Abstract
Background
Multisystem inflammatory syndrome in children (MIS-C) associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a new entity affecting a small percentage of children during the COVID-19 pandemic.
Materials and methods
Demography, clinical, and laboratory variables of children admitted from April to September 2020 with MIS-C were studied retrospectively at eight hospitals in Delhi, India.
Results
We identified 120 patients [median age: 7 years (interquartile range (IQR): 4–10)] with male-to-female ratio of 2.3:1. Overall, 73 out of 120 children (60.8%) presented with shock, 63 (52.5%) required inopressor support, and 51 (43%) required respiratory support. We categorized the cohort into three observed clinical phenotypes: MIS-C with shock (n = 63), MIS-C with Kawasaki disease (KD) (n = 23), and MIS-C without shock and KD (n = 34). Atypical presentations were hypothermia, orchitis, meningoencephalitis, demyelination, polyneuropathy, pancreatitis, and appendicitis. Ninety-four percent had laboratory evidence of SARS-CoV-2 (78.3%, seropositive and 15.8%, RT-PCR positive). The median C-reactive protein (CRP) was 136 mg/L (IQR, 63.5–212.5) and ferritin was 543 ng/mL (IQR, 225–1,127). More than 90% received immunomodulatory therapy (intravenous immunoglobulins and/or steroids) with an excellent outcome (96% survived). CRP and absolute neutrophil count (ANC) were correlated statistically with severity.
Conclusion
MIS-C data from Delhi are presented. Rising CRP and ANC predict the severe MIS-C.
How to cite this article
Mehra B, Pandey M, Gupta D, Oberoi T, Jerath N, Sharma R, et al. COVID-19-associated Multisystem Inflammatory Syndrome in Children: A Multicentric Retrospective Cohort Study. Indian J Crit Care Med 2021;25(10):1176–1182.
Keywords: Corticosteroids, Hyperinflammation, Intravenous Immunoglobulin—IVIG, Kawasaki disease, Multisystem inflammatory syndrome in children (MIS-C), SARS-CoV-2
Introduction
Multisystem inflammatory syndrome in children (MIS-C) associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a novel entity being reported in children with increasing frequency from countries where the coronavirus disease- 2019 (COVID-19) pandemic has peaked or fading.1,2 Children with this multisystem illness have been described to have overlapping features of Kawasaki disease (KD), toxic shock syndrome, and macrophage activation syndrome. The initial cases from India were reported in May 2020,3,4 and as the number of COVID-19 cases grew exponentially across the country, clinicians identified this new entity more frequently. In view of the paucity of data from the Asian countries, we conducted this retrospective study with the objective to study the patient characteristics, clinical and laboratory variables, and their analysis for severity prediction.
Study Design and Data Collection
We conducted a retrospective multicenter cohort study at eight pediatric centers in Delhi, an early epicenter of the COVID-19 pandemic in India. After getting institutional ethical clearance, MIS-C cases were identified retrospectively at each participating site, for the study period from April 1 to September 30, 2020, using Centers for Disease Control and Prevention criteria for MIS-C.2 Organ system involvement was defined as described by Feldstein et al. in their study on MIS-C in the United States.5 Data were extracted for demographics, clinical presentation, radiological findings, underlying comorbidities, echocardiographic (ECHO) findings, laboratory investigations, treatment modalities, and outcome.
Statistics
We described the patient characteristics as numbers and percentages for categorical variables, and median with interquartile range (IQR) for quantitative ones. The Chi-square test was used for assessing the statistical significance of the difference in the percentage of cases with different characteristics between the groups. KruskalWallis test was used for the difference in medians of quantitative data in view of a highly skewed distribution of these measurements. For severity prediction, two models were used, viz. “Binary logistic regression” model and “Random-Forest” model. Receiver operating characteristic (ROC) curves were used to obtain the optimized cutoff value for the score maximizing sensitivity and specificity to distinguish severe and non-severe patients. SPSS 21 was used for calculations.
Results
Initially, 141 cases were identified. Twenty-one cases were excluded (evidence of other tropical infections along with COVID-19, duplicate patients, detailed investigations not done, and those who expired within 24 hours of hospital stay). Finally, 120 cases were enrolled. The surge of MIS-C cases in our cohort was observed during July and August (Fig. 1), a month after the first surge of acute COVID-19 cases in Delhi, which was observed during the end of May and early June.6
Fig. 1.
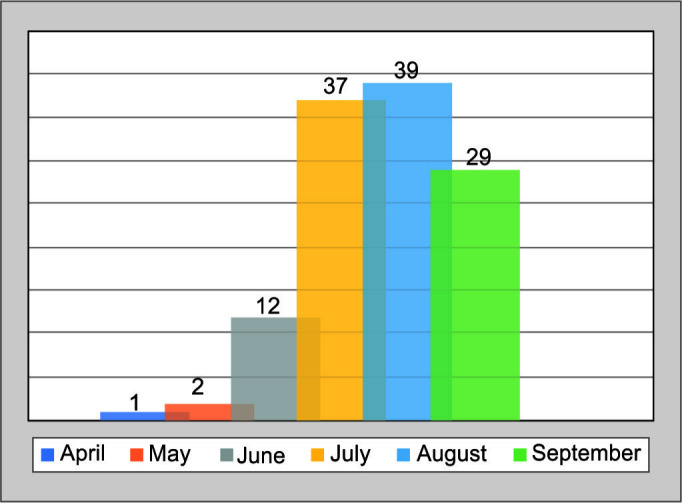
Month-wise trend of MIS-C cases during COVID-19 pandemic
Clinical Characteristics, Organ System Involvement, Treatment, and Outcome
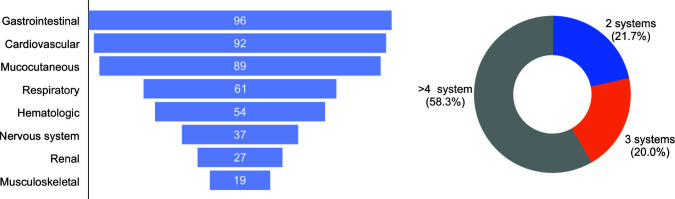
In our cohort, there was a male preponderance (70%), and the majority (n = 73; 61%) were from 5 to 12 years of age-group (Table 1). Twelve children were <1 year old, 20 were between 1 and 4 years, and the rest 15 were >13 years old. The youngest case of MIS-C was a child of 6 weeks of age. Figure 2 describes the organ system involvement.
Table 1.
Demographic, clinical features, treatment, and outcome of MIS-C cohort
| Clinical variables | TOTAL (n = 120) |
Clinical phenotype of MIS-C
|
p value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group 1 Shock (n = 63) | Group 2 KD complete (n = 23) | Group 3 No Shock/KD (n = 34) | ||||||||
| Demographics | ||||||||||
| Median age, y (IQ-R) | 7 | (4–10) | 8 | (5–10) | 4 | (8 months– 6 years) | 7 | (4–11) | 0.002 | |
| Males | 84 | (70%) | 42 | (66.7%) | 19 | (82.6%) | 23 | (67.6%) | 0.339 | |
| BMI >95th centile | 34 | (28.3%) | 17 | (27%) | 4 | (17.4%) | 13 | (38.2%) | 0.217 | |
| Comorbidity | 8 | (6.7%) | 5 | (7.9%) | 0 | 3 | (8.8%) | |||
| Clinical features at admission | ||||||||||
| Fever duration | <5 days | 37 | (30.8%) | 21 | (33%) | 0 | 13 | (38.2%) | ||
| ≥5 days | 82 | (68.3%) | 41 | (65%) | 23 | (100%) | 21 | (61.8%) | ||
| GI symptoms | 86 | (71.7%) | 48 | (76.2%) | 16 | (69.6%) | 22 | (64.7%) | 0.26 | |
| Rash | 79 | (65.8%) | 39 | (61.9%) | 20 | (87%) | 20 | (58.8%) | 0.126 | |
| Conjunctivitis | 68 | (56.6%) | 31 | (49.2%) | 22 | (95.7%) | 15 | (44%) | 0.326 | |
| Shock* | 73 | (60.8%) | 63 | (100%) | 10 | (43.5%) | 0 | <0.001 | ||
| Respiratory distress | 52 | (43.3%) | 40 | (63.5%) | 3 | (13%) | 9 | (26.5%) | <0.001 | |
| ARDS@ | 23 | (18.3%) | 16 | (25.4%) | 1 | (4.3%) | 6 | (17.6%) | 0.078 | |
| Abnormal ECHO | 63/108 | (58.3%) | 48/63 | (76.1%) | 12/23 | (52.1%) | 3/22 | (13.6%) | ||
| • LV dysfunction | 37 | (30.8%) | 32 | (50.8%) | 4 | (17.4%) | 1 | (2.9%) | <0.001 | |
| • CA abnormality | 16 | (13.3%) | 5 | (7.9%) | 9 | (39.1%) | 2 | (5.9%) | 0.001 | |
| • Pericardial effusion | 15 | (12.5%) | 13 | (20.6%) | 1 | (4.3%) | 1 | (2.9%) | ||
| Encephalopathy | 32 | (26.7%) | 22 | (34.9%) | 5 | (21.7%) | 5 | (14.7%) | 0.454 | |
| Seizures | 7 | (5.8%) | 5 | (7.9%) | 0 | 2 | (5.9%) | 0.072 | ||
| Acute kidney injury# | 27 | (22.5%) | 23 | (36.5%) | 0 | 4 | (11.8%) | <0.001 | ||
| Abnormal CXR | 45 | (37.5%) | 32 | (50.8%) | 2 | (8.7%) | 11 | (32.4%) | 0.001 | |
| • Infiltrate/opacity | 37 | (30.8%) | 26 | (41.3%) | 2 | (8.7%) | 9 | (26.5%) | ||
| • Pleural effusion | 25 | (20.8%) | 19 | (30.2%) | 1 | (4.3%) | 5 | (14.7%) | ||
| • Pneumothorax | 1 | 1 | 0 | 0 | ||||||
| ≥4 Organ system involved | 70 | (58.3%) | 51 | (81%) | 10 | (43.5%) | 9 | (26.5%) | 0.001 | |
| Treatment | ||||||||||
| PICU care >48 hours | 108 | (90%) | 53 | (84.1%) | 20 | (86.9%) | 25 | (73.5%) | 0.047 | |
| HHFNC/NIV | 25 | (20.8%) | 16 | (25.4%) | 2 | (8.7%) | 7 | (20.6%) | 0.245 | |
| Invasive ventilation | 26 | (22.5%) | 25 | (39.7%) | 0 | 1 | (2.9%) | <0.001 | ||
| Vasoactive infusion | 63 | (52.5%) | 56 | (88.9%) | 7 | (30.4%) | 0 | <0.001 | ||
| RRT | 12 | (8.3%) | 9 | (14.3%) | 0 | 3 | (8.4%) | 0.814 | ||
| ECMO | 1 | 1 | 0 | 0 | ||||||
| Pharmacotherapy | ||||||||||
| Steroids >3 days | 95 | (79.1%) | 55 | (87.3%) | 19 | (82.6%) | 21 | (61.7%) | ||
| IV-IG | 82 | (68.3%) | 44 | (69.8%) | 22 | (95.7%) | 16 | (47.1%) | ||
| No IV-IG/steroids | 10 | (8.3%) | 4 | (6.3%) | 0 | 0 | 6 | (17.6%) | ||
| Aspirin | 36 | (30%) | 17 | (27%) | 16 | (69.6%) | 3 | (8.8%) | ||
| Enoxaparin/heparin | 59 | (49.2%) | 37 | (58.7%) | 19 | (82.6%) | 21 | (61.7%) | ||
| Remdesivir | 4 | (3.3%) | 4 | (6.3%) | 0 | 0 | ||||
| Outcome | ||||||||||
| Survived todischarge | 116 | (96.7%) | 60 | (95.2%) | 23 | (100%) | 33 | (97.1%) | ||
| Death | 4 | 3 | 0 | 1 | ||||||
ARDS, acute respiratory distress syndrome; BMI, body mass index; CA, coronary artery; CXR, chest X-ray; ECMO, extracorporeal membrane oxygenation; ECHO, echocardiography; GI, gastrointestinal; HHFNC, humidified high-flow nasal cannula; IQR, interquartile range; IV-IG, intravenous immunoglobulin; KD, Kawasaki disease; LV, left ventricle; PICU, pediatric intensive care unit; NIV, noninvasive ventilation; RRT, renal replacement therapy. *Shock was defined as requirement of fluid bolus of ≥20 mL/kg or need for vasoactive medication. _ARDS was defined as per Pediatric Acute Lung Injury Consensus Conference Group (2015). #Acute kidney injury was defined as a creatinine level above the values for age as follows: 1 month – <1 year, 0.6 mg/dL; 1–10 years, 1.05 mg/dL; ≥11 years, >1.5 mg/dL
Fig. 2.
Organ system involvement of the MIS-C cohort
The most common symptom was fever (99%), although one infant presented with severe hypothermia (31°C) with multi-organ dysfunction. Mucocutaneous involvement was noted in the form of rash (maculopapular, morbilliform, urticarial, and erythroderma), non-purulent conjunctivitis, oral mucositis, and non-pitting edema in hands and feet. We categorized the cohort based on the major clinical phenotype. The first group (n = 63; 52.5%) had features of shock during the stay. The second group fulfilled the criteria for KD with or without shock (n = 23; 19.2%), and the last group had features of multisystem involvement but did not have shock or KD (n = 34; 28.3%). Abnormal ECHO findings [such as left ventricular (LV) dysfunction, pericardial effusion, and abnormal coronaries] were observed in 63 patients (58.3%) out of 108 ECHOs performed. Coronary artery dilatation (defined as coronary artery diameter z score >2)7 was found in 11 patients. In five patients, it was reported as prominent and echogenic. Acute respiratory distress syndrome (ARDS) was observed in 23 patients (10, RT-PCR positive; 13, antibody positive).
Regarding nervous system involvement, 32 patients had encephalopathy (defined as confusion, irritability, or GCS <14 despite correction of shock or hypoxemia). Significant neurological involvement was observed in four patients [one case each of acute disseminated encephalomyelitis, Guillain–Barré syndrome, polyneuropathy, and meningoencephalitis (cerebrospinal fluid and nasopharyngeal swab positive for SARS-CoV-2)]. Ultrasonography of the abdomen was performed in 58 cases, and gall bladder edema with or without sludge was observed in 39 cases. Unusual findings noted were orchitis (n = 1), pancreatitis (n = 2), and inflamed appendix (n = 2).
More than 90% of cohorts (110/120) received some form of immunomodulatory therapy [intravenous immunoglobulins (IV-IG) and/or steroids]. None of the patients in our cohort received biologic agents, such as tocilizumab or anakinra.
The overall outcome was excellent with 96.6% of survival rate. Among four deaths, two cases were RT-PCR positive, one was antibody positive, and one had the epidemiological link in family. One was an adolescent with acute COVID-19 (positive for RT-PCR)-related cytokine release syndrome with severe cardiogenic shock and ARDS, who later succumbed to pancreatitis and peritonitis. Others were: 10-year-old girl from a COVID-19 hotspot (but RT-PCR-negative), who presented with vasoplegic shock, ARDS, and renal failure; a 3-month-old infant with acute COVID-19 related severe ARDS and shock, and last one was a 3-year-old with seizures and acute renal shutdown followed by multi-organ failure.
Laboratory Parameters
Table 2 describes the values of various laboratory parameters done within first 48 hours of admission. One-hundred and thirteen out of 120 patients had laboratory evidence of exposure to SARS-CoV-2 (94 cases seropositive, 16 cases RT-PCR positive, and 3 patients with both RT-PCR- and antibody positive). Rest seven patients were included based on the epidemiological link (out of these, five could not be tested for antibody as it was not available during that time). All patients had one or more elevated biomarkers of inflammation [C-reactive protein (CRP) and ferritin]. When compared across the three clinical phenotypes, median platelet count and absolute lymphocyte count (ALC) were lower, and the incidence of thrombocytopenia (defined as platelet count <120 x 109/L) was significantly higher in MIS-C with shock. Similarly, the values of CRP, D-dimer, ferritin, neutrophil-to-lymphocyte ratio (NLR), and absolute neutrophil count (ANC) were significantly higher ingroup 1 (MIS-C with shock).
Table 2.
Laboratory parameters of MIS-C cohort
| TOTAL (n = 120) | Clinical phenotype of MIS-C | p value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Group 1 Shock (n = 63) | Group 2 KD complete (n = 23) | Group 3 No Shock/KD (n = 34) | |||||||
| Evidence of SARS-CoV-2 | |||||||||
| Only antibodypositive* | 94 | (78.3%) | 51 | (81%) | 20 | (87%) | 23 | (67.6%) | |
| RT-PCR positive$ | 19 | (15.8%) | 9 | (14.3%) | 3 | (13%) | 7 | (20.6%) | |
| Epidemiological link | 25 | (20.8%) | 12 | (19%) | 4 | (17.4%) | 9 | (26.5%) | |
| Laboratory values in the first 24 hours, median (IQR) | |||||||||
| Total leukocyte count, x109/L | 12.5 (7.5–19.6) | 13,100 (7,030–20,500) | 11.7 (9.0–18.3) | 9.0 (7–17.8) | 0.397 | ||||
| ANC,x109/L | 9.5 (5.5–15.6) | 11.0 (5.7–16.8) | 9.4 (6.2–12.1) | 6.8 (3.9–12.3) | 0.077 | ||||
| ALC,/μL | 1,912 (892–3,515) | 1,500 (862–3,110) | 2,600 (1,120–4,860) | 2,200 (820–3,636) | 0.166 | ||||
| NL ratio (NLR) | 4.6 (2.2–10.1) | 6.9 (2.9–11.0) | 2.8 (1.5–10.4) | 3.4 (1.9–5.7) | 0.014 | ||||
| Platelet count, x109/L | 170 (106–265) | 120 (52–206) | 247 (162–480) | 195 (158–322) | <0.001 | ||||
| CRP, mg/L | 136 (63.5–212.5) | 160 (78–225) | 113.5 (46.6–196) | 106 (33.5–161) | 0.028 | ||||
| Ferritin, ng/mL | 543 (225–1,127) | 559 (283–977) | 319.5 (139–651) | 250 (122–1,204) | 0.289 | ||||
| D-dimer, ng/mL | 2,075 (1,120–5972) | 3,230 (1,400–7,034) | 710 (460–1,900) | 1,660 (857–6,000) | 0.002 | ||||
| Thrombocytopenia** | 43 (35.8%) | 34 (54%) | 3 (13%) | 6 (17.6 %) | <0.001 | ||||
| Lymphopenia# | 51 (42.5%) | 26 (41.3%) | 11 (47.8%) | 14 (41.1%) | 0.516 | ||||
| Cardiac marker elevated_ | 59/104 (56.7%) | 38/55 (67.2%) | 8/21 (38.1%) | 13/28 (46.4%) | 0.036 | ||||
| ALT>40 IU/L | 64 (53.3%) | 35 (55.6%) | 7 (30.4%) | 22 (64.7%) | 0.034 | ||||
| INR>1.1 | 38 (31.7%) | 25 (39.7%) | 4 (17.4%) | 9 (26.5%) | 0.068 | ||||
ALT, alanine aminotransferase; INR, international normalized ratio; NL ratio, neutrophil-to-lymphocyte ratio; RT-PCR, real-time polymerase chain reaction. *Tested for either total antibody or anti-S1- and anti-S2-specific IgG antibody by chemiluminescent immunoassay (CLIA). $Out of total 19 positive for RT-PCR, 3 were also positive for antibody. **Thrombocytopenia: Platelet count less than 120 x 109/L; #Lymphopenia: Absolute lymphocyte count of less than 1,500/μL in patients 8 months of age or older and of less than 4,500/μL in patients younger than 8 months of age._Increased cardiac markers (troponin-I, CPKMB, NT-ProBNP) were defined on the basis of the hospital cutoff for the upper limit of the normal range
Predictors of Severity
Among 120 cases, severe MIS-C cases (n = 71, 59%) were identified as (presence of any of the following):
Use of inotropes
Use of invasive or noninvasive ventilation
ARDS
Use of renal replacement therapy
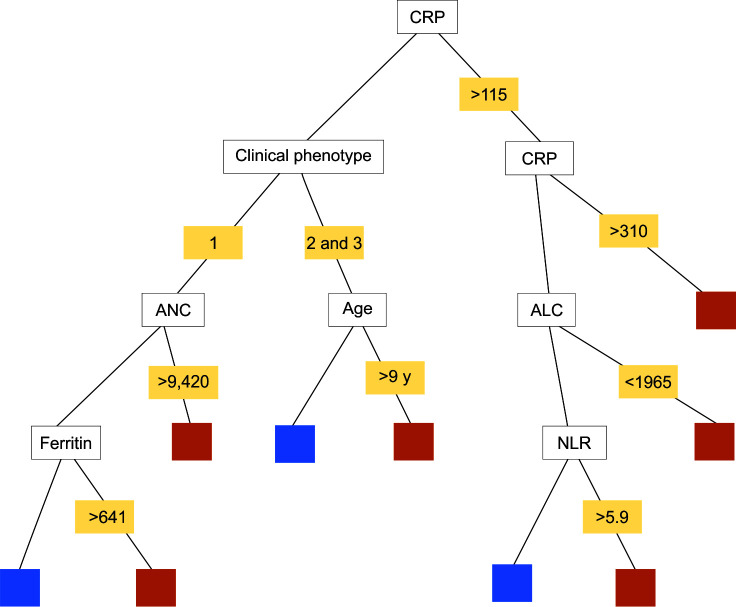
Logistic regression analysis of the whole cohort for severity versus age and obesity did not show a statistically significant association. Laboratory parameters (TLC, ANC, ALC, platelet count, CRP, D-dimer and ferritin) were analyzed for severity prediction for 100 patients (20 cases got excluded as they were not tested for either ferritin or D-dimer). Two models were used for severity prediction. In the first model (logistic regression), statistically significant (p <0.05) predictors of severity were rising CRP and rising neutrophil count (Table 3). In the second model (random forest), CRP was considered as the first split attribute 204 times(out of 322 times = 63%) and the second split attribute 308 times and thus was found to be the most significant predictor of severity. The best cutoff for various laboratory parameters was identified using ROC curves (Table 4). Using the results from logistic regression and random forest method, five-node “Classification And Regression Tree” (CART) model was developed to identify the early predictors of severity (Fig. 3).
Table 3.
Logistic regression model for severity prediction
| aOR | (95% C.I. for aOR) | p value | |
|---|---|---|---|
| Age (per 1-year increase) | 1.094 | (0.983–1.218) | 0.099 |
| Male gender (reference = female) | 0.300 | (0.034–2.671) | 0.280 |
| Obesity (reference = no obesity) | 2.255 | (0.862–5.898) | 0.097 |
| TLC (per unit increase in 109/L) | 1.000 | (0.999–1.001) | 0.787 |
| Neutrophil count (per unit increase in 109/L) | 1.000 | (1.000–1.001) | 0.036 |
| Lymphocyte count (per unit decrease in 109/L) | 1.000 | (0.999–1.001) | 0.167 |
| NL RATIO (per unit increase) | 0.987 | (0.867–1.123) | 0.838 |
| CRP (per unit increase in mg/L) | 1.007 | (1.002–1.018) | 0.010 |
| Ferritin (per unit increase in ng/mL) | 1.000 | (0.999–1.000) | 0.450 |
| Platelet count (per unit decrease in 109/L) | 0.997 | (0.994–1.001) | 0.096 |
| D-dimer (per unit increase in ng/mL) | 1.000 | (1.000–1.001) | 0.554 |
Table 4.
“Best cutoffs coordinates of the Curve” for severity prediction
| Variable (s) | Value | Sensitivity | 1-specificity | AUC |
|---|---|---|---|---|
| Logistic regression model (n = 100) | ||||
| CRP (mg/L) | >138.50 | 0.710 | 0.237 | 0.742 |
| Ferritin (ng/mL) | >217.50 | 0.903 | 0.658 | 0.597 |
| D-dimer (ng/mL) | >2170.00 | 0.581 | 0.342 | 0.622 |
| TLC (x106/L) | >10085.00 | 0.710 | 0.395 | 0.635 |
| Neutrophil count (x106/L) | >8387.00 | 0.710 | 0.342 | 0.684 |
| NL ratio | >4.150 | 0.661 | 0.368 | 0.677 |
| Random forest model (n = 100) | ||||
| CRP (mg/L) | >115 | 0.611 | 0.500 | 0.766 |
| Ferritin (ng/mL) | >435 | 0.522 | 0.367 | 0.82 |
| D-dimer (ng/mL) | >1,815 | 0.551 | 0.467 | 0.58 |
| Neutrophil count (x106/L) | >9,500 | 0.680 | 0.411 | 0.70 |
| Lymphocyte count (x106/L) | <1,965 | 0.389 | 0.767 | 0.935 |
| NL ratio | >2.25 | 0.811 | 0.600 | 0.727 |
Fig. 3.
Five-node CART classification model for severity prediction with splits. Orange squares—severe case; blue squares—non-severe cases
Discussion
This study describes the characteristics and outcomes of 120 children with MIS-C admitted to hospitals that serve diverse geographical regions of Delhi. This is so far the largest cohort of MIS-C described from India. Similar to the trends observed in the United States, United Kingdom, and European cohorts of MIS-C, we witnessed the surge of MIS-C a month later to the peak observed in adults.5,8–10 This trend further reaffirms the temporal association of this syndrome to SARS-CoV-2.
Whereas in adults, severe COVID-19 is usually observed in second week of illness, MIS-C can occur both during the acute COVID-19 (when RT-PCR comes positive) and 2 to 6 weeks later when the antibodies have been formed.11 The latter scenario was much more common in our cohort (78.3%), and similar findings have been reported across all case series described so far.5,8–10 Many of our children who were seropositive did not report any prior COVID-19-like illness in the family or the child. This shows that even asymptomatic COVID-19 infection in children can trigger an immune dysregulation weeks later, resulting in multi-organ dysfunction.
Though the febrile phase precedes the presentation, one infant presented with severe hypothermia and was included in the study because all other clinical and laboratory findings were consistent with MIS-C. Hypothermia at presentation has not been described in any of the previous studies of MIS-C. There is a reported case of a 62-year-old man with COVID-19 who presented with hypothermia and lethargy.12 The underlying mechanism could be similar to rare presentation of systemic inflammatory response with hypothermia (instead of fever) in small children.
We observed three clinical phenotypes, although some degree of overlap between the groups can always be argued. The first group (MIS-C shock, 52%) with hemodynamic instability at presentation was relatively sicker, with a higher incidence of multi-organ dysfunction, with higher use of respiratory and cardiac support. In the UK cohort, the overall shock was observed in 50% of children, and complete KD was observed in 22.4%.9 Italy reported 30-fold increase in the incidence of “KD like illness” in children during the peak of the COVID-19 pandemic, with 50% of cases fulfilling complete KD criteria, and shock was present in 50% of children.8 Contrary to the findings in European countries where coronary artery abnormalities were found in around 30% of children, our study showed lesser involvement of coronaries (13%), with more of LV dysfunction (31%).10 Small cohorts of pediatric multisystem inflammatory syndrome temporally related to SARS-CoV-2 from Chennai and Mumbai, India, reported coronary involvement in 16 and 26% of patients, respectively, whereas LV dysfunction was observed in one-third of children in the Mumbai cohort.13,14 None of the patients in our cohort had arrhythmia, as compared to 12% reported in the US cohort.5
Unlike adults, only 18% of our cohort had ARDS. The incidence of ARDS in the spectrum of MIS-C has not been previously reported. The need to highlight this finding is because the chest X-ray (CXR) or computerized tomography findings in MIS-C resemble the late-stage adult COVID-19, with negative results for respiratory pathogens, and they respond very well to the immunomodulatory therapy, particularly steroids.15 Serious neurological insult to the nervous system as spectrum of MIS-C, though rare, needs to be highlighted. Abdel-Mannan et al. reported four children with severe MIS-C having encephalopathy with myopathic changes. Neuroimaging revealed lesions in the splenium of the corpus callosum in all patients and T2-hyperintense lesions with restricted diffusion in three children.16Our cohort had four cases of serious neurological involvement, with two cases already published.17,18
Literature on severity predictors in MIS-C is scarce till the time of writing this article. Fernandes et al. studied the predictors of severity on 281 children with COVID-19 related respiratory illness and MIS-C.19 Their criteria for the severe disease was ICU care >48 hours. In their MIS-C group, lower ALC and increasing CRP were the predictors of severity. Pouletty et al. compared 16 children with KD-like illness following SARS-CoV-2 infection with a historical cohort of “classical” KD and found higher age and high ferritin associated with severe disease.20 In our cohort, age and obesity were not associated with severe disease. CRP and neutrophil count served as predictors of severity. The difference in findings could be due to the different criteria used for defining the severity.
Limitations
Clinical management, laboratory testing protocols, laboratory techniques, and units for reporting of various parameters differed among different centers. Therefore, we may not have captured certain variables uniformly and accurately. For severity prediction, only values taken at admission were measured; however, their values may have changed during the course of illness as the disease progressed.
Conclusion
The young population is not universally spared from the morbidity of COVID-19 and that even previously healthy children can develop severe disease requiring supportive therapy. The major gain of this study was identifying severity predictors. CRP and ANC are simple laboratory tests that predict the severity.
Acknowledgment
The authors greatly acknowledge the contribution of Ms. Nidhi Mittal in the statistical analysis of data.
Orcid
Bharat Mehra https://orcid.org/0000-0001-8194-7623
Mukul Pandey https://orcid.org/0000-0003-4544-7423
Dhiren Gupta https://orcid.org/0000-0002-8244-0768
Tania Oberoi https://orcid.org/0000-0001-5868-268X
Nameet Jerath https://orcid.org/0000-0002-1688-3317
Rachna Sharma https://orcid.org/0000-0003-2282-3801
Naresh Lal https://orcid.org/0000-0003-1806-3803
Chandrasekhar Singha https://orcid.org/0000-0002-1620-9963
Bhavana Malhotra https://orcid.org/0000-0002-6169-6887
Vinamra Manocha https://orcid.org/0000-0002-4844-2524
Ashish K Simalti https://orcid.org/0000-0002-8227-9038
Yogesh Arya https://orcid.org/0000-0002-4918-7385
Sandeep K Dugaya https://orcid.org/0000-0003-2154-6144
Swati Kalra https://orcid.org/0000-0002-9714-5710
Amar J Chitkara https://orcid.org/0000-0002-7066-8407
Anil Sachdev https://orcid.org/0000-0002-7624-6985
Neeraj Gupta https://orcid.org/0000-0002-7131-4985
Footnotes
Source of support: Nil
Conflict of interest: None
References
- 1.2020. Multisystem inflammatory syndrome in children and adolescents temporally related to COVID-19. Scientific brief: World Health Organization. Available from: https://www.who.int/news-room/commentaries/detail/multisystem-inflammatorysyndrome-in-children-and-adolescents-with. covid-19 [Accessed December18, 2020] [Google Scholar]
- 2.2020. Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with Coronavirus Disease 2019. Centers for Disease Control and Prevention. Emergency preparedness and response: Health alert network. Available from: https://emergency.cdc.gov/han/2020/han00432.asp. [Accessed December 15, 2020] [Google Scholar]
- 3.Balasubramanian S, Nagendran TM, Ramachandran B, Ramanan AV. Hyper-inflammatory syndrome in a child with COVID-19 treated successfully with intravenous immunoglobulin and tocilizumab. Indian Pediatr. 2020;57(7):681–683. doi: 10.1007/s13312-020-1901-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Acharyya BC, Acharyya S, Das D. Novel coronavirus mimicking Kawasaki disease in an infant. Indian Pediatr. 2020;57(8):753–754. doi: 10.1007/s13312-020-1924-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feldstein LR, Rose EB, Horwitz SM, Collins JP, Newhams MM, Son MBF, et al. Multisystem inflammatory syndrome in US. children and adolescents. NEJM. 2020;383:334–336. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Press Trust of India. Delhi added 64,000 fresh Covid-19 cases in June; 47,489 patients recovered. Business standard. 2020. Available from: https://www.business-standard.com/article/current-affairs/delhi-added-64-000-fresh-covid-19-cases-in-june-47-489-patients-recovered-120063001938_1.html .
- 7.McCrindle BW, Li JS, Minich LL, Colan SD, Atz AM, Takahashi M, et al. Pediatric Heart Network Investigators. Coronary artery involvement in children with Kawasaki disease: risk factors from analysis of serial normalized measurements. Circulation. 2007;116(2):174–179. doi: 10.1161/CIRCULATIONAHA.107.690875. [DOI] [PubMed] [Google Scholar]
- 8.Verdoni L, Mazza A, Gervasoni A, Martelli L, Ruggeri M, Ciuffreda M, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395(10239):1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whittaker E, Bamford A, Kenny J, Kaforou M, Jones CE, Shah P, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324(3):259–269. doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Götzinger F, Santiago-García B, Noguera-Julián A, Lanaspa M, Lancella L, Calò Carducci FI, et al. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet. 2020;4(9):653–661. doi: 10.1016/S2352-4642(20)30177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kabeerdoss J, Pilania RK, Karkhele R, Kumar TS, Danda D, Singh S. Severe COVID-19, multisystem inflammatory syndrome in children, and Kawasaki disease: immunological mechanisms, clinical manifestations and management. Rheumatol Int. 2020:1–14. doi: 10.1007/s00296-020-04749-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allard N, Maruani A, Cret C, Ameri A. Acute hypothermia in Covid-19: a case report. eNeurologicalSci. 2020;20:100248. doi: 10.1016/j.ensci.2020.100248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dhanalakshmi K, Venkataraman A, Balasubramanian S, Madhusudan M, Amperayani S, Putilibai S, et al. Epidemiological and clinical profile of pediatric inflammatory multisystem syndrome - temporally associated with SARS-CoV-2 (PIMS-TS) in Indian children. Indian Pediatr. 2020;57(11):1010–1014. doi: 10.1007/s13312-020-2025-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jain S, Sen S, Lakshmivenkateshiah S, Bobhate P, Venkatesh S, Udani S, et al. Multisystem Inflammatory Syndrome in Children With COVID-19 in Mumbai, India. Indian Pediatr. 2020;57(11):1015–1019. doi: 10.1007/s13312-020-2026-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Winant AJ, Blumfield E, Liszewski MC, Kurian J, Foust A, Lee EY. Thoracic imaging findings of Multisystem Inflammatory Syndrome in Children (MIS-C) associated with COVID-19: what radiologists need to know now. Radiol Cardiothorac Imaging. 2020;2(4):e200346. doi: 10.1148/ryct.2020200346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdel-Mannan O, Eyre M, Löbel U, Bamford A, Eltze C, Hameed B, et al. Neurologic and radiographic findings associated with COVID-19 infection in children. JAMA Neurol. 2020;77(11):1–6. doi: 10.1001/jamaneurol.2020.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehra B, Aggarwal V, Kumar P, Kundal M, Gupta D, Kumar A, Dugaya SK. COVID-19 associated severe multisystem inflammatory syndrome in children with encephalopathy and neuropathy in an adolescent girl with the successful outcome: an unusual presentation. Indian J Crit Care Med. 2020;24(12):1276–1278. doi: 10.5005/jp-journals-10071-23685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pandey M. Acute Meningoencephalitis in a Child Secondary to SARS-CoV-2 Virus. Indian Pediatr. 2021 Feb 15;58(2):183–184. doi: 10.1007/s13312-021-2140-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernandes DM, Oliveira CR, Guerguis S, Eisenberg R, Choi J, Kim M, et al. J Pediatr. 20. 2020. Severe acute respiratory syndrome coronavirus 2 clinical syndromes and predictors of disease severity in hospitalized children and youth. pp. S0022–3476. 31393-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pouletty M, Borocco C, Ouldali N, Caseris M, Basmaci R, Lachaume N, et al. Paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 mimicking Kawasaki disease (Kawa-COVID-19): a multicentre cohort. Ann Rheum Dis. 2020;79(8):999–1006. doi: 10.1136/annrheumdis-2020-217960. [DOI] [PMC free article] [PubMed] [Google Scholar]