Abstract
Background
We aimed to study organizational aspects, case mix, and practices in Indian intensive care units (ICUs) from 2018 to 2019, following the Indian Intensive Care Case Mix and Practice Patterns Study (INDICAPS) of 2010–2011.
Methods
An observational, 4-day point prevalence study was performed between 2018 and 2019. ICU, patient characteristics, and interventions were recorded for 24 hours, and ICU outcomes till 30 days after the study day. Adherence to selected compliance measures was determined. Data were analyzed for 4,669 adult patients from 132 ICUs.
Results
On the study day, mean age, acute physiology and chronic health evaluation (APACHE II), and sequential organ failure assessment (SOFA) scores were 56.9 ± 17.41 years, 16.7 ± 9.8, and 4.4 ± 3.6, respectively. Moreover, 24% and 22.2% of patients received mechanical ventilation (MV) and vasopressors or inotropes (VIs), respectively. On the study days, 1,195 patients (25.6%) were infected and 1,368 patients (29.3%) had sepsis during their ICU stay. ICU mortality was 1,092 out of 4,669 (23.4%), including 737 deaths and 355 terminal discharges (TDs) from ICU. Compliance for process measures related to MV ranged between 62.7 and 85.3%, 11.2 and 47.4% for monitoring delirium, sedation, and analgesia, and 7.7 and 25.3% for inappropriate transfusion of blood products. Only 34.8% of ICUs routinely used capnography. Large hospitals with ≥500 beds, closed ICUs, the APACHE II and SOFA scores, medical admissions, the presence of cancer or cirrhosis of the liver, the presence of infection on the study day, and the need for MV or VIs were independent predictors of mortality.
Conclusions
Hospital size and closed ICUs are independently associated with worse outcomes. The proportion of TDs remains high. There is a scope for improvements in processes of care.
Registered at clinicaltrials.gov (NCT03631927).
How to cite this article
Divatia JV, Mehta Y, Govil D, Zirpe K, Amin PR, Ramakrishnan N, et al. Intensive Care in India in 2018–2019: The Second Indian Intensive Care Case Mix and Practice Patterns Study. Indian J Crit Care Med 2021;25(10):1093–1107.
Keywords: Adult, Health care, India, Intensive care units, Mortality, Patients, Process assessment
Introduction
The Indian Intensive Care Case Mix and Practice Patterns Study (INDICAPS) was the first large-scale, multicenter survey that gathered information about intensive care units and practices in India.1 This multicenter study of 4,038 adult patients from 120 ICUs conducted between July 2010 and April 2011 provided a snapshot of intensive care in India. Highlights included a moderate severity of illness with relatively high mortality in patients with severe sepsis and septic shock, and those receiving vasopressors or inotropes (VIs) or mechanical ventilation (MV). Self-paying patients, public hospital ICUs, and inadequately equipped ICUs were independently associated with ICU mortality, and terminal discharge (TD) from the ICU was widely practiced. Over the next several years, there has been a significant change in the delivery of intensive care services, critical care education, socioeconomic indicators, antibiotic use, resistance patterns, and other aspects of practices in Indian ICUs. Hence the second Indian Intensive Care Case Mix and Practice Patterns Study (INDICAPS-II) was performed to revisit and study the practice of intensive care in India in the years 2018 and 2019.
Patients and Methods
This was a multicenter, observational, staggered point prevalence study performed on four separate days: August 23, 2018; October 25, 2018; December 13, 2018; and April 11, 2019. All ICUs in India, including participants from INDICAPS, were invited to participate through announcements on social media, at conferences, and on the website of the Indian Society of Critical Care Medicine (ISCCM). All investigators obtained approval from their respective hospital ethics committees. The study is registered at clinicaltrials.gov (NCT03631927).
The study protocol, forms, and instructions were uploaded on the study website (http://indicaps.isccm.org). Individual sites could contribute on any or all days of the study. All patients present in the ICU on the study days were included in the study. Data were recorded for all patients present in the ICU during the 24 hours starting from 08.00 a.m. on the study day to 08.00 a.m. the next day. Neonatal and pediatric ICUs were not included. There were no other exclusion criteria. All data were anonymized and submitted online through a dedicated website.
The first time an ICU joined the study, demographic data about the ICU were recorded. A closed ICU was defined as one in which final orders for the patient were written only by the ICU team; all other ICUs, where orders could be written by either the ICU team or the primary team, were considered as open ICUs. A center was considered adequately equipped if all the following facilities were available: renal replacement therapy (RRT) and echocardiography available in the ICU, computed tomography scan, microbiology, biochemistry and hematology laboratories, blood bank, and cardiac catheterization laboratory available in the hospital.
Primary reasons for ICU admission, source of admission, demographics, patient characteristics, and comorbidities were recorded. Admission was defined as surgical if the patient was admitted to the ICU from the operation theater or recovery room. Elective surgery was defined as a surgical procedure that was planned more than 24 hours before ICU admission. Emergency surgery was defined as a surgical procedure before ICU admission that was planned <24 hours in advance. The primary reason for ICU admission was the single most applicable diagnostic category based on the acute physiology and chronic health evaluation (APACHE) III classification.2
Age, physiological parameters, and comorbidities were collected and used to calculate the APACHE II score3 and sequential organ failure assessment (SOFA) score.4 Physiological variables used for the calculation were the worst recorded values during the 24-hour study period. When data for any parameter required calculation of the APACHE II and SOFA score were missing, that parameter was assumed to be normal. A SOFA score of 3 or 4 for any individual organ was used to identify organ failure.
The presence of infection (suspected or proven infection at ICU admission or during the 24-hour study period) was recorded. Tropical infection (malaria, dengue, leptospirosis, or scrub typhus) was diagnosed based on a positive laboratory test. Sepsis was diagnosed if the investigators entered a diagnostic code for sepsis, or if the patient had a SOFA score ≥2 and suspected or confirmed infection on the study day,5 or confirmed tropical infection. Septic shock was recorded if the investigator entered a diagnosis of septic shock or when vasopressors were used in patients with sepsis as defined above.5 Data on cultured microorganisms were also recorded.
ICU survival status was recorded up to 30 days from the day of the study. Patients discharged alive from ICU were followed till hospital discharge, or 30 days from the day of the study, whichever was earlier. For patients dying in the ICU, investigators were asked to record whether any form of limitation of treatment occurred. TDs from ICU to a location outside the hospital, either on family or patient request, as well as those documented as left against medical advice,6 were recorded.
The primary outcome was ICU mortality, which included patients who died in the ICU, as well as TDs, up to 30 days from the day of the study. Secondary outcomes included hospital mortality (including patients who died in the hospital and TDs from the ICU within 30 days from the day of the study), and ICU and hospital lengths of stay, till 30 days from the study day.
The standardized mortality ratio (SMR) using the ratio of observed hospital mortality to the hospital-predicted mortality by APACHE II was calculated for those patients who were admitted to the ICU within 24 hours of the study day.
We also determined the adherence to selected process measures, including the presence of written protocols in the ICU, capnography to confirm tracheal intubation, use of subglottic suction and closed tracheal suction systems, administration of deep venous thrombosis (DVT) prophylaxis, and maintaining plateau pressure or peak airway pressure (during volume-controlled or pressure-controlled ventilation, respectively) ≤30 cm H2O in patients receiving invasive MV, and monitoring of sedation, analgesia, and delirium.7 We also determined the proportion of patients with inappropriate transfusion triggers of hemoglobin (Hb) >9 g/dL for packed red blood cell transfusion, international normalized ratio (INR) ≤1.5 and activated partial thromboplastin time (APTT) ≤ 45 for fresh frozen plasma (FFP), and platelet count >50 × 103/mm3 for platelet transfusion.
Prior training of investigators and verification of source data were not performed. However, an online investigator's discussion forum was formed to deal with queries and problems during the data entry. Investigators were contacted by E-mail to complete missing data.
Analysis
Analysis was performed for adult patients (≥16 years of age), for whom ICU mortality was available. Continuous variables were compared with the use of the Student's t-test, analysis of variance, Mann–Whitney test, or the Kruskal–Wallis test. Categorical variables were compared using the chi-square test. A two-tailed p <0.05 was considered statistically significant. Multivariable binary logistic regression analysis (method Enter) was performed to determine the independent predictors of ICU mortality using ICU characteristics, patient factors, and interventions (RRT, MV, and VIs in the ICU) with a p-value of ≤0.1 in the univariate analysis. All analyses were performed using IBM® SPSS® Statistics version 20.0.0.
Results
A total of 5,222 patients from 141 ICUs were enrolled inclusive of all the four study days, of whom 5,094 were adults (≥16 years of age). Data for the primary outcome were not available in 425 adults, resulting in the exclusion of these patients and nine ICUs. Data analysis was done for adult patients (n = 4,669) from 132 ICUs. Details of participation are provided in Supplementary Tables S1 to S4. Missing data for patient-related variables are summarized in Supplementary Table S5.
Table 1 summarizes the facilities available in the ICU or the hospital. In the study, the number per bed {median [interquartile range, (IQR)]} of invasive ventilators, noninvasive ventilators, and high-flow nasal oxygen (HFNO) were 0.51 (0.36–0.76), 0.14 (0.08–0.25), and 0 (0–0.08), respectively. Sixty-two centers (47%) were considered adequately equipped, whereas 70 (53%) were categorized as “not adequately equipped.”
Table 1.
Facilities available in the ICU and in the hospital in 132 centers
| Facility | Available in ICU | Available in hospital | Not available |
|---|---|---|---|
| Chest X-ray | 107 (81.1) | 25 (18.9) | 0 (0.0) |
| Blood gas analysis | 96 (72.7) | 35 (26.5) | 1 (0.8) |
| Ultrasonography (excluding echocardiography) | 104 (78.8) | 26 (19.7) | 2 (1.5) |
| Echocardiography | 103 (78.0) | 28 (21.2) | 1 (0.8) |
| Hemodialysis | 103 (78.0) | 24 (18.2) | 5 (3.8) |
| Continuous renal replacement therapy | 61 (46.2) | 19 (14.4) | 52 (39.4) |
| Fiber-optic bronchoscope | 76 (57.6) | 50 (37.9) | 6 (4.5) |
| Blood bank | Not applicable | 109 (82.6) | 23 (17.4) |
| Platelet pheresis | Not applicable | 97 (73.5) | 35 (26.5) |
| Microbiology laboratory | Not applicable | 126 (95.5) | 6 (4.5) |
| Computed tomography | Not applicable | 125 (94.7) | 7 (5.3) |
| Magnetic resonance imaging | Not applicable | 109 (82.5) | 23 (17.4) |
| Cardiac catheterization laboratory | Not applicable | 117 (88.6) | 15 (11.4) |
| High-flow nasal cannula oxygen | 62 (46.7) | Not applicable | 70 (53.3) |
| Videolaryngoscope | 48 (36.4) | Not applicable | 84 (63.6) |
| Extracorporeal membrane oxygenation | 33 (25) | 32 (24.2) | 67 (50.8) |
Table 2 summarizes the characteristics of the 132 ICUs. The median (IQR) number of hospital beds, ICU beds, nurse: patient ratio, and full-time consultants per bed were 338(200–650), 20 (13.25–32.75), 0.55 (0.43–0.67), and 0.15(0.08–0.23), respectively. Most ICUs were in hospitals that were not accredited to the National Accreditation Board for Hospitals and Healthcare Providers (NABH) or the Joint Commission International (JCI). The vast majority of ICUs and patients were from private hospitals.
Table 2.
Characteristics of the participating intensive care units
| Characteristic | Number of ICUs (%) | Number of patients (%) | APACHE II score (mean ± SD) | ICU mortality (%) [terminal discharges, %] | Hospital mortality (n = 4,594) |
|---|---|---|---|---|---|
| Overall | 132 (100) | 4,669 (100) | 16.7 ± 9.8 | 1,092 (23.4) [355, 7.6%] | 1,164 (25.3) |
| Type of ICU | |||||
| Open | 112 (84.8) | 3,939 (84.4) | 16.7 ± 9.7 | 877 (22.3) [274, 7.0%] | 939 (24.3) |
| Closed | 20 (15.2) | 730 (15.6) | 16.8 ± 10.0 | 215 (29.5) [81, 11.1%] | 225 (31.0) |
| p | 0.884 | 0.000 | 0.0001 | ||
| ICU specialty | |||||
| 1. Mixed medical–surgical | 108 (81.8) | 4,082 (87.4) | 16.8 ± 9.8* | 962 (23.6) [304, 7.4%] | 1,025 (26.0) |
| 2. All other specialcty ICUs | 24 (18.2) | 587 (12.6) | 16.6 ± 9.3 | 130 (22.1) [51, 8.7%] | 139 (23.7) |
| a. Neuro-intensive care | 6 (4.5) | 120 (2.6) | 16.0 ± 8.6 | 23 (19.2) [18, 15%] | 27 (22.5) |
| b. Surgical | 7 (5.3) | 141 (3.0) | 12.2 ± 6.7 | 16 (11.3) [2, 1.4%] | 16 (11.4) |
| c. Coronary care | 1 (0.8) | 45 (0.96) | 11.8 ± 6.7 | 5 (11.1) [0] | 5 (11.1) |
| d. Medical | 8 (6.1) | 266 (5.7) | 19.6 ± 9.9 | 84 (31.6) [31, 11.7%] | 88 (33.3) |
| e. Cardiac surgical | 1 (0.8) | 5 (0.11) | 16.2 ± 6.3 | 0 (0) | 0 (0) |
| f. Other | 1 (0.8) | 10 (0.21) | 28.0 ± 5.7 | 2 (20.0) [0] | 3 (30.0) |
| p | 0.7 (1 vs 2) | 0.45 (1 vs 2) | 0.11 (1 vs 2) | ||
| Number of beds in ICU | |||||
| A. 1–20 beds | 67 (50.8) | 1,490 (31.9) | 17.1 ± 9.6 | 349 (23.4) [114, 7.6%] | 380 (25.5) |
| B. >20 beds | 65 (49.2) | 3,179 (68.1) | 16.6 ± 9.8 | 743 (23.4) [241, 7.6%] | 784 (25.1) |
| p | 0.09 (A vs B) | 0.920 (A vs B) | 0.20 (A vs B) | ||
| Number of hospital beds | |||||
| A. 1–499 | 84 (63.6) | 2,453 (52.5) | 17.1 ± 9.8 | 521 (21.2) [178, 7.3%] | 560 (22.3) |
| a. 1–199 | 32 (24.2) | 777 (16.6) | 15.4 ± 9.6 | 169 (21.8) [71, 9.1%] | 190 (24.7) |
| b. 200–499 | 52 (39.4) | 1,676 (35.9) | 17.8 ± 9.8 | 352 (21.0) [107, 6.4%] | 370 (22.5) |
| B. ≥500 | 48 (36.4) | 2,216 (47.5) | 16.4 ± 9.7 | 571 (25.8) [177, 8.0%] | 604 (27.7) |
| p | 0.01 (A vs B) | 0.000 (A vs B) | 0.002 (A vs B) | ||
| Nurse to patient ratio | |||||
| <1:2 (less than 1 nurse per two patients) | 45 (34.1) | 1,205 (25.8) | 16.8 ± 9.6 | 265 (21.2) [101, 8.4%] | 289 (24.1) |
| ≥1:2 (1 or more nurses per two patients) | 87 (65.9) | 3,464 (74.2) | 16.7 ± 9.8 | 827 (23.9) [254, 7.3%] | 874 (25.7) |
| p | 0.672 | 0.182 | 0.278 | ||
| Hospital | |||||
| Public hospital ICUs | 6 (4.5) | 111 (2.4) | 18.6 ± 11.1 | 31 (27.9) [2, 1.8%] | 33 (30.0) |
| Private hospital ICUs | 126 (95.5) | 4,558 (97.6) | 16.7 ± 9.7 | 1,061 (23.3) [353, 7.7%] | 1,131 (25.2) |
| p | 0 | 0 | 0.04 | 0.253 | 0.255 |
| Postgraduate teaching/training program in intensive care | |||||
| None | 37 (28.0) | 812 (17.4) | 15.9 ± 9. 5 | 168 (20.7) [60, 7.4%] | 186 (23.8) |
| Present | 95 (72.0) | 3,857 (82.6) | 16.9 ± 9.8 | 924 (23.9) [295, 7.6%] | 978 (25.7) |
| p | 0.006 | 0.046 | 0.264 | ||
| Equipment and facilities | |||||
| Adequate | 62 (47.0) | 2,855 (61.1) | 17.2 ± 9.9 | 692 (24.2) [229, 8.0%] | 738 (26.3) |
| Not adequate | 70 (53.0) | 1,814 (38.9) | 16.0 ± 9.4 | 400 (22.1) [126, 6.9%] | 426 (23.8) |
| p | 0.004 | 0.089 | 0.055 | ||
| NABH/JCI accreditation | |||||
| Not accredited | 109 (82.6) | 4,136 | 16.5 ± 9.7 | 965 (23.3) [304, 7.4%] | 1,023 (25.2) |
| Accredited | 23 (17.4) | 533 | 18.3 ± 10.2 | 127 (23.8) [51, 9.6%] | 141 (26.6) |
| p | 0.000 | 0.748 | 0.846 | ||
| Written protocols | |||||
| Present | 122 (92.4) | 4,486 | 16.8 ± 9.8 | 1,051 (23.4) [338, 7.5%] | 1,119 (25.4) |
| Absent | 10 (7.6) | 183 | 15.4 ± 9.0 | 41 (22.4) [17, 9.3%] | 45 (24.7) |
| p | 0.059 | 0.748 | 0.846 | ||
APACHE, acute physiology and chronic health evaluation; ICU, intensive care unit; NABH, national accreditation board for hospitals and healthcare providers; JCI, joint commission international
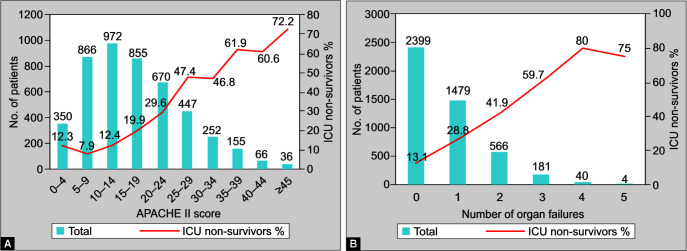
The primary APACHE III diagnostic categories are summarized in Table 3, and patient demographics, the severity of illness, and outcomes are detailed in Table 4. Overall, 737 out of 4,669 patients (15.8%) died in the ICU, and an additional 355 patients were TDs from the ICU. Thus total ICU mortality including TDs was 1,092 out of 4,669 (23.4%). The total hospital mortality was 25.3%, which included 809 patients who died in the hospital and 355 TDs. The median (IQR) length of ICU stay was 6 (3–13) days, with significantly longer stays in nonsurvivors (Table 2). Figures 1A and 1B show the distribution of APACHE II scores and the number of organ failures with the associated ICU mortality. Almost 51% of patients did not have any organ failure on the study day; ICU mortality was 13.1% in these patients and 32% of patients did not have any comorbidities.
Table 3.
Primary reason for ICU admission
| Primary reason for ICU admission | Number of patients | APACHE II score | ICU nonsurvivors N (%) |
|---|---|---|---|
| Medical | 3,993 | 17.9 ± 9.4* | 1,030 (25.8)* |
| Cardiovascular | 580 | 15.2 ± 9.4 | 105 (18.1) |
| Respiratory | 884 | 19.6 ± 8.9 | 260 (29.4) |
| Gastrointestinal | 462 | 17.5 ± 9.0 | 146 (31.6) |
| Neurological | 723 | 16.3 ± 8.8 | 163 (22.5) |
| Sepsis | 587 | 20.7 ± 9.4 | 202 (34.4) |
| Trauma | 205 | 13.9 ± 8.8 | 31 (15.1) |
| Metabolic | 123 | 17.2 ± 9.7 | 21 (17.1) |
| Hematological | 84 | 16.5 ± 9.3 | 20 (23.8) |
| Renal | 247 | 22.2 ± 8.9 | 59 (23.9) |
| Unclassified | 100 | 14.1 ± 9.3 | 23 (23.0) |
| Surgical | 676 | 11.5 ± 6.5 | 62 (9.2) |
| Cardiovascular | 126 | 10.3 ± 5.2 | 4 (3.2) |
| Respiratory | 63 | 13.1 ± 7.7 | 8 (12.7) |
| Gastrointestinal | 187 | 11.5 ± 5.7 | 19 (10.2) |
| Neurological | 113 | 12.0 ± 7.9 | 16 (14.2) |
| Trauma | 34 | 12.2 ± 7.5 | 7 (20.6) |
| Renal | 58 | 11.8 ± 6.4 | 4 (6.9) |
| Obstetric | 44 | 10.5 ± 5.2 | 1 (2.3) |
| Hip or extremity fracture | 46 | 11.4 ± 6.3 | 3 (6.5) |
| Unclassified | 3 | 12.0 ± 7.9 | 0 (0) |
| Type of Surgery | |||
| Elective surgery | 462 | 10.8 ± 6.0 | 37 (8.0)* |
| Emergency surgery | 214 | 12.3 ± 7.9 | 25 (11.7) |
*p <0.001 comparing medical vs surgical admissions, and elective vs emergency surgery; ICU, intensive care unit; APACHE, acute physiology and chronic health evaluation
Table 4.
Patient demographics, ICU admission characteristics and severity of illness*
| All patients | ICU survivors | ICU nonsurvivors | p | |
|---|---|---|---|---|
| Patient demographics | ||||
| Number of patients (%) | 4,669 (100) | 3,577 (76.6%) | 1,092 (23.4%) | |
| Age (years) (mean ± SD) | 56.9 ± 17.4 | 56.4 ± 17.6 | 58.5 ± 16.6 | 0.01 |
| Male [number of patients, (%)] | 2,973 (63.7) | 2,271 (76.4) | 702 (23.6) | 0.632 |
| Female [number of patients, (%)] | 1,696 (36.3) | 1,306 (77.0) | 390 (23.0) | |
| Financial resources | 0.193 | |||
| Self-paying [number of patients, (%)] | 3,010 (64.5) | 2,288 (76.0) | 722 (24.0) | |
| Not self-paying (payment by employer, insurance, etc.) [number of patients, (%)] | 1,659 (35.5) | 1,289 (77.7) | 370 (22.3) | |
| Type of ICU admission [number of patients, (%)] | <0.001 | |||
| Medical/nonoperative | 3,993 (85.5) | 2,963 (74.2) | 1,030 (25.8) | |
| Surgical | 676 (14.5) | 614 (90.8) | 62 (9.2) | |
| Elective postoperative | 462 (9.9) | 425 (92.0) | 37 (8.0) | |
| Unscheduled/emergent postoperative | 214 (4.6) | 189 (88.3) | 25 (11.7) | |
| Source of admission [number of patients, (%)] | <0.001 | |||
| Home | 913 (19.6) | 716 (78.4) | 197 (21.6) | |
| Emergency department | 1,596 (34.2) | 1,202 (75.3) | 394 (24.7) | |
| Ward of same hospital | 703 (15.1) | 479 (68.1) | 224 (31.9) | |
| ICU of other hospital | 435 (9.3) | 307 (70.6) | 128 (29.4) | |
| Ward of other hospital | 283 (6.1) | 212 (74.9) | 71 (25.1) | |
| From operation theater | 676 (14.5) | 614 (90.8) | 62 (9.2) | |
| Not known/missing | 63 (1.3) | 47 (74.6) | 16 (25.4) | |
| Comorbidities [number of patients, (%)] | ||||
| Chronic obstructive pulmonary disease | 363 (7.8) | 267 (73.6) | 96 (26.4) | 0.152 |
| Diabetes mellitus (IDDM and NIDDM) | 1,555 (33.3) | 1,170 (77.3) | 385 (22.7) | 0.118 |
| Hypertension | 2,055 (44.0) | 1,589 (77.3) | 466 (22.7) | 0.308 |
| Heart failure | 362 (7.8) | 245 (67.7) | 117 (32.3) | <0.001 |
| Any cancer | 598 (12.8) | 417 (69.7) | 181 (30.3) | <0.001 |
| Hematological malignancy | 80 (1.7) | 41 (51.3) | 39 (48.7) | <0.001 |
| Metastatic cancer | 200 (4.3) | 118 (59.0) | 82 (41.0) | <0.001 |
| Dialysis-dependent renal failure | 280 (6.0) | 179 (63.9) | 101 (36.1) | 0.001 |
| Cirrhosis of the liver | 195 (4.2) | 102 (52.3) | 93 (47.7) | <0.001 |
| Immunosuppressive treatment | 354 (7.6) | 237 (66.9) | 117 (33.1) | 0.001 |
| Number of comorbidities [number of patients, (%)] | <0.001 | |||
| 0 | 1,496 (32.0) | 1,203 (80.4) | 293 (19.6) | |
| 1 | 1,364 (29.2) | 1,049 (76.9) | 315 (23.1) | |
| 2 | 1,186 (25.4) | 912 (76.9) | 214(23.1) | |
| 3 | 483 (10.3) | 325 (67.3) | 158 (32.7) | |
| 4 | 125 (2.7) | 83 (66.4) | 42 (33.6) | |
| 5 | 13 (0.3) | 4 (30.8) | 9 (69.2) | |
| 6 | 2 (0.0) | 1 (50.0) | 1 (50.0) | |
| Patients with suspected or confirmed infection on the study day | 1,195 (25.6) | 740 (61.9) | 455 (38.1) | <0.001 |
| Patients in whom infection developed during the ICU stay | 121 (2.6) | 68 (56.2) | 53 (43.8) | <0.001 |
| Sepsis and/or septic shock during ICU stay | 1,368 (29.3) | 863 (63.1) | 505 (36.9) | <0.001 |
| Septic shock during ICU stay | 590 (12.6) | 275 (46.6) | 315 (53.4) | <0.001 |
| Confirmed tropical infection | 135 (2.9) | 110 (81.5) | 25 (18.5) | 0.175 |
| Acute respiratory failure with PaO2/FiO2 ratio <300 | 2,395 (51.3) | 1,661 (69.4) | 734 (30.6) | <0.001 |
| Poisoning or overdose | 68 (1.5) | 55 (81.9) | 13 (19.1) | 0.54 |
| Severity of illness | ||||
| APACHE II score (mean ± SD) | 16.7 ± 9.8 | 14.8 ± 8.6 | 23.1 ± 10.5 | <0.001 |
| SOFA score (mean ± SD) | 4.4 ± 3.6 | 3.7 ± 3.2 | 6.7 ± 4.1 | <0.001 |
| No. of organ failures [median, (IQR)] | 0 [0–1] | 0 [0–1] | 1 [0–2] | <0.001 |
| ICU stay, days [median, (IQR)] N = 4,137 |
6 [3–13] | 6 [3–12] | 9 [4–17] | <0.001 |
| Hospital stay, days [median, (IQR)] N = 3,842 |
12 [7–20] | 12 [7–20] | 12 [6–21] | 0.225 |
| ICU admission to study day interval, days [median, (IQR)] | 2.0 [1–6] | 2.0 [1–5] | 3.0 [1–8] | <0.001 |
Figures represent the number of patients (percent) unless otherwise indicated; p values compare survivors vs nonsurvivors; ICU, intensive care unit; IDDM, insulin-dependent diabetes mellitus; NIDDM, non-insulin-dependent diabetes mellitus; APACHE, acute physiology and chronic health evaluation; SOFA, sequential organ failure assessment; IQR, interquartile range
Figs 1A and B.
(A) APACHE II score on the study day; (B) Number of organ failures on the study day and ICU nonsurvivors
A subset of 1,854 patients was admitted within 24 hours of a study day, of which 1,819 had data for hospital outcomes. Of these, 398 patients were predicted to die in the hospital, whereas the observed hospital mortality was 368. The SMR was thus 0.92. The APACHE II score generally predicted mortality well, except at scores ≥30, when it overpredicted mortality, as seen in Figure 2.
Fig. 2.
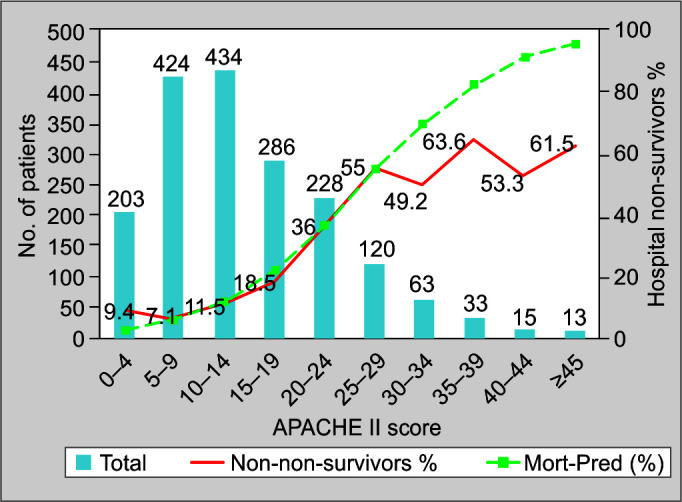
Predicted vs actual hospital mortality for 1,819 patients
Medical admissions accounted for 85.5% of admissions; they had a higher severity of illness than surgical admissions and significantly higher mortality. Mortality was significantly higher for admissions after emergency surgery than elective surgery (Table 4).
Sepsis with or without septic shock during the ICU stay was present in 1,368 patients (29.3%), with ICU mortality of 36.9%. During the 24-hour study period, 1,195 patients had a suspected or confirmed infection. A total of 4,609 microbiological cultures were obtained in 2,275 patients, and 1,304 organisms were identified in 902 patients. Gram-negative organisms accounted for 75.6%, while gram-positive organisms, fungi, mycobacteria, and anaerobes accounted for 13.6, 9.7, and 0.46% of organisms identified, respectively. In addition, 87 patients (1.99%) had a positive laboratory test for dengue, 78 (1.67%) for H1N1 influenza virus, one for cytomegalovirus, three for other viruses, 23 (0.49%) for scrub typhus, 18 (0.39%) for leptospirosis, and 14 (0.30%) patients for malaria.
On the study day, 3,263 patients (69.9%) received antimicrobials. In patients receiving antimicrobials, a median of 2.0 (IQR 1, 2) antimicrobials was given, and 16.5% of patients received three or more antimicrobials and 68 patients (1.5%) were admitted after poisoning or drug overdose, including 36 organophosphorus or organochlorine poisoning, 9 corrosive poisonings, and 4 snake bites. ICU mortality in this group was 19.1%.
Various interventions in the ICU are detailed in Table 5. Patients receiving invasive MV, VIs, and RRT had significantly higher mortality than those who did not (44.4 vs 16.7%, p <0.001; 44.0 vs 17.5%, p <0.001; and 41.7 vs 21.5%, p <0.001, respectively). Arterial and central venous catheters were inserted in 25.4 and 34.3% of all patients, respectively, and 50.3 and 64.3% of 1,033 patients receiving VIs. Echocardiography in the ICU was performed in 21.4% of patients, and cardiac output was measured in 81 patients (1.7%). In 727 patients who received fluid boluses, normal saline was used in more than 86% of patients, balanced crystalloids were used in 58.4%, and album in 6.6% of patients.
Table 5.
Interventions
| All patients | ICU survivors | ICU nonsurvivors (mortality %) | p | |
|---|---|---|---|---|
| Number of patients | 4,669 (100) | 3,577 (76.6) | 1,092 (23.4) | |
| Infectious disease | ||||
| Patients receiving antibiotics | 3,263 (69.9) | 2,417 (74.1) | 846 (25.9) | <0.001 |
| One antibiotic | 1,321 (28.3) | 1,084 (82.1) | 237 (17.9) | |
| Two antibiotics | 1,172 (25.1) | 861 (73.5) | 311 (26.5) | |
| Three antibiotics | 527 (11.3) | 344 (65.3) | 183 (34.7) | |
| Four or more antibiotics | 243 (5.2) | 128 (52.7) | 115 (47.3) | |
| Procalcitonin measured | 528 (11.3) | 352 (66.7) | 176 (33.3) | <0.001 |
| Ventilation and airway | ||||
| High-flow nasal oxygen | 165 (3.5) | 110 (66.7) | 55 (33.3) | <0.001 |
| Mechanical ventilation | 1,539 (33.0) | 974 (7.2) | 635 (58.2) | <0.001 |
| Noninvasive ventilation | 484 (10.4) | 349 (72.1) | 135 (27.9) | <0.001 |
| Invasive ventilation | 1,125 (24.1) | 625 (55.6) | 500 (44.4) | <0.001 |
| Prone position | 175 (3.7) | 114 (65.1) | 61 (34.9) | 0.001 |
| Neuromuscular blockade | 272 (5.8) | 147 (54.0) | 125 (46.0) | <0.001 |
| Tracheal intubation | 1,006 (21.5) | 552 (54.9) | 454 (41.1) | <0.001 |
| Tracheostomy | 392 (8.4) | 274 (69.9) | 118 (30.1) | 0.02 |
| Surgical tracheostomy | 201 (4.3) | 139 (69.2) | 62 (30.8) | |
| Percutaneous tracheostomy | 191 (4.1) | 135 (70.7) | 56 (29.3) | |
| High-frequency oscillation | 40 (0.9) | 25 (62.5) | 15 (37.5) | 0.03 |
| Extracorporeal membrane oxygenation (veno-venous) | 20 (0.4) | 19 (95.0) | 1 (5.0) | |
| Extracorporeal membrane oxygenation (veno-arterial) | 4 (0.1) | 3 (75.0) | 1 (25.0) | |
| Capnography | 432 (9.1) | 276 (63.9) | 156 (36.1) | <0.001 |
| Renal | ||||
| Renal replacement therapy | 434 (9.3) | 253 (58.3) | 181 (41.7) | <0.001 |
| Continuous | 34 (0.7) | 18 (52.9) | 16 (47.1) | |
| Intermittent hemodialysis | 177 (3.8) | 117 (66.1) | 60 (33.9) | |
| Sustained low-efficiency daily dialysis | 187 (4.0) | 96 (51.3) | 91 (48.7) | |
| Ultrafiltration | 17 (0.4) | 11 (64.7) | 6 (35.3) | |
| Cardiovascular and hemodynamic | ||||
| Vasopressors/inotropes | 898 (22.2) | 574 (63.9) | 324 (36.1) | <0.001 |
| Invasive blood pressure monitoring | 1,185 (25.4) | 751 (63.4) | 434 (36.6) | <0.001 |
| Central venous catheter inserted | 1,603 (34.3) | 1,048 (65.4) | 555 (34.6) | <0.001 |
| Central venous pressure monitoring | 566 (12.1) | 398 (70.3) | 168 (29.7) | <0.001 |
| Hourly urine output monitoring | 3,253 (69.7) | 2,412 (74.1) | 841 (25.9) | <0.001 |
| Echocardiography in ICU | 1,000 (21.4) | 674 (67.4) | 326 (32.6) | <0.001 |
| Pulse pressure variation monitoring | 279 (6.0) | 186 (66.7) | 93 (33.3) | <0.001 |
| Cardiac output monitoring | 81 (1.7) | 61 (75.3) | 20 (24.7) | 0.30 |
| Passive leg raising test | 108 (2.3) | 72 (66.7) | 36 (33.3) | <0.001 |
| Blood lactate measured | 1,477 (31.6) | 952 (64.5) | 525 (35.5) | <0.001 |
| ScvO2 measured | 69 (1.5) | 41 (59.4) | 28 (40.6) | 0.002 |
| Intra-aortic balloon pump | 76 (1.6) | 46 (60.5) | 30 (39.5) | 0.001 |
| Fluid therapy, blood and blood products | ||||
| Fluid boluses | 727 (15.6) | 503 (69.2) | 224 (30.8) | <0.001 |
| Normal saline | 627 (13.4) | 4,426 (70.5) | 185 (29.5) | <0.001 |
| Lactated Ringers’ | 215 (4.6) | 161 (74.9) | 54 (25.1) | 0.18 |
| Plasmalyte™ | 210 (4.5) | 143 (68.1) | 67 (31.9) | 0.002 |
| Gelatins | 25 (0.5) | 20 (80.0) | 5 (20.0) | 0.27 |
| Starches | 18 (0.4) | 15 (83.3) | 3 (16.7) | 0.37 |
| Albumin | 48 (1.0) | 33 (68.8) | 15 (31.2) | 0.06 |
| Whole blood/packed red blood cells | 297 (6.4) | 200 (67.3) | 97 (32.7) | <0.001 |
| Fresh frozen plasma | 99 (2.1) | 45 (45.5) | 54 (54.5) | <0.001 |
| Platelets | 76 (1.6) | 37 (48.7) | 39 (51.3) | <0.001 |
| Random donor platelets | 38 (0.8) | 17 (44.7) | 21 (55.3) | |
| Single donor platelets | 38 (0.8) | 20 (52.6) | 18 (47.4) | |
| Neurological | ||||
| Intracranial pressure monitoring | 25 (0.5) | 14 (56.0) | 11 (44.0) | <0.001 |
| EEG monitoring | 147 (3.1) | 114 (77.6) | 33 (22.4) | 0.004 |
| Transcranial Doppler | 32 (0.7) | 22 (68.8) | 10 (31.2) | 0.006 |
| General care | ||||
| Stress ulcer prophylaxis | 3,635 (90.0) | 2,953 (89.2) | 682 (93.6) | <0.001 |
| Low-molecular-weight heparin for deep venous thrombosis prophylaxis | 1,384 (29.6) | 1,060 (76.6) | 324 (23.4) | 0.25 |
| Unfractionated heparin | 365 (7.8) | 266 (72.9) | 99 (27.1) | 0.10 |
| Compression stockings | 547 (11.7) | 414 (75.7) | 133 (24.3) | 0.30 |
| Intermittent calf compression | 1,175 (25.2) | 836 (71.1) | 339 (28.9) | <0.001 |
| Enteral nutrition | 2,823 (60.5) | 2,085 (73.9) | 738 (26.1) | <0.001 |
| Parenteral nutrition | 230 (4.9) | 152 (66.1) | 78 (33.9) | <0.001 |
| Sedation, analgesia, delirium | ||||
| Sedation measured | 800 (17.1) | 519 (64.9) | 281 (35.1) | <0.001 |
| Ramsay sedation score | 228 (4.9) | 172 (75.4) | 56 (24.6) | |
| RASS | 592 (12.7) | 369 (62.3) | 223 (37.7) | |
| Bispectral index | 88 (1.9) | 54 (61.4) | 34 (38.6) | |
| Pain measured | 2,215 (47.4) | 1,703 (76.9) | 512 (23.1) | 0.36 |
| Behavioral pain scale | 373 (8.0) | 249 (66.8) | 124 (33.2) | |
| Critical care pain observation tool | 200 (4.3) | 141 (70.5) | 59 (29.5) | |
| Numeric rating scale | 242 (5.2) | 199 (82.2) | 43 (17.8) | |
| Visual analog scale | 1,244 (26.6) | 979 (78.7) | 265 (21.3) | |
| Delirium monitored | 522 (11.2) | 397 (76.1) | 125 (23.9) | 0.27 |
| CAM-ICU | 500 (10.7) | 379 (75.8) | 121 (24.2) | |
| IDSC | 11 (0.2) | 10 (90.9) | 1 (9.1) |
ICU, intensive care unit; RASS, Richmond agitation-sedation scale; CAM, confusion assessment method; IDSC, intensive care delirium screening checklist
The degree of compliance with selected process measures is outlined in Table 6. Almost all ICUs (92%) had written protocols. Compliance for process measures related to MV ranged from 62.7– 85.3%, whereas for monitoring delirium, sedation, and analgesia, it ranged from 11.2–47.4%. Inappropriate triggers for transfusion of blood products, based only on the laboratory values (Hb >9 g/dL), were observed in 7.7–25.3% of patients (Table 6).
Table 6.
Compliance with process measures
| Indicator | Compliance |
|---|---|
| ICUs having written protocols | 122 (92.4%) |
| ICUs that always use capnography to confirm tracheal intubation | 46 (34.8%) |
| Patents receiving invasive mechanical ventilation | 1,055 |
| Subglottic suction via endotracheal or tracheostomy tube | 814 (77.2%) |
| Closed tracheal suction system | 661 (62.7%) |
| Receiving DVT prophylaxis | 807 (76.5%) |
| Receiving stress ulcer prophylaxis | 900 (85.3%) |
| Patients with plateau pressure <30 cm H2O* | 750 (71.1) |
| Sedation monitored | 800 (17.1) |
| Analgesia monitored | 2,215 (47.4%) |
| Delirium monitored | 522 (11.2) |
| Patients receiving packed red blood cell transfusion | 297 |
| Hb (g/dL) at transfusion [Median, (IQR)] N = 265 | 7.0 [6.2–7.9] |
| Patients with Hb >9 g/dL** | 23 (7.7%) |
| Patients receiving fresh frozen plasma | 99 |
| INR at transfusion [Median, (IQR)] N = 92 | 2.25 [1.67–3.43] |
| APTT at transfusion [Median, (IQR)] N = 92 | 42.85 [33.85–58.5] |
| Patients with INR ≤1.5 and APPT ≤45** | 25 (25.3%) |
| Patients receiving platelet transfusions | 76 |
| Platelet count at transfusion (median, [IQR]) (N = 72) | 18.0 [8.63–40] |
| Patients with platelet count >50 x 103/mm3** | 14 (19.4%) |
ICU, intensive care unit; DVT, deep venous thrombosis; Hb, hemoglobin; IQR, interquartile range; INR, international normalized ratio; APTT, activated partial thromboplastin time; *Plateau pressure during volume-controlled ventilation, peak airway pressure during pressure-controlled ventilation; **Inappropriate use of blood product based on the laboratory values; the clinical context was not available
The results of the multivariable analysis of organizational and patient characteristics, severity of illness, and need for interventions are summarized in Table 7. Closed ICUs and ICUs in hospitals with ≥500 beds were independently associated with increased ICU mortality. In addition, the APACHE II and SOFA scores on the study day, medical admissions, the presence of cancer or cirrhosis of the liver, the presence of infection on the study day, and the need for invasive or noninvasive ventilation or VIs were independent predictors of mortality.
Table 7.
Multivariable analysis for independent predictors of mortality
| p | Odds ratio for ICU mortality | 95% CI (lower) | 95% CI (upper) | |
|---|---|---|---|---|
| Biopsy-proven cirrhosis | 0.000 | 2.523 | 1.815 | 3.508 |
| Medical admission (vs surgical admission) | 0.000 | 2.081 | 1.547 | 2.800 |
| Mechanical ventilation | 0.000 | 1.707 | 1.441 | 2.022 |
| Vasopressors or inotropes | 0.000 | 1.587 | 1.317 | 1.913 |
| Any cancer | 0.000 | 1.567 | 1.236 | 1.986 |
| Infection on the study day | 0.031 | 1.404 | 1.032 | 1.910 |
| Closed ICU (vs open ICU) | 0.002 | 1.398 | 1.131 | 1.727 |
| Hospital size (≥500 beds vs 1–499 beds) | 0.000 | 1.355 | 1.143 | 1.607 |
| SOFA score | 0.000 | 1.077 | 1.039 | 1.117 |
| APACHE II score | 0.000 | 1.044 | 1.029 | 1.058 |
a. Variable not significant: age, immunosuppressive therapy, presence of heart failure, dialysis-dependent, sepsis, need for RRT, adequately equipped, respiratory system dysfunction or failure, ICU teaching, number of ICU beds
CI, confidence interval; ICU, intensive care unit; SOFA, sequential organ failure assessment; APACHE, acute physiology and chronic health evaluation
Discussion
The study provides a snapshot of adult critical care in India between August 2018 and May 2019. Patients had moderate severity of illness and the ICU mortality, including TDs, was 23.4%.
While we attempted to describe the change in intensive care practices and outcomes over the 9 years between INDICAPS and INDICAPS-II (Table 8), direct comparisons of the results of the two studies may not be appropriate. The participating ICUs were different; ICUs that participated in both studies may have changed in their structure, organization, and staffing in the intervening period, and criteria used to classify open and closed ICUs, adequately equipped ICUs, sepsis, and tropical infections differed between the two studies.
Table 8.
ICU and patient characteristics and outcomes in INDICAPS1 and INDICAPS-II
| Characteristic | INDICAPS | INDICAPS-II | Remarks |
|---|---|---|---|
| Number of ICUs | 120 | 132 | |
| Number of patients | 4,038 | 4,669 | |
| Age (years) (mean ± SD) | 54.1 ± 17.1 | 56.9 ± 17.4 | |
| Male patients (%) | 66.1 | 63.7 | |
| APACHE II score | 17.4 ± 9.2 | 16.7 ± 9.8 | |
| SOFA score (mean ± SD) | 3.8 ± 3.6 | 4.4 ± 3.6 | |
| ICU stay, days [median, (IQR)] | 6 [3–13] | 6 [3–13] | |
| Patients dying in ICU (%) | 13.5 | 15.8 | |
| Terminal discharges (%) | 4.5 | 7.6 | |
| Total ICU mortality (%) | 18.1 | 23.4 | |
| Open ICUs (%)/patients in open ICUs (%) | 74.2/78.0 | 84.8/84.4 | |
| Mixed medical-surgical ICUs (%)/patients in mixed medical-surgical ICUs (%) | 80.8/83.1 | 81.8/87.4 | |
| ICUs with >20 beds (%)/patients in ICUs with >20 beds (%) | 25.0/37.0 | 49.2/68.1 | |
| Hospitals with ≥500 beds (%)/patients in hospitals with ≥500 beds (%) | 35/46.6 | 36.4/47.5 | |
| ICUs with nurse:patient ratio <1:2 (%)/patients in ICUs with nurse:patient ratio <1:2 (%) | 30.8/45.6 | 34.1/25.8 | |
| Public hospital ICUs (%)/patients in public hospital ICUs (%) | 10.8/9.7 | 4.5/2.4 | |
| ICUs with a postgraduate teaching program in intensive care (%)/patients in ICUs with a postgraduate teaching program in intensive care (%) | 39.2/64.9 | 72.0/82.6 | |
| Adequately equipped ICUs (%)/patients in adequately equipped ICUs (%) | 67.5/87.4 | 47.0/61.1 | Criteria for adequately equipped ICUs were different between the two studies |
| Self-paying patients, (%) | 80.5 | 64.5 | |
| Medical or nonoperative patients | 77.1 | 85.5 | |
| Patients with suspected or confirmed infection on the study day (%) | 36.0 | 25.6 | |
| Sepsis and/or septic shock during ICU stay (%) | 28.3 | 29.3 | Criteria for diagnosis of sepsis were different between the two studies |
| Poisoning or overdose | 3.1 | 1.5 | |
| Invasive mechanical ventilation (%) | 31.1 | 24.1 | |
| ICU mortality in patients receiving invasive mechanical ventilation (%) | 35.6 | 44.4 | |
| Patients receiving renal replacement therapy (%) | 12.0 | 9.3 | |
| ICU mortality in patients receiving renal replacement therapy (%) | 31.5 | 41.7 | |
| Patients receiving vasopressors/inotropes (%) | 22.2 | 22.2 | |
| ICU mortality in patients receiving vasopressors/inotropes (%) | 36.1 | 44.0 | |
| Invasive blood pressure monitoring (%) | 19.5 | 25.4 | |
| Central venous catheter inserted (%) | 34.6 | 34.3 | |
| Blood lactate measured (%) | 11.3 | 31.6 |
INDICAPS, Indian intensive care unit case-mix and practice patterns study; ICU, intensive care unit; APACHE, acute physiology and chronic health evaluation; SOFA, sequential organ failure assessment
Overall ICU mortality of 23.4% appears to be higher than the 18.1% mortality observed in the previous study. The proportion of patients dying in the ICU was 15.8%, and TDs constituted a significant percentage of total nonsurvivors (32.5%) in the present study, as compared to 25.1% in INDICAPS. The increase in the proportion of TDs is an area of concern and may reflect more defensive practice after the Aruna Shanbaugh case, where the Supreme Court ruled that “passive euthanasia” was permissible, but required prior approval from the High Court.8 We assumed that all TDs from the ICU eventually died. A single-center study from a tertiary level private hospital in South India found that 23 and 14% of patients were alive 30 and 90 days after being discharged against medical advice, respectively. However, only 9% of their patients were discharged because of an overall poor prognosis.9 Thus classifying all TDs does overestimate mortality, but excluding them would grossly underestimate mortality.
Public hospital ICUs, self-paying patients, and inadequately equipped ICUs were independently associated with ICU mortality in INDICAPS but were not associated with ICU mortality in INDICAPS-II on univariate analysis. Only six (4.5%) public hospital ICUs accounting for 111 (2.4%) patients participated in the study. The proportion of self-paying patients was smaller in this study as compared to INDICAPS (64.5 vs 80.5%).1 This may be the result of increasing penetration of insurance as well as central and state government schemes. While 53.0% of ICUs were inadequately equipped in this study, as opposed to only 32.5% in the INDICAPS study, this may be because we changed the definition of adequately equipped ICUs to include the presence of a blood bank in the hospital and have facilities for RRT and echocardiography in the ICU, rather than in the ICU or hospital. While there was a median of 0.55 invasive ventilators per ICU bed, HFNO capability was available in only 47% of ICUs.
In INDICAPS, we found no difference in outcome between open and closed ICUs, where an open ICU was defined as one in which care of the patient was directed by non-ICU doctor teams, and orders could be written by non-ICU team doctors.1 A striking finding in INDICAPS-II was the association of closed ICUs with higher mortality on multivariable analysis. A closed ICU was defined as one in which final orders for the patient were written only by the ICU team; all other ICUs, where orders could be written by either the ICU team or the primary team, were considered as open ICUs. Thus an open ICU could include not only those ICUs where the care of the patient was directed by non-ICU teams but also the “hybrid” or mandatory consult model, where all patients admitted to the ICU are seen by the intensive care team as well as by the primary consultant, both of them have the privileges to write orders.10–12 An overwhelming majority of ICUs (84.8%) were classified as open ICUs. Since the data in this study were contributed by intensivists, we believe that most open ICUs followed a “hybrid” model, which may have resulted in better interaction between the ICU and primary referring teams, with a beneficial impact on the outcome.13 Two other surveys of Indian ICUs in 2018 found that only 20 and 14% were closed ICUs. However, they did not evaluate association with mortality.14,15 A study based on the Project IMPACT database of 1,01,832 patients in 123 ICUs in the United States had also found that even after adjusting for disease severity, patients managed by critical care specialists showed higher mortality.16 They speculated that some routine critical care practices and procedures may not be beneficial or that the presence of confounders not included in the model may account for worse outcomes. Another study of 69 ICUs in the USA found higher crude mortality (but no difference in adjusted mortality) for closed ICUs compared to open ICUs,13 while an analysis of data from the EPIC study found no difference in outcome between closed and open ICUs.17 Unlike these studies,13,17 we did not find a higher nurse: patient ratio to be associated with a better outcome. We believe that further studies specifically directed at practice patterns in open and closed ICUs are required, and a separate analysis of the hybrid model needs to be performed.
The SMR observed was 0.92, higher than the 0.68 observed in INDICAPS. However, this was obtained in a subset of patients admitted within 24 hours of a study day. A formal evaluation of APACHE II, as well as other scoring systems, is necessary.
This study confirms that gram-negative infections are predominant in India (75.6%), much higher than in Western countries.18 The ICU mortality in patients with sepsis (including septic shock) was higher than that in INDICAPS, but criteria for identifying sepsis were different; in INDICAPS, the diagnosis of sepsis was at the discretion of the investigator.1 Compared to INDICAPS, fewer patients received MV and RRT and a similar proportion received VIs; however, ICU mortality in patients receiving these interventions was higher compared to INDICAPS (Table 8).
We looked at select process measures in patients who received invasive MV. Less than 80% compliance was observed with most, except stress ulcer prophylaxis. In particular, monitoring for delirium and sedation was done in less than 20% of patients, while pain assessment was performed in less than half of the patients. Despite the findings of the National Audit Project-4 that failure to use capnography contributed to 74% of cases of death or persistent neurological injury related to airway management in the ICU or emergency department,19 capnography was routinely used after intubation in less than 35% of ICUs. Triggers for transfusion of RBCs, FFP, and platelets appeared to be inappropriate in up to 25.3% of patients. However, this determination was based only on laboratory parameters; data on clinical circumstances that may have necessitated transfusions, e.g., ongoing hemorrhage and perioperative or periprocedural transfusion, were not available. Thus, further improvements are required in the organization and delivery of critical care in Indian ICUs.
There are limitations to our study. Participation was purely voluntary, and ICUs that were motivated and willing to share data contributed to the study. Participation of public hospital ICUs was negligible, and only 14.5% of patients were surgical admissions. These are even lower than in INDICAPS.1 Source data verification was not performed.
The strengths of this study include a large number of ICUs and patients from all regions of the country and different types of ICUs. Updated definitions were used to classify patients with sepsis, and tropical infections were diagnosed based on confirmatory laboratory tests. Data from this study can be used as a benchmark of structure, process, and outcome of Indian ICUs for comparative, quality assurance, and audit purposes. This may also help the regulatory and planning authorities for resource allocation and also in planning future research studies. Future studies could focus on details of ICU organization, costs of care, and antibiotic utilization.
Conclusion
Patients in this study had moderate severity of illness with relatively high mortality in patients with sepsis, patients on VIs, or receiving MV. Closed ICUs were independently associated with a worse outcome, and the proportion of TDs from the ICU has increased compared to INDICAPS. Public hospital ICUs, self-paying patients, and inadequately equipped ICUs were not associated with increased ICU mortality. Analgesia, sedation, and delirium are infrequently monitored, and the use of capnography after tracheal intubation is uncommon, suggesting scope for improvements in process of care. The role of open and hybrid ICUs requires further study, and legal and procedural issues related to end-of-life care need to be resolved.
Author Contributions
Conception or design of the work: Jigeeshu V Divatia, Yatin Mehta, Deepak Govil, Kapil Zirpe, Pravin R Amin, Nagarajan Ramakrishnan, Farhad N Kapadia, Subhash K Todi.
Acquisition, analysis, or interpretation of data for the work; Jigeeshu V Divatia, Yatin Mehta, Deepak Govil, Kapil Zirpe, Pravin R Amin, Nagarajan Ramakrishnan, Farhad N Kapadia, Mrinal Sircar, Samir Sahu, Pradip K Bhattacharya, Sheila N Myatra, Srinivas Samavedam, Subhal Dixit, Rajesh K Pande, Sujata N Mehta, Ramesh Venkatraman, Khusrav Bajan, Vivek Kumar, Rahul Harne, Leelavati Thakur, Darshana Rathod, Prachee Sathe, Sushma Gurav, Carol D'Silva, Shaik A Pasha, Subhash K Todi.
Drafting the work or revising it critically for important intellectual content: All authors.
Final approval of the version to be published: All authors.
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: All authors.
Footnotes
Source of support: This study was funded by the Indian Society of Critical Care Medicine
Conflict of interest: None
Contributor Information
the INDICAPS-II investigators:
Neeraj Kumar, Neeraj Kumar, Amarjeet Kumar, Dipak Kumar Agarwal, Sarat Kumar Behera, Susruta Bandyopadhyay, Rajarshi Roy, Samir Sahu, Rajeshree Nandy, Sushmita Basu, Subhash Kumar Todi, Saswati Sinha, Lawni Goswami, Manoj Kumar Singh, Jay Kothari, Vilas Kushare, Pravin Tajane, Banambar Ray, Sharmili Sinha, Saroj Pattnaik, Nagarajan Ramakrishnan, Ramesh Venkataraman, Reshma Tewari, Sujan Dey, Ruchira Khasne, Prasanna Kumar Mishra, Sampat Dash, Sandip Bhattacharyya, Vandana Sinha, Anup Jyoti Dutta, Praveen Kumar Koppula, Krishna Prabhakar Kasam, Basanth Kumar Rayani, Abhijit Sukumaran Nair, Jignesh Navinchandra Shah, Prashant Jedge, A Chakravarthi, Amol Hartalkar, Rajesh Kumar Pande, Abhishek Vishnu, Pravin R Amin, Sujata N Mehta, Kalpesh Bhoyar, Joanne Mascarenhas, Madhusudan R Jaju, Venkat Raman Kola, Hariprasad, T Mohan S Maharaj, Lakshmi Rani Takkellapati, Sunil T Pandya, M Kiran, Akshay Shrivastava, Pallavi Shrivastava, Pradip K Bhattacharya, Nimita Deora, Anand Sanghi, Abhishek Samprathi, Kishore Pichamuthu, Bhagyesh Shah, Shuchi Kaushik, Palepu B Gopal, Ch. Balasubrahmanyam, Deepak Jeswani, Deepti Jeswani, Shruti Sharma, Gunchan Paul, Prasad Rajhans, Safal Sable, Chaitri Shah, JD Lakhani, Arpita Dwivedy, Priteema Chanana, Vaibhav Bhargava, Pramod Sarwa, Kishore Mangal, Yatendra Kumar Gupta, Vivek Nangia, Amina Mobashir, Mrinal Sircar, Saurabh Mehra, Arun Kumar, Amit Kumar Mandal, VK Thakur, Sandeep Patil, Bhushan Kinholkar, Neeta Bose, Dhara Tanna, Bhavik V Shah, Priyanka Khatri, Nishchil H Patel, Sanjoy Joseph George, Sanghamitra Mishra, Basanta Kumar Pati, Rajesh Chawla, Sudha Kansal, Atul Kumar Singh, Sulakshana, Leelavati Thakur, Shruti Tandan, Varun Deshmukh, Gyanendra Agrawal, Deepak Singhal, Narendra Rungta, Neena Rungta, Om Prakash Shrivastava, Satnam Singh, Vivek Kumar, Srinivasan Ramananthan, Dipak Aghara, Jayendra Aghara, Rajesh Mohan Shetty, Manjunath Thimmappa, Shantanu Belwal, Bhupesh Uniyal, Rekha Gupta, Mudit Garg, Yatin Mehta, Deepak Govil, Shaleen Bhatnagar, Chitra Mehta, Prashant Kumar, Tariq Ali, Rahul Harne, Payel Bose, Saurabh Debnath, Lalit Singh, Nipun Agrawal, Ajay A Bulle, Ajita Annachhatre, Yogesh Belapurkar, Kanwalpreet Sodhi, Harmanpreet Kaur, AS Ansari, Sourabh Phadtare, Ranjit Sousa, Minal Jariwala, Yuti Sheth, Gunjan Chanchalani, Harish Mallapura Maheshwarappa, BM Ramya, Sachin Gupta, Deeksha Singh Tomar, Utpal Sarma, Vipul Mishra, Sultana Teslima Begum, Ajit Deka, Sunitha Binu Varghese, Ajit Yadav, Shaik Arif Pasha, V Chittaranjan Naidu, Lakshmi Prasannam, Pankaj Patil, Farhad N Kapadia, Khusrav Bajan, Ajoy Krishna Sarkar, Diptimala Agarwal, Simant Kumar Jha, Shiv Kumar, Kapil Zirpe, Sushma Gurav, Prajakta Wankhede, Prachee Sathe, Prashant Sakhavalkar, TR Jadhav, Shilpa Kulkarni, Saurabh Shaha, Sanjith Saseedharan, Roopa Karanam, Promise Jain, Subhal Dixit, Priyanka Khalate, Nikhil Ajmera, Geetesh Mangal, AS Arunkumar, MS Kalaiselvan, A Mohana Rao, V Kuchela Babu, Vishal Sadatia, Tushar Patel, Abhishek Prajapati, Deepak S Sharma, Krutika Tandon, Nitinkumar B Agarwal, Basavraj Pujari, Sudhir Khunteta, Darshana Rathod, Ankur Bhavsar, NK Vinod, KV Bharath, Carol Dsilva, Bhuvana Krishna, Harjit Dumra, Mansi Dandnaik, Anand V Joshi, Nirmal Jaiswal, Shivam Chopra, Ranvir Singh Tyagi, Rakesh Kumar Tyagi, Milap Mashru, Jayeshkumar Dobariya, Jigeeshu V Divatia, Sheila Nainan Myatra, Atul P Kulkarni, Anjana Shrivastava, Amit Narkhede, Bharat Jagiasi, Pallavi Patekar, Shyam Sunder Tipparaju, Yalavarthy Swathi, Himansu Sekhar Mishra, N Srinivas, Srinivas Samavedam, and Narmada Aluru
Orcid
Jigeeshu V Divatia https://orcid.org/0000-0001-7384-4886
Yatin Mehta https://orcid.org/0000000208884774
Deepak Govil https://orcid.org/0000-0002-4624-1614
Kapil Zirpe https://orcid.org/0000-0002-8140-727X
Pravin R Amin https://orcid.org/0000-0002-9865-2829
Nagarajan Ramakrishnan https://orcid.org/0000-0001-5208-4013
Farhad N Kapadia https://orcid.org/0000-0003-1837-1144
Mrinal Sircar https://orcid.org/0000-0002-2199-3318
Samir Sahu https://orcid.org/0000-0003-1246-3187
Pradip Kumar Bhattacharya https://orcid.org/0000-0002-0219-385X
Sheila Nainan Myatra https://orcid.org/0000-0001-6761-163X
Srinivas Samavedam https://orcid.org/0000-0001-6737-8663
Subhal Dixit https://orcid.org/0000-0002-1441-0807
Rajesh Kumar Pande https://orcid.org/0000-0002-0149-727X
Sujata N Mehta https://orcid.org/0000-0003-0306-538X
Ramesh Venkataraman https://orcid.org/0000-0003-1949-3979
Khusrav Bajan https://orcid.org/0000-0002-7339-4288
Vivek Kumar https://orcid.org/0000-0002-6914-5422
Rahul Harne https://orcid.org/0000-0002-0178-2628
Leelavati Thakur https://orcid.org/0000-0002-1592-7592
Darshana Rathod https://orcid.org/0000-0002-5446-6768
Prachee Sathe https://orcid.org/0000-0002-1236-1669
Sushma Gurav https://orcid.org/0000-0001-6875-2071
Carol D'Silva https://orcid.org/0000-0002-3920-1366
Shaik Arif Pasha https://orcid.org/0000-0001-6314-8473
Subhash Kumar Todi https://orcid.org/0000-0003-2306-6080
Supplementary Material
All the supplementary material from Supplementary tables 1–5 are available online on the website of www.IJCCM.org
References
- 1.Divatia JV, Amin PR, Ramakrishnan N, Kapadia FN, Todi S, Sahu S, et al. Intensive care in India: The Indian intensive care case mix and practice patterns study. Indian J Crit Care Med. 2016;20(4):216–225. doi: 10.4103/0972-5229.180042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knaus WA, Wagner DP, Draper EA, Zimmerman JE, Bergner M, Bastos PG, et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100:1619–1636. doi: 10.1378/chest.100.6.1619. [DOI] [PubMed] [Google Scholar]
- 3.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. [PubMed] [Google Scholar]
- 4.Ferreira FL, Bota DP, Bross A, Mélot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286(14):1754–1758. doi: 10.1001/jama.286.14.1754. [DOI] [PubMed] [Google Scholar]
- 5.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mani RK. End-of-life care in India. Intensive Care Med. 2006;32:1066–1068. doi: 10.1007/s00134-006-0185-7. [DOI] [PubMed] [Google Scholar]
- 7.Devlin JW, Skrobik Y, Gélinas C, Needham DM, Slooter AJC, Pandharipande PP, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46(9):e825–e873. doi: 10.1097/CCM.0000000000003299. [DOI] [PubMed] [Google Scholar]
- 8.2009. Aruna Ramachandra Shanbaug vs the Union of India & Ors. WRIT Petition (CRIMINAL) No. 115 of 2009 (Supreme Court of India Proceedings);
- 9.Ramakrishnan N, Ranganathan L, Abraham BK, Rajagopalan S, Venkataraman R. What happens to patients discharged against medical advice? Indian J Crit Care Med. 2018;22(8):580–584. doi: 10.4103/ijccm.IJCCM_101_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brilli RJ, Spevetz A, Branson RD, Campbell GM, Cohen H, Dasta JF, et al. Critical care delivery in the intensive care unit: Defining clinical roles and the best practice model. Crit Care Med. 2001;29(10):2007–2019. doi: 10.1097/00003246-200110000-00026. [DOI] [PubMed] [Google Scholar]
- 11.Critical care delivery in intensive care units in India: defining the functions, roles and responsibilities of a consultant intensivist: recommendations of the Indian Society of Critical Care Medicine Committee on defining the functions, roles and responsibilities of a consultant intensivist. Available from: https://isccm.org/pdf/ISCCM%20Intensivist%20guidelines.pdf. [Last cited on June 07, 2021]
- 12.Rungta N, Zirpe KG, Dixit SB, Mehta Y, Chaudhry D, Govil D, et al. Indian Society of Critical Care Medicine experts committee consensus statement on ICU planning and designing, 2020. Indian J Crit Care Med. 2020;24(Suppl. 1):S43–S60. doi: 10.5005/jp-journals-10071-G23185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Checkley W, Martin GS, Brown SM, Chang SY, Dabbagh O, Fremont RD, et al. Structure, process, and annual ICU mortality across 69 centers: United States critical illness and injury trials group critical illness outcomes study. Crit Care Med. 2014;42(2):344–356. doi: 10.1097/CCM.0b013e3182a275d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kashyap R, Vashistha K, Saini C, Dutt T, Raman D, Bansal V, et al. Critical care practice in India: results of the intensive care unit need assessment survey (ININ2018). World J Crit Care Med. 2020;9(2):31–42. doi: 10.5492/wjccm.v9.i2.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kartik M, Gopal PBN, Amte R. Quality indicators compliance survey in Indian intensive care units. Indian J Crit Care Med. 2017;21(4):187–191. doi: 10.4103/ijccm.IJCCM_164_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levy MM, Rapoport J, Lemeshow S, Chalfin DB, Phillips G, Danis M. Association between critical care physician management and patient mortality in the intensive care unit. Ann Intern Med. 2008;148(11):801–809. doi: 10.7326/0003-4819-148-11-200806030-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakr Y, Moreira CL, Rhodes A, Ferguson ND, Kleinpell R, Pickkers P, et al. The impact of hospital and ICU organizational factors on outcome in critically ill patients: results from the extended prevalence of infection in intensive care study. Crit Care Med. 2015;43(3):519–526. doi: 10.1097/CCM.0000000000000754. [DOI] [PubMed] [Google Scholar]
- 18.Vincent JL, Sakr Y, Singer M, Martin-Loeches I, Machado FR, Marshall JC, et al. Prevalence and Outcomes of infection among patients in intensive care units in 2017. JAMA. 2020;323(15):1478–1487. doi: 10.1001/jama.2020.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cook TM, Woodall N, Harper J, Benger J Fourth National Audit Project. Major complications of airway management in the UK: results of the Fourth National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society. Part 2: intensive care and emergency departments. Br J Anaesth. 2011;106(5):632–642. doi: 10.1093/bja/aer059. [DOI] [PubMed] [Google Scholar]