Abstract
Oxygen is an essential molecule for animal respiration, growth, and survival. Unlike in terrestrial environments, contamination and climate change have led to the frequent occurrence of hypoxia in aquatic environments, thus impacting aquatic animal survival. However, the adaptative mechanisms underlying fish responses to environmental hypoxia remain largely unknown. Here, we used large yellow croaker (Larimichthys crocea) and large yellow croaker fry (LYCF) cells to investigate the roles of the Hif-1α/Hsf1/Hsp70 signaling pathway in the regulation of cellular redox homeostasis, and apoptosis. We confirmed that hypoxia induced the expression of Hif-1α, Hsf1, and Hsp70 in vivo and in vitro. Genetic Hsp70 knockdown/overexpression indicated that Hsp70 was required for maintaining redox homeostasis and resisting oxidative stress in LYCF cells under hypoxic stress. Hsp70 inhibited caspase-dependent intrinsic apoptosis by maintaining normal mitochondrial membrane potential, enhancing Bcl-2 mRNA and protein expression, inhibiting Bax and caspase3 mRNA expression, and suppressing caspase-3 and caspase-9 activation. Hsp70 suppressed caspase-independent intrinsic apoptosis by inhibiting nuclear translocation of apoptosis-inducing factor (AIF) and disturbed extrinsic apoptosis by inactivating caspase-8. Genetic knockdown/overexpression of Hif-1α and dual-luciferase reporter assay indicated that Hif-1α activated the Hsf1 DNA promoter and enhanced Hsf1 mRNA transcription. Hsf1 enhanced Hsp70 mRNA transcription in a similar manner. In summary, the Hif-1α/Hsf1/Hsp70 signaling pathway plays an important role in regulating redox homeostasis and anti-apoptosis in L. crocea under hypoxic stress.
Keywords: Hypoxia, Larimichthys crocea, Apoptosis, Redox homeostasis, Hif-1α/Hsf1/Hsp70
INTRODUCTION
The large yellow croaker (Larimichthys crocea), which is primarily cultured in offshore cages, is an important economic mariculture fish in China. In recent years, however, climate change and environmental pollution have led to the frequent occurrence of marine hypoxia (Breitburg et al., 2018; Diaz & Rosenberg, 2008). Hypoxia in marine culture areas has become an unfavorable factor affecting the healthy and sustainable development of the L. crocea breeding industry (Liu et al., 2018). Therefore, studying the effects of hypoxic stress on fish and exploring the adaptative mechanisms of L. crocea to hypoxia are critical.
Hypoxic stress can induce excessive reactive oxygen species (ROS) production in aerobic organisms (Leonarduzzi et al., 2010). In response to the increase in ROS, organisms are equipped with a defense system consisting of antioxidant enzymes and non-enzymatic antioxidant small molecules that regulate cellular redox homeostasis (Guérin et al., 2001; Ming et al., 2019). The inability of the antioxidant defense system to neutralize excessive ROS will lead to an imbalance in intracellular redox homeostasis, with excess ROS attacking lipids, proteins, and DNA to produce malondialdehyde (MDA), protein carbonyl (PCO), and 8-hydroxy-2 deoxyguanosine (8-OHdG), respectively, leading to oxidative stress (Klein & Ackerman, 2003). The effects of hypoxic stress on redox homeostasis have been reported in Micropogonias undulatus (Rahman & Thomas, 2011) and Leiostomus xanthurus (Cooper et al., 2002), but studies on the responses of L. crocea under hypoxic stress remain scarce. In our previous study, superoxide dismutase (SOD) and catalase (CAT) activity in the liver of L. crocea was significantly higher than that in the normoxic group after 96 h of acute hypoxic stress (Wang et al., 2017), implicating the involvement of the antioxidant defense system ofL. crocea in response to hypoxic stress. We further showed that hypoxic stress can induce ROS overproduction and oxidative stress in L. crocea and large yellow croaker fry (LYCF) cells, whereas the ROS scavenger N-acetylcysteine (NAC) can significantly reduce ROS levels and attenuate oxidative stress in LYCF cells under hypoxic stress (Luo et al., 2021). Hypoxia can also induce apoptosis through the intrinsic (mitochondrial) and extrinsic (death receptor) pathways (Grilo & Mantalaris, 2019; Lohberger et al., 2016; Pan et al., 2014). The effects of hypoxic stress on apoptosis in fish have also been reported for M. undulatus (Ondricek & Thomas, 2018), Danio rerio (Williams et al., 2017), and Ictalurus punctatus (Yuan et al., 2016). However, studies on the impact of stress conditions on apoptosis in L. crocea are limited. Wang et al. (2020) reported on the effects of hydrogen peroxide (H2O2) on oxidative stress and apoptosis in large yellow croaker head kidney cells and we recently found that hypoxic stress can induce apoptosis in L. crocea via the intrinsic and extrinsic pathways (unpublished data).
Heat shock protein 70 (Hsp70) is an inducible stress protein that is highly conserved in both prokaryotes and eukaryotes and plays a significant role in maintaining intracellular environmental homeostasis (Azad et al., 2011; Diao et al., 2012). In particular, Hsp70 exerts antioxidative stress effects by increasing antioxidant enzyme activity, e.g., SOD, glutathione peroxidase (GPx), and CAT (Broome et al., 2006; Gu et al., 2012; Xu et al., 2018), binding and antagonizing polymerized glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Lazarev et al., 2016), promoting SOD2 transport to mitochondria (Afolayan et al., 2014), maintaining glutathione (GSH) levels in cells (Broome et al., 2006), and regulating nicotinamide adenine dinucleotide phosphate oxidase (NOX) enzymatic activity (Chen et al., 2012; Troyanova et al., 2015). The antioxidative stress effects of Hsp70 have been explored in humans (Homo sapiens) (Afolayan et al., 2014; Jiang et al., 2020; Yurinskaya et al., 2017), rats (Rattus norvegicus) (Lazarev et al., 2016; Liu et al., 2015), mice (Mus musculus) (Broome et al., 2006; Hernández-Santana et al., 2014), chickens (Gallus gallus) (Gu et al., 2012), and fruit flies (Drosophila melanogaster) (Gupta et al., 2007). To date, however, regulation of redox homeostasis by Hsp70 in fish under stressful conditions has only been reported in rainbow trout (Oncorhynchus mykiss) (Zeng et al., 2014). Hsp70 is also known to play a regulatory role in the intrinsic and extrinsic apoptosis pathways. For example, it is reported that Hsp70 can inhibit the activation of caspase-3/9 (Giffard et al., 2008; Ueng et al., 2013), prevent the translocation of Bcl2 associated X (Bax) proteins from the cytoplasm to mitochondria (Saini & Sharma, 2018; Stankiewicz et al., 2005), up-regulate the expression of Bcl-2 (Yenari et al., 2005) and maintain its stability (Jiang et al., 2011), and inhibit the nuclear translocation of apoptosis-inducing factor (AIF) (Ravagnan et al., 2001), thus regulating mitochondria-mediated apoptosis. In addition, Hsp70 can bind to the death receptor and inhibit formation of the death-inducing signaling complex (DISC), thus inhibiting extrinsic apoptosis (Gao et al., 2015; Guo et al., 2005). The anti-apoptotic role of Hsp70 in fish under stressful conditions has been reported in Mugil cephalus (Padmini & Tharani, 2014), O. mykiss (Zeng et al., 2014), Acanthopagrus schlegelii (Deane et al., 2012), Sparus sarba (Deane et al., 2006, 2012), and Prochilodus argenteus (Domingos et al., 2013). However, its role in the maintenance of redox homeostasis and regulation of apoptosis in L. crocea under stress remains unclear.
Hypoxia-inducible factor 1α (Hif-1α), a regulatory subunit of Hif-1, is essential for maintaining normal cellular function under hypoxic stress (Bruick & McKnight, 2002). As a transcription factor, Hif-1 regulates the transcription of various target genes (Ema et al., 1999), which are involved in the regulation of biological processes such as energy metabolism, erythropoiesis, angiogenesis, cell proliferation, extracellular matrix formation, and apoptosis (Semenza, 2004; Wenger et al., 2005; Zhong et al., 2002). Under hypoxic stress, Hsp70 expression is closely correlated with that of Hif-1α (Tsuchida et al., 2014). Research has indicated that Hif-1 indirectly regulates the expression of Hsp70 in Drosophila melanogaster Kc167 tissue culture cells (Baird et al., 2006) and Crassostrea gigas (Kawabe & Yokoyama, 2011) under hypoxic stress by activating the transcription of heat shock factor 1 (Hsf1) mRNA. Moreover, Hif-1α is reported to bind to the hypoxia response element (HRE) in the Hsp70 promoter and activate Hsp70 transcription expression in hepatocellular carcinoma (HCC) cells under hypoxic stress (Xia et al., 2009). However, the mechanism underlying Hif-1α-induced regulation of Hsp70 in fish, especially marine fish, under hypoxic stress has not yet been elucidated.
Given that Hif-1α can enhance the transcription of the Hsp70 gene through Hsf1 in fish under hypoxic stress and that Hsp70 has antioxidative and anti-apoptotic functions in other animals, we hypothesized that Hif-1α may regulate Hsp70 expression via Hsf1 to maintain cellular redox homeostasis and enhance anti-apoptotic ability, thereby improving the adaptation of L. crocea to hypoxic stress. To test this hypothesis, we explored the regulatory role of Hsp70 in redox homeostasis and apoptosis in LYCF cells under hypoxic stress and investigated the transcriptional regulatory relationships among Hif-1α, Hsf1, and Hsp70. This study provides basic biological information for elucidating the hypoxia-response mechanism of L. crocea and provides a theoretical basis for the selective breeding of hypoxia-tolerant L. crocea.
MATERIALS AND METHODS
Fish experiments and sample collection
Large yellow croakers (length, 15.90±1.52 cm; body weight, 63.61±6.63 g) were provided by Fufa Aquatic Products Co., Ltd. (Ningde, China). All fish were subjected to 2 weeks of acclimation in aerated natural seawater (dissolved oxygen (DO), 7.8±0.5 mg/L; salinity, 29; temperature, 22±0.5 °C; pH, 8.1). Briefly, 240 fish were randomly divided into six tanks (800 L per tank; three tanks each for hypoxic and normoxic groups). For the hypoxia experiment, the DO in each tank was maintained at 2.0±0.1 mg/L for 96 h using a HACH DO probe system (HACH LDO II, HACH, USA) to control the duration and dose of the nitrogen injection in real time. Liver samples were collected after 0, 3, 6, 12, 24, 48, and 96 h of hypoxic stress. The dissected liver samples were stored at −80 °C. All sampling method principles and procedures were conducted in strict accordance with the requirements of the Governing Regulation for the Use of Experimental Animals in Zhejiang Province (Zhejiang Provincial Government Order No. 263, released on 17 August 2009, effective from 1 October 2010) and approved by the Animal Care and Use Committee of Ningbo University.
Cloning and bioinformatics analysis of full-length cDNA of LcHif-1α and LcHsp70
Total RNA was isolated using TRIzol reagent (Invitrogen, China) according to the manufacturer’s instructions. First-strand cDNA was synthesized using a SMARTer® RACE 5'/3' kit (Takara, Japan) as per the manufacturer’s protocols. Gene-specific primers (Supplementary Table 1) were designed based on genome assembly data from the National Center for Biotechnology Information (NCBI, https://www.ncbi.nlm.nih.gov/). The desired PCR products were cloned into the pMD19-T simple vector (Takara, Japan) and sequenced at GENEWIZ (China).
The deduced amino acid (aa) sequence was analyzed using the Expert Protein Analysis System (http://www.expasy.org/). Conserved domains were searched using the respective NCBI module (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi). Multiple sequence alignment was performed using Vector NTI software (Invitrogen, USA). Phylogenetic and molecular evolutionary analyses were conducted using MEGA v5.0 (Tamura et al., 2011).
Cell culture and hypoxia challenge
The LYCF cell line was kindly provided by Dr. You-Hua Huang (South China Agricultural University, Guangzhou). The LYCF cells were cultured in Leibovitz’s-15 Medium (Gibco, USA) supplemented with 10% (v/v) fetal bovine serum (Gibco, USA) and 200 µg/mL penicillin-streptomycin (Gibco) at 27 °C. For the hypoxic challenge, LYCF cells were cultured in a MIC-101 modular incubator (Billups Rothenberg Inc., USA) with 1% O2 and 99% N2 for 0, 3, 6, 12, 24, and 48 h.
Reverse-transcription quantitative polymerase chain reaction (RT-qPCR)
The expression levels of LcHif-1α, LcHsp70, LcBax, LcBcl-2, and Lccaspase3 were assessed by RT-qPCR using a LightCycler 480 instrument (Roche, Switzerland). The primers used for RT-qPCR are listed in Supplementary Table 1. cDNA synthesis and RT-qPCR analysis were performed as described previously (Luo et al., 2019).
Prokaryotic expression and preparation of mouse anti-LcHsp70 polyclonal antibodies
The ATPase domain (located at 1–1326 bp of the open reading frame (ORF)) of LcHsp70 was amplified using specific primers (Supplementary Table 1). The amplified PCR products were ligated into the pEASY-Blunt E1 expression vector (TransGen Biotech, China). The recombinant plasmid was sequenced to confirm the insert and then transformed into Escherichia coli Rosetta (DE3) (TransGen Biotech, China). Subsequently, bacteria were induced with 1 mmol/L isopropyl-β-d-thiogalactoside (IPTG, Solarbio, China). The obtained target proteins were purified as described in our previous study (Gao et al., 2019). Purified recombinant proteins were renatured according to the method described in Lu et al. (2017). Mouse immunization was performed as described previously (Lv et al., 2015). Antisera were extracted for subsequent experiments.
Western blotting
RIPA buffer supplemented with protease inhibitor phenylmethanesulfonyl fluoride (PMSF; Beyotime, China) was used to isolate total proteins from tissues and cells. The proteins were then subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (20 μg of protein per lane) and electrophoretically transferred to polyvinylidene difluoride (PVDF, Solarbio, China) membranes. After blocking with 5% non-fat dry milk, the membranes were sequentially incubated with the AIF rabbit polyclonal antibody (1:1 000; Beyotime, China), Bcl-2 rabbit polyclonal antibody (1:1 000; Dia-An Biotech, China), LcHsp70 mouse polyclonal antibody (1:1 000), and Actin mouse monoclonal antibody (1:1 000; Abmart, China) overnight at 4 °C. The membranes were then washed thrice with Tris-buffered saline with Tween (TBST; 20 mmol/L Tris-HCl, 150 mmol/L NaCl, and 0.05% Tween-20) and incubated with horseradish peroxidase (HRP)-labeled goat anti-rabbit IgG (1:2 000; Beyotime, China) or HRP-labeled goat anti-mouse IgG (1:2 000; Beyotime, China) second antibodies for 1 h at 37 °C. Membranes were visualized using a chemiluminescence imaging analysis system (Tanon 5200, Tanon, China). Data were normalized to the level of the Actin protein.
Small interfering RNA (siRNA)-mediated RNA silencing in vitro
Specific siRNAs targeting LcHif-1α (siLcHif-1α) and LcHsp70 (siLcHsp70) (Supplementary Table 1) were synthesized by Genepharma (China) and dissolved in RNase-free water (20 μmol/L). Small interfering negative control (siNC) RNA, which was not homologous to any gene in the L. crocea genome, was used as a negative control. Both siRNA (1.5 μL) and siRNA-mate transfection reagent (Genepharma, China) were mixed and added to each well of a 24-well plate containing 500 μL of LYCF cells, then cultured for an additional 24 h.
Overexpression vector construction and transfection
The ORFs of LcHif-1α, LcHsp70, and LcHsf1 were amplified using specific primers (Supplementary Table 1) and ligated into pcDNA3.1 to construct the oeLcHif-1α, oeLcHsp70, and oeLcHsf1 overexpression vectors, respectively. The LYCF cells were seeded into 6-well plates and cultured at 27 °C for 12 h prior to transfection. For overexpression analysis, the LYCF cells were transfected with the overexpression vectors using the Lipo6000™ agent (Beyotime, China). Cells transfected with an empty pcDNA3.1 vector served as the negative control.
Measurement of intracellular ROS and superoxide
The levels of intracellular ROS and superoxide in LYCF cells were assayed using 2',7'-dichlorofluorescein diacetate (DCFH-DA, Sigma, USA) and a superoxide assay kit (Beyotime, China), respectively.
Determination of oxidative stress markers MDA, PCO, and 8-OHdG
The levels of MDA, PCO, and 8-OHdG were measured in vivo and in vitro using an MDA kit (Nanjing Jiancheng Institute of Bioengineering, China), PCO content detection kit (Solarbio, China), and fish 8-OHdG ELISA kit (Chenglinbio, China), respectively, in accordance with the manufacturers’ instructions.
Annexin V apoptosis assay
LYCF cells were stained using an Annexin V-FITC-PI apoptosis detection kit (Beyotime, China) according to the manufacturer’s instructions and immediately photographed under a laser confocal microscope (LSM880, Carl Zeiss, Germany) or subjected to flow cytometry (Becton Dickinson, USA) to detect apoptotic rates. The acquired data were analyzed using FlowJo v10 software (Ashland, USA).
Detection of mitochondrial membrane potential (MMP) in LYCF cells
The MMP of LYCF cells was measured using the potentiometric dye tetramethyl rhodamine methyl ester (TMRM; MedChem Express, USA) at a final concentration of 0.5 μmol/L for 20 min at 27 °C.
Measurement of caspase-3, -8, and -9 activities
The activities of caspase-3, -8, and -9 in LYCF cells were assessed using caspase-3, -8, and -9 activity assay kits (Beyotime), respectively, as per the manufacturer’s protocols.
Nucleoplasm distribution of AIF
Nuclear and cytoplasmic proteins of LYCF cells were separated using a nuclear and cytoplasmic protein extraction kit (Beyotime, China) and subjected to western blot analysis to identify the nucleoplasmic distribution of AIF.
Dual-luciferase reporter assay
The promoter fragments of the LcHsf1 (Gene ID: 104931900) and LcHsp70 (Gene ID: 104926754) genes were amplified with specific primers (Supplementary Table 1) using genomic DNA as a template and ligated into a pGL3-basic vector to construct luciferase reporter vectors pGL3-prom-LcHsf1 and pGL3-prom-LcHsp70, respectively. The LYCF cells were cotransfected with the pGL3-promoter vectors (or pGL3 basic), pRL-TK Renilla luciferase vector, and siLcHif-1α/siNC (or pcDNA3.1-Hif-1α/pcDNA3.1 or pcDNA3.1-Hsf1/pcDNA3.1) using Lipo6000™ agent (Beyotime, China). After 24 h, the cells were harvested and subjected to measurement of luciferase activity using a dual-luciferase reporter gene assay kit (Yeasen, China).
Statistical analysis
Data are expressed as mean±standard error of the mean (SEM). All statistical analyses were carried out using SPSS software (v21.0; IBM, USA). Significant differences between two groups were determined using the two-tailed independent samples t-test. Significant differences among three or more groups were determined using one-way analysis of variance (ANOVA), followed by Tukey’s post hoc test. In all figures, line charts, and histograms, “*” and “**” indicate significant differences (P<0.05) and extremely significant differences (P<0.01) compared with another set of data.
RESULTS
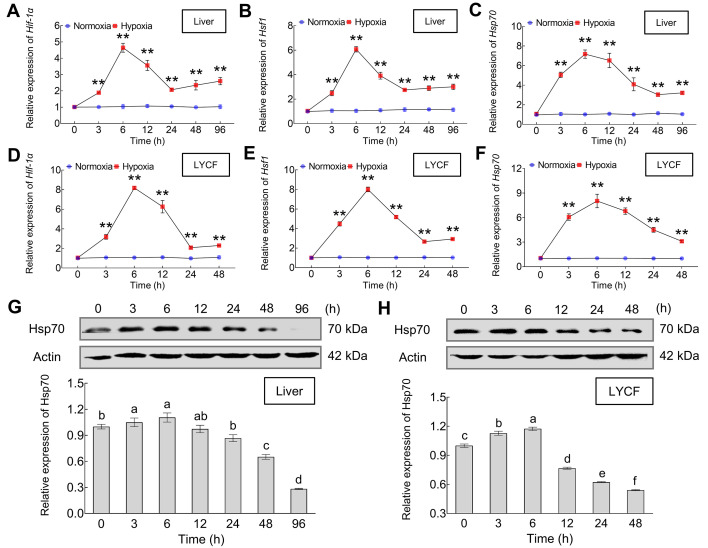
Hypoxic stress induced LcHif-1α, LcHsf1, and LcHsp70 expression in vivo and in vitro
We obtained the full-length cDNA of LcHif-1α (GenBank accession No.: MZ197829) and LcHsp70 (GenBank accession No.: MZ197830) using the RACE technique. The respective sequences and bioinformatic characteristics are detailed in Supplementary Text. We observed that the Hif-1α, Hsf1, and Hsp70 mRNA and Hsp70 protein expression levels initially increased and then decreased in the liver of L. crocea following 96 h of hypoxic stress (Figure 1A–C, G) and in LYCF cells following 48 h of hypoxic stress (Figure 1D–F, H). Interestingly, levels were significantly higher than those in the normoxic group after 3 h of hypoxic stress, reaching a peak after 6 h of hypoxic stress.
Figure 1.
Expression patterns of LcHif-1α, LcHsf1, and LcHsp70 in Larimichthys crocea liver and LYCF cells under hypoxic stress
A, B, and C illustrate expression patterns of LcHif-1α, LcHsf1, and LcHsp70 mRNA in liver of L. crocea under hypoxic stress, respectively. D, E, and F illustrate expression patterns of LcHif-1α, LcHsf1, and LcHsp70 mRNA in LYCF cells under hypoxic stress, respectively. G: Changes in level of LcHsp70 protein expression in liver of L. crocea after 0, 3, 6, 12, 24, 48, and 96 h of hypoxic stress. H: Changes in level of LcHsp70 protein expression in LYCF cells after 0, 3, 6, 12, 24, and 48 h of hypoxic stress.
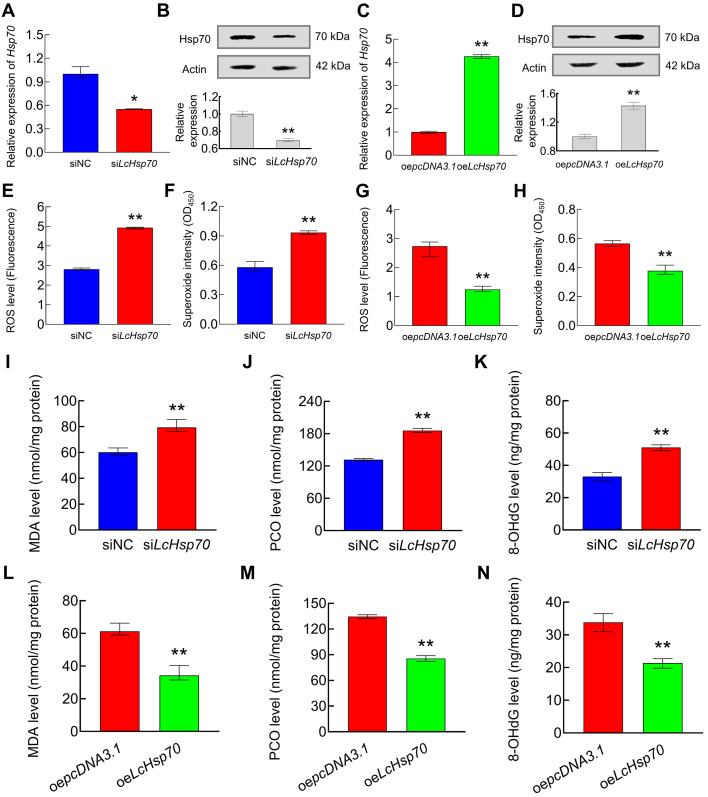
LcHsp70 reduced ROS levels and attenuated oxidative stress in LYCF cells exposed to hypoxia
After 24 h of hypoxic stress, the LcHsp70 mRNA and protein expression levels decreased by 45% (Figure 2A) and 30% (Figure 2B), respectively, in the siLcHsp70 group compared with the siNC group. After 24 h of hypoxic stress, the LcHsp70 mRNA and protein expression levels in the oeLcHsp70 group increased 4.26-fold (Figure 2C) and 1.43-fold (Figure 2D), respectively, compared with levels in the oepcDNA3.1 group. In addition, the ROS (Figure 2E), superoxide (Figure 2F), MDA (Figure 2I), PCO (Figure 2J), and 8-OHdG levels (Figure 2K) were significantly higher in the siLcHsp70 group compared with the siNC group but were lower in the oeLcHsp70 group compared with the oepcDNA3.1 group (Figure 2G, H, L, M).
Figure 2.
Changes in ROS and oxidative stress levels in LYCF cells under hypoxic stress following knockdown/overexpression of Hsp70
A, B: Changes in Hsp70 mRNA (A) and protein levels (B) in LYCF cells after transfection with siHsp70 (LcHsp70-interference) and hypoxic stress for 24 h. C, D: Changes in Hsp70 mRNA (C) and protein levels (D) in LYCF cells after overexpression of LcHsp70 and hypoxic stress for 24 h. E, F: Changes in ROS (E) and superoxide levels (F) in LYCF cells after LcHsp70-interference and hypoxic stress for 24 h. G, H: Changes in ROS (G) and superoxide levels (H) in LYCF cells after overexpression of LcHsp70 and hypoxic stress for 24 h. I–K: Changes in MDA (I), PCO (J), and 8-OHdG levels (K) in LYCF cells after LcHsp70-interference and hypoxic stress for 24 h. L–N: Changes in MDA (L), PCO (M), and 8-OHdG levels (N) in LYCF cells after overexpression of LcHsp70 and hypoxic stress for 24 h.
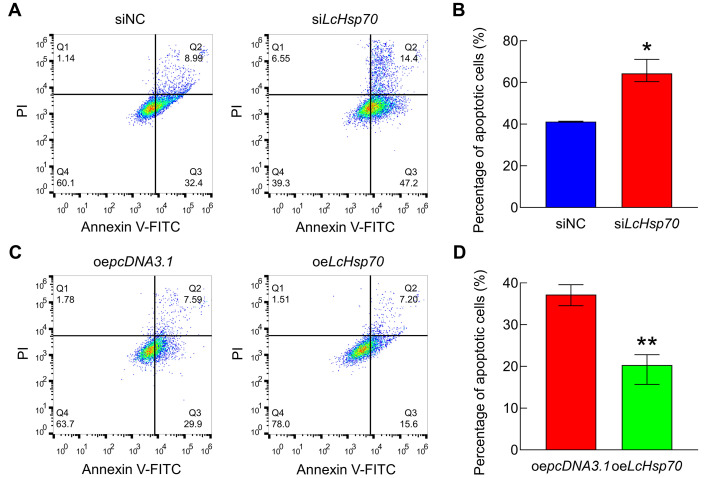
LcHsp70 played an anti-apoptotic role in LYCF cells under hypoxic stress
The apoptotic rate of LYCF cells in the siLcHsp70 group (64.37%) was significantly higher than that in the siNC group (41.26%) (Figure 3A, B). In contrast, the apoptotic rate of cells in the oeLcHsp70 group (20.30%) was significantly lower than that in the oepcDNA3.1 group (37.20%) (Figure 3C, D).
Figure 3.
LcHsp70 suppresses LYCF cell apoptosis under hypoxic stress
A, B: Changes in apoptotic rate of LYCF cells after LcHsp70-interference and hypoxic stress for 24 h. C, D: Changes in apoptotic rate of LYCF cells after overexpression of LcHsp70 and hypoxic stress for 24 h.
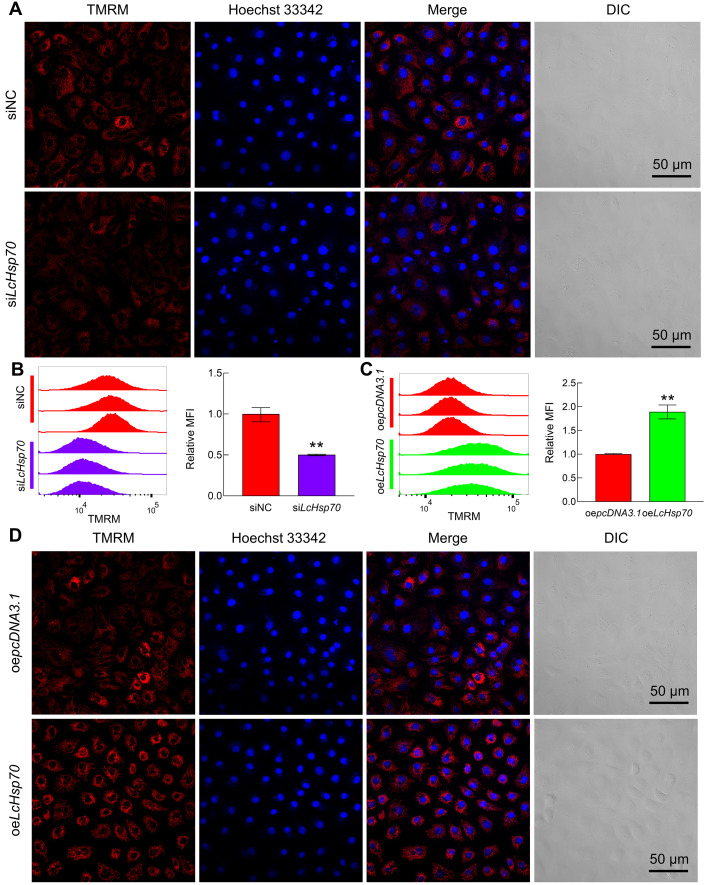
LcHsp70 suppressed caspase-dependent intrinsic apoptosis in LYCF cells exposed to hypoxia
Based on laser confocal microscopy analysis, the MMP (red fluorescence intensity) of cells in the siLcHsp70 group was significantly lower than that in the siNC group (Figure 4A), whereas the MMP of cells in the oeLcHsp70 group was significantly higher than that in the oepcDNA3.1 group (Figure 4D). In addition, flow cytometry analysis revealed that the relative mean fluorescence intensity (TMRM) of cells was reduced by 50% in the siLcHsp70 group compared with the siNC group (Figure 4B) but increased by 89% in the oeLcHsp70 group compared with the oepcDNA3.1 group (Figure 4C). These results indicate that LcHsp70 is involved in maintaining normal MMP in LYCF cells exposed to hypoxia.
Figure 4.
Changes in MMP of LYCF cells after interference/overexpression of LcHsp70 and hypoxic stress for 24 h
A, B: Changes in MMP of LYCF cells after LcHsp70-interference and hypoxic stress for 24 h. A: Laser confocal microscopy analysis. B: Flow cytometry analysis. C, D: Changes in MMP of LYCF cells after overexpression of LcHsp70 and hypoxic stress for 24 h. C: Flow cytometry analysis. D: Laser confocal microscopy analysis.
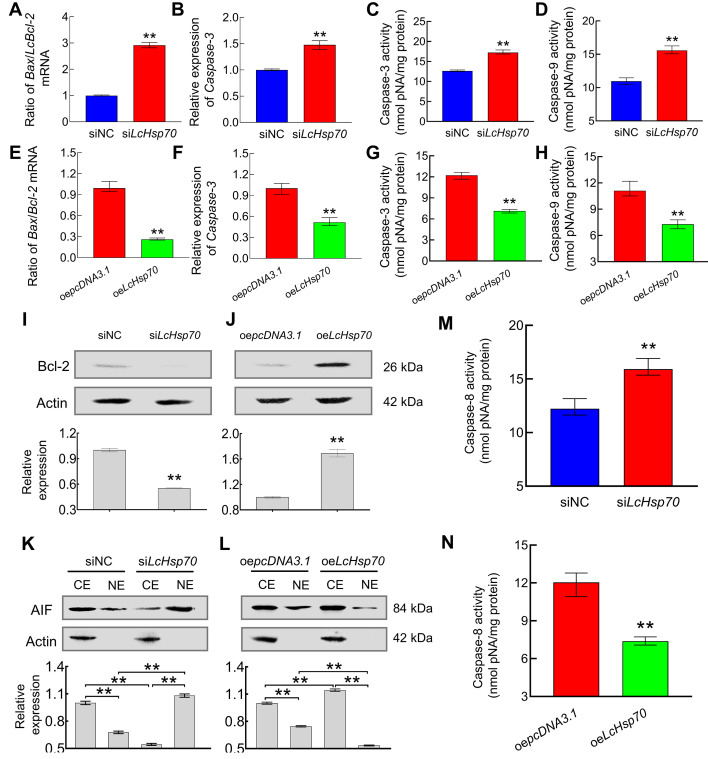
Results also showed that the LcBax/LcBcl-2 mRNA ratio, Lccaspase-3 mRNA expression level, and caspase-3 and -9 activities increased 2.92-fold (P<0.01) (Figure 5A), 1.48-fold (P<0.01) (Figure 5B), 1.36-fold (P<0.01) (Figure 5C), and 1.42-fold (P<0.01) (Figure 5D), respectively, in the siLcHsp70 group relative to the siNC group. In contrast, Bcl-2 protein expression significantly declined by 45% (P<0.01) (Figure 5I).
Figure 5.
LcHsp70 up-regulates Bcl-2 mRNA and protein expression, down-regulates Bax and caspase-3 mRNA expression, inhibits caspase-3, -9, and -8 activity, and prevents AIF translocation in LYCF cells under hypoxic stress
Changes in LcBax/LcBcl-2 mRNA ratio (A), Lccaspase-3 mRNA expression (B), caspase-3 activity (C), caspase-9 activity (D), Bcl-2 protein level (I), caspase-8 activity (M), and nucleoplasmic distribution of AIF protein (K) in LYCF cells after LcHsp70-interference and hypoxic stress for 24 h. Changes in LcBax/LcBcl-2 mRNA ratio (E), Lccaspase-3 mRNA expression (F), caspase-3 activity (G), caspase-9 activity (H), Bcl-2 protein level (J), caspase-8 activity (N), and nucleoplasmic distribution of AIF protein (L) in LYCF cells after overexpression of LcHsp70 and hypoxic stress for 24 h.
Furthermore, the LcBax/LcBcl-2 mRNA ratio, Lccaspase-3 mRNA expression level, and caspase-3 and -9 activities decreased by 74% (P<0.01) (Figure 5E), 49% (P<0.01) (Figure 5F), 42% (P<0.01) (Figure 5G), and 35% (P<0.01) (Figure 5H), respectively, in the oeLcHsp70 group relative to the siNC group. In contrast, Bcl-2 protein expression significantly increased by 1.69-fold (P<0.01) in the oeLcHsp70 group compared with the oepcDNA3.1 group (Figure 5J).
LcHsp70 repressed caspase-independent intrinsic apoptosis and inhibited extrinsic apoptosis in LYCF cells under hypoxic stress
Compared with the siNC group, the AIF protein expression level was significantly reduced in the cytoplasm (P<0.01) and significantly increased in the nucleus (P<0.01) (Figure 5K) of the LYCF cells under hypoxic stress in the siLcHsp70 group. These findings indicated that knockdown of LcHsp70 expression promoted nuclear translocation of the AIF protein in LYCF cells under hypoxic stress. Conversely, enhancing the expression of LcHsp70 resulted in a decrease in the nuclear translocation of the AIF protein in LYCF cells under hypoxic stress (Figure 5L). These results suggest that LcHsp70 represses the caspase-independent intrinsic apoptosis pathway in LYCF cells under hypoxic stress.
Our results also showed that caspase-8 activity was 1.30-fold higher in the siLcHsp70 group compared with the siNC group (P<0.01) (Figure 5M). Caspase-8 activity declined by 39% in the oeLcHsp70 group relative to the oepcDNA3.1 group (P<0.01) (Figure 5N). These findings indicate that LcHsp70 inhibits the extrinsic apoptosis pathway in LYCF cells under hypoxic stress.
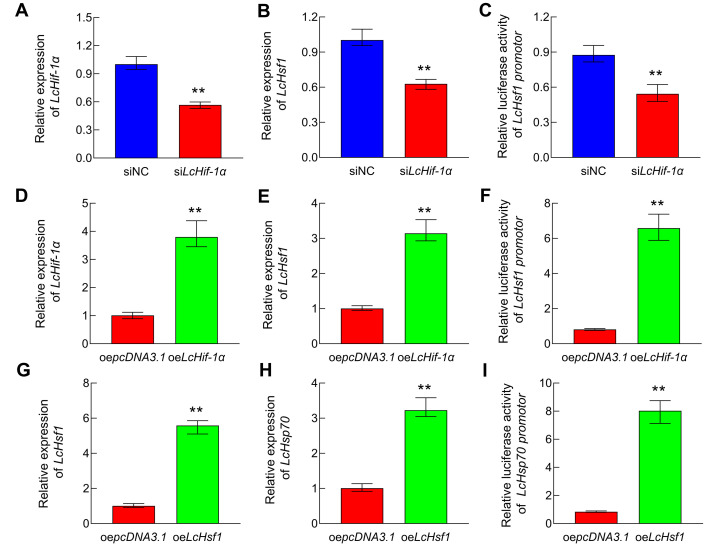
LcHif-1α activated LcHsf1 DNA promoter and initiated LcHsf1 mRNA transcription in LYCF cells under hypoxic stress
The LcHif-1α mRNA expression level was reduced by 44% in the siLcHif-1a group relative to the siNC group (P<0.01) (Figure 6A). Likewise, the LcHsf1 mRNA expression level also decreased by 37% in the siLcHif-1a group (P<0.01) (Figure 6B). In addition, the LcHif-1α and LcHsf1 mRNA expression levels were 3.78-fold (P<0.01) (Figure 6D) and 3.14-fold (P<0.01) (Figure 6E) higher, respectively, in the oeLcHif-1α group than in the oepcDNA3.1 group. The dual-luciferase reporter gene assay revealed that LcHsf1 promoter activity decreased by 38% (P<0.01) afterLcHif-1α-interference (Figure 6C) but increased by 7.09-fold (P<0.01) afterLcHif-1α overexpression (Figure 6F) in LYCF cells under hypoxic conditions. These results indicate that LcHif-1α enhances LcHsf1 DNA promoter activity and promotes LcHsf1 mRNA transcription in LYCF cells exposed to hypoxia.
Figure 6.
LcHif-1α activates LcHsf1 DNA promoter and initiates LcHsf1 mRNA transcription, which activates LcHsp70 DNA promoter and initiates LcHsp70 mRNA transcription in LYCF cells under hypoxic stress
Changes in LcHif-1α (A) and LcHsf1 mRNA levels (B) in LYCF cells after LcHif-1α-interference and hypoxic stress for 24 h. Changes inLcHsf1 promoter activity (C) after 24 h of LcHif-1α-interference in LYCF cells cotransfected with LcHsf1 promoter plasmid. Changes in LcHif-1α (D) and LcHsf1 mRNA levels (E) in LYCF cells after overexpression of LcHif-1α and hypoxic stress for 24 h. Changes in LcHsf1 promoter activity (F) after 24 h of overexpression of LcHif-1α in LYCF cells cotransfected with LcHsf1 promoter plasmid. Changes in LcHsf1 (G) and LcHsp70 mRNA levels (H) in LYCF cells after overexpression of LcHsf1 and hypoxic stress for 24 h. Changes in LcHsp70 promoter activity (I) in LYCF cells after overexpression of LcHsf1 for 24 h and cotransfection with LcHsp70 promoter plasmid.
LcHsf1 activated LcHsp70 DNA promoter and initiated LcHsp70 mRNA transcription in LYCF cells under hypoxic stress
After 24 h of hypoxic stress, the LcHsf1 mRNA expression level was 5.56-fold higher (P<0.01) in the oeLcHsf1 group compared with the oepcDNA3.1 group (Figure 6G). In addition, the LcHsp70 mRNA expression level was 3.21-fold higher in the oeLcHsf1 group relative to the oepcDNA3.1 group (P<0.01) (Figure 6H). Furthermore, compared with the negative control, LcHsp70 promoter activity increased significantly by 9.57-fold after LcHsf1 overexpression in LYCF cells under hypoxic conditions (P<0.01) (Figure 6I). These findings suggest that enhancement of LcHsf1 expression results in an increase in LcHsp70 DNA promoter activity and facilitation of LcHsp70 mRNA transcription.
DISCUSSION
Effects of hypoxic stress on Hif-1α, Hsf1, and Hsp70 expression
Hif-1α is a hypoxic stress-induced nuclear transcription factor that regulates the transcription of many target genes (Semenza, 2009). In higher animals, Hif-1α enhances adaptation to hypoxia by regulating the expression of genes related to biological processes, such as energy metabolism, erythropoiesis, angiogenesis, cell proliferation, extracellular matrix formation, and apoptosis (Semenza, 2004; Wenger et al., 2005; Zhong et al., 2002). Several studies have reported variations in the expression patterns of the Hif-1α gene in fish under hypoxic stress. For instance, Rimoldi et al. (2012) found that Hif-1α mRNA expression is significantly increased in the brain and liver of Perca fluviatilis under hypoxic stress. Likewise, Mohindra et al. (2013) reported that the Hif-1α mRNA level is significantly increased in the brain, liver, and kidney of Clarias batrachus under short-term hypoxic stress and in the spleen under long-term hypoxic stress. Yang et al. (2017) found that Hif-1α mRNA expression is significantly elevated in the liver, gills, and brain of largemouth bass (Micropterus salmoides) under acute hypoxic stress. Thus, these studies suggest that the significant increase in Hif-1α mRNA expression in fish under hypoxic stress may be an adaptative strategy of the organism to such stress. In this study, the LcHif-1α mRNA expression level in the liver of L. crocea initially showed an increasing trend, with a peak at 6 h, and then a decreasing trend over the 96 h of hypoxic stress. Nevertheless, compared with the normoxic group, LcHif-1α expression was significantly higher in the livers of the hypoxic group over the stress period. Likewise, the LcHif-1α mRNA expression levels in the LYCF cells showed an increasing and then decreasing trend over the 48 h of hypoxic stress, highly consistent with the in vivo experimental results. Thus, the elevated Hif-1α mRNA expression levels may be a common response to hypoxic stress in fish, resembling its function in higher animals. Therefore, Hif-1α may play a key role in the adaptation of fish to hypoxia.
The molecular chaperone Hsp70 plays a key role in maintaining intracellular environmental homeostasis under stressful conditions (Evans et al., 2010; Mashaghi et al., 2014). The expression of Hsp70 is dependent on Hsf1, which binds to the promoter region of the Hsp70 gene and enhances its transcription (Calderwood et al., 2010; Wu, 1995). Interestingly, both Hsf1 and Hsp70 play a protective role in organisms in response to stressful conditions (Doubrovin et al., 2012; Lin et al., 2016; Peng et al., 2010; Yang et al., 2020). Accordingly, several studies have evaluated the changes in Hsf1 and Hsp70 expression in model animals under hypoxic stress. Michaud et al. (2011) showed that Hsp70 mRNA expression is significantly increased in Sarcophaga crassipalpis under hypoxic stress, whereas Baird et al. (2006) found that the Hsf1 protein and Hsp70 mRNA expression levels are significantly increased in Kc167 cells under hypoxic stress. Baek et al. (2001) showed that both Hsf1 transcriptional activity and Hsp70 protein expression are significantly increased in radiation-induced murine fibrosarcoma tumor cells under hypoxic stress, while Park et al. (2003) found that the ability of Hsf1 to bind to DNA and the Hsp70 mRNA and protein expression levels are significantly increased in colon cancer clone A cells under hypoxic stress. However, relatively few studies on changes in Hsf1 and Hsp70 expression have been studied in aquatic organisms under hypoxic stress. Kawabe & Yokoyama ( 2011) reported a significant increase in Hsf1 and Hsp70 mRNA expression in the gills of Pacific oysters under hypoxic stress, suggesting this may be a common adaptative mechanism of organisms in response to such stress. In the present study, the expression patterns of Hsf1 and Hsp70 mRNA in the L. crocea liver and LYCF cells under hypoxic stress were highly consistent. Both showed an initial upward trend, reached a peak at 6 h, and then showed a downward trend. Nevertheless, compared with the normoxic group, the Hsf1 and Hsp70 mRNA expression levels were significantly higher in the liver of the hypoxic group over the stress period, suggesting that Hsf1 and Hsp70 may function in the adaptation of L. crocea to hypoxia. In addition, we found that the Hsp70 protein expression in the L. crocea liver and LYCF cells under hypoxic stress initially increased and then decreased to a significantly lower level than that in the normoxic group. Based on this, we assumed that short-term hypoxic stress (<6 h) may induce a rapid increase in Hsp70 protein expression inL. crocea to facilitate adaptation to hypoxic stress, whereas prolongation of stress time (>6 h) led to a continuous disruption of the internal environmental homeostasis of the organism, eventually resulting in a significant decrease in the level of Hsp70 due to excessive depletion. Of note, the expression patterns of Hsf1 and Hsp70 under hypoxic stress were consistent with those of Hif-1α in our study. Therefore, Hif-1α, Hsf1, and Hsp70 may exhibit synergistic effects in the response ofL. crocea to hypoxia.
Role of Hsp70 in regulating redox homeostasis
Interestingly, Hsp70 plays various roles in the regulation of cellular redox homeostasis (Afolayan et al., 2014; Broome et al., 2006; Chen et al., 2012; Gu et al., 2012; Lazarev et al., 2016; Troyanova et al., 2015; Xu et al., 2018). The role of Hsp70 in maintaining redox homeostasis and inhibiting oxidative stress in organisms and cells under stressful conditions has been reported in several higher animals and model organisms. Yurinskaya et al. (2017) found that endotoxin can induce an increase in ROS levels in cultured human macrophages, but the addition of exogenous recombinant human Hsp70 results in a significant reduction. Jiang et al. (2020) showed that cytoplasmic exosome-induced Hsp70 significantly reduces ROS levels in mice with cerebral ischemia/reperfusion, thereby attenuating cerebral ischemia/reperfusion injury. Similarly, Russo et al. (2001) found that Hsp70 significantly attenuates the effects of alcohol-induced oxidative stress in rat astrocytes. Hernández-Santana et al. (2014) found that Hsp70 significantly reduces the level of oxidative stress in H2O2-treated C2C12 skeletal muscle cells, while Yurinskaya et al. (2015) showed that Hsp70 protects human neuroblastoma cells from amyloid isoAsp7-Aβ(1-42)-induced oxidative stress. Gu et al. (2012) found that enhanced Hsp70 expression significantly reduces the level of MDA in the intestines of chickens under acute high-temperature stress. To date, however, studies on the regulation of redox homeostasis by Hsp70 in aquatic animals under stressful conditions remain limited. Zeng et al. (2014) reported a significant increase in the levels of ROS in the rainbow trout gill epithelial cell line (RTgill-W1) following 2-phenylethynesulfonamide (PES) treatment after inhibition of Hsp70 expression. In our study, the levels of ROS, superoxide, MDA, PCO, and 8-OHdG were significantly increased in the LYCF cells after LcHsp70-interference and hypoxic stress for 24 h, indicating that knockdown of LcHsp70 expression significantly increased the levels of ROS, causing oxidative stress in LYCF cells under hypoxia. In contrast, overexpression of LcHsp70 significantly decreased the levels of ROS, superoxide, MDA, PCO, and 8-OHdG in the LYCF cells after hypoxic stress for 24 h, indicating that enhancing LcHsp70 expression may significantly attenuate oxidative stress-induced changes. Thus, we suggest that Hsp70 may be involved in the hypoxic response of L. crocea through regulation of redox homeostasis and prevention of oxidative stress; however, the specific mechanism underlying this regulation requires further investigation.
Anti-apoptotic effects of Hsp70
Under normal physiological conditions, apoptosis plays an important role in cellular self-renewal and maintenance of homeostasis; however, excessive apoptosis under stressful or pathological conditions can lead to a significant reduction in the number of cells in an organism (Majno & Joris, 1995). In mammals, Hsp70 inhibits apoptosis in multiple ways. For instance, Hsp70 inhibits the apoptosis of H2O2-treated C2C12 cells by up-regulating Bcl-2 protein expression and decreasing caspase-3 activity (Jiang et al., 2011). Ueng et al. (2013) found that Hsp70 prevents chondrocyte apoptosis by inhibiting the activation of caspase-3 in chondrocytes subjected to nitric oxide (NO) stress. In 293T and Jurkat T-lymphoma cells, Hsp70 directly binds to Apaf-1, preventing apoptosome assembly and inhibiting procaspase-9 activation, thus inhibiting apoptosis (Beere et al., 2000). However, only a few studies have explored the regulation of apoptosis by Hsp70 in aquatic organisms under stressful conditions. In one such study, Padmini & Tharani ( 2014) showed that up-regulation of Hsp70 in the hepatocytes of M. cephalus inhabiting contaminated waters suppresses apoptosis signal-regulated kinase 1 (Ask-1) protein expression, thus suggesting that Hsp70 is involved in the regulation of apoptosis of hepatocytes under stressful conditions through suppression of Ask-1. Zeng et al. (2014) reported that PES treatment in rainbow trout RTgill-W1 cells results in a significant decrease in Hsp70 protein expression, significant increase in the apoptotic rate, significant decrease in MMP, and significant increase in caspase-3 and -9 activities. Accordingly, the authors speculated that Hsp70 may play an inhibitory role in the mitochondrial pathway of apoptosis in RTgill-W1 cells under stressful conditions. In this study, Hsp70 knockdown led to a significant increase in the apoptotic rate of LYCF cells under hypoxic stress, whereas Hsp70 overexpression significantly decreased the apoptotic rate of the LYCF cells, indicating the anti-apoptotic effects of Hsp70 in LYCF cells under hypoxic stress. In addition, Hsp70 knockdown in LYCF cells under hypoxic stress resulted in a significant decrease in MMP, significant increase in Bax/Bcl-2 mRNA ratio, significant decrease in Bcl-2 protein expression, significant increase in caspase-3 mRNA expression, and significant increase in caspase-3 and -9 activities, whereas Hsp70 overexpression showed the opposite effects. These results indicate that Hsp70 may inhibit the caspase-dependent mitochondrial pathway of apoptosis in LYCF cells under hypoxic stress by maintaining normal MMP, enhancing Bcl-2 mRNA and protein expression, inhibiting Bax and caspase-3 mRNA expression, and inhibiting caspase-3 and -9 activation.
AIF is a caspase-independent apoptosis “executor”. Upon enhanced MMP, AIF translocates from the mitochondria to the cytoplasm and then to the nucleus, where it promotes the condensation of chromatin and fragmentation of genomic DNA (Susin et al., 1999). Ravagnan et al. (2001) demonstrated that Hsp70 directly binds to the AIF proapoptotic factor and inhibits its nuclear translocation, thereby inhibiting caspase-independent apoptosis in a cell-free system. To date, however, no studies have been reported on the regulation of AIF protein-mediated apoptosis by Hsp70 in fish under stressful conditions. Our study is the first to explore the regulatory role of LcHsp70 in AIF protein-mediated apoptosis in LYCF cells under hypoxic stress. Our results showed that LcHsp70 knockdown significantly increased the nuclear translocation of the AIF protein in LYCF cells under hypoxic stress, whereas LcHsp70 overexpression inhibited the nuclear translocation of AIF. These results suggest that Hsp70 may inhibit the caspase-independent mitochondrial apoptotic pathway in LYCF cells under hypoxic stress via inhibition of AIF nuclear translocation.
Of note, Hsp70 can also bind to the death receptor and inhibit the death-inducing signaling complex, and thus indirectly inhibit the procaspase-8 cleavage and downstream apoptotic cascade response (Gao et al., 2015; Guo et al., 2005). Several studies have reported on the regulation of caspase-8 activation by Hsp70 in higher animals under stressful or pathological conditions. For example, Matsumori et al. (2006) found that Hsp70 overexpression significantly reduces the active caspase-8 subunit (cleaved-caspase-8) in rats after hypoxic/ischemic treatment. In addition, Gao et al. (2015) reported that overexpression of Hsp70 inhibits caspase-8 activity in norepinephrine-treated rat cardiomyocytes (H9C2), while Kong et al. (2016) found that overexpression of Hsp70 significantly reduces cleaved-caspase-8 levels in H2O2-treated Buffalo rat liver (BRL) cells. However, as no studies have reported on the regulation of caspase-8 activation by Hsp70 in fish under stressful or pathological conditions, we explored this in the current study. We found that LcHsp70 knockdown in LYCF cells under hypoxic stress resulted in a significant increase in caspase-8 activity, whereas LcHsp70 overexpression had the opposite effect. These findings indicate that LcHsp70 may inhibit the extrinsic apoptosis pathway in LYCF cells under hypoxic stress via inhibition of caspase-8 activity.
Involvement of Hif-1α/Hsf1 in transcriptional regulation of Hsp70
The expression of Hsp70 under hypoxic stress is closely related to that of Hif-1α. In human hepatocellular carcinoma cells (HepG2) under hypoxic stress, Hif-1α expression is significantly inhibited after treatment with the Hif-1α-specific inhibitor 3-(5'-hydroxymethyl-2'-furyl)-1-benzylindazole (YC-1) or Hif-1α-specific siRNA, with concomitant down-regulation of the Hsp70 protein (Xia et al., 2009). In human articular chondrocytes under hypoxic stress, Hif-1α induces a significant increase in the expression of both Hsp70 mRNA and protein (Tsuchida et al., 2014). The transcription of Hsp70 mRNA is primarily mediated by Hsf1 (Doubrovin et al., 2012; Lin et al., 2016; Peng et al., 2010; Yang et al., 2020). Accordingly, several studies have explored the transcriptional regulation of Hif-1α, Hsf1, and Hsp70 under hypoxic stress. Baird et al. (2006) reported that the Hif-1α, Hsf1, and Hsp70 protein expression levels are significantly increased in Kc167 cells under hypoxic stress but are significantly decreased after Hif-1α knockdown, indicating Hif-1α-induced regulation of Hif-1α and Hsp70 expression in Kc167 cells under hypoxic stress. Knockdown of Hsf1 under hypoxic stress is also reported to reduce Hsp70 protein expression, thus suggesting Hsf1-induced regulation of Hsp70 expression in Kc167 cells under hypoxic stress. Chromatin immunoprecipitation analysis has also shown that, under hypoxic stress, Hif-1α directly binds to the HRE in the Hsf1 promoter, suggesting that Hif-1α activates its expression in Kc167 cells under hypoxic stress, with Hsf1 acting as a transcription factor to further activate Hsp70 expression (Baird et al., 2006). Kawabe & Yokoyama ( 2011) showed that the transcription of Hsp70 in Pacific oysters under hypoxic stress is regulated by the Hif-1/Hsf1 pathway. In contrast, Xia et al. (2009) found that Hif-1α directly binds to the HRE in the Hsp70 promoter independent of Hsf1 and activates the transcription of Hsp70 in HCC cells under hypoxic stress. In this study, LcHif-1α knockdown significantly reduced the expression of LcHsf1 mRNA and activity of the LcHsf1 DNA promoter in LYCF cells under hypoxic stress, whereas LcHif-1α overexpression had the opposite effect, indicating that Hif-1α positively regulates Hsf1 transcription in LYCF cells under hypoxic stress. Likewise, LcHsf1 overexpression significantly increased LcHsp70 mRNA expression and LcHsp70 DNA promoter activity in LYCF cells under hypoxic stress, suggesting Hsf1-induced positive regulation of Hsp70 transcription in LYCF cells under hypoxic stress. Thus, in agreement with our proposed hypothesis, Hif-1α appears to enhance Hsp70 gene transcription in L. crocea under hypoxic stress via Hsf1.
CONCLUSIONS
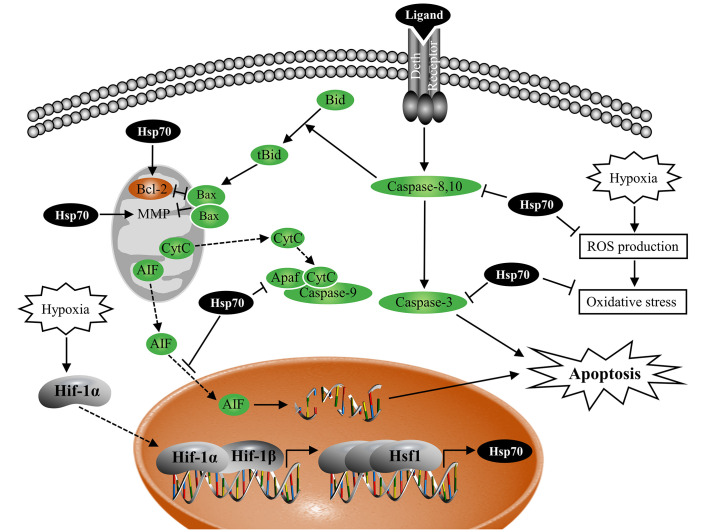
In this study, hypoxic stress induced the expression levels of Hif-1α, Hsf1, and Hsp70 in L. crocea. Notably, Hsp70 was involved in maintaining redox homeostasis and resistance to oxidative stress in LYCF cells under hypoxic stress. Hsp70 inhibited the caspase-dependent mitochondrial apoptotic pathway in LYCF cells under hypoxic stress by maintaining normal MMP, enhancing Bcl-2 mRNA and protein expression, inhibiting Bax and caspase3 mRNA expression, and suppressing caspase-3 and -9 activation. In addition, Hsp70 inhibited the caspase-independent mitochondrial and extrinsic apoptosis pathways of LYCF cells under hypoxic stress by inhibiting AIF nuclear translocation and caspase-8 activity, respectively. Of note, in the LYCF cells under hypoxic stress, Hif-1α activated the Hsf1 DNA promoter and enhanced the transcription of Hsf1 mRNA, whereas Hsf1 promoted the transcription of Hsp70 mRNA by binding to and activating its promoter. Conclusively, the Hif-1α/Hsf1/Hsp70 signaling pathway is involved in the regulation of redox homeostasis and anti-apoptosis in L. crocea under hypoxic stress (Figure 7).
Figure 7.
Regulation mode of Hif-1α/Hsf1/Hsp70 on redox homeostasis and apoptosis under hypoxic stress
Hif-1α protein accumulates in the nucleus under hypoxic stress and polymerizes with Hif-1β to form transcriptionally active Hif-1, which binds to the hypoxia response element in the Hsf1 DNA promoter and enhances Hsf1 mRNA transcription. Hsf1 homotrimer enters the nucleus and binds to the heat shock response element in the Hsp70 DNA promoter and enhances Hsp70 mRNA transcription. Hsp70 plays a role in maintaining redox homeostasis and antioxidative stress in LYCF cells under hypoxic stress; Hsp70 enhances Bcl-2 mRNA and protein expression, inhibits Bax and Caspase-3 mRNA expression, suppresses caspase-3, -8, and -9 activation, maintains normal MMP, and inhibits AIF entry into the nucleus to suppress apoptosis in LYCF cells under hypoxic stress.
SUPPLEMENTARY DATA
Supplementary data to this article can be found online.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
S.Y.L., J.Q.Z., and B.L. designed the research. S.Y.L., J.Q.W., C.L., Y.B.Z., J.D., C.D.Z., and D.J.T. performed the research. S.Y.L., C.L., and X.M.G. analyzed the data. S.Y.L. and J.Q.Z. wrote the paper. S.Y.L., X.M.G., C.C.H., X.F.W., B.L., and W.L.S. modified the manuscript. All authors read and approved the final version of the manuscript.
The authors thank Dr. You-Hua Huang for providing the LYCF cell line. The authors would also like to thank Editage (www.editage.cn) for English language editing.
ACKNOWLEDGEMENTS
The authors thank Dr. You-Hua Huang for providing the LYCF cell line. The authors would also like to thank Editage (www.editage.cn) for English language editing.
Funding Statement
This work was supported by the National Key Research and Development Program of China (2018YFC1406300), NSFC-Zhejiang Joint Fund for the Integration of Industrialization and Informatization (U1809212), Scientific and Technical Project of Zhejiang Province (2021C02069-1, 2016C02055-7), Scientific and Technical Project of Ningbo City (2021Z002, 2015C110005), Ningbo Science and Technology Plan Projects (2018A610228), Teaching and Research Project of Ningbo University (XYL19023), Collaborative Innovation Center for Zhejiang Marine High-Efficiency and Healthy Aquaculture, K.C. Wong Magna Fund in Ningbo University
Contributor Information
Jun-Quan Zhu, Email: zhujunquan@nbu.edu.cn.
Bao Lou, Email: loubao6577@163.com.
References
- 1.Afolayan AJ, Teng RJ, Eis A, Rana U, Broniowska KA, Corbett JA, et al Inducible HSP70 regulates superoxide dismutase-2 and mitochondrial oxidative stress in the endothelial cells from developing lungs. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2014;306(4):L351–L360. doi: 10.1152/ajplung.00264.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azad P, Ryu J, Haddad GG Distinct role of Hsp70 in Drosophila hemocytes during severe hypoxia . Free Radical Biology and Medicine. 2011;51(2):530–538. doi: 10.1016/j.freeradbiomed.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baek SH, Lee UY, Park EM, Han MY, Lee YS, Park YM Role of protein kinase Cδ in transmitting hypoxia signal to HSF and HIF-1. Journal of Cellular Physiology. 2001;188(2):223–235. doi: 10.1002/jcp.1117. [DOI] [PubMed] [Google Scholar]
- 4.Baird NA, Turnbull DW, Johnson EA Induction of the heat shock pathway during hypoxia requires regulation of heat shock factor by hypoxia-inducible factor-1. Journal of Biological Chemistry. 2006;281(50):38675–38681. doi: 10.1074/jbc.M608013200. [DOI] [PubMed] [Google Scholar]
- 5.Beere HM, Wolf BB, Cain K, Mosser DD, Mahboubi A, Kuwana T, et al Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nature Cell Biology. 2000;2(8):469–475. doi: 10.1038/35019501. [DOI] [PubMed] [Google Scholar]
- 6.Breitburg D, Levin LA, Oschlies A, Grégoire M, Chavez FP, Conley DJ, et al Declining oxygen in the global ocean and coastal waters. Science. 2018;359(6371):eaam7240. doi: 10.1126/science.aam7240. [DOI] [PubMed] [Google Scholar]
- 7.Broome CS, Kayani AC, Palomero J, Dillmann WH, Mestril R, Jackson MJ, et al Effect of lifelong overexpression of HSP70 in skeletal muscle on age-related oxidative stress and adaptation after nondamaging contractile activity. The FASEB Journal. 2006;20(9):1549–1551. doi: 10.1096/fj.05-4935fje. [DOI] [PubMed] [Google Scholar]
- 8.Bruick RK, McKnight SL Transcription. Oxygen sensing gets a second wind. Science. 2002;295(5556):807–808. doi: 10.1126/science.1069825. [DOI] [PubMed] [Google Scholar]
- 9.Calderwood SK, Xie Y, Wang X, Khaleque MA, Chou SD, Murshid A, et al Signal transduction pathways leading to heat shock transcription. Signal Transduction Insights. 2010;2:13–24. doi: 10.4137/STI.S3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen F, Yu YF, Qian J, Wang YS, Cheng B, Dimitropoulou C, et al Opposing actions of heat shock protein 90 and 70 regulate nicotinamide adenine dinucleotide phosphate oxidase stability and reactive oxygen species production. Arteriosclerosis, Thrombosis, and Vascular Biology. 2012;32(12):2989–2999. doi: 10.1161/ATVBAHA.112.300361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooper RU, Clough LM, Farwell MA, West TL. 2002. Hypoxia-induced metabolic and antioxidant enzymatic activities in the estuarine fish Leiostomus xanthurus. Journal of Experimental Marine Biology and Ecology, 279(1–2): 1–20.
- 12.Deane EE, Jia A, Qu Z, Chen JX, Zhang XH, Woo NYS Induction of apoptosis in sea bream fibroblasts by Vibrio harveyi haemolysin and evidence for an anti-apoptotic role of heat shock protein 70 . Journal of Fish Diseases. 2012;35(4):287–302. doi: 10.1111/j.1365-2761.2012.01346.x. [DOI] [PubMed] [Google Scholar]
- 13.Deane EE, Zhou LR, Woo NYS Cortisol can be pro-or anti-apoptotic in sea bream cells: Potential role of HSP70 induction for cytoprotection. Molecular and Cellular Endocrinology. 2006;259(1-2):57–64. doi: 10.1016/j.mce.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Diao LW, Zhao LL, Qi F, Sun ZD, Zhang QH, Wu NS Heat shock protein 70 induced by heat stress protects heterotopically transplanted hearts in rats. Molecular Medicine Reports. 2012;6(4):729–732. doi: 10.3892/mmr.2012.982. [DOI] [PubMed] [Google Scholar]
- 15.Diaz RJ, Rosenberg R Spreading dead zones and consequences for marine ecosystems. Science. 2008;321(5891):926–929. doi: 10.1126/science.1156401. [DOI] [PubMed] [Google Scholar]
- 16.Domingos FFT, Thomé RG, Martinelli PM, Sato Y, Bazzoli N, Rizzo E Role of HSP70 in the regulation of the testicular apoptosis in a seasonal breeding teleost Prochilodus argenteus from the São Francisco River, Brazil . Microscopy Research & Technique. 2013;76(4):350–356. doi: 10.1002/jemt.22173. [DOI] [PubMed] [Google Scholar]
- 17.Doubrovin M, Che JT, Serganova I, Moroz E, Solit DB, Ageyeva L, et al Monitoring the induction of heat shock factor 1/heat shock protein 70 expression following 17-allylamino-demethoxygeldanamycin treatment by positron emission tomography and optical reporter gene imaging. Molecular Imaging. 2012;11(1):67–76. [PMC free article] [PubMed] [Google Scholar]
- 18.Ema M, Hirota K, Mimura J, Abe H, Yodoi J, Sogawa K, et al Molecular mechanisms of transcription activation by HLF and HIF1α in response to hypoxia: their stabilization and redox signal-induced interaction with CBP/p300. The EMBO Journal. 1999;18(7):1905–1914. doi: 10.1093/emboj/18.7.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evans CG, Chang L, Gestwicki JE Heat shock protein 70 (Hsp70) as an emerging drug target. Journal of Medicinal Chemistry. 2010;53(12):4585–4602. doi: 10.1021/jm100054f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao XJ, Liu WL, Huang LS, Zhang T, Mei ZS, Wang XX, et al HSP70 inhibits stress-induced cardiomyocyte apoptosis by competitively binding to FAF1. Cell Stress and Chaperones. 2015;20(4):653–661. doi: 10.1007/s12192-015-0589-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao XM, Mu DL, Hou CC, Zhu JQ, Jin S, Wang CL. 2019. Expression and putative functions of KIFC1 for nuclear reshaping and midpiece formation during spermiogenesis of Phascolosoma esculenta. Gene, 683: 169–183.
- 22.Giffard RG, Han RQ, Emery JF, Duan M, Pittet JF Regulation of apoptotic and inflammatory cell signaling in cerebral ischemia: the complex roles of heat shock protein 70. Anesthesiology. 2008;109(2):339–348. doi: 10.1097/ALN.0b013e31817f4ce0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grilo AL, Mantalaris A Apoptosis: a mammalian cell bioprocessing perspective. Biotechnology Advances. 2019;37(3):459–475. doi: 10.1016/j.biotechadv.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 24.Gu XH, Hao Y, Wang XL Overexpression of heat shock protein 70 and its relationship to intestine under acute heat stress in broilers: 2. Intestinal oxidative stress. Poultry Science. 2012;91(4):790–799. doi: 10.3382/ps.2011-01628. [DOI] [PubMed] [Google Scholar]
- 25.Guérin P, El Mouatassim S, Ménézo Y Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Human Reproduction Update. 2001;7(2):175–189. doi: 10.1093/humupd/7.2.175. [DOI] [PubMed] [Google Scholar]
- 26.Guo F, Sigua C, Bali P, George P, Fiskus W, Scuto A, et al Mechanistic role of heat shock protein 70 in Bcr-Abl–mediated resistance to apoptosis in human acute leukemia cells. Blood. 2005;105(3):1246–1255. doi: 10.1182/blood-2004-05-2041. [DOI] [PubMed] [Google Scholar]
- 27.Gupta SC, Siddique HR, Mathur N, Vishwakarma AL, Mishra RK, Saxena DK, et al Induction of hsp70, alterations in oxidative stress markers and apoptosis against dichlorvos exposure in transgenic Drosophila melanogaster: modulation by reactive oxygen species . Biochimica et Biophysica Acta (BBA)-General Subjects. 2007;1770(9):1382–1394. doi: 10.1016/j.bbagen.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 28.Hernández-Santana A, Pérez-López V, Zubeldia JM, Jiménez-del-Rio M A rhodiola rosea root extract protects skeletal muscle cells against chemically induced oxidative stress by modulating heat shock protein 70 (HSP70) expression. Phytotherapy Research. 2014;28(4):623–628. doi: 10.1002/ptr.5046. [DOI] [PubMed] [Google Scholar]
- 29.Jiang BM, Liang PF, Deng GH, Tu ZZ, Liu MD, Xiao XZ Increased stability of Bcl-2 in HSP70-mediated protection against apoptosis induced by oxidative stress. Cell Stress and Chaperones. 2011;16(2):143–152. doi: 10.1007/s12192-010-0226-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang YB, He RY, Shi YJ, Liang J, Zhao L Plasma exosomes protect against cerebral ischemia/reperfusion injury via exosomal HSP70 mediated suppression of ROS. Life Sciences. 2020;256:117987. doi: 10.1016/j.lfs.2020.117987. [DOI] [PubMed] [Google Scholar]
- 31.Kawabe S, Yokoyama Y. 2011. Novel isoforms of heat shock transcription factor 1 are induced by hypoxia in the Pacific oyster Crassostrea gigas. Journal of Experimental Zoology Part A: Ecological and Integrative Physiology, 315(7): 394–407.
- 32.Klein JA, Ackerman SL Oxidative stress, cell cycle, and neurodegeneration. The Journal of Clinical Investigation. 2003;111(6):785–793. doi: 10.1172/JCI200318182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kong FZ, Wang H, Guo JR, Peng ML, Ji H, Yang HM, et al Hsp70 suppresses apoptosis of BRL cells by regulating the expression of Bcl-2, cytochrome C, and caspase 8/3. In Vitro Cellular & Developmental Biology-Animal. 2016;52(5):568–575. doi: 10.1007/s11626-016-0005-5. [DOI] [PubMed] [Google Scholar]
- 34.Lazarev VF, Nikotina AD, Mikhaylova ER, Nudler E, Polonik SG, Guzhova IV, et al Hsp70 chaperone rescues C6 rat glioblastoma cells from oxidative stress by sequestration of aggregating GAPDH. Biochemical and Biophysical Research Communications. 2016;470(3):766–771. doi: 10.1016/j.bbrc.2015.12.076. [DOI] [PubMed] [Google Scholar]
- 35.Leonarduzzi G, Sottero B, Poli G Targeting tissue oxidative damage by means of cell signaling modulators: the antioxidant concept revisited. Pharmacology & Therapeutics. 2010;128(2):336–374. doi: 10.1016/j.pharmthera.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 36.Lin PY, Folorunso O, Taglialatela G, Pierce A Overexpression of heat shock factor 1 maintains TAR DNA binding protein 43 solubility via induction of inducible heat shock protein 70 in cultured cells. Journal of Neuroscience Research. 2016;94(7):671–682. doi: 10.1002/jnr.23725. [DOI] [PubMed] [Google Scholar]
- 37.Liu SG, Ren PY, Wang GY, Yao SX, He XJ Allicin protects spinal cord neurons from glutamate-induced oxidative stress through regulating the heat shock protein 70/inducible nitric oxide synthase pathway. Food & Function. 2015;6(1):320–329. doi: 10.1039/c4fo00761a. [DOI] [PubMed] [Google Scholar]
- 38.Liu W, Liu XX, Wu CW, Jiang LH. 2018. Transcriptome analysis demonstrates that long noncoding RNA is involved in the hypoxic response in Larimichthys crocea. Fish Physiology and Biochemistry, 44(5): 1333–1347.
- 39.Lohberger B, Steinecker-Frohnwieser B, Stuendl N, Kaltenegger H, Leithner A, Rinner B The proteasome inhibitor bortezomib affects chondrosarcoma cells via the mitochondria-caspase dependent pathway and enhances death receptor expression and autophagy. PLoS One. 2016;11(12):e0168193. doi: 10.1371/journal.pone.0168193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu JK, Yu ZB, Mu CK, Li RH, Song WW, Wang CL. 2017. Characterization and functional analysis of a novel C-type lectin from the swimming crab Portunus trituberculatus. Fish & Shellfish Immunology, 64: 185–192.
- 41.Luo SY, Gao XM, Ding J, Liu C, Du C, Hou CC, et al Transcriptome sequencing reveals the traits of spermatogenesis and testicular development in large yellow croaker (Larimichthys crocea) . Genes. 2019;10(12):958. doi: 10.3390/genes10120958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luo SY, Liu C, Ding J, Gao XM, Wang JQ, Zhang YB, et al Scavenging reactive oxygen species is a potential strategy to protect Larimichthys crocea against environmental hypoxia by mitigating oxidative stress . Zoological Research. 2021;42(5):592–605. doi: 10.24272/j.issn.2095-8137.2021.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lv ZM, Li CH, Zhang PJ, Wang ZH, Zhang WW, Jin CH. 2015. miR-200 modulates coelomocytes antibacterial activities and LPS priming via targeting Tollip in Apostichopus japonicus. Fish & Shellfish Immunology, 45(2): 431–436.
- 44.Majno G, Joris I Apoptosis, oncosis, and necrosis. An overview of cell death. The American Journal of Pathology. 1995;146(1):3–15. [PMC free article] [PubMed] [Google Scholar]
- 45.Mashaghi A, Kramer G, Lamb DC, Mayer MP, Tans SJ Chaperone action at the single-molecule level. Chemical Reviews. 2014;114(1):660–676. doi: 10.1021/cr400326k. [DOI] [PubMed] [Google Scholar]
- 46.Matsumori Y, Northington FJ, Hong SM, Kayama T, Sheldon RA, Vexler ZS, et al Reduction of caspase-8 and -9 cleavage is associated with increased c-FLIP and increased binding of Apaf-1 and Hsp70 after neonatal hypoxic/ischemic injury in mice overexpressing Hsp70. Stroke. 2006;37(2):507–512. doi: 10.1161/01.STR.0000199057.00365.20. [DOI] [PubMed] [Google Scholar]
- 47.Michaud MR, Teets NM, Peyton JT, Blobner BM, Denlinger DL Heat shock response to hypoxia and its attenuation during recovery in the flesh fly. Sarcophaga crassipalpis. Journal of Insect Physiology. 2011;57(1):203–210. doi: 10.1016/j.jinsphys.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 48.Ming JH, Ye JY, Zhang YX, Yang X, Shao XP, Qiang J, et al Dietary optimal reduced glutathione improves innate immunity, oxidative stress resistance and detoxification function of grass carp (Ctenopharyngodon idella) against microcystin-LR . Aquaculture. 2019;498:594–605. doi: 10.1016/j.aquaculture.2018.09.014. [DOI] [Google Scholar]
- 49.Mohindra V, Tripathi RK, Singh RK, Lal KK Molecular characterization and expression analysis of three hypoxia-inducible factor alpha subunits, HIF-1α, -2α and -3α in hypoxia-tolerant Indian catfish, Clarias batrachus [Linnaeus, 1758] . Molecular Biology Reports. 2013;40(10):5805–5815. doi: 10.1007/s11033-013-2685-1. [DOI] [PubMed] [Google Scholar]
- 50.Ondricek K, Thomas P Effects of hypoxia exposure on apoptosis and expression of membrane steroid receptors, ZIP9, mPRα, and GPER in Atlantic croaker ovaries. Comparative Biochemistry and Physiology Part A:Molecular & Integrative Physiology. 2018;224:84–92. doi: 10.1016/j.cbpa.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 51.Padmini E, Tharani J. 2014. Heat-shock protein 70 modulates apoptosis signal-regulating kinase 1 in stressed hepatocytes of Mugil cephalus. Fish Physiology and Biochemistry, 40(5): 1573–1585.
- 52.Pan WL, Wong JH, Fang EF, Chan YS, Ng TB, Cheung RCF Preferential cytotoxicity of the type I ribosome inactivating protein alpha-momorcharin on human nasopharyngeal carcinoma cells under normoxia and hypoxia. Biochemical Pharmacology. 2014;89(3):329–339. doi: 10.1016/j.bcp.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park EM, Lee IJ, Kim SH, Song GY, Park YM Inhibitory effect of a naphthazarin derivative, S64, on heat shock factor (Hsf) activation and glutathione status following hypoxia. Cell Biology and Toxicology. 2003;19(5):273–284. doi: 10.1023/B:CBTO.0000004935.81879.d7. [DOI] [PubMed] [Google Scholar]
- 54.Peng W, Zhang Y, Zheng M, Cheng HP, Zhu WZ, Cao CM, et al Cardioprotection by CaMKII-δB is mediated by phosphorylation of heat shock factor 1 and subsequent expression of inducible heat shock protein 70. Circulation Research. 2010;106(1):102–110. doi: 10.1161/CIRCRESAHA.109.210914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rahman MS, Thomas P Characterization of three IGFBP mRNAs in Atlantic croaker and their regulation during hypoxic stress: potential mechanisms of their upregulation by hypoxia. American Journal of Physiology-Endocrinology and Metabolism. 2011;301(4):E637–E648. doi: 10.1152/ajpendo.00168.2011. [DOI] [PubMed] [Google Scholar]
- 56.Ravagnan L, Gurbuxani S, Susin SA, Maisse C, Daugas E, Zamzami N, et al Heat-shock protein 70 antagonizes apoptosis-inducing factor. Nature Cell Biology. 2001;3(9):839–843. doi: 10.1038/ncb0901-839. [DOI] [PubMed] [Google Scholar]
- 57.Rimoldi S, Terova G, Ceccuzzi P, Marelli S, Antonini M, Saroglia M HIF-1α mRNA levels in Eurasian perch (Perca fluviatilis) exposed to acute and chronic hypoxia . Molecular Biology Reports. 2012;39(4):4009–4015. doi: 10.1007/s11033-011-1181-8. [DOI] [PubMed] [Google Scholar]
- 58.Russo A, Palumbo M, Scifo C, Cardile V, Barcellona ML, Renis M Ethanol-induced oxidative stress in rat astrocytes: role of HSP70. Cell Biology and Toxicology. 2001;17(3):153–168. doi: 10.1023/A:1011936313510. [DOI] [PubMed] [Google Scholar]
- 59.Saini J, Sharma PK Clinical, prognostic and therapeutic significance of heat shock proteins in cancer. Current Drug Targets. 2018;19(13):1478–1490. doi: 10.2174/1389450118666170823121248. [DOI] [PubMed] [Google Scholar]
- 60.Semenza GL Hydroxylation of HIF-1: oxygen sensing at the molecular level. Physiology. 2004;19(4):176–182. doi: 10.1152/physiol.00001.2004. [DOI] [PubMed] [Google Scholar]
- 61.Semenza GL Regulation of cancer cell metabolism by hypoxia-inducible factor 1. Seminars in Cancer Biology. 2009;19(1):12–16. doi: 10.1016/j.semcancer.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 62.Stankiewicz AR, Lachapelle G, Foo CPZ, Radicioni SM, Mosser DD Hsp70 inhibits heat-induced apoptosis upstream of mitochondria by preventing Bax translocation. Journal of Biological Chemistry. 2005;280(46):38729–38739. doi: 10.1074/jbc.M509497200. [DOI] [PubMed] [Google Scholar]
- 63.Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, et al Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397(6718):441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- 64.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution. 2011;28(10):2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Troyanova NI, Shevchenko MA, Boyko AA, Mirzoev PP, Pertseva MA, Kovalenko EI, et al Modulating effect of extracellular hsp70 on generation of reactive oxigen species in populations of phagocytes. Russian Journal of Bioorganic Chemistry. 2015;41(3):271–279. doi: 10.1134/S1068162015030097. [DOI] [PubMed] [Google Scholar]
- 66.Tsuchida S, Arai Y, Takahashi KA, Kishida T, Terauchi R, Honjo K, et al HIF-1α-induced HSP70 regulates anabolic responses in articular chondrocytes under hypoxic conditions. Journal of Orthopaedic Research. 2014;32(8):975–980. doi: 10.1002/jor.22623. [DOI] [PubMed] [Google Scholar]
- 67.Ueng SWN, Yuan LJ, Lin SS, Niu CC, Chan YS, Wang IC, et al Hyperbaric oxygen treatment prevents nitric oxide-induced apoptosis in articular cartilage injury via enhancement of the expression of heat shock protein 70. Journal of Orthopaedic Research. 2013;31(3):376–384. doi: 10.1002/jor.22235. [DOI] [PubMed] [Google Scholar]
- 68.Wang QF, Shen WL, Hou CC, Liu C, Wu XF, Zhu JQ Physiological responses and changes in gene expression in the large yellow croaker Larimichthys crocea following exposure to hypoxia . Chemosphere. 2017;169:418–427. doi: 10.1016/j.chemosphere.2016.11.099. [DOI] [PubMed] [Google Scholar]
- 69.Wang XH, Li QH, Mu PF, Guan YY, Chen XH, Ao JQ Large yellow croaker peroxiredoxin IV protect cells against oxidative damage and apoptosis. Molecular Immunology. 2020;127:150–156. doi: 10.1016/j.molimm.2020.08.019. [DOI] [PubMed] [Google Scholar]
- 70.Wenger RH, Stiehl DP, Camenisch G Integration of oxygen signaling at the consensus HRE. Science's STKE:Signal Transduction Knowledge Environment. 2005;2005(306):re12. doi: 10.1126/stke.3062005re12. [DOI] [PubMed] [Google Scholar]
- 71.Williams TA, Bergstrome JC, Scott J, Bernier NJ CRF and urocortin 3 protect the heart from hypoxia/reoxygenation-induced apoptosis in zebrafish. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2017;313(2):R91–R100. doi: 10.1152/ajpregu.00045.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu C Heat shock transcription factors: structure and regulation. Annual Review of Cell and Developmental Biology. 1995;11:441–469. doi: 10.1146/annurev.cb.11.110195.002301. [DOI] [PubMed] [Google Scholar]
- 73.Xia LM, Tian DA, Zhang Q, Yan W, Zhu Q, Luo M, et al Hypoxia induces heat shock protein HSP70-2 expression in a HIF-1 dependent manner. Chinese Journal of Hepatology. 2009;17(3):207–212. [PubMed] [Google Scholar]
- 74.Xu NW, Chen Y, Liu WE, Chen YJ, Fan ZM, Liu M, et al Inhibition of JAK2/STAT3 signaling pathway suppresses proliferation of Burkitt's lymphoma raji cells via cell cycle progression, apoptosis, and oxidative stress by modulating HSP70. Medical Science Monitor. 2018;24:6255–6263. doi: 10.12659/MSM.910170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang S, Yan T, Wu H, Xiao Q, Fu HM, Luo J, et al Acute hypoxic stress: Effect on blood parameters, antioxidant enzymes, and expression of HIF-1alpha and GLUT-1 genes in largemouth bass (Micropterus salmoides) . Fish & Shellfish Immunology. 2017;67:449–458. doi: 10.1016/j.fsi.2017.06.035. [DOI] [PubMed] [Google Scholar]
- 76.Yang XR, Gao YJ, Zhao MH, Wang XY, Zhou H, Zhang AY Cloning and identification of grass carp transcription factor HSF1 and its characterization involving the production of fish HSP70. Fish Physiology and Biochemistry. 2020;46(6):1933–1945. doi: 10.1007/s10695-020-00842-4. [DOI] [PubMed] [Google Scholar]
- 77.Yenari MA, Liu JL, Zheng Z, Vexler ZS, Lee JE, Giffard RG Antiapoptotic and anti-inflammatory mechanisms of heat-shock protein protection. Annals of the New York Academy of Sciences. 2005;1053:74–83. doi: 10.1196/annals.1344.007. [DOI] [PubMed] [Google Scholar]
- 78.Yuan ZH, Liu SK, Yao J, Zeng QF, Tan SX, Liu ZJ Expression of Bcl-2 genes in channel catfish after bacterial infection and hypoxia stress. Developmental & Comparative Immunology. 2016;65:79–90. doi: 10.1016/j.dci.2016.06.018. [DOI] [PubMed] [Google Scholar]
- 79.Yurinskaya MM, Kochetkova OY, Shabarchina LI, Antonova OY, Suslikov AV, Evgen'ev MB, et al Encapsulated Hsp70 decreases endotoxin-induced production of ROS and TNFα in human phagocytes. Cell Stress and Chaperones. 2017;22(1):163–171. doi: 10.1007/s12192-016-0743-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yurinskaya MM, Mitkevich VA, Kozin SA, Evgen'ev MB, Makarov AA, Vinokurov MG HSP70 protects human neuroblastoma cells from apoptosis and oxidative stress induced by amyloid peptide isoAsp7-Aβ(1–42) . Cell Death & Disease. 2015;6(11):e1977. doi: 10.1038/cddis.2015.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zeng FX, Tee C, Liu M, Sherry JP, Dixon B, Duncker BP, et al The p53/HSP70 inhibitor, 2-phenylethynesulfonamide, causes oxidative stress, unfolded protein response and apoptosis in rainbow trout cells. Aquatic Toxicology. 2014;146:45–51. doi: 10.1016/j.aquatox.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 82.Zhong H, Mabjeesh NJ, Willard MT, Simons JW Nuclear expression of hypoxia-inducible factor 1α protein is heterogeneous in human malignant cells under normoxic conditions. Cancer Letters. 2002;181(2):233–238. doi: 10.1016/S0304-3835(02)00053-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data to this article can be found online.