Highlights
-
•
Okara peptides were prepared by high-pressure homogenization-assisted double enzymes.
-
•
The method can improve the extraction rate of okara proteins by 69% and 51%.
-
•
Antioxidant and immune activity were compared, and HPH-VAP was screened out.
-
•
116 okara peptides were identified from HPH-VAP by HPLC-MS/MS.
-
•
The release of cytokines IL-6, TNF-α and IFN-γ was elevated.
Keywords: High-pressure homogenization, Double enzyme hydrolysis, Okara peptides, Immunity, Antioxidation
Abstract
In this study, a method for preparing low molecular weight peptides (HPH-VAP) from okara using high-pressure homogenization assisted double enzymes was proposed. In order to explore its advantages, the effects of various methods on protein extraction rate and on the structure, antioxidant and immune properties of peptides were compared. The results showed that the protein extraction rate of this method was increased by 69% and 51% compared with other methods, and the structure only led to changes in the hydrogen bonds between peptide chains. HPH-VAP was screened out through functional characteristics, its structure was identified by HPLC-MS/MS, and further immunological activity analysis was carried out. The results showed that it promoted cell phagocytic ability, NO level and release of cytokines IL-6, IFN- γ, TNF-α. Therefore, this method is an effective and applicable method for industrial preparation of okara peptides, and has a positive effect on the reuse of okara resources.
Introduction
Okara is the waste produced during the production of soy milk, tofu, and soybean protein isolate in the food industry (Ma, Liu, Kwok, & Kwok, 1996). Each kilogram of soybeans can produce wet okara 1.2 times its weight (Santos et al., 2019). The world's okara production (1.4 billion tons) is dominated by countries with high soybean consumption in Asia (W. K. Mok, Tan, Lee, Kim, & Chen, 2019). Especially in China, the tofu processing sector produced 2,800,000 tons of okara (Lee et al., 2020, Mok et al., 2020). Although its main ingredients have high nutritional value, most of them are treated as livestock feed or waste, resulting in waste of resources and environmental pollution (Vong & Liu, 2016). Therefore, the effective use of okara is still a challenge facing the current okara market, so that it is urgent to propose a method to utilize the nutritional resources of okara.
Traditionally, okara are hydrolyzed by enzymes; but soon after, high-pressure and high temperature methods are adopted. These approaches will affect the structure and molecular weight of protein, enhance the action of enzymes, and thus affect the nutritional quality. Therefore, different processing approaches are used in the world, including physical methods such as high-pressure homogenization (Fayaz et al., 2019, Li et al., 2019), processing technologies such as enzymatic treatment, and advanced fermented methods (yeast, fungus or germs) (Yang, Fu, & Yang, 2020) to improve the restrictions on foods made from okara and okara to increase the release of biologically active substances and the utilization of nutrients. In addition to physical methods, the importance of enzymatic hydrolysis has also been emphasized by recent studies. de Figueiredo et al. (de Figueiredo, Yamashita, Vanzela, Ida, & Kurozawa, 2018) used Viscozyme multi-enzyme complex to pretreat okara, which improved the traditional alkaline preparation method to increase the protein extraction rate.
Biologically active peptides are usually prepared by protease hydrolysis, and their technology is safe, natural and environmentally friendly, so they are more and more popular. For peptides, and especially for oligopeptides, many biological activities have been recorded. These biological activities include anticancer, antioxidative, anti-fatigue and immunomodulatory, anti-aging activities (Karami and Akbari-adergani, 2019, Nielsen et al., 2017). Studies have shown that soybean hydrolysates have the ability of scavenging free radicals (Moure, Domínguez, & Parajó, 2006; L. Zhang, Li, & Zhou, 2010). Studies have pointed out that soybean peptides have a variety of the ability of immune regulation on both congenital immunity and adaptive immune response (Chalamaiah, Yu, & Wu, 2018). Immune activity of biologically active peptides is determined by its amino acid composition, hydrophobicity, molecular mass, sequence and other factors. Although the activities of immune regulation of soybean peptides have been reported in other literatures (Wang et al., 2008, Yimit et al., 2012), the research on obtaining antioxidant and immunologically active peptides from okara and the information about its complete peptide structure and regulation mechanism are scarce.
Therefore, the main purpose of our research is to propose three methods for preparing low molecular weight peptides from okara, that is, method one: use high-pressure homogenization and mixed enzyme hydrolysis followed by alkaline protease hydrolysis, method two: use mixed enzyme hydrolysis followed by alkaline protease hydrolysis, method three: alkali-dissolved acid precipitation method to extract protein and carry out alkaline protease hydrolysis. The effects of the three methods on the structure, antioxidant and immune activity of the peptides were also discussed. Finally, method one was screened to explore the immune activity. The research results will help to widely use soybean waste to obtain biologically active peptides, and to improve the added value of okara.
Materials and methods
Materials
Okara was a by-product of preparing soybean protein isolate, with a protein content of 10–15%, kindly given by Shandong Jiahua Health Care Products Co., Ltd (Liaocheng, China). LPS (Escherichia coli 055: B5) was obtained from Sigma-Aldrich (Shanghai, China). Dulbecco’s modified Eagle’s high glucose medium (DMEM), and penicillin–streptomycin (P/S) were offered from Gibco (Grand Island, NY, USA). Fetal bovine serum (FBS) was bought from Clark Bioscience (Virginia, U.S.). NO assay kit was offered from Nanjing Jiancheng Bioengineering Institute (Nanjing, Jiangsu, China). IFN-γ, IL-6 and TNF-α ELISA kits were offered by mlbio (Shanghai, China). Ferrous sulfate and salicylic acid were purchased from Tianjin Guangfu Technology Development Co., Ltd. (Tianjin, China). Anhydrous ethanol and hydrogen peroxide were obtained from Beijing Chemical Works (Beijing, China). Viscozyme L and Alcalase 2.4L were obtained from Novozymes (Bagsvaerd, Denmark).
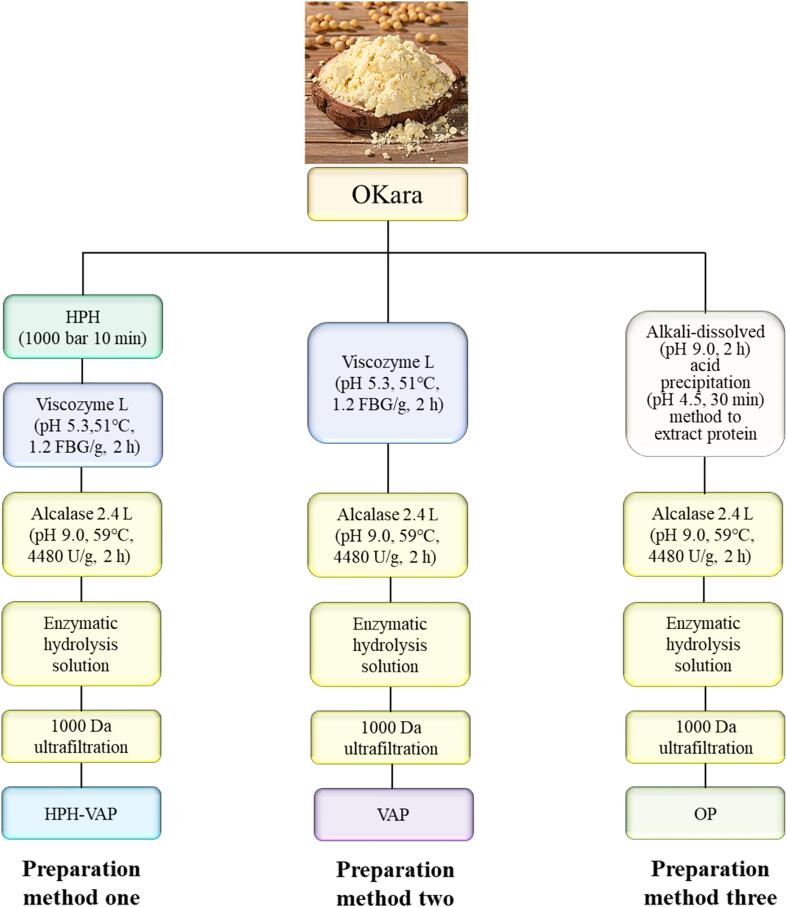
Preparation of okara peptides
Three different preparation methods are used to obtain peptides from Okara (Fig. 1). The detailed description of these technologies is as follows.
Fig. 1.
Okara peptide obtained by different processing methods. Method one: HPH-VAP was pretreated by high pressure homogenization, and then directly prepared by enzymatic hydrolysis with Viscozyme L and Alcalase 2.4 L; method two: VAP was directly prepared by enzymatic hydrolysis of Viscozyme L and Alcalase 2.4 L successively; method three: OP was alkali-dissolved acid precipitation method to prepare protein and then enzymatic hydrolysis.
Preparation method one: Preparation of HPH-VAP
Refer to the method of GolyFayaz (Fayaz, Plazzotta, Calligaris, Manzocco, & Nicoli, 2019) and Vitória Ribeiro Garcia de Figueiredo (de Figueiredo, Yamashita, Vanzela, Ida, & Kurozawa, 2018)with slight modifications. Okara suspension (material-to-liquid ratio is 1:50), which prepared and stirred evenly at room temperature, was treated by high pressure homogenization (1000 bar 10 min) and Viscozyme L enzyme. The treated okara suspension was centrifuged to obtain the supernatant, its protein content was measured. Alcalase 2.4 L was added for enzymatic hydrolysis and then ultrafiltration to obtain HPH-VAP.
Preparation method two: Preparation of VAP
Refer to the method of Vitória Ribeiro Garcia de Figueiredo (de Figueiredo, Yamashita, Vanzela, Ida, & Kurozawa, 2018) with a slight modification. Okara suspension (material-to-liquid ratio was 1:50), which prepared and stirred evenly at room temperature, was treated by Viscozyme L. The treated okara suspension was centrifuged to obtain the supernatant, its protein content was measured. Alcalase 2.4 L was added for enzymatic hydrolysis and then ultrafiltration to obtain VAP.
Preparation method three: Preparation of OP
Refer to Xia Tao's method (Tao et al., 2019) with a slight modification. Okara suspension (material-to-liquid ratio was 1:50) was prepared and stirred. The pH value of the okara suspension was adjusted to 9.5 with NaOH solution (2 M), the alkali solution acid precipitation method was used, and the continuous magnetic stirring was used for 2 h. The supernatant was obtained by centrifugation at 14000 × g, 4 °C for 30 min, and the pH was adjusted to 4.5 by HCl solution (2 M). It was centrifuged under the same conditions. The collected precipitate was washed several times with water, adjusted to neutral with NaOH solution (2 M), centrifuged under the same conditions again, and the supernatant was taken. Its protein content was measured. Alcalase 2.4L was added for enzymatic hydrolysis and then ultrafiltration to obtain OP.
Structural Characterization of okara peptides
SEM analysis
The microstructures of the three samples were observed using a SEM (Zeiss evo18, Germany). The sample was sputtered coating with gold. Then the sample was observed at a voltage of 20 kV.
Particle size distribution and zeta-potential analysis
5 mg/mL peptide solution was prepared, and the solvent was distilled water. Then, the sample (1 mL) was taken out of the polystyrene cell, and the particle size and zeta potential of the samples were detected by using a nanoparticle size potential analyzer (Zetasizer Nano-ZS 90, Malvern Instruments Co., LTD., UK).
FT-IR analysis
The three peptide samples were mixed uniformly with KBr at a ratio of 1/100 (w/w) and pressed into tablets. A fourier-infrared spectrometer (Thermo Fisher, Massachusetts, USA) was used to scan each sample at a wave range of 4000 cm−1 to 400 cm−1.
Circular dichroism
Three samples (1 mg/mL) were subjected to circular dichroism analysis under continuous nitrogen flow conditions with a fixed wavelength between 180 and 260 nm.
Endogenous fluorescence spectroscopy
According to the method of NaSun et al. (Sun, Wang, Bao, Cui, Wang, & Lin, 2020), slightly modified. The endogenous fluorescence spectrum of peptides was measured by F-7000 fluorescence spectrophotometer. A sample solution of the peptide (0.25 mg/mL) was prepared, and the solvent was water. The selected excitation wavelength was 290 nm, slit width was 5 nm, scanning speed was 200 nm/min, scanning interval was 20 ms, reaction time was 0.1 s, and scanning range was 300–400 nm.
Antioxidant activity of okara peptides
ABTS free radical scavenging ability
With reference to the national standard GB/T 39100–2020, the ABTS free radical scavenging activity of okara peptides was measured, and glutathione (GSH) was used as a positive control. A microplate reader (VICTOR Nivo, Perkin Elmer, USA) was used to measure the absorbance. Each sample was analyzed in three replicates. The calculation formula for scavenging ability is as follows:
where Ab was the absorbance of sample solvent and ABTS solution mixture, As was the absorbance of the mixture of the solution to be tested and the ABTS solution. EC50 is the concentration at which the sample scavenges 50% of free radicals.
DPPH free radical scavenging ability
With reference to the national standard GB/T 39100-2020, the DPPH free radical scavenging activity of okara peptides was measured, and glutathione (GSH) was used as a positive control. A microplate reader (VICTOR Nivo, Perkin Elmer, USA) was used to measure the absorbance. Each sample was analyzed in three replicates. The calculation formula for scavenging ability is as follows:
where As was the absorbance of the sample to be tested; Ac was the blank absorbance; Ab was the control absorbance. EC50 is the concentration at which the sample scavenges 50% of free radicals.
Hydroxyl radical scavenging ability
According to the method of Cao et al. (Cao, Chu, & Ye, 2003), with slight modifications, the Hydroxyl radical scavenging activity of peptides was measured, and glutathione (GSH) was used as a positive control. FeSO4 solution (6 mM, 1 mL), salicylic acid solution (6 Mm,1 mL), various concentrations of okara peptides (1 mL), and H2O2 solution (6 mM, 1 mL) were added in a colorimetric test tube. They were shaken well, placed at 37 °C for 30 min. Absorbance of it was detected at 510 nm. The calculation formula for scavenging ability is as follows:
where A0 was the blank absorbance, A1 was the absorbance of the mixture of samples, and A2 was the control absorbance. EC50 is the concentration at which the sample scavenges 50% of free radicals.
Immune activity of okara peptides
Cell culture
RAW264.7 macrophages were obtained from Solarbio (Beijing, China). The cells were seeded in high glucose DMEM medium (Gibco, USA) containing 10% FBS and 1% P/S and stored in a carbon dioxide incubator (5%CO2, 37℃).
Cytotoxicity assay
A CCK-8 kit was used to determine the cytotoxicity of okara peptides (Wen et al., 2020). In short, RAW 264.7 macrophages were maintained for 24 h in a 96-well microplate. The cells from which the medium was removed were treated with a series of concentrations (0–2.0 mg/mL) of OP, VAP, and HPH-VAP for another 24 h. After that, the medium was discarded, and the CCK-8 solution was added. Incubated for 2 h before detecting the absorbance at 450 nm.
Phagocytic ability
Cells (1 × 105 cells/mL) were seeded in a 96-well microplate with 100 μL/well. After they resumed adherent growth, a series of concentrations (0.5, 1.0 and 2.0 mg/mL) of OP, VAP, HPH-VAP were added, Lipopolysaccharide (LPS 1 μg/mL) was served as the positive control group. Then, the cells were cultured for 24 h in a carbon dioxide incubator. After the okara peptide treatment was over, 0.33% neutral red dye (100 μL) was added. After incubating for 1 h, the neutral red dye was discarded. The RAW 264.7 cells were washed several times with 37 °C PBS, and then acetic acid–ethanol lysate was added. Incubate at 4 °C for 2 h before detecting the absorbance at 540 nm with a microplate reader. The phagocytic ability was calculated as shown below:
where A0 and A1 were the absorbance of the blank and test samples, respectively.
Identification of peptide structure by HPLC-MS/MS
The HPH-VAP was desalted by phoenix kit (PreOmics, Germany, Art.No.P.O.00023). The samples were analyzed by LC-MS/MS equipped with an online nano jet ion source. The entire system is a Q Exactive™ Plus mass spectrometer (Thermo Fisher Scientific, MA, USA) connected in series with EASY-nano LC 1200. A total of 3 μL samples were loaded (analysis column: Acclaim PepMap C18, 75 μm × 25 cm), the column flow was controlled at 300 nL/min, the column temperature was 40 °C, the electrospray voltage was 2 kV, and the peptides were eluted in a gradient. The score of each peptide sequence was evaluated by Peptide Ranker (http://distilldeep.ucd.ie/PeptideRanker/) to predict its possible biological activity.
Immunological activity of HPH-VAP
NO level
The 5-10th passage RAW264.7 macrophages were trained in a 96-well microplate, incubated with different concentrations (0.5, 1.0 and 2.0 mg/mL) of HPH-VAP for 24 h, then the supernatant was collected. A NO detection kit (Nanjing, Jiangsu, China) was used to measure the NO production, that is, the absorbance of the supernatant was measured at 540 nm by a multimode plate reader. LPS was served as a positive control.
Cytokine secretion
According to the manufacturer's protocol, ELISA kits (mlbio, Shanghai, China) were used to determine the content of IL-6, TNF-α and IFN-γ in the culture supernatant. RAW264.7 macrophages were incubated with different concentrations (0.5, 1.0 and 2.0 mg/mL) of HPH-VAP for 24 h. The cell supernatant was collected, then the cytokine content was determined, that is, the absorbance was detected at 450 nm by a multi-mode plate reader. LPS was served as a positive control.
Statistical analysis
Each treatment was repeated 3 times, and the data results were expressed as mean ± standard deviation. SPSS Statistics 24.0 software and GraphPad Prism 6 software were used to analyze the significance of the results. P < 0.05 indicated that the results were significant.
Results and discussion
The influence of different processing methods on protein preparation rate
It can be seen from Fig. S1 that three different treatment methods have a significant impact on the protein content in the supernatant. The protein content of okara extracted by the alkaline solution and acid precipitation of preparation method three was 0.30 mg/mL. The protein content in the supernatant after treatment with Viscozyme L of preparation method two was 0.72 mg/mL, and the protein content in the supernatant after treatment with high pressure homogenization and Viscozyme L of preparation method one was 1.97 mg/mL, compared with preparation method three and preparation method two, its protein extraction rate increased by 69%, 51%.
Characterization of the structure of okara peptides
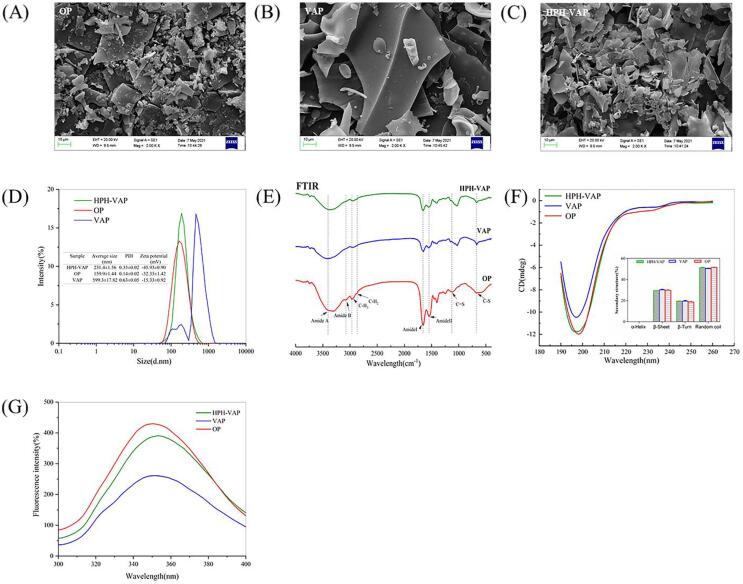
Scanning electron microscope analysis
The scanning electron microscope results of the three samples were shown in Fig. 2((A)–(C)). Fig. 2(B) showed that VAP had the characteristics of large size, complete and compact structure, and smooth surface. However, it can be seen from Fig. 2(A) and (C) that OP and HPH-VAP showed relatively rough structure and small size. It can be concluded that protease hydrolysis destroyed the surface structure of the protein, and HPH-VAP was more effective than VAP due to high pressure, but lower than OP. This also explained that high pressure can destroy the surface structure of the protein and have a positive effect on the hydrolyzed protein. The research was similar to Carullo (Carullo, Donsì, & Ferrari, 2020). The effects of high-pressure homogenization on the structural properties and enzymatic hydrolysis of milk protein were studied, and it was pointed out that it changed the protein structure. The use of enzymatic hydrolysis and high-pressure homogenization was related to the microstructure of the protein, which was shown by the scanning electron microscope results.
Fig. 2.
Characterization of the structure of okara peptide. (A)-(C) SEM of okara peptides; (D) Particle size distribution and zeta-potential of okara peptides;(E) FT-IR of okara peptides; (F) Circular dichroism of okara peptides; (G) Fluorescence spectroscopy of okara peptides.
Particle size distribution and zeta potential measurement
It can be seen from Fig. 2(D) that OP had the smallest particle size, possibly because it was obtained by direct protein hydrolysis, so it had the smallest average particle size (159.9 nm), followed by HPH-VAP (231.4 nm), and VAP had the largest particle size (599.3 nm). After the three peptides were dissolved in distilled water, the polydispersity index (PDI) of the solution had the same trend as the particle size. The zeta potentials of the three polypeptides were quite different. The potential of VAP was relatively small (−15.33 mV). The absolute potentials of the other two polypeptides were greater than 30 mV, which usually meant that the system was stable.
FT-IR
As shown in Fig. 2(E), all three samples showed unique spectra of typical protein molecules, and the FT-IR spectra of the three samples did not change significantly, indicating that the three processing methods did not add functional groups. The functional characteristic peaks at 1700–1600 cm−1, 1500–1400 cm−1, and 3400–3300 cm−1 indicated the presence of amide I and amide II bands, as well as –OH stretching vibration or –NH stretching, CO = NH bond and peptide bond (Bhimrao Muley, Bhalchandra Pandit, Satishchandra Singhal, & Govind Dalvi, 2021). If the peak position of the amide A band dropped to about 3300 cm−1 or below, there may be amino groups in the peptide involved in the formation of hydrogen bonds (Cardamone, 2010). It can be seen from Fig. 2(E) that the characteristic peaks of the amide A band of the polypeptides obtained by three different processing methods had changed, indicating that the hydrogen bond had changed.
Circular dichroism
Circular dichroism analysis technology is commonly used in the secondary structure of proteins and the changes in protein conformation by the external environment (Kelly, Jess, & Price, 2005). In the far ultraviolet range, the circular dichroism spectrum often reflects the circular dichroism of peptide bonds. Generally, the circular dichroism of natural proteins contains a positive peak (around 190 nm) and a negative groove (205–235 nm interval). As shown in Fig. 2(F), there was a negative peak at 190 nm and the greater the absolute value, the less α-helix and β-sheet content. This was almost the same as the results of Zhao et al. (Zhao, Xiong, & McNear, 2013). The secondary structure of the peptides prepared by the three methods was different from that of the natural protein. And the contents of α-helix, β-sheet, β-turn and random coil in the peptides obtained by the three methods were analyzed. It can be seen from the Fig. 2(F) that the three peptides had no α-helix, and there was no significant difference in β-sheet, β-turn and random coil. Compared with VAP, the increased content of random coil of HPH-VAP can further conclude that high pressure can destroy protein secondary structure. The results were similar to the experimental results of Carullo et al. (Carullo, Donsì, & Ferrari, 2020).
Fluorescence spectroscopy
When the fluorescent probe is combined with a protein or peptide, the formed system can emit light with a wavelength greater than the excitation wavelength after being excited by ultraviolet light or visible light. The number of residues of aromatic amino acids (tryptophan, tyrosine and phenylalanine) exposed on the surface of the peptides will cause changes in fluorescence intensity. The fluorescence emission spectrum produced was mainly tryptophan because the fluorescence of tyrosine residues was quenched and the fluorescence of tryptophan residues was enhanced. It can be known from Fig. 2(G) that the γmax of HPH-VAP was 353 nm, followed by VAP (351 nm), and the smallest was OP (350 nm). Fluorescence intensity showed different trends, with the highest fluorescence intensity was OP, followed by HPH-VAP, and the smallest was VAP. According to Condurache et al. (2020), they compared the fluorescence intensity of Lactoferrin (LF) and LF peptides and found that the fluorescence intensity of LF peptides was higher than of LF. The reason may be that Trp residues were involved in the hydrolysis and were more exposed to the solution. In this study, the fluorescence intensity of OP was the largest, and it was also possible that more Trp residues were involved in the hydrolysis, making it more exposed. According to the research of Mao et al. (Mao, Tu, Fan, Wu, Yu, & Du, 2020), PHM and PHMX were processed at a lower pressure of 400 bar, resulting in a decrease in relative fluorescence intensity. Previous studies had also shown that after high-pressure homogenization at a pressure lower than 1000 bar, the relative fluorescence intensity was significantly reduced, which was lower than that of natural peptides (Zhang, Li, & Mittal, 2010). This may be due to collision quenching, energy transfer, ground state complex formation, molecular rearrangement and other molecular interactions leading to fluorescence quenching. Therefore, the fluorescence intensity of HPH-VAP was lower than OP in this study, it may be due to other effects such as aggregation or rearrangement caused by high pressure homogenization, which caused the fluorescence intensity to decrease. However, VAP may be due to its larger particle size and less hydrolysis of Trp residues, so its fluorescence intensity decreases.
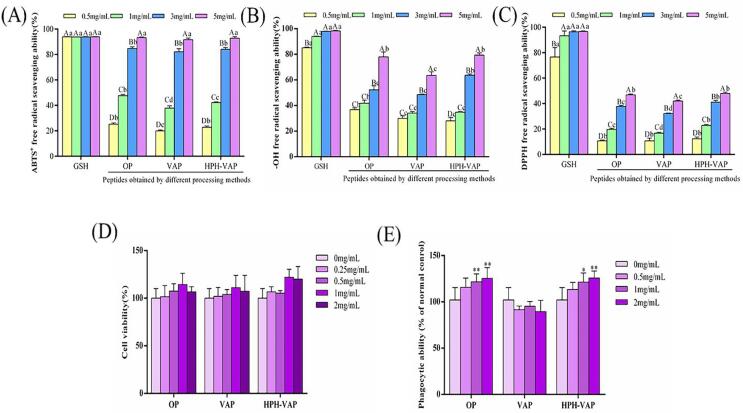
Antioxidant properties of okara peptides
The scavenging abilities of ABTS radicals, hydroxyl radicals and DPPH radicals were shown in Fig. 3((A)–(C)). The detection results of the peptides obtained by the three different treatments all showed a dose-dependent increase trend. The results showed that all three samples had antioxidant activity, for ABTS free radical scavenging ability, at 0.5 mg/mL and 1 mg/mL, the scavenging ability of OP (25.24%, 46.10%) and HPH-VAP (22.80%, 42.23%) were significantly higher than that of VAP (19.97%, 37.85%), there was no significant difference in the scavenging ability of the three samples at 3 mg/mL and 5 mg/mL, and their concentration is higher than the concentration of the sample that scavenges 50% of free radicals (EC50), at this time, the clearance rates of OP were 84.71% and 93.30%; the clearance rates of VAP were 82.17% and 91.60%; the clearance rates of HPH-VAP were 85.15% and 92.89%. For the scavenging ability of hydroxyl free radicals, the scavenging ability of OP (36.8%, 40.31%) was significantly higher than that of VAP (29.97%, 34.09%) and HPH-VAP (27.21%, 34.76%) at 0.5 mg/mL and 1 mg/mL. At 3 mg/mL, the scavenging ability of HPH-VAP (63.73%) was significantly higher than that of OP (52.29%) and VAP (48.55%), it can be seen that the concentrations of HPH-VAP and OP at this time were both higher than the concentration of the sample that scavenges 50% of free radicals (EC50). For DPPH free radical scavenging ability, at 0.5 mg/mL, there was no difference in scavenging ability of the three samples, at 3 mg/mL and 5 mg/mL, the free radical scavenging ability of HPH-VAP(41.15%, 48.45%) and OP(37.75%, 46.86%)were higher than that of VAP(32.2%, 42.01%). In summary, the in vitro antioxidant capacity of HPH-VAP and OP were better than that of VAP. Sierra et al. (Sierra, Fan, Zapata, & Wu, 2021) prepared peptides with antioxidant activity from red tilapia scale hydrolysates, and verified that they had the effect of reducing oxidative stress through cell experiments, and identified 20 antioxidant peptides after separation and purification. The research results of Liying Wang et al. (Wang, Ma, Yu, & Du, 2021) showed that the cottonseed peptides prepared by enzymatic hydrolysis had antioxidant properties. Habinshuti et al. (Habinshuti, Mu, & Zhang, 2020) proved that the antioxidant activity of the sweet potato antioxidant peptide prepared by the ultrasonic-microwave-assisted enzymatic hydrolysis method was improved.
Fig. 3.
Comparison of antioxidant and immune activity of okara peptides. Different letters indicate significant differences, A-D (same treatment), a-d (same concentration) (P < 0.05), mean values labelled with (*) are significantly different from the blank control group (i.e., *P < 0.05; ** P < 0.01; ***P < 0.001).
Immune activity of okara peptides
The results were indicated in the Fig. 3((D) and (E)). The effects of three okara peptides at different concentrations on the cell viability and phagocytic ability of macrophages were tested. It can be seen from the Fig. 3(D) that the three okara peptides at different concentrations had no cytotoxicity on macrophages and can promote the proliferation of macrophages. Phagocytosis is a crucial indicator of macrophages, which can resist infection caused by foreign biological and non-biological attacks (Wunderlich et al., 2015). When treated with OP (0.5 mg/mL), its phagocytic ability was significantly higher than that of the blank group (P < 0.05), and when treated with HPH-VAP (1 mg/mL), its phagocytic ability was significantly higher than that of the blank group (P < 0.05), and they were observed to increase their phagocytic ability as the sample dose increased. There was no significant difference in the phagocytic ability of VAP compared with the blank group. These data showed that HPH-VAP and OP can play a part in immunomodulatory activity through macrophages. Yihan Yu et al. (Yu et al., 2021) used macrophage phagocytic ability and NO release as indicators to isolate the immunologically active peptide KSPLY from Hericium erinaceus. And though the determination of cytokines in macrophages to explore the immune activity, and preliminarily judge its immune activity. Kang He et al. (He et al., 2021) extracted low molecular weight peptides from Mytilus coruscus and found that it had the ability to promote the phagocytosis of macrophages and the ability to release NO without cytotoxicity. Therefore, this study found that the peptides had antioxidant properties and at the same time measured the peptide’s immune activity.
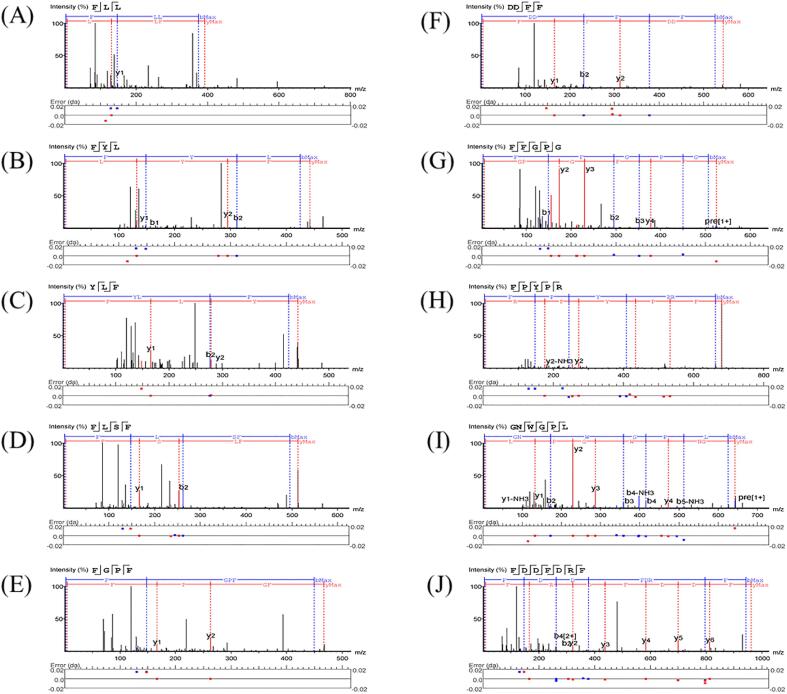
Identification of peptide sequences
Studies have shown that peptides that may have biological activity have a Peptide Ranker score higher than 0.5 (Shang et al., 2018). In this experiment, using nano HPLC-MS/MS, 116 peptide sequences with scores above 0.8 were identified, of which all were designated as having antioxidant activity, and 54 peptides (Table 1) were designated as having immunomodulatory activity. The 10 peptides with the highest predicted scores of potential antioxidant and immune activity, the corresponding sequences are Phe-Leu-Leu (FLL, score of 0.953945), Phe-Tyr-Leu (FYL, score of 0.950547), and Tyr-Leu-Phe (YLF, score of 0.950152), Phe-Gly-Pro-Phe (FGPF, score of 0.995241), Phe-Leu-Ser-Phe (FLSF, score of 0.965539), Asp-Asp-Phe-Phe (DDFF, Score 0.965214), Phe-Phe-Gly-Pro-Gly (FFGPG, score 0.98344), Phe-Pro-Tyr-Pro-Arg (FPYPR, score 0.960542), Gly-Asn-Trp-Gly-Pro-Leu (GNWGPL, score of 0.951482), Phe-Asp-Asp-Phe-Asp-Arg-Phe (FDDFDRF, score of 0.954218). These peptides are not in the BIOPEP database (http://www.uwm.edu.pl/biochemia/index.php/pl/biopep/). The MS/MS spectra of 10 synthetic peptides were shown in Fig. 4. Di et al. (Di, Wang, Wang, Chi, Jian-Yin, & Deng, 2013) reported that hydrophobic amino acids such as Leu, Val, Ala, Pro, and Phe, aromatic amino acids such as Tyr, Trp, and His, sulfur-containing amino acids such as Cys and Met, and Glu, Lys acidic and basic amino acids all contributed to the antioxidant activity of peptides. EAA (for example, Phe, Leu, Ile, and Trp) plays a crucial part in intestinal inflammation by strengthening the immune defense system and improving the integrity of the intestinal morphology. He et al. (He, Wu, Li, Li, Zhang, Zhu, et al., 2018) suggested that the supplement of Pro, Asp or Asn played a vital part in improving SOD activity, restoring intestinal barrier function and reducing intestinal damage. As important components of immune regulation, Ser and Ala played a vital part in intestinal inflammation (Li, Yin, Li, Kim, & Wu, 2007).
Table 1.
The peptides were scored by Peptide Ranker for predicting the potential for immune activity.
| No. | Sequence | Score |
|---|---|---|
| 1 | FLL | 0.953949 |
| 2 | FYL | 0.950547 |
| 3 | YLF | 0.950152 |
| 4 | EFW | 0.947113 |
| 5 | LLF | 0.938925 |
| 6 | AYF | 0.915352 |
| 7 | SWL | 0.913341 |
| 8 | FIL | 0.898768 |
| 9 | WNL | 0.898033 |
| 10 | DLF | 0.893079 |
| 11 | YIF | 0.892272 |
| 12 | FHL | 0.88902 |
| 13 | ILF | 0.884427 |
| 14 | FLI | 0.882207 |
| 15 | FDL | 0.870302 |
| 16 | LIF | 0.865651 |
| 17 | FYI | 0.858249 |
| 18 | FLD | 0.820681 |
| 19 | FLK | 0.805333 |
| 20 | FGPF | 0.995241 |
| 21 | FLSF | 0.965539 |
| 22 | DDFF | 0.965214 |
| 23 | FISF | 0.949218 |
| 24 | GWLG | 0.948294 |
| 25 | GWIG | 0.921154 |
| 26 | SLGF | 0.915554 |
| 27 | GDLF | 0.90902 |
| 28 | SIGF | 0.886873 |
| 29 | FGSL | 0.861457 |
| 30 | EDFF | 0.857384 |
| 31 | GDIF | 0.855158 |
| 32 | EPGF | 0.842858 |
| 33 | FFGPG | 0.98344 |
| 34 | FPYPR | 0.960542 |
| 35 | SPSPF | 0.928005 |
| 36 | HFDAF | 0.903091 |
| 37 | DDGPF | 0.88928 |
| 38 | SVPPF | 0.885107 |
| 39 | WWDAK | 0.864797 |
| 40 | WGEDW | 0.85414 |
| 41 | KDWVF | 0.825197 |
| 42 | AFTPL | 0.812467 |
| 43 | FYADP | 0.802557 |
| 44 | KDFLPF | 0.943593 |
| 45 | EFFDRF | 0.937159 |
| 46 | SFWDGK | 0.905982 |
| 47 | DDFDRF | 0.898629 |
| 48 | TFPYPR | 0.869334 |
| 49 | KDFPPR | 0.851136 |
| 50 | GYDDGF | 0.833562 |
| 51 | SWGEDW | 0.818031 |
| 52 | DFFDGK | 0.817769 |
| 53 | FEPPRY | 0.811904 |
| 54 | DYHDLF | 0.811285 |
Fig. 4.
MS/MS spectra showing the fragments for ten peptides. (A). FLL; (B) FYL; (C) YLF; (D) FLSF; (E) FGPF; (F) DDFF; (G) FFGPG; (H) FPYPR; (I) GNWGPL; (J) FDDFDRF.
Immune activity of HPH-VAP
NO level
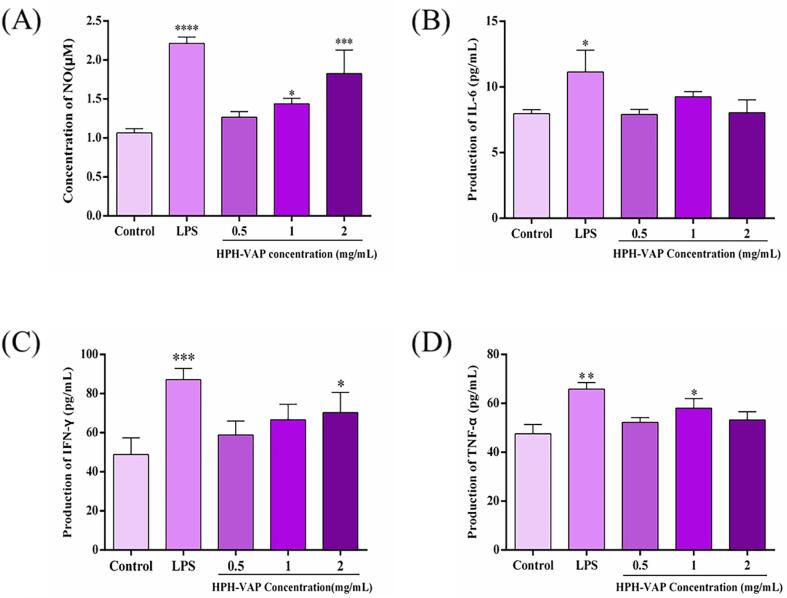
In order to further study whether HPH-VAP can activate RAW264.7 macrophages, the content of NO was also measured in macrophages. As known in Fig. 5(A), higher concentrations (1.0–2.0 mg/mL) of HPH-VAP can significantly improve the content of NO (P < 0.05), compared with the blank group, which indicated that higher concentrations of peptides can induce the activation of macrophages, and the production of NO increased with the increase of peptide concentration. And when treated with 2.0 mg/mL HPH-VAP, the content of NO was significantly higher than that of the blank group (P < 0.05) and lower than that of the LPS group.
Fig. 5.
The immunomodulatory activity of HPH-VAP and its effects on the production of inflammatory cytokines RAW264.7 macrophages. (A) Determination of NO production. Cells were treated with DMEM (Control), LPS (Model, 1 μg/mL) and HPH-VAP for 24 h. (B, C and D) Determination of IL-6, IFN-γ and TNF-α contents in the medium supernatants. Cells were treated with DMEM (Control), LPS (Model, 1 μg/mL) and HPH-VAP for 24 h, mean values labelled with (*) are significantly different from the blank control group (i.e., *P < 0.05; ** P < 0.01; ***P < 0.001; ****P < 0.0001).
The production of cytokines
The results were shown in the Fig. 5((B)–(D)). The content of cytokines TNF-α, IL-6, and IFN-γ were detected in the medium. They are released into the medium where HPH-VAP was induced. The levels of cytokine IL-6 had no significant difference compared with the blank group, but there was still an increasing trend. When HPH-VAP (1.0 mg/mL) was added, the levels of TNF-α were significantly greater than the blank group (P < 0.05). When the concentration of HPH-VAP was 2.0 mg/mL, the levels of IFN-γ were significantly greater than the blank group (P < 0.05), and its content increased with the dose-dependent increase of HPH-VAP. The levels of the three cytokines are lower than the LPS induction group, which may be because LPS activated macrophages, induced local inflammation and antibody production, and then promoted the release of their cytokines. However, excessive activation of immune cells leaded to an excessive increase in the content of pro-inflammatory cytokines, which may cause the chronic inflammation (Chen, Bozec, Ramming, & Schett, 2019). In this study, HPH-VAP can induce macrophages to secrete cytokines, it is worth mentioning that the release of cytokines induced by it was lower than that of the LPS induction group, suggesting that HPH-VAP didn’t induce excessive production of pro-inflammatory cytokines. The data obtained in this work indicated that HPH-VAP was a potential source of immunomodulatory nutrients.
Conclusion
In this study, we suggested an effective approach for preparing peptides from soybean dregs (Okara), a by-product of soy protein isolate. Its protein preparation rate was compared with method three and method two, and the preparation rates were increased by 69% and 51%, respectively. The obtained three peptides were analyzed by SEM, zeta potential, particle size distribution, FT-IR, fluorescence spectroscopy, and circular dichroism, which showed that the apparent particle size of HPH-VAP was relatively small and caused changes in the hydrogen bonds between peptide chains. And no new groups were produced. We further measured the antioxidant properties and immune properties of the three peptides and found that the in vitro antioxidant and immune properties of HPH-VAP and OP were better than those of VAP. Due to the high yield of HPH-VAP, HPH-VAP was identified by HPLC-MS/MS and its immune activity was further studied. It was found that HPH-VAP can promote the production of NO by macrophages and the release of cytokines. And this method can make the high-value utilization of okara, by-product of soybean protein isolate, as an effective way for sustainable development. Hence, our research results highlighted the process of using high-pressure homogenization-assisted double enzymatic hydrolysis to increase the utilization rate of okara, and showed its application potential in industrial environments.
CRediT authorship contribution statement
Jiaqi Fang: Conceptualization, Software, Writing – review & editing. Jiahong Lu: Data curation, Writing – original draft. Ying Zhang: Visualization. Jinyu Wang: Visualization. Sainan Wang: Methodology. Hongliang Fan: Investigation. Jiarui Zhang: Investigation. Weichang Dai: Investigation. Junpeng Gao: Investigation. Hansong Yu: Funding acquisition, Project administration.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This work was supported by the China Agriculture Research System of MOF and MARA (CARS-04).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2021.100175.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Bhimrao Muley A., Bhalchandra Pandit A., Satishchandra Singhal R., Govind Dalvi S. Production of biologically active peptides by hydrolysis of whey protein isolates using hydrodynamic cavitation. Ultrasonics Sonochemistry. 2021;71 doi: 10.1016/j.ultsonch.2020.105385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Chu Q., Ye J. Determination of hydroxyl radical by capillary electrophoresis and studies on hydroxyl radical scavenging activities of Chinese herbs. Analytical and Bioanalytical Chemistry. 2003;376(5):691–695. doi: 10.1007/s00216-003-1961-7. [DOI] [PubMed] [Google Scholar]
- Cardamone J.M. Investigating the microstructure of keratin extracted from wool: Peptide sequence (MALDI-TOF/TOF) and protein conformation (FTIR) Journal of Molecular Structure. 2010;969(1-3):97–105. [Google Scholar]
- Carullo D., Donsì F., Ferrari G. Influence of high-pressure homogenization on structural properties and enzymatic hydrolysis of milk proteins. LWT. 2020;130:109657. doi: 10.1016/j.lwt.2020.109657. [DOI] [Google Scholar]
- Chalamaiah M., Yu W., Wu J. Immunomodulatory and anticancer protein hydrolysates (peptides) from food proteins: A review. Food Chemistry. 2018;245:205–222. doi: 10.1016/j.foodchem.2017.10.087. [DOI] [PubMed] [Google Scholar]
- Chen Z., Bozec A., Ramming A., Schett G. Anti-inflammatory and immune-regulatory cytokines in rheumatoid arthritis. Nature Reviews Rheumatology. 2019;15(1):9–17. doi: 10.1038/s41584-018-0109-2. [DOI] [PubMed] [Google Scholar]
- Condurache N.N., Aprodu I., Grigore-Gurgu L., Petre B.A., Enachi E., Râpeanu G.…Stănciuc N. Fluorescence spectroscopy and molecular modeling of anthocyanins binding to bovine lactoferrin peptides. Food Chemistry. 2020;318:126508. doi: 10.1016/j.foodchem.2020.126508. [DOI] [PubMed] [Google Scholar]
- de Figueiredo V.R.G., Yamashita F., Vanzela A.L.L., Ida E.I., Kurozawa L.E. Action of multi-enzyme complex on protein extraction to obtain a protein concentrate from okara. Journal of Food Science and Technology. 2018;55(4):1508–1517. doi: 10.1007/s13197-018-3067-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di, Y. U., Wang, Y. M., Wang, B., Chi, C. F., Jian-Yin, M. A., & Deng, S. G. J. O. e. L. S. (2013). PREPARATION AND EVALUATION OF AN ANTIOXIDANT PEPTIDE FROM PROTEIN HYDROLYSATE OF SEPIA ESCULENTA.
- Fayaz G., Plazzotta S., Calligaris S., Manzocco L., Nicoli M.C. Impact of high pressure homogenization on physical properties, extraction yield and biopolymer structure of soybean okara. LWT. 2019;113:108324. doi: 10.1016/j.lwt.2019.108324. [DOI] [Google Scholar]
- Habinshuti I., Mu T.-H., Zhang M. Ultrasound microwave-assisted enzymatic production and characterisation of antioxidant peptides from sweet potato protein. Ultrasonics Sonochemistry. 2020;69:105262. doi: 10.1016/j.ultsonch.2020.105262. [DOI] [PubMed] [Google Scholar]
- He F., Wu C., Li P., Li N., Zhang D., Zhu Q.…Peng Y. Functions and Signaling Pathways of Amino Acids in Intestinal Inflammation. BioMed Research International. 2018;2018:1–13. doi: 10.1155/2018/9171905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He K., Zeng Y.u., Tian H., Zhang Z., Zhang H., Huang F., Yu F. Macrophage immunomodulatory effects of low molecular weight peptides from Mytilus coruscus via NF-κB/MAPK signaling pathways. Journal of Functional Foods. 2021;83:104562. doi: 10.1016/j.jff.2021.104562. [DOI] [Google Scholar]
- Karami Z., Akbari-adergani B. Bioactive food derived peptides: A review on correlation between structure of bioactive peptides and their functional properties. Journal of Food Science and Technology. 2019;56(2):535–547. doi: 10.1007/s13197-018-3549-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly S.M., Jess T.J., Price N.C. How to study proteins by circular dichroism. Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics. 2005;1751(2):119–139. doi: 10.1016/j.bbapap.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Lee D.P.S., Gan A.X., Kim J.E. Incorporation of biovalorised okara in biscuits: Improvements of nutritional, antioxidant, physical, and sensory properties. LWT. 2020;134:109902. doi: 10.1016/j.lwt.2020.109902. [DOI] [Google Scholar]
- Li B., Yang W., Nie Y., Kang F., Goff H.D., Cui S.W. Effect of steam explosion on dietary fiber, polysaccharide, protein and physicochemical properties of okara. Food Hydrocolloids. 2019;94:48–56. [Google Scholar]
- Li P., Yin Y.-L., Li D., Woo Kim S., Wu G. Amino acids and immune function. British Journal of Nutrition. 2007;98(2):237–252. doi: 10.1017/S000711450769936X. [DOI] [PubMed] [Google Scholar]
- Ma C.-Y., Liu W.-S., Kwok K.C., Kwok F. Isolation and characterization of proteins from soymilk residue (okara)∗. Food Research International. 1996;29(8):799–805. [Google Scholar]
- Mao F., Tu M., Fan F., Wu C., Yu C., Du M. Beneficial effects of high-pressure homogenization on the dispersion stability of aqueous hydrolysate from Mytilus edulis. Food Science and Human Wellness. 2020;9(4):394–401. [Google Scholar]
- Mok W.K., Tan Y.X., Lee J., Kim J., Chen W.N. A metabolomic approach to understand the solid-state fermentation of okara using Bacillus subtilis WX-17 for enhanced nutritional profile. AMB Express. 2019;9(1):60. doi: 10.1186/s13568-019-0786-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok W.K., Tan Y.X., Lyu X.M., Chen W.N. Effects of submerged liquid fermentation of Bacillus subtilis WX-17 using okara as sole nutrient source on the composition of a potential probiotic beverage. Food Science & Nutrition. 2020;8(7):3119–3127. doi: 10.1002/fsn3.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moure A., Domínguez H., Parajó J. Antioxidant properties of ultrafiltration-recovered soy protein fraction from industrial effluents and their hydrolysates. Process Biochemistry. 2006;41:447–456. [Google Scholar]
- Nielsen S.D., Beverly R.L., Qu Y., Dallas D.C. Milk bioactive peptide database: A comprehensive database of milk protein-derived bioactive peptides and novel visualization. Food Chemistry. 2017;232:673–682. doi: 10.1016/j.foodchem.2017.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos D.C.D., Oliveira Filho J.G.d., Silva J.d.S., Sousa M.F.d., Vilela M.d.S., Silva M.A.P.d.…Egea M.B. Okara flour: Its physicochemical, microscopical and functional properties. Nutrition & Food Science. 2019;49(6):1252–1264. [Google Scholar]
- Shang W.-H., Tang Y., Su S.-Y., Han J.-R., Yan J.-N., Wu H.-T., Zhu B.-W. In silico assessment and structural characterization of antioxidant peptides from major yolk protein of sea urchin Strongylocentrotus nudus. Food & Function. 2018;9(12):6435–6443. doi: 10.1039/c8fo01668b. [DOI] [PubMed] [Google Scholar]
- Sierra L., Fan H., Zapata J., Wu J. Antioxidant peptides derived from hydrolysates of red tilapia (Oreochromis sp.) scale. LWT. 2021;146:111631. doi: 10.1016/j.lwt.2021.111631. [DOI] [Google Scholar]
- Sun N.a., Wang Y., Bao Z., Cui P., Wang S., Lin S. Calcium binding to herring egg phosphopeptides: Binding characteristics, conformational structure and intermolecular forces. Food Chemistry. 2020;310:125867. doi: 10.1016/j.foodchem.2019.125867. [DOI] [PubMed] [Google Scholar]
- Tao X., Cai Y., Liu T., Long Z., Huang L., Deng X.…Zhao M. Effects of pretreatments on the structure and functional properties of okara protein. Food Hydrocolloids. 2019;90:394–402. [Google Scholar]
- Vong W.C., Liu S.-Q. Biovalorisation of okara (soybean residue) for food and nutrition. Trends in Food Science & Technology. 2016;52:139–147. [Google Scholar]
- Wang L., Ma M., Yu Z., Du S.-K. Preparation and identification of antioxidant peptides from cottonseed proteins. Food Chem. 2021;352:129399. doi: 10.1016/j.foodchem.2021.129399. [DOI] [PubMed] [Google Scholar]
- Wang W., Bringe N.A., Berhow M.A., Gonzalez de Mejia E. β-conglycinins among sources of bioactives in hydrolysates of different soybean varieties that inhibit Leukemia cells in vitro. Journal of agricultural and food chemistry. 2008;56(11):4012–4020. doi: 10.1021/jf8002009. [DOI] [PubMed] [Google Scholar]
- Wen L., Shi D., Zhou T., Tu J., He M., Jiang Y., Yang B. Identification of two novel prenylated flavonoids in mulberry leaf and their bioactivities. Food Chemistry. 2020;315:126236. doi: 10.1016/j.foodchem.2020.126236. [DOI] [PubMed] [Google Scholar]
- Wunderlich R., Ernst A., Rödel F., Fietkau R., Ott O., Lauber K.…Gaipl U.S. Low and moderate doses of ionizing radiation up to 2 Gy modulate transmigration and chemotaxis of activated macrophages, provoke an anti-inflammatory cytokine milieu, but do not impact upon viability and phagocytic function. Clinical & Experimental Immunology. 2015;179(1):50–61. doi: 10.1111/cei.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L.-C., Fu T.-J., Yang F.-C. Biovalorization of soybean residue (okara) via fermentation with Ganoderma lucidum and Lentinus edodes to attain products with high anti-osteoporotic effects. Journal of Bioscience and Bioengineering. 2020;129(4):514–518. doi: 10.1016/j.jbiosc.2019.10.003. [DOI] [PubMed] [Google Scholar]
- Yimit D., Hoxur P., Amat N., Uchikawa K., Yamaguchi N. Effects of soybean peptide on immune function, brain function, and neurochemistry in healthy volunteers. Nutrition. 2012;28(2):154–159. doi: 10.1016/j.nut.2011.05.008. [DOI] [PubMed] [Google Scholar]
- Yu Y., Hu Q., Liu J., Su A., Xu H., Li X.…Yang W. Isolation, purification and identification of immunologically active peptides from Hericium erinaceus. Food and Chemical Toxicology. 2021;151:112111. doi: 10.1016/j.fct.2021.112111. [DOI] [PubMed] [Google Scholar]
- Zhang H., Li L., Mittal G.S. EFFECTS OF HIGH PRESSURE PROCESSING ON SOYBEAN BETA-CONGLYCININ. Journal of Food Process Engineering. 2010;33(3):568–583. [Google Scholar]
- Zhang L., Li J., Zhou K. Chelating and radical scavenging activities of soy protein hydrolysates prepared from microbial proteases and their effect on meat lipid peroxidation. Bioresource Technology. 2010;101(7):2084–2089. doi: 10.1016/j.biortech.2009.11.078. [DOI] [PubMed] [Google Scholar]
- Zhao J., Xiong Y.L., McNear D.H. Changes in structural characteristics of antioxidative soy protein hydrolysates resulting from scavenging of hydroxyl radicals. Journal of Food Science. 2013;78(2):C152–C159. doi: 10.1111/1750-3841.12030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.