Highlights
-
•
Fluorescent and non-fluorescent species of Pseudomonas are important for plant growth promotion, phytopathogenic control and plant disease management.
-
•
Pseudomonas belong to Pseudomonadaceae family (10 groups on the basis of rRNA-DNA hybridization) classified into 6-subgroups of rRNA gene homology and RFLP.
-
•
Pseudomonas species produce antagonistic mechanism such as ISR and compounds like cell wall degradation enzymes, and antibiotics to maintain a mutualistic relationship with the associated plant.
-
•
Pseudomonas sp. synthesize auxins having properties similar to phytohormones like IAA, which act as signaling molecules for regulating plant growth.
Keywords: Pseudomonas, Plant growth promoting rhizobacteria (PGPR), Siderophores, Phenazines, Induced systemic resistance (ISR), Disease management
Abstract
Numerous microbial communities show synergistic and antagonistic interactions among themselves, resulting in benefit and harm to either or both the associated members. The association holds accountability for nutrients recycling and energy drift, resulting in the availability of macronutrients unavailable and insoluble forms of rhizospheric nutrients, crucial for vital processes in plants, e.g., act as co-factors of various phyto-enzyme and redox mediators. Plant growth promoting rhizobacteria are known to enhance plant growth by increasing these macronutrients availability during their plant root colonization. In comparison to any other genera, Pseudomonas is the most favored bioinoculant due to its significant properties in both plant growth and phytopathogen control during its synergistic association with the host plant. These properties include siderophore production, phosphate solubilization, nitrogen fixation, phenazines, antibiotics, and induced systemic resistance carried out by various Pseudomonas species like Pseudomonas fluorescens, Pseudomonas putida, and Pseudomonas syringae. The association of Pseudomonas with crop plants procures several secretory and electron-based feedback mechanisms in order to regulate the plant growth and phytopathogen control activities through the secretion of several phytohormones (auxins, gibberellins, Indole-3-acetic acid), secondary metabolites (flavonoids) and enzymes (aminocyclopropane-1-carboxylate, phenylalanine ammonia-lyase). Ecologically significant applications of Pseudomonas in biocontrol and bioaugmentation are crucial for maintaining food security.
Graphical abstract
1. Introduction
Soil ecosystem possesses rich diversity than any other ecosystem with complex interactions between living and non-living entities. These living forms habituated in soil are crucial for supporting qualitative plant growth (Uppal et al., 2017). Plants get their nourishment by absorbing nutrients from various sources such as (i) decayed plants and animals composed of organic matter; (ii) soil that occurs as silt, sand, and clay of different particle sizes and water holding capacities.
Over utilization of chemical fertilizers and insecticides throughout the years had shown adverse effect on soil quality, fertility and ecology (Garrido-Sanz et al., 2017). Other than this, several competitive agricultural practices like overgrazing and traditional tilling are responsible for the deterioration of cultivable topsoil. So as to combat this situation, various biotechnological methods manage the fusion of conventional organic harvest techniques to chief elements required for promoting rhizobial competency. Plant promotional rhizobial modification methods (like sustainable, active rhizobia and self-regulation) are called biocontrol, bioaugmentation, and biostimulation (Bhadauria et al., 2010). The additional microbial strains present in the rhizobial regions may interact positively or negatively with local microbes to raise active metabolism and colonization by decreasing various microbial infections caused by parasites (Garcia-Seco et al., 2013).
Approximately 1 billion species of bacteria exist on Earth. Out of them, 5000 species identified yet (Sah and Singh, 2016). Moreover, surface soil microbes present on the Earth consider around 10 billion microbes per gram. One gram of that soil contains approximately 4000 distinct bacterial genomes. These units estimate an enormous mass diversity significance while showing the progressive competition in the rhizospheric complement (Sah and Singh, 2016). The total percentage value of the microbes present in the soil constituents is <0.4–0.5% w/w. This microflora defines the rhizobial interactions by forming a vibrant assembly of various beneficial microbes interacting: (i) with each other and (ii) with plants, forming positive interactions to promote their growth in order to get protection (Kumar et al., 2016b). Pseudomonadales, on the basis of physiological and biological characteristics (1973), are most abundantly reported among all the other classified orders such as Enterobactera, Xanthomonadales, Gammaproterbacteria, Alteromonadales, and Legionellale (Chu et al., 2019). Several strains of Pseudomonas veronii abundantly found, approx 38%, with a proportion of all bacterial communities present in the rhizosphere (Qessaoui et al., 2019).
1.1. Pseudomonas and its classification
The widespread occurrence of Pseudomonas indicates its adaptability through molecular, environmental, and physiological diversity (Sah and Singh, 2016). The classification of Pseudomonas covered as a part of Bergy's Manual (in 1923), based on its phenotypic characteristics, described various media presenting comparable plate counts select different bacterial types reaching several diversity estimates within the same soil. Pseudomonas taxonomy has always been questionable for decades since numerous bacterial taxa were primarily classified into the genus, later incorporated within various other genus of diverse classes of proteobacteria. That was due to advancements in phylogenetic, molecular characterization and distribution procedures for classification of microbes (Chu et al., 2019). Pseudomonadaceae family includes genera like Pseudomonas, Zoogloea, Frateuria, and Xanthomonas. The genus Pseudomonas holds gram-negative, rod-shaped straight or slightly curved cells with polar flagella. The taxonomic situation of Pseudomonas has been changed drastically since its first classification report. On the basis of genomic data, Pseudomonas sensu stricto has been segregated from proteobacteria subclass Beta and reclassified as Proteobacterial subclass Gamma (Chu et al., 2019).
Blagodatskaya and Kuzyakov (2008) summarized the dietary properties of >200 strains approximately on >100 various organic composites, along with an extensive modification of components for simplifying Pseudomonas taxonomy. Although the classification of this genera never got mentioned in Bergey's manual because Pseudomonas distribution, solely based on phenotype with% GC content as only molecular information mentioned during species description. The most significant contribution in the characterization of Pseudomonas genetically was stretched in the 1970s. The classification of the various groups of bacteria into 5-subgroups of rRNA and the relative DNA to RNA quantities separated based on their phylogeny describing the remains of the Pseudomonas genera (Chu et al., 2019).
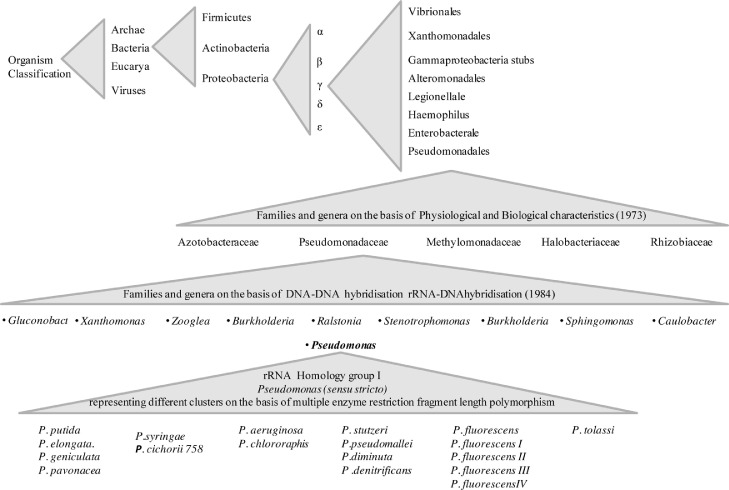
In a genotypic strategy known as the ribosomal RNA cataloging method initially, the rRNA partial sequence leads to the pseudomonads classification and its grouping based on phylogeny coinciding with 5-subgroups of rRNA (Sundar et al., 2021). Bacterial phylogenetic classification carried out on the basis of its 16S rRNA sequencing technique directed three subdivisions known as Proteobacteria, α, β, and γ. Currently, there are >10 classified genera in Pseudomonadales, including Stenotrophomonas, Burkholderia, Sphingomonas, Caulobacter, Xanthomonas, and Ralstonia (Chu et al., 2019) (Fig. 1).
Fig. 1.
An overall classification of Pseudomonas showing >10 classified genera in Pseudomonadales and their further classified species representing different clusters on the basis of multiple enzyme RFLP.
Pseudomonas fluorescent species, subdivided into seven biotypes as A to F, later known as biovars bv., I, II, III, IV, and V. Biovar D and E shifted to P. chlororaphis and P. aureofaciens, clustered as the P. chlororaphis sp. Although this approach introduces flaws like (i) associates significance of some characteristics and (ii) quantitative analysis of characteristics is less. Pseudomonas has simple nutritional requirements due to their properties in the degradation of distinct substrates, aromatic chemicals, halogenated derivatives, and recalcitrant organic residues.(Sah and Singh, 2016) studied the growth of various Pseudomonas strains on several different organic substrates, reporting great diversity in genus Pseudomonas. Taxonomic characterization of the Pseudomonas with the advancement in the DNA fingerprinting techniques like RFLP, 16S rDNA, and ARDRA of different groups with monophyla and several before included Pseudomonas species moved to distinct another genus yet, Pseudomonas sensu-stricto persists influential diversity, for example, P. plecoglossicida, P. simiae, P. salomonii, P. palleroniana and P. costantinii (Verma et al., 2019a). Pseudomonas brassicacearum and Pseudomonas thivervalensis isolated from rice and garlic, Pseudomonas rhizosphaerae, P. lutea and P. argentinensis isolated from rhizosphere of grass, P. lurida isolated from grass phyllosphere, P. duriflava from desert soil, P. guinea isolated from Antarctic soil, P. psychrotolerans from the veterinary clinic, and P. thermotolerans isolated from clinical samples of animals (Chu et al., 2019).
2. Pseudomonas mediated establishment of rhizospheric competency
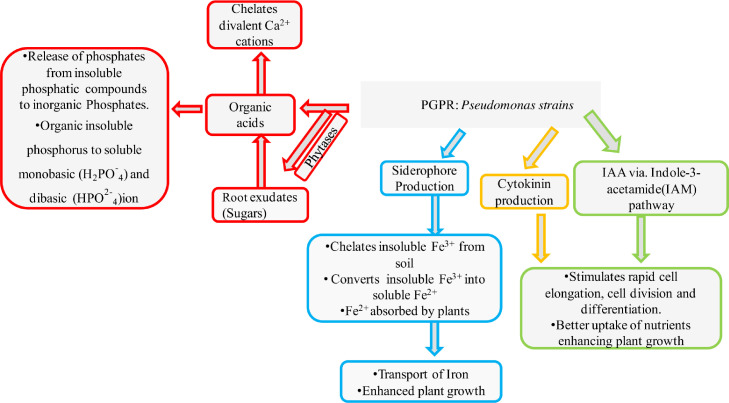
Quantitative comparative analysis of the rhizo-microbial population and total existing bacteria scattered around (per gram of soil) explicates enormous metabolic activities inside the root ranges (Curtis and Sloan, 2004). The introduction of artificial rhizobacteria is crucial for the (i) stabilization of huge microbial populations by forming a constant feedback mechanism inside rhizobacteria and associated plants and (ii) reducing initial inoculum because of abrupt resource depletion. The mechanism of selecting the rhizobial root colonizers by plants is through induced resistance (IR) or the process of rhizodeposition by specific secretions of exudes crucial for microbial certainty (Fig. 2). These accumulative pathways establish various constant feedback mechanisms for the maintenance of competent rhizosphere. Various Pseudomonas species flexibly grow on agro-fields proving their stimulation, sustainability, and remediating properties. The interactions and uptake of nutrients in Pseudomonas spp. are essential for establishing successively competent colonization (Saharan and Nehra, 2011). Different strains of Pseudomonas exhibit several ecological qualified characteristics, like biofilm formation, antifungal metabolite production, quorum sensing mediation, synergistic attachment with the plant root system, chemotactic mediation, uptake, and catabolism of various plant secretions (Saharan and Nehra, 2011). Pseudomonas species, generally regarded as plant elongation promoters (root and shoot), appending at the molecular level, and have several phytobenefits. Numerous strains of fluorescent Pseudomonas exhibit health improvement through enhanced plant growth and biological suppressions, such as Fusarium wilts in wheat pathogenesis and root rot in tobacco (Garcia-Seco et al., 2013).
Fig. 2.
Pseudomonas mediated transport of nutrients to the plant involving various organic acids, producton of siderophores, cytokines, IAA through IAM pathway.
The correlation of the level of suppression of disease with the rhizobial Pseudomonas population density i.e. competency may be accessed through a model strain approach, e.g. corresponding rhizosphere competitiveness of Pseudomonas fluorescens C7R12 (model strain) is assessed with nitrate reductase and pyoverdine synthesizing properties (Saharan and Nehra, 2011). The properties of rhizospheric competency display several observable characteristics for the continuation of life, like the ability to utilize substrates, N2 dissimilation, iron acquisition through siderophore production, N-acyl-homoserine lactones, and antibiotic production (Ashrafuzzaman et al., 2009). Several Pseudomonas strains, assessed for antibiotic resistance (like rifampin-resistant derivatives) on various crop parts like tomato seedlings with reduced concentration of iron, show the insufficient to moderate survival rate (Forney et al., 2004). The data concludes the capability of strains to actively compete in the harsh environment with available nitrogen and siderovars. Mechanisms like N2 fixation, (Plant growth regulators) PGR production, phytohormone production, H3PO4 solubilization, and exudate production are essential to optimize the overall plant growth and health (Table 1) (Ashrafuzzaman et al., 2009).
Table 1.
Distribution of Pseudomonas species based on rRNA homology cluster group-I and their role in plant growth promotional activities in association with specific crops.
| S. No. | rRNA homology group-I cluster | Pseudomonas species | Crop plants | Outcome | Type of inoculum | % enhancement in growth of plant | Refs. |
|---|---|---|---|---|---|---|---|
| 1. | Pseudomonas fluorescens; Pseudomonas fluorescens I; Pseudomonas fluorescens II; Pseudomonas fluorescens III; Pseudomonas fluorescens IV | Pseudomonas fluorescens | Turmeric | Growth and curcumin content | Root inoculum; | Plant height increased by 12.2% | Jaleel et al. (2007) |
| Wheat | Seed yield and shoot dry mass | Rhizobacterial inoculants | Increase in iron content of plants 40% to 30% | Garcia-Seco et al. (2013) | |||
| Mustard | Growth and yield attributes | Rhizobacterial inoculants | Plant height increased- 17.14% to 21.43% | Hou and Oluranti (2013) | |||
| Opium poppy | Morphine, thebaine, codeine | Leaf inoculums | Plant length increased 15.0% to 37.5% | Sharma et al. (2013) | |||
| Marigold | Shoot fresh weight, root dry weight, leaf number, node number | Root inoculums | Growth increased by 20–30% | Bonilla et al. (2014) | |||
| Blackberries | Fruit quality | Leaf inoculums; bacterial inoculums | Mineral uptake increased by 12% | Kumar et al. (2016b) | |||
| 2. | Pseudomonas aeruginosa; Pseudomonas chlororaphis | Pseudomonas aeruginosa | Tomato | Fruit yield | PGPR inoculums | 20% yield in tomato and maize crop | Dashti et al. (2012) |
| Pseudomonas corrugate | Maize | Grain yield | |||||
| 3. | Pseudomonas tolassi | Pseudomonas sp., Burkholderia caryophylli | Wheat | Growth and yield of wheat | PGPR inoculums | fresh and dry weight increased by 19.95% to 47.41% | Cappellari et al. (2013; Bouaichi et al. (2021) |
| 4. | Pseudomonas putida; Pseudomonas elongata; Pseudomonas geniculata; Pseudomonas pavonacea | Pseudomonas putida | Tomato | Plant growth, yield | Inoculum with different carbon sources Hexa-chlorocyclohexane-degrading consortium |
Mineral uptake enhancement by 12–19% with respect to control | Shen et al. (2012), Tang et al. (2021b) |
| Cherry trees | Fruit set, plant vegetative growth | ||||||
| Maize | Grain yield | ||||||
| 5. | Undefined (general) | Pseudomonas sp. | Wheat | Tomato | Seed inoculums | Enhanced whole plant fresh and dry weight by approximately 9–10% | Combes-Meynet et al. (2011) |
| Growth | Root growth | ||||||
| Onion | Onion bud | Rhizobacterial inoculums | |||||
| Maize | Plant height, dry weight, Compatible solutes, antioxidant |
There are different responses of Pseudomonas while competitive colonization in the rhizosphere which can be categorized as:
2.1. Chemical-induced responses
The mutualistic nature of PGPR in Pseudomonas spp. to plants and rhizobia is a constant physiological feedback system that reverts to the rhizobial microbes holds its dependency (of colonization) on the number of organic components of exudates secreted from the host. Initiation, absorption, and metabolism of chemicals by N2 fixers, through H3PO4 solubilization, including various determining factors associated with bacteria attachment on root cells (Forney et al., 2004). Soil fertility improvement is a limiting factor crucial for improving the uptake of readily available nitrogen and phosphorous for crop production (Perez-Montano et al., 2014). Nitrogen is required to increase soil fertility, and phosphorus is a necessary macro-nutrient for host plant developmental growth. Nitrogen and phosphorous are crucial for the constant development in chemically formed supplements and developing various biological tools to improve soil fertility for maintaining microbial ecological competency (Pattnaik et al., 2019).
The capability of these microbes in transforming insoluble phosphorous to its soluble form is one of their crucial characteristics for enhancing crop yield. These microbes are known as plant growth-promoting bacteria. They are present in the rhizospheric region contributing to plant growth-promoting activities, therefore, used as biofertilizers for sustainable agriculture practices (Pattnaik et al., 2019).
The communication between microbe and associated plant roots is carried out by chemical signaling molecules resulting in the establishment of a mutualistic relationship (Mhlongo et al., 2018). This communication leads to various physiological changes in plant growth-promoting rhizobacteria such as biofilm formation necessary for primary defense and induced resistance in the plant. The primary defense stimulus activates various signaling molecules, metabolic regulators, and finally, activates the genes that code for enzymes required for secondary defense metabolite production. The plant secretes various primary and secondary metabolites in response to the altered gene expression. A similar process yet intensified is repeated after the production of secondary stimuli to minimize the impact on the plant. Metabolomics studies interpretating the role of physiological metabolites (endo/exo) help to describe the mutualistic association via chemical/ biochemical signals between rhizobacteria and associated plant roots (Mhlongo et al., 2018).
2.2. Pseudomonas-plant surface interactions
The interactions of Pseudomonas with associated plants require several preparatory steps for their attachment through the surface and cellular appendages resulting in the node formation. In Pseudomonas fluorescens interaction with the corn plant roots, the primary structures involved in the appendage are fimbriae and pili (Forney et al., 2004). Type-4 pili present in Pseudomonas aeruginosa initiates the first contact of epithelial cell surface to rhizospheric bacteria by implying various structure functions in locomotion (like twitching motility). Twitching motility is a property that enables bacterial attachment to the abiotic surface for biofilm formation. The pilA gene is responsible for prepilin encoding, retraction of pili, and twitching motility. The pilA gene promoter expression has an association with the tomato crop plant and effectively establishes competitive root tip colonization showing the intermittent function of type-4 pili (Pattnaik et al., 2019).
During the interaction of rhizobacteria to its associated plants, the primary step of their interaction is the biofilm formation initiation on plant root for effective colonization, governed through several mechanisms and surface molecules (Pattnaik et al., 2019). The estimated presence of plant lectin molecules (until now found in >600 legume plant species) is approximately 9–10% of total protein in mature plant parts (both root and shoot systems), associates with the binding of rhizobacteria through receptors (polysaccharides) for the effective plant surface attachment (Dun-chun et al., 2016). The rhicadhesin is Ca+2 binding with proteins, indicating the bacteria-legume plant attachment by adhering to the plant cell surface. The protein binds Ca+2 at the bacterial cellular end and root hair initiates agglutination depending on Ca+2 concentration. There are >30 diverse Pseudomonas strains isolated from the rhizosphere of tomato, potato, and grasses with no agglutination-dependency adherence observed, suggesting the involvement of several genetic determinants within the bacterial-plant association and several feedback governing pathways (Pattnaik et al., 2019).
2.3. Chemo-tactic behavior
As nutrients serve as the limiting factor, essential normal microflora of the rhizosphere and phytopathogens excavate the rhizoplane and compete for nutrients in order to achieve reproduction and active metabolic processes (Palmgren et al., 2015). Several strains of Pseudomonas actively colonize roots because of their mineral uptake techniques, initiating fast root colonization, host-bacteria feedback mechanism, and strategic defense mechanism that hinder the invading phytopathogen. Pseudomonas fluorescens is very adaptive in nature (in terms of root colonization) and are actively involved in plant stress responses, mineral recovery, and various root secretions (George et al., 2016). Secretions of roots exudates comprise high concentrations yet low molecular weight organic compounds like sugar, organic, and amino acids present within the cytoplasm (Singh and Nagar, 2021). Pseudomonas fluorescens secretes different enzymes like protease, glucanase, chitinase etc. to obtain energy from complex carbon and nitrogen residual components present in the environment which in turn supplements the minerals in soil. Ecological factors that affect the variation in the root exudate composition directly or indirectly affect the composition and activity of rhizosphere bacteria (Albareda et al., 2006).
Ecological circumstances representing dry soils promote intense penetrable reserves, where minerals penetrate and are least available for plant and rhizobial microbes. The roots of the plants are attracted to the upper subsurface areas for extending their tubes in order to achieve available minerals, despite water holding nutrients and minerals penetration by soil perforation lower to the center areas (George et al., 2016). The regions rich in mineral nutrients from deeper porous zones are hard to reach due to constricted plant root anatomy that make them incapable of achieving the root length required for harvesting nutrients. In deep hard areas, the phenomenon like evaporation, and high transpiration rates dehydrate the plant leading to reduction in porosity and cysts generation (Tang et al., 2021a). The Pseudomonas and its associated PGPR induce root stimulation resulting in elongation to promote plant-mineral feedback mechanism by mutualistic cooperation. Several Pseudomonas strains adequately organize themselves physiologically by chemotactic locomotion and O-antigen production for successive integration within numerous soil layer zones. Various strains of Pseudomonas sp. are known as progressive colonizers due to the presence of 9-polar flagella per cell (Godinho and Bhosle, 2013a; Pattnaik et al., 2019). Berg and Smalla (2009) have also indicated the role of cheA gene in transport and feedback pathways for bacteria-root cell interactions in wild-type Pseudomonas fluorescens.
3. Pseudomonas mediated induction of biological mechanisms of plant growth and disease resistance
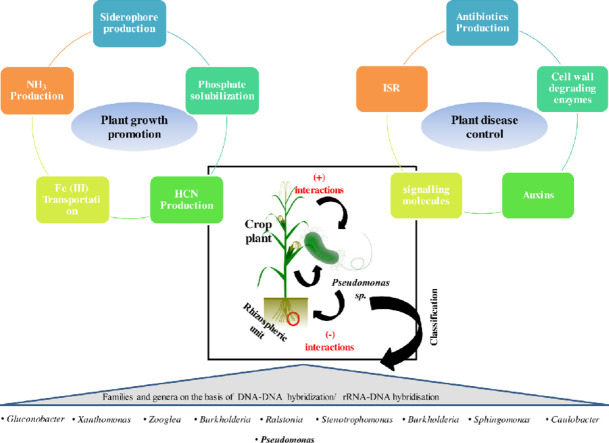
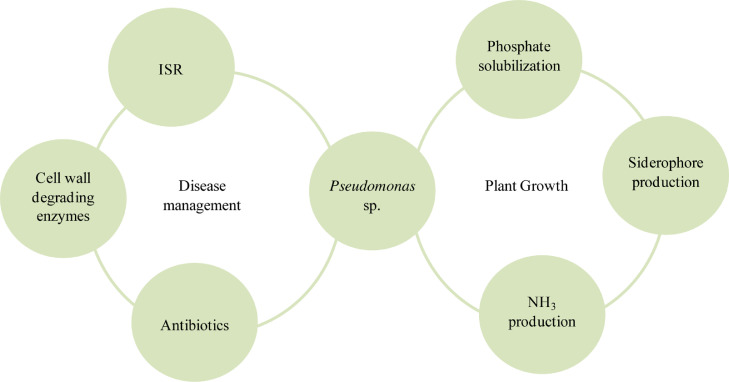
Pseudomonas regulate phytopathogenic activity of other microbes present in rhizospheric zones by different metabolic activity like siderophore production, antibiotic production, and (Hydrogen cyanide) HCN (Gray and Smith, 2005). The discharge of the growth factors by Pseudomonas help the plant for rhizospheric compentency, effective microbe colonization, and synergistic cooperations. Several Pseudomonas species, like, Pseudomonas aeruginosa, Pseudomonas fluorescens, Pseudomonas jessenii, Pseudomonas putida, and Pseudomonas vancouverensis, are known for their roles in the biosupression of plant pathogens (Yu et al., 2011). Lately, various Pseudomonas spp., derived marketable products produced from resultant antimicrobial compounds, siderophores, and ortho-hydroxybenzoic acid, proven efficacious toward tobacco plant rhizospheric microbe (Peronospora tabacina), Pseudoperonospora cubensis within Cucumis sativus and Alternaria solani inside Solanum lycopersicum (Costa-Gutierrez et al., 2020). Pseudomonas species can acts both as PGR and in phytopathogen control due to their ability to secrete specific compounds having essential roles in phosphate solubilizing compound production, siderophore production, and nitrogen fixation as a part of its plant growth-promoting activities (Fig. 3). Pseudomonas species produce antagonistic compounds such as cell wall degradation enzymes, antibiotics etc., for plant disease management and maintain a mutualistic relationship with the associated plant (Kour et al., 2019).
Fig. 3.
Contributive role of Pseudomonas in both plant growth regulation and phytopathogenic control through various mechanisms.
3.1. PGPR properties of pseudomonas
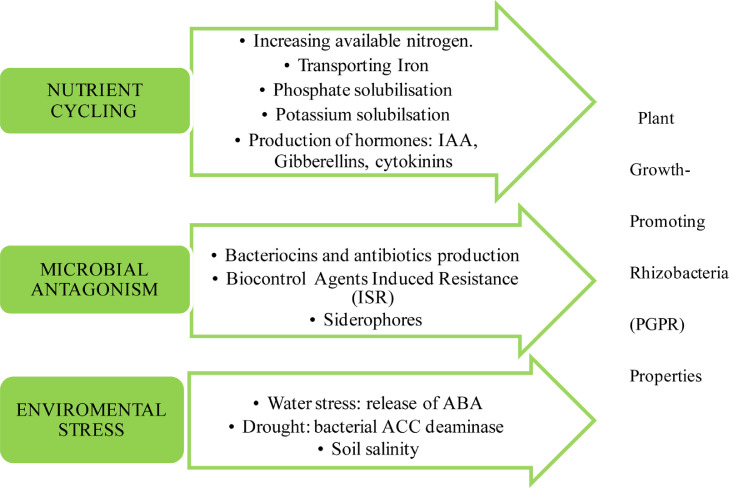
Pseudomonas is a very distinct and ecologically important group of bacteria on the Earth. They have an exceptionally significant role in the carbon and nitrogen cycles ((Zhang et al., 2020)Zhuang et al., 2021). Pseudomonas species control plant growth because of its various beneficial characteristics like the presence of siderophores, phosphorous solubilization, and antagonistic compounds secretion for several plant pathogens (Fig. 4). Fluorescent Pseudomonas spp. is one of the most effective strains of Pseudomonas that has enormous potential. Distinct Pseudomonas fluorescens strains correlated with the putida group acts as seed inoculants for crops resulting in enhanced yield (Yan et al., 2008; Costa-Gutierrez et al., 2020).
Fig. 4.
Plant Growth Promoting Properties of Pseudomonas through nutrient cycling, microbial antagonism, and environmental stress.
3.2. Role of pseudomonas in plant growth regulation
The plant growth regulators (PGR) are hormones or chemotactic messengers, also known as phytohormones, are actively controlling the environmental stressors through positive (synergism) or negative (antagonistic) associations (Gamez et al., 2019). This mechanism of communication is called cross-talk signaling. Phytohormones undertake the root system and initiate cell growth, reproduction, and specialization. Phytohormones directly/obliquely invade roots via ACC deaminase catalysis for improving the growth of the side root system. The prolongation of the root system and its development promote minerals separation of local and far regions, whereas plant ACC hydrolyzed by ACC deaminase blocks the phytohormone ethylene level acting antagonistically to the plant (Khan et al., 2013). A novel bacterium Pseudomonas putida strain UW4 attached to ACC deaminase possesses various properties to support phyto development beneath harsh environments e.g., drought and attacking plant pathogens (Godinho and Bhosle, 2013a). Phytohormone synthesis is linked with production of auxin similar to indole-3-acetic acid (IAA) which act as signaling molecules for regulating plant developmental growth, organogenesis stimulation, cell growth, division, and differentiation. Pseudomonas fluorescens, Pseudomonas putida, and Pseudomonas syringae are the most distinguished and active strains which synthesize auxin and utilize L-Trp (precursor molecule) toward PGPR activities ((Costacurta and Vanderleyden, 1995); Sah and Singh, 2016). A high concentration of L-Trp-corresponds to high IAA production, which inhibits the effective parameters required for plant growth (Costacurta and Vanderleyden, 1995; Mia and Shamsuddin, 2010).
3.2.1. Pseudomonas mediated siderophore production
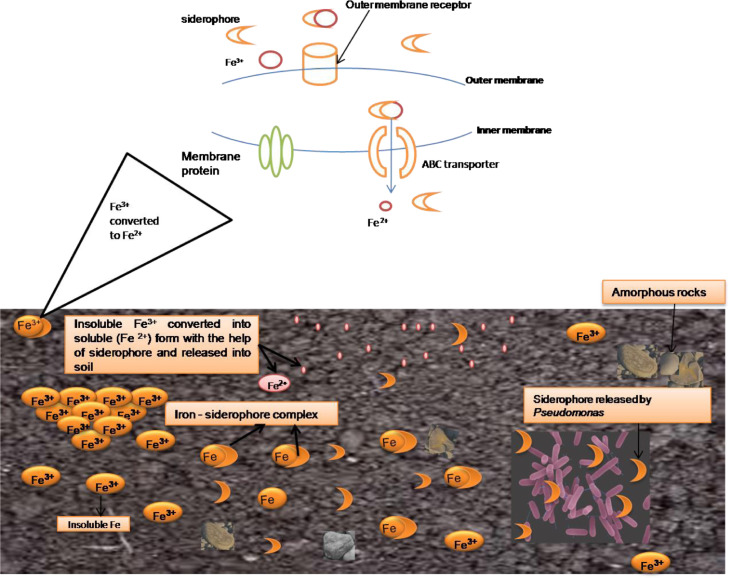
Siderophores are low molecular weight, water-soluble, organic ligands having affinity towards iron-binding compounds or iron carriers (Fig. 5). The fourth most abundant element on the earth is iron (Fe), present as Fe (II) in the native form but, under aerobic conditions, Fe (II) oxidization occurs, leading to its conversion to Fe (III) (Sah et al., 2017a). This form of iron is insoluble, causing iron unavailable for metabolism in living organisms. Siderophores produced majorly by gram-negative bacteria, such as genera of Enterobacter and Pseudomonas, possess significant molecular and physiological indication for biocontrol of rhizospheric phytopathogens (Haas and Défago, 2005). Higher production of heterologous siderophores in the rhizospheric Pseudomonas sp. through Fe (III) chelation indicates the blocking of other microorganisms with less iron affinity in the rhizosphere. Moreover, the binding of Ferric ion in phytopathogens reduces the competition in the rhizosphere (Sah et al., 2017b).
Fig. 5.
Transport of iron in Pseudomonas through production of siderophore depcting the conversion of insolule ferric (Fe+3) to its soluble ferrous (Fe+2) form.
The Pseudomonas aeruginosa strain GRC1 produces hydroxamate siderophores, pyoverdine in an iron-deficit environment (Sah and Singh, 2015). The spectral enhancement of siderophores improves the rhizospheric competence of Pseudomonas sp. B24 in sugar beet using rif (pCUP2) gene, carrying a unit replica of gene pbuA acting as a receptor on Ps114 membrane to provide siderophores (FeIII). Transport of Fe (III) siderophore within the bacterial cell initiates with the Fe-complex binding mediated by specific receptors. The recepter's expression level is regulated by the available iron concentration in the environment. Several ferric pseudobactin receptors encoded by genes in fluorescent Pseudomonas like pupA were sequenced for promoting Fe- complex binding with distinct Pseudomonas strains (Godinho and Bhosle, 2013b). Pyoverdine is a fluorescent siderophore that symbolizes its function in stimulating plant growth. A mutant strain of Pseudomonas aeruginosa with no ability of siderophore production was examined for its biocontrol activity against Pythium sp. in tomato plants showed infection rendering with inactive Pseudomonas aeruginosa siderophore complex (Fig. 6) (Pattnaik et al., 2019). The siderophore integration mechanism indicates the sustainable synthesis at moderate ferric concentrations due to their dependent and independent uptake mechanisms. Siderophores production through various antagonist Pseudomonas strains, upon other microbes through chelation and producing secondary metabolites like IAA, HCN, and 2,4-DAPG for direct antagonistic activating of ISR against the phytopathogen (Meliani et al., 2017). The potential antagonistic rhizospheric phytopathogens of the saprophytic grade possibly consume available iron in the rhizosphere region (Sah et al., 2017a).
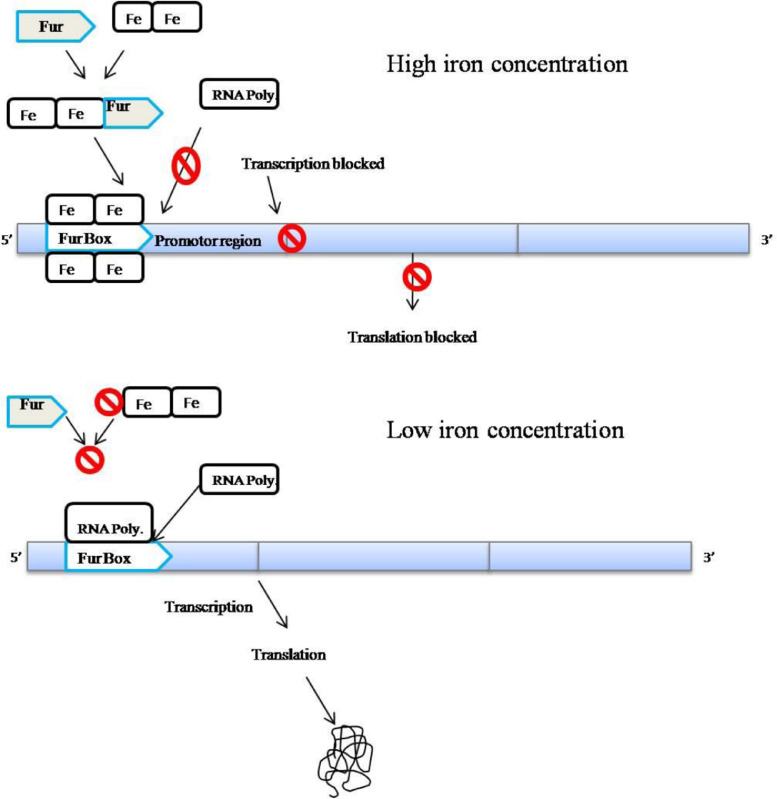
Fig. 6.
Regulation of siderophore product through the Fur Box mechanism and the RNA polymerase mediated actions.
3.2.2. Pseudomonas mediated phosphate solubilization
Phosphorous is available in the rhizosphere at a low level or sometimes unavailable. It occurs in its insoluble phosphate forms that plants cannot uptake and utilize. Most of the soils on the Earth are phosphorous deficient and only 1–5% of phosphorous is available for plants (Cheng et al., 2017). The phosphorous availability is limited due to its rapid fixation followed by oxides production like Calcium hydroxide [Ca (OH)2], Aluminium hydroxide [Al (OH)3], and Ferrous hydroxide [Fe(OH)2]. Availability of phosphorous serves as a limiting factor for the plant growth, certain microbes known as phosphate solubilizing bacteria (PSB), have capability to convert insoluble Phosphorous to soluble. Most PSB such as Pseudomonas, genera Bacilli, Rhizobia, and Azotobacter are industrially very important (Verma et al., 2019b).
The PSB such as Pseudomonas aeruginosa and Pseudomonas fluorescens can produce cyanide as secondary metabolites. This metabolite is crucial as it provides specific benefits to PSB through phytopathogen and disease management. Pseudomonas fluorescens intensify groundnut plant germination by 29–30% with an enhancing >70% grain yield. In order to know the biocontrol potential of Pseudomonas fluorescens against Macrophomina phaseolina, Rodriguez-Navarro et al. (2007) demonstrated that phytopathogen results in a grain yield reduction by half, which was recovered by Pseudomonas fluorescens implying its role in protection against Macrophomia phaseolina. Pseudomonas fluorescens also has characteristics as PSB to improve peanut plant growth with an increased harvest of approximately 20% (dry weight of nodule, N, and phosphorous content in the rhizosphere) (Zabihi et al., 2011). Charcoal rot and collar rot which are rhizobial fungal diseases by Aspergillus niger, observed decreasing after Pseudomonas fluorescens inoculation. Pseudomonas fluorescens associated secondary control activity like ACC deaminase, IAA synthesis, siderophores production, and antifungal compounds synthesis regulates the PGPR. Pseudomonas spp. and PGPR mixture in an inoculum shows coupled activity and forms a mutualistic association aiming to increase plant growth. Pseudomonas putida strains 4, 108, 153, and 169 act as PSB as observed during enhanced cereal production of up to 80–90%. Pseudomonas as a coupled inoculant with arbuscular mycorrhizal (AM) promotes plant growth, mineral uptake (such as nitrogen), harvest index, high soluble phosphate content, and enhanced root colonization capacity under stressful conditions (Zabiha et al., 2011). Competition with AM is highly controlled due to development of regional dominancy of PGPR while AM inside the system regulates the activity within the plant and rhizobial normal microflora. Therefore, PSB can enhance crop yields by approximately 60–70%, enhancing phosphorus solubilization that stimulates plant growth by developing biological nitrogen fixation (Rodríguez et al., 2020).
3.2.3. Pseudomonas mediated nitrogen fixation
Nitrogen is crucial for various biological molecules for constructing some significant proteins and nucleic acid required for life. It is present in the atmosphere in the gaseous form (nitrogen gas, N2) (Adhikari et al., 2021). The occurrence of its inorganic forms like nitrate (NO3−) and ammonium (NH4+) are very restricted in land and water regions responsible for limiting plant and crop growth. Plants provide beneficiary secretions for N2 fixers for the conversion of atmospheric N2 and revert a portion of nitrogen fixed straight for the host in the form of NH4+ (Gage, 2004). The root nodule symbiotic association by nitrogen fixer microbes, during the limited nitrogen conditions, incompetently includes leguminous plants with an increased ability to take up nitrogen (Mia et al., 2013). Nevertheless, utilization of diazotrophic bacteria as initial shows successful attributes of host plants able to fix nitrogen in the close association with their mutualistic diazotroph (Rodríguez et al., 2020). Strain A15, used as inoculum in rice that is ecologically associating with the rice endophyte. Association of Pseudomonas stutzeri strains A1501 (the nif and rnf genes) require the transportation of electrons toward nitrogenase enzyme within Rhodobacter capsulatus running laterally along with the combination of three genes associated with the N2-fixation in Azotobacter vinelandii. The verification of N2-fixation through nif and rnf genes indicating systemic mutagenesis stabilizes these genes in the A1501 strain undergoing N2-fixation (Kumar et al., 2007). The Pseudomonas stutzeri is an N2 fixing microbe analog to Pseudomonas stutzeri DSM4166, isolated from the rhizosphere of Indian grass. These changes in distinct techniques of N2 fixation with no nitrate reduction conversion, denitrification, and consumption of carbohydrate molecules and organic acids in Pseudomonas sp. strains that are non-nitrogen-fixers (Dudeja et al., 2011).
3.3. Pseudomonas in plant disease control management
There is an estimation regarding an enormous loss in food commodity or crop losses every year because of the infections induced via bacterium, fungus, and Nematoda (Siddiqui et al., 2006). Disease in the plants is an influential factor affecting food safety and anthropogenic development over centuries. Efficiency of the plant-growth-promoting-bacteria (PGPB) acting as biocontrol processes holds various benefits upon traditional chemical control methods due to their nontoxic and naturally occurring nature. Another use of PGPB is its distinct array of activities, such as antibiotic secretion, disruption of cell walls, enzyme secretion, rhamnolipid secretion, including initiation of ISR (Kang et al., 2020). Biocontrol describes infection control occurring throughout the growth phases of the plants along with various other steps of food security. Biocontrol studies of the plant pathogens by Rhizospheric bacteria usually focus on infectious microbes. Cereals are the most affected crops by rhizospheric pathogens. An efficient method of disease control through PGPR by farmers was reported effective in different cereals and crops (Oksinska et al., 2011).
Various strains are used as a starter culture for biocontrol in cereals (Table 2). Pseudomonas extensively related group of bacteria that serve as biological control mediators within plants. Several strains of Pseudomonas act as a biocontrol agent like Pseudomonas fluorescens, Pseudomonas putida, Pseudomonas aeruginosa, Pseudomonas syringae produce several antibiotic compounds such as oomycin A, pyrrolnitrin, pyoluteorin, and 2,4-diacetylphloroglucinol. The molecular basis of biological control resistance in species of Pseudomonas indicates synergism among infection reduction to antibiotic compounds secretion as observed in the wheat plant through phytopathogen Gaeumannomyces graminis by antibiotic 2,4-diacetylphloroglucinol that defeat rhizospheric pathogens as its part of the biological control agent (Conrath et al., 2002).
Table 2.
Distribution of Pseudomonas species based on rRNA homology cluster group-I and their role in phyto-pathogen control in association with specific crops.
| S. No. | rRNA homology group-I cluster | Pseudomonas species | Phyto-pathogen species | Crop plants | Disease caused | % enhancement | Refs. |
|---|---|---|---|---|---|---|---|
| 1. | Pseudomonas fluorescens; Pseudomonas fluorescens I; Pseudomonas fluorescens II; Pseudomonas fluorescens III; Pseudomonas fluorescens IV | Pseudomonas fluorescens | Ralstonia solanacearum; Bemisia tabaci | Tomato | Leaf folder pest | Significant suppression of ∼ 60% of diseases | Uppal et al. (2017), Jiménez et al. (2020) |
| Ewingella americana NWU59 | Mung bean | Root rot | |||||
| Fusarium graminearum | Barley | Leaf folder insect | |||||
| Fusarium solani | Lupine | sheath blight disease | |||||
| Fusarium oxysporum | Chickpea | Blast disease | |||||
| Sarocladium oryzae | Rice | Sheath rot; | |||||
| Tobacco mosaic virus | Tobacco | collar rot pathogen | |||||
| 2. | Pseudomonas aeruginosa; Pseudomonas chlororaphis | Pseudomonas aeruginosa | Cucumber mosaic virus CMV | Tomato | |||
| Pseudomonas chlororaphis | Heterodera cajani | Sesame and Wheat | |||||
| 3. | Pseudomonas tolassi | Pseudomonas rhodesiae | Xanthomonas axonopodis | ∼ 12% antibiotic activity with respect to the control | Kumar et al. (2015) | ||
| 4. | Undefined (general) | Pseudomonas sp. | Fusarium graminearum | Cucumber | disease suppression of ∼ 20–35% | Garrido-Sanz et al., (2017) | |
| Fusarium solani | Cucurbit | Powdery mildew | |||||
| Fusarium oxysporum | Tomato | Blossom-end rot | |||||
| Heterodera cajani | Tea | Blister blight disease |
The bacterial isolates inhibit pathogen by different mechanisms such as by secreting antibiotics, toxins, and surface-active compounds (biosurfactants), and production of metabolites that trigger the induction of SAR (Joshi and Chitanand, 2020).The most advanced biocontrol mechanism is suppression of pathogens by secreting siderophores that efficiently sequester iron and deprive the pathogen of this element. Pseudomonas syringae strain CE 01 (genetically modified) also provide protection against frost in various crop plants (Singh et al., 2009).
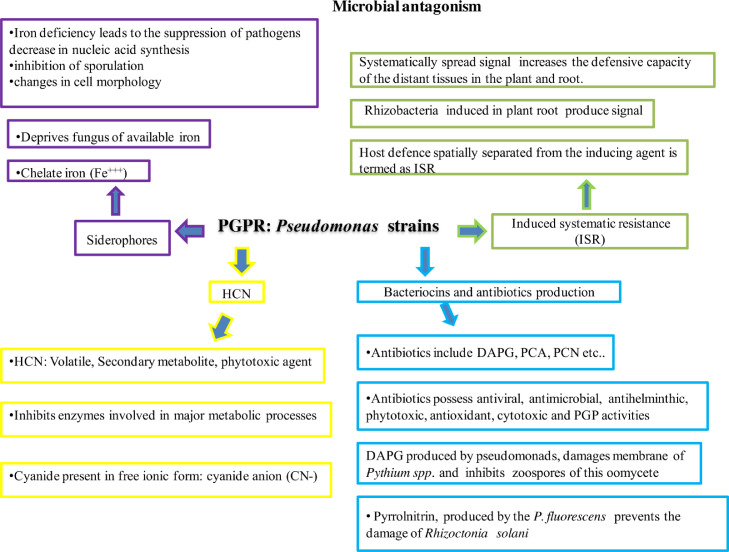
3.3.1. Pseudomonas mediated induced systemic resistance (ISR)
ISR is a pathway of phytopathogen control and management via expression of several underlying signal mechanisms for substantial resistance against various microbial strains (Fig. 7). Inherited diseases in plants stimulate the production of salicylic-dependent signaling cascade increasing the production of SA within local and systemic disease (Vicente and Plasencia, 2011). Salicylic acid plays an important role in establishing systemic acquired resistance as seen in tobacco plants. Salicylic acid-binding protein-2 (SABP2) mediated methyl salicylate esterase activity is essential for the secretion of active defense plant hormones and conversion of salicylic acid from methyl salicylate assists long-distance signal for SAR. A new gene has been identified from the organism Arabidopsis thaliana encoding hydrolases showing identity with SABP2. The genes encoding methyl salicylate esterases were transcriptionally upregulated during infection of Arabidopsis with avirulent Pseudomonas spp. Hence it was concluded that methyl salicylate is a conserved SAR signal in Arabidopsis thaliana and tobacco plant (Vicente and Plasencia, 2011; Vlot et al., 2008). Pseudomonad PGPR isolates also control early blight disease of tomato caused by Alternaria solani through ISR (Rajkumar and Freitas, 2008). ISR consistently provide optimal suppression of phytopathogens and secretion of molecular metabolites (siderophores and phytohormones) that assist in plant-microbe interaction and induced systemic resistance. ISR similarity specifies physiological and biochemical reactions of the host plant, such as synthesis and secretion of defense chemicals like phenolic compounds, peroxidase, and phenylalanine ammonia-lyase (PALase) enzymes (Elena and de Villegas, 2007). ISR-expressing plants can alter aminocyclopropane-1-carboxylate (ACC) which acts as a suppressant against phytopathogens. Signaling compounds like SA and ET play impotant role in inducing and regulating basal resistance (Rajkumar and Freitas, 2008). Decrease in the production of ethylene in several regions of plant and inadequate biosynthetic enzyme, ACC synthase, and oxidase expressions imply the significance of ISR expression in the systemic transduction pathway (Kumar et al., 2015). Various applications of Pseudomonas fluorescens showing ISR activities have been showed to trigger the suppression of pathogens (Elena and de Villegas, 2007) with secretion of metabolites like siderophores and phytohormones to aid plant-microbial interactions in rhizospheric regions (Guerrieri et al., 2020).
Fig. 7.
Pseudomonas mediated antagonism through induced systemic resistance, siderophore production and HNC mediated actions.
3.3.2. Pseudomonas mediated phenazines production
Phenazines infers as the N2 bearing heterocyclic compounds with various antibiotic properties. Phenazines are biologically competent and ecologically active in the Pseudomonas spp (both beneficial and pathogenic strains). Rhizospheric opportunistic pathogen, Pseudomonas aeruginosa, secretes numerous phenazines, like pyocyanin, associated with pathogenicity. Pseudomonas chlororaphis and Pseudomonas fluorescens are root colonizers actively producing phenazine molecules to suppress fungal disease in plants (Guerrieri et al., 2020). The expression of the phz gene (phenazine) determines the root development and responds to the exogenous nutrients. Pseudomonas aeruginosa PNA1, rhizospheric strain of chickpea, exhibits self-biocontrol of phytopathogenic fungi (Fusarium spp. on chickpea/ pigeon pea, Pythium splendens on bean and Pythium myriotylum on cocoyam) (Khan and Parmar, 2013). The mechanism of action of phenazines in antifungal interactions is a due to genetic interactions and signaling mechanism for cell density-dependent regulation. The phzR encodes a transcriptional activator inducing the expression of phenazines in response to phzI production. Numerous Pseudomonas strains are sold commercially as biocontrol agents due to their self-regulation capabilities in the seed treatment to treat the seed-borne pathogens through phenazine production by Pseudomonas chlororaphis (Pham et al., 2017).
3.3.3. Pseudomonas mediated antibiotics production
Antibiotics are the secondary metabolites used as a natural control of plant-based pathogens and unwanted microbes by blocking the pathogen growth or killing it. Antibiosis through antibiotics and bacteriocins signifies the well-known biocontrol mechanism (Singh et al., 2005). Fluorescent pseudomonads exhibit a unique system of antibiosis opposing root-pathogenic microbes such as Pseudomonas fluorescens inhibit various rhizobacterial microbes by producing various antibiotics on roots in the rhizosphere. Phenazine compounds are chemical siblings of Pseudomonas aeruginosa whereas appropriate phrase will be "Phenazine derivatives like 2,4 diacetyl phloroglucinol (DAPG) that secreted from Pseudomonas aeruginosa specifically get activated against the Triticum aestivum pathogens (Ahemad and Khan, 2010). These classes of antibiotics are nonvolatile in nature secreted through Pseudomonas fluorescens, broad spectrum of action upon pathogenic microorganisms. Active suppression by antibiotic gene expression through Pseudomonas fluorescens comprises transmitting the signaling compounds like N-acyl-homoserine lactones (AHL). The compound 2, 4-DAPG is also observed as the black root rot of tobacco suppressor through Pseudomonas spp. in rhizospheric soil (Rodríguez et al., 2020). The mechanism of self-biocontrol initiated through DAPG biosynthesis with diffusible signal to induce DAPG biosynthetic genes and its expression (Shahid et al., 2017). The strain migula F113 of Pseudomonas fluorescens shows the control of soft rot disease in potato plants through DAPG production. A mutant with rifampicin resistance, Pseudomonas fluorescens strain NBRI1303R, observed to control phytopathogen through rapid root colonization. These types of mutations are found to be beneficial for the development of more effective biocontrol agents (Singh et al., 2014).
4. Conclusion
Pseudomonas species are notable colonizers residing either as a part of local or exotic microflora within the rhizosphere holding an extensive significance in soil health improvement, sustainable agricultural practices, and maintenance of rhizospheric microbial diversity. Various factors such as soil pH, temperature, water holding capacity, nutrient availability, oxygenic conditions, and soil type determine the growth of this essential rhizospheric Pseudomonas (Lami et al., 2020). Various fluorescent and non-fluorescent strains of the genus Pseudomonas have been rearranged by modern molecular classification and are kept in the RNA homology group-I. Applications of the Pseudomonas species are extensively used in numerous industrial sectors such as food, pharmaceutical, ecological. Several species of fluorescent Pseudomonas acts as PGPR possess properties as both provide plant growth regulation and disease resistance. Properties of Pseudomonas in providing plant growth include nitrogen fixation, siderophore production, and phosphate solubilization while in disease resistance includes induced systemic resistance (ISR), phenazine production, and antibiotic production (Desnoues et al., 2003). The increase in siderophore and secondary metabolite production, phosphate solubilization and chemotactic responses between the host plant crop and the Pseudomonas species triggers the colonization process leading to enhanced growth in the host plant. Various chemically mediated feedback mechanisms are also involved in proper growth regulation of plant by Pseudomonas sp. (Rodríguez et al., 2020). The overall vision of this review is based on the importance of Pseudomonas in food security and safe agricultural practices. Prospects supporting the transgenic crop production and technologies based on their generation are yet to be optimized, until now bioavailability of minerals in crops via microbial intervention are giving positive results.
CRediT authorship contribution statement
Stuti Sah: Resources, Writing – original draft. Shweena Krishnani: . Rajni Singh: Conceptualization, Visualization, Writing – original draft, Writing – review & editing.
CRediT authorship contribution statement
Stuti Sah: Resources, Writing – original draft. Shweena Krishnani: Writing – original draft. Rajni Singh: Conceptualization, Visualization, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
None.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Adhikari P., Jain R., Sharma A., Pandey A. Plant growth promotion at low temperature by phosphate-solubilizing Pseudomonas Spp. Isolated from high-altitude himalayan soil. Microb. Ecol. 2021:1–11. doi: 10.1007/s00248-021-01702-1. [DOI] [PubMed] [Google Scholar]
- Ahemad M., Khan M.S. Phosphate-solubilizing and plant-growth-promoting Pseudomonas aeruginosa PS1 improves greengram performance in quizalafop-p-ethyl and clodinafop amended soil. Arch Environ. Contam. Toxicol. 2010;58:361–372. doi: 10.1007/s00244-009-9382-z. [DOI] [PubMed] [Google Scholar]
- Albareda M., Dardanelli M.S., Sousa C., Megías M., Temprano F., Rodríguez-Navarro D.N. Factors affecting the attachment of rhizospheric bacteria to bean and soybean roots. FEMS Microbiol. Lett. 2006;259:67–73. doi: 10.1111/j.1574-6968.2006.00244.x. [DOI] [PubMed] [Google Scholar]
- Ashrafuzzaman M., Hossen F.A., Ismail M.R., Hoque A., Islam M.Z., Shahidullah S.M., Meon S. Efficiency of plant growth-promoting rhizobacteria (PGPR) for the enhancement of rice growth. Afr. J. Biotechnol. 2009;8:1247–1252. ISSN 1684–5315. [Google Scholar]
- Berg G., Smalla K. Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere. FEMS Microbiol. Ecol. 2009;68:1–13. doi: 10.1111/j.1574-6941.2009.00654.x. [DOI] [PubMed] [Google Scholar]
- Bhadauria S., Sengar R.M.S., Mohan D., Singh C., Kushwah B.S. Sustainable land use planning through utilization of alkaline wasteland by biotechnological intervention. Am. Eurasian J. Agric. Environ. Sci. 2010;9:325–337. ISSN 1818-6769. [Google Scholar]
- Blagodatskaya Е., Kuzyakov Y. Mechanisms of real and apparent priming effects and their dependence on soil microbial biomass and community structure: critical review. Biol. Fertil. Soils. 2008;45:115–131. doi: 10.1007/s00374-008-0334-y. [DOI] [Google Scholar]
- Bonilla A., Sarria A.L.F., Algar E., Ledesma F.M., Solano B.R., Fernandes J.B., Mañero F.G. Microbe associated molecular patterns from rhizosphere bacteria trigger germination and Papaver somniferum metabolism under greenhouse conditions. Plant Physiol. Biochem. 2014;74:133–140. doi: 10.1016/j.plaphy.2013.11.012. [DOI] [PubMed] [Google Scholar]
- Bouaichi A., Benkirane R., El-kinany S., Habbadi K., Lougraimzi H., Sadik S., Benbouazza A., Achbani E.H. Potential effect of antagonistic bacteria in the management of olive knot disease caused by Pseudomonas savastanoi pv. savastanoi. J. Microbiol. Biotechnol. Food Sci. 2021;2021:1035–1040. doi: 10.15414/jmbfs.2019.8.4.1035-1040. [DOI] [Google Scholar]
- Cheng Xu, Etalo DW., Mortel JE., Dekkers E., Nguyen L., Medema MH., Raaijmakers JM. Genome-wide analysis of bacterial determinants of plant growth promotion and induced systemic resistance by Pseudomonas fluorescens. Environ. Microbiol. 2017;19(no. 11):4638–4656. doi: 10.1111/1462-2920.13927. [DOI] [PubMed] [Google Scholar]
- Chu T.N., Tran B.T.H., Hoang M.T.T. Plant growth-promoting rhizobacterium Pseudomonas PS01 induces salt tolerance in Arabidopsis thaliana. BMC Res. Notes. 2019;12:1–7. doi: 10.1186/s13104-019-4046-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combes-Meynet E., Pothier J.F., Moënne-Loccoz Y., Prigent-Combaret C. The Pseudomonas secondary metabolite 2, 4-diacetylphloroglucinol is a signal inducing rhizoplane expression of Azospirillum genes involved in plant-growth promotion. Mol. Plant Microb. Interact. 2011;24:271–284. doi: 10.1094/MPMI-07-10-0148. [DOI] [PubMed] [Google Scholar]
- Conrath U., Pieterse C.M., Mauch-Mani B. Priming in plant–pathogen interactions. Trends Plant Sci. 2002;7:210–216. doi: 10.1016/S1360-1385(02)02244-6. [DOI] [PubMed] [Google Scholar]
- Costacurta A, Vanderleyden J. Synthesis of phytohormones by plant-associated bacteria. Crit Rev Microbiol . 1995;21(1):1–18. doi: 10.3109/10408419509113531. [DOI] [PubMed] [Google Scholar]
- Costa-Gutierrez S.B., Lami M.J., Caram-Di Santo M.C., Zenoff A.M., Vincent P.A., Molina-Henares M.A., Espinosa-Urgel M., de Cristóbal R.E. Plant growth promotion by Pseudomonas putida KT2440 under saline stress: role of eptA. Appl. Microbiol. Biotechnol. 2020;104:4577–4592. doi: 10.1007/s00253-020-10516-z. [DOI] [PubMed] [Google Scholar]
- Curtis T.P., Sloan W.T. Prokaryotic diversity and its limits: microbial community structure in nature and implications for microbial ecology. Curr. Opin. Microbiol. 2004;7:221–226. doi: 10.1016/j.mib.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Dashti N.H., Ali N.Y., Cherian V.M., Montasser M.S. Application of plant growth-promoting rhizobacteria (PGPR) in combination with a mild strain of Cucumber mosaic virus (CMV) associated with viral satellite RNAs to enhance growth and protection against a virulent strain of CMV in tomato. Can. J. Plant Pathol. 2012;34:177–186. doi: 10.1080/07060661.2012.685495. [DOI] [Google Scholar]
- del Rosario Cappellari L., Santoro M.V., Nievas F., Giordano W., Banchio E. Increase of secondary metabolite content in marigold by inoculation with plant growth-promoting rhizobacteria. Appl. Soil Ecol. 2013;70:16–22. doi: 10.1016/j.apsoil.2013.04.001. [DOI] [Google Scholar]
- Desnoues N., Lin M., Guo X., Ma L., Carreño-Lopez R., Elmerich C. Nitrogen fixation genetics and regulation in a Pseudomonas stutzeri strain associated with rice. Microbiol. 2003;149:2251–2262. doi: 10.1099/mic.0.26270-0. [DOI] [PubMed] [Google Scholar]
- Dudeja S.S., Singh N.P., Sharma P., Gupta S.C., Chandra R., Dhar B., Bansal R.K., Brahmaprakash G.P., Potdukhe S.R., Gundappagol R.C., Gaikawad B.G. Bioaugmentation, Biostimulation and Biocontrol. Springer; Berlin, Heidelberg: 2011. Biofertilizer technology and pulse production; pp. 43–63. [DOI] [Google Scholar]
- Elena M., de Villegas D. Microbial Siderophores. Springer; Berlin, Heidelberg: 2007. Biotechnological production of siderophores; pp. 219–231. [DOI] [Google Scholar]
- Forney L.J., Zhou X., Brown C.J. Molecular microbial ecology: land of the one-eyed king. Curr. Opin. Microbiol. 2004;7:210–220. doi: 10.1016/j.mib.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Gage D.J. Infection and invasion of roots by symbiotic, nitrogen-fixing rhizobia during nodulation of temperate legumes. Microbiol. Mol. Biol. Rev. 2004;68:280–300. doi: 10.1128/MMBR.68.2.280-300.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamez R., Cardinale M., Montes M., Ramirez S., Schnell S., Rodriguez F. Screening, plant growth promotion and root colonization pattern of two rhizobacteria (Pseudomonas fluorescens Ps006 and Bacillus amyloliquefaciens Bs006) on banana cv. Williams (Musa acuminata Colla) Microbiol. Res. 2019;220:12–20. doi: 10.1016/j.micres.2018.11.006. [DOI] [PubMed] [Google Scholar]
- García-Seco D., Bonilla A., Algar E., García-Villaraco A., Mañero J.G., Ramos-Solano B. Enhanced blackberry production using Pseudomonas fluorescens as elicitor. Agron. Sustain. Dev. 2013;33:385–392. doi: 10.1007/s13593-012-0103-z. [DOI] [Google Scholar]
- Garrido-Sanz D., Arrebola E., Martínez-Granero F., García-Méndez S., Muriel C., Blanco-Romero E., Martín M., Rivilla R., Redondo-Nieto M. Classification of isolates from the Pseudomonas fluorescens complex into phylogenomic groups based in group-specific markers. Front. Microbiol. 2017;8:413. doi: 10.3389/fmicb.2017.00413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George R., Bahadur N., Singh N., Singh R., Verma A., Shukla A.K. Environmentally benign TiO2 nanomaterials for removal of heavy metal ions with interfering ions present in tap water. Mater. Today Proc. 2016;3:162–166. doi: 10.1016/j.matpr.2016.01.051. [DOI] [Google Scholar]
- Godinho A., Bhosle S. Bacteria in agrobiology: Crop productivity. Springer; Berlin, Heidelberg: 2013. Rhizosphere bacteria from coastal sand dunes and their applications in agriculture; pp. 77–96. [DOI] [Google Scholar]
- Godinho A., Bhosle S. Bacteria in agrobiology: Crop productivity. Springer; Berlin, Heidelberg: 2013. Rhizosphere bacteria from coastal sand dunes and their applications in agriculture; pp. 77–96. [DOI] [Google Scholar]
- Gray E.J., Smith D.L. Intracellular and extracellular PGPR: commonalities and distinctions in the plant–bacterium signaling processes. Soil Biol. Biochem. 2005;37:395–412. doi: 10.1016/j.soilbio.2004.08.030. [DOI] [Google Scholar]
- Guerrieri M.C., Fanfoni E., Fiorini A., Trevisan M., Puglisi E. Isolation and screening of extracellular PGPR from the rhizosphere of tomato plants after long-term reduced tillage and cover crops. Plants. 2020;9:668. doi: 10.3390/plants9050668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas D., Défago G. Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat. Rev. Microbiol. 2005;3:307–319. doi: 10.1038/nrmicro1129. [DOI] [PubMed] [Google Scholar]
- Hou M.P., Oluranti B.O. Evaluation of plant growth promoting potential of four rhizobacterial species for indigenous system. J. Cent. South Univ. 2013;20:164–171. doi: 10.1007/s11771-013-1472-4. [DOI] [Google Scholar]
- Jaleel C.A., Manivannan P., Sankar B., Kishorekumar A., Gopi R., Somasundaram R., Panneerselvam R. Pseudomonas fluorescens enhances biomass yield and ajmalicine production in Catharanthus roseus under water deficit stress. Colloids Surf. B. 2007;60:7–11. doi: 10.1016/j.colsurfb.2007.05.012. [DOI] [PubMed] [Google Scholar]
- Jiménez J.A., Novinscak A., Filion M. Pseudomonas fluorescens LBUM677 differentially increases plant biomass, total oil content and lipid composition in three oilseed crops. J. Appl. Microbial. 2020;128:1119–1127. doi: 10.1111/jam.14536. [DOI] [PubMed] [Google Scholar]
- Joshi A., Chitanand M. Complete genome sequence of plant growth promoting Pseudomonas aeruginosa AJ D 2 an isolate from monocropic cotton rhizosphere. Genomics. 2020;112:1318. doi: 10.1016/j.ygeno.2019.07.022. [DOI] [PubMed] [Google Scholar]
- Kang S.M., Asaf S., Khan A.L., Khan A., Mun B.G., Khan M.A., Gul H., Lee I.J. Complete genome sequence of Pseudomonas psychrotolerans CS51, a plant growth-promoting bacterium, under heavy metal stress conditions. Microorganisms. 2020;8:382. doi: 10.3390/microorganisms8030382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan H., Parmar N. Bacteria in Agrobiology: Crop Productivity. Springer; Berlin, Heidelberg: 2013. Bioinoculants: understanding chickpea rhizobia in providing sustainable agriculture; pp. 185–215. [DOI] [Google Scholar]
- Khan M.S., Ahmad E., Zaidi A., Oves M. Bacteria in Agrobiology: Crop Productivity. Springer; Berlin, Heidelberg: 2013. Functional aspect of phosphate-solubilizing bacteria: importance in crop production; pp. 237–263. [DOI] [Google Scholar]
- Kour D., Rana K.L., Sheikh I., Kumar V., Yadav A.N., Dhaliwal H.S., Saxena A.K. Proceedings of the National Academy of Sciences, India Section B - Biological Sciences. 2019. Alleviation of drought stress and plant growth promotion by Pseudomonas libanensis EU-LWNA-33, a drought-adaptive phosphorus-solubilizing bacterium; pp. 1–11. [DOI] [Google Scholar]
- Kumar A., Bahadur I., Maurya B.R., Raghuwanshi R., Meena V.S., Singh D.K., Dixit J. Does a plant growth-promoting rhizobacteria enhance agricultural sustainability? J. Pure Appl. Microbiol. 2015;9:715–724. [Google Scholar]
- Kumar B., Trivedi P., Pandey A. Pseudomonas corrugata: a suitable bacterial inoculant for maize grown under rainfed conditions of Himalayan region. Soil Biol. Biochem. 2007;39:3093–3100. doi: 10.1016/j.soilbio.2007.07.003. [DOI] [Google Scholar]
- Lami M.J., Adler C., Caram-Di Santo M.C., Zenoff A.M., de Cristóbal R.E., Espinosa-Urgel M., Vincent P.A. Pseudomonas stutzeri MJL19, a rhizosphere-colonizing bacterium that promotes plant growth under saline stress. J. Appl. Microbial. 2020;129:1321–1336. doi: 10.1111/jam.14692. [DOI] [PubMed] [Google Scholar]
- Meliani A., Bensoltane A., Benidire L., Oufdou K. Plant growth-promotion and IAA secretion with Pseudomonas fluorescens and Pseudomonas putida. Res. Rev. J. Bot. Sci. 2017;6:16–24. p-ISSN:2347-2308. [Google Scholar]
- Mhlongo Msizi I., Piater Lizelle A., Madala Ntakadzeni E., Labuschagne Nico, Dubery Ian A. The Chemistry of Plant–Microbe Interactions in the Rhizosphere and the Potential for Metabolomics to Reveal Signaling Related to Defense Priming and Induced Systemic Resistance. Front. Plant Sci. 2018;2 doi: 10.3389/fpls.2018.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mia M.A.B., Hossain M.M., Shamsuddin Z.H., Islam M.T. Plant-associated bacteria in nitrogen nutrition in crops, with special reference to rice and banana. Bact. Agrobiol. Crop Product. 2013:97–126. doi: 10.1007/978-3-642-37241-4_5. [DOI] [Google Scholar]
- Mia M.B., Shamsuddin Z.H. Nitrogen Fixation and Transportation by Rhizobacteria. Int. J. Bot. 2010;6:235–242. ISSN 1811-9700. [Google Scholar]
- Oksinska M.P., Wright S.A., Pietr S.J. Colonization of wheat seedlings (Triticum aestivum L.) by strains of Pseudomonas spp. with respect to their nutrient utilization profiles. Eur. J. Soil Biol. 2011;47:364–373. doi: 10.1016/j.ejsobi.2011.08.005. [DOI] [Google Scholar]
- Palmgren M.G., Edenbrandt A.K., Vedel S.E., Andersen M.M., Landes X., Østerberg J.T., Falhof J., Olsen L.I., Christensen S.B., Sandøe P., Gamborg C. Are we ready for back-to-nature crop breeding. Trends Plant Sci. 2015;20:155–164. doi: 10.1016/j.tplants.2014.11.003. [DOI] [PubMed] [Google Scholar]
- Pattnaik S., Mohapatra B., Kumar U., Pattnaik M., Samantaray D. Microbe-mediated plant growth promotion: a mechanistic overview on cultivable plant growth-promoting members. Biofertil. Sustain. Agric. Environ. 2019:435–463. doi: 10.1007/978-3-030-18933-4_20. [DOI] [Google Scholar]
- Pérez-Montaño F., Alías-Villegas C., Bellogín R.A., Del Cerro P., Espuny M.R., Jiménez-Guerrero I., López-Baena F.J., Ollero F.J., Cubo T. Plant growth promotion in cereal and leguminous agricultural important plants: from microorganism capacities to crop production. Microbiol. Res. 2014;169:325–336. doi: 10.1016/j.micres.2013.09.011. [DOI] [PubMed] [Google Scholar]
- Pham V.T., Rediers H., Ghequire M.G., Nguyen H.H., De Mot R., Vanderleyden J., Spaepen S. The plant growth-promoting effect of the nitrogen-fixing endophyte Pseudomonas stutzeri A15. Arch. Microbiol. 2017;199:513–517. doi: 10.1007/s00203-016-1332-3. [DOI] [PubMed] [Google Scholar]
- Qessaoui R., Bouharroud R., Furze J.N., El Aalaoui M., Akroud H., Amarraque A., Van Vaerenbergh J., Tahzima R., Mayad E.H., Chebli B. Applications of new rhizobacteria Pseudomonas isolates in agroecology via fundamental processes complementing plant growth. Sci. Rep. 2019;9:1–10. doi: 10.1038/s41598-019-49216-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkumar M., Freitas H. Influence of metal resistant-plant growth-promoting bacteria on the growth of Ricinus communis in soil contaminated with heavy metals. Chemosphere. 2008;71:834–842. doi: 10.1016/j.chemosphere.2007.11.038. [DOI] [PubMed] [Google Scholar]
- Rivas-San Vicente M., Plasencia J. Salicylic acid beyond defence: its role in plant growth and development. J. Exp. Bot. 2011;62(10):3321–3338. doi: 10.1093/jxb/err031. [DOI] [PubMed] [Google Scholar]
- Rodríguez M., Torres M., Blanco L., Béjar V., Sampedro I., Llamas I. Plant growth-promoting activity and quorum quenching-mediated biocontrol of bacterial phytopathogens by Pseudomonas segetis strain P6. Sci. Rep. 2020;10:1–12. doi: 10.1038/s41598-020-61084-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Navarro D.N., Dardanelli M.S., Ruíz-Saínz J.E. Attachment of bacteria to the roots of higher plants. FEMS Microbiol. Lett. 2007;272:127–136. doi: 10.1111/j.1574-6968.2007.00761.x. [DOI] [PubMed] [Google Scholar]
- Sah S., Singh R. Siderophore: structural and functional characterization-a comprehensive review. Agriculture. 2015;61:97. doi: 10.1515/agri-2015-0015. [DOI] [Google Scholar]
- Sah S., Singh R. Phylogenetical coherence of Pseudomonas in unexplored soils of Himalayan region. 3 Biotech. 2016;6(2):1–10. doi: 10.1007/s13205-016-0493-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah S., Sahgal M., Singh R. Concomitant ability of siderophore against iron paucity and fusarium wilt in lycopersicon esculentum. Biosci. Biotechnol. Res. Asia. 2017;14:319–328. doi: 10.13005/bbra/2449. [DOI] [Google Scholar]
- Sah S., Singh N., Singh R. Iron acquisition in maize (Zea mays L.) using Pseudomonas siderophore. 3 Biotech. 2017;7:1–7. doi: 10.1007/s13205-017-0772-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saharan B.S., Nehra V. Plant growth promoting rhizobacteria: a critical review. Life Sci. Med. Res. 2011;21:30. doi: 10.1094/PHP-2002-0510-01-RV. [DOI] [Google Scholar]
- Shahid I., Rizwan M., Baig D.N., Saleem R.S., Malik K.A., Mehnaz S. Secondary metabolites production and plant growth promotion by Pseudomonas chlororaphis and P. aurantiaca strains isolated from cactus, cotton, and para grass. J. Microbiol. Biotechnol. 2017;27:480–491. doi: 10.4014/jmb.1601.01021. [DOI] [PubMed] [Google Scholar]
- Sharma A., Shankhdhar D., Shankhdhar S.C. Enhancing grain iron content of rice by the application of plant growth promoting rhizobacteria. Plant Soil Environ. 2013;590:89–94. doi: 10.17221/683/2012-PSE. [DOI] [Google Scholar]
- Shen M., Kang Y.J., Wang H.L., Zhang X.S., Zhao Q.X. Effect of plant growth-promoting rhizobacteria (PGPRs) on plant growth, yield, and quality of tomato (Lycopersicon esculentum Mill.) under simulated seawater irrigation. J. Gen. Appl. Microbiol. 2012;58:253–262. doi: 10.2323/jgam.58.253. [DOI] [PubMed] [Google Scholar]
- Siddiqui I.A., Shaukat S.S., Sheikh I.H., Khan A. Role of cyanide production by Pseudomonas fluorescens CHA0 in the suppression of root-knot nematode, Meloidogyne javanica in tomato. World J. Microbiol. Biotechnol. 2006;22:641–650. doi: 10.1007/s11274-005-9084-2. [DOI] [Google Scholar]
- Singh M., Nagar R.S.D. In vitro compatibility of Pseudomonas fluorescens with different systemic fungicides. J. Pharma. Phytochem. 2021;10:735–738. doi: 10.22271/tpi.2021.v10.i3l.5900. [DOI] [Google Scholar]
- Singh P.B., Sharma S., Saini H.S., Chadha B.S. Biosurfactant production by Pseudomonas sp. and its role in aqueous phase partitioning and biodegradation of chlorpyrifos. Lett. Appl. Microbiol. 2009;49:378–383. doi: 10.1111/j.1472-765X.2009.02672.x. [DOI] [PubMed] [Google Scholar]
- Singh R., Jain A., Panwar S., Gupta D., Khare S.K. Antimicrobial activity of some natural dyes. Dyes Pigment. 2005;66:99–102. doi: 10.1016/j.dyepig.2004.09.005. [DOI] [Google Scholar]
- Singh R., Smitha M.S., Singh S.P. The role of nanotechnology in combating multi-drug resistant bacteria. J. Nanosci. Nanotechnol. 2014;14:4745–4756. doi: 10.1166/jnn.2014.9527. [DOI] [PubMed] [Google Scholar]
- Sundar H., Mohan S.S., Sornam A., Sivanandam G., Govindan C. Effects of three strains of Pseudomonas fluorescens to soil-borne fungal pathogens and silkworm, Bombyx mori. Int. J. Trop. Insect Sci. 2021:1–8. doi: 10.1007/s42690-021-00506-7. [DOI] [Google Scholar]
- Tang G., Tian Y., Niu J., Tang J., Yang J., Gao Y., Chen X., Li X., Wang H., Cao Y. Development of carrier-free self-assembled nanoparticles based on fenhexamid and polyhexamethylene biguanide for sustainable plant disease management. Green Chem. 2021;23:2531–2540. doi: 10.1039/D1GC00006C. [DOI] [Google Scholar]
- Tang J., Tang G., Niu J., Yang J., Zhou Z., Gao Y., Chen X., Tian Y., Li Y., Li J., Cao Y. Preparation of a porphyrin metal–organic framework with desirable photodynamic antimicrobial activity for sustainable plant disease management. J. Agric. Food Chem. 2021;69:2382–2391. doi: 10.1021/acs.jafc.0c06487. [DOI] [PubMed] [Google Scholar]
- Uppal, H., Kayal, N., Chawla, S., Tripathy, S.S., Gupta, S., Singh, R., Sharma, B. and Singh, N., 2017. Surface modified alumina compact: a potential material for decontamination of trivalent and hexavalent chromium and growth inhibitor of microbes from water. 10.5185/amlett.2017.6475.
- Verma M., Mishra J., Arora N.K. Environmental biotechnology: For Sustainable Future. Springer; Singapore: 2019. Plant growth-promoting rhizobacteria: diversity and applications; pp. 129–173. [DOI] [Google Scholar]
- Verma M., Mishra J., Arora N.K. Environmental biotechnology: For Sustainable Future. Springer; Singapore: 2019. Plant growth-promoting rhizobacteria: diversity and applications; pp. 129–173. [DOI] [Google Scholar]
- Vlot A.C., Liu P.P., Cameron R.K., Park S.W., Yang Y., Kumar D., Zhou F., Padukkavidana T., Gustafsson C., Pichersky E., Klessig D.F. Identification of likely orthologs of tobacco salicylic acid-binding protein 2 and their role in systemic acquired resistance in Arabidopsis thaliana. Plant J. 2008;56(3):445–456. doi: 10.1111/j.1365-313X.2008.03618.x. [DOI] [PubMed] [Google Scholar]
- Yan Y., Yang J., Dou Y., Chen M., Ping S., Peng J., Lu W., Zhang W., Yao Z., Li H., Liu W. Nitrogen fixation island and rhizosphere competence traits in the genome of root-associated Pseudomonas stutzeri A1501. Proc. Natl Acad. Sci. 2008;105:7564–7569. doi: 10.1073/pnas.0801093105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Yuan M., Lu W., Yang J., Dai S., Li Q., Yang Z., Dong J., Sun L., Deng Z., Zhang W. Complete genome sequence of the nitrogen-fixing and rhizosphere-associated bacterium Pseudomonas stutzeri strain DSM4166. J. Bacterial. 2011;193:3422. doi: 10.1128/JB.05039-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabihi H.R., Savaghebi G.R., Khavazi K., Ganjali A., Miransari M. Pseudomonas bacteria and phosphorous fertilization, affecting wheat (Triticumaestivum L.) yield and P uptake under greenhouse and field conditions. Acta Physiol. Plant. 2011;33:145–152. doi: 10.1007/s11738-010-0531-9. [DOI] [Google Scholar]
- Zhang L., Chen W., Jiang Q., Fei Z., Xiao M. Genome analysis of plant growth-promoting rhizobacterium Pseudomonas chlororaphis subsp. aurantiaca JD37 and insights from comparasion of genomics with three Pseudomonas strains. Microbiol. Res. 2020;237 doi: 10.1016/j.micres.2020.126483. [DOI] [PubMed] [Google Scholar]
- Zhuang L., Li Y., Wang Z., Yu Y., Zhang N., Yang C., Zeng Q., Wang Q. Synthetic community with six Pseudomonas strains screened from garlic rhizosphere microbiome promotes plant growth. Micro Biotechnol. 2021;14:488–502. doi: 10.1111/1751-7915.13640. [DOI] [PMC free article] [PubMed] [Google Scholar]