Abstract
Purpose
To underscore the importance of histopathological evaluation in cases presenting with a constellation of unusual ocular inflammation and physical findings.
Observation
A 51-year-old male, presented with a chief complaint of worsening visual field loss due to droopy eyelids two months post excision of a right upper eyelid squamous cell carcinoma. His past medical history included chronic edematous facial features, chronic sinusitis, unexplained peripheral neuropathy, and worsening fatigue. Pre-blepharoplasty work-up revealed mechanical ptosis from lid edema, madarosis, a concave nasal bridge, pancytopenia, and numerous burn marks due to inadvertent injuries. Bilateral blepharoplasty was performed, and the excised tissue submitted for histopathological evaluation that revealed non-caseating granulomatous perineural inflammation with numerous acid-fast bacilli in dermal layers and nerves. These findings prompted a diagnosis of lepromatous leprosy with suspected bone marrow involvement. The source of the infection was unknown. The blepharoplasty restored his visual fields and multi-drug therapy (MDT) improved his general health and wellbeing with concomitant reductions of pancytopenia, fatigue, and facial edema.
Conclusions and importance
Biopsy histopathology, in patients with longstanding ocular adnexal inflammation, can facilitate diagnosis and treatment. To the authors’ knowledge, this is an unusual ocular leprosy presentation and represents the first leprosy case diagnosed via blepharoplasty.
Keywords: Acid-fast bacillus, Eye, Hansen's disease, Mycobacterium leprae, Neuropathy, Pancytopenia
1. Introduction
Leprosy, also known as Hansen's disease, is a chronic bacterial infection caused by Mycobacterium leprae (M. leprae).1,2 Since its introduction into the United States,1 the number of new Hansen's disease cases has remained around 140 per year.2 The mode of transmission is not clear (possibly airborne droplets or prolonged contact with an untreated person), and it continues to spread resulting in about 200,000 new cases each year worldwide.2,3 Leprosy typically affects the integumentary system, the nervous system, the nasal mucosa, and the eyes.2 The eyes are targeted due to the cooler environmental preference of M. leprae.4,5 Ocular manifestations in leprosy patients are estimated to be 70–75%; 10–50% have severe ocular symptoms and 5% become blind.4 Ocular manifestations can range from adnexal involvement resulting in lagophthalmos, orbicularis oculi weakness, ectropion, entropion, trichiasis, loss of eyebrows, and blepharoptosis, to anterior segment involvement resulting in impaired corneal sensation, corneal ulceration, corneal opacity, and cataracts, to inflammatory changes producing episcleritis, scleritis, iridocyclitis, iris atrophy, uveitis, as well as other symptoms.4, 5, 6, 7, 8 We present a patient who complained of vision loss due to bilateral droopy eyelids. The bilateral ptosis presentation alone was unusual, but the persistent clinical manifestations, acid-fast bacilli histopathology post blepharoplasty, and treatment response were consistent with lepromatous leprosy.
2. Case report
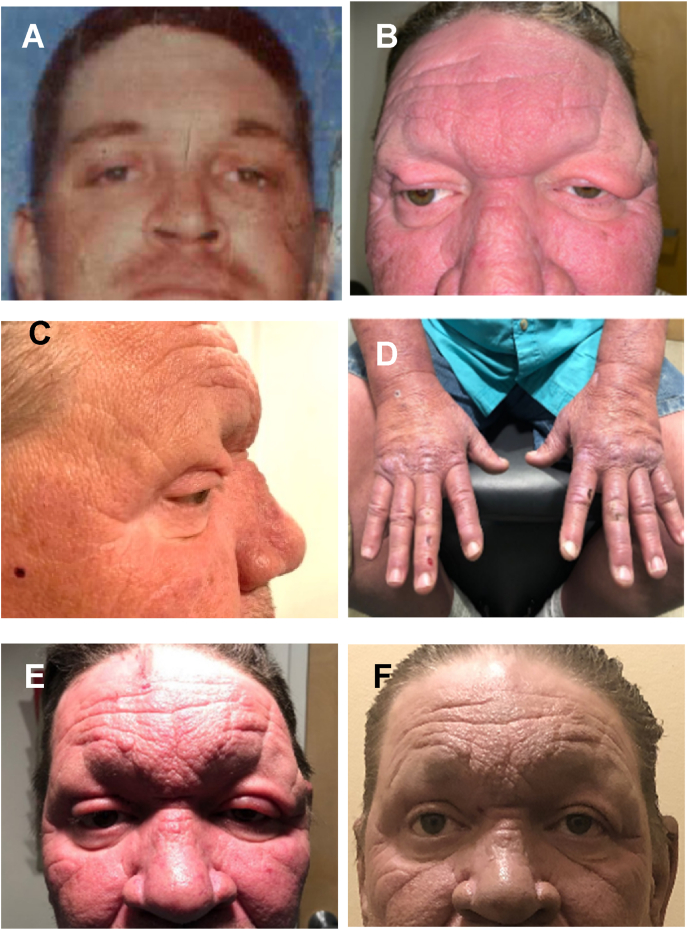
A 51-year-old male was referred to our oculoplastics service with a 1-2-year history of worsening bilateral visual field loss due to droopy eyelids. His immediate past medical history was significant for squamous cell carcinoma of the right upper eyelid excised 2 months earlier. His past medical history included hypertension, obesity, alcohol dependence, tobacco use, unexplained peripheral neuropathy and fatigue, chronic sinusitis, polyarthralgia, and a 40-pound unintentional weight loss over the past 2 years. His external physical exam upon presentation was significant for coarse facial features, facial edema, redundant eyelid skin, thickened forehead and eyelid skin, madarosis and a concave nasal bridge that had developed over the last 25 years (Fig. 1A–C). A right upper eyelid scar was noted consistent the carcinoma excision. He recounted no personal or family history of ocular disease, diabetes, cancer, or immunosuppression. He denied international travel, but his spouse of 15 years is a Naval Commanding Officer and served overseas as needed. Currently unemployed, he previously worked as a farmer, welder, and mechanic. As a welder, he reports suffering from numerous inadvertent burn injuries to his extremities due to his inability to perceive pain or changes in temperature. Numerous burn injury scars on his extremities and overall body swelling and erythema were noted (Fig. 1D) as well as a marked decrease in sensation to both pinprick and dull touch in his upper and lower extremities but normal reflexes and strength. A previous evaluation had concluded the neuropathy was likely the result of a “peripheral vascular anomaly/neuropathy worsened by alcohol dependence” for which a lower extremity color doppler with ankle brachial index (ABI) was scheduled. A blood analysis including hemoglobin and hematocrit, comprehensive metabolic panel, hemoglobin A1c, and thyroid-stimulating hormone was ordered and all returned within normal limits.
Fig. 1.
Clinical presentations pre and post blepharoplasty. (A) Photo of patient in mid-twenties. (B) Coarse facial features, bilateral ptosis, excessive and redundant eyelid skin, loss of eyelashes and eyebrows, and wide nasal bridge. (C) Side facial view showing his concave nasal bridge, excessive/redundant eyelid skin, and loss of eyelashes. (D) Thickened/edematous features of his hands with scars/burn marks. (E) Five days post surgery. (F) Six-months post surgery/MDT.
His ophthalmic exam at presentation revealed edematous and thickened eyelids, bilateral ptosis with levator function, and dermatochalasis (blepharochalasis) (Fig. 1B and C). His visual acuities were 20/25 OD and 20/30 OS and intraocular pressures were 10 mmHg OD and 11 mmHg OS. His pupils were equally round, and reactive and extraocular movements were full. His confrontational visual fields were limited temporally OS>OD. His palpebral fissures measured 6 mm OD and 4.5 mm OS. Marginal reflex distance-1 was 0.5 mm OD and 0 mm OS. His lid creases were 9 mm OD and 11 mm OS. Slit lamp examination revealed bilateral Meibomian gland dysfunction, posterior blepharitis, and bilateral early nuclear sclerotic cataracts. He denied a prior history of ptosis, repeated eye rubbing, intraocular surgery, contact lens use, eye pain, trauma, or muscular dystrophy. The bilateral ptosis was unresponsive to eyelid and jaw movement and did not fluctuate throughout the day. The patient was diagnosed with blepharoptosis with excessive upper eyelid skin obstructing his field of vision prompting a bilateral blepharoplasty treatment plan.
His preoperative labs, performed a week prior to surgery, were within normal limits except for pancytopenia [WBC: 3.14 K/μl (Neutrophils 1.74 K/μl, Lymphocytes 0.99 K/μl), RBC: 3.89 m/μl, platelets 92] and an elevated mean corpuscular volume (MCV; 109 fL). Additional labs were prompted by the elevated MCV but were within normal limits.
On the day of blepharoplasty surgery, the patient was noted to have diffuse tarsal and facial erythema, as well as bilateral eyelids swelling, but no levator deficiency or dehiscence. Due to pre-operation detection of increased erythema, lid swelling, and recent history of squamous cell carcinoma and the broad constellation of unexplained signs and systemic symptoms, and undiagnosed pancytopenia, an intraoperative decision was made to send surgical specimens for histopathological interpretation. The patient reported peripheral vision improvement and was very satisfied with his results 5 days post surgery (Fig. 1E).
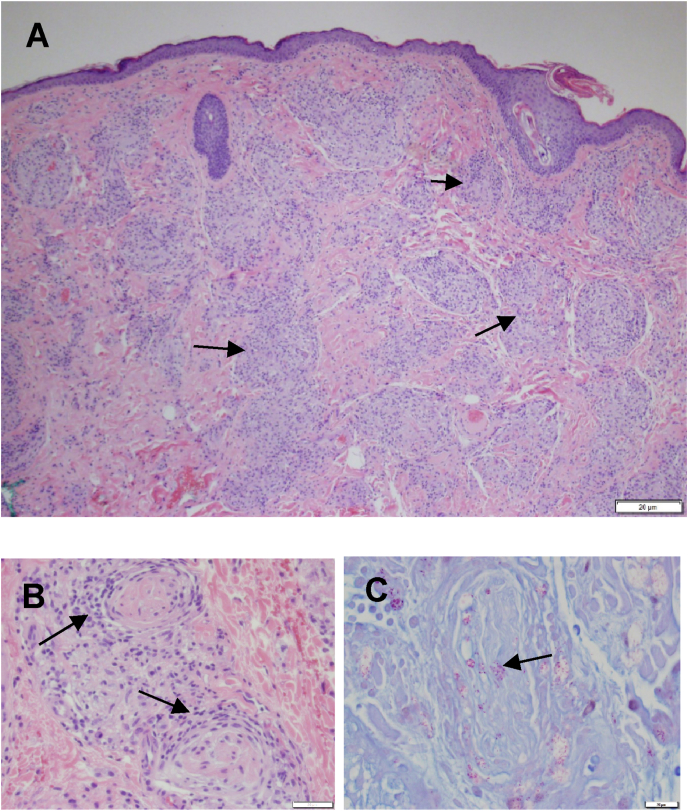
The left upper eyelid skin histopathology report (obtained 4 d post surgery) was significant for non-caseating granulomatous inflammation in the superficial and deep dermal layers (Fig. 2A) that differ from regular granuloma due to the lymphocytes surrounding the thickened nerve fibers (Fig. 2B). Numerous groups and singularly dispersed acid-fast bacilli were detected in the superficial and deep dermis, as well as nerve fibers (Fig. 2C), consistent with lepromatous leprosy. The non-caseating granulomatous inflammation differs from regular granulomas due to the lymphocytes surrounding the thickened nerve fibers (Fig. 2B). In consideration of the acid-fast bacilli and histology, his undiagnosed signs and symptoms, peripheral neuropathy, and his pancytopenia there was high suspicion for lepromatous leprosy with bone marrow involvement. The patient was referred to the Hansen's Disease Infectious Disease Center, where the diagnosis was confirmed, and he was started on standard MDT (rifampicin 600 mg once monthly, Clofazimine 50mg daily plus 300mg once monthly, and Dapsone 100 mg daily for 12 months). His general health improved over 6 months with reductions of fatigue, pancytopenia, and facial edema (Fig. 1F). The patient and spouse are being followed at the Hansen's Disease Specialty Center, Baton Rouge, LA.
Fig. 2.
Biopsy histopathology. (A) Hematoxylin-stained eyelid skin section showing numerous non-caseating granulomas (arrows). (B) Granulomatous inflammatory cells surrounding nerve fibers (arrows). (C) Numerous acid-fast bacilli seen in groups and singly dispersed within the dermis. Note the acid-fast bacilli in the nerve (arrow).
3. Discussion
The ocular leprosy presentation of bilateral ptosis/blepharitis was initially misdiagnosed, as with many leprosy cases, delaying treatment.7 The peripheral neuropathy and thickened skin are suspicious for leprosy, but the histopathologic findings of non-caseating granuloma, thickened nerve fibers, and acid-fast bacilli are pathognomonic for M. leprae infection. In retrospect, the classical manifestations of Hansen's disease7 were present, such as a prolonged facial swelling, madarosis, concave nasal bridge, numbness and tingling in the hands and feet, skin with decreased sensation, painless skin burns or wounds, and polyarthralgia. Ocular manifestations in leprosy patients are common4 and eyelid diseases in long-standing leprosy includes eyelash ptosis, lagophthalmos, and lower lid ectropion.6,8 While bilateral ptosis is rare, Guimaraes and Cruz reported a 12.2% incidence of blepharoptosis in leprosy patients.6
The source of infection in our patient is uncertain. The transmission mode of M. leprae is not fully established, but there is an increased risk for individuals living in close contact with leprosy patients. Anthroponotic (mosquito), or zoonotic (armadillo) transmission9 cannot be ruled since he resides in an endemic area.7 However, he reported no armadillo consumption or contact. His asymptomatic spouse's military assignments were unobtainable, but military personnel deployed to endemic areas are at risk for M. leprae infection.8
Blepharoplasty treatment of his vision complaint was successful, and he responded very well to the WHO recommended lepromatous leprosy MDT. MDT rapidly interrupts M. leprae transmission but does not guarantee ocular symptom resolution or prevent ocular complications (leprosy reaction).3,4,8 Long-term monitoring is advised due to relapse because of incomplete treatment, drug resistance, drug sensitivity, and late-onset leprosy reactions.3,4,9,10 There is no approved leprosy vaccine, but a vaccine is being evaluated to inhibit infection in at-risk persons in disease-endemic regions.11
Hansen's disease is endemic in several countries worldwide including the United States.7 Comparative genomic and phylogeographic analysis of M. leprae has linked the global spread of leprosy with human migration.1,9 New leprosy cases are rare, but generally occur in travelers from endemic areas. Leprosy remains endemic among native-born Americans and immigrant populations from the world's endemic areas.1,8, 9, 10 The range and complexity of zoonotic leprosy is expanding, and new active and latent cases are arising, making an early diagnosis and MDT crucial to effectively stopping transmission, aiding normal function restoration, and identifying contact persons. The correct diagnosis in patients with longstanding orbit and ocular adnexa inflammation may necessitate aspiration or biopsy histopathology.12
4. Conclusions
The challenge for eye care providers in identifying ocular leprosy is overcoming the stigma of the diagnosis. A differential diagnosis should include leprosy if the person gives a history of long-standing edematous inflammation in exposed skin with associated peripheral sensory neuropathy, resides in or traveled from an endemic area, or lives in contact with a person with Hansen's disease. The lepromatous leprosy diagnosis in a suspect patient is substantiated by detection of acid-fast bacilli in affected tissue.
Patient consent
The IRB waiver and patient's consent to publish identifiable photographs and pathological images are on file. Collection and evaluation of protected patient health information complied with HIPPA regulations and adhered to the ethical principles of the 2013 amended Declaration of Helsinki.
Funding
No funding or grant support.
Authorship
All authors attest they meet current ICMJE criteria for Authorship.
Intellectual property
We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property.
Research ethics
We further confirm that any aspect of the work covered in this manuscript that has involved human patients has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript.
IRB approval was obtained (required for studies and series of 3 or more cases)
Case report was deemed non-research investigation by the IRB and the notification letter is available.
Written consent to publish potentially identifying information, such as details or the case and photographs, was obtained from the patient(s) or their legal guardian(s).
The signed and witnessed consent is on file and a certified copy rendered upon request.
Declaration of competing interest
The authors have not conflicts of interest to disclose.
Acknowledgements
The authors thank our dedicated pathology department for their assistance in this diagnosis. Histopathology was crucial in coming to this diagnosis.
References
- 1.Monot M., Honore N., Garnier T., et al. On the origin of leprosy. Science. 2005;308(5724):1040–1042. doi: 10.1038/ng.477. [DOI] [PubMed] [Google Scholar]
- 2.Nolen L., Haberling D., Scollard D., et al. Centers for disease control and prevention. 2014. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5779477/ Incidence of Hansen’s Disease--United States. 1994,2011.
- 3.Franco-Paredes C., Montes de Oca Sanchez G., White C. Global leprosy status in 2020: still losing touch. Ann Acad Med Singapore. 2020;49(1):1–2. http://www.annals.edu.sg/pdf/49VolNo1Jan2020/V49N1p1.pdf [PubMed] [Google Scholar]
- 4.Grzybowski A., Nita M., Virmond M. Ocular leprosy. Clin Dermatol. 2015;33(1):79–89. doi: 10.1016/j.clindermatol.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Yudkin A.M. Leprosy with especial references to ophthalmologic finding. Am J Ophthalmol. 1918;1:303–310. [Google Scholar]
- 6.Guimarães F.C., Cruz A.A. Eyelid changes in long-standing leprosy. Ophthalmic Plast Reconstr Surg. 1998;14(4):239–243. doi: 10.1097/00002341-199807000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Joel Chandranesan A.S., Mada P.K., Ramos-Herberth F., et al. Leprosy in Northwest Louisiana: a case series. Int J Mycobacteriol. 2018;7(2):173–177. doi: 10.4103/ijmy.ijmy_21_18. [DOI] [PubMed] [Google Scholar]
- 8.Wroblewski K.J., Hidayat A., Neafie R., Meyers W. The AFIP history of ocularleprosy. Saudi J Ophthalmol. 2019;33(3):255–259. doi: 10.1016/j.sjopt.2019.09.003. PMCID: PMC6819722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ploemacher T., Faber W.R., Menke H., et al. Reservoirs and transmission routes of leprosy; A systematic review. PLoS Neglected Trop Dis. 2020;14(4) doi: 10.1371/journal.pntd.0008276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh A.V., Chauhan D.S. Mycobacterium lepromatosis. Lepromatous leprosy in US citizen who traveled to disease-endemic areas. Emerg Infect Dis. 2018;24(5):951–952. doi: 10.3201/eid2405.171972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duthie M.S., Frevol A., Day T., et al. A phase 1 antigen dose escalation trial to evaluate safety, tolerability and immunogenicity of the leprosy vaccine candidate LepVax (LEP-F1 + GLA-SE) in healthy adults. Vaccine. 2020;38(7):1700–1707. doi: 10.1016/j.vaccine.2019.12.050. [DOI] [PubMed] [Google Scholar]
- 12.Dhaliwal U., Arora V.K., Singh N., Bhatia A. Clinical and cytopathologic correlation in chronic inflammations of the orbit and ocular adnexa: a review of 55 cases. Orbit. 2004;23(4):219–225. doi: 10.1080/01676830490512260. [DOI] [PubMed] [Google Scholar]