) | 55 (47, 59) | 60 (53, 65) |
| Sex, male | 55 (86) | 18 (86) | 37 (86) |
| Body mass index (kg/m2) | 29.9 (26.6, 33.8) | 28.7 (26.6, 32.6) | 30.6 (26.6, 35.2) |
| Aortic valve indications | |||
| Aortic insufficiency, mod-to-severe | 24 (41) | 4 (24) | 20 (48) |
| Aortic stenosis, mod-to-severe | 13 (25) | 2 (14) | 11 (28) |
| Bicuspid aortic valve | 23 (36) | 1 (4.8) | 22 (51) |
| Thoracic aortic aneurysm | |||
| Root | 26 (41) | 7 (33) | 19 (44) |
| Ascending | 43 (67) | 8 (38) | 35 (81) |
| Arch | 16 (25) | 4 (19) | 12 (28) |
| Descending | 1 (16) | 0 (0) | 1 (2.3) |
| Max diameter (cm) | 5.3 (4.7, 5.6) | 5.1 (4.5, 5.5) | 5.3 (4.7, 5.7) |
| Risk Factors | |||
| Hypertension | 50 (78) | 17 (81) | 33 (77) |
| Dyslipidemia | 39 (61) | 7 (33) | 32 (74) |
| Peripheral vascular disease | 13 (20) | 9 (43) | 4 (9.3) |
| Smoking history, former/current | 35 (55) | 12 (57) | 23 (53) |
| Diabetes | 10 (16) | 1 (4.8) | 9 (21) |
| Chronic lung disease | 6 (9.4) | 0 (0) | 6 (14) |
| Chronic kidney disease | 4 (6.2) | 2 (9.5) | 2 (4.6) |
| On dialysis | 2 (3.1) | 1 (4.8) | 1 (2.3) |
| Coronary artery disease | 20 (31) | 3 (14) | 17 (40) |
| History of myocardial infarction | 6 (9.4) | 0 (0) | 6 (14) |
| History of stroke | 3 (4.7) | 0 (0) | 3 (7.0) |
| History of heart failure | 8 (12) | 1 (4.8) | 7 (16) |
| Heart failure within 2 weeks of operation | 2 (3.1) | 0 (0) | 2 (4.7) |
| NYHA classification | |||
| I | 41 (64) | 18 (86) | 23 (53) |
| II | 17 (27) | 2 (9.5) | 15 (35) |
| III | 5 (7.8) | 1 (4.8) | 4 (9.3) |
| IV | 1 (1.6) | 0 (0) | 1 (2.3) |
| Previous cardiac intervention | 14 (22) | 2 (9.5) | 12 (28) |
| Previous cardiac surgery | 10 (16) | 2 (9.5) | 8 (19) |
Data presented as median (25%, 75%) for continuous data and n (%) for categorical data.
Abbreviations: ATAAD=acute type A aortic dissection; NYHA=New York heart association; pTAA=thoracic aortic aneurysm.
Table 2.
Intraoperative Details and Postoperative Outcomes
| All Participants n=64 | ATAAD n=21 | pTAA n=43 | |
|---|---|---|---|
|
| |||
| Intraoperative | |||
| Valve/Root Procedure | |||
| Aortic valve replacement | 13 (20) | 0 (0) | 13 (30) |
| Root replacement | 30 (47) | 8 (38) | 22 (51) |
| Aortic valve/root repair | 17 (27) | 11 (52) | 6 (14) |
| Ascending replacement | 60 (94) | 21 (100) | 39 (91) |
| Arch Procedure | |||
| Hemiarch replacement | 24 (37) | 14 (67) | 10 (23) |
| Zone 1/2/3 replacement | 13 (20) | 6 (29) | 7 (16) |
| Concomitant surgery | |||
| CABG | 5 (7.8) | 1 (4.8) | 4 (9.3) |
| Mitral valve | 2 (3.1) | 0 (0) | 2 (4.7) |
| Tricuspid valve | 0 (0) | 0 (0) | 0 (0) |
| CPB time, minutes | 212 (172, 256) | 216 (188, 267) | 212 (166, 250) |
| Cross-clamp time, minutes | 153 (123, 186) | 156 (109, 191) | 152 (128, 186) |
| Hypothermic circulatory arrest | 37 (58) | 21 (100) | 16 (37) |
| HCA time, minutes | 27 (21, 35) | 28 (21, 41) | 26 (19, 34) |
| Cerebral Perfusion | |||
| None | 27 (42) | 0 (0) | 27 (63) |
| Antegrade | 22 (34) | 18 (86) | 4 (9.3) |
| Retrograde | 8 (12) | 1 (4.8) | 7 (16) |
| Both | 7 (11) | 2 (9.5) | 5 (12) |
|
| |||
| Postoperative | |||
| Reoperation for bleeding | 1 (1.6) | 1 (4.8) | 0 (0) |
| Myocardial infarction | 0 (0) | 0 (0) | 0 (0) |
| Cerebrovascular accident | 2 (3.1) | 2 (9.5) | 0 (0) |
| TIA | 0 (0) | 0 (0) | 0 (0) |
| Atrial fibrillation | 18 (28) | 4 (19) | 14 (33) |
| Pneumonia | 2 (3.1) | 2 (9.5) | 0 (0) |
| New-onset renal failure | 4 (6.2) | 2 (9.5) | 2 (4.6) |
| Requiring dialysis | 2 (3.1) | 2 (9.5) | 0 (0) |
| On dialysis at discharge | 1 (1.6) | 1 (4.8) | 0 (0) |
| Deep sternal wound infection | 0 (0) | 0 (0) | 0 (0) |
| Sepsis | 0 (0) | 0 (0) | 0 (0) |
| Gastrointestinal complications | 0 (0) | 0 (0) | 0 (0) |
| Hours ventilated | 8.4 (5.6, 13.1) | 11.6 (7.2, 26.6) | 7.6 (5.0, 12.6) |
| Prolonged ventilation, >24 hrs | 8 (12) | 6 (29) | 2 (4.6) |
| Hours intubated | 17.3 (14.3, 23.0) | 18.6 (16.4, 34) | 16.5 (12.5, 20.6) |
| Reintubation | 0 (0) | 0 (0) | 0 (0) |
| Postoperative length of stay (days) | 6 (5, 9) | 9 (6, 11) | 6 (5, 8) |
| Total length of stay (days) | 7 (5, 11) | 11 (7, 14) | 6 (5, 9) |
Data presented as median (25%, 75%) for continuous data and n (%) for categorical data.
Abbreviations: ATAAD=acute type A aortic dissection; CABG=coronary artery bypass graft; CPB=cardiopulmonary bypass; HCA=hypothermic circulatory arrest; pTAA=thoracic aortic aneurysm; TIA=transient ischemic attack.
Zone 1 arch replacement=the arch is divided between the innominate and LCC arteries with replacement of the innominate artery to its bifurcation; Zone 2 arch replacement=the arch is divided between the LCC and left subclavian arteries with replacement of the innominate and LCC arteries; and Zone 3 arch replacement (total arch replacement)=the arch is divided distal to the left subclavian artery with replacement of all arch branch vessels.
Cardiorespiratory, Hemodynamics, and Functional Capacity
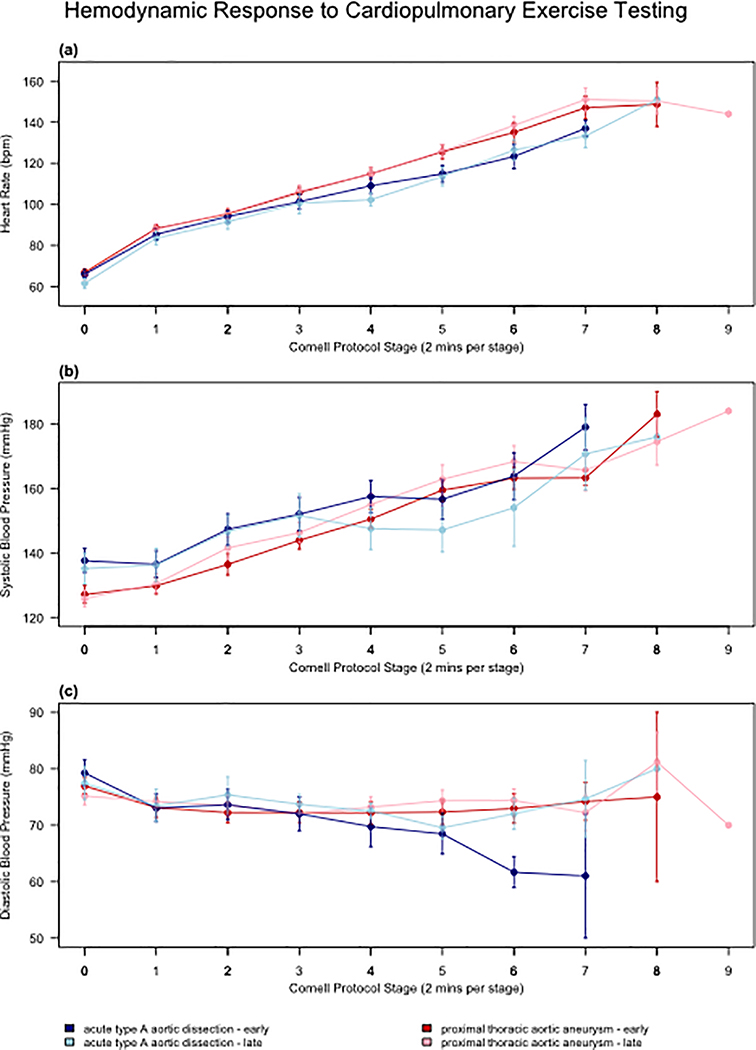
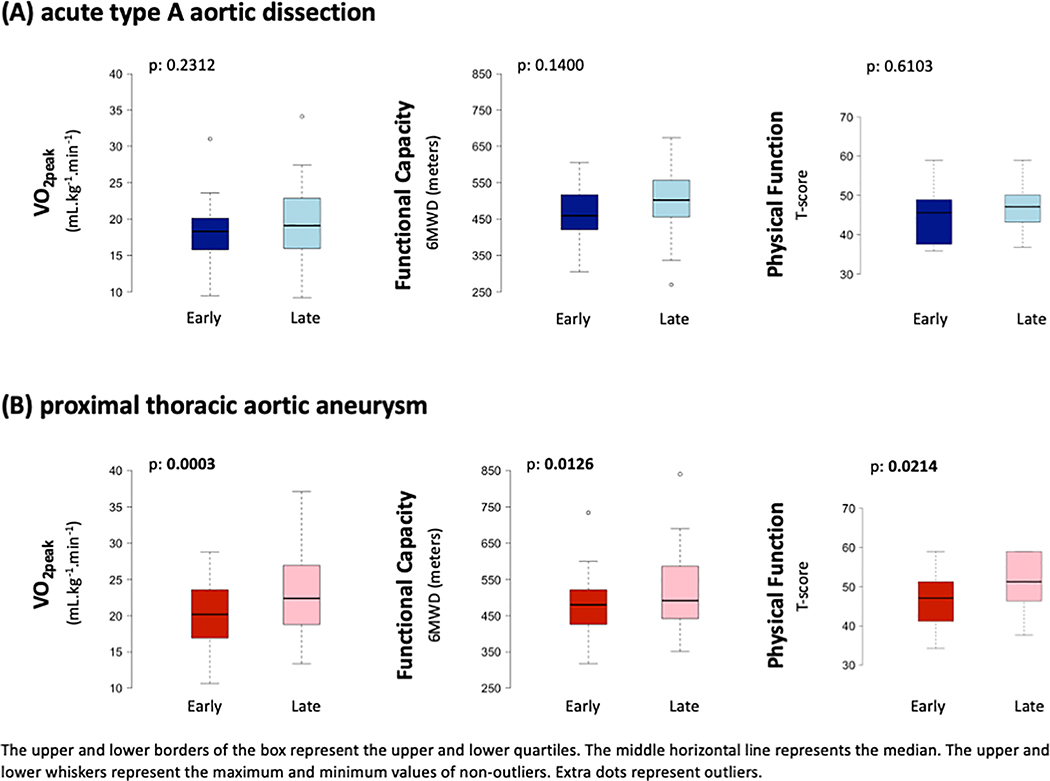
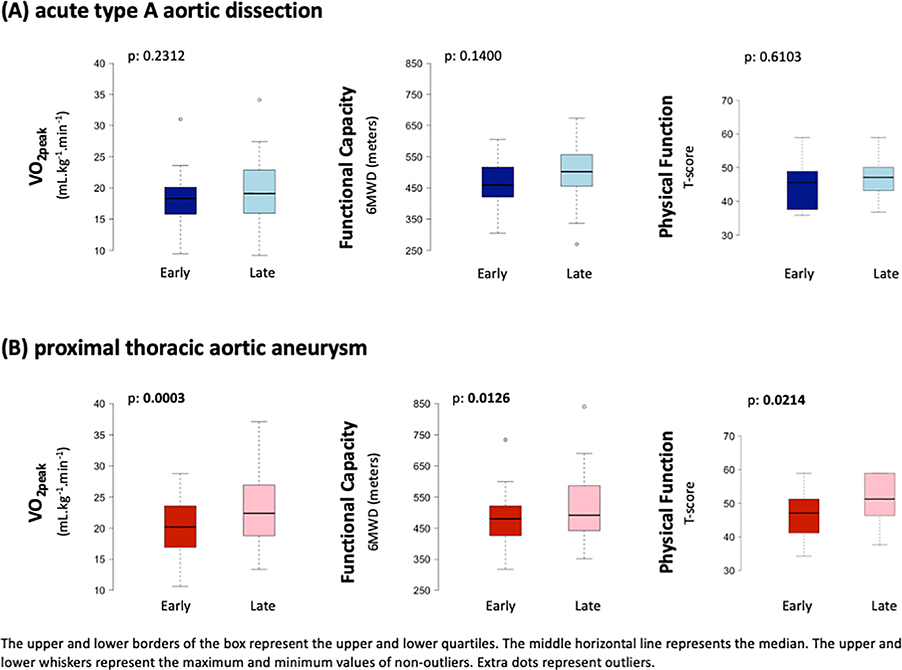
Body mass index significantly increased between early and late timepoints (28.7 vs 30.6 kg·m−2, p=0.0245), with 56% of participants classified as obese at the late timepoint. There was no change in median VO2peak between early and late timepoints (18.3 to 19.1 mLO2·kg−1·min−1, p=0.2312). The average VO2peak was 43% and 36% below age- and sex-predicted normative values at the early and late timepoints, respectively. There were no other significant changes in cardiorespiratory outcomes over time. The median RER was 1.1 with 62% (early) and 75% (late) of CPX studies meeting “peak” criteria as defined by a RER≥1.05. There were no significant differences over time for peak HR, systolic blood pressure, or diastolic blood pressure (Figure 1). The 1-minute HR recovery did not improve between the early and late timepoints (13 vs 14 bpm, p=0.11). There was no change in median 6MWD between the early and late timepoints (459 vs 502 m, p=0.14), with 80% of participants achieving age- and sex-predicted distance (Table 3).
Figure 1: Heart rate and blood pressure responses to cardiopulmonary exercise testing.
Heart rate and blood pressure responses to cardiopulmonary exercise testing for ATAAD and pTAA groups from Timepoints Early and Late. ATAAD=acute type A aortic dissection; DBP=diastolic blood pressure; pTAA=proximal thoracic aortic aneurysm; SBP=systolic blood pressure.
Table 3.
Cardiopulmonary Exercise Test Outcomes Among Groups
| ATAAD (N=21) | pTAA (N=43) | Group by Time Interaction | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Variables | Early | Late | p-value* | Early | Late | p-value* | p-value† |
| Resting Outcomes | |||||||
| BMI, kg·m−2 | 28.7 (26.1, 30.1) | 30.6 (27.4, 32.9) | 0.0245 | 30.4 (26.6, 34.5) | 31.2 (27.7, 37.7) | 0.0045 | 0.3131 |
| Anaerobic Threshold Outcomes | |||||||
| VO2, mL.kg−1.min−1 | 12.4 (10.9, 15.4) | 13.9 (12.4, 15.4) | 0.1025 | 13.5 (12.6, 15) | 14.4 (13, 15.8) | 0.0132 | 0.8021 |
| RER | 0.9 (0.8, 0.9) | 0.9 (0.8, 0.9) | 0.0958 | 0.9 (0.8, 0.9) | 0.9 (0.8, 1) | 0.3034 | 0.5697 |
| VE/VCO2 slope | 28 (25.5, 33.5) | 27.8 (24.2, 30) | 0.2338 | 28.5 (25, 30) | 28 (25.2, 30) | 0.4758 | 0.2990 |
| Peak Outcomes | |||||||
| VO2peak, mL.kg−1.min−1 | 18.3 (15.8, 20.1) | 19.1 (16.8, 22.8) | 0.2312 | 20.2 (17, 23.5) | 22.4 (18.8, 26.9) | 0.0003 | 0.1405 |
| VO2, mL.min−1 | 1638 (1281, 2070) | 1849.5 (1539.8, 2235.2) | 0.0833 | 1924 (1498.8, 2221) | 2120.5 (1926.5, 2683.5) | 0.0001 | 0.1489 |
| RER | 1.1 (1, 1.1) | 1.1 (1.1, 1.2) | 0.6908 | 1.2 (1.1, 1.2) | 1.2 (1.1, 1.3) | 0.3958 | 0.4220 |
| VEpeak | 52.7 (36.6, 58.6) | 55 (43.2, 66.5) | 0.8603 | 66.5 (51.5, 82.8) | 73.9 (63.7, 86.2) | 0.0162 | 0.2144 |
| VE/ VCO2 slope | 24.7 (22.8, 29.6) | 24.4 (22.7, 27) | 0.0744 | 27.2 (23.6, 30.5) | 25.9 (24, 28.7) | 0.2944 | 0.5655 |
| Predicted VO2peak-FRIEND | 33.84 (27.71, 36.04) | 31.86 (26.35, 33.90) | 0.0213 | 28.91 (24.22, 31.51) | 27.96 (23.80, 31.74) | 0.0052 | 0.3900 |
| % predicted, VO2peak-FRIEND | 0.60 (0.49, 0.65) | 0.68 (0.57, 0.74) | 0.0788 | 0.73 (0.66, 0.82) | 0.81 (0.70, 0.96) | 0.0004 | 0.4758 |
| METs | 5.2 (4.5, 5.9) | 6 (4.9, 8.4) | 0.1705 | 5.8 (4.9, 7) | 6.4 (5.5, 8) | 0.0128 | 0.7647 |
| RPE | 15 (13, 16) | 14 (12, 16) | 0.7500 | 16 (15, 17) | 15.5 (15, 17) | 0.7424 | 0.6377 |
| PRP | 183.8 (162, 244) | 188.7 (156.3, 250.2) | 0.9799 | 228.2 (173.9, 258.5) | 236.5 (201.2, 278.5) | 0.5043 | 0.2423 |
| CPX Time (min:sec) | 545 (461, 684) | 615 (450, 723.5) | 0.7173 | 643 (590, 790.8) | 720 (600, 836) | 0.0720 | 0.2775 |
| Heart Rate, bpm | 115 (107, 133) | 121.5 (104.8, 133) | 0.7331 | 131 (118, 150) | 137.5 (124.5, 159) | 0.2561 | 0.2788 |
| SBP, mmHg | 170 (148, 182) | 167 (147.5, 190) | 0.4496 | 164 (158, 173) | 167 (156, 185.5) | 0.8530 | 0.7902 |
| DBP, mmHg | 68 (62, 80) | 75 (69.5, 80) | 0.1658 | 70 (63, 80) | 70 (68, 80) | 0.8288 | 0.1561 |
| Recovery Outcomes | |||||||
| 1 min-HR, bpm | 13 (7, 18) | 14 (10.8, 19.2) | 0.1108 | 11 (7, 15) | 15.5 (11.2, 20.8) | 0.0280 | 0.7652 |
| % abnormal, 1 min-HR | 9 (43%) | 5 (31%) | 0.0833 | 22 (54%) | 9 (26%) | 0.0522 | 0.5727 |
| 6 Minute Walk Test Outcomes | |||||||
| 6MWD, meters | 459 (420, 517) | 502 (456, 556) | 0.1400 | 480 (426, 522) | 492 (443, 585) | 0.0126 | 0.4214 |
| % predicted, 6MWD | 0.8 (0.6, 0.8) | 0.8 (0.7, 0.9) | 0.1164 | 0.9 (0.8, 1) | 0.9 (0.8, 1) | 0.0089 | 0.3371 |
| Pulmonary Function Outcomes | |||||||
| MVV | 116 (96, 141) | 138 (109.5, 152.5) | 0.9818 | 132.5 (108.8, 146.8) | 137 (114.8, 148) | 0.1945 | 0.1023 |
| Breathing Reserve, L | 66 (32.3, 86.7) | 75.5 (59.8, 92.4) | 0.4060 | 59.3 (49.2, 71.7) | 57.4 (41.4, 74.7) | 0.2114 | 0.0246 |
| % Breathing Reserve | 55 (37, 65) | 59 (46, 68) | 0.1633 | 48 (37, 54) | 43 (36, 52) | 0.0979 | 0.0201 |
Abbreviations: ATAAD, acute type A aortic dissection; BMI, body mass index; CPX, cardiopulmonary exercise testing; DBP, diastolic blood pressure; FRIEND, fitness registry and the importance of exercise national database; HR, heart rate; MET, metabolic equivalent; MVV, maximal voluntary ventilation; PRP, pressure-rate product; RER, respiratory exchange ratio; RPE, rated perceived exertion; SBP, systolic blood pressure, pTAA, thoracic aortic aneurysm; VO2peak, peak oxygen uptake; 6MWD, 6-minute walk test distance.
Data are reported as median (interquartile range) or n (%).
P-values for continuous and categorical variables were derived from paired Wilcoxon signed-rank test and McNemar’s test, respectively.
P-values for group by time interactions were derived from linear mixed model.
Adverse Events and Diagnostic Imaging Outcomes
No serious adverse events requiring hospitalization were observed during CPX. A total of two participants at both timepoints required early CPX termination due to non-serious hypotensive responses (early, n=1; late, n=1) or electrocardiogram abnormalities (early, n=1; late, n=1) The same participants’ experienced similar non-serious responses during CPX 1 and CPX 2 (Supplemental Table 1). There were no differences in LVEF or aortic insufficiency over time. Root, ascending, arch, and thoracic descending aortic diameters remained similar; however, the abdominal aortic diameter significantly increased between the early and late timepoints (30 vs 31.9 mm, p=0.005) (Table 4).
Table 4.
Time Differences in Diagnostic Imaging Outcomes Among Groups
| ATAAD (N=21) | pTAA (N=43) | Group by Time Interaction | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Variables | Early | Late | p-value* | Early | Late | p-value* | p-value† |
|
| |||||||
| LVEF | 60 (56.2, 60) | 60 (60, 62.8) | 1.0000 | 60 (54.2, 60) | 60 (55, 65) | 0.2129 | 0.4818 |
| Aortic Insufficiency | 1 (6%) | 2 (18%) | 0.3173 | 1 (3%) | 0 (0%) | 1.0000 | 0.0558 |
|
| |||||||
| Aortic Diameters (mm) | |||||||
| Sinus | 38.3 (33.9, 42.2) | 37.6 (34.4, 42.7) | 0.6659 | 38.5 (35.2, 42.5) | 38.6 (35.6, 42.3) | 0.1339 | 0.1590 |
| Mid Ascending | 30 (29.6, 32.5) | 30.1 (28.5, 33.3) | 0.4973 | 32.8 (31, 33.3) | 32 (30.8, 34) | 0.9904 | 0.5250 |
| Proximal Arch | 32 (29.1, 33) | 31.9 (28.5, 33.4) | 0.6462 | 33 (32, 36.2) | 34 (32, 36.9) | 0.6567 | 0.6265 |
| Mid Arch | 32 (29.4, 36) | 33.9 (32, 35.2) | 0.0711 | 31 (28.9, 34.1) | 31 (28, 33.4) | 0.8190 | 0.1756 |
| Mid Descending | 31.8 (27.1, 36.5) | 31.4 (28.6, 34.9) | 0.8445 | 27 (25.2, 30.3) | 26.6 (24, 28.8) | 0.0880 | 0.6145 |
| Abdominal | 30 (24, 32.5) | 31.9 (26.8, 40) | 0.0049 | 26.2 (24, 29) | 24.7 (23.2, 28.1) | 0.2291 | 2*10−5 |
Abbreviations: ATAAD, acute type A aortic dissection; CT, computed tomography; LVEF, left ventricular ejection fraction; pTAA, proximal aortic aneurysm.
Data are reported as median (interquartile range) or n (%)
P-values for continuous and categorical variables were derived from paired Wilcoxon signed-rank test and McNemar’s test, respectively.
P-values for group by time interactions were derived from linear mixed model.
Health-Related Quality of Life Outcomes
Thirty-seven percent of participants reported moderate-to-severe anxiety at the early timepoint compared to 16% at the late timepoint (p=0.0061, Figure 2 and 3; Supplemental Table 2).
Figure 2: Health-related quality of life outcomes.
Health-related quality of life outcomes for ATAAD and pTAA groups for timepoints Early and Late. P-values for differences between early and late timepoints in social activities and physical function in the pTAA group were derived from paired Wilcoxon signed-rank tests. ATAAD=acute type A aortic dissection; pTAA=proximal thoracic aortic aneurysm.
Figure 3: (Central Picture):
VO2peak, functional capacity, and physical function remained similar from three months fifteen months following aortic repair in the ATAAD group; however, VO2peak, functional capacity, and physical function significantly improved from three to fifteen months after open aortic repair in the pTAA group. The upper and lower borders of the box represent the upper and lower quartiles. The middle horizontal line represents the median. The upper and lower whiskers represent the maximum and minimum values of non-outliers.
Study Outcomes for pTAA
Clinical Characteristics and Operative Outcomes
The pTAA group was predominantly male (86%) with a median age of 60 years. Eightyone percent of participants in the pTAA had ascending aortic aneurysms. At time of open repair, 48% of participants had moderate-to-severe aortic insufficiency and 28% had moderate-to-severe aortic stenosis. Regarding cardiovascular disease risk, 77% of participants had hypertension, 9.3% had peripheral vascular disease, and 19% had previous cardiac surgery (Table 1). Fifty-one percent of participants in the pTAA group underwent aortic root replacement and 16% underwent a zone 1/2/3 arch replacement. The median cardiopulmonary bypass time was 212 minutes with hypothermic circulatory arrest utilized in 37% of participants. Postoperative complication rates were low as 0% of participants suffered a stroke or had renal failure requiring dialysis; the median postoperative length of stay was six days (Table 2).
Cardiorespiratory, Hemodynamics and Functional Capacity
Body mass index significantly increased the between early and late timepoints (30.4 vs 31.2 kg·m−2, p=0.0045) with 54% of participants classified as obese at the late timepoint. There was a significant increase in median VO2peak between early and late timepoints (20.2 to 22.4 mL.kg−1.min−1, p=0.0003). The average VO2peak was 21% and 13% below age- and sex-predicted normative values at the early and late timepoints, respectively. There were significant increases in peak ventilation (66.5 vs 73.9 L.min-1, p=0.0162) and METs (5.8 vs 6.4, p=0.0128) between the early and late timepoints. Median RER at both timepoints was 1.2 with 90% (early) and 94% (late) of CPX studies meeting “peak” criteria as defined by a RER≥1.05. There were no significant differences over time for peak HR, systolic blood pressure, or diastolic blood pressure (Figure 1). There was a significant increase in median 6MWD between the early and late timepoints, (480 vs 492 m, p=0.01), with 90% achieving age- and sex-predicted distance (Table 3).
Adverse Events and Diagnostic Imaging Outcomes
No serious adverse events requiring hospitalization were observed during CPX. A total of three participants at the early timepoint and two participants at the late timepoint required early CPX termination due to non-serious hypotensive responses (early, n=2; late, n=1) or electrocardiogram abnormalities (early, n=1; late, n=1). The same participants experienced similar non-serious responses during CPX 1 and CPX 2 (Supplemental Table 1). There were no differences in LVEF or aortic insufficiency between timepoints. Root, ascending, arch, thoracic descending, and abdominal aortic diameters remained similar between timepoints (Table 4).
Health-Related Quality of Life Outcomes
The pTAA group significantly improved PROMIS measures of satisfaction with social roles and activities (p=0.0105) and physical function (p=0.0214) between the early and late timepoints (Figure 2; Supplemental Table 2). PROMIS measure of satisfaction with social roles and activities was positively associated with improvements in VO2peak within the pTAA group (correlation=0.57; p=0.005) using Pearson correlation test. Marginal significance was identified using Spearman correlation between PROMIS measure of satisfaction with social roles and activities and improvement of VO2peak (p=0.064) within pTAA group. There were no significant correlations between PROMIS measures of satisfaction and participants’ improvements in 6MWD between timepoints, but non-statistically significant correlations of improvement in 6MWD with higher satisfaction were observed across all PROMIS measurements (e.g., psychosocial, social activities, and physical function, and fatigue) (Figure 3).
DISCUSSION
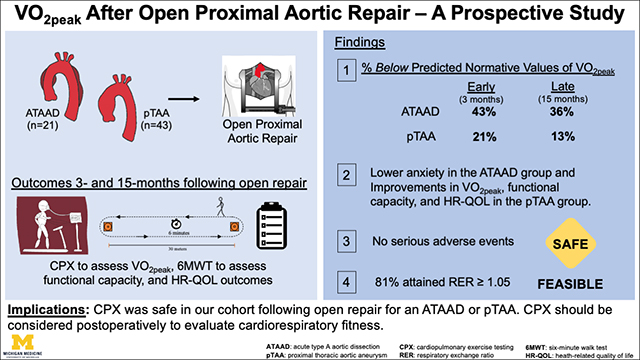
We prospectively evaluated VO2peak response following open proximal aortic repair for an ATAAD or pTAA. Our findings show the following: i) significantly lower anxiety and increased BMI with no change in VO2peak among the ATAAD group between 3- and 15-months postoperatively. VO2peak remained 36% below age- and sex-matched normative values > 1 year following open repair; ii) no serious adverse events during CPX procedures, providing additional evidence to support the safety and feasibility of CPX and mild-to-moderate aerobic exercise in this setting for participants cleared by a cardiac surgeon or cardiologist; and iii) significant improvements in VO2peak, functional capacity, and health-related quality of life measures were noted among the pTAA group between 3- and 15-months following open repair (Figure 4).
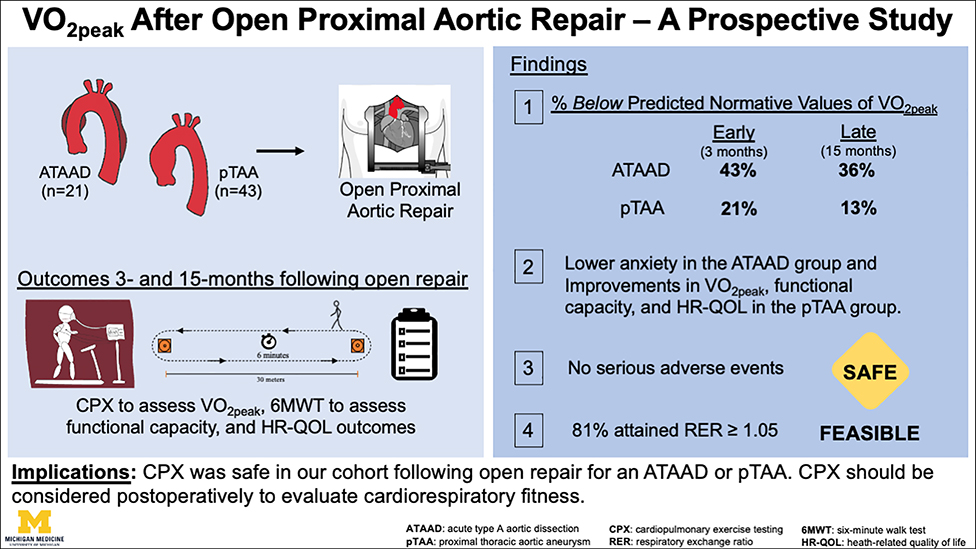
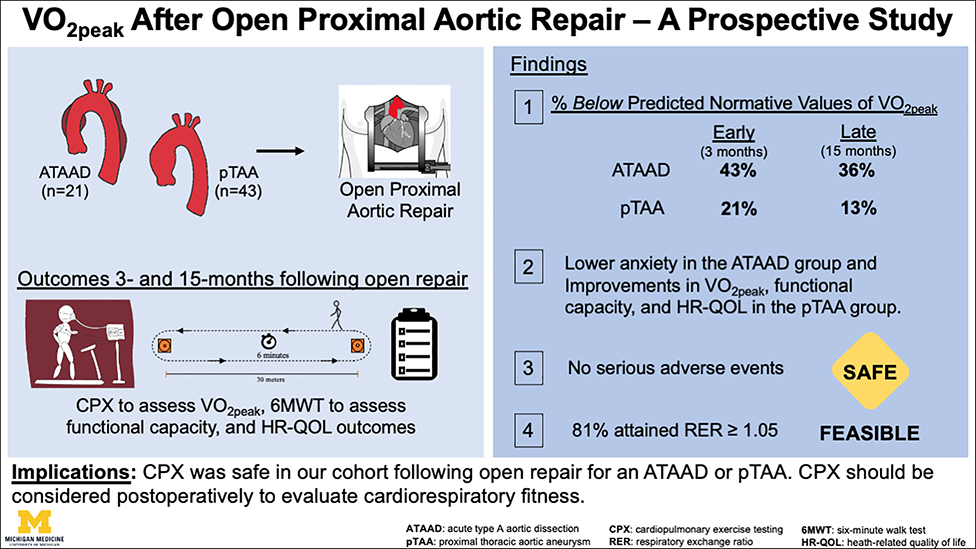
Figure 4: (Graphial Abstract):
Peak oxygen consumption (VO2peak), functional capacity, and health-related quality of life outcomes were prospectively (3- and 15-months) evaluated following open repair. Significant improvements in VO2peak, functional capacity, and HR-QOL metrics (social roles and activities, physical function) were observed within the pTAA group. There were no serious adverse events during CPX procedures. P-values were derived from paired Wilcoxon signed-rank tests. The upper and lower borders of the box represent the upper and lower quartiles. The middle horizontal line represents the median. The upper and lower whiskers represent the maximum and minimum values of non-outliers.
ATAAD=acute type A aortic dissection; pTAA=proximal thoracic aortic aneurysm; CPX=cardiopulmonary exercise test; 6MWT=Six minute walk test; HR-QOL=health-related quality of life; cardiorespiratory fitness=VO2peak; RER=respiratory exchange ratio.
VO2peak was on average 43% less than predicted normative values at the 3-month follow-up after open repair for an ATAAD. Over the course of one year, body mass index significantly increased and no significant change in VO2peak was observed (mean, 18.4 to 19.5 mL.kg−1 min−1, 36% below predicted normative values). Our findings corroborate the initial work of Delsart et al. that reported a mean VO2peak of 19.2 mL.kg−1.min−1 among 105 type A and B aortic dissection participants approximately two years following hospitalization or open repair6. At this juncture, preliminary studies show reduced VO2peak and increased BMI one to two years following an aortic dissection5,6,15. These outcomes typically are interwoven although the effects are cumulative leading to an increased risk of cardiovascular morbidity and mortality25–28. Recently, Delsart et al. showed that a low VO2peak (<70% of predicted) among 165 type A and B dissection survivors was an independent predictor of a major cardiac event occurrence (i.e., cardiovascular death, stroke, acute coronary syndrome, hospitalization for cardiac failure, and coronary or peripheral artery revascularization) over a 39 month follow-up period29.
VO2peak provides an objective assessment of the integrative capacity of the cardiovascular, pulmonary, hematopoietic, and musculoskeletal systems to transport and use oxygen11, and thus, it is widely considered a reflection of overall health2, with numerous empirical studies highlighting the association between reduced VO2peak and future risk of cardiovascular morbidity and mortality25,30,31. The exact mechanisms of reduced VO2peak among ATAAD survivors was not assessed in this study, although all ATAAD participants (with the exception of one, LVEF=43%) had a normal LVEF (>50%, range 50–60%), normal pulmonary function (>20% breathing reserve)16, and 80% had no history of peripheral vascular disease. Indirect causes of reduced VO2peak could be that: i) the acute event and complex surgical repair likely exacerbated declines in exercise leading to weight gain and exercise intolerance, and ii) fear that exercise may increase the risk of disease progression32,33,34. We observed significantly lower generalized anxiety between timepoints; however, we did not directly assess anxiety related to exercise and thus, further investigation is warranted. Current recommendations advise against strenuous resistance or isometric exercise and competitive sports due to concerns that elevations in blood pressure may cause progression of the patent false lumen, aortic expansion, and rupture8. At present, there is no confirmatory data to show that low-to-moderate intensity exercise is unsafe4.
Aerobic exercise training is a prototypical strategy that maintains and restores homeostasis and can prevent or reduce the risk of numerous chronic diseases as observed in numerous epidemiological studies35–38. Post-dissection rehabilitation appears justified given findings of reduced VO2peak and increased BMI > 1 year following open repair. Additional findings from this cohort show that participants are able to perform incremental exercise (81% attained a RER ≥1.05) without serious adverse events, which aligns with tolerability (i.e., more than 80% of participants achieve peak criteria) and safety (i.e., no exercise-related deaths and an approximate 15% nonserious adverse event rate) data observed in other populations39. Preliminary findings from Corone et al.40 and Fuglsang et al.15 show beneficial effects of moderate-intensity exercise-based cardiac rehabilitation following open repair for an ATAAD. However, these studies are limited by sample size, lack of randomization, minimal details outlining the exercise prescription, and quantitative assessment of VO2peak.
We contend that the path forward involves a well-designed randomized controlled trial, utilizing submaximal CPX to assess exercise tolerance to guide low-to-moderate-intensity aerobic exercise prescriptions. Careful and continued monitoring of systolic and diastolic blood pressures (for hyper- and hypotensive responses) during CPX procedures and aerobic exercise are paramount to ensure safety. We recommend that intervention align with the post-operative clinical follow-up and utilize available diagnostic imaging data (echocardiography and CTA) to ensure optimal aortic valve function and stable aortic diameters, by comparing serial CTA imaging, prior to CPX procedures to ensure safety.
Novel data on a cohort of pTAA participants is also presented, showing that pTAA participants appear to more spontaneously recover based on significant improvements in VO2peak, functional capacity, and patient-reported quality of life – PROMIS metrics: social roles/activities and physical function over time, which were not observed among the ATAAD group. It is unclear why VO2peak recovers more exponentially after open repair for a pTAA as neither diagnosis meet eligibility criteria for insurance reimbursement of cardiac rehabilitation and exercise recommendations in this setting vary among cardiac surgeons. The pTAA group had a substantial percentage of patients with preoperative aortic valve disease (Table 1). Thus, improvements in VO2peak, functional capacity and health-related quality of life may be secondary to improved hemodynamic function as the majority of these patients now have “normal” aortic valves and aortas and can perform activities of daily living with far fewer symptoms and/or fear.
There are limitations that need to be considered when interpreting the findings. First, the findings are from a single center and the sample size is relatively small, possibly limiting the statistical power to assess changes in VO2peak. Second, selection biases may exist given that participants were cleared by their cardiac surgeon prior to participating and only participants motivated to voluntarily participate in supervised CPX to evaluate cardiorespiratory fitness were recruited. These factors may affect the generalizability of our findings to all postsurgical participants in this setting. It is possible that the general population of ATAAD participants could be worse. Thirdly, three participants (n=2, pTAA; n=1, ATAAD) completed cardiac rehabilitation which may have also resulted in study bias. Lastly, it can be argued that VO2peak was reduced compared with normative values since participants attaining an RER of <1.05 were included. To address this point, we evaluated participants achieving only an RER ≥1.05 (range RER 1.07–1.36), and VO2peak remained 36% below normative values (among peak CPX) >1 year following open repair for an ATAAD.
CONCLUSION
Cardiorespiratory fitness among the ATAAD group remained 36% below predicted normative values >1 year after repair, whereas the pTAA group experienced a significant improvement in cardiorespiratory fitness, which was only 13% below predicted normative values > 1 year after repair. Cardiopulmonary exercise testing was safe and feasible following open aortic repair in this setting for patients cleared to participate by their cardiac surgeons. Cardiac surgeons and cardiologists managing ATAAD participants post-operatively should consider CPX to assess exercise tolerance and blood pressure response to determine whether mild-to-moderate aerobic exercise should be recommended to reduce future risk of morbidity and mortality (Video).
Supplementary Material
Video Legend
Discussion of cardiorespiratory fitness and physical function following open repair for an acute type A aortic dissection or proximal thoracic aortic aneurysm..
CENTRAL PICTURE.
VO2peak, functional capacity, and physical function improved in pTAA but not ATAAD group.
CENTRAL MESSAGE
VO2peak remained 36% below normative values >1 year after ATAAD repair. CPX should be considered to evaluate exercise tolerance and blood pressure response to determine if exercise should be recommended.
PERSPECTIVE STATEMENT.
Cardiopulmonary exercise testing (CPX) is safe and feasible following open repair for an ATAAD or pTAA in patients cleared by their cardiac surgeons. Cardiac surgeons and cardiologists should consider CPX to evaluate exercise tolerance and blood pressure response and determine if mild-to-moderate aerobic exercise should be recommended to reduce future morbidity/mortality in post-repair ATAAD patients.
Acknowledgements
We thank the study participants, aortic surgeons, and clinical staff at the University of Michigan, Michigan Medicine for their contributions. We appreciate the valuable efforts of the exercise physiologists from Preventive Cardiology at the University of Michigan, Michigan Medicine, Ann Arbor (Jacob Sitzmann, Samantha Fink, and Steven Walsh). We also acknowledge the contributions from Linda Farhat and Marc Thomas. We acknowledge the Aikens Aortic Discovery Program of the Samuel and Jean Frankel Cardiovascular Center
Sources of Funding
Bo Yang is supported by the National Institutes of Health (K08-HL130614, R01-HL141891, and R01-HL151776) and The Phil Jenkins and Darlene & Stephen J. Szatmari Funds. Cristen J. Willer is supported by the National Institutes of Health (R01-HL127564, R35-HL135824, and R01-HL142023).
Whitney E. Hornsby, Bo Yang, Cristen J. Willer, and Sara Saberi are supported by The University of Michigan, Michigan Medicine, Frankel Cardiovascular Center, Ann Arbor, MI, Aikens Fund for Aortic Research. Sara Saberi is the site principal investigator for two clinical trials funded by Myokardia and receives financial support from Myokardia.
Glossary of Abbreviations:
- (ATAAD)
acute type A aortic dissection
- (CPX)
cardiopulmonary exercise testing
- (FRIEND Registry)
Fitness Registry and the Importance of Exercise National Database
- (HR)
heart rate
- (LVEF)
left ventricular ejection fraction
- (PROs)
patient reported outcomes
- (pTAA)
proximal thoracic aortic aneurysm
- (VO2peak)
peak oxygen consumption
- (RER)
respiratory exchange ratio
- (6MWD)
six minute walk distance
Footnotes
Disclosures: Dr. Willer’s husband works for Regeneron. No other relevant disclosures.
Date and Number of IRB Approval: HUM00110749, 1/30/2016
Informed consent was obtained from all participants.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Anderson L, Oldridge N, Thompson DR, Zwisler AD, Rees K, Martin N, et al. Exercise-Based Cardiac Rehabilitation for Coronary Heart Disease: Cochrane Systematic Review and Meta-Analysis. Journal of the American College of Cardiology. 2016;67:1–12. [DOI] [PubMed] [Google Scholar]
- 2.Ross R, Blair SN, Arena R, Church TS, Després JP, Franklin BA, et al. Importance of Assessing Cardiorespiratory Fitness in Clinical Practice: A Case for Fitness as a Clinical Vital Sign: A Scientific Statement From the American Heart Association. Circulation. 2016;134:e653–e699. [DOI] [PubMed] [Google Scholar]
- 3.Scott JM, Stene G, Edvardsen E, Jones LW. Performance Status in Cancer: Not Broken, But Time for an Upgrade? J Clin Oncol 2020;38:2824–2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ehrman JK, Fernandez AB, Myers J, Oh P, Thompson PD, Keteyian SJ. Aortic Aneurysm: DIAGNOSIS, MANAGEMENT, EXERCISE TESTING, AND TRAINING. J Cardiopulm Rehabil Prev. 2020;40:215–223. [DOI] [PubMed] [Google Scholar]
- 5.Hornsby WE, Norton EL, Fink S, Saberi S, Wu X, McGowan CL, et al. Cardiopulmonary Exercise Testing Following Open Repair for a Proximal Thoracic Aortic Aneurysm or Dissection. J Cardiopulm Rehabil Prev. 2020;40:108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delsart P, Maldonado-Kauffmann P, Bic M, Boudghene-Stambouli F, Sobocinski J, Juthier F, et al. Post aortic dissection: Gap between activity recommendation and real life patients aerobic capacities. Int J Cardiol. 2016;219:271–276. [DOI] [PubMed] [Google Scholar]
- 7.Yang B, Norton EL, Hobbs R, Farhat L, Wu X, Hornsby WE, et al. Short- and long-term outcomes of aortic root repair and replacement in patients undergoing acute type A aortic dissection repair: Twenty-year experience. J Thorac Cardiovasc Surg. 2019;157:2125–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE Jr., et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease. Circulation. 2010;121:e266–369. [DOI] [PubMed] [Google Scholar]
- 9.Wolford BN, Hornsby WE, Guo D, Zhou W, Lin M, Farhat L, et al. Clinical Implications of Identifying Pathogenic Variants in Individuals With Thoracic Aortic Dissection. Circ Genom Precis Med. 2019;12:e002476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fletcher GF, Ades PA, Kligfield P, Arena R, Balady GJ, Bittner VA, et al. Exercise standards for testing and training: a scientific statement from the American Heart Association. Circulation. 2013;128:873–934. [DOI] [PubMed] [Google Scholar]
- 11.ATS/ACCP Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167:211–277. [DOI] [PubMed] [Google Scholar]
- 12.Myers J, Kaminsky LA, Lima R, Christle JW, Ashley E, Arena R. A Reference Equation for Normal Standards for VO(2) Max: Analysis from the Fitness Registry and the Importance of Exercise National Database (FRIEND Registry). Prog Cardiovasc Dis. 2017;60:21–29. [DOI] [PubMed] [Google Scholar]
- 13.Saberi S, Wheeler M, Bragg-Gresham J, Hornsby W, Agarwal PP, Attili A, et al. Effect of Moderate-Intensity Exercise Training on Peak Oxygen Consumption in Patients With Hypertrophic Cardiomyopathy: A Randomized Clinical Trial. Jama. 2017;317:1349–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guazzi M, Adams V, Conraads V, Halle M, Mezzani A, Vanhees L, et al. EACPR/AHA Scientific Statement. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation. 2012;126:2261–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuglsang S, Heiberg J, Hjortdal VE, Laustsen S. Exercise-based cardiac rehabilitation in surgically treated type-A aortic dissection patients. Scand Cardiovasc J. 2017;51:99–105. [DOI] [PubMed] [Google Scholar]
- 16.Balady GJ, Arena R, Sietsema K, Myers J, Coke L, Fletcher GF, et al. Clinician’s Guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation. 2010;122:191–225. [DOI] [PubMed] [Google Scholar]
- 17.Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med. 1999;341:1351–1357. [DOI] [PubMed] [Google Scholar]
- 18.Goldstein SA, Evangelista A, Abbara S, Arai A, Asch FM, Badano LP, et al. Multimodality imaging of diseases of the thoracic aorta in adults: from the American Society of Echocardiography and the European Association of Cardiovascular Imaging: endorsed by the Society of Cardiovascular Computed Tomography and Society for Cardiovascular Magnetic Resonance. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2015;28:119–182. [DOI] [PubMed] [Google Scholar]
- 19.ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. [DOI] [PubMed] [Google Scholar]
- 20.Magasi S, Ryan G, Revicki D, Lenderking W, Hays RD, Brod M, et al. Content validity of patient-reported outcome measures: perspectives from a PROMIS meeting. Qual Life Res. 2012;21:739–746. [DOI] [PubMed] [Google Scholar]
- 21.Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092–1097. [DOI] [PubMed] [Google Scholar]
- 22.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singap. 1994;23:129–138. [PubMed] [Google Scholar]
- 23.Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. [DOI] [PubMed] [Google Scholar]
- 24.Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Canadian journal of applied sport sciences. Journal canadien des sciences appliquees au sport. 1985;10:141–146. [PubMed] [Google Scholar]
- 25.Myers J, McAuley P, Lavie CJ, Despres JP, Arena R, Kokkinos P. Physical activity and cardiorespiratory fitness as major markers of cardiovascular risk: their independent and interwoven importance to health status. Prog Cardiovasc Dis. 2015;57:306–314. [DOI] [PubMed] [Google Scholar]
- 26.Myers J, Kokkinos P, Nyelin E. Physical Activity, Cardiorespiratory Fitness, and the Metabolic Syndrome. Nutrients. 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clarke SL, Reaven GM, Leonard D, Barlow CE, Haskell WL, Willis BL, et al. Cardiorespiratory Fitness, Body Mass Index, and Markers of Insulin Resistance in Apparently Healthy Women and Men. Am J Med. 2020;133:825–830.e822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Myers J, Kokkinos P, Arena R, LaMonte MJ. The impact of moving more, physical activity, and cardiorespiratory fitness: Why we should strive to measure and improve fitness. Prog Cardiovasc Dis. 2020. [DOI] [PubMed] [Google Scholar]
- 29.Delsart P, Delahaye C, Devos P, Domanski O, Azzaoui R, Sobocinski J, et al. Prognostic value of aerobic capacity and exercise oxygen pulse in postaortic dissection patients. Clin Cardiol. 2021;44:252–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. Jama. 2009;301:2024–2035. [DOI] [PubMed] [Google Scholar]
- 31.Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346:793–801. [DOI] [PubMed] [Google Scholar]
- 32.Chaddha A, Eagle KA, Braverman AC, Kline-Rogers E, Hirsch AT, Brook R, et al. Exercise and Physical Activity for the Post-Aortic Dissection Patient: The Clinician’s Conundrum. Clin Cardiol. 2015;38:647–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chaddha A, Kline-Rogers E, Braverman AC, Erickson SR, Jackson EA, Franklin BA, et al. Survivors of Aortic Dissection: Activity, Mental Health, and Sexual Function. Clin Cardiol. 2015;38:652–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE Jr., et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: executive summary. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. 2010;76:E43–86. [DOI] [PubMed] [Google Scholar]
- 35.Lear SA, Hu W, Rangarajan S, Gasevic D, Leong D, Iqbal R, et al. The effect of physical activity on mortality and cardiovascular disease in 130 000 people from 17 high-income, middle-income, and low-income countries: the PURE study. Lancet (London, England). 2017;390:2643–2654. [DOI] [PubMed] [Google Scholar]
- 36.Kokkinos P, Myers J. Exercise and physical activity: clinical outcomes and applications. Circulation. 2010;122:1637–1648. [DOI] [PubMed] [Google Scholar]
- 37.Saint-Maurice PF, Coughlan D, Kelly SP, Keadle SK, Cook MB, Carlson SA, et al. Association of Leisure-Time Physical Activity Across the Adult Life Course With All-Cause and Cause-Specific Mortality. JAMA Netw Open. 2019;2:e190355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaminsky LA, Arena R, Ellingsen Ø, Harber MP, Myers J, Ozemek C, et al. Cardiorespiratory fitness and cardiovascular disease - The past, present, and future. Prog Cardiovasc Dis. 2019;62:86–93. [DOI] [PubMed] [Google Scholar]
- 39.Jones LW, Eves ND, Haykowsky M, Joy AA, Douglas PS. Cardiorespiratory exercise testing in clinical oncology research: systematic review and practice recommendations. Lancet Oncol. 2008;9:757–765. [DOI] [PubMed] [Google Scholar]
- 40.Corone S, Iliou MC, Pierre B, Feige JM, Odjinkem D, Farrokhi T, et al. French registry of cases of type I acute aortic dissection admitted to a cardiac rehabilitation center after surgery. Eur J Cardiovasc Prev Rehabil. 2009;16:91–95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video Legend
Discussion of cardiorespiratory fitness and physical function following open repair for an acute type A aortic dissection or proximal thoracic aortic aneurysm..