Abstract
Background
Cabozantinib is a tyrosine kinase inhibitor with a substantial efficacy in metastatic renal cell carcinoma, and is associated with a challenging toxicity profile leading to frequent drug discontinuations. Whereas an exposure/safety relationship was demonstrated for this drug, an exposure/efficacy relationship is still unknown.
Patients and methods
We carried out a monocentric, observational, pharmacokinetics/pharmacodynamics (PK/PD) study in patients with metastatic renal cell carcinoma (INDS MR 5612140520). We used measured blood concentrations of cabozantinib (Cmeas) to determine the area under the curve (AUC), apparent clearance (Cl/F) and residual blood concentration (Ctrough). Best overall response according to RECIST 1.1 and relevant toxicity (adverse event grade 3-4 or grade 2 requiring dose reduction or discontinuation) were assessed according to Cmeas, Ctrough, AUC and Cl/F.
Results
We enrolled 76 patients, including 35 who experienced disease progression and 30 with grade 3-4 toxicity. Patients with progressive disease had a significantly lower median Ctrough (406 versus 634 ng/ml, P = 0.001), Cl/F (2 versus 2.9 l/h, P = 0.002) and AUC (16 versus 20 μg h/ml, P = 0.037) compared with patients who had disease control as best response. Patients with relevant toxicity had a significantly higher Cmeas (732 versus 531 ng/ml, P = 0.006), Ctrough (693 versus 521 ng/ml, P = 0.005) and AUC (21 versus 16 μg h/ml, P = 0.046) compared with patients who did not experience any grade relevant toxicity. Receiver operating characteristic curves obtained from our study defined a threshold for drug efficacy of 536.8 ng/ml and of 617.7 ng/ml for toxicity.
Conclusion
We first demonstrate the PK/PD relationship for cabozantinib. Severe toxicities are associated with a higher drug exposure, whereas inefficacy is associated with a lower drug exposure. Cabozantinib plasma drug monitoring may be useful to optimize clinical practice.
Key words: cabozantinib, dose-exposure, toxicity, therapeutic failure, kidney cancer, pharmacokinetics
Highlights
-
•
Cabozantinib is a widely used TKI for mRCC for which there is no clearly noted pharmacokinetics/pharmacodynamics relationship.
-
•
We demonstrated that a lower Ctrough AUC and a higher drug apparent clearance (Cl/F) are associated with progressive disease.
-
•
We demonstrated that a dose-limiting toxicity is associated with a higher Ctrough and a lower clearance.
Introduction
Cabozantinib is a tyrosine kinase inhibitor (TKI) that exerts its activity mainly by inhibiting the vascular endothelial growth factor receptor 2, but also further inhibits other tyrosine kinases, such as MET, AXL, RET, KIT, FLT3 and TYRO 1.1 Cabozantinib is a weak base, and is considered as a Biopharmaceutics Classification System Class II compound, characterized by a low aqueous solubility and a high cellular permeability in vivo.2
In patients with metastatic renal cell carcinoma (mRCC), cabozantinib is European Medicines Agency (EMA) and Food and Drug Administration (FDA) approved as a second- and further-line treatment of all comers mRCC patients, and as a first-line treatment in patients with high- and intermediate-risk features per the international mRCC database consortium (IMDC) criteria,3 following the results of the METEOR and CABOSUN trials, respectively. In METEOR, cabozantinib demonstrated superiority over everolimus in terms of progression-free survival (PFS), overall response rate (ORR) and overall survival (OS).4 In the CABOSUN trial for first-line mRCC patients, cabozantinib was superior to first-line standard sunitinib, in terms of both PFS and ORR.5 Finally, the combination of cabozantinib with nivolumab, an immune checkpoint inhibitor (ICI), improved outcomes PFS, 12 months OS, ORR and health-related quality of life compared with sunitinib as a first-line treatment in mRCC patients.6
Toxicity remains a key issue for cabozantinib, however, with a grade (G) 3-4 adverse event (AE) rate of 74% and 67%, a dose reduction (DR) rate of 60% and 46% and a drug discontinuation (DD) rate of 9% and 16%, respectively, in the METEOR and CABOSUN trials.4,5 Furthermore, in the Checkmate 9ER trial, the G3-4 treatment-related AE rate for the nivolumab plus cabozantinib arm was 60.6%, with a DD rate of 19.7% (7.5% of patients discontinued cabozantinib only whereas 5.6% of patients discontinued both drugs).6
Few data are available regarding the pharmacokinetics/pharmacodynamics (PK/PD) relation in mRCC patients treated with cabozantinib. Actually, in the METEOR trial, assessment of cabozantinib PK was carried out immediately before the first drug administration (day 0) and then on days 29 and 57 from drug start. PK blood draws were carried out at least 8 hours from the last drug administration.4 In the population PK model obtained using the PK data from the METEOR trial, the individual predicted average cabozantinib concentration at steady state was 375 ng/ml for 20 mg, 750 ng/ml for 40 mg and 1125 ng/ml for 60 mg daily, respectively.7 In this model, a lower cabozantinib starting dose was associated with a higher risk of progression when compared with the recommended starting dose of 60 mg daily [hazard ratio (HR) 1.10 for 40 mg and 1.39 for 20 mg). Conversely, a higher starting dose was associated with a higher risk of developing several G3-4 AEs, such as hand-foot syndrome (HFS), fatigue/asthenia, hypertension and diarrhea.7 Furthermore, an association between the plasmatic apparent total clearance (Cl/F) of cabozantinib after oral administration and the risk of dose modification (DM) was demonstrated, as patients with a lower Cl/F (1.3 l/h) had an increased risk of DM when compared with patients with a standard Cl/F (2.3 l/h), HR 2.07.7 Nevertheless, PK data deriving from the METEOR trial were collected in a very early timeframe (up to 2 months from treatment start), which may not mirror daily clinical practice, where patients are treated with cabozantinib for a longer time interval. Hence, it has been observed for several oral compounds that chronic exposure may be associated with a decrease in circulating concentration of the drug and therefore a reduced efficacy. This phenomenon, known as ‘tachyphylaxis’, may explain some therapeutic failures to TKIs, such as sorafenib or pazopanib.8, 9, 10 Particularly for pazopanib, first-order decay kinetics were noted in a population PK model.11
The development of a PK/PD relationship for cabozantinib using data derived from daily clinical practice is therefore of key importance to inform clinical practice. We developed a PK/PD therapeutic drug monitoring (TDM) protocol, aiming to assess (i) whether a lower plasma exposure to cabozantinib is associated with cabozantinib failure and (ii) whether a higher plasma exposure is associated with the onset of severe toxicity.
Patients and methods
Study procedures
We carried out an observational PK monitoring study on mRCC patients treated at Gustave Roussy, Villejuif, starting from 1 October 2019. A data cut-off was set at 31 January 2021 for this analysis. This study is registered at the French National Health Data agency (INDS) with protocol number: MR 5612140520.
Patients
All patients aged >18 years affected by mRCC either with clear-cell or with non-clear-cell histology and currently on treatment with cabozantinib were eligible. Key exclusion criteria were concurrent treatment with immune checkpoint inhibitors (ICIs) and cabozantinib and the documented refusal of the patient to use personal data. Patients’ demographics, disease features and previous oncologic treatments were collected at study inclusion. Patients were followed up according to our institutional clinical practice with clinical examination and blood tests (complete blood cell count, blood chemistry, serum albumin, serum proteins, thyroid-stimulating hormone) every 4-6 weeks. A periodic radiological assessment was carried out every 12 weeks or earlier if a symptomatic disease progression was suspected. Data regarding concurrent medications, anthropometric measurements and AEs were collected at each visit. Cabozantinib therapeutic adherence in the last 28 days before PK blood draw was assessed through a direct patient interview. AEs were graded with Common Terminology Criteria for Adverse Events (CTCAE) v5.0 criteria.
Blood draws
Blood draws for cabozantinib PK were performed in all patients treated at our center with cabozantinib as a single agent, in any line of treatment, who experienced at least one G1 toxicity. The blood draws were carried out at least 8 h from the last drug administration and at steady state, e.g. at least at 2 weeks from drug start.
PK sample analysis
Blood samples were collected in lithium heparinate tubes and centrifuged at 2250 g for 10 min before storage at −20°C. After thawing, a protein precipitation was carried out by mixing 200 μl of the blood sample and 400 μl of acetonitrile spiked with the internal standard (cabozantinib d-4) in a 1 ml Eppendorf tube. This mix was vortexed for 15 s and then centrifuged for 10 min at 4000 g. The supernatant was then injected on an Acquity UPLC I-Class system (Waters, Milford, MA) where the separation was carried out using an Acquity BEH C18 1.7 × 2.1 × 100 mm analytical column with a vanguard pre-column. The detection was carried out by a Xevo TQ-D MS/MS (Waters, Milford, MA) system using electrospray ionization and multiple reaction monitoring detection with mass transition (m/z) of 502.2 → 391.1 and 506.3 → 391.2 for cabozantinib and its internal standard, respectively. This method was fully validated according to the EMA guidelines with a range of calibration from 25 to 2500 ng/ml, accuracy of 95.5%-105.3%, intra-day precision 2.3%-5.1% and inter-day precision 7.0%-8.8% for four QC samples (50, 187.5, 1000 and 2250 ng/ml).
Endpoints
Endpoints definition
Drug efficacy was assessed radiologically using disease response as measured per RECIST 1.1 criteria. Progressive disease (PD) per RECIST v1.1 criteria was the efficacy endpoint used for this analysis.
Relevant toxicity was defined as either G3-4 toxicity per CTCAE v 5.0 or as a G2 toxicity that required a DR or a DD.
Association between plasma concentration, efficacy and toxicity
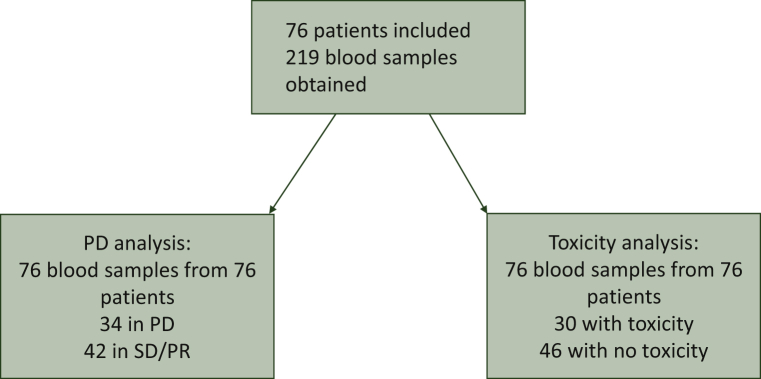
Plasma concentration of cabozantinib was measured at the time of PD, and compared with that of non-progressive patients [patients with radiological evidence of either stable disease or partial response (PR) according to RECIST 1.1]. Similarly, each time a relevant toxicity was registered, as defined above, the cabozantinib plasma concentration was measured and compared with that of patients not experiencing a relevant toxicity. Each patient included in the study was considered once for efficacy assessment and once for toxicity assessment (see STROBE diagram, Figure 1).
Figure 1.
STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) diagram for patients included in this report.
PD, progressive disease; PR, partial response; SD, stable disease.
PK analysis
For the PK/PD analysis, a blood draw was considered eligible if it was collected at least 8 h from the last drug dose, as already carried out in the METEOR trial, and if it was performed at steady state (i.e. at least 15 days from the first drug administration). A PK population model was constructed through a non-linear mixed effect model, using the measured plasma concentration (Cmeas), which will be described in a separate paper. Cl/F and area under the curve (AUC) were obtained from the population PK model departing from the Cmeas. Conversely, residual blood concentration (Ctrough), i.e. the lowest plasma drug concentration reached before the next dose, was estimated by the following formula:
where DI is the dosing interval, the time between the last intake of the drug and collection of the PK sample, and t½ is the elimination half-life of the drug (i.e. 99 h).12 The PK population analysis was conducted with Monolix (Lixoft, Anthony, France).
Statistical analysis
Descriptive statistics used numbers and percentages for qualitative variables, and median and interquartile range (IQR) for quantitative variables. Baseline characteristics for each group were compared using the Student’s t-test for continuous variables or the chi-square or Fisher’s exact test for categorical variables. The difference between medians was evaluated by a Wilcoxon signed-rank test and a Mann–Whitney U-test. Receiver operating characteristic (ROC) curves were plotted in order to define a significant statistical threshold for both toxicity and PD. Statistical analysis was carried out with R version 3.04 (R Foundation for Statistical Computing, Vienna, Austria) and PRISM vers 6.0 (GraphPad, San Diego, CA).
Results
Study population
At data cut-off, 219 PK blood draws were obtained from 76 patients (median blood draw number per patient: 2, range 1-11). Patients were mostly male (80.3%), affected mostly by mRCC of clear-cell type (75%), had a median age of 58 years (IQR 47-67 years) and had an intermediate or poor IMDC risk at cabozantinib start of 43.0% and 18.4%, respectively. Most patients received cabozantinib as a third-line treatment (range 1-10), received ICIs as a previous line of treatment (46.1%) and received cabozantinib at a median daily dose of 40 mg (IQR 40-60). Median time on cabozantinib treatment was 38.3 weeks. Therapeutic adherence in our study was 93.1%, meaning that patients reported taking 93% of the theoretically administered dose. No patient received either CYP3A4 inhibitors or CYP3A4 inducers, while 32 patients received proton pump inhibitors (PPI) as a comedication.
Mean Cmeas was 598.3 ng/ml, mean Ctrough was 597.7 ng/ml, mean Cl/F was 2.5 l/h and mean AUC was 20.9 μg h/ml for the whole population. Other relevant parameters are summarized in Table 1. Intraindividual variability was obtained through a population PK model that will be discussed in a separate paper. In our population, it is 0.089 with a relative standard error of 25.3%.
Table 1.
Summary of population characteristics
| Variable | |
|---|---|
| Median age, years (range) | 58 (22-79) |
| Sex, n (%) | |
| Male | 61 (80.3) |
| Female | 15 (19.7) |
| Histology, n (%) | |
| Clear-cell | 57 (75) |
| Type 1 papillary | 4 (5.3) |
| Type 2 papillary | 7 (9.2) |
| Chromophobe | 3 (3.9) |
| Other | 5 (6.6) |
| IMDC prognostic group, n (%) | |
| Good | 19 (25) |
| Intermediate | 33 (43.4) |
| Poor | 14 (18.4) |
| Unknown | 10 (13.2) |
| Line before cabozantinib, n (%) | |
| First | 6 (7.9) |
| Nivolumab/ipilimumab + nivolumab | 35 (46.1) |
| Sunitinib | 5 (6.6) |
| Axitinib | 16 (21.1) |
| Everolimus | 3 (3.9) |
| Sorafenib | 4 (5.3) |
| ICI + TKI | 6 (7.9) |
| Other TKI (tivozanib, crizotinib) | 2 (2.6) |
| Median line of treatment with cabozantinib (range) | 3 (1-10) |
| Cabozantinib starting dose, n (%) | |
| 60 mg daily | 46 (64) |
| 40 mg daily | 25 (35) |
| 20 mg daily | 1 (1) |
| Median dose intensity, mg (IQR) | 40 (40-60) |
| Mean Ctrough, ng/ml (SD) | 597.7 (363.9) |
| Median Ctrough, ng/ml (IQR) | 500.2 (365.5-742.5) |
| Mean Cl/F, l/h (SD) | 2.5 (1.2) |
| Median Cl/F, l/h (IQR) | 2.3 (1.7-3.2) |
| Mean AUC, μg h/ml (SD) | 20.9 (11.1) |
| Median AUC, μg h/ml (IQR) | 18 (13.8-25.5) |
| Mean time from day 1, cycle 1 to blood draw, weeks (SD) | 50 (45.8) |
| Median time from day 1, cycle 1 to blood draw, weeks (IQR) | 38.3 (13.4-69.1) |
| Mean serum albumin, g/l (SD) | 38.2 (4.5) |
| Mean total serum protein, g/l (SD) | 66.8 (7.5) |
| Mean Ctrough at 60 mg daily, ng/ml (SD) | 667 (375) |
| Median Ctrough at 60 mg daily, ng/ml (IQR) | 564 (402-777) |
| Mean Ctrough at 40 mg daily, ng/ml (SD) | 565 (283) |
| Median Ctrough at 40 mg daily, ng/ml (IQR) | 477 (366-649) |
AUC, area under the curve; Cmeas, measured blood concentration; Ctrough, residual blood concentration; Cl/F, apparent clearance; ICI, immune checkpoint inhibitor; IMDC, International Metastatic RCC Database Consortium; IQR, Interquartile range; SD, standard deviation; TKI, tyrosine kinase inhibitor.
PK/PD relation for efficacy
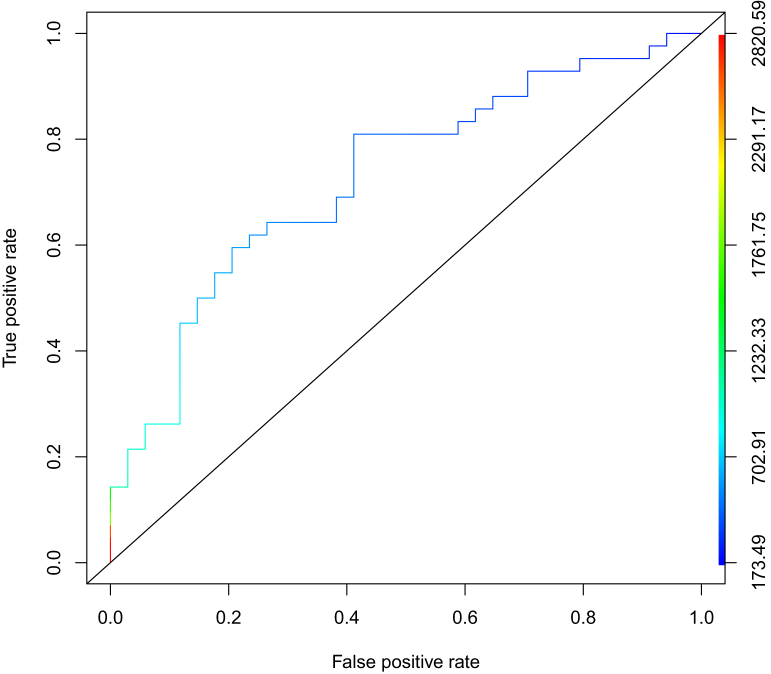
At data cut-off, 34 patients developed a PD. Patients with PD showed a lower mean Ctrough [mean 465.6 ng/ml, standard deviation (SD) 213 versus 788.5 ng/ml, SD 557.7, P = 0.001), and AUC (17.7 μg h/ml, SD 6.8 versus 24.6 μg h/ml, SD 16.8, P = 0.037), and conversely a higher mean Cl/F (2.86 l/h, SD 1.04 versus 2.01 l/h, SD 1.1, P = 0.002) when they were compared with both PR and stable disease patients (n = 42). Other patients’ key features are summarized in Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2021.100312. Finally, to better establish the PK threshold for prediction of disease progression on cabozantinib, we plotted ROC curves using data obtained from these patients. We found that a threshold of 536.8 ng/ml for Ctrough had a 64.3% sensitivity and a 73.5% specificity to detect disease progression (Figure 2).
Figure 2.
Receiver operating characteristic curve (ROC) for prediction of progression to cabozantinib in mRCC patients, based on the assessment of cabozantinib Ctrough.
A threshold of 536.8 ng/ml for Ctrough had a 64.3% sensitivity and a 73.5% specificity to detect disease progression.
PK/PD relation for toxicity
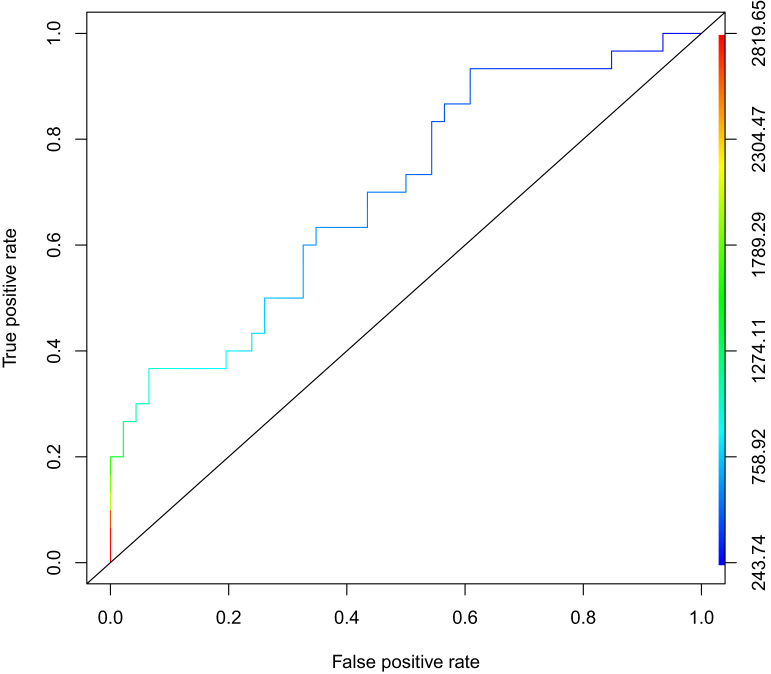
At data cut-off, 30 patients overall experienced a relevant toxicity, of whom 14 (46.7%) had at least one G2 event requiring a DD (8 patients, 26.7%) or a DR (6 patients, 20%) and 16 (53.3%) experienced at least one G3 toxicity. No patient in our cohort experienced a G4 toxicity. The most frequent G3 toxicities were hypertension in 15 patients (50%), HFS in 8 (26.7%), cutaneous rash in 5 (16.7%) and vomiting in 3 (10%). Patients experiencing a relevant toxicity exhibited higher mean Ctrough (950.2 ng/ml, SD 652.1 versus 574.1 ng/ml, SD 268.3, P = 0.005) and AUC (28.6 μg h/ml, SD 18.9 versus 19.8 μg h/ml, SD 9.0, P = 0.046) than patients who did not. Patients’ key features are summarized in Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2021.100312. Furthermore, in order to better assess the safety threshold for cabozantinib, we plotted ROC curves using data obtained from these patients. We found that a threshold of 617.7 ng/ml for Ctrough had a 63.3% sensitivity and a 65.3% specificity to detect relevant toxicity as defined above (Figure 3).
Figure 3.
Receiver operating characteristic curve (ROC) for prediction of relevant toxicity to cabozantinib in mRCC patients.
A threshold of 617.7 ng/ml for Ctrough had a 63.3% sensitivity and a 65.3% specificity to detect relevant toxicity (i.e. G3-4 toxicities and G2 toxicities determining a dose reduction or a drug discontinuation).
Discussion
In this real-life PK study of cabozantinib in mRCC patients, we demonstrated that in real-life patients, the plasma concentration of cabozantinib is lower than that of participants in the registrative clinical trial, a lower plasma concentration of cabozantinib is associated with PD and a higher plasma concentration of cabozantinib is associated with clinically relevant toxicity.
First, our study highlights the difference between clinical trial patients and real-life patients. In fact, patients included in this cohort were exposed to cabozantinib at a median daily dose of 40 mg, for a median time of 38 weeks and exhibited a median measured cabozantinib plasma concentration of 500 ng/ml. In our cohort, the cabozantinib plasma concentration of 40 mg daily is lower than the one from the METEOR trial (700 ng/ml)7 that was predicted from the PK assessment carried out up to 2 months from treatment start.7 The plasma concentrations found in our study, however, are lower than previously reported, but conversely the median time of exposure to the drug is >8 weeks. For patients with chronic long-term drug exposure, the plasmatic drug concentration could decrease over the time. This phenomenon has already been demonstrated for sorafenib and pazopanib,9, 10, 11 two TKIs with PK features comparable to those of cabozantinib and this is relevant for clinical practice, as in the case of progression or toxicity it could allow dose modifications or other pharmacological adjustments according to pharmacological drug monitoring. Finally, as a relevant comedication potentially interfering with cabozantinib PK, 44% of the patients included in our study received a PPI.13 Nevertheless, we previously demonstrated that there is no relevant effect of PPI administration on cabozantinib efficacy and clinical PK.12
We then demonstrated that a lower cabozantinib plasma concentration is associated with disease progression. This finding is novel and somehow contrasting with those derived from current literature. As an example, in a recent PK/PD study carried out in patients included in the CheckMate 9ER trial, the average cabozantinib concentration (Cavg) measured up to day 1 of cycle 7 had no effect on PFS.14 Nevertheless, these data are not comparable with ours, as the co-administration of cabozantinib with nivolumab in patients included in the trial6 may per se be relevant for PFS. Furthermore, we measured cabozantinib plasma concentration at clinically relevant time points rather than at fixed time points, thus we did not obtain a Cavg but a Ctrough which is a different PK parameter. Finally, the primary endpoint of our study was PD and not PFS, hence, for all the previously cited factors, the results of the two studies are not comparable.
Even if a threshold value for Ctrough and response to cabozantinib is not available, some interventions may be put into practice, to increase cabozantinib Ctrough and potentially induce response in a patient with PD. A simple intervention may be a drug dose increase (e.g. an increase from 40 to 60 mg daily) in order to increase cabozantinib Ctrough. This intervention should be associated with monitoring cabozantinib PK.
Furthermore, for toxicity we found that higher Cmeas, Ctrough and AUC are associated with clinically relevant toxicities. These findings may justify the drug dosing adjustments that occur in routine clinical practice. Noticeably, only a trend towards a lower Cl/F (P = 0.1) was associated with relevant toxicity in our study, whereas a low Cl/F has been previously described as a key determinant of toxicity.7, 15 This finding may be explained both by our definition of relevant toxicity as a combined endpoint that pooled poorly tolerated G2 and G3 toxicities, whereas most PK/PD studies published considered only G3-4 toxicity, and by the relatively small number of patients included.
Taking into account our results, blood monitoring of cabozantinib Ctrough may be routinely integrated into the management of mRCC patients treated with cabozantinib in a real-life setting. Blood draws for PK assessment should be carried out at clinically relevant time points (e.g. PD or intolerable toxicity) rather than at fixed time points. The consequential interventions put into practice (e.g. dose adjustments) may also be monitored with timely blood draws. In addition, the liquid chromatography/mass spectrometry method is generally available at every tertiary clinical center and at least in France, it is not expensive (estimated cost is 37 euros in France, completely covered by the national health system).
Furthermore, we tried to establish a therapeutic window for cabozantinib using real-life clinical data. We found a lower threshold for efficacy of 536.8 ng/ml and a higher threshold for relevant toxicity of 617.2 ng/ml. Those data depict a narrow therapeutic window comprised within 80 ng/ml. This is compatible with clinical data available for cabozantinib, i.e. of a drug with a high efficacy but with a high toxicity burden.
Nevertheless, this narrow therapeutic window may be explained by several factors. The first one relies on the combined endpoint used for toxicity. Actually, we defined a relevant toxicity either as a G3-4 toxicity or as a G2 toxicity that led to a DD or a DR. In the population PK models specific to mRCC patients, an increase in average cabozantinib concentrations was associated with increased risk of HFS, fatigue (grade ≥3), hypertension (grade ≥3) and diarrhea (grade ≥3).7 Therefore, patients with a G2 toxicity may have a lower cabozantinib concentration when compared with patients with G3-4 toxicity and this may explain the low threshold for toxicity found in our study. Another reason may be the inclusion into the group of patients with PD who are ‘primary resistant’ (i.e. patients who experienced a PD as a best objective response to cabozantinib). Those patients may not benefit from TKI therapy despite its plasma concentration falling into an optimal therapeutic range. Furthermore, due to the large intra-individual variability of cabozantinib (i.e. 34% in our population), this narrow therapeutic window implementation in routine TDM is impossible. It seems relevant to say, however, that firstly, a PK/PD relationship for cabozantinib does exist and secondly, regarding efficacy, a Ctrough >530 ng/ml in mRCC could be proposed as a target for Ctrough in ‘real life’ TDM. Indeed, to the best of our knowledge, no therapeutic window has been defined for cabozantinib TDM. Thus, current cabozantinib TDM is founded on the mean Ctrough obtained from the METEOR clinical trial. This Ctrough, however, was determined until week 8 from treatment start and therefore, does not consider the decrease of exposure over time which may result in an under-exposure for many patients taking long-term cabozantinib.
In addition to this narrow therapeutic range, the sensitivity and specificity for both efficacy and toxicity found with our data were not optimal, as they were all <80%.
Hence, it clearly appears that these data are part of preliminary results and they need to be validated in a larger cohort, especially for the narrow therapeutic window depicted.
Finally, our results are generally comparable to those previously obtained for other TKIs used in mRCC, namely sunitinib, axitinib and pazopanib (summarized in Table 2), which also exhibit comparable PK/PD relationships.16, 17, 18, 19, 20, 21, 22, 23 In particular, for both sunitinib and pazopanib a concentration lower than a determined threshold was associated with an increased risk for progression, while conversely a blood concentration higher than a determined threshold is associated with an increased risk of toxicity, especially hypertension, hypothyroidism and asthenia.
Table 2.
Exposure-toxicity and exposure-efficacy relationship in anti-vascular endothelial growth factor receptor 2 tyrosine kinase inhibitors in metastatic renal cell carcinoma
| Drug | PK parameter | Safety | Efficacy |
|---|---|---|---|
| Axitinib16,17 | AUC at 4 weeks from treatment start | — |
|
| Css,trough |
|
|
|
| Pazopanib18,19 | Css,trough |
|
|
| Sunitinib20, 21, 22, 23 | Css,trough |
|
|
| AUCss |
|
|
AUC, area under the curve; AUCss, AUC at steady state; Css,trough, residual concentration at steady state; OS, overall survival; PD, pharmacodynamics; PFS, progression-free survival; PK, pharmacokinetics; PR, partial response; SD, stable disease; TTF, time to treatment failure.
The main limitations of this paper lie in the intrinsic design of an observational real-life study. We prospectively included in our study all mRCC patients treated with cabozantinib at Gustave Roussy. Most of the patients in our study had already started treatment with cabozantinib several weeks before the study was initiated and before the blood draws for PK assessment were carried out. Thus, most of the patients included in our study had already experienced a response outside of our PK monitoring window. This led both to the low number of PR observed (7, 9.2% of patients) and conversely to the high number of patients with PD observed (42, 55.6%). Furthermore, unlike clinical trials in which the compulsory starting dose of cabozantinib was 60 mg daily and dose reductions were applied in case of an intolerable toxicity, in real-life clinical practice, cabozantinib is often started at a lower dose of 40 mg daily. This factor may have further contributed to the low number of G4 toxicities highlighted, to the lower number of PR observed and to the lower plasma concentration measured. Nevertheless, the design of our study reflects general clinical practice, in which cabozantinib is started at a dose of 40 mg daily in up to 30% of patients, but with a demonstrated dismal prognostic outcome.24,25
Conclusion
In this observational PK/PD study we demonstrated that a lower plasma concentration of cabozantinib and a consequent lower exposure to the drug is associated with PD, whereas a higher blood concentration and a higher exposure is associated with a higher rate of relevant toxicity. Therefore, cabozantinib seems to be a good candidate for TDM and we propose to target a Ctrough >530 ng/ml to ensure its efficacy.
Acknowledgments
Funding
None declared.
Disclosures
EA reports personal financial interest from Genzyme and Mundipharma, outside the submitted work. EC reports personal financial interest from Bristol Myers Squibb (BMS), Brazil Clovis Oncology, GlaxoSmithKline, Ipsen, Merck, Pfizer outside the submitted work. OM reports personal financial interest from Bayer, Blueprint Medicines, BMS, Eli Lilly, Ipsen, Merck Sharp & Dohme (MSD), Pfizer, Roche, Servier and institutional financial interest from Bayer, Blueprint Medicines, Eli Lilly and Epizyme outside the submitted work. BE reports personal financial interest from AVEO, BMS, EUSA Pharma, Ipsen, MSD, Novartis, Oncorena, Pfizer; Roche/Genentech and institutional consulting fees compensated to their institution from BMS France, all outside the submitted work. LA reports consulting fees compensated to their institution from Amgen, Astellas, AstraZeneca, BMS, Corvus Pharmaceuticals, Exelixis, Ipsen, Merck KGaA, Merck & Co., Novartis, Peloton Therapeutics, Roche and Pfizer outside the submitted work. All other authors have declared no conflicts of interest.
Study approval/ethics
This study was approved from the institutional ethical committee and registered at French national health data institute (INDS) with the following number: MR5612140520.
Supplementary data
References
- 1.Yakes F.M., Chen J., Tan J., et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther. 2011;10:2298–2308. doi: 10.1158/1535-7163.MCT-11-0264. [DOI] [PubMed] [Google Scholar]
- 2.Lacy S.A., Miles D.R., Nguyen L.T. Clinical pharmacokinetics and pharmacodynamics of cabozantinib. Clin Pharmacokinet. 2017;56:477–491. doi: 10.1007/s40262-016-0461-9. [DOI] [PubMed] [Google Scholar]
- 3.Heng D.Y., Xie W., Regan M.M., et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol. 2009;27(34):5794. doi: 10.1200/JCO.2008.21.4809. [DOI] [PubMed] [Google Scholar]
- 4.Choueiri T.K., Escudier B., Powles T., et al. Cabozantinib versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1814–1823. doi: 10.1056/NEJMoa1510016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choueiri T.K., Halabi S., Sanford B.L., et al. Cabozantinib versus sunitinib as initial targeted therapy for patients with metastatic renal cell carcinoma of poor or intermediate risk: the alliance A031203 CABOSUN trial. J Clin Oncol. 2017;35(6):591–597. doi: 10.1200/JCO.2016.70.7398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choueiri T.K., Powles T., Burotto M., et al. CheckMate 9ER Investigators. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2021;384(9):829–841. doi: 10.1056/NEJMoa2026982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lacy S., Nielsen J., Yang B., et al. Population exposure–response analysis of cabozantinib efficacy and safety endpoints in patients with renal cell carcinoma. Cancer Chemother Pharmacol. 2018;81:1061–1070. doi: 10.1007/s00280-018-3579-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lehne R.A. Vol. 81. Saunders; Philadelphia: 2013. “Tachyphylaxis”. (Pharmacology for Nursing Care). [Google Scholar]
- 9.Arrondeau J., Mir O., Boudou-Rouquette P., et al. Sorafenib exposure decreases over time in patients with hepatocellular carcinoma. Invest New Drugs. 2012;30(5):2046–2049. doi: 10.1007/s10637-011-9764-8. [DOI] [PubMed] [Google Scholar]
- 10.Boudou-Rouquette P., Ropert S., Mir O., et al. Variability of sorafenib toxicity and exposure over time: a pharmacokinetic/pharmacodynamic analysis. Oncologist. 2012;17:1204–1212. doi: 10.1634/theoncologist.2011-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu H., van Erp N., Bins S., et al. Development of a pharmacokinetic model to describe the complex pharmacokinetics of pazopanib in cancer patients. Clin Pharmacokinet. 2017;56(3):293–303. doi: 10.1007/s40262-016-0443-y. [DOI] [PubMed] [Google Scholar]
- 12.Rassy E., Cerbone L., Auclin E., et al. The effect of concomitant proton pump inhibitor and cabozantinib on the outcomes of patients with metastatic renal cell carcinoma. Oncologist. 2021;26(5):389–396. doi: 10.1002/onco.13711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguyen L., Holland J., Mamelok R., et al. Evaluation of the effect of food and gastric pH on the single-dose pharmacokinetics of cabozantinib in healthy adult subjects. J Clin Pharmacol. 2015;55(11):1293–1302. doi: 10.1002/jcph.526. [DOI] [PubMed] [Google Scholar]
- 14.Shah A.Y., Motzer R.J., Apolo A.B., et al. Cabozantinib (C) exposure-response (ER) analysis for the phase 3 CheckMate 9ER (CM 9ER) trial of nivolumab plus cabozantinib (N+C) versus sunitinib (S) in first-line advanced renal cell carcinoma (1L aRCC) J Clin Oncol. 2021;39(suppl 15):abstr 4561. [Google Scholar]
- 15.Castellano D., Pablo Maroto J., Benzaghou F., et al. Exposure-response modeling of cabozantinib in patients with renal cell carcinoma: implications for patient care. Cancer Treat Rev. 2020;89:102062. doi: 10.1016/j.ctrv.2020.102062. [DOI] [PubMed] [Google Scholar]
- 16.Rini B.I., Garrett M., Poland B., et al. Axitinib in metastatic renal cell carcinoma: results of a pharmacokinetic and pharmacodynamic analysis. J Clin Pharmacol. 2013;53(5):491–504. doi: 10.1002/jcph.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Igarashi R., Inoue T., Fujiyama N., et al. Contribution of UGT1A1 genetic polymorphisms related to axitinib pharmacokinetics to safety and efficacy in patients with renal cell carcinoma. Med Oncol Northwood Lond Engl. 2018;35:51. doi: 10.1007/s12032-018-1113-8. [DOI] [PubMed] [Google Scholar]
- 18.Hurwitz H.I., Dowlati A., Saini S., et al. Phase I trial of pazopanib in patients with advanced cancer. Clin Cancer Res. 2009;15:4220–4227. doi: 10.1158/1078-0432.CCR-08-2740. [DOI] [PubMed] [Google Scholar]
- 19.Suttle A.B., Ball H.A., Molimard M., et al. Relationships between pazopanib exposure and clinical safety and efficacy in patients with advanced renal cell carcinoma. Br J Cancer. 2014;111:1909–1916. doi: 10.1038/bjc.2014.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mendel D.B., Laird A.D., Xin X., et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res. 2003;9:327–337. [PubMed] [Google Scholar]
- 21.Houk B.E., Bello C.L., Poland B., et al. Relationship between exposure to sunitinib and efficacy and tolerability endpoints in patients with cancer: results of a pharmacokinetic/pharmacodynamic meta-analysis. Cancer Chemother Pharmacol. 2010;66:357–371. doi: 10.1007/s00280-009-1170-y. [DOI] [PubMed] [Google Scholar]
- 22.Noda S., Otsuji T., Baba M., et al. Assessment of sunitinib-induced toxicities and clinical outcomes based on therapeutic drug monitoring of sunitinib for patients with renal cell carcinoma. Clin Genitourin Cancer. 2015;13:350–358. doi: 10.1016/j.clgc.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 23.Cabel L., Blanchet B., Thomas-Schoemann A., et al. Drug monitoring of sunitinib in patients with advanced solid tumors: a monocentric observational French study. Fundam Clin Pharmacol. 2018;32:98–107. doi: 10.1111/fcp.12327. [DOI] [PubMed] [Google Scholar]
- 24.Albiges L., Fléchon A., Chevreau C., et al. Real-world evidence of cabozantinib in patients with metastatic renal cell carcinoma: results from the CABOREAL Early Access Program. Eur J Cancer. 2021;142:102–111. doi: 10.1016/j.ejca.2020.09.030. [DOI] [PubMed] [Google Scholar]
- 25.Procopio G., Prisciandaro M., Iacovelli R., et al. Safety and efficacy of cabozantinib in metastatic renal-cell carcinoma: real-world data from an Italian Managed Access Program. Clin Genitourin Cancer. 2018;16(4):e945–e951. doi: 10.1016/j.clgc.2018.03.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.