Abstract
Objectives:
Dorsal root ganglion (DRG) stimulation is effective in treating chronic pain. While burst stimulation has been proven to enhance the therapeutic efficacy in spinal cord stimulation, currently only a tonic stimulation waveform is clinically used in DRG stimulation. We hypothesized that burst DRG stimulation might also produce analgesic effect in a preclinical neuropathic pain model. We evaluated both the therapeutic effects of burst DRG stimulation and the possible effects of DRG stimulation upon inflammation within the DRG in a preclinical neuropathic pain model.
Materials and Methods:
Rats received either a painful tibial nerve injury or sham surgery. Analgesic effects of DRG stimulation were evaluated by testing a battery of evoked pain-related behaviors as well as measuring the positive affective state associated with relief of spontaneous pain using conditioned place preference. Histological evidence for neuronal trauma or neuroinflammation was evaluated.
Results:
All of the waveforms tested (20 Hz-tonic, 20 Hz-burst, and 40 Hz-burst) have similar analgesic effects in sensory tests and conditioned place preference. Long-term DRG stimulation for two weeks does not change DRG expression of markers for nerve injury and neuroinflammation.
Conclusions:
DRG stimulation using burst waveform might be also suitable for treating neuropathic pain.
Keywords: Burst stimulation, dorsal root ganglion, neuropathic pain, rat, tonic stimulation
INTRODUCTION
Neuromodulation approaches such as spinal cord stimulation (SCS) and dorsal root ganglion (DRG) stimulation are established methods to alleviate chronic pain while avoiding the risks of opioid addiction and overdose. DRG stimulation is achieved with electrodes placed adjacent to the DRG in the intervertebral foramen. DRG stimulation has proven effective for treating chronic neuropathic and non-neuropathic pain (1–3). Compared to SCS, DRG stimulation has advantages of directly targeting nociceptive sensory neurons. In the case of SCS, the highly conductive cerebrospinal fluid between the stimulation electrode and the target spinal cord make it less efficient to deliver electrical currents to elicit a modulatory effect on the spinal cord. However, in DRG stimulation, proximity of the electrode to the targeted neuronal structure provides a therapeutic benefit with less electric current. This energy efficiency permits use of a smaller implantable pulse generator (IPG) and provides prolonged battery life. Moreover, DRG stimulation may provide pain relief for conditions in which SCS is ineffective and for patients for whom SCS has failed (4,5).
A computational modeling study suggested that action potentials (APs) of pseudo-unipolar nociceptive fibers can be blocked at the T-junction with DRG stimulation (6). This use-dependent AP blockade has been confirmed by electrophysiological examination in a rodent model, which showed blocking effects of DRG stimulation that is selective for nonmyelinated nociceptive C-type neurons (7). These require high stimulation intensity to elicit APs at the level of their axons in peripheral nerves but are readily activated by electrical stimulation delivered to their somata within the DRG (7). These findings indicate that DRG stimulation with clinically relevant levels of intensity provides analgesia by regulating AP conduction in nociceptive C-fibers directly. This dependence on inducing neuronal activity raises the issue that DRG stimulation with different intensities may have different effectiveness in initiating sensory neuron activity and thereby generating analgesia.
Compared to recent advances in the development of different stimulation waveforms for SCS, only the tonic waveform is being used for clinical DRG stimulation. Considering the enhanced therapeutic efficacy of burst or DeRidder burst in SCS, which has been shown to be effective not only in mitigating pain intensity but also in modulating the affective aspect of pain (8), a burst stimulation waveform may also provide better therapeutic efficacy compared to traditional tonic stimulation waveform in DRG stimulation. Furthermore, effectiveness of tonic DRG stimulation in preclinical animal experiments is frequency dependent (7). Therefore, in this study, we compared tonic stimulation to burst stimulation at two different frequencies and assessed their analgesic effectiveness using behavioral tests of both somatosensory and affective/motivational aspects of pain in a tibial nerve injury (TIN) rat model (9), which is a variant of spared nerve injury model. In addition, we conducted postmortem immunohistochemistry analysis to evaluate whether DRG stimulation may limit neuropathy-related neuroinflammation or may itself produce inflammation.
MATERIALS AND METHODS
Animals
Male Sprague Dawley rats weighing 200–250 g were obtained from the Taconic Farms Biosciences (Rensselaer, NY, USA) and were maintained and used according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All animal experiments were performed according to protocols approved by the Institutional Animal Care and Use Committee of the Medical College of Wisconsin (animal protocol AUA0454). Animals were housed in a pathogen-free facility, two animals per ventilated cage, in a room maintained at 25 ± 1°C at 35–45% humidity, with a normal 12/12-hour day/night cycle. Animals had free access to food and water, and bedding was aspen wood chips. At the termination of the study, euthanasia was performed by decapitation during deep isoflurane anesthesia.
Neuropathic Pain Model
Tibial nerve injury (TNI) surgery was performed as described in our previous report (9). Briefly, animals were anesthetized with 2% isoflurane/O2 mixture, a 2 cm incision was made on the lateral mid-thigh of right hind limb, and the sciatic nerve was exposed at the point at which it divides into its distal branches. At a distance 5 mm distal to this branch point, the tibial nerve was ligated with 5.0 silk sutures and 2–3 mm of the nerve was removed distal to the ligation. Contact with the preserved sural and common peroneal nerves was avoided. Muscle and fascia were closed in layers, and skin was closed with staples. Sham TNI control rats had only exposure of the nerves without further handling.
Dorsal Root Ganglion Stimulation Electrode Implantation
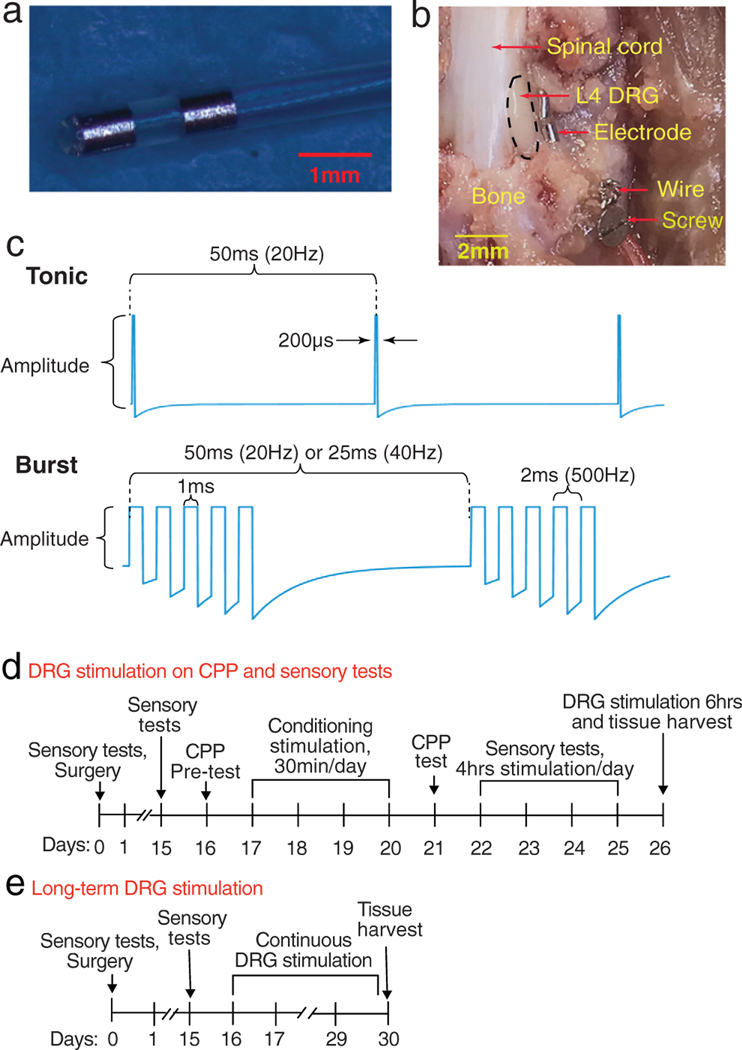
Cylindrical bipolar stimulation electrodes with a diameter of 0.45 mm (Fig. 1a) were modified from that in our previous report (10) and manufactured by Abbott. The implantation of the electrode was performed as described in our previous report (10). Briefly, rats were anesthetized with 2% isoflurane/O2 while maintaining body temperature at 36.5°C. A dorsal paramecia incision was made to expose the external aspect of the intervertebral foramen at the level of the fourth lumbar (L4) spinal nerve, and the accessory process overhanging the foramen was removed. A probe with a 0.4-mm diameter was inserted into the intervertebral foramen lateral to the DRG in the middle of dorsal and ventral parts of DRG (Fig. 1b), to create a space into which the electrode was inserted in juxtaposition to the DRG. A stainless-steel ligature was used to bind the electrode to a screw inserted into the transverse process caudal to the foramen (Fig. 1b). The leads were tunneled to the head, where the connection hub was secured to the skull with dental cement and screws.
Figure 1.
Study design. (a) Bipolar stimulating electrode. (b) In situ position of stimulating electrode three weeks after implantation, following laminectomy for exposure. Black dotted line circles DRG. (c) Different stimulation waveforms. (d) Sequency of events for CPP test and mechanical sensory behaviors. (e) Sequency of events with two-week continuous stimulation.
Dorsal Root Ganglion Stimulation
Animals received DRG stimulation while awake and freely moving via a tethering cable and commutator. In the clinic setting, the stimulation intensity used is that which may or may not produce paresthesia (1,4,11), but at current amplitude levels that do not produce motor activity. Therefore, in these experiments on rats, we tested the effectiveness of stimulation amplitudes at 50%, 70%, and 90% of the motor threshold with different stimulating waveforms (Fig. 1c). The motor threshold (MT) was determined as the current amplitude at which any further increase resulted in perceptible hind limb movement for ganglion stimulation during stimulation at 2 Hz for tonic waveform and 5 Hz for burst waveforms with a pulse width of 200 sec and 1 msec, respectively (Fig. 1C). This was established at each testing session. Stimulation was delivered from an implantable pulse generator (IPG, Proclaim DRG Neurostimulator) provided by Abbott.
Behavioral Tests
Sensory testing of the plantar skin included eliciting punctate mechanically evoked behaviors induced at threshold intensity (von Frey test) and at noxious intensity (Pin test). Affective dimension of spontaneous pain relief was assessed using the conditioned place preference (CPP) test (12).
Threshold Punctate Mechanical Stimulation (von Frey)
The von Frey test was performed using calibrated monofilaments (Patterson Medical, Bolingbrook, IL, USA). Briefly, beginning with the 2.8-g filament, the tip of filament was applied perpendicularly to the glabrous skin on the lateral third of the plantar aspect of the hind paw for 1 sec, with just enough force to bend the fiber. If a paw withdrawal response was observed, then the next weaker filament was applied, and if no response was observed, then the next stiffer fiber was applied, until a reversal occurred. After a reversal event, four more stimulations were performed following the same pattern with at least 10 sec intervals between applications. The forces of the filaments applied before and after the reversal, and those of the four filaments applied after the reversal, were used to calculate the 50% withdrawal threshold (13). Rats not responding to any filament were assigned a score of 25 g. In the absence of a hypersensitivity state, animals often default to the 25 g score.
Noxious Punctate Mechanical Stimulation (Pin Test)
Pin testing was performed using the point of a 22-gauge spinal anesthesia needle that was applied to the lateral third of the plantar surface of hind paw with enough force to indent the skin but not puncture it. This was repeated for five applications, with intervals of at least 10 sec between applications, and this set of applications was repeated after 1 min, making a total of ten touches. Each application induced a behavior that was categorized as one of the following two types. The typical response of uninjured rats in the Pin test consisted of a very brief (<1 sec) withdrawal and immediate return of the foot to the cage floor. An alternate behavior that we termed a hyperalgesic response consisted of a complex event with sustained elevation of the foot for at least 1 sec, variably combined with grooming that included licking and chewing of the paw, and with shaking of the limb (14). This hyperalgesic behavior is specifically associated with place avoidance (15), indicating that it represents an aversive experience. Hyperalgesia was quantified by tabulating the number of hyperalgesic responses as a percentage of total ten touches.
Conditioned Place Preference
This was performed as described in our previous report (10). A 3-chamber CPP apparatus (Med associates, Fairfax, VT, USA) was used. On the preconditioning day, animals were allowed to explore both sides of chambers for 15 min and the time spent in each side was recorded. Animals that showed a preference for one chamber (≥67% of total time) were be excluded from further study. On the next four days following the baseline assessment, place conditioning was conducted using an unbiased procedure. Specifically, on each day animals received two 30 min sessions separated by six hours in which either DRG stimulation or sham stimulation. The chamber paired with DRG stimulation was consistent on all four days for a given animal but was randomly assigned for different animals. Acquisition of place preference was tested on the day after the last conditioning session. At these final sessions, after each animal was placed in the central chamber, it was allowed to freely explore the chambers for 15 min, and the time spent on each side was recorded. A preference score was calculated as the total time spent in the chamber paired with DRG stimulation minus the total time spent in the other chamber paired with sham stimulation. Each rat had only a single CPP test.
Immunohistochemistry
During anesthesia, the animals were perfused via a cardiac cannula, using 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS). L4 DRGs were harvested during perfusion and fixed in the same solution overnight at 4°C, and sequentially dehydrated by 20% and 30% sucrose at 4°C for one day, respectively, then embedded with OCT and fast frozen in isopentane and liquid N2. DRG was sectioned (10 μm) with Cryostat (Cm1800; Leica, Germany) and mounted onto precleaned slides for immunohistochemistry. Frozen DRG slices were incubated with 5% goat and donkey serum in 0.01 M PBS for one hour. The sections were then incubated with primary antibodies: rabbit anti glial fibrillary acidic protein (GFAP) (Cell Signaling, Danvers, MA, USA; #12716, 1:1000), ionized calcium-binding adapter molecule 1 (Iba-1) (Cell Signaling, #17198, 1:1000), myelin basic protein (MBA) (Cell Signaling, #12716, 1:1000), rabbit anti-activating transcription factor 3 (ATF3) (Cell Signaling, #18665, 1:1000), and mouse anti-β3 Tubulin (Santa Cruz, Dallas, TX, USA; 1:200) at 4°C overnight. Then, sections were rinsed and incubated with second antibodies: goat anti-rabbit IgG conjugated with Alexa Fluor® 488 (Cell Signaling, 1:1000) and goat anti-mouse IgG conjugated with Alexa Fluor® 594 (Cell Signaling, 1:1000) for one hour at room temperature. Finally, the sections were mounted with anti-fad medium with DAPI (Cell Signaling). Comparable DRG sections from DRG stimulation-treated ipsilateral and contralateral L4 DRG were selected for analysis. Images of a field (20×) from three pre-determined DRG sections with dense cells at least 30 μm internal was captured by an Olympus fluorescence microscopy (Olympus IX73, Japan). The average intensity of GFAP, Iba1 and MBP staining immunofluorescence was determined in a fixed area (250 μm × 250 μm) with dense β3-Tubulin positive DRG neurons in the 20× field in a blinded fashion using NIH ImageJ. The intensity of immunostaining was subtracted by the average of adjacent unstained area in the field. ATF3 was used as a marker of neuronal axotomy (16). The number of ATF3 and β3-Tubulin positive cells was counted in the 20× field, and the percentage of ATF positive neurons was calculated from the number of β3-Tubulin+ neurons divided by ATF3+/β3-Tubulin+ neurons. The investigator analyzing those images was blinded to the animal’s treatment.
Protocol Design
Surgery for TNI and ganglion stimulation electrode implantation was performed after baseline (day 0) sensory testing. After the sensory tests to confirm the hypersensitivity to mechanical stimulation on days 7 and 15, rats were randomly assigned to one of four groups: Sham DRG stimulation, 20 Hz-tonic, 20 Hz-burst, and 40 Hz-burst. CPP testing was performed on days 16 through 21 after TNI. This consisted of one preconditioning test day, four days conditioning (starting one day after the preconditioning test), and one test day. On 22–25 days after electrodes implantation surgery, ganglion stimulation was provided for four hours. The ensemble of pain behavior tests (von Frey, Pin test, all done within 5 min) was performed 15 min before DRG stimulation, and again 15, 30, and 60 min after the initiation of ganglion stimulation (i.e., during stimulation), and again 0, 15, 30, and 60 min after the end of DRG stimulation. On these four days, each rat received DRG stimulation with the same waveform but at different stimulation intensities of 0, 50, 70, or 90% of MT in a random order. The first batch of 11 rats of this study experienced four days DRG stimulation first with sensory tests on metal mesh floors before CPP test and showed preference to CPP chambers with mesh floor on the pretest day. They were excluded from further CPP test, and we changed the behavioral sequence to CPP first and then sensory tests during DRG stimulation for all other rats (Fig. 1d). Some rats experienced two weeks continuous stimulation by connecting IPGs to leads via commutator with tethering cables that rats could move freely in their cages. At the end of stimulation, DRGs were harvested for immunohistochemistry (Fig. 1e). All behavioral tests were performed between 9:00 AM and 5:00 PM, during which nociceptive behaviors are relatively stable (17). The investigator performing those behavioral tests was blinded to the animal’s treatment, that is, different stimulation waveforms and intensities.
The sample size was based on our previous experience with this design (9,10). A total of 40 rats were used for behavioral tests. After behavioral tests, 12 rats were randomly chosen to evaluate effects of six hours DRG stimulation on expression of GFAP, Iba-1, MBP, and ATF3 using immunohistochemistry. Fourteen rats without TNI were used for testing effects of long-term DRG stimulation on expression of GFAP, Iba-1, MBP, and ATF3. Six rats with TNI were used for testing effects of long-term DRG stimulation on expression of GFAP, Iba-1, and MBP.
Statistical Analysis
All data including outliers are included. Statistical analyses were performed with Prism 8 (GraphPad Software, La Jolla, CA, USA). To compare changes from baseline prior to simulation, responses to Pin were evaluated nonparametrically using two-way repeated measures analysis of variance (ANOVA) with post hoc Dunnett’s test. Responses to von Frey test were evaluated using two-way repeated measures ANOVA, with post hoc comparisons using Dunnett’s test. The area under the curve (AUC) of behavior values during and after the stimulation was calculated in reference to the baseline value just before the initiation of DRG stimulation for each animal. AUC thus represents the integrated response to DRG stimulation across time and was used to compare groups using two-way ANOVA with post hoc comparison Tukey test. CPP scores were analyzed with paired t-tests for each treatment group and one-way ANOVA with post hoc Tukey test was used for comparison between treatments. A two-tailed test was use for comparison between two treatments. Results are reported as mean ± SEM, or median where appropriate. p < 0.05 were considered statistically significant.
RESULTS
Tonic and Burst DRG Stimulation Relieve Allodynia and Hyperalgesia in an Intensity-Dependent Fashion
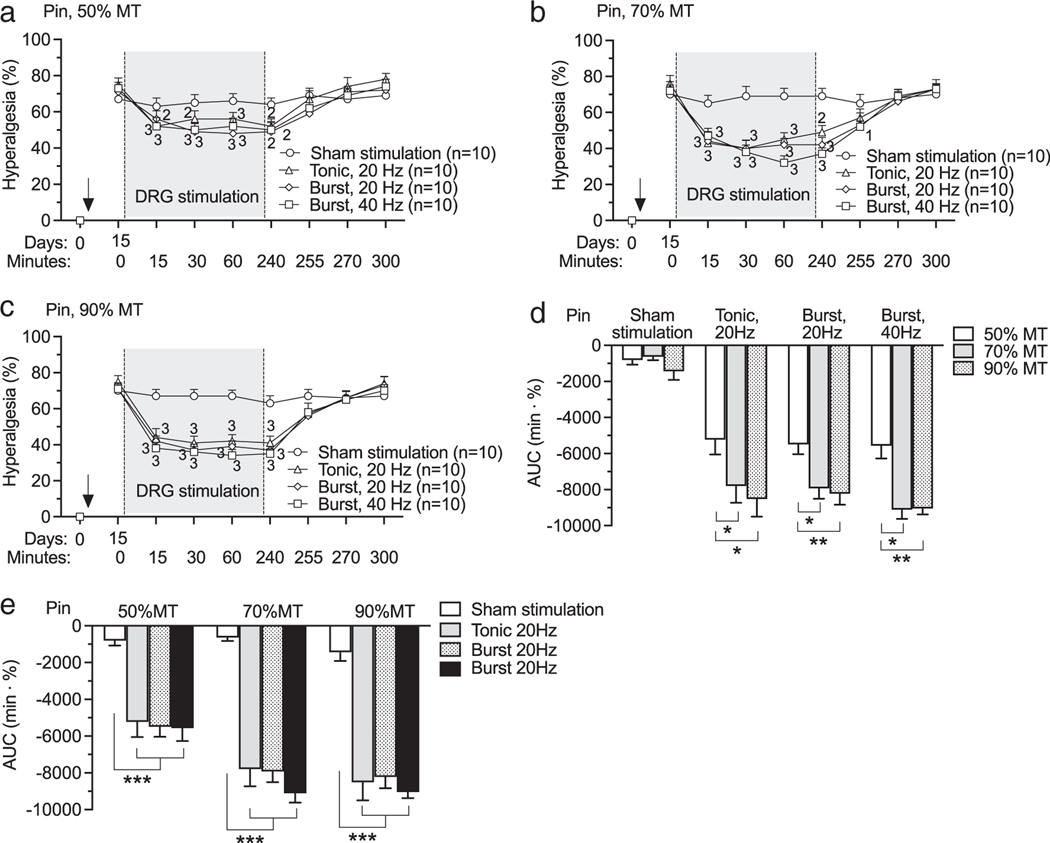
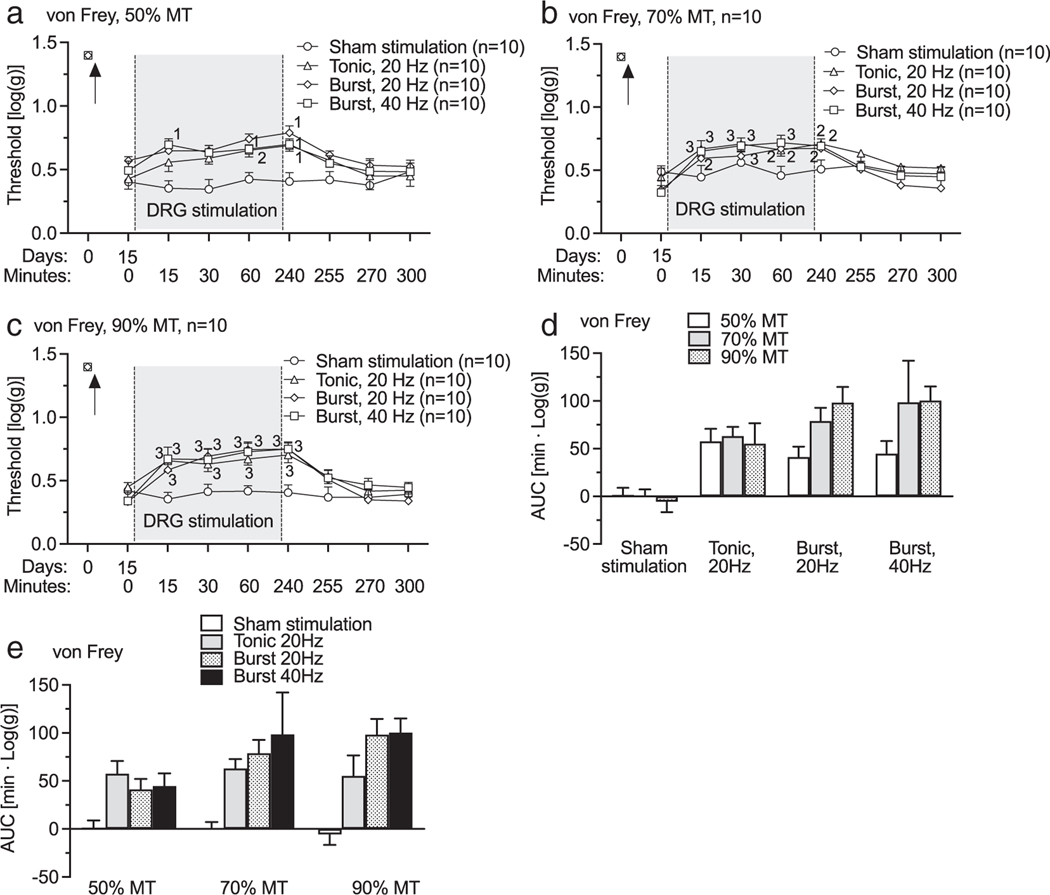
In clinical DRG stimulation for pain treatment, optimal analgesic effect with tonic stimulation is reached with 20 Hz DRG stimulation (3). Similarly, our preclinical animal models showed that maximal analgesic effect is reached with 20 Hz-tonic DRG stimulation (7,9,10,18). Here, we examined the effectiveness of burst DRG stimulation at two different frequencies compared to 20 Hz-tonic DRG stimulation. Two weeks after TNI and electrode implantation, mechanical hypersensitivity was evident by elevated rates of hyperalgesia responses to noxious mechanical stimulation (Pin, Fig. 2a). Following this baseline testing session, testing was again performed at 15, 30, and 60 min timepoints during DRG stimulation. Residual effects were tested at 0, 15, 30, and 60 min timepoints after stopping the stimulation. DRG stimulation with both tonic (20 Hz) and burst (20 and 40 Hz) stimulation waveforms with intensities of 50%MT (Fig. 2a; F21,252 = 5.26, p < 0.0001), 70%MT (Fig. 2b; F21,252 = 9.51, p < 0.0001), 90MT (Fig. 2c; F21,252 = 10, p < 0.0001) showed analgesic effects compared to pre-DRG stimulation baseline. Comparison between groups by AUC analysis confirmed analgesic effect of treatments was enhanced by increasing stimulation intensity (Fig. 2d; F6,72 = 2.89, p = 0.014), but no statistically significant difference was observed between DRG stimulation with different waveforms though there was difference between sham DRG stimulation and DRG stimulation with all stimulation waveforms with different intensities (Fig. 2e). TNI also reduced the threshold for withdrawal from non-noxious mechanical stimulation (von Frey, Fig. 3a). DRG stimulation with both tonic (20 Hz) and burst (20 and 40 Hz) stimulation waveforms with intensities of 50%MT (Fig. 3a; F21,252 = 2.83, p < 0.0001), 70%MT (Fig. 3b; F21,252 = 2, p = 0.0005), 90%MT (Fig. 3c; F21,252 = 3, p < 0.0001) showed analgesic effects compared to pre-DRG stimulation baseline. Comparison between groups by AUC analysis showed no difference in analgesic effects between different stimulation intensities within the same waveform (Fig. 3d; F6,73 = 1.32, p = 0.26), and between different waveforms within the same intensity (Fig. 3e). Motor threshold amplitudes tested with burst waveforms were lower than that by 20 Hz-tonic waveform (Supporting Information Fig. S1).
Figure 2.
Effects of DRG stimulation with different waveforms and intensities on sensation to noxious stimulation (Pin) on rats with TNI. (a) Time course for effects of DRG stimulation with intensities of 50% MT. (b) Time course for effects of DRG stimulation with intensities of 70% MT. (c) Time course for effects of DRG stimulation with intensities of 90% MT. (d) AUC analysis shows that analgesic effects within each frequency patterns are intensity-dependent. (e) AUC analysis shows that analgesic effects of DRG stimulation with same intensity are frequency pattern independent. Results are means ± SEM. “1” p < 0.05, “2” p < 0.01, “3” p < 0.001 compared to data immediately before DRG stimulation by the Dunnett’s test following two-way repeated-measures ANOVA on ranks. *p < 0.05, **p < 0.01, ***p < 0.001 by the Tukey test following two-way ANOVA. n represents the number of animals.
Figure 3.
Effects of DRG stimulation with different waveforms and intensities on sensation to non-noxious stimulation (von Frey) on rats with TNI. (a) Time course for effects of DRG stimulation with intensities of 50% MT. (b) Time course for effects of DRG stimulation with intensities of 70% MT. (c) Time course for effects of DRG stimulation with intensities of 90% MT. (d) AUC analysis shows that analgesic effects within burst stimulations are intensity independent. (e) AUC analysis shows that analgesic effects of DRG stimulation with same intensity are frequency pattern independent. Results are means ± SEM. “1” p < 0.05, “2” p < 0.01, “3” p < 0.001 compared to data immediately before DRG stimulation by the Dunnett’s test following two-way repeated-measures ANOVA. n represents the number of animals.
Spontaneous Pain-Related Behavior After TNI Was Relieved by DRG Stimulation
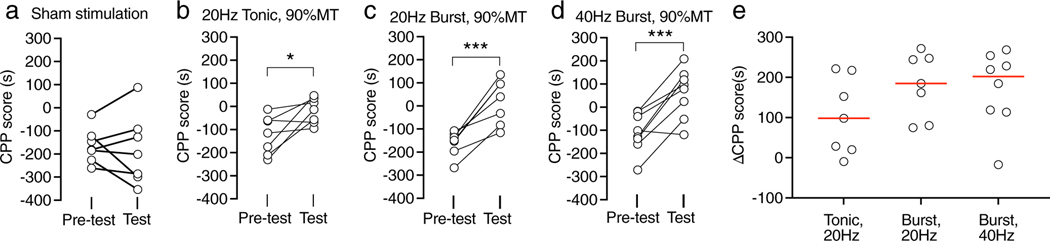
To determine whether DRG stimulation analgesia with different waveforms is effective in motivating a place choice by relieving spontaneous pain (9), we measured the ability of DRG stimulation to condition a place preference. A 20 Hz-tonic DRG stimulation has been shown to be effective to induce place preference in rats with TNI and did not induce place preference and avoidance in rats without painful nerve injury in our prior study (9). Similar to that, 40 Hz-burst DRG stimulation also did not induce place preference and avoidance in rats without painful nerve injury (Supporting Information Fig. S2). Sham DRG stimulation did not induce a preference in rats with TNI (Fig. 4a). TNI rats developed a preference for the chamber paired with L4 DRG stimulation with different stimulating waveforms at 90%MT (Fig. 4b–d), which indicates the effectiveness of DRG stimulation in alleviating spontaneous pain. We compared the effectiveness of the different treatments using the time differences between the preference on test day and preconditioning day for each group (Fig. 4e). Although there was no statistical significance among those treatments (F2,19 = 1.5, Pp= 0.25), there was a trend that burst stimulation might have stronger effects than tonic stimulation.
Figure 4.
Effects of DRG stimulation with 90%MT on conditioned place preference test in rats with tibial nerve injury. (a) Sham DRG stimulation could not induce CPP. A 20 Hz-tonic (b), 20 Hz-burst (c), and 40 Hz-burst (d) with intensities of 90%MT-induced CPP. (e) Comparison between treatments with different stimulation waveforms by normalizing the test to the pretest baseline. Red bars represent means. *p < 0.05, ***p < 0.001 by the paired t-test (a–d). Each dot represents one animal.
Long-Term DRG Stimulation Did Not Alter Markers of Neuroinflammation and Injury
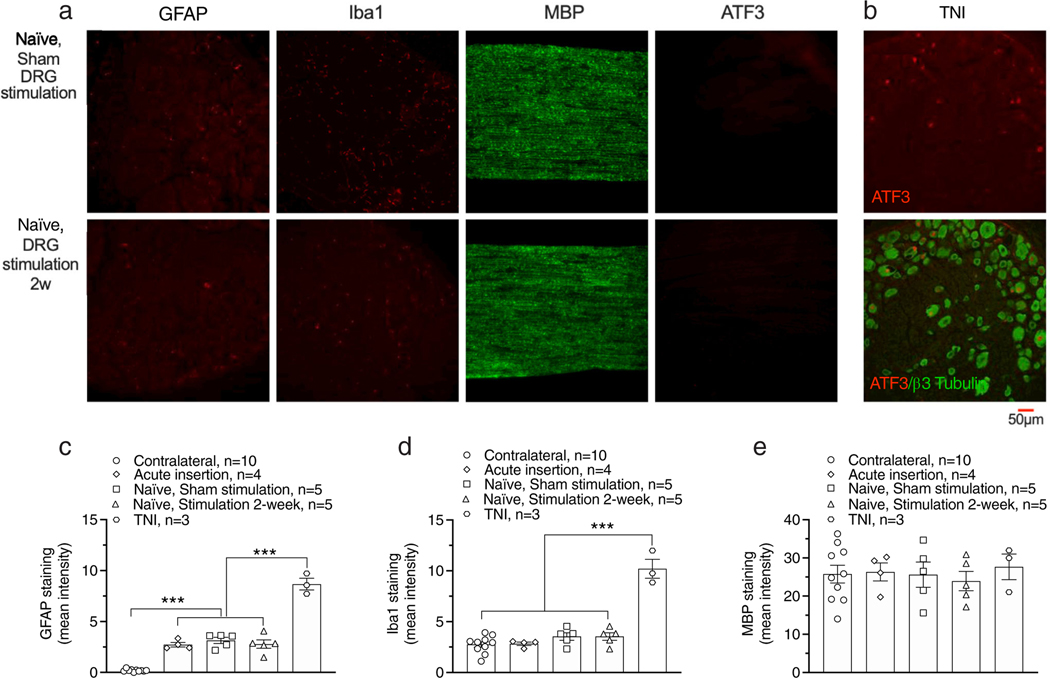
To test whether long-term DRG stimulation may directly harm sensory neurons, we used rats without nerve injury and examined the effects of two-week DRG stimulation on the expression of GFAP, Iba-1, ATF3, and MBP, which are markers of activated astrocytes, activated microglia, injury, and process of myelination respectively. Electrode implantation alone elevated GFAP expression (Fig. 5a,b; F4,22 = 119.6, p < 0.0001), but at a level much below that induced by TNI (Fig. 5b–e). Iba-1, MBP, and ATF3 were unaffected by electrode insertion (Fig. 5a,c,d). A two-week 40 Hz-burst DRG stimulation did not change expression of these markers (Fig. 5a,c,d,e).
Figure 5.
Effects of DRG stimulation with 40 Hz-burst-90%MT on expression of GFAP, Iba-1, MBP, and ATF3 in rats without nerve injury. (a) Samples showing expression of GFAP, Iba-1, MBP, and ATF3 with two-week sham DRG stimulation (top) and two-week DRG stimulation with pattern of 40 Hz-burst-90%MT (bottom). (b) Expression of ATF3 in DRG from rat with TNI as positive control. Summary of expression of GFAP (c), Iba-1 (d), MBP (e), and ATF3 (f). Results are means ± SEM. ***p < 0.001 by the Tukey test following one-way ANOVA. n represents the number of animals.
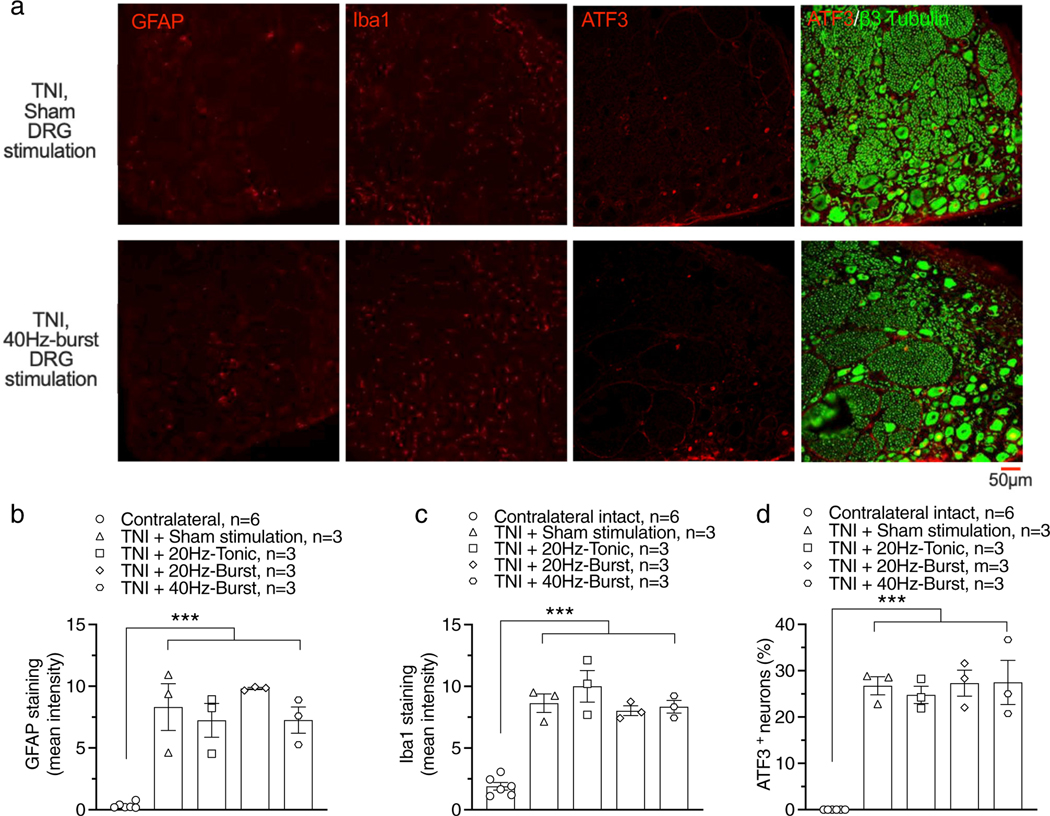
Long-Term DRG Stimulation Did Not Affect TNI-Induced Expression of Neuroinflammation and Injury Markers
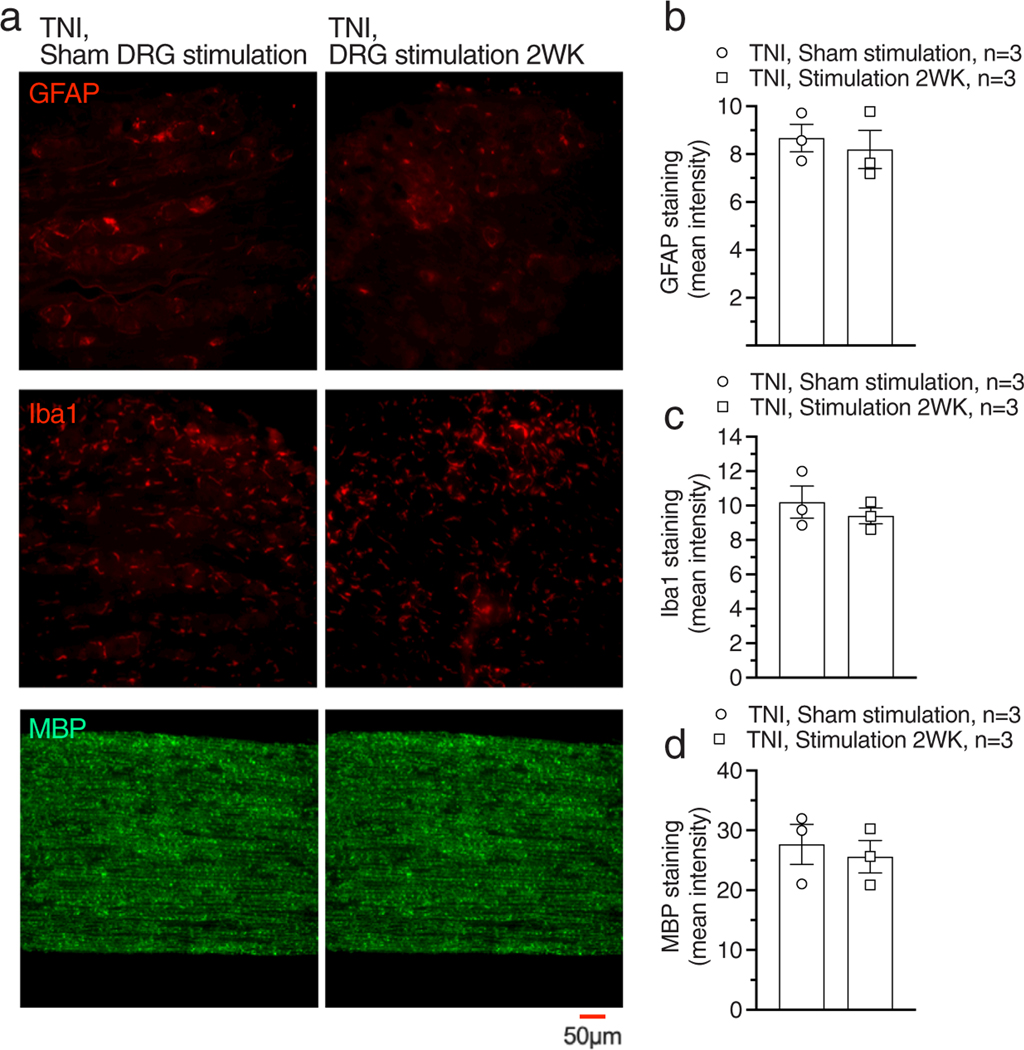
In a previous study, we found that long-term continuous DRG stimulation prevented inflammation and joint damage in an rat arthritis model (18). This raises the possibility that DRG stimulation might similarly reduce expression of inflammatory changes in the DRG that follow painful nerve injury. To test this, we first examined the effects of DRG stimulation (four hours stimulation/day for four days, another six hours DRG stimulation before tissue harvesting) on expression of GFAP, Iba-1, and ATF3. After TNI, expression of GFAP (F4,13 = 21.07, p < 0.0001), Iba-1 (F4,13 = 34.04, p < 0.0001), or ATF3 (F4,13 = 40.66, p < 0.0001) was elevated comparing with contralateral controls (Fig. 6a–d). DRG stimulation with different stimulating waveforms had no effect on expression of GFAP, Iba-1, or ATF3 comparing with sham DRG stimulation (Fig. 6a–d). We further tested whether extending the duration of 20 Hz-tonic stimulation to two weeks would alter those markers but still found no effect on expression of GFAP, Iba-1, and MBP (Fig. 7a,d).
Figure 6.
Effects of six hours DRG stimulation with different waveforms on expression of GFAP, Iba-1, and ATF3 in rats with TNI. (a) Samples showing expression of GFAP, Iba-1, and ATF3 with two-week sham DRG stimulation (top) and two-week DRG stimulation with pattern of 40 Hz-burst-90%MT (bottom). Summary of expression of GFAP (b), Iba-1 (c), and ATF3 (d). n represents the number of animals.
Figure 7.
Effects of two-week continuous 20 Hz-Tonic DRG stimulation on expression of GFAP, Iba-1, and MBP in rats with TNI. (a) Samples showing expression of GFAP, Iba-1, and ATF3 with two-week sham DRG stimulation (top) and two-week DRG stimulation with pattern of 40 Hz-burst-90%MT (bottom). Summary of expression of GFAP (b), Iba-1 (c), and BMP (d). n represents the number of animals.
DISCUSSION
In this study, we tested analgesic effects of DRG stimulation with 20 Hz-burst and 40 Hz-burst waveforms in an animal model of painful nerve injury. We found that DRG stimulation with 20 Hz-burst, and 40 Hz-burst can relieve hyperalgesia measured by sensory tests and spontaneous pain measured by the CPP test. Additionally, immunohistochemistry results indicate that DRG stimulation does not result in evidence of tissue damage. These findings suggest that DRG stimulation with a burst waveform might be as effective as DRG stimulation with a tonic waveform in treating neuropathic pain.
DRG stimulation is effective for treating patients with chronic neuropathic pain and also possibly for non-neuropathic pain (1–3,19) and 20 Hz-tonic DRG stimulation has also demonstrated its effectiveness in alleviating neuropathic pain in preclinical animal model studies (9,10). A previous preclinical study also demonstrated that DRG 20 Hz-tonic stimulation can relieve both somatosensory and affective components of neuropathic pain and osteoarthritis pain (9). In addition to 20 Hz-tonic stimulation, a study using a rodent painful diabetic peripheral neuropathic model showed that DRG stimulation with a 40 Hz-burst waveform was effective in relieving hypersensitivity to non-noxious stimulation measured with von Frey test (20). However, the effects of DRG stimulation on hyperalgesia with noxious stimulation and the affective aspect of pain have not been investigated for stimulation with a burst waveform. SCS with a burst stimulation waveform has been proven to be more effective in treating medically intractable chronic pain than tonic stimulation waveform by modulating the ascending medial and lateral pain pathway and descending inhibitory pathway (8,21,22). In the present study, both tonic and burst stimulation have demonstrated therapeutic effects on somatosensory and affective components of neuropathic pain in a stimulation intensity-dependent manner. Although there was no statistical significance between tonic and burst, burst DRG stimulation has a trend for a stronger effect in affective component of pain (Fig. 4). Intensity of the stimulation waveform is another important factor relevant to analgesic efficacy of DRG stimulation. In our previous study, 20 Hz-tonic DRG stimulation with higher intensity below the motor threshold amplitude showed stronger neuromodulatory effects in both sensory test and CPP in rats with TNI (9). Consistent with that study, 20/40 Hz-burst DRG stimulation with higher intensity (70% and 90% of MT) show better analgesic effects than DRG stimulation with lower stimulating intensity (50% of MT) (Fig. 2). A future study with a finer stimulation amplitude resolution would provide more information about this aspect of stimulation.
The mechanism of analgesic effects of DRG stimulation is actively being explored. We have found that tonic DRG stimulation produces analgesic effects by activating somata of sensory neurons, leading to enhanced T-junction filtering that blocks AP propagation from the periphery in a frequency-dependent manner (7). Use-dependent accumulation of intracellular Ca2+ induced by AP trains can decrease membrane excitability by activating Ca2+-sensitive K+ channels (23,24). This accumulated intracellular Ca2+ ([Ca2+]c) also activates Ca2+-calmodulin kinase II (25), which further amplifies Ca2+ influx, and retains its activated state long after intracellular Ca2+ levels return to baseline (26). The kinetics of Ca2+ extrusion and sequestration after Ca2+ influx are such that [Ca2+]c levels climb during AP trains in a rate-dependent fashion, with maximal [Ca2+]c levels being achieved at lower rates for C-type units than for Aδ and Aβ-type neurons (27). Thus, different stimulation frequencies and patterns may have selective effects on subsets of neurons. Future study will be required to explore the influences on [Ca2+]c and resulting neuronal excitability in response to tonic and burst stimulations.
In our previous study, long-term continuous DRG stimulation prevented peripheral tissue inflammation and joint damage in an arthritis rat model (18). However, in the present study, DRG stimulation with various waveforms and duration were unable to reduce TNI-induced changes within the DRG of injury and inflammation biomarkers (Figs. 6 and 7). This suggests that analgesic effects of DRG stimulation may not be related to regulation of inflammatory or neuronal soma response to axonal injury. Continuous DRG stimulation in naïve animals for two weeks with 20 Hz tonic waveform also did not affect biomarkers relevant to injury or inflammation (Fig. 5), which reflects the safety of DRG stimulation.
CONCLUSION
All stimulation modes (20 Hz-tonic, 20 Hz-burst, and 40 Hz-burst) alleviated neuropathic pain in a manner dependent on stimulation intensity. We also note that both tonic and burst waveforms provided positive motivation as evidence of analgesia for spontaneous pain in the CPP test. One limitation of this study is that we tested only tonic stimulation waveform for continuous two week stimulation on expression of markers of neuroinflammation and injury. Future studies with extended stimulation parameter variations along with longer stimulation duration will provide more detailed information about the therapeutic possibilities of burst waveform in DRG stimulation.
Supplementary Material
Acknowledgments
Source(s) of financial support: This work was supported by Abbott and National Institutes of Health Grant R01NS103812.
Footnotes
For more information on author guidelines, an explanation of our peer review process, and conflict of interest informed consent policies, please go to http://www.wiley.com/WileyCDA/Section/id-301854.html
SUPPORTING INFORMATION
Additional supporting information may be found online in the supporting information tab for this article.
COMMENT
This is a necessary step forward for merging the two therapies and seeing how they could potentially work synergistically in the treatment of pain.
Conflict of Interest: Mr. Ian Segel, Mr. Hai Tran, Dr. Quinn H. Hogan, Dr. Bin Pan, and Dr. Guoliang Yu report grants from Abbott and grants from the National Institutes of Health during the conduct of the study. Dr. Hyun-Joo Park and Dr. Erika Ross are employees of Abbott.
REFERENCES
- 1.Liem L, Russo M, Huygen FJ et al. One-year outcomes of spinal cord stimulation of the dorsal root ganglion in the treatment of chronic neuropathic pain. Neuromodulation. 2015;18:41–48. discussion 48–49. [DOI] [PubMed] [Google Scholar]
- 2.Eldabe S, Burger K, Moser H et al. Dorsal root ganglion (DRG) stimulation in the treatment of phantom limb pain (PLP). Neuromodulation. 2015;18:610–616. discussion 616–617. [DOI] [PubMed] [Google Scholar]
- 3.Deer TR, Levy RM, Kramer J et al. Dorsal root ganglion stimulation yielded higher treatment success rate for complex regional pain syndrome and causalgia at 3 and 12 months: a randomized comparative trial. Pain 2017;158:669–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liem L, Russo M, Huygen FJ et al. A multicenter, prospective trial to assess the safety and performance of the spinal modulation dorsal root ganglion neurostimulator system in the treatment of chronic pain. Neuromodulation. 2013; 16:471–482. discussion 482. [DOI] [PubMed] [Google Scholar]
- 5.Deer TR, Grigsby E, Weiner RL, Wilcosky B, Kramer JM. A prospective study of dorsal root ganglion stimulation for the relief of chronic pain. Neuromodulation. 2013;16:67–71. discussion 71–62. [DOI] [PubMed] [Google Scholar]
- 6.Kent AR, Min X, Hogan QH, Kramer JM. Mechanisms of dorsal root ganglion stimulation in pain suppression: a computational modeling analysis. Neuromodulation. 2018; 21:234–246. [DOI] [PubMed] [Google Scholar]
- 7.Chao D, Zhang Z, Mecca CM, Hogan QH, Pan B. Analgesic dorsal root ganglionic field stimulation blocks conduction of afferent impulse trains selectively in nociceptive sensory afferents. Pain 2020;161:2872–2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Ridder D, Vanneste S. Burst and tonic spinal cord stimulation: different and common brain mechanisms. Neuromodulation. 2016;19:47–59. [DOI] [PubMed] [Google Scholar]
- 9.Yu G, Segel I, Zhang Z, Hogan QH, Pan B. Dorsal root ganglion stimulation alleviates pain-related behaviors in rats with nerve injury and osteoarthritis. Anesthesiology 2020;133:408–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan B, Yu H, Fischer GJ, Kramer JM, Hogan QH. Dorsal root ganglionic field stimulation relieves spontaneous and induced neuropathic pain in rats. J Pain 2016; 17:1349–1358. [DOI] [PubMed] [Google Scholar]
- 11.Van Buyten JP, Smet I, Liem L, Russo M, Huygen F. Stimulation of dorsal root ganglia for the management of complex regional pain syndrome: a prospective case series. Pain Pract 2015;15:208–216. [DOI] [PubMed] [Google Scholar]
- 12.King T, Vera-Portocarrero L, Gutierrez T et al. Unmasking the tonic-aversive state in neuropathic pain. Nat Neurosci 2009;12:1364–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994;53:55–63. [DOI] [PubMed] [Google Scholar]
- 14.Hogan Q, Sapunar D, Modric-Jednacak K, McCallum JB. Detection of neuropathic pain in a rat model of peripheral nerve injury. Anesthesiology 2004;101:476–487. [DOI] [PubMed] [Google Scholar]
- 15.Wu HE, Gemes G, Zoga V, Kawano T, Hogan QH. Learned avoidance from noxious mechanical simulation but not threshold semmes weinstein filament stimulation after nerve injury in rats. J Pain 2010;11:280–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Linda H, Skold MK, Ochsmann T. Activating transcription factor 3, a useful marker for regenerative response after nerve root injury. Front Neurol 2011;2:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang J, Li H, Teng H et al. Regulation of peripheral clock to oscillation of substance P contributes to circadian inflammatory pain. Anesthesiology 2012;117:149–160. [DOI] [PubMed] [Google Scholar]
- 18.Pan B, Zhang Z, Chao D, Hogan QH. Dorsal root ganglion field stimulation prevents inflammation and joint damage in a rat model of rheumatoid arthritis. Neuromodulation. 2018;21:247–253. [DOI] [PubMed] [Google Scholar]
- 19.Antony AB, Schultheis BC, Jolly SM, Bates D, Hunter CW, Levy RM. Neuromodulation of the dorsal root ganglion for chronic postsurgical pain. Pain Med 2019;20:S41–S46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franken G, Debets J, Joosten EAJ. Nonlinear relation between burst dorsal root ganglion stimulation amplitude and behavioral outcome in an experimental model of painful diabetic peripheral neuropathy. Neuromodulation. 2020;23:158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Ridder D, Perera S, Vanneste S. Are 10 kHz stimulation and burst stimulation fundamentally the same? Neuromodulation 2017;20:650–653. [DOI] [PubMed] [Google Scholar]
- 22.Kirketeig T, Schultheis C, Zuidema X, Hunter CW, Deer T. Burst spinal cord stimulation: a clinical review. Pain Med 2019;20:S31–S40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hogan Q, Lirk P, Poroli M et al. Restoration of calcium influx corrects membrane hyperexcitability in injured rat dorsal root ganglion neurons. Anesth Analg 2008; 107:1045–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lirk P, Poroli M, Rigaud M et al. Modulators of calcium influx regulate membrane excitability in rat dorsal root ganglion neurons. Anesth Analg 2008;107:673–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kostic S, Pan B, Guo Y et al. Regulation of voltage-gated ca(2+) currents by ca(2+)/calmodulin-dependent protein kinase II in resting sensory neurons. Mol Cell Neurosci 2014;62:10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang Q, Bangaru ML, Kostic S et al. Ca(2)(+)-dependent regulation of ca(2)(+) currents in rat primary afferent neurons: role of CaMKII and the effect of injury. J Neurosci 2012;32:11737–11749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gemes G, Rigaud M, Koopmeiners AS, Poroli MJ, Zoga V, Hogan QH. Calcium signaling in intact dorsal root ganglia: new observations and the effect of injury. Anesthesiology 2010;113:134–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.