Abstract
This review aims to give an insight into the main hazards currently found in smoked meat and fish products. Literature research was carried out on international databases such as Access to Global Online Research in Agriculture (AGORA) database, Science direct, and Google scholar to collect and select 92 relevant publications included in this review. The smoking process was described and five hazards mostly found in smoked fish and meat were presented. The heat‐induced compounds such as polycyclic aromatic hydrocarbons, heterocyclic amines, and nitrosamines were found in smoked fish and meat. Other hazards such as biogenic amines and heavy metals were also present in smoked fish and meat. The levels of these hazards reported from the literature exceeded the maximal limits of European Union. A brief description of risk assessment methodology applicable to such toxic compounds and risk assessment examples was also presented in this review. As most of the hazards reported in this review are toxic and even carcinogenic to humans, actions should be addressed to reduce their presence in food to protect consumer health and to prevent public health issue.
Keywords: benzo(a)pyrene, food safety, heat‐induced compounds, nitrosamines, systematic review
‐Main hazards reported in smoked meat and fish are toxic and carcinogenic to humans
‐Levels of chemical hazards reported from literature exceeded the maximal limits of European Union
‐Consumption of smoked meat and fish presents risk for consumers ‘health
1. INTRODUCTION
Fish and meat preservation is a big challenge in different regions of the world. Among fish preservation methods, smoking is the mostly used method (Berkel et al., 2005; Nout et al., 2003; Toth & Potthast, 1984). It extends shelf life and confers special taste and aroma to the end products (Igwegbe et al., 2015; Yusuf et al., 2015). Despite these advantages, the consumption of smoked fish or meat products presents health concern due to process contaminants. Several authors reported acrolein, acrylamide, furan, heterocyclic amines, monochloropropanediol (MCPD), nitrosamine, and polycyclic aromatic hydrocarbons (PAHs) as heat‐induced toxic compounds in foods and dealt with their risk assessment (Akpambang et al., 2009; Alomirah et al., 2011; Domingo & Nadal, 2015; Larsen, 2006; Mey et al., 2014; Skog, Johansson, & Jaègerstad, 1998; Stadler & Lineback, 2009; Swann, 1977; Yurchenko & Molder, 2007). Among these various heat‐induced compounds, PAHs and heterocyclic amines are mainly associated with smoking or grilling process. Moreover, due to the amino acid composition of fish and meat, some toxic compounds like biogenic amines and even nitrosamines may be formed. Other environmental hazards like heavy metals can also be found in fish and meat. The consumption of food contaminated with these compounds could result in adverse effects on human health including cancer (EFSA (European Food Safety Authority), 2008; EFSA (European Food Safety Authority), 2011). This literature review aims to focus on chemical hazards (nitrosamines, heterocyclic amines, PAHs, heavy metals, and biogenic amines) commonly reported in smoked fish and meat.
2. METHODOLOGY
Publications included in this review were from international databases such as Access to Global Online Research in Agriculture (AGORA) database, Science direct, and google scholar. Original and review papers were collected on December 2019 and updated on July 2021 using key words such as “smoking,” “heterocyclic amines,” “PAHs,” “benzo(a)pyrene (BaP),” “heavy metals,” “nitrosamine,” “biogenic amines,” “histamine,” “risk assessment,” “fish,” and “meat.” Only 112 relevant papers in accordance with the topic of this review were included on the basis of their keywords. The software Endnote, version 13 was used to manage and rank the collected publications in different subgroups according to each topic.
3. RESULTS
3.1. Smoking process
Smoking is a food processing method used as a preservation method to extend shelf life of food by reducing moisture content and microorganism load (Köse, 2010). Smoking is also used to improve sensorial characteristics including taste, aroma, and appearance of smoked fish and meat (Berkel et al., 2005; Codex Alimentarius, 2009). Two types of smoking processes are commonly used. The “cold” smoking process in which the temperature of the product does not exceed 30°C and “hot” smoking process during which food such as fish is well cooked and temperature in the center of the product may reach up to 60–85°C (Berkel et al., 2005; Stołyhwo & Sikorski, 2005). According to Berkel et al. (2005), there is a third smoking process called smoke‐drying which is hot smoking followed by a drying step carried out in the smoking equipment. Hot smoking and smoke‐drying are frequently used to preserve fish in African countries (Assogba et al., 2019). According to Codex Alimentarius (2013), the smoke‐drying process enables us to obtain dried products with a water activity lower or equal to 0.75, allowing keeping the end product at room temperature and to control bacterial and fungi alteration.
During smoking, fish or meat and their products are submitted directly or indirectly to smoke produced by partial burning of wood. Direct smoking is a process during which fish or meat is laid on mesh trays above the embers, whereas in indirect smoking, smoke is produced in a separate chamber and fish is smoked in another chamber (Codex Alimentarius, 2009). Traditional fish smoking is carried out in kilns (barrel of locally made clay) using fuel such as wood, charcoal, wood sawdust, wood chips, bagasse, corn cobs, coconut husks, and shells (Assogba et al., 2019; Codex Alimentarius, 2009; Kpoclou et al., 2014; Stołyhwo & Sikorski, 2005). Smoke is composed of a mixture of about 380 compounds, mainly phenols, aldehydes, ketones, organic acids, alcohols, esters, hydrocarbons, and various heterocyclic compounds (Codex Alimentarius, 2009; Toth & Potthast, 1984). Some of them such as phenolic, carbonyls, furan derivatives, organic acids, and their esters affect the sensory quality, but could also improve the shelf life of the product by inhibiting the growth of spoilage bacteria (Ciecierska & Obiedzinski, 2007; Gomez‐Guillén et al., 2009; Igwegbe et al., 2015; Stołyhwo & Sikorski, 2005; Yusuf et al., 2015). However, carcinogenic compounds such as PAHs, nitrosamines, and heterocyclic amines may be formed during the smoking process either from pyrolysis of organic matter and transferred inside the food or directly produced inside the food as a result of reactions between food composition and heat (Skog et al., 1998; Stołyhwo & Sikorski, 2005; Viegas et al., 2012; Yurchenko & Molder, 2007). Figure 1 shows different smoking methods of fish and meat reported from the literature.
FIGURE 1.
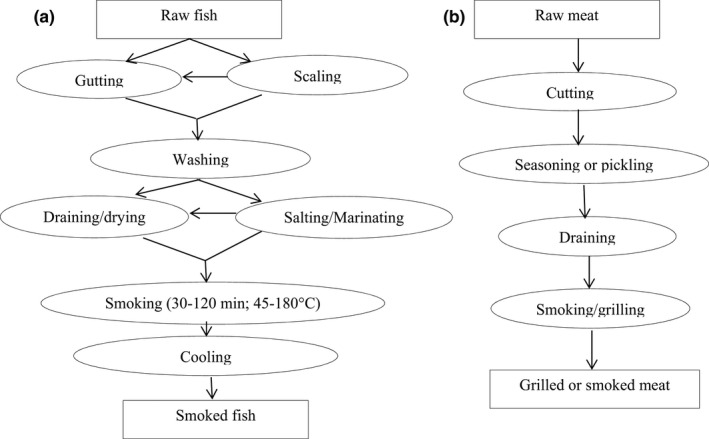
(a) Flow chart of smoked fish production (Adeyemi et al., 2013; Adeyeye et al., 2015; Assogba et al., 2019; Dègnon et al., 2013; Goulas & Kontominas, 2005; Ubwa et al., 2015) and (b) smoked meat production (Poligné et al., 2001; Roseiro et al., 2011)
3.2. Nitrosamine, heterocyclic amines, and polycyclic aromatic hydrocarbons
3.2.1. Nitrosamines
Nitrosamines (Figure 2) or N‐nitroso compounds (N‐nitrosodiméthylamine (NDMA), N‐nitrosométhyléthylamine (NMEA), N‐nitrosodiethylnitrosoamine (NDEA), Nitrosodipropylamine (NDPA), N‐nitrosodibutylamine (NDBA), N‐nitrosomorpholine (NMOR), 1‐nitrosopiperidine (NPIP), 1‐nitrosopyrrolidine (NPYR), N‐nitrosodiéthanolamine (NDELA), 1‐methyl‐3‐nitro‐1‐nitrosoguanidine (MNNG), N‐nitroso‐N‐ethylbutylamine (NEBA), N'‐nitrosoanabasine (NAB), and 4‐(N‐nitrosomethylamino)‐1‐(3‐pyridyl)‐1‐butanone (NNK)) are a group of toxic compounds produced mainly in meat products during heat processing (Belitz et al., 2009; Domanska & Kowalski, 2003; Reinik, 2007).
FIGURE 2.
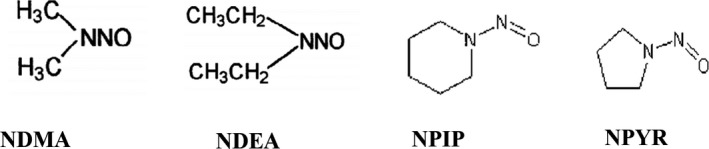
Chemical structure of four examples of nitrosamines: N‐nitrosodiméthylamine (NDMA); N‐nitrosodiethylnitrosoamine (NDEA); 1‐nitrosopiperidine (NPIP) and 1‐nitrosopyrrolidine (NPYR) (PubChem, 2020).
N‐nitroso compounds can be subdivided into two groups (Herrmann et al., 2015): volatile nitrosamines (NDMA, NMOR, NMEA, NPYR, NDEA, and NPIP) and nonvolatile nitrosamines (N‐nitrososarcosine (NSAR), N‐nitrosoproline (NPRO), N‐nitrosomethylaniline (NMA), N‐nitroso‐thiazolidine‐4‐carboxylic (NTCA) acid, and N‐nitroso‐2‐methylathiazolidine‐4‐carboxylic acid (NMTCA)).
The human exposure to N‐nitroso compounds is from environment, tobacco smoke, and the diet which has been identified to be the main source (Jakszyn et al., 2005). N‐nitroso compounds formation requires substrates (primary amine, secondary amine, tertiary amine, amides, secondary amino acids, quaternary ammonium salts, etc.) and a nitrosating agent (nitrite, nitrates, and nitrogen oxides) through several reactions (Filho et al., 2003; INERIS (Institut National de l'Environnement Industriel et des RISques), 2014; Reinik, 2007). Nitrogen oxides are formed either from the addition of nitrate and/or nitrite to foods or from the heating process of food such as smoking, during which nitrogen molecular can be oxidized or present in the smoke (INERIS (Institut National de l'Environnement Industriel et des RISques), 2014; Jakszyn et al., 2005). Al Bulushi et al. (2009) reported NPYR and NPIP in vitro formation at high temperature (160°C, 2 h). Microorganisms (Aspergillus sp.; Pseudomonas sp.; P. stutzeri; E. coli) can be involved in N‐nitrosamine formation by reducing nitrates to nitrites, by degrading proteins to amines and amino acids, or by producing enzymes working at a suitable pH (2–4) for nitrosation (Al Bulushi et al., 2009; Ayanaba & Alexander, 1973; Drabik‐Markiewicz et al., 2009; Jägerstad & Skog, 2005; Jägerstad et al., 1998; Mills & Alexander, 1976; Rostkowska et al., 1998; Yurchenko & Molder, 2007). Nitrite and nitrate are frequently used in meat preservation and lead to nitrosamines formation due to reaction with amino compounds either in the stomach or within the food product (Filho et al., 2003; Pan et al., 2011; Sebranek & Bacus, 2007; Swann, 1977). It is the case of meat products such as sausages, ham, and salami where the addition of nitrite and nitrate was used to inhibit the formation of spoilage bacteria (Drabik‐Markiewicz et al., 2009; Filho et al., 2003; Hustad et al., 1973). The nitrosamines are found in smoked meat, grilled meat, canned meat, and pickled meat at different levels (Table 1), but not in raw meat where there is not enough nitrite and amines for its production (Yurchenko & Molder, 2007). Studies carried out on nitrosamine determination in fish products mostly focused on NDMA determination because of its precursor dimethylamine (DMA) which is widely formed in marine fish (Al Bulushi et al., 2009). NDMA is classified in Group 2A (probably carcinogenic to humans) by the International Agency for Research on Cancer (IARC (International Agency for Research on Cancer), 2010), whereas N‐nitrosonornicotine (NNN) and 4‐(N‐nitrosomethylamino)‐1‐(3‐pyridyl)‐1‐butanone (NNK) are classified in Group 1 (carcinogenic to humans). Belitz et al. (2009) reported NDMA in cured meat processed with pickling with levels ranging between 0.5 and 15 μg/kg (Table 1). Herrmann et al. (2014) also reported NDMA in smoked pork fillet (1.3 µg/kg) and smoked ham (2.1 µg/kg).
TABLE 1.
Concentrations of volatile N‐nitrosamines in various smoked or grilled fish and meat as reported from the literature
| Processing | N‐nitrosamines (µg/kg) | References | |||||
|---|---|---|---|---|---|---|---|
| NDMA | NDEA | NPYR | NPIP | NDBA | NDPA | ||
| Smoked meat | 0.2–1.4 | 0.3–0.7 | 0.2–19.5 | 0.4–2.3 | 0.4–0.9 | 0.3 | Al‐Kaseem et al. (2014); Reinik (2007); Yurchenko and Molder (2007) |
| Cured meat (pickling salt) | 0.5–15 | nd | 3.2–4.2 | nd | Nd | nd | Belitz et al. (2009) |
| Grilled meat | 0.2–3.2 | 0.3–0.6 | 0.8–14.6 | 1.0–2.8 | 0.2–0.4 | ˂0.1–0.3 | Al‐Kaseem et al. (2014); Reinik (2007); Yurchenko and Molder (2007) |
| Smoked fish | ˂0.1–2.8 | ˂0.1–0.5 | 0.4–25.4 | ˂0.2–7.8 | ˂0.2–6.0 | nd | Reinik (2007) |
| Smoked chicken | 1.2–2.1 | ˂0.1–0.3 | 0.5–22.1 | ˂0.1–5.3 | 0.1–6.3 | nd | |
Abbreviations: nd, not determined;NDBA, N‐nitrosodibuthylnitrosamine; NDEA, N‐nitrosodiethylnitrosamine; NDMA, N‐nitrosodimethylnitrosamine; NDPA, N‐nitrosodipropylamine; NPIP, N‐nitrosopiperidine; NPYR, N‐nitrosopyrrolidine.
Different analytical methods were used to analyze nitrosamines. The method of gas chromatography and mass spectrometry detection with ion monitoring using different columns has been used by several authors to identify and quantify nitrosamines (Filho et al., 2003; Herrmann et al., 2014; Swann et al., 1977; Yurchenko & Molder, 2007). However, only Thermal Energy Analyzer (TEA) detection is recognized as specifically for nitrosamines but expensive (Filho et al., 2003). Filho et al. (2003) developed methods for nitrosamine compounds analysis (extraction, preconcentration, and analysis) which allowed their determination even at trace levels. The separation of nitrosamines was performed using micellar electrokinetic chromatography and confirmation was achieved using gas chromatography coupled with mass spectrometry detection (Filho et al., 2003; Herrmann et al., 2014).
3.2.2. Heterocyclic amines
Heterocyclic amines are toxic compounds produced in meat and fish during processing at temperature over 150°C (Haskaraca et al., 2014; Jägerstad & Skog, 2005; Puangsombat et al., 2011; Sinha et al., 1998; Solyakov & Skog, 2002). According to their chemical structures, two groups of heterocyclic amines can be distinguished: pyrolytic heterocyclic amines also known as amino‐carboline heterocyclic amines and thermic heterocyclic amines composed of imidazo‐quinolines (e.g., IQ ((2‐Amino‐3,4‐dimethylimidazo[4,5‐f]quinolone)), imidazoquinoxalines (e.g., MeIQx (MeIQx (2‐Amino‐3,8‐dimethylimidazo[4,5‐f]quinoxaline)), and imidazopyridines (e.g., PhIP (2‐Amino‐1‐methyl‐6‐phenylimidazo[4,5‐b]pyridine)) (Jägerstad & Skog, 2005; Viegas, Novo, Pinto, et al., 2012). The imidazo‐quinolines, imidazoquinoxalines, and imidazopyridines are three groups of precursors present in raw meat and fish muscle and could be produced from creatine or creatinine, free amino acids, and sugars through the Maillard reaction (Jägerstad & Skog, 2005; Viegas, Novo, Pinto, et al., 2012). The IARC (International Agency for Research on Cancer) classified MeIQx, MeIQ, and PhiP as possibly carcinogenic to humans (Group 2B). The 2‐amino‐3,8‐dimethylimidazo[4,5‐f]quinoxaline (8‐MeIQx) and 2‐amino‐1‐methyl‐6‐ phenylimidazo[4,5‐b]pyridine (PhIP) (Figure 3) are the most abundant heterocyclic amines formed in grilled beef, bacon, fish, and poultry (Turesky, 2007). Skog et al. (1998) reported the presence of heterocyclic amines in smoked fish and fried meat products. The authors showed that the use of wood charcoal induced high production of heterocyclic amines (1.6–4 ng/g MeIQx; 1.5–7.8 ng/g PhIP) contrary to coconut charcoal (0.7–1 ng/g MeIQx; 0.9–3 ng/g PhIP) (data not shown) in grilled salmon and beef samples (Viegas, Novo, Pinto, et al., 2012). Gibis (2016) reported high temperature (180°C and 220°C) and duration as key factors of heterocyclic amines production, mainly IQ, MeIQ, MeIQx, 4,8‐DiMeIQx, and PhIP. Table 2 shows different concentrations of some heterocyclic amines reported from the literature. Very few studies reported the presence of IQ in grilled or smoked foods. Levels of 1.6–2 ng/g were reported in grilled beef (Table 2). However, MeIQx was reported in several foods such as processed bacon and pork (Sinha et al., 1998) with levels ranging from 0.4 to 5.4 ng/g (Table 2). High levels of PhIP (till 480 ng/g) were reported from the literature (Table 2). Even though no maximal limit of heterocyclic amines was reported in the literature, their presence in food is a health concern and adequate food preparation procedures should be implemented having the ALARA (ALARA = as low as reasonably achievable) principle in mind. Lu et al. (2018) reported that the use of different spices (Garlic, onion, red chili, paprika, black pepper, and ginger) before deep‐frying of beef and chicken had inhibitory effects (43%–87%) on the formation of heterocyclic amines (data not shown).
FIGURE 3.
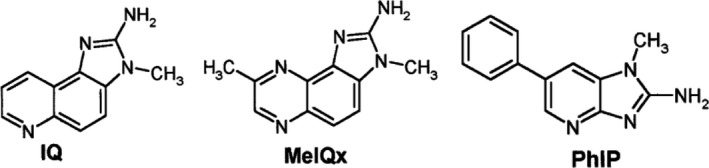
Chemical structure of three examples of heterocyclic amines: IQ ((2‐Amino‐3,4‐dimethylimidazo[4,5‐f]quinolone)); MeIQx (2‐amino‐3,8‐dimethylimidazo[4,5‐f]quinoxaline ) and PhIP (2‐amino‐1‐methyl‐6‐phenylimidazo[4,5‐b]pyridine) (PubChem, 2020)
TABLE 2.
Concentration of main heterocyclic amines in cooked meat, as reported from the literature
| Products | Cooking methods. | Heterocyclic amines (µg/kg) | References | ||||
|---|---|---|---|---|---|---|---|
| MeIQx | DiMeIQx | PhIP | IQ | MeIQ | |||
| Bacon | Pan‐fried | 0.4–4.3 | nd | 0.7–4.8 | nd | nd | Sinha et al. (1998) |
| Oven‐broiled | 1.5–4 | nd | 1.4–30.3 | nd | nd | ||
| microwaved | 0.4–1.5 | nd | 3.1 | nd | nd | ||
| Grilled | 1.0–27 | nd−9.3 | nd−36 | nd | nd |
Knize et al. (1997); Skog et al. (1998). |
|
| Pork | Pan‐fried | 0.4–5.4 | nd | 0.1–2.3 | nd | nd | Sinha et al. (1998) |
| Oven‐broiled | nd | nd | nd | nd | nd | ||
| Beef | Grilled | 0.5–6 | 0.1–1.2 | 0.6–27 | 0.2 | nd |
Fay et al., (1997); |
| Barbecued | 4.4 | 2.7 | 38 | 1.6 | nd | Skog et al. (1998) | |
| Chicken | Barbecued | 0.3–9 | 0.1–3.1 | 27–480 | nd | nd | Knize et al. (1996); Murray and Lynch (1993); Sinha et al. (1995) |
| Grilled | 0.6–2.3 | 0.5–3.1 | 21–315 | nd | nd | Knize, Salmon, Hopmans, et al. (1997); Knize et al. (1997); Wakabayashi et al. (1993) | |
Abbreviations: nd, not determined; MeIQx = 2‐amino‐3,8‐dimethylimidazo[4,5‐f] quinoxaline; DiMeIQx = 2‐amino‐3,4,8‐trimethylimidazo[4,5‐f]quinoxaline; PhIP = 2‐amino‐1‐methyl‐6‐phenylimidazo[4,5‐b]pyridine; IQ = 2‐amino‐3‐methylimidazo[4,5‐f ]quinoline; MeIQ = 2‐amino‐3,4‐dimethylimidazo[4,5‐f]quinoline.
Heterocyclic amine determination was performed according to methods including extraction, purification injection, and quantification using high‐performance liquid chromatography coupled with diode array and fluorescence detectors (HPLC‐DAD/FLD) (Melo et al., 2008). Heterocyclic amines can also be extracted by solid‐phase extraction and analyzed by reverse phase HPLC or LC/MS (Oz & Yuze, 2016; Santos et al., 2004; Sinha et al., 1998; Viegas, Novo, Pinto, et al., 2012).
3.2.3. Polycyclic aromatic hydrocarbons
Polycyclic aromatic hydrocarbons are toxic compounds having a low solubility in water and constitute a large class of organic compounds, containing 2 or more fused aromatic rings composed of carbon and hydrogen atoms (EFSA (European Food Safety Authority), 2008; SCF (Scientific Committee on Food), 2002). They are produced from incomplete combustion of the organic matter when foods such as fish or meat are processed by smoking, grilling, or roasting (Battisti et al., 2015; EFSA (European Food Safety Authority), 2008; Ingenbleek et al., 2019; Yusuf et al., 2015). Several studies reported that fat dropping in the flame during grilling processing contributes to PAHs formation (Chen et al., 2013; Viegas, Novo, Pinto, et al., 2012). Additionally, studies showed that PAHs formation depends on the type of raw material, smoking methods, fuel and kiln type, smoke composition and degree of exposure to smoke, and combustion temperature (Chen et al., 2013; Codex Alimentarius, 2009; Kpoclou et al., 2014; Stołyhwo & Sikorski, 2005). Traditional smoking or grilling is responsible for the production of high amounts of PAH in meat and fish as reported by Forsberg et al. (2012); Onyango et al. (2012); Iko Afé et al. (2020); Ubwa et al. (2015).
Consumers are exposed to PAHs according to three possible ways: by inhalation, contact with the skin, and consumption of contaminated food (EFSA (European Food Safety Authority), 2008; Silva et al., 2011). Likewise, foods are contaminated with PAHs either by environment (exhaust fumes of the engines, bush fires, etc.) or by traditional food processing (drying, smoking, grilling, etc.) (ANSES (Agence nationale de sécurité sanitaire de l’alimentation, de l’environnement et du travail), 2011). The main route of human exposure to PAHs is diet (ANSES (Agence nationale de sécurité sanitaire de l’alimentation, de l’environnement et du travail), 2011; EFSA (European Food Safety Authority), 2008). PAHs are genotoxic, carcinogenic, and mutagen (EFSA (European Food Safety Authority), 2008; SCF (Scientific Committee on Food), 2002). Due to their genotoxicity, sixteen PAHs have been included in a priority list of the European Union (EU) (SCF (Scientific Committee on Food), 2002). Among these 16 priority EU PAHs, benzo[a]anthracene (BaA), chrysene (CHR), benzo[a]pyrene (BaP), and benzo[b]fluoranthene (BbF) are four PAHs (named PAH4) (Figure 4) relevant in food due to their toxicity and occurrence (EFSA (European Food Safety Authority), 2008). PAHs are metabolized in the liver by cytochrome P450 (CYP1A1 in particular) into compounds named epoxides, which are able to bind to macromolecules such as proteins and nucleic acids (EFSA (European Food Safety Authority), 2008). After ingestion, before to reach the liver, PAHs come in contact with the intestinal microbiota, which can also have a metabolization role. Van de Wiele et al. (2005) evaluated the possible ways of biotransformation of PAHs in the human intestine using a simulator of the human intestinal microbial ecosystem (SHIME). These authors showed that PAHs are bioactivated in colon digestion into estrogenic metabolites, whereas the digestion of the stomach and small intestine does not generate any estrogenic metabolite. Moreover, the inactivation of the colon microbiote eliminated these estrogenic effects, which suggests that the estrogenic activity would be related to the bio‐activation of PAHs by the microbiote of the colon (Van de Wiele & Al, 2005). In addition to be carcinogenic, PAHs can thus be qualified of endocrine disrupters.
FIGURE 4.
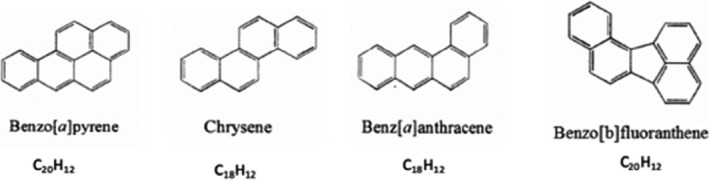
Chemical structure of the PAH4 for which a maximum limit in food has been set in EU (PubChem, 2020).
Among PAHs, BaP is the mostly used one for in vivo toxicological studies. After giving female mice BaP at doses >10 mg/kg b.w. (body weight) per day, impaired fertility was observed in their offspring. The studies on carcinogenicity of PAHs showed that the type of cancer developed after PAHs exposure depends on the exposure way. Indeed, a dermic exposure would induce tumors on the skin, whereas an exposure by oral way would induce gastric tumors. After oral exposure of laboratory animals to BaP, gastrointestinal tract, liver, and lung tumors were reported (EFSA (European Food Safety Authority), 2008). After feeding female mice with diets containing BaP at a concentration of 0, 5, 25, or 100 mg/kg of diet for 2 years, papillomas and carcinomas were observed in the forestomach, oesophagus, and tongue (Culp et al., 1998). Several authors also associate colorectal cancer with meat consumption and some of them established colorectal cancer (Gunter et al., 2007; Ronco et al., 2011; Sinha et al., 2005). Sinha et al. (2005) reported an increased risk of colorectal adenomas resulting from high BaP intake from both meat consumption and other food sources.
Benzo(a)pyrene is classified as carcinogenic to humans (Group 1), and CHR, BaA, and BbF are classified as possibly carcinogenic to humans (Group 2B) (IARC (International Agency for Research on Cancer), 2010).
The European commission set maximum levels of 2 and 12 μg/kg for benzo(a)pyrene (BaP) and the PAH4, respectively, in smoked meat and smoked fish products (EC (European Commission), 2006). Table 3 shows some examples of PAH4 levels reported from the literature (between 2015 and 2020), far above the EU limit of 12 µg/kg (25 times (Iko Afé et al., 2020) or 52 times (Rozentale et al., 2018) this limit).
TABLE 3.
Examples of levels of PAH4 above the maximal EU limit of 12 µg/kg in various smoked or grilled meat and fish
| Products | PAH4 (µg/kg) | References |
|---|---|---|
| Smoked fish | 198 | Ingenbleek et al. (2019) |
| Smoked sprats | 25.6 | Gheorghe et al. (2019) |
| Grilled pork | 53.8–300.6 | Iko Afé et al. (2020) |
| Slavonska kobasica, smoked pork sausage | 12.8–42.6 | Mastanjević et al. (2020) |
| Smoked meat | 56.2–628 | Rozentale et al. (2018) |
| Smoked meat | 34.6 | Rozentale et al. (2015) |
| Barbecued pork | 25.2 | Duedahl‐Olesen et al. (2015) |
| Barbecued beef | 48 |
PAH4: sum of benzo[a]pyrene, chrysene, benzo[b]fluoranthene, and benz[a]anthracene.
Determination of PAH in food can be performed after using an accelerated solvent extractor (ASE) for the extraction, and HPLC coupled with fluorescence and photo diode array detectors (FLD/PDA) or gas chromatography coupled with mass spectrometry (GC/MS) for quantification (Brasseur et al., 2007; Kendirci et al., 2014; Saito et al., 2014; Viegas et al., 2012).
During these past decades, several studies dealt with PAHs in processed food, especially in smoked meat and fish. Some of these studies reported in Tables 3 and 4 were from different continents: Asia (15.8%), Africa (42.1%), and Europe (42.1%). In Africa, Nigeria is the country in which more studies were carried out on PAHs. The PAHs data reported in Table 4 showed that most studies are recent (published between 2017 and 2021), showing that there is a new interest for scientists to update data on the presence of PAHs in smoked or grilled fish and meat. However, for countries such as Benin and Egypt, very few relevant data were available on PAHs contamination in fish and meat products before 2016 (Table 4). Most of the reported concentrations were above the EU maximal limit for BaP, and the highest BaP level (288 µg/kg) was about one hundred and forty‐four times above this limit, showing that consumers could be highly exposed to PAHs through the consumption of this kind of food.
TABLE 4.
Examples of polycyclic aromatic hydrocarbon levels found in smoked or grilled fish and meat products in these past decades
| Country | Type of food | Benzo(a)pyrene (µg/kg) | PAH4 (µg/kg) | References |
|---|---|---|---|---|
| Benin | Smoked Scomber Scombrus | 5.6 ± 2.4 | 52.6 ± 20.4 | Assogba et al. (2021) |
| Smoked Cypselurus cyanopterus | 23.0 ± 19.3 | 90.1 ± 93.3 | ||
| Smoked‐dried Cypselurus cyanopterus | 30.9 ± 16.2 | 153.8 ± 85.8 b | ||
| Grilled pork | 28.9 ± 18.0 | 161.8 ± 87.2 | Iko Afé et al. (2020) | |
| Smoked fish | 21.8 ± 21.2 | 119.3 ± 107.5 | Iko Afé et al. (2021) | |
| Smoked‐dried fish | 78.5 ± 53.8 | 484.2 ± 305.6 | ||
| Croatia | Smoked sprat | 2.2 ± 0.5 | 12.5 ± 1.9 | Racovita et al. (2021) |
| Egypt | Grilled beef meat | 2.7 ± 0.4 | 4.8 ± 0.9 | Darwish al. (2019 |
| Grilled beef (kebab) | 9.2 | ‐ | Eldaly et al. (2016) | |
| Grilled beef (kofta) | 26 | ‐ | ||
| Estonia | Smoked meat products | 3.9 | 26.3 | Rozentale et al. (2018) |
| France | Smoked boucané (pork product) | 6.9 ± 2.4 | ‐ | Poligné et al. (2001) |
| Ghana | Smoked Atlantic chub mackerel (Scomber colias) | 15.5 ± 16.6 | 121.6 ± 98.9 | Asamoah et al. (2021) |
| Smoked barracuda (Sphyraena Sphyraena) | 1.3 ± 2.1 | 68 ± 32.6 | ||
| Ivory Cost | Smoked Cyprinus carpio | 16.9 | ‐ | Ake Assi (2012) |
| Smoked Esox lucius | 56.5 | ‐ | ||
| Smoked Pagellus erythrinus | 36.7 | ‐ | ||
| Smoked Sarda spp. | 55.4 | ‐ | ||
| Smoked Sarpa salpa | 18.0 | ‐ | ||
| Korea | Charcoal broiled pork | 2.6 ± 0.3 | ‐ | Kim et al. (2014) |
| Kuwait | Meat tikka | 2.5 | ‐ | Alomirah et al. (2011) |
| Latvia | Smoked pork | 35.1 | ‐ | Stumpe‐Viksna et al. (2008) |
| Smoked meat products | 8.1 | 53.8 | Rozentale et al. (2018) | |
| Lithuania | Smoked meat products | 1.9 | 9.5 | Rozentale et al. (2018) |
| Nigeria | Smoked Arius heude loti | 5.7 | ‐ | Ubwa et al. (2015) |
| Smoked Mud minnow | 5.4 | ‐ | ||
| Smoked Scomber scombrus | 2.4 | ‐ | Amos‐Tautua et al. (2013) | |
| Smoked Clarias gariepinus | 204 ± 20 | ‐ | Tongo et al., 2017; Zachara et al. (2017) | |
| Smoked Ethmalosa fimbriata | 288 ± 230 | ‐ | ||
| Smoked Scomber scombrus | 7 ± 13 | ‐ | ||
| Smoked Pseudotolithus elongates | 44 | ‐ | Akpan et al. (1994) | |
| Smoked Pomadasys perotati | 25 | ‐ | ||
| Smoked Heterotis niloticus | 19.4 | ‐ | ||
| Grilled suya* | 10.1 | ‐ | Akpambang et al. (2009) | |
| Grilled antelope* | 7.9 | ‐ | ||
| Smoked Clarias gariepinus * | 38.0 | ‐ | ||
| Smoked Selar crumenophthalmus * | 3.0 | ‐ | ||
| Smoked Scomber scombrus * | 6.6 | ‐ | ||
| Smoked Pseudotolithus senegalensis * | 21.5 | ‐ | ||
| Poland | Smoked sprat | 1 | 10.3 | Zachara et al. (2017) |
| Smoked sausage | 3 | 24.3 | ||
| Smoked pork hams | 1.8 | 15.5 | ||
| Portugal | Chouriço grosso, dry‐cured fermented pork sausages* | 3.3 | ‐ | Roseiro et al. (2011) |
| Grilled Salmon | 4.7 ± 0.8 | ‐ | Viegas, Novo, Pinto, et al. (2012) | |
| Chicken | 8.7 ± 0.3 | ‐ | ||
| Spain | Chorizo, Spanish smoked pork meat | 3.2 | ‐ | Ledesma et al. (2015) |
| Turkey | Grilled anchovy fish (Engraulis encrasicolus) | 0.7 ± 0.04 | 3.3 ± 0.1 | Sahin et al. (2020) |
| Grilled chicken | <LOD (0.05) | 2.1 ± 0.1 |
Abbreviations: ‐, data not presented in the cited paper; PAH4, sum of benzo[a]pyrene, chrysene, benzo[b]fluoranthene and benz[a]anthracene.
Data of this author were presented in dry weight.
3.3. Other hazards in grilled or smoked fish and meat
3.3.1. Heavy metals
Trace elements include environmental contaminants (heavy metals such as cadmium, mercury, and lead) which can have toxic effects on human health (Aina et al., 2012; Ismail et al., 2015) and oligo‐elements (copper, nickel, iron, cobalt, zinc, manganese, etc.) which play important physiological roles when they are at low concentrations. Heavy metals such as cadmium (Cd), mercury (Hg), and lead (Pb) are toxic even at low concentrations (Amos‐Tautua et al., 2013; Daniel et al., 2013; Ersoy et al., 2006; Şireli et al., 2006). Cadmium and arsenic are classified as carcinogenic for humans (Group 1) and lead is classified as possibly carcinogenic for humans (Group 2B) by the IARC (International Agency for Research on Cancer) (2010). Environmental pollution is the main way of food contamination with heavy metals (Costa et al., 2016; EFSA (European Food Safety Authority), 2010). In 2010, the European Food Safety Authority reported that human exposure to lead through diet results in its bioaccumulation responsible for adverse effects on the cardiovascular, renal, endocrine, gastrointestinal, immune, and reproductive systems. The Codex Alimentarius reported that lead was responsible for the low intellectual quotient based on lead exposure studies in children (Codex Alimentarius, 2004). European Commission set a maximum limit of 0.1 mg/kg for lead in meat (excluding offal) of bovine animals, sheep, pig, and poultry and 0.3 mg/kg in muscle of fish (EC (European Commission), 2006). For cadmium, the maximal limit is 0.1 mg/kg in muscle of mackerel (Scomber spp.), tuna (Thunnus spp., Katsuwonus pelamis, Euthynnus spp.), and bichique (Sicyopterus lagocephalus), whereas in meat products, the maximal limits range between 0.05 and 1 mg/kg, depending on the species and the tissue of the animal.
Several authors reported the presence of trace elements in smoked fish (Anigboro et al., 2011; Ibanga et al., 2019; Inobeme et al., 2018). Şireli et al. (2006) reported the presence of lead (0.01–0.8 mg/kg) in vacuum packaged smoked fish marketed on the Ankara market in Turkey (Table 5). In that study, 37% of the smoked fish samples were not compliant to the Turkish acceptable limit of 0.2 mg/kg. Likewise, Anigboro et al. (2011) reported high levels of lead (13–59 mg/kg) in smoked fish samples (Table 5) collected from different local markets in Nigeria. Arsenic was found in smoked Dicentrarchus labrax (0.4 mg/kg), Scomber scombrus (0.4 mg/kg), Clarias gariepinus (0.02 mg/kg), and Ethmalosa fimbriata (0.02 mg/kg) (Table 5). For cadmium, examples of concentration reported from the literature are shown in Table 5.
TABLE 5.
Mean concentrations of heavy metals in smoked or grilled fish (a) and meat (b) products as reported from the literature
| (a) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Country | Fish species | Heavy metals (mg/kg) | References | |||||
| Pb | Cd | Hg | Ni | As | Cr | |||
| Egypt | Ctenopharyngodon idella | nd | 0.2 | nd | 7.7 | nd | nd | Abbas et al. (2021)* |
| Iran | Rutilus frissi | 0.003 | 0.002 | nd | nd | nd | 0.002 | Mehdipour et al. (2018)* |
| Nigeria | Scomber scombrus | nd | nd | nd | nd | 0.40 | 0.1 | Aremu et al. (2014) |
| Clarias gariepinus | 0.2 | 2.5 | 0.02 | 12.8 | 0.02 | nd | Ibanga et al. (2019)* | |
| Ethmalosa fimbriata | 0.2 | 19.5 | 0.02 | 12.4 | 0.02 | nd | ||
| Heteroclaria | 18.7 | 1 | nd | 123.3 | nd | 50.3 | Anigboro et al. (2011) | |
| Ethmalosa fimbriata | 21.3 | 2.2 | nd | 120.7 | nd | 54.3 | ||
| Tilapia guineensis | 43.7 | nd | nd | 148.7 | nd | 71 | ||
| Poland | Herring | 0.04 | 0.004 | nd | nd | nd | nd | Rajkowska‐Myśliwiec et al. (2021) |
| Sprats | 0.02 | 0.02 | nd | nd | nd | nd | ||
| Spain | Sardine | 0.04 | 0.002 | 0.03 | 3.3 | nd | Perello et al. (2008) | |
| Hake | 0.02 | nd | 0.2 | 1.4 | nd | |||
| Tuna | 0.03 | 0.002 | 0.4 | 1.6 | nd | |||
| Turkey | Dicentrarchus labrax | 0.3 | nd | nd | 0.2 | 0.4 | 0.05 | Ersoy et al. (2006) |
| Salmo salar | 0.2 | 0.02 | nd | nd | nd | nd | Şireli et al. (2006)* | |
| Oncorhynhus mykiss | 0.1 | 0.01 | nd | nd | nd | nd | ||
| Mackerel | 0.05 | 0.01 | nd | nd | nd | nd | ||
| Oncorhynhus mykiss | 0.4 | 0.02 | nd | nd | nd | nd | ||
| (b) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Country | Meat products | Heavy metals (mg/kg) | References | |||||
| Pb | Cd | Hg | Ni | As | Cr | |||
| Burkina‐Faso | Braised chicken | 0.2 | 0.5 | nd | nd | Nd | Bazié et al. (2021) | |
| Flamed chicken | 0.1 | 0.2 | nd | nd | Nd | |||
| Ghana | Bush meat | 3.6 | 0.1 | nd | nd | nd | nd | Kobia et al. (2016) |
| Spain | Veal steak | 0.02 | nd | nd | nd | 0.2 | nd | Perello et al. (2008) |
| Loin pork | nd | nd | nd | nd | 0.2 | nd | ||
| Chicken | nd | nd | nd | nd | 0.1 | nd | ||
| Lamb | nd | nd | nd | nd | 0.2 | nd | ||
Abbreviation: nd, not determined.
Reported data were expressed in dry matter.
Perello et al. (2008) reported the increase of Pb, As, and Hg contents in fish and meat products processed with grilling, frying, boiling, and roasting, compared to the raw products collected from Spain markets (data not shown). Even though an increase of heavy metal levels was recorded after processing in different studies, this increase could be due to the absorption phenomenon or environmental contamination as the culinary practices were not carried out in controlled close space. It could also be a concentration of the contaminants due to water loss during smoking and drying.
Heavy metal concentrations can be measured by a graphite furnace atomic absorption spectrometer (GFAAS) or an atomic absorption spectrophotometer (Anigboro et al., 2011; Şireli et al., 2006). They could also be determined using atomic absorption spectrometry after microwave digestion and inductively coupled plasma mass spectrometry (ICP/MS) (Kabir et al., 2011; Uluozlu et al., 2009). The studies on the occurrence of heavy metals in smoked or grilled fish and meat reported in this section were mainly from Africa. Indeed, although some studies were from Turkey (Ersoy et al., 2006; Şireli et al., 2006), Spain (Perello et al., 2008) and Poland (Rajkowska‐Myśliwiec et al., 2021), the majority of them were from Nigeria (Amos‐Tautua et al., 2013; Anigboro et al., 2011; Aremu et al., 2014; Daniel et al., 2013; Ersoy et al., 2006; Ibanga et al., 2019) and other African countries such as Egypt (Abbas et al., 2021), Ghana (Kobia et al., 2016) and Burkina Faso (Bazié et al., 2021). Heavy metals contamination data (Table 5) showed that before 2015 (2006–2014) many studies from different countries especially Turkey and Nigeria were carried out on the occurrence of these environmental contaminants in smoked or grilled fish and meat. From 2015 to 2021, additional studies from Nigeria were carried out again on these compounds showing the necessity to update contamination data in smoked or grilled fish and meat. However, for other countries such as Burkina‐Faso or Egypt, very few relevant data were available on heavy metals contamination in smoked or grilled fish and meat before 2015 (Table 5).
3.3.2. Biogenic amines
Biogenic amines are found in protein‐rich foods such as fish and meat products (Chong et al., 2011; Latorre‐Moratalla et al., 2017; Sagratini et al., 2012). Despite the important role of some biogenic amines in human and animal physiology, the consumption of a high amount of these amines can result in food intoxication (EFSA (European Food Safety Authority), 2011; Lehane & Olley, 2000). They are usually produced from decarboxylation of free amino acids by bacterial enzymes (Table 6), before or after processing. They are also heat resistant, so not destroyed by the cooking practices. Among biogenic amines, histamine received particular attention due to its toxicity. Several authors reported histamine as responsible for foodborne intoxication in reference to scombroid fish poisoning (EFSA (European Food Safety Authority), 2011; Latorre‐Moratalla et al., 2017). Intoxication with histamine is associated with symptoms such as hypertension, headache, and allergy reactions including reddening on the face, neck and upper chest, vomiting, sweating, nausea, abdominal cramps, diarrhea, rash, dizziness, palpitations, spasm of bronchi, and flushing (Hassan, El‐ Shater, & Waly, 2017; Marissiaux et al., 2018; da Silva, Pinho, Ferreira, Plestilova, & Gibbs, 2002; Zaman et al., 2010). Several papers reported biogenic amines in smoked fish and grilled meat products (Douny et al., 2019; Köse et al., 2012; Ntzimani et al., 2008; Simunovic et al., 2019). The histamine concentrations reported by several authors in these kinds of food are summarized in Table 7. The presence of histamine was reported in smoked salmon at levels ranging between 2.5 and 171 mg/kg, in smoked Sardinella sp. (18 mg/kg), and in hot smoked bonito (98.7 ± 0.6 mg/kg) (Table 7). The presence of histamine was also reported in grilled pork (<11.2–81.5 mg/kg) and in smoked turkey (32.9 ± 1.4 mg/kg) (Table 7).
TABLE 6.
Structure, precursors, and microorganisms producing decarboxylase of some biogenic amines
| Amino acid precursors | Biogenic amine | Chemical structure and formula | Main microorganisms producing amino acid decarboxylase |
|---|---|---|---|
| Histidine | Histamine |
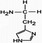 C5H9N3 C5H9N3
|
Hafnia alvei, Morganella morganii, Klebsiella pneumonia, Morganella psychrotolerans, Photobacterium phosphoreum, Photobacterium psychrotolerans |
| Tryptophan | Tryptamine |
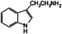 C10H12N2 C10H12N2
|
‐ |
| Tyrosine | Tyramine |
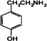 C8H11NO C8H11NO |
Enterococcus (Ent. faecalis, Ent. faecium) Lactobacillus (Lact. curvatus; Lact. brevis) Leuconostoc spp, Carnobacterium spp Staphylococcus spp |
| Phenylalanine | 2‐Phenylethylamine |
|
Enterococcus, Lactobacillus curvatus, Staphylococcus (S. carnosus) |
| Hydroxytryptophan | Serotonine | nd | ‐ |
| Lysine | Cadavérine |
 NH2(CH2)5NH2 NH2(CH2)5NH2
|
Enterobacteriaceae (Citrobacter, Klebsiella, Escherichia, Proteus, Salmonella et Shigella) Pseudomonadaceae, Shewanellaceae |
| Ornithine; arginine | Putrescine |
 NH2(CH2)4NH2 NH2(CH2)4NH2
|
Enterobacteriaceae (Citrobacter, Klebsiella, Escherichia, Proteus, Salmonella et Shigella) Pseudomonadaceae, Shewanellaceae |
| Ornithine; arginine | Spermine |
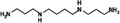 C10H26N4 C10H26N4
|
‐ |
| Ornithine; arginine | Spermidine |
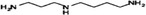 C7H19N3 C7H19N3
|
‐ |
TABLE 7.
Histamine levels in smoked or grilled fish and meat products
| Product | Concentration (mg/kg) | Analytical method | References |
|---|---|---|---|
| Smoked salmon | 2.5–171 | Extraction with Trichloroacetic acid; LC‐MS/MS | Simunovic et al. (2019) |
| Smoked Sardinella sp. | 18 |
Extraction trichloroacetic acid ion‐exchange chromatography |
Plahar et al. (1999) |
| Cold‐smoked salmon | 30.9 ± 0.4 |
Extraction with perchloric acid high‐performance liquid chromatography with a diode array detector |
Köse et al. (2012) |
| Hot‐smoked Bonito (Tuna fish) | 98.7 ± 0.6 | ||
| Grilled tuna | 4,400 | Not mentioned | Marissiaux et al. (2018) |
| Smoked fish from different species | 11–63 | Quantification colorimetrically at 495 nm using a spectrophotometer. | CSIR (2017) |
| Smoked turkey breast fillets stored at 4°C after 30 days | 32.9 ± 1.4 |
Extraction trichloroacetic acid With liquid chromatography. Quantification was performed coupled with a UV detector |
Ntzimani et al. (2008) |
| Grilled pork | <11.2–81.5 | Extraction with perchloric acid and injection on UPLC coupled with a fluorescence detector | Douny et al. (2019) |
European Commission set maximal limits for histamine (100–200 mg/kg) in fish and fishery products from fish species associated with a high amount of histidine (EC (European Commission), 2005). No maximal limit of histamine is available for meat products. However, several authors reported the use of biogenic index (sum of putrescine, tyramine, cadaverine, and histamine levels) to assess the freshness and quality of pork (Cheng et al., 2016; Douny et al., 2019).
The highest histamine concentration in fish reported in this review was 44 times over the authorized European limit and resulted in histamine fish poisoning (HFP) (Marissiaux et al., 2018). Similar concentration (4,384.2 mg/kg) was also reported in smoked‐dried fish from Benin (Table 8). Regarding the geographical location, the selected paper reported in the Tables 7 and 8 was from America (5%), Asia (20%), Africa (30%), and Europe (45%). Although several studies dealt with the production of biogenic amines in fish, very few studies were available about grilled and/or smoked fish and meat products. From 2015 to 2021, studies dealing with biogenic amines in grilled or smoked fish and meat mainly focused on their occurrence (Tables 7 and 8).
TABLE 8.
Mean (maximum) concentrations of histamine and tyramine in fish (a) and meat products (b) from different countries
| (a) | ||||
|---|---|---|---|---|
| Country | Type of food | Histamine (mg/kg) | Tyramine (mg/kg) | References |
| Austria | Smoked tuna |
|
‐ | Rauscher‐Gabernig et al. (2009) |
| Smoked mackerel |
|
‐ | ||
| Smoked salmon |
|
‐ | ||
| Belgium | Grilled tuna fish |
|
‐ | Marissiaux et al. (2018) |
| Benin | Smoked Cypselurus cyanopterus | 471.7 (1,139.4) | 810.9 (1766.5) | Assogba et al. (2021) |
| Smoked‐dried Cypselurus cyanopterus | 754.3 (2,255.1) | 19.1 (20.6) | ||
| Smoked fish | <10 (1,511.3) | 151.9 (700.9) | Iko Afé et al. (2021) | |
| Smoked‐dried fish | 1,340.2 (4,384.2) | 33.1 (45.8) | ||
| Cambodia | Smoked fish | 16.6 (24.2) | 9.9 (38.4) | Douny et al. (2021) |
| Denmark | Cold‐smoked tuna | 4,548 (‐) | 150 (‐) | Emborg and Dalgaard (2006) |
| (b) | ||||
|---|---|---|---|---|
| Country | Type of food | Histamine (mg/kg) | Tyramine (mg/kg) | References |
| Benin | Grilled pork | 59.7 (86.4) | 2 (3.8) | Douny et al. (2019) |
| Egypt | Beef shawarma | 80.2 (30) | ‐ | Sallam et al. (2021) |
| Chicken shawarma | 103 (36) | ‐ | ||
| Spain | Dry fermented sausages | 27 (475) | 139 (742) | Latorre‐Moratalla et al. (2017) |
Abbreviation: ‐, data not presented in the cited paper.
Numbers in parentheses represent the maximum value.
3.4. Risk assessment
3.4.1. Risk assessment methodology applicable to toxic compounds
The risk assessment is part of the risk analysis concept, which, as reported by Larsen (2006), includes risk assessment, risk evaluation, and risk communication. These three elements are separate tasks, performed by different actors, but should be part of an interactive process (Larsen, 2006; Stadler & Lineback, 2009). Risk assessment is a scientific process used to quantify the risk linked to a hazard and requires expertise in toxicology and nutrition (for the intake assessment). It is used to determine whether a particular chemical poses a significant risk to human health (FASFC (Federal Agency for the Safety of the Food Chain), 2005; Larsen, 2006; Reinik, 2007; Stadler & Lineback, 2009; Scholl et al., 2012). Risk assessment follows four steps (EFSA (European Food Safety Authority), 2008; FASFC (Federal Agency for the Safety of the Food Chain), 2005; FASFC (Federal Agency for the Safety of the Food Chain), 2006; Larsen, 2006) which are as follows:
Hazard identification : It will indicate which dangers can be associated with the consumption of a specific foodstuff and what harmful effects they can cause for consumers.
Hazard characterization : This step aims to describe and evaluate the dose–response relationship, the mode of action, including dynamic and kinetic aspects, and how to establish an acceptable daily intake (ADI) or a tolerable daily intake (TDI) using a safety factor to consider for the intra‐ and inter‐species variation.
Exposure assessment : To assess the exposure, consumption data and contamination data are needed to calculate the estimated daily intake (EDI) by multiplying the concentration of hazard by the daily consumption of food contaminated with this hazard. EDI can be calculated for several categories of population (i.e., babies, children, teenager, and adults). EDI can be calculated either following a deterministic approach using median, mean, or maximum of consumption or contamination data, or following a probabilistic approach using distributions of consumption and contamination data.
Risk characterization : This step consists of comparing the calculated EDI with a toxicological reference dose (classical way) which can be a tolerable daily intake (TDI) or an acceptable daily intake (ADI). For carcinogenic compounds such as PAHs, the margin of exposure (MoE) suggested by EFSA (European Food Safety Authority) (2005) and Constable and Barlow (2009) is used. MOE is calculated as follows:
where BMDL10 is the 95% lower confidence limit of the benchmark dose causing 10% extra risk of cancer in laboratory animals (in case of PAHs, of rat hepatocellular adenomas, and carcinoma), and EDI is the estimated daily intake. For carcinogenic compounds such as PAHs, the risk may be considered as negligible or very low only when MOE is above 10,000.
3.4.2. Examples reported from the literature of risk assessment for some chemical hazards (PAHs, heavy metals, and biogenic amines)
Examples of risk assessments related to PAH ingestion through consumption of grilled and/or smoked fish and meat (Table 9) pointed out a health concern for consumers of several countries such as Cambodia (Douny et al., 2021), Benin (Iko Afé et al., 2020, 2021), Turkey (Sahin et al., 2020), Nigeria (Akpambang et al., 2009), and Latvia (Rozentale et al., 2018). Before 2015, the mean values of MoE associated with the consumption of smoked or grilled fish (Table 9a) and meat products (Table 9b) contaminated with PAHs including BaP and PAH4 were globally above 10,000, showing a very low concern for the consumers of these products. After 2015, the studies reported showed MoE globally below 10,000 for consumers of smoked or grilled fish and meat products from different countries such as Benin, Cambodia, Turkey, and Latvia (Table 9). MOE below 10,000 indicates a high concern (risk of cancer) for consumers for carcinogenic compounds such as PAHs.
TABLE 9.
Estimated daily intakes (EDI) and margin of exposure (MOE) for polycyclic aromatic hydrocarbons (PAH) through consumption of smoked or grilled fish (a) and meat (b) products, in different countries
| (a) | ||||
|---|---|---|---|---|
| Country | Type of food | Estimated daily intake (ng/kg bw/day) | Margin of exposure | References |
| Benin | Smoked fish | BaP: 2.3‐809.9 | BaP: 30,978‐86 | Iko Afé et al. (2021) |
| PAH4: 12.0‐4,314.9 | PAH4: 28,241‐79 | |||
| Smoked‐dried fish | BaP: 2.5‐1,974.8 | BaP: 27,718‐35 | ||
| PAH4: 17.4‐13,627.2 | PAH4: 19,510‐25 | |||
| Cambodia | Smoked fish | BaP: 1,407 | BaP: 50 | Douny et al. (2021) |
| PAH4: 5,773 | PAH4: 59 | |||
| China | Grilled fish | BaP: 0.2 | BaP: 333,000 | Wang et al. (2021) |
| PAH4: 1.0 | PAH4: 336,000 | |||
| Croatia | Shellfish products | BaP: ‐ | BaP: 1,643,906 | Bogdanovic et al. (2019) |
| PAH4: ‐ | PAH4: 298,900 | |||
| Nigeria | Smoked fish | BaP: 4‐52 | BaP: 17,722‐1,346 | Akpambang et al. (2009) |
| PAH4: ‐ | PAH4: ‐ | |||
| Turkey | Grilled fish | BaP: 0.2 | BaP: 389 | Sahin et al. (2020) |
| PAH4: 0.8 | PAH4: 425 | |||
| (b) | ||||
|---|---|---|---|---|
| Country | Type of food | Estimated daily intake (ng/kg bw/day) | Margin of exposure | References |
| Benin | Grilled pork | BaP: 07‐235.7 | BaP: 96,871‐297 | Iko Afé et al. (2020) |
| PAH4: 4.1‐1,321.3 | PAH4: 83,925‐257 | |||
| China | Grilled meat | BaP: 0.5 | BaP: ‐ | Jiang et al. (2018) |
| PAH4: 4.0 | PAH4: ‐ | |||
| Croatia | Smoked meat products | BaP: ‐ | BaP: 280,657 | Bogdanovic et al. (2019) |
| PAH4: ‐ | PAH4: 66,213 | |||
| Denmark | Home grilled meat (beef, pork, and chicken) | BaP: ‐ | BaP: ‐ | Duedahl‐Olesen et al. (2015) |
| PAH4: 10 | PAH4: 33,800 | |||
| Egypt | Grilled beef meat | BaP: 290.5 | BaP: ‐ | Darwish al. (2019 |
| PAH4: ‐ | PAH4: ‐ | |||
| France | Foodstuffs (28 different foods) | BaP: 0.2 | BaP: ‐ | Veyrand et al. (2013) |
| PAH4: 1.5 | PAH4: 230,000 | |||
| Korea |
Smoked meat products (bacon, chicken, duck, pork, salmon, tuna, and turkey). |
BaP: 0.014 | BaP: 666,667 | Kim et al. (2014) |
| PAH4: 0.038 | PAH4: 265,957 | |||
| Kuwait | Grilled chicken | BaP: 15.6 | BaP: ‐ | Alomirah et al. (2011) |
| PAH4: ‐ | PAH4: ‐ | |||
| Latvia | Smoked meat products | BaP: 5.4 | BaP: 12,952 | Rozentale et al. (2018) |
| PAH4: 35.9 | PAH4: 9,475 | |||
| Smoked meat (pork, pork breast, chop, speck, ham, and chicken) and meat products (sausages, small sausages, semi‐dry sausages, and roulette) | BaP: 2.3 | BaP: 30,606 | Rozentale et al. (2015) | |
| PAH4: 13.7 | PAH4: 24,776 | |||
| Nigeria | Grilled meat | BaP: 10.5‐14.0 | BaP: 5,015‐6,652 | Akpambang et al. (2009) |
| PAH4: ‐ | PAH4: ‐ | |||
| Turkey | Grilled chicken | BaP: ‐ | BaP: ‐ | Sahin et al. (2020) |
| PAH4: 1.8 | PAH4: 190 | |||
Abbreviations: ‐, data not presented in the cited paper; PAH4, sum of benzo[a]pyrene, chrysene, benzo[b]fluoranthene, and benz[a]anthracene; bw, body weight.
Regarding consumers exposure to heavy metals from consumption of smoked or grilled fish and meat products, very few data were available from the literature. Recently, two papers have been published on exposure of consumers from Burkina‐Faso (Bazié et al., 2021) and Poland (Rajkowska‐Myśliwiec et al., 2021).
The cancer risk index linked to lead exposure calculated for consumers of braised and flamed chicken processed in Burkina Faso ranged between 7 × 10−7 and 3 × 10−6 (Table 10). None of the index risk values was above the threshold set by US‐EPA (IR > 10−4). Similar to the cancer risk index, a noncancer risk index was calculated using the median consumption level of braised and flamed chicken. This Hazard Index (HI), which is the sum of individual metal hazard (Ag, Cd, Pb, Zn, Ni, Co, Fe, Mn, Cu, and Cr) quotients, ranged between 0.07 and 0.15. These values were below the reference value (HI = 1) (Hough et al., 2004) showing also the absence of noncancer risk linked to heavy metals exposure for Burkina‐Faso consumers of braised and flamed chicken (Bazié et al., 2021). However, the HI (sum of hazard quotient of Zn, Fe, Mn, Cu, Al, Pb, and Cd) calculated for polish consumers was 1.4 (Table 10), so above the reference value of 1. The HI obtained for polish consumers was similar to HI values reported for Ugandan consumers of heat‐processed meat which ranged from 1.2 to 1.9 for different types of meats (Table 10).
TABLE 10.
Cancer and noncancer risks related to heavy metals through consumption of smoked or grilled fish and meat products reported from the literature
| Country | Type of food | Noncancer risk: Hazard index (HI) | Cancer index risk (IR) | References |
|---|---|---|---|---|
| Burkina‐Faso | Flamed chicken | 0.2 | Pb: 7 × 10−7 to 3 × 10−6 | Bazié et al. (2021) |
| Braised chicken | 0.1 | Pb: 7 × 10−7 to 3 × 10−6 | ||
| Poland | Smoked fish | 1.4 | ‐ | Rajkowska‐Myśliwiec et al. (2021) |
| Uganda | Roasted pork | 1.7 | Pb: 4.5 × 10–5 | Bamuwamye et al. (2015) |
| Cd: 1.0 × 10–3 | ||||
| As: 7.4 × 10–5 | ||||
| Roasted beef | 1.7 | Pb: 3.92 × 10–5 | ||
| Cd: 6.30 × 10–4 | ||||
| As: 2.00 × 10–4 | ||||
| Roasted goat | 1.2 | Pb: 2.95 × 10–6 | ||
| Cd: 2.60 × 10–3 | ||||
| As: 9.94 × 10–5 | ||||
| Roasted chicken | 1.9 | Pb: 2.50 × 10–5 | ||
| Cd: 2.00 × 10–3 | ||||
| As: 3.00 × 10–4 |
Abbreviation: ‐, data not presented in the cited paper.
Among biogenic amines, histamine and tyramine are two dietary biogenic amines which are present in food are undesirable due to their adverse effects on consumer's health such as hypertension, headache, and allergic reactions (EFSA, 2011; Marissiaux et al., 2018). To our best knowledge, there are few relevant studies showing the exposure to histamine or tyramine for consumers of smoked or grilled fish and meat products. Four studies dealing with histamine exposure were reported in Table 11. These four studies were published during the period of 2017–2021. The mean histamine intake calculated from the consumption of smoked fish and smoked‐dried fish marketed in Benin was 146 mg/meal and 116 mg/meal, respectively, whereas the acute reference dose (ARfD) of histamine suggested by the European Food Safety Authority (EFSA) is 50 mg histamine/meal (EFSA, 2011). In Spain, Cambodia, and Egypt, the mean histamine exposure (Table 11) was well below this ARfD. Based on the limited published data, no adverse health effects have been observed in healthy volunteers exposed to a level of 25–50 mg of histamine per person per meal (EFSA, 2011). The mean histamine exposure reported in Table 11 revealed a health concern for Beninese consumers of smoked fish and smoked‐dried fish (Iko Afé et al., 2021). Although the mean histamine exposure reported for consumers of Cambodia, Spain, and Egypt showed an absence of intoxication risk, there is risk of histamine poisoning in case of extreme consumption of smoked or grilled fish and meat products during the same meal or for sensitive consumers.
TABLE 11.
Histamine and tyramine exposure from consumption of fish and meat products
| Country | Type of food | Histamine exposure (mg/meal) | Tyramine exposure (mg/meal) | References |
|---|---|---|---|---|
| Benin | Smoked fish | 145.6 (1,019.1)* | ‐ | Iko Afé et al. (2021) |
| Smoked‐dried fish | 115.9 (1,236.2) | ‐ | ||
| Cambodia | Smoked fish | <50 (‐) | ‐ | Douny et al. (2021) |
| Egypt | Beef shawarma | 16.0 | ‐ | Sallam et al. (2021) |
| Chicken shawarma | 31 | ‐ | ||
| Spain | Dry fermented sausages | 1.4 (45.8) | 6.2 (92.5) | Latorre‐Moratalla et al. (2017) |
Abbreviation: ‐, data not presented in the cited paper.
Numbers in parentheses represent the maximum value.
4. CONCLUSION
Smoked fish and meat products may be contaminated by various toxic compounds including carcinogenic compounds. Most of the chemical hazards reported in this review are processing contaminants. Some of them can be formed when high temperature is reached inside the product (heterocyclic amines and nitrosamines) and others during pyrolysis of the fuel during processing (PAHs). Biogenic amines are not related to the smoking process but can be present in raw or smoked fish due to decarboxylation of free amino acids occurring after microbial contamination. In case of heavy metals, they are environmental pollutants found in raw and processed food. In traditionally smoked fish or grilled meat, most of the chemical hazards mentioned in this review exceed the maximal limits established by EU. Several actions should be addressed to decrease them in smoked fish and meat as they are highly consumed products.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
ETHICAL APPROVAL
This study does not involve any human or animal testing.
ACKNOWLEDGMENTS
This work was fully supported by QualiSani project through ARES CCD (Académie de Recherche et d'Enseignement Supérieur, Commission de la Coopération au Développement).
Iko Afé, O. H. , Kpoclou, Y. E. , Douny, C. , Anihouvi, V. B. , Igout, A. , Mahillon, J. , Hounhouigan, D. J. , & Scippo, M.‐L. (2021). Chemical hazards in smoked meat and fish. Food Science & Nutrition, 9, 6903–6922. 10.1002/fsn3.2633
DATA AVAILABILITY STATEMENT
All the data used in this study can be made available upon reasonable request.
REFERENCES
- Abbas, M. M. M. , Shehata, S. M. , Talab, A. S. , & Mohamed, M. H. (2021). Effect of traditional processing methods on the cultivated fish species, Egypt. Part I. Mineral and heavy metal concentrations. Biological Trace Element Research, 1‐15. 10.1007/s12011-021-02840-w [DOI] [PubMed] [Google Scholar]
- Adeyemi, K. D. , Ahmed El‐Imam, A. M. , Dosunmu, O. O. , & Lawal, O. K. (2013). Effect of Moringa oleifera marinade on microbial stability of smoke‐dried African catfish (Clarias gariepinus). Ethiopian Journal of Environmental Studies and Management, 6(1), 104–109. [Google Scholar]
- Adeyeye, S. A. O. , Oyewole, O. B. , Obadina, A. O. , & Omemu, A. M. (2015). Evaluation of microbial safety and quality of traditional smoked Bonga Shad (Ethmalosa frimbriata) fish from Lagos State, Nigeria. The Pacific Journal of Science and Technology, 16(1), 286–294. [Google Scholar]
- Aina, M. P. , Degila, H. , Chikou, A. , Adjahatode, F. , & Matejka, G. (2012). Risk of intoxication by heavy metals (Pb, Cd, Cu, Hg) connected to the consumption of some halieutic species in Lake Nokoué: Case of the Penaeus shrimps and the Sarotherodon melanotheron . British Journal of Science, 5(1), 104–118. [Google Scholar]
- Ake Assi, Y. (2012). Validation of a method for the quantification of polycyclic aromatic hydrocarbons in fish. European Journal of Scientific Research, 74(1), 69–78. [Google Scholar]
- Akpambang, V. O. E. , Purcaro, G. , Lajide, L. , Amoo, I. A. , Conte, L. S. , & Moret, S. (2009). Determination of polycyclic aromatic hydrocarbons (PAHs) in commonly consumed Nigerian smoked/grilled fish and meat. Food Additives & Contaminants (A), 26(7), 1096–1103. 10.1080/02652030902855406 [DOI] [PubMed] [Google Scholar]
- Akpan, V. , Lodovici, M. , & Dolara, P. (1994). Polycyclic Aromatic Hydrocarbons in fresh and smoked fish samples from three Nigerian cities. Bulletin of Environmental and Toxicology, 53, 246–253. 10.1007/BF00192040 [DOI] [PubMed] [Google Scholar]
- Al Bulushi, I. , Poole, S. , Deeth, H. C. , & Dykes, G. A. (2009). Biogenic amines in fish: Roles in intoxication, spoilage, and nitrosamine formation—A review. Critical Reviews in Food Science and Nutrition, 49(4), 369–377. 10.1080/10408390802067514 [DOI] [PubMed] [Google Scholar]
- Ali, A. , Waheed, K. N. , Hadaiyt, A. , Begum, I. , & Hayat, S. (2016). Determination of histamine levels by LC‐MS/MS in various fish species available in the local markets of Punjab, Pakistan. International Journal of Fisheries and Aquatic Studies, 4(6), 128–132. [Google Scholar]
- Al‐Kaseem, M. , Al‐Assaf, Z. , & Karabeet, F. (2014). Determination of seven volatile N‐nitrosamines in fast food. Pharmacology & Pharmacy, 5, 195–203. 10.4236/pp.2014.52026 [DOI] [Google Scholar]
- Alomirah, H. , Al‐Zenki, S. , Al‐Hooti, S. , Zaghloul, S. , Sawaya, W. , Ahmed, N. , & Kannan, K. (2011). Concentrations and dietary exposure to polycyclic aromatic hydrocarbons (PAHs) from grilled and smoked foods. Food Control, 22, 2028–2035. 10.1016/j.foodcont.2011.05.024 [DOI] [Google Scholar]
- Amos‐Tautua, B. M. W. , Inengite, A. K. , Abasi, C. Y. , & Amirize, G. C. (2013). Evaluation of polycyclic aromatic hydrocarbons and some heavy metals in roasted food snacks in Amassoma, Niger Delta, Nigeria. African Journal of Environmental Science and Technology, 7(10), 961–966. [Google Scholar]
- Anigboro, F. O. , Akpoveta, O. V. , & Aweatefe, K. J. (2011). Determination of concentration levels of heavy metals in different species of smoked fishes from three markets in Agbor, Delta state, Nigeria. Agbor Journal of Science Education, 4, 81–88. [Google Scholar]
- ANSES (Agence nationale de sécurité sanitaire de l’alimentation, de l’environnement et du travail) (2011). Étude de l’alimentation totale française 2 (EAT 2), Tome 2: Résidus de pesticides, additifs, acrylamide, hydrocarbures aromatiques polycycliques [French Total Food Study 2 (TFS 2), Volume 2: Pesticide residues, additives, acrylamide, polycyclic aromatic hydrocarbons]. Retrieved from https://www.anses.fr/fr/system/files/PASER2006sa0361Ra2.pdf [Google Scholar]
- Aremu, M. O. , Namo, B. S. , Oko, O. J. , Adelagun, R. O. A. , & Yebpella, G. G. (2014). Compositional evaluation of local smoked Nigerian mackerel (Scomber scombrus). Food Science and Quality Management, 24, 42–50. [Google Scholar]
- Asamoah, E. K. , Nunoo, F. K. E. , Addo, S. , Nyarko, J. O. , & Hyldig, G. (2021). Polycyclic aromatic hydrocarbons (PAHs) in fih smoked using traditional and improved kilns: Levels and human health risk implications through dietary exposure in Ghana. Food Control, 121, 1–9. [Google Scholar]
- Assogba, M. F. , Anihouvi, D. G. H. , Iko Afé, O. H. , Kpoclou, Y. E. , Mahillon, J. , Scippo, M.‐L. , Hounhouigan, D. J. , & Anihouvi, V. B. (2019). Processing methods, preservation practices and quality attributes of smoked and smoked‐dried fishes consumed in Benin. Cogent Food and Agriculture, 5(1), 1–13. 10.1080/23311932.2019.1641255 [DOI] [Google Scholar]
- Assogba, M. F. , Iko Afé, O. H. , Ahouansou, R. H. , Anihouvi, D. G. H. , Kpoclou, Y. E. , Djago, D. , Douny, C. , Igout, A. , Mahillon, J. , Hounhouigan, D. J. , Scippo, M.‐L. , & Anihouvi, V. B. (2021). Performances of barrel kiln used in cottage industry for fish processing and effects on physicochemical characteristics and safety of smoked fish products. Journal of the Science of Food and Agriculture, 1–40. 10.1002/jsfa.11421 [DOI] [PubMed] [Google Scholar]
- Ayanaba, A. , & Alexander, M. (1973). Microbial formation of nitrosamines in vitro. Applied Microbiology, 25(6), 862–868. 10.1128/am.25.6.862-868.1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamuwamye, M. , Ogwok, P. , & Tumuhairwe, V. (2015). Cancer and non‐cancer risks associated with heavy metal exposures from street foods: Evaluation of roasted meats in an urban setting. Journal of Environment Pollution and Human Health, 3(2), 24–30. [Google Scholar]
- Battisti, C. , Girelli, A. M. , & Tarola, A. M. (2015). Polycyclic aromatic hydrocarbons (PAHs) in yogurt samples. Food Additives & Contaminants (A), 8(1), 50–55. 10.1080/19393210.2014.968880 [DOI] [PubMed] [Google Scholar]
- Bazié, B. S. R. , Bougma, A. , Séré, A. , Ouilly, J. T. , Kabré, E. , Hounhouigan, D. J. , Scippo, M.‐L. , Savadogo, A. , & Bassolé, I. H. N. (2021). Concentrations and health risk assessment of metallic trace elements in ready‐to‐eat braised and flamed chickens in Burkina Faso. Biological Trace Element Research, 199, 1556–1565. [DOI] [PubMed] [Google Scholar]
- Belitz, H. D. , Grosch, W. , & Schieberle, P. (2009).Food Contamination In Belitz H. D., Grosch W., & Schieberle P. (Eds.), Food Chemistry (4th edn, 467–496). Berlin: Springer‐Verlag. [Google Scholar]
- Berkel, B. M. V. , Boogaard, B. V. D. , & Heijnen, C. (2005). Smoking. In Goffau‐Markusse M. (Ed.), Preservation of fish and meat, Agrodokseries 12.86 (3rd edn.). Agromisa Foundation. Retrieved from http://www.agromisa.org/wp‐content/uploads/Agrodok‐12‐Preservation‐of‐fish‐and‐meat_sample.pdf [Google Scholar]
- Bogdanovic, T. , Pleadin, J. , Petricevic, S. , Listes, E. , Sokolic, D. , Markovic, K. , Ozogul, F. , & Simat, V. (2019). The occurrence of polycyclic aromatic hydrocarbons in fish and meat products of Croatia and dietary exposure. Journal of Food Composition and Analysis, 75, 49–60. 10.1016/j.jfca.2018.09.017 [DOI] [Google Scholar]
- Brasseur, C. , Brose, F. , Pirlot, A. , Douny, C. , Eppe, G. , Maghuin‐Rogister, G. , & Scippo, M.‐L. (2007). Validation of the analytical procedure for the determination of polyaromatic hydrocarbons in smoke flavorings using high performance liquid chromatography coupled to an ultraviolet, diode array or fluorescence detector. Accreditation and Quality Assurance, 12, 535–542. [Google Scholar]
- Chen, S. , Kao, T. H. , Chen, C. J. , Huang, C. W. , & Chen, B. H. (2013). Reduction of carcinogenic polycyclic aromatic hydrocarbons in meat by sugar‐smoking and dietary exposure assessment in Taiwan. Journal of Agricultural and Food Chemistry, 61, 7645–7653. 10.1021/jf402057s [DOI] [PubMed] [Google Scholar]
- Cheng, W. , Sun, D.‐W. , & Cheng, J.‐H. (2016). Pork biogenic amine index (BAI) determination based on chemometric analysis of hyperspectral imaging data. LWT ‐ Food Science and Technology, 73, 13–19. 10.1016/j.lwt.2016.05.031 [DOI] [Google Scholar]
- Chong, C. Y. , Abu Bakar, F. , Russly, A. R. , Jamilah, B. , & Mahyudin, N. A. (2011). The effects of food processing on biogenic amines formation. MiniReview. International Food Research Journal, 18(3), 867–876. [Google Scholar]
- Ciecierska, M. , & Obiedzinski, M. (2007). Influence of smoking process on Polycyclic Aromatic Hydrocarbons'content in meat products. Acta Scientiarum Polonorum Technologia Alimentaria, 6(4), 17–28. [Google Scholar]
- Codex Alimentarius . (2004). Code of practice for the prevention and reduction of lead contamination in foods. CAC/RCP 56‐2004. Retrieve from http://www.fao.org/input/download/standards/10099/CXP_056e.pdf [Google Scholar]
- Codex Alimentarius . (2009). Code d’usages pour la réduction de la contamination des aliments par les hydrocarbures aromatiques polycycliques (HAP) issus des processus de fumage et de séchage direct. CAC/RCP 68‐2009. Retrieve from http://www.fao.org/input/download/standards/11257/CXP_068f.pdf [Google Scholar]
- Codex Alimentarius . (2013). Norme pour le poisson fumé, le poisson aromatisé à la fumée et le poisson fumé‐séché [Standard for smoked fish, smoke‐flavored fish and smoked‐dried fish] (Codex stan 311. 2013). Retrieved from http://www.fao.org/fao‐who‐codexalimentarius/shproxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao [Google Scholar]
- Constable, A. , & Barlow, S. (2009). Application of the Margin of Exposure Approach to Compounds in Food which are both Genotoxic and Carcinogenic. In Summary Report of a Workshop held in October 2008, Organized by the International Life Sciences Institute Europe Risk Assessment of Genotoxic Carcinogens in Food Task Force. Retrieve from http://www.ilsi.org/Publications/MOE%20WS%20Report.pdf [Google Scholar]
- Costa, F. D. N. , Korn, M. G. A. , Brito, G. B. , Ferlin, S. , & Fostier, A. H. (2016). Preliminary results of mercury levels in raw and cooked seafood and their public health impact. Food Chemistry, 192, 837–841. 10.1016/j.foodchem.2015.07.081 [DOI] [PubMed] [Google Scholar]
- CSIR (Council for Scientific and Industrial Research) (2017). Histamine levels in smoked fin fishes from Ghanaian coastal waters. Report, Food research institute, 12. Retrieved from https://www.academia.edu/35536721/HISTAMINE_LEVELS_IN_SMOKED_FINFISHES_FROM_GHANAIAN_COASTAL_WATERS [Google Scholar]
- Culp, S. J. , Gaylor, D. W. , Sheldon, W. G. , Goldstein, L. S. , & Beland, F. A. (1998). A comparison of the tumours induced by coal tar and benzo[a]pyrene in a 2‐year bioassay. Carcinogenesis, 19, 117–124. [DOI] [PubMed] [Google Scholar]
- da Silva, M. V. , Pinho, O. , Ferreira, I. , Plestilova, L. , & Gibbs, P. A. (2002). Production of histamine and tyramine by bacteria isolated from Portuguese vacuum‐packed cold‐smoked fish. Food Control, 13, 457–461. 10.1016/S0956-7135(01)00081-0 [DOI] [Google Scholar]
- Darwish, W. S. , Chiba, H. , El‐Ghareeb, W. R. , Elhelaly, A. E. , & Hui, S.‐P. (2019). Determination of polycyclic aromatic hydrocarbon content in heat‐treated meat retailed in Egypt: Health risk assessment, benzo[a]pyrene induced mutagenicity and oxidative stress in human colon (CaCo‐2) cells and protection using rosmarinic and ascorbic acids. Food Chemistry, 290, 114–124. [DOI] [PubMed] [Google Scholar]
- Daniel, E. O. , Ugwueze, A. U. , & Igbegu, H. E. (2013). Microbiological quality and some heavy metals analysis of smoked fish sold in Benin City, Edo State, Nigeria. World Journal of Fish and Marine Sciences, 5(3), 239–243. [Google Scholar]
- Dègnon, R. G. , Agossou, V. , Adjou, E. S. , Dahouenon‐Ahoussi, E. , Soumanou, M. M. , & Sohounhloué, C. K. D. (2013). Évaluation de la qualité microbiologique du chinchard (Trachurus trachurus) au cours du processus de fumage traditionnel [Assessment of the microbiological quality of horse mackerel (Trachurus trachurus) during the traditional smoking process]. Journal of Applied Biosciences, 6(67), 5210–5218. [Google Scholar]
- Domanska, K. , & Kowalski, B. (2003). Occurrence of volatile N‐nitrosamines in polish processed meat products. Bulletin of the Veterinary Institute in Pulawy, 47, 507–514. [Google Scholar]
- Domingo, J. L. , & Nadal, M. (2015). Human dietary exposure to polycyclic aromatic hydrocarbons: A review of the scientific literature. Food and Chemical Toxicology, 86, 144–153. 10.1016/j.fct.2015.10.002 [DOI] [PubMed] [Google Scholar]
- Douny, C. , Benmedjadi, S. , Brose, F. , Iko Afé, O. H. , Igout, A. , Hounhouigan, D. J. , Anihouvi, V. B. , & Scippo, M.‐L. (2019). Development of an analytical method for the simultaneous measurement of 10 biogenic amines in meat: Application to beninese grilled pork samples. Food Analytical Methods, 12(9), 2392–2400. 10.1007/s12161-019-01587-4 [DOI] [Google Scholar]
- Douny, C. , Mith, H. , Igout, A. , & Scippo, M.‐L. (2021). Fatty acid intake, biogenic amines and polycyclic aromatic hydrocarbons exposure through the consumption of nine species of smoked freshwater fish from Cambodia. Food Control, 130, 1–10. 10.1016/j.foodcont.2021.108219 [DOI] [Google Scholar]
- Drabik‐Markiewicz, G. , Maagdenberg, K. V. , De Mey, E. , Deprez, S. , Kowalska, T. , & Paelinck, H. (2009). Role of proline and hydroxyproline in N‐nitrosamine formation during heating in cured meat. Meat Science, 81, 479–486. 10.1016/j.meatsci.2008.10.002 [DOI] [PubMed] [Google Scholar]
- Duedahl‐Olesen, L. , Aaslyng, M. , Meinert, L. , Christensen, T. , Jensen, A. H. , & Binderup, M.‐L. (2015). Polycyclic aromatic hydrocarbons (PAH) in Danish barbecued meat. Food Control, 57, 169–176. 10.1016/j.foodcont.2015.04.012 [DOI] [Google Scholar]
- EC (European Commission) (2005). No 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. Official Journal of the European Union L, 338, 1–32. Retried from https://eur‐lex.europa.eu/legal‐content/EN/TXT/PDF/?uri=CELEX:32005R2073&from=EN [Google Scholar]
- EC (European Commission) (2006). Regulation No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Official Journal of the European Union L, 364, 1–40. Retried from https://eur‐lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2006:364:0005:0024:EN:PDF [Google Scholar]
- EFSA (European Food Safety Authority) (2005). Opinion of the Scientific Committee on a request from EFSA related to A Harmonised Approach for Risk Assessment of Substances Which are both Genotoxic and Carcinogenic. The European Food Safety Authority Journal, 282, 1–31. [Google Scholar]
- EFSA (European Food Safety Authority) (2008). Polycyclic Aromatic Hydrocarbons in food: Scientific opinion of the panel on contaminants in the food chain. The European Food Safety Authority Journal, 724, 1–114. Retrieved from 10.2903/j.efsa.2008.724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) (2010). Scientific Opinion on Lead in Food. The European Food Safety Authority Journal, 8(4), 1–151. 10.2903/j.efsa.2010.1570 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) (2011). Scientific Opinion on risk based control of biogenic amine formation in fermented foods. The European Food Safety Authority Journal, 9(10), 1–93. Retrieved from 10.2903/j.efsa.2011.2393 [DOI] [Google Scholar]
- Eldaly, E. A. , Hussein, M. A. , El‐Gaml, A. M. A. , El‐hefny, D. E. , & Mishref, M. A. (2016). Polycyclic aromatic hydrocarbons (PAHs) in charcoal grilled meat (Kebab) and Kofta and the effect of marinating on their existence. Zagazig Veterinary Journal, 44(1), 40–47. 10.21608/zvjz.2016.7830 [DOI] [Google Scholar]
- Emborg, J. , & Dalgaard, P. (2006). Formation of histamine and biogenic amines in cold‐smoked Tuna: An investigation of psychrotolerant bacteria from samples implicated in cases of histamine fish poisoning. Journal of Food Protection, 69(4), 897–906. 10.4315/0362-028X-69.4.897 [DOI] [PubMed] [Google Scholar]
- Ersoy, B. , Yanar, Y. , Kucukgulmez, A. , & Celik, M. (2006). Effects of four cooking methods on the heavy metal concentrations of sea bass fillets (Dicentrarchus labrax Linne, 1785). Food Chemistry, 99, 748–751. 10.1016/j.foodchem.2005.08.055 [DOI] [Google Scholar]
- FASFC (Federal Agency for the Safety of the Food Chain) (2006). Application of risk assessment in the food chain. Sci Com Workshop. p98. Retrieved from http://www.afsca.be/comitescientifique/publications/brochures/_documents/2007‐11_WS_SciCOM_Fr.pdf [Google Scholar]
- FASFC (Federal Agency for the Safety of the Food Chain) (2005). Terminology for hazard and risk analysis according to the codex alimentarius. p46. Retrieved from http://www.afsca.be/comitescientifique/publications/brochures/_documents/2005‐09_SciCom_Term_Fr.pdf [Google Scholar]
- Fay, L. B. , Ali, S. , & Gross, G. A. (1997). Determination of heterocyclic aromatic amines in food products: Automation of the sample preparation method prior to HPLC and HPLC‐MS quantification. Mutation Research, 376, 29–35. 10.1016/S0027-5107(97)00022-5 [DOI] [PubMed] [Google Scholar]
- Filho, P. J. S. , Rios, A. , Valcarcel, M. , Zanin, K. D. , & Caramao, E. B. (2003). Determination of nitrosamines in preserved sausages by solid‐phase extraction–micellar electrokinetic chromatography. Journal of Chromatography A, 985, 503–512. 10.1016/S0021-9673(02)01825-3 [DOI] [PubMed] [Google Scholar]
- Forsberg, N. D. , Stone, D. , Harding, A. , Harper, B. , Harris, S. , Matzke, M. M. , Cardenas, A. , Waters, K. M. , & Anderson, K. A. (2012). Effect of Native American fish smoking methods on dietary exposure to Polycyclic Aromatic Hydrocarbons and possible risks to human health. Journal of Agricultural and Food Chemistry, 60, 6899–6906. 10.1021/jf300978m [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheorghe, P. , Iulia, D. , Valentin, N. , Valter, E. D. , & Carmen, I. (2019). Research on the detection of polycyclic aromatic hydrocarbons (PAHs) from fish and smoked fish samples, the values obtained and the significance of their presence on human health. Romanian Biotechnological Letters, 24(4), 697–704. 10.25083/rbl/24.4/697.704 [DOI] [Google Scholar]
- Gibis, M. (2016). Heterocyclic aromatic amines in cooked meat products: Causes, formation, occurrence, and risk assessment. Comprehensive Reviews in Food Science and Food Safety, 15, 269–302. 10.1111/1541-4337.12186 [DOI] [PubMed] [Google Scholar]
- Gomez‐Guillén, M. C. , Gomez‐Estaca, J. , Giménez, B. , & Montero, P. (2009). Alternative fish species for cold‐smoking process. International Journal of Food Science and Technology, 44, 1525–1535. 10.1111/j.1365-2621.2008.01762.x [DOI] [Google Scholar]
- Goulas, A. E. , & Kontominas, M. G. (2005). Effect of salting and smoking‐method on the keeping quality of chub mackerel (Scomber japonicus): Biochemical and sensory attributes. Food Chemistry, 93, 511–520. 10.1016/j.foodchem.2004.09.040 [DOI] [Google Scholar]
- Gunter, M. J. , Divi, R. L. , Kulldorff, M. , Vermeulen, R. , Haverkos, K. J. , Kuo, M. M. , Strickland, P. , Poirier, M. C. , Rothman, N. , & Sinha, R. (2007). Leukocyte polycyclic aromatic hydrocarbon–DNA adduct formation and colorectal adenoma. Carcinogenesis, 28(7), 1426–1429. 10.1093/carcin/bgm022 [DOI] [PubMed] [Google Scholar]
- Haskaraca, G. , Demirok, E. , Kolsarıcı, N. , Öz, F. , & Özsaraç, N. (2014). Effect of green tea extract and microwave pre‐cooking on the formation of heterocyclic aromatic amines in fried chicken meat products. Food Research International, 63, 373–381. 10.1016/j.foodres.2014.04.001 [DOI] [Google Scholar]
- Hassan, M. A. , El‐ Shater, M. A. , & Waly, H. A. (2017). Histamine as biogenic amine residue in imported frozen fish. Benha Veterinary Medical, 32(1), 75–78. 10.21608/bvmj.2017.31115 [DOI] [Google Scholar]
- Herrmann, S. S. , Duedahl‐Olesen, L. , & Granby, K. (2014). Simultaneous determination of volatile and non‐volatile nitrosamines in processed meat products by liquid chromatography tandem mass spectrometry using atmospheric pressure chemical ionization and electrospray ionization. Journal of Chromatography A, 1330, 20–29. [DOI] [PubMed] [Google Scholar]
- Herrmann, S. S. , Duedahl‐Olesen, L. , & Granby, K. (2015). Occurrence of volatile and non‐volatile N‐nitrosamines in processed meat products and the role of heat treatment. Food Control, 48, 163–169. 10.1016/j.foodcont.2014.05.030 [DOI] [Google Scholar]
- Hough, R. L. , Breward, N. , Young, S. D. , Crout, N. M. J. , Tye, A. M. , Moir, A. M. , & Thornton, I. (2004). Assessing potential risk of heavy metal exposure from consumption of home‐produced vegetables by urban populations. Environmental Health Perspectives, 112(2), 215–221. 10.1289/ehp.5589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hustad, G. O. , Cerveny, J. G. , Trenk, H. , Deibel, R. H. , Kautter, D. A. , Fazio, T. , Johnston, R. W. , & Kolari, O. E. (1973). Effect of sodium nitrite and sodium nitrate on botulinal toxin production and nitrosamine formation in wieners. Applied Microbiology, 26(1), 22–26. 10.1128/am.26.1.22-26.1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- IARC (International Agency for Research on Cancer) (2010). Monographs on the evaluation of carcinogenic risks to humans Lyon, (France). http://monographs.iarc.fr/ENG/Monographs/vol92/mono92.pdf [Google Scholar]
- Ibanga, I. J. , Moses, E. A. , Edet, E. J. , & Moses, A. E. (2019). Microbial and some heavy metals analysis of smoked fihes sold in urban and rural markets in Akwa Ibom State. Nigeria. Calabar Journal of Health Sciences, 3(3), 73–79. [Google Scholar]
- Igwegbe, A. O. , Negbenebor, C. A. , Chibuzo, E. C. , Badau, M. H. , & Agbara, G. I. (2015). Effect of season and fish smoking on heavy metal contents of selected fish species from three locations in Borno State of Nigeria. Asian Journal of Science and Technology, 6(2), 110–1019. [Google Scholar]
- Iko Afé, O. H. , Saegerman, C. , Kpoclou, Y. E. , Anihouvi, V. B. , Douny, C. , Igout, A. , Mahillon, J. , Hounhouigan, D. J. , & Scippo, M.‐L. (2020). Polycyclic aromatic hydrocarbons contamination of traditionally grilled pork marketed in South Benin and health risk assessment for the Beninese consumer. Food Additives & Contaminants (A), 7(5), 742–752. 10.1080/19440049.2020.1726502 [DOI] [PubMed] [Google Scholar]
- Iko Afé, O. H. , Saegerman, C. , Kpoclou, Y. E. , Douny, C. , Igout, A. , Mahillon, J. , Anihouvi, V. B. , Hounhouigan, D. J. , & Scippo, M.‐L. (2021). Contamination of smoked fish and smoked‐dried fish with polycyclic aromatic hydrocarbons and biogenic amines and risk assessment for the Beninese consumers. Food Control, 126, 1–10. [Google Scholar]
- INERIS (Institut National de l'Environnement Industriel et des RISques) (2014). Données technico‐économiques sur les substances chimiques en France: Nitrosamines [Technical and economic data on chemical substances in France: Nitrosamines]. Retrieved from https://substances.ineris.fr/fr/substance/getDocument/9578 [Google Scholar]
- Ingenbleek, L. , Veyrand, B. , Adegboye, A. , Hossou, S. E. , Koné, A. Z. , Oyedele, A. D. , Kisito, C. S. K. J. , Dembélé, Y. K. , Eyangoh, S. , Verger, P. , Leblanc, J.‐C. , Durand, S. , Venisseau, A. , Marchand, P. , & Bizec, B. L. (2019). Polycyclic aromatic hydrocarbons in foods from the first regional total diet study in Sub‐Saharan Africa: Contamination profile and occurrence data. Food Control, 103, 133–144. 10.1016/j.foodcont.2019.04.006 [DOI] [Google Scholar]
- Inobeme, A. , Ajai, A. I. , Mann, A. , & Iyaka, Y. A. (2018). Determination of polycyclic aromatic hydrocarbons and heavy metal contents of barbecue beef, fish and chicken. Journal of Faculty of Food Engineering, 17(4), 395–403. [Google Scholar]
- Ismail, A. , Riaz, M. , Akhtar, S. , Ismail, T. , Ahmad, Z. , & Hashmi, M. S. (2015). Estimated daily intake and health risk of heavy metals by consumption of milk. Food Additives & Contaminants (B), 8(4), 260–265. 10.1080/19393210.2015.1081989 [DOI] [PubMed] [Google Scholar]
- Jägerstad, M. , & Skog, K. (2005). Genotoxicity of heat‐processed foods. Mutation Research, 574, 156–172. 10.1016/j.mrfmmm.2005.01.030 [DOI] [PubMed] [Google Scholar]
- Jägerstad, M. , Skog, K. , Arvidsson, P. , & Solyakov, A. (1998). Chemistry, formation and occurrence of genotoxic heterocyclic amines identified in model systems and cooked foods. Zeitschrift Für Lebensmitteluntersuchung und‐Forschung A, 207, 419–427. 10.1007/s002170050355 [DOI] [Google Scholar]
- Jakszyn, P. , Agudo, A. , Berenguer, A. , Ibanez, R. , Amiano, P. , Pera, G. , Ardanaz, E. , Barricarte, A. , Chirlaque, M. D. , Dorronsoro, M. , Larranaga, N. , Martinez, C. , Navarro, C. , Quiros, J. R. , Sanchez, M. J. , Tormo, M. J. , & Gonzalez, C. A. (2005). Intake and food sources of nitrites and N‐nitrosodimethylamine in Spain. Public Health Nutrition, 9(6), 785–791. 10.1079/PHN2005884 [DOI] [PubMed] [Google Scholar]
- Jiang, D. , Wang, G. , Li, L. , Wang, X. , Li, W. , Li, X. , Shao, L. , & Li, F. (2018). Occurrence, dietary exposure, and health risk estimation of polycyclic aromatic hydrocarbons in grilled and fried meats in Shandong of China. Food Science & Nutrition, 6, 2431–2439. 10.1002/fsn3.843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabir, E. , Kim, K.‐H. , & Yoon, H. O. (2011). Trace metal contents in barbeque (BBQ) charcoal products. Journal of Hazardous Materials, 185, 1418–1424. 10.1016/j.jhazmat.2010.10.064 [DOI] [PubMed] [Google Scholar]
- Kendirci, P. , Icier, F. , Kor, G. , & Onogur, T. A. (2014). Influence of infrared final cooking on polycyclic aromatic hydrocarbon formation in ohmically pre‐cooked beef meatballs. Meat Science, 97, 123–129. 10.1016/j.meatsci.2014.01.020 [DOI] [PubMed] [Google Scholar]
- Kim, M.‐J. , Hwang, J.‐H. , & Shin, H.‐S. (2014). Evaluation of polycyclic aromatic hydrocarbon contents and risk assessment for fish and meat products in Korea. Food Science and Biotechnoly, 23(3), 991–998. 10.1007/s10068-014-0134-0 [DOI] [Google Scholar]
- Kim, S. H. , Price, R. J. , Morrissey, M. T. , Field, K. G. , Wei, C. I. , & An, H. (2002). Histamine production by Morganella morganii in mackerel, albacore, Mahi‐mahi, and Salmon at various storage temperatures. Journal of Food Science, 67(4), 1522–1528. 10.1111/j.1365-2621.2002.tb10316.x [DOI] [Google Scholar]
- Knize, M. G. , Salmon, C. P. , Hopmans, E. C. , & Felton, J. S. (1997). Analysis of foods for heterocyclic aromatic amine carcinogens by solid‐phase extraction and high‐performance liquid chromatography. Journal of Chromatography A, 763, 179–185. 10.1016/S0021-9673(96)00720-0 [DOI] [PubMed] [Google Scholar]
- Knize, M. G. , Salmon, C. P. , Mehta, S. S. , & Felton, J. S. (1997). Analysis of cooked muscle meats for heterocyclic aromatic amine carcinogens. Mutation Research, 376, 129–134. 10.1016/S0027-5107(97)00035-3 [DOI] [PubMed] [Google Scholar]
- Knize, M. G. , Sinha, R. , Salmon, C. P. , Mehta, S. S. , Dewhirst, K. P. , & Felton, J. S. (1996). Formation of heterocyclic amine mutagens/carcinogens during home and commercial cooking of muscle foods. Journal of Muscle Foods, 7, 271–279. 10.1111/j.1745-4573.1996.tb00603.x [DOI] [Google Scholar]
- Kobia, J. , Emikpe, B. , Asare, D. , Asenso, T. , Yeboah, R. , Jarikre, T. , & Jagun‐Jubril, A. (2016). Effects of different cooking methods on heavy metals level in fresh and smoked game meat. Journal of Food Processing and Technology, 7(9), 1–3. [Google Scholar]
- Köse, S. (2010). Evaluation of seafood safety health hazards for traditional fish products: Preventive measures and monitoring issues. Turkish Journal of Fisheries and Aquatic Sciences, 10, 139–160. [Google Scholar]
- Köse, S. , Koral, S. , Tufan, B. , Pompe, M. , Scavniçar, A. , & Koçar, D. (2012). Biogenic amine contents of commercially processed traditional fish products originating from European countries and Turkey. European Food Research and Technology, 235, 669–683. 10.1007/s00217-012-1794-8 [DOI] [Google Scholar]
- Kpoclou, E. Y. , Anihouvi, V. B. , Azokpota, P. , Soumanou, M. M. , Douny, C. , Brose, F. , Hounhouigan, D. J. , Scippo, M.‐L. (2014). Effect of fuel and kiln type on the polycyclic aromatic hydrocarbon (PAH) levels in smoked shrimp, a Beninese food condiment. Food Additives & Contaminants (A), 31(7), 1212–1218. 10.1080/19440049.2014.916422 [DOI] [PubMed] [Google Scholar]
- Larsen, J. C. (2006). Risk assessment of chemicals in European traditional foods. Trends in Food Science & Technology, 17, 471–481. 10.1016/j.tifs.2006.04.007 [DOI] [Google Scholar]
- Latorre‐Moratalla, M. L. , Comas‐Baste, O. , Bover‐Cid, S. , & Vidal‐Carou, M. C. (2017). Tyramine and histamine risk assessment related to consumption of dry fermented sausages by the Spanish population. Food and Chemical Toxicology, 99, 78–85. 10.1016/j.fct.2016.11.011 [DOI] [PubMed] [Google Scholar]
- Ledesma, E. , Rendueles, M. , & Diaz, M. (2015). Spanish smoked meat products: Benzo(a)pyrene (BaP) contamination and moisture. Journal of Food Composition and Analysis, 37, 87–94. 10.1016/j.jfca.2014.09.004 [DOI] [Google Scholar]
- Lehane, L. , & Olley, J. (2000). Histamine fish poisoning revisited. International Journal of Food Microbiology, 58, 1–37. 10.1016/S0168-1605(00)00296-8 [DOI] [PubMed] [Google Scholar]
- Lu, F. , Kuhnle, G. K. , & Cheng, Q. (2018). The effect of common spices and meat type on the formation of heterocyclic amines and polycyclic aromatic hydrocarbons in deep‐fried meatballs. Food Control, 92, 399–411. 10.1016/j.foodcont.2018.05.018 [DOI] [Google Scholar]
- Marissiaux, L. , Scippo, M.‐L. , Daube, G. , Denayer, S. , Botteldoorn, N. , Gobert, S. , Scholtes, B. , & Ghuysen, A. (2018). An awkward fishing expedition. Acta Anæsthesiologica Belgica, 69(2), 1–4. [Google Scholar]
- Mastanjević, K. M. , Kartalović, B. D. , Vranešević, J. M. , Novakov, N. J. , & Habschied, K. J. (2020). Polycyclic aromatic hydrocarbons in traditionally smoked Slavonska kobasica . Food Additives & Contaminants (B), 3(2), 82–87. [DOI] [PubMed] [Google Scholar]
- Mehdipour, S. Z. , Shokrzadeh, M. , Khanzadi, S. , & Shahsavani, D. (2018). Effects of cooking methods on the concentrations of lead, chromium and cadmium in whitefish (Rutilus frissi kutum) from the Caspian Sea, Iran. Iranian Journal of Chemistry and Chemical Engineering, 37(1), 141–147. [Google Scholar]
- Melo, A. , Viegas, O. , Eça, R. , Petisca, C. , Pinho, O. , & Ferreira, I. M. P. L. V. O. (2008). Extraction, detection, and quantification of heterocyclic aromatic amines in Portuguese meat dishes by HPLC/diode array. Journal of Liquid Chromatography & Related Technologies, 31, 772–787. 10.1080/10826070701855987 [DOI] [Google Scholar]
- Mey, E. D. , Klerck, K. D. , Maere, H. D. , Dewulf, L. , Derdelinckx, G. , Peeters, M.‐C. , Fraeye, I. , Heyden, Y. V. , & Paelinck, H. (2014). The occurrence of N‐nitrosamines, residual nitrite and biogenic amines in commercial dry fermented sausages and evaluation of their occasional relation. Meat Science, 96, 821–828. 10.1016/j.meatsci.2013.09.010 [DOI] [PubMed] [Google Scholar]
- Mills, L. , & Alexander, M. (1976). Nitrosamine formation by cultures of several microorganisms. Applied and Environmental Microbiology, 31(6), 892–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray, S. , & Lynch, A. M. (1993). Quantification of the carcinogens 2‐amino‐3,8‐dimethyland 2‐amino‐3,4,8‐trimethylimidazo[4,5‐f]quinoxaline and 2‐amino‐1‐methyl‐6‐phenylimidazo[4,5‐b]pyridine in food using a combined assay based on gas chromatographynegative ion mass spectrometry. Journal of Chromatography, 616, 211–219. 10.1016/0378-4347(93)80388-K [DOI] [PubMed] [Google Scholar]
- Nout, R. , Hounhouigan, J. D. , & Van Boekel, T. (2003). Les Aliments: Transformation, conservation et qualité [Foods: Processing, conservation and quality]. The Netherlands: Backhuys Publishers. [Google Scholar]
- Ntzimani, A. G. , Paleologos, E. K. , Savvaidis, I. N. , & Kontominas, M. G. (2008). Formation of biogenic amines and relation to microbial flora and sensory changes in smoked turkey breast fillets stored under various packaging conditions at 4 °C. Food Microbiology, 25, 509–517. 10.1016/j.fm.2007.12.002 [DOI] [PubMed] [Google Scholar]
- Onal, A. (2007). A review: Current analytical methods for the determination of biogenic amines in foods. Food Chemistry, 103, 1475–1486. 10.1016/j.foodchem.2006.08.028 [DOI] [Google Scholar]
- Onyango, A. A. , Lalah, J. O. , & Wandiga, S. O. (2012). The effect of local cooking methods on polycyclic aromatic hydrocarbons (PAHs) contents in beef, goat meat, and pork as potential sources of human exposure in Kisumu City, Kenya. Polycyclic Aromatic Compounds, 32, 656–668. org%252Fsites%252Fcodex%252FStanda rds%252FCXS%2B311‐2013%252FCXS_311f.pdf. 10.1080/10406638.2012.725195 [DOI] [Google Scholar]
- Oz, F. , & Yuzer, M. O. (2016). The effects of cooking on wire and stone barbecue at different cooking levels on the formation of heterocyclic aromatic amines and polycyclic aromatic hydrocarbons in beef steak. Food Chemistry, 203, 59–66. 10.1016/j.foodchem.2016.02.041 [DOI] [PubMed] [Google Scholar]
- Pan, A. , Sun, Q. , Bernstein, A. M. , Schulze, M. B. , Manson, J. E. , Willett, W. C. , & Hu, F. B. (2011). Red meat consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta‐analysis1–3 . American Journal of Clinical Nutrition, 94, 1088–1096. 10.3945/ajcn.111.018978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perello, G. , Marti‐Cid, R. , Llobet, J. M. , & Domingo, J. L. (2008). Effects of various cooking processes on the concentrations of arsenic, cadmium, mercury, and lead in foods. Journal of Agricultural and Food Chemistry, 56, 11262–11269. 10.1021/jf802411q [DOI] [PubMed] [Google Scholar]
- Plahar, W. A. , Nerquaye‐Tetteh, G. A. , & Annan, N. T. (1999). Development of an integrated quality assurance system for the traditional Sardinella sp. and anchovy fish smoking industry in Ghana. Food Control, 10, 15–25. 10.1016/S0956-7135(98)00150-9 [DOI] [Google Scholar]
- Poligné, I. , Collignan, A. , & Trystram, G. (2001). Characterization of traditional processing of pork meat into boucané . Meat Science, 59, 377–389. 10.1016/S0309-1740(01)00090-0 [DOI] [PubMed] [Google Scholar]
- Puangsombat, K. , Gadgil, P. , Houser, T. A. , Hunt, M. C. , & Smith, J. S. (2011). Heterocyclic amine content in commercial ready to eat meat products. Meat Science, 88, 227–233. 10.1016/j.meatsci.2010.12.025 [DOI] [PubMed] [Google Scholar]
- PubChem (2020). National Center for Biotechnology Information, PubChem Database. Retrieved from https://pubchem.ncbi.nlm.nih.gov/compound [Google Scholar]
- Racovita, R. C. , Secuianu, C. , & Israel‐Roming, F. (2021). Quantification and risk assessment of carcinogenic polycyclic aromatic hydrocarbons in retail smoked fish and smoked cheeses. Food Control, 121, 1–9. 10.1016/j.foodcont.2020.107586 [DOI] [Google Scholar]
- Rajkowska‐Myśliwiec, M. , Pokorska‐Niewiada, K. , Witczak, A. , Balcerzak, M. , & Ciecholewska‐Juśko, D. (2021). Health benefits and risks associated with elements uptake from grilled fish and fish products. Journal of the Science of Food and Agriculture, 1–8. 10.1002/jsfa.11429 [DOI] [PubMed] [Google Scholar]
- Rauscher‐Gabernig, E. , Grossgut, R. , Bauer, F. , & Paulsen, P. (2009). Assessment of alimentary histamine exposure of consumers in Austria and development of tolerable levels in typical foods. Food Control, 20, 423–429. 10.1016/j.foodcont.2008.07.011 [DOI] [Google Scholar]
- Reinik, M. (2007). Nitrates, nitrites, N‐nitrosamines and Polycyclic Aromatic Hydrocarbons in food: Analytical methods, occurrence and dietary intake (Doctoral dissertation). Retrieved from https://www.academia.edu/23501371/Nitrates_nitrites_N_nitrosamines_and_polycyclic_aromatic_hydrocarbons_in_food_analytical_methods_occurrence_and_dietary_intake [Google Scholar]
- Ronco, A. L. , Stefani, E. D. , Correa, P. , DeneoPellegrini, H. , Boffetta, P. , Acosta, G. , & Mendilaharsu, M. (2011). Dietary benzo[a]pyrene, alcohol drinking, and risk of breast cancer: A case‐control study in Uruguay. Asian Pacific Journal of Cancer Prevention, 12, 1463–1467. [PubMed] [Google Scholar]
- Roseiro, L. C. , Gomes, A. , & Santos, C. (2011). Influence of processing in the prevalence of polycyclic aromatic hydrocarbons in a Portuguese traditional meat product. Food and Chemical Toxicology, 49, 1340–1345. 10.1016/j.fct.2011.03.017 [DOI] [PubMed] [Google Scholar]
- Rostkowska, K. , Zwierz, K. , Różański, A. , Moniuszko‐Jakoniuk, J. , & Roszczenko, A. (1998). Formation and Metabolism of N‐Nitrosamines. Polish Journal of Environmental Studies, 7(6), 321–325. [Google Scholar]
- Rozentale, I. , Stumpe‐Vıksna, I. , Zacs, D. , Siksna, I. , Melngaile, A. , & Bartkevics, V. (2015). Assessment of dietary exposure to polycyclic aromatic hydrocarbons from smoked meat products produced in Latvia. Food Control, 54, 16–22. 10.1016/j.foodcont.2015.01.017 [DOI] [Google Scholar]
- Rozentale, I. , Zacs, D. , Bartkiene, E. , & Bartkevics, V. (2018). Polycyclic aromatic hydrocarbons in traditionally smoked meat products from the Baltic States. Food Additives & Contaminants (B), 11(2), 138–145. 10.1080/19393210.2018.1440637 [DOI] [PubMed] [Google Scholar]
- Sagratini, G. , Fernández‐Franzón, M. , Berardinis, F. D. , Font, G. , Vittori, S. , & Mañes, J. (2012). Simultaneous determination of eight underivatised biogenic amines in fish by solid phase extraction and liquid chromatography–tandem mass spectrometry. Food Chemistry, 132, 537–543. 10.1016/j.foodchem.2011.10.054 [DOI] [PubMed] [Google Scholar]
- Sahin, S. , Ulusoy, H. I. , Alemdar, S. , Erdogan, S. , & Agaoglu, S. (2020). The presence of polycyclic aromatic hydrocarbons (PAHs) in grilled beef, chicken and fish by considering dietary exposure and risk assessment. Food Science of Animal Resources, 40(5), 675–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito, E. , Tanaka, N. , Miyazaki, A. , & Tsuzaki, M. (2014). Concentration and particle size distribution of polycyclic aromatic hydrocarbons formed by thermal cooking. Food Chemistry, 153, 285–291. 10.1016/j.foodchem.2013.12.055 [DOI] [PubMed] [Google Scholar]
- Sallam, K. I. , Morgan, S. E. M. , Sayed‐Ahmed, M. Z. , Alqahtani, S. S. , & Abd‐Elghany, S. M. (2021). Health hazard from exposure to histamine produced in ready‐to‐eat Shawarma widely consumed in Egypt. Journal of Food Composition and Analysis, 97, 1–8. [Google Scholar]
- Santos, F. J. , Barceló‐Barrachina, E. , Toribio, F. , Puignou, L. , Galceran, M. T. , Persson, E. , Skog, K. , Messner, C. , Murkovic, M. , Nabinger, U. , & Ristic, A. (2004). Analysis of heterocyclic amines in food products: Interlaboratory studies. Journal of Chromatography B, 802, 69–78. 10.1016/j.jchromb.2003.09.030 [DOI] [PubMed] [Google Scholar]
- Santos, M. H. S. (1996). Biogenic amines: Their importance in foods. International Journal of Microbiology, 29, 213–231. 10.1016/0168-1605(95)00032-1 [DOI] [PubMed] [Google Scholar]
- SCF (Scientific Committee on Food) (2002). Opinion of the Scientific Committee on Food on the risks to human health of polycyclic aromatic hydrocarbons in food expressed on 4 December 2002. Retrieved from https://ec.europa.eu/food/sites/food/files/safety/docs/sci‐com_scf_out153_en.pdf [Google Scholar]
- Scholl, G. , Humblet, M.‐F. , Scippo, M.‐L. , Pauw, E. D. , Eppe, G. , & Saegerman, C. (2012). Risk assessment of Belgian adults for furan contamination through the food chain. Food Additives & Contaminants: Part A, 29(3), 345–353. [DOI] [PubMed] [Google Scholar]
- Sebranek, J. G. , & Bacus, J. N. (2007). Cured meat products without direct addition of nitrate or nitrite: What are the issues? Meat Science, 77, 136–147. 10.1016/j.meatsci.2007.03.025 [DOI] [PubMed] [Google Scholar]
- Silva, B. O. , Adetunde, O. T. , Oluseyi, T. O. , Olayinka, K. O. , & Alo, B. I. (2011). Effects of the methods of smoking on the levels of polycyclic aromatic hydrocarbons (PAHs) in some locally consumed fishes in Nigeria. African Journal of Food Science, 5(7), 384–391. [Google Scholar]
- Simunovic, S. , Jankovic, S. , Baltic, T. , Nikolic, D. , Djinovic‐Stojanovic, J. , Lukic, M. , & Parunovic, N. (2019). Histamine in canned and smoked fishery products sold in Serbia. Meat technology, 60(1), 8–16. [Google Scholar]
- Sinha, R. , Knize, M. G. , Salmon, C. P. , Brown, E. D. , Rhodes, D. , Felton, J. S. , Levander, O. A. , & Rothman, N. (1998). Heterocyclic amine content of pork products cooked by different methods and to varying degrees of doneness. Food and Chemical Toxicology, 36, 289–297. 10.1016/S0278-6915(97)00159-2 [DOI] [PubMed] [Google Scholar]
- Sinha, R. , Kulldorff, M. , Gunter, M. J. , Strickland, P. , & Rothman, N. (2005). Dietary benzo[a]pyrene intake and risk of colorectal adenoma. Cancer Epidemiology, Biomarkers & Prevention, 14(8), 2030–2034. 10.1158/1055-9965.EPI-04-0854 [DOI] [PubMed] [Google Scholar]
- Sinha, R. , Rothman, N. , Brown, E. D. , Salmon, C. P. , Knize, M. G. , Swanson, C. A. , Rossi, S. C. , Mark, S. D. , Levander, O. A. , & Felton, J. S. (1995). High concentrations of the carcinogen 2‐amino‐1‐methyl‐6‐phenylimidazo [4,5‐b]pyridine (PhIP) occur in chicken but are dependent on the cooking method. Cancer Research, 55(20), 4516–4519. [PubMed] [Google Scholar]
- Şireli, U. T. , Göncüoğlu, M. , Yıldırım, Y. , Gücükoğlu, A. , & Çakmak, Ö. (2006). Assessment of heavy metals (Cadmium and Lead) in vacuum packaged smoked fish species (Mackerel, Salmo salar and Oncorhynhus mykiss) Marketed in Ankara (Turkey). Journal of Fisheries & Aquatic Sciences, 23(3–4), 353–356. [Google Scholar]
- Skog, K. I. , Johansson, M. A. E. , & Jaègerstad, M. I. (1998). Carcinogenic heterocyclic amines in model systems and cooked foods: A review on formation, occurrence and intake. Food and Chemical Toxicology, 36(9–10), 879–896. 10.1016/S0278-6915(98)00061-1 [DOI] [PubMed] [Google Scholar]
- Solyakov, A. , & Skog, K. (2002). Screening for heterocyclic amines in chicken cooked in various ways. Food and Chemical Toxicology, 40, 1205–1211. 10.1016/S0278-6915(02)00054-6 [DOI] [PubMed] [Google Scholar]
- Stadler, R. H. , & Lineback, D. R. (2009). Process‐Induced food toxicants: Occurrence, Formation, Mitigation, and Health Risks. John Wiley & Sons, Inc. p. 57. [Google Scholar]
- Stołyhwo, A. , & Sikorski, Z. E. (2005). Polycyclic aromatic hydrocarbons in smoked fish – a critical review. Food Chemistry, 91, 303–311. 10.1016/j.foodchem.2004.06.012 [DOI] [Google Scholar]
- Stumpe‐Viksna, I. , Bartkevics, V. , Kukare, A. , & Morozovs, A. (2008). Polycyclic aromatic hydrocarbons in meat smoked with different types of wood. Food Chemistry, 110, 794–797. 10.1016/j.foodchem.2008.03.004 [DOI] [Google Scholar]
- Swann, P. F. (1977). Carcinogenic risk from nitrite, nitrate and N‐nitrosamines in food. Proceedings of the Royal Society of Medicine, 70, 113–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tongo, I. , Ezemonye, L. , & Akpeh, K. (2017). Distribution, characterization, and human health risk assessment of polycyclic aromatic hydrocarbons (PAHs) in Ovia River, Southern Nigeria. Environmental Monitoring and Assessment, 189(247), 1–16. 10.1007/s10661-017-5931-5 [DOI] [PubMed] [Google Scholar]
- Tosukhowong, A. , Visessanguan, W. , Pumpuang, L. , Tepkasikul, P. , Panya, A. , & Valyasevi, R. (2011). Biogenic amine formation in Nham, a Thai fermented sausage, and the reduction by commercial starter culture, Lactobacillus plantarum BCC 9546. Food Chemistry, 129, 846–853. 10.1016/j.foodchem.2011.05.033 [DOI] [PubMed] [Google Scholar]
- Toth, L. , & Potthast, K. (1984). Chemical aspects of the smoking of meat and meat products. Advances in Food Research, 29, 87–158. [Google Scholar]
- Turesky, R. J. (2007). Formation and biochemistry of carcinogenic heterocyclic aromatic amines in cooked meats. Toxicology Letters, 168, 219–227. 10.1016/j.toxlet.2006.10.018 [DOI] [PubMed] [Google Scholar]
- Ubwa, S. T. , Abah, J. , Tarzaa, L. , Tyohemba, R. L. , & Ahile, U. J. (2015). Effects of traditional smoking methods on the concentrations of polynuclear aromatic hydrocarbons (PAHs) in some species of smoked fish traded in Benue State, Nigeria. Journal of Food Research, 4(2), 119–127. 10.5539/jfr.v4n2p119 [DOI] [Google Scholar]
- Uluozlu, O. D. , Tuzen, M. , Mendil, D. , & Soylak, M. (2009). Assessment of trace element contents of chicken products from turkey. Journal of Hazardous Materials, 163, 982–987. 10.1016/j.jhazmat.2008.07.050 [DOI] [PubMed] [Google Scholar]
- Van de Wiele, T. , Vanhaecke, L. , Boeckaert, C. , Peru, K. , Headley, J. , Verstraete, W. , & Siciliano, S. (2005). Human colon microbiota transform polycyclic aromatic hydrocarbons to estrogenic metabolites. Environmental Health Perspectives, 113(1), 6–10. 10.1289/ehp.7259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veyrand, B. , Sirot, V. , Durand, S. , Pollono, C. , Marchand, P. , Dervilly‐Pinel, G. , Tard, A. , Leblanc, J.‐C. , & Bizec, B. L. (2013). Human dietary exposure to polycyclic aromatic hydrocarbons: Results of the second French Total Diet Study. Environment International, 54, 11–17. 10.1016/j.envint.2012.12.011 [DOI] [PubMed] [Google Scholar]
- Viegas, O. , Novo, P. , Pinho, O. , & Ferreira, I. M. P. L. V. O. (2012). A comparison of the extraction procedures and quantification methods for the chromatographic determination of polycyclic aromatic hydrocarbons in charcoal grilled meat and fish. Talanta, 88, 677–683. 10.1016/j.talanta.2011.11.060 [DOI] [PubMed] [Google Scholar]
- Viegas, O. , Novo, P. , Pinto, E. , Pinho, O. , & Ferreira, I. M. P. L. V. O. (2012). Effect of charcoal types and grilling conditions on formation of heterocyclic aromatic amines (HAs) and polycyclic aromatic hydrocarbons (PAHs) in grilled muscle foods. Food and Chemical Toxicology, 50, 2128–2134. 10.1016/j.fct.2012.03.051 [DOI] [PubMed] [Google Scholar]
- Wakabayashi, K. , Ushiyama, H. , Takahashi, M. , Nukaya, H. , Kim, S.‐B. , Hirose, M. , Ochiai, M. , Sugimura, T. , & Nagao, M. (1993). Exposure to heterocyclic amines. Environmental Health Perspectives, 99, 129–133. 10.1289/ehp.9399129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Jiao, Y. , Kong, Q. , Zheng, F. , Shao, L. , Zhang, T. , Jiang, D. , & Gao, X. (2021). Occurrence of polycyclic aromatic hydrocarbons in fried and grilled fish from Shandong China and health risk assessment. Environmental Science and Pollution Research, 28, 32802–32809. 10.1007/s11356-021-13045-y [DOI] [PubMed] [Google Scholar]
- Yurchenko, S. , & Molder, U. (2007). The occurrence of volatile N‐nitrosamines in Estonian meat products. Food Chemistry, 100, 1713–1721. 10.1016/j.foodchem.2005.10.017 [DOI] [Google Scholar]
- Yusuf, K. A. , Ezechukwu, L. N. , Faykoya, K. A. , Akintola, S. L. , Agboola, J. I. , & Omoleye, T. O. (2015). Influence of fish smoking methods on polycyclic aromatic hydrocarbons content and possible risks to human health. African Journal of Food Science, 9(3), 126–135. 10.5897/AJFS2014.1227 [DOI] [Google Scholar]
- Zachara, A. , Gałkowska, D. , & Juszczak, L. (2017). Contamination of smoked meat and fish products from Polish market with polycyclic aromatic hydrocarbons. Food Control, 80, 45–51. 10.1016/j.foodcont.2017.04.024 [DOI] [Google Scholar]
- Zaman, M. Z. , Bakar, F. A. , Selamat, J. , & Bakar, J. (2010). Occurrence of Biogenic Amines and Amines Degrading Bacteria in Fish Sauce. Czech Journal of Food Sciences, 28(5), 440–449. 10.17221/312/2009-CJFS [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data used in this study can be made available upon reasonable request.



 C8H11N
C8H11N