Abstract
Two diagnostic tests, an Aspergillus-specific PCR and an enzyme-linked immunosorbent assay (ELISA) for the quantitative determination of galactomannan, were compared for diagnosing and monitoring invasive pulmonary aspergillosis. Persistently neutropenic rats with left-sided invasive pulmonary aspergillosis were sacrificed at regular intervals after inoculation. Blood samples and bronchoalveolar lavage (BAL) fluid were cultured and tested by PCR as well as by ELISA. Disseminated fungal infection in extrapulmonary organs was determined. The sensitivity of the ELISA was higher than that of the PCR on all days of measurements, in both blood and BAL fluid. Positive PCR or ELISA results in blood were not significantly associated with disseminated fungal infection. Serial testing in a separate group of rats showed consistently increasing concentrations of circulating galactomannan during the course of disease, while a positive PCR could be followed by negative results. The concentration of galactomannan was highly predictive for the time of survival (P < 0.0001). It was concluded that, in this model, quantitative galactomannan detection is superior to PCR in diagnosing and monitoring invasive pulmonary aspergillosis.
The incidence of invasive pulmonary aspergillosis (IPA) has increased considerably in the past decade, and this infection is now a major cause of morbidity and mortality in immunocomprised hosts (9). Patients with prolonged chemotherapy-induced neutropenia and transplant recipients receiving long-term, high-dose corticoid therapy are at greatest risk (2). Although mortality rates of IPA remain high despite the use of antifungal therapy, observations suggest that the mortality rate may be reduced by early diagnosis and treatment (1, 6). However, no method has proven sufficiently sensitive and specific to allow a diagnosis at an early stage (22), and new diagnostic methods are therefore under investigation.
Methods for the molecular and serological diagnosis of IPA in blood or bronchoalveolar lavage (BAL) fluid have drawn particular attention. Using a PCR method for detecting Aspergillus-specific nucleotide sequences, Einsele et al. (7) found a 77% sensitivity in patients with IPA prior to antifungal therapy, the sensitivity increasing to 100% when two blood samples were analyzed. Among the techniques based on antigen detection, the sandwich enzyme-linked immunosorbent assay (ELISA) for the detection of galactomannan (GM) is currently the most promising. Studies in neutropenic patients report sensitivities of between 70 and 90% when applying the test to serum (4, 19, 21). It must be noted, however, that in these clinical studies it is often not indicated how early during the course of the disease the test becomes positive in relation to the development of clinical and radiological signs. Actually, clinical investigations into the sensitivity and specificity of tests for IPA are hampered by the absence of proven infection in many patients and by the fact that the time of onset of infection cannot be determined.
In the present study, PCR (two separate assays) and GM detection were evaluated in a rat model of IPA that has been developed in our laboratory (12). Using this animal model, we were able to compare both tests in the early phase of the disease, with a known time of onset of the infection. In addition, we determined the value of these tests for monitoring the course of the disease.
MATERIALS AND METHODS
Infection model of IPA.
The animal model used was as described previously (12), with some modifications to lengthen the survival time. Specific-pathogen-free female RP strain albino rats (18 to 25 weeks old, 185 to 225 g) were used. Neutropenia was induced by intraperitoneal (i.p.) administration of cyclophosphamide (Sigma-Aldrich Chemie, Steinheim, Germany) at 75 mg/kg 5 days before inoculation, followed by repeated doses of cyclophosphamide at 60 mg/kg i.p. 1 day before and 3 and 7 days after inoculation. This protocol resulted in granulocyte counts of less than 108/liter on the day of inoculation. To prevent bacterial superinfections, animals received ciprofloxacin (660 mg/liter) and polymyxin B (100 mg/liter) in their drinking water during the entire experiment. Starting 1 day before inoculation, daily intramuscular (i.m.) doses of amoxicillin (40 mg/kg/day) were added to this regimen for the remainder of the experiment. On the day of inoculation, gentamicin (6 mg/kg) was added i.m. to the regimen. For infection of the rats a strain of Aspergillus fumigatus was used that was originally isolated from an immunocompromised patient with IPA.
While the animals were under general anesthesia, the left main bronchus was intubated. A cannula was passed through the tube, and the left lung was inoculated with 2 × 104 A. fumigatus conidia. This resulted in a one-sided IPA. The mortality rate was ±50% on day 7 and 90 to 100% on day 12 after inoculation. At the end of the experiments or at the indicated intervals, rats were sacrificed and the left lung, as well as the right lung, liver, spleen, and brain, were homogenized and cultured to determine the presence of disseminated fungal infection. In approximately half of the rats, fungal dissemination to extrapulmonary organs occurred, especially to the liver. Blood cultures for Aspergillus species always remained negative in this model.
Blood sampling and BAL.
Groups of rats were sacrificed to obtain blood for PCR and GM detection and to determine the presence of disseminated fungal infection. While the animals were under CO2 anesthesia, blood samples were taken by cardiac puncture. Bronchoalveolar lavage (BAL) was performed by exposing the trachea and lavaging the lungs three times with 5 ml of phosphate-buffered saline (PBS). Of the BAL sample, 2 ml was used for culture, 1 ml was used for PCR, and 300 μl was used for GM detection. To monitor the course of disease in individual rats, sequential blood sampling was performed by puncture of the orbital plexus.
PCR.
Two different methods were used for the extraction of fungal DNA from fluids: an in-house method developed in our laboratory by van Deventer et al. (21) and a method of Einsele et al. (7, 13, 14), with some modifications.
DNA extraction from fungal suspensions and BAL fluid.
In the in-house method, 1 ml of fungal suspension or BAL fluid was centrifuged at 16,000 × g for 5 min. Pellets were resuspended in 0.2 ml of TEG buffer (50 mM glucose, 25 mM Tris-HCl [pH 8.0], 10 mM EDTA) containing 1.5 μl of lyticase (900 U/ml; Sigma Chemical Co., St. Louis, Mo.) and then incubated for 1 h at 37°C. Subsequently, 3.0 μl of pronase (15 mg/ml; Boehringer GmbH, Mannheim, Germany) and 10 μl of 10% sodium dodecyl sulfate (SDS) were added, followed by incubation for 1 h at 37°C. The sample containing fungal DNA was further purified.
In the method according to Einsele et al., 1 ml of fungal suspension or BAL fluid was centrifuged (16,000 × g, 10 min) and the pellet resuspended in 0.2 ml of white blood cell lysis buffer (WCLB; 10 mM Tris [pH 7.6], 10 mM EDTA, 50 mM NaCl, 0.2% SDS, 200 μg of proteinase K per ml), followed by incubation at 65°C for 45 min. After centrifugation (1,500 × g, 10 min), the pellet was resuspended in 0.2 ml of zymolyase buffer (50 mM Tris [pH 7.5], 10 mM EDTA, 28 mM β-mercaptoethanol, and 300 μg of zymolyase [20T; ICN, Costa Mesa, Calif.] (per ml) and incubated at 37°C for 45 min. The solution was centrifuged (1,500 × g, 10 min), and the pellet containing fungal DNA was further purified.
DNA extraction from blood specimens.
In the in-house method of van Deventer, 0.5 ml of lysis buffer (0.32 M sucrose, 10 nM Tris-HCl [pH 7.5], 5 mM MgCl2, 1% Triton X-100) was added to 0.5 ml of EDTA-blood. After lysis, samples were centrifuged (5 min, 16,000 × g) and the supernatant was discarded. The pellet was resuspended in 0.2 ml of lysis buffer. To remove free, nonfungal DNA, 7 μl of DNase 1 (10 mg/ml; Boehringer GmbH) was added and the samples were incubated at 37°C for 1 h. After centrifugation at 16,000 × g for 5 min, pellets were resuspended in 0.2 ml of TEG buffer containing 1.5 μl of lyticase (900 U/ml) and incubated for a further 1 h at 37°C. Subsequently, 3.0 μl of pronase and 10 μl of 10% SDS were added, followed by incubation for 1 h at 37°C. The sample containing fungal DNA was further purified.
In the method of Einsele et al., 1.5 ml of red blood cell lysis buffer (RCLB; 10 mM Tris [pH 7.6], 5 mM MgCl2, 10 mM NaCl) was added to 0.5 ml of EDTA-blood, and then the mixture was incubated on a shaking platform for 10 min. The sample was centrifuged (1,200 × g, 10 min), and the pellet was treated again with 1.5 ml of RCLB and centrifuged. Subsequently, the pellet was resuspended in 0.2 ml of WCLB and incubated at 65°C for 45 min. After centrifugation (1,500 × g, 10 min), the pellet was resuspended in 0.2 ml of zymolyase buffer and incubated at 37°C for 45 min. The sample was centrifuged (1,600 × g, 10 min), and the pellet containing fungal DNA was used for further purification.
Purification and amplification of DNA.
DNA was purified according to the method of Boom et al. (3). Briefly, 1 ml of lysis buffer (0.1 M Tris-HCl [pH 6.4], 40 mM EDTA [pH 8.0], 1% Triton X-100, 4 M guanidine isothiocyanate) and 50 μl of a Celite suspension (200 mg/ml; Aoroa Organics, Grel, Belgium) was added to the sample or pellet containing fungal DNA, and this suspension was shaken vigorously by hand, followed by incubation at room temperature for 10 min. The suspension was centrifuged (1 min, 15,000 × g), and the pellet was washed two times with a second lysis buffer (0.1 M Tris-HCl [pH 6.4], 4 M guanidine isothiocyanate), two times with 70% ethanol, and one time with acetone, in succession. After it was dry, the pellet was resuspended in 100 μl of bidistilled water and incubated for 10 min at 56°C. The sample was centrifuged (15,000 × g, 10 min), and 10 μl of the supernatant was used for amplification.
The following primer set, amplifying a sequence of the multicopy 18S rRNA gene, was used: 5′-ATTGGAGGGCAAGTCTGGTG-3′ and 5′-CCGATCCCTAGTCGGCATAG-3′ (7). PCR was performed in 100 μl of PCR solution containing 50 mM KCl, 10 mM Tris-HCl (pH 8.3), 2.5 mM MgCl2, 200 μM concentrations of each deoxynucleoside triphosphate, 50 pmol of each primer, 0.08 U of Taq polymerase (SuperTAQ; Sphaero Q, Leiden, The Netherlands), and a 10-μl sample specimen. Forty cycles of amplification were performed with a PCR processor (9600; Perkin-Elmer). Each cycle consisted of a denaturation step at 95°C for 30 s, a primer-annealing step at 55°C for 30 s, and a chain elongation step at 72°C for 45 s.
Southern blot analysis of products.
Aliquots (20 μl) of each amplification product were electrophoretically separated on a 1.5% agarose gel in 0.5× Tris-borate-EDTA buffer. The DNA was transferred from agarose to Hybond-Plus nylon filters (Amersham International, Amersham, United Kingdom) by electrophoretic transfer (17). The PCR products were analyzed with an Aspergillus-specific DNA probe (CATGGCCTTCACTGGCTGTGGGGGGAACCA) (7). Hybridization was detected by the ECL3 oligolabeling and detection system (Amersham International).
Sandwich ELISA for detection of GM.
The sandwich ELISA was performed as described by Stynen et al. (18) and was used to measure the concentrations of GM quantitatively. Some minor modifications were made in the protocol to reduce the standard deviation in series of samples that were spiked with the same concentration of GM. Briefly, 300 μl of each serum or BAL fluid sample was mixed with 100 μl of treatment solution (4% EDTA), and the mixture was subsequently boiled for 5 min. After centrifugation (20,000 × g, 10 min), the supernatant was used for further testing. Then, 50 μl of conjugate was added to each well of an anti-GM immunoglobulin M-coated microtiter plate (Platelia Aspergillus; Sanofi Diagnostics Pasteur), followed by the addition of 50 μl of the treated sample. The plates were incubated at 37°C for 90 min and then washed five times with washing buffer (Tris NaCl [pH 7.4] containing 1% Tween 20 and 0.01% sodium merthiolate). Next, 200 μl of substrate buffer containing orthophenylenediamine dihydrochloride was added to each well, and the plates were incubated for 30 min at room temperature in darkness. To stop the reaction, 100 μl of 1.5 M sulfuric acid was added, and the optical density was measured at 450 and 620 nm. Each plate contained a calibration curve consisting of rat serum samples containing 0, 1, 1.5, 2, 3, 4, 6, 8, and 12 ng of GM (the kind gift of Marc Tabouret, Sanofi Diagnostics Pasteur, Steenvoorde, France) per ml. A test sample was considered positive when the optical density at 450 nm was higher than the cutoff sample (i.e., 1.0 ng). The concentration of GM in positive test samples was expressed as the nanograms of GM per milliliter.
Statistical methods.
Associations between GM concentrations and PCR results or disseminated fungal infection were analyzed by the Mann-Whitney test. The association between the PCR result and the disseminated fungal infection was analyzed by using the chi-square test. Spearman's correlation was used to analyze relations between GM concentrations and time to death.
RESULTS
Validation of two DNA isolation methods.
Two methods were used for isolating Aspergillus DNA from blood: an in-house-developed method (21) and the method described of Einsele et al. (7), with modifications. The in vitro sensitivities of both methods were compared by isolating fungal DNA from blood spiked with 10-fold serial dilutions of A. fumigatus conidia. The isolated DNA was then amplified, and the amplification product was hybridized with an Aspergillus-specific probe. Using both DNA isolation methods, 10 CFUs per ml of rat blood could be detected. The sensitivity was not influenced by using larger blood volumes: when volumes of 0.1, 0.5, or 2.5 ml of blood were spiked with equal concentrations of conidia, no increase in sensitivity was seen.
Validation of quantitative ELISA.
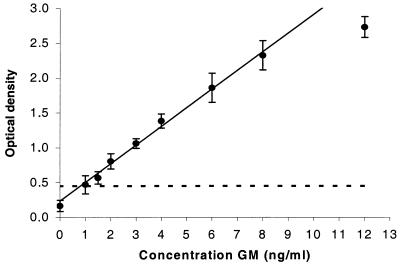
The commercially available “Platelia” sandwich ELISA for detecting GM was validated for quantitative use in rat serum. Concentrations of 0, 1, 1.5, 2, 3, 4, 6, 8, and 12 ng of GM per ml were spiked sixfold into rat serum. In Fig. 1 the resulting calibration curve is presented. In all samples tested, linear concentration response curves were obtained at between 1 and 8 ng/ml (r = 0.994). The detection limit, defined as the concentration corresponding to the mean optical density of the blank plus three standard deviations (SD), was 1.0 ng/ml.
FIG. 1.
Representative callibration curve of the sandwich ELISA, prepared in sixfold dilutions with known concentrations of GM in rat serum. Each point represents the mean ± the SD (bars) optical density at 450 nm. The detection limit of the assay is indicated as a dashed line.
PCR and ELISA in blood and association with disseminated fungal infection in rats with IPA.
Five groups of rats (the number of animals in each group varied from 11 to 29) were sacrificed on days 1, 2, 3, 5, and 7 after inoculation (Table 1). Control animals (three to nine rats per group) were inoculated with PBS. From each rat a blood sample was taken for PCR as well as for ELISA. The presence of disseminated fungal infection was determined by the culture of organs. In all 97 blood samples taken from infected rats, the in-house DNA isolation method was used, and in 68 of these samples the Einsele et al. method was also used. Both methods showed the same results in 66 samples. Results of the in-house PCR are shown in Table 1. PCR had a considerably lower sensitivity than ELISA, especially between days 2 and 5 after inoculation. The highest rates of positivity for both tests were found on the last day of sampling (day 7): 41% for PCR and 100% for ELISA. The median concentrations of GM increased from below the detection limit in rats on day 1 to 46 ng/ml in rats on day 7. Of all 97 samples, 62% were positive for ELISA; 18% were positive for PCR, all of these latter samples being also positive for ELISA. Specificity was high for both tests: of the 31 blood samples taken from uninfected animals, none were found positive by the ELISA and only 1 of 31 samples was positive by PCR.
TABLE 1.
PCR and ELISA analyses of blood and association with disseminated fungal infection in rats with IPA after dissection
| Day | No. of animals | No. of animals positive (%)
|
Median concn (ng/ml) of GM (range) | Disseminationb (no. of rats positive [%]) | |
|---|---|---|---|---|---|
| PCRa | ELISA | ||||
| 1 | 24 | 0 (0) | 2 (8) | <1 (<1–3) | 0 (0) |
| 2 | 11 | 0 (0) | 3 (27) | <1 (<1–7) | 0 (0) |
| 3 | 18 | 2 (22) | 16 (89) | 7 (<1–31) | 0 (0) |
| 5 | 15 | 3 (20) | 14 (93) | 19 (<1–217) | 0 (0) |
| 7 | 29 | 12 (41) | 29 (100) | 46 (1.9–600) | 11 (38) |
In-house PCR method.
Extrapulmonary disseminated fungal infection.
Results for rats on day 7 were further analyzed to investigate associations between PCR, the concentration of GM, and the presence of disseminated fungal infection. The median concentration of GM in blood samples that were positive by PCR was higher (48.0 ng/ml; range, 8.3 to 602 ng/ml) than the median concentration in PCR-negative samples (30.4 ng/ml; range, 1.9 to 480 ng/ml). Although there was a trend, this difference was not significant (P = 0.09). On day 7, 38% of the rats showed disseminated infection in the extrapulmonary organs; in all cases the liver was the affected organ. The percentage of rats with a positive PCR result was higher (57%) in rats with disseminated fungal infection than in rats with pulmonary infection alone (27%). In addition, higher concentrations of GM were found in rats with disseminated fungal infection (median, 50.1 ng/ml; range, 1.9 to 602 ng/ml) than in rats without disseminated infection (median, 15.8 ng/ml; range, 21.0 to 294 ng/ml). However, both associations were not significant (P = 0.35 and 0.14, respectively).
PCR, ELISA, and fungal culture of BAL fluid and blood in rats with IPA.
Four groups of rats (five to eight animals per group) were sacrificed on days 1, 3, 5, and 7 after inoculation, respectively (Table 2). From each rat, blood and BAL fluid were used for PCR, ELISA, and fungal culture analyses. The blood cultures were all negative. The fungal cultures of BAL fluid were all positive on day 1 after inoculation, with the numbers of CFUs decreasing over time. Most BAL fluid cultures obtained from rats on days 5 and 7 after inoculation remained negative. The PCR results were not related to the culture findings: one to two positive samples were found on all days. Four rats (two on day 1, one on day 5, and one on day 7) were found to be negative in blood and positive in BAL fluid by PCR. ELISA of BAL fluid was more often positive on days 5 and 7, with increasing titers of GM over time, despite the negative cultures. Three rats (two on day 1 and one on day 5) were negative by ELISA of blood but positive for the BAL fluid.
TABLE 2.
PCR, ELISA, and fungal culture analyses of BAL fluid and blood of rats with IPA after dissectiona
| Day | No. of animals | BAL fluid
|
Blood
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| PCR (no. of rats positive) | ELISA (no. of rats positive) | Median concn (ng/ml) of GM (range) | Culture (no. of rats positive [mean CFU/ml]) | PCR (no. of rats positive) | ELISA (no. of rats positive) | Median concn (ng/ml) of GM (range) | Culture (no. of rats positive [mean CFU/ml]) | ||
| 1 | 8 | 2 | 2 | <1 (<1–2.7) | 8 (4) | 0 | 0 | <1 (<1) | 0 (0) |
| 3 | 5 | 1 | 2 | <1 (<1–11) | 3 (1) | 1 | 3 | 4.4 (<1–6.2) | 0 (0) |
| 5 | 5 | 2 | 5 | 11.6 (5.4–157) | 0 (0) | 1 | 4 | 10.2 (<1–26.2) | 0 (0) |
| 7 | 5 | 1 | 5 | 8.8 (3.9–114) | 1 (0) | 2 | 5 | 19.2 (7.6–48) | 0 (0) |
The in-house PCR method was used.
Monitoring the course of disease by PCR.
Ten rats with IPA were sequentially sampled on days 1, 3, 5, and 7 after inoculation for PCR (Table 3). All rats were found to be negative by PCR on day 1 after inoculation. On day 3 four of ten rats were found to be positive by PCR; on day 5 two of eight and on day 7 two of four were found to be positive. No clear increase in the rate of positive PCR tests was seen during the course of the disease. Some rats (animals 1 and 10) remained negative for PCR at all time points, even immediately prior to death. Two other rats (animals 6 and 8) were positive at day 3 but were found to be negative thereafter.
TABLE 3.
PCR analysis of blood of 10 individual rats with IPA after sequential sampling
| Rat | PCR results on:
|
||||
|---|---|---|---|---|---|
| Day 1 | Day 3 | Day 5 | Day 7 | Day 8+ | |
| 1 | − | − | − | Died on day 7 | |
| 2 | − | − | + | Died on day 7 | |
| 3 | − | + | Died on day 5 | ||
| 4 | − | − | + | Died on day 7 | |
| 5 | − | + | + | + | Died on day 8 |
| 6 | − | + | − | − | Died on day 8 |
| 7 | − | − | − | + | Died on day 13 |
| 8 | − | + | − | Died on day 6 | |
| 9 | − | − | NDa | − | Died on day 10 |
| 10 | − | − | − | Died on day 6 | |
ND, not determined.
Monitoring the course of disease by GM concentrations.
Nine infected rats were sequentially sampled for GM detection (Fig. 2). A consistent increase in signal was seen during the course of disease. On day 1 all rats were GM negative; on day 3 all rats were positive by ELISA, and the median concentration of GM was 9.3 ng/ml. On days 5 and 7 the median concentrations increased to 25.8 and 53.0 ng/ml, respectively. Two rats with relatively high concentrations of GM (71 and 240 ng/ml) on day 5 died the next day. One rat with a relatively low concentration of GM on day 7 survived relatively long compared to other rats and ultimately died on day 11. We investigated the relationship between the concentration of GM and the time to death. An inverse relation was found that was highly significant (P < 0.0001) (Fig. 2).
FIG. 2.
GM in the blood of individual rats with IPA after sequential sampling. One line represents one rat. (Inset) Relationship between GM concentrations and time to death.
DISCUSSION
In the present study, we compared two diagnostic tests, Aspergillus-specific PCR and a sandwich ELISA for detecting GM, with respect to their value in diagnosing and monitoring IPA in a rat model. By using an animal model, both tests could be evaluated in a controlled fashion in the early phase of the disease, with a known time of onset of infection.
For the diagnosis of IPA in blood, we used two PCR methods, including the method described by Einsele et al. (7). Those authors found a 77 to 100% sensitivity in patients with IPA. In our model of severe IPA, the maximum sensitivity that was found using both methods was only 41% at a moment at which more than 50% of the rats had already died (i.e., day 7 after inoculation). One explanation for this difference in sensitivity might be the relatively low blood volume (0.5 ml) used for PCR in our model compared to the blood volume of 3 to 5 ml used in the study of Einsele et al. Those authors suggested that a larger blood volume might help to increase the sensitivity of the assay due to the higher yield of fungal DNA. However, when we compared different blood volumes (range, 0.1 to 2.5 ml) obtained from infected rats, we did not observe any increase in sensitivity. Moreover, a higher blood volume may contain more competing DNA or other inhibiting substances which may interfere with the specific PCR signal (5). In addition, the in vitro sensitivities of both of the PCR methods we used was 10 CFUs per ml, which was similar to the test results as described by Einsele et al.
Compared to PCR, the sensitivity of the sandwich ELISA in serum was high in our rat model: up to 100% on day 7. In addition, the ELISA was positive earlier than was the PCR assay. No blood samples from infected animals were PCR positive and ELISA negative. This leads to the conclusion that, at least in our rat model, PCR is not only less sensitive than ELISA but also has no additional value to the ELISA in the diagnosis of IPA.
In monitoring the course of the disease, PCR showed inconsistent results in the sequentially sampled rats. No clear increase in the fraction of positive animals was seen over time with this assay. Also, an on-off phenomenon was observed: some rats that were positive by PCR became negative at a later stage in their disease. In the PCR methods we used, fungal DNA was extracted from the pellet obtained after blood cell lysis. Since the pellet contains fungal elements, whereas free DNA may be present in the serum, our method could fail to detect circulating free DNA. It is possible that a PCR assay in which circulating DNA is detected in serum gives a better correlation with fungal load and severity of disease thus gives more consistent results (4, 10). Comparison of PCR assays in serum with PCR methods as used in our study should be investigated in future studies.
In contrast to PCR in our model, concentrations of GM increased consistently in the course of the disease, which has also been reported by others in a rabbit model (15). In addition, we found a highly significant inverse relation between the concentration of GM and the time to death of the rats. These findings indicate that the concentrations of GM in serum are correlated with the severity of the disease in our model and, possibly, with the fungal load.
Several other studies have compared PCR and GM detection for the diagnosis of IPA in blood. Hashimoto et al. compared PCR, a (1 → 3) β-d-glucan assay, and GM detection in a rat model of IPA (10). In their study the sensitivity of PCR was higher (80 to 87%) in the early phase of the disease than that of the (1 → 3) β-d-glucan assay (60 to 75%) or of GM detection (71 to 80%). They also found similar results in a study in patients: 70% sensitivity for PCR and 60% for GM detection (23). However, comparison of their findings with our data is difficult, since they used a latex agglutination test for detecting circulating GM, an assay which is about 10 times less sensitive than the sandwich ELISA that we used (21). Also, they used a different PCR method, i.e., a nested PCR in serum. Possibly, a nested PCR is more sensitive than conventional PCR. However, it has been stated that nested PCR is more prone to contamination in a routine hospital laboratory when it is used as a diagnostic tool (16). Bretagne et al. compared GM detection by sandwich ELISA with a PCR in the sera of patients with IPA (4). These authors, as we found in our animal study, reported a higher sensitivity for the ELISA than for the PCR: of the 18 patients with positive mycological data, 78% had at least two ELISA-positive sera and 50% had at least one PCR-positive serum. They also found only one sample that was positive for PCR and negative for ELISA, and they noted that a PCR-positive signal was usually obtained when ELISA was highly positive. This result is in accordance with our own data: we found no samples that were ELISA negative and PCR positive, and the median concentration of GM tended to be higher in PCR-positive samples. Finally, Roth et al. compared PCR by using the method described by Einsele et al. and GM detection by sandwich ELISA in 34 neutropenic patients, of whom 6 had proven IPA (J. Roth, E. Engelmann, M. Mielke, and D. Huhn, Abstr. 9th Eur. Congr. Clin. Microbiol. Infect. Dis., abstr. O45, 1999). In that study GM detection provided both a higher sensitivity and more consistent results during the course of disease than did PCR.
In our model, the yield of fungal cultures of BAL fluid significantly decreased during the course of disease. Positive results of early cultures were probably related to conidia inoculated into the left lung. In contrast, the GM assay was more often positive later in the disease. Francis et al. found comparable results in a rabbit model of IPA. In their model, cultures of BAL fluid were rarely positive, in contrast to elevated levels of mannitol and GM (8). These findings are in accordance with results reported by Kauffman et al. (11), who investigated the nature of antigenic determinants released by conidia and hyphae. These authors found that components that are released spontaneously from conidia are only weakly positive or negative in immunologic assays, in contrast to components released from hyphae. Thus, it is likely that a strongly immunogenic molecule such as GM is released predominantly from hyphae and in much lesser amounts from conidia. Therefore, the presence of GM in BAL fluid is likely to be a better diagnostic indicator for hyphal growth than is routine mycological culture of the organism.
In conclusion, we demonstrated that quantitative GM detection in our model of IPA is superior to PCR in diagnosing as well as in monitoring the disease in both blood and BAL fluid.
ACKNOWLEDGMENT
We thank Marc Tabouret, Sanofi Diagnostics Pasteur (Steenvoorde, France), for kindly providing the GM.
REFERENCES
- 1.Aisner J, Wiernik P H, Schimpff S C. Treatment of invasive aspergillosis: relation of early diagnosis and treatment to response. Ann Intern Med. 1977;86:539–543. doi: 10.7326/0003-4819-86-5-539. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong D. Overview of invasive fungal infections and clinical presentation. In: Meunier F, et al., editors. Balliere's clinical infectious diseases. Vol. 2. London, United Kingdom: Balliere Tindal; 1995. pp. 17–24. [Google Scholar]
- 3.Boom R, Sol C J, Salimans M M, Jansen C L, Wertheim-van Dillen P M, van der Noordaa J. Rapid and simple method for purification of nucleic acids. J Clin Microbiol. 1990;28:495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bretagne S, Costa J M, Bart-Delabesse E, Dhedin N, Rieux C, Cordonnier C. Comparison of serum galactomannan antigen detection and competitive polymerase chain reaction for diagnosing invasive aspergillosis. Clin Infect Dis. 1998;26:1407–1412. doi: 10.1086/516343. [DOI] [PubMed] [Google Scholar]
- 5.Crampin A C, Matthews R C. Application of the polymerase chain reaction to the diagnosis of candidosis by amplification of an HSP 90 gene fragment. J Med Microbiol. 1993;39:233–238. doi: 10.1099/00222615-39-3-233. [DOI] [PubMed] [Google Scholar]
- 6.Denning D W. Therapeutic outcome in invasive aspergillosis. Clin Infect Dis. 1996;23:608–615. doi: 10.1093/clinids/23.3.608. [DOI] [PubMed] [Google Scholar]
- 7.Einsele H, Hebart H, Roller G, Loffler J, Rothenhofer I, Muller C A, Bowden R A, van Burik J, Engelhard D, Kanz L, Schumacher U. Detection and identification of fungal pathogens in blood by using molecular probes. J Clin Microbiol. 1997;35:1353–1360. doi: 10.1128/jcm.35.6.1353-1360.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Francis P, Lee J W, Hoffman A, Peter J, Francesconi A, Bacher J, Shelhamer J, Pizzo P A, Walsh T J. Efficacy of unilamellar liposomal amphotericin B in treatment of pulmonary aspergillosis in persistently granulocytopenic rabbits: the potential role of bronchoalveolar D-mannitol and serum galactomannan as markers of infection. J Infect Dis. 1994;169:356–368. doi: 10.1093/infdis/169.2.356. [DOI] [PubMed] [Google Scholar]
- 9.Groll A H, Shah P M, Mentzel C, Schneider M, Just-Nuebling G, Huebner K. Trends in the postmortem epidemiology of invasive fungal infections at a university hospital. J Infect. 1996;33:23–32. doi: 10.1016/s0163-4453(96)92700-0. [DOI] [PubMed] [Google Scholar]
- 10.Hashimoto A, Yamakami Y, Kamberi P, Yamagata E, Karashima R, Nagaoka H, Nasu M. Comparison of PCR, (1→3)-beta-d-glucan and galactomannan assays in sera of rats with experimental invasive aspergillosis. J Clin Lab Anal. 1998;12:257–262. doi: 10.1002/(SICI)1098-2825(1998)12:5<257::AID-JCLA1>3.0.CO;2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kauffman H F, Beaumont F, Meurs H, van der Heide S, de Vries K. Comparison of antibody measurements against Aspergillus fumigatus by means of double-diffusion and enzyme-linked immunosorbent assay (ELISA) J Allergy Clin Immunol. 1983;72:255–261. doi: 10.1016/0091-6749(83)90029-5. [DOI] [PubMed] [Google Scholar]
- 12.Leenders A C, de Marie S, ten Kate M T, Bakker-Woudenberg I A, Verbrugh H A. Liposomal amphotericin B (AmBisome) reduces dissemination of infection as compared with amphotericin B deoxycholate (Fungizone) in a rat model of pulmonary aspergillosis. J Antimicrob Chemother. 1996;38:215–225. doi: 10.1093/jac/38.2.215. [DOI] [PubMed] [Google Scholar]
- 13.Loffler J, Hebart H, Schumacher U, Reitze H, Einsele H. Comparison of different methods for extraction of DNA of fungal pathogens from cultures and blood. J Clin Microbiol. 1997;35:3311–3312. doi: 10.1128/jcm.35.12.3311-3312.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loffler J, Hebart H, Sepe S, Schumacher U, Klingebiel T, Einsele H. Detection of PCR-amplified fungal DNA by using a PCR-ELISA system. Med Mycol. 1998;36:275–279. doi: 10.1080/02681219880000441. [DOI] [PubMed] [Google Scholar]
- 15.Patterson T F, Miniter P, Ryan J L, Andriole V T. Effect of immunosuppression and amphotericin B on Aspergillus antigenemia in an experimental model. J Infect Dis. 1988;158:415–422. doi: 10.1093/infdis/158.2.415. [DOI] [PubMed] [Google Scholar]
- 16.Reichard U, Margraf S, Hube B, Ruchel R. A method for recovery of Candida albicans DNA from larger blood samples and its detection by polymerase chain reaction on proteinase genes. Mycoses. 1997;40:249–253. doi: 10.1111/j.1439-0507.1997.tb00228.x. [DOI] [PubMed] [Google Scholar]
- 17.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 18.Stynen D, Goris A, Sarfati J, Latge J P. A new sensitive sandwich enzyme-linked immunosorbent assay to detect galactofuran in patients with invasive aspergillosis. J Clin Microbiol. 1995;33:497–500. doi: 10.1128/jcm.33.2.497-500.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sulahian A, Tabouret M, Ribaud P, Sarfati J, Gluckman E, Latge J P, Derouin F. Comparison of an enzyme immunoassay and latex agglutination test for detection of galactomannan in the diagnosis of invasive aspergillosis. Eur J Clin Microbiol Infect Dis. 1996;15:139–45. doi: 10.1007/BF01591487. [DOI] [PubMed] [Google Scholar]
- 20.van Deventer A J M, Goessens W H F, van Belkum A, van Vliet H J A, van Etten E W M, Verbrugh H A. Improved detection of Candida albicans by PCR in blood of neutropenic mice with systemic candidiasis. J Clin Microbiol. 1995;33:625–628. doi: 10.1128/jcm.33.3.625-628.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verweij P E, Stynen D, Rijs A J, de Pauw B E, Hoogkamp-Korstanje J A, Meis J F. Sandwich enzyme-linked immunosorbent assay compared with Pastorex latex agglutination test for diagnosing invasive aspergillosis in immunocompromised patients. J Clin Microbiol. 1995;33:1912–1914. doi: 10.1128/jcm.33.7.1912-1914.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walsh T J, Lyman C A, Pizzo P A. Laboratory diagnosis of invasive fungal infections in patients with neoplastic diseases. In: Meunier F, et al., editors. Balliere's clinical infectious diseases. Vol. 2. London, United Kingdom: Balliere Tindal; 1995. pp. 25–70. [Google Scholar]
- 23.Yamakami Y, Hashimoto A, Tokimatsu I, Nasu M. PCR detection of DNA specific for Aspergillus species in serum of patients with invasive aspergillosis. J Clin Microbiol. 1996;34:2464–2468. doi: 10.1128/jcm.34.10.2464-2468.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]