Abstract
Background
We determined the prognostic impact of lymphovascular invasion (LVI) in a large, national, multicenter, retrospective cohort of patients with early breast cancer (BC) according to numerous factors.
Patients and methods
We collected data on 17 322 early BC patients treated in 13 French cancer centers from 1991 to 2013. Survival functions were calculated using the Kaplan–Meier method and multivariate survival analyses were carried out using the Cox proportional hazards regression model adjusted for significant variables associated with LVI or not. Two propensity score-based matching approaches were used to balance differences in known prognostic variables associated with LVI status and to assess the impact of adjuvant chemotherapy (AC) in LVI-positive luminal A-like patients.
Results
LVI was present in 24.3% (4205) of patients. LVI was significantly and independently associated with all clinical and pathological characteristics analyzed in the entire population and according to endocrine receptor (ER) status except for the time period in binary logistic regression. According to multivariate analyses including ER status, AC, grade, and tumor subtypes, the presence of LVI was significantly associated with a negative prognostic impact on overall (OS), disease-free (DFS), and metastasis-free survival (MFS) in all patients [hazard ratio (HR) = 1.345, HR = 1.312, and HR = 1.415, respectively; P < 0.0001], which was also observed in the propensity score-based analysis in addition to the association of AC with a significant increase in both OS and DFS in LVI-positive luminal A-like patients. LVI did not have a significant impact in either patients with ER-positive grade 3 tumors or those with AC-treated luminal A-like tumors.
Conclusion
The presence of LVI has an independent negative prognostic impact on OS, DFS, and MFS in early BC patients, except in ER-positive grade 3 tumors and in those with luminal A-like tumors treated with AC. Therefore, LVI may indicate the existence of a subset of luminal A-like patients who may still benefit from adjuvant therapy.
Key words: lymphovascular invasion, luminal A subtype, breast cancer, multicenter study
Highlights
-
•
In a study of 17 322 early BC patients, LVI had a significant independent negative prognostic impact on survival.
-
•
LVI negatively impacted survival in almost every patient category and cancer subtype, with and without AC.
-
•
LVI did not have a negative survival impact in patients with ER+ grade 3 or with luminal A-like tumors with chemotherapy.
-
•
Results suggest a possible benefit of AC in LVI-positive luminal A-like patients.
Introduction
Breast cancer (BC) accounts for almost 12% of all new cancer cases worldwide each year and is the first most common cause of cancer death in women.1 However, patient management appears to have improved over the past decade as disease mortality has steadily decreased during the same period.2 Indeed, at the 16th St. Gallen International Breast Cancer Conference, the panel specifically acknowledged the potential impact of adjuvant therapy on the risk of BC recurrence or overall survival (OS) and highlighted the importance of prognostic factors in prescribing individualized treatments with regard to the magnitude of clinical benefit.3 The most commonly accepted prognostic factors for proposing adjuvant therapy include patient age, tumor size, axillary lymph node status, tumor pathology including grade, endocrine receptor (ER) (estrogen and progesterone) status, human epidermal growth factor receptor 2 (HER2) status, and proliferation assays such as the Ki67 labeling index.3,4 Lymphovascular invasion (LVI) is also a known negative prognostic factor and is defined as tumor cells present within a definite endothelial-lined space (either lymphatic or blood vessels) in the area surrounding the invasive carcinoma.5 LVI is an early indicator of the potential for metastatic dissemination.6, 7, 8 However, its use when recommending adjuvant therapy is very limited or not recognized in most guidelines.3,9, 10, 11, 12 The lack of consensus on the importance of LVI in the decision for adjuvant therapy has been further emphasized by the recent and steadily increasing utilization of gene expression signature (genomic) assay results in adjuvant decision making, particularly for patients with ER-positive/HER2-negative tumors.8 Yet LVI could be considered as an independent prognostic factor that extends beyond the contentious position of a high-risk indicator in early, ER-positive/HER2-negative or lymph node-negative tumors,13, 14, 15 and may, in fact, have a wider prognostic impact that has been overlooked in the modern era of genomic assays. This supposition is further supported by recent studies whose findings indicate that the detection of LVI adds significant prognostic information to the 21-gene recurrence score (RS).16,17 However, to our knowledge, no other study with a large sample population has investigated the association of LVI with survival according to both adjuvant chemotherapy (AC) and molecular BC subtypes. Therefore, we have retrospectively analyzed a large national multicenter cohort of 17 322 early BC patients for the independent prognostic impact of LVI in both the entire population and in subgroups of clinical interest including molecular BC subtypes and treatment.
Patients and methods
The medical records of 23 000 patients who were treated from January 1991 to December 2013 were retrieved from the clinical databases of 13 cancer centers in France for retrospective analysis. Of this initial cohort, 17 322 patients were included based on tumor size ≥2 mm and known LVI, lymph node, and ER status, while 5678 patients with unknown status were excluded. Data were collected on patient and tumor characteristics, treatments received, time periods, and clinical outcomes (Table 1) with a follow-up of 5 or more years. Patients without events were censored as of the date of last follow-up. Informed consent was waived since all data were de-identified and collected retrospectively from each center.
Table 1.
Association of LVI with clinical and pathological characteristics
| Lymphovascular invasion status |
|||||
|---|---|---|---|---|---|
| Negative |
Positive |
χ2 |
|||
| n | % | n | % | P | |
| All patients | 13 117 | 75.7 | 4205 | 24.3 | |
| Age, years | <0.0001 | ||||
| ≤40 | 673 | 57.6 | 495 | 42.38 | |
| 40.1-50 | 2724 | 70.8 | 1123 | 29.19 | |
| 50.1-74.9 | 8621 | 79.0 | 2295 | 21.02 | |
| ≥75 | 1094 | 79.0 | 291 | 21.01 | |
| T size, mm | <0.0001 | ||||
| ≤5 | 1016 | 94.3 | 61 | 5.66 | |
| 5.1-10 | 4105 | 89.0 | 505 | 10.95 | |
| 10.1-19.9 | 4909 | 75.8 | 1569 | 24.22 | |
| 20-50 | 3087 | 59.9 | 2070 | 40.14 | |
| T histology | <0.0001 | ||||
| Ductal | 10 049 | 72.8 | 3757 | 27.21 | |
| Lobular | 1896 | 88.6 | 244 | 11.40 | |
| Mixed | 229 | 73.4 | 83 | 26.60 | |
| Others | 943 | 88.6 | 121 | 11.37 | |
| Grade | <0.0001 | ||||
| 1 | 5317 | 88.6 | 681 | 11.35 | |
| 2 | 5893 | 73.8 | 2091 | 26.19 | |
| 3 | 1907 | 57.1 | 1433 | 42.90 | |
| pN status | <0.0001 | ||||
| pN0 | 9785 | 85.3 | 1686 | 14.70 | |
| pN0 (i+) | 376 | 66.1 | 193 | 33.92 | |
| pN1mi | 994 | 70.5 | 415 | 29.45 | |
| pN1 macro | 1962 | 50.7 | 1911 | 49.34 | |
| Breast surgery | <0.0001 | ||||
| Conservative | 11 138 | 77.8 | 3171 | 22.16 | |
| Mastectomy | 1710 | 64.1 | 958 | 35.91 | |
| Unknown | 269 | 78.0 | 76 | 22.03 | |
| Axillary surgery | <0.0001 | ||||
| SLNB | 7888 | 87.8 | 1092 | 12.16 | |
| SLNB + ALND | 3046 | 67.4 | 1476 | 32.64 | |
| ALND | 2181 | 57.1 | 1637 | 42.88 | |
| Periods | <0.0001 | ||||
| <2005 | 6346 | 71.7 | 2499 | 28.25 | |
| ≥2005 | 6771 | 79.9 | 1705 | 20.12 | |
| T location | <0.0001 | ||||
| Outer/equatorial | 6282 | 73.7 | 2241 | 26.29 | |
| Inner | 2906 | 74.7 | 982 | 25.26 | |
| Unknown | 3929 | 80.0 | 982 | 20.00 | |
| Endocrine therapy | <0.0001 | ||||
| No | 3082 | 72.5 | 1168 | 27.48 | |
| Yes | 10 029 | 76.8 | 3034 | 23.23 | |
| Chemotherapy | <0.0001 | ||||
| No | 8917 | 86.2 | 1427 | 13.80 | |
| Yes | 4200 | 60.2 | 2778 | 39.81 | |
| RNI | <0.0001 | ||||
| No | 7285 | 84.2 | 1367 | 15.80 | |
| Yes | 3379 | 61.1 | 2153 | 38.92 | |
| Postmastectomy Radiotherapy | <0.0001 | ||||
| No | 699 | 83.7 | 136 | 16.29 | |
| Yes | 1009 | 55.1 | 821 | 44.86 | |
| Subtypes | <0.0001 | ||||
| Luminal A | 7127 | 84.0 | 1362 | 16.04 | |
| Luminal B HER2− G3 | 611 | 56.4 | 473 | 43.63 | |
| Luminal B HER2+ | 448 | 66.7 | 224 | 33.33 | |
| HER2 | 231 | 64.2 | 129 | 35.83 | |
| Triple negative | 737 | 74.4 | 254 | 25.63 | |
ALND, axillary lymph node dissection; HER2, human epidermal growth factor receptor 2; LVI, lymphovascular invasion; RNI, regional node irradiation; SLNB, sentinel lymph node biopsy; T, tumor.
Pathological assessment
ER and HER2 status were determined according to French guidelines [immunohistochemistry (IHC) detection of estrogen and/or progesterone receptors with a 10% threshold for ER positivity; HER2 positivity with a 3+ IHC score and/or HER2 amplification identified by in situ hybridization]. Patients with unknown HER2 status were included in the overall analyses of the entire patient population but excluded in analyses of BC subtypes defined as surrogates for intrinsic BC molecular subtypes based on tumor grade, ER and HER2: triple negative (HER2−/ER−), HER2 positive (non-luminal, HER2+/ER−), luminal A like (ER+/HER2−/grade 1 or 2), luminal B like/HER2 negative (ER+/HER2−/grade 3), and luminal B like/HER2 positive (ER+/HER2+/all grades).18 LVI was determined to be present when lymphovascular emboli, defined as tumor cells present in an endothelium-lined space within the peritumoral area, were detected by trained pathologists via examination of hematoxylin-eosin-saffron (HES) slides in accordance with the policies/guidelines of each individual center. Only specimens with definite LVI were classified as LVI positive. When LVI was rare, uncertain, or detected by IHC only, this was considered as negative to account for variability in reporting concordance between pathologists, since BC cases either without LVI or those involving the invasion of multiple vessels have reportedly had the highest LVI detection rates and concordance.19
Statistical analysis
Analyses were carried out separately for all patients or patients with ER-positive or -negative tumors on factors associated with the presence or not of LVI according to patient, disease, and clinical characteristics such as age; tumor size, histology, Scarff–Bloom–Richardson grade, and subtypes (triple negative, HER2 positive, luminal A like, luminal B like/HER2 negative, and luminal B like/HER2 positive; pN status [four categories: pN0, pN0(i+), pN1mi, and pNmacro (any pN+ >2 mm)]; breast and axillary surgery, endocrine therapy (ET), AC, and radiotherapy; and periods before and after 2005 (according to availability of trastuzumab therapy). OS, disease-free survival (DFS), and metastasis-free survival (MFS) were defined as the time interval from the date of surgery to death or last follow-up, to an event (recurrence, metastasis, or death) or last follow-up, and to the date of a distant recurrence as a first event or last follow-up, respectively. Patients lost to follow-up were considered as alive as of the date of last contact.
The associations between categorical values were evaluated via χ2 tests. Factors significantly associated with LVI were determined by binary logistic regression adjusted for all significant variables determined by univariate analysis. Survival functions were calculated using the Kaplan–Meier method with differences assessed via the log-rank test. Multivariate survival analyses were carried out using the Cox proportional hazards regression model adjusted for significant variables associated with LVI. To balance differences in prognostic variables associated with LVI status, we generated 1 : 1 LVI-positive and LVI-negative matched cohorts. Coefficients of a logistic regression adjusted on age, tumor size, ERs, HER2, pN status, AC, ET, and tumor grade were used to compute a propensity score. LVI-positive patients were then matched on this score to LVI-negative patients using nearest-neighbor matching without replacement.20,21 The impact of LVI on DFS, MFS, and OS was assessed on this matched population by log-rank tests stratified on the pairs.22 A second propensity score-based matching approach was designed to assess the impact of AC specifically in LVI-positive luminal A-like patients. To reduce confounding biases associated with AC prescription, coefficients of a logistic regression adjusted on age, tumor size, pN status, and ET were used to compute a second propensity score. LVI-positive luminal A-like patients with AC were then matched on this score to LVI-positive luminal A-like patients without AC using nearest-neighbor matching without replacement. The impact of AC on DFS, MFS, and OS of LVI-positive luminal A patients was then assessed in this matched population by log-rank tests stratified on the pairs.22 Further details of both propensity score matching approaches have been provided. (Supplementary Figures S1 and S2, available at https://doi.org/10.1016/j.esmoop.2021.100316). Statistical significance was set as P ≤ 0.05. Analyses were carried out with SPSS 16.0 (SPSS Inc., Chicago, IL) and R version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Association of LVI with other clinical and pathological features
LVI was present in 24% (4205/17 322) of patients overall (Table 1), was significantly associated with all clinical and pathological characteristics analyzed in the entire population, and was most clearly prevalent in the following categories: patient age ≤40 years (42%); tumor size 20-50 mm (40%); grade 3 (G3, 43%); pN1macro status (49%); patients treated before 2005 (28%); and patients treated via mastectomy (36%), axillary lymph node dissection (ALND) (43%), chemotherapy (40%), regional nodal irradiation (RNI) (39%), and postmastectomy radiotherapy (45%), but not ET (no = 28%). Additionally, LVI was most prevalent in patients presenting the luminal B-like subtype, specifically HER2-negative G3 (44%). When analyzed according to ER-positive or -negative status (Table 2), where 22% (3279/14 655) and 35% (926/2667) of patients were LVI positive, respectively, LVI was most prevalent in the following categories: patient age ≤40 years (41%, 45%); tumor size 20-50 mm (39%, 46%); grade 3 (G3; 46%, 38%); pN1macro status (47%, 62%); patients treated before 2005 (26%, 38%); and patients treated via mastectomy (35%, 44%), ALND (42%, 45%), ET (23%, 44%), chemotherapy (40%, 39%), RNI (37%, 46%), and postmastectomy radiotherapy (43%, 55%), respectively. Additionally, LVI was most prevalent in ER-negative patients presenting HER2+ BC and in ER-positive patients presenting the luminal B-like subtype, specifically HER2-negative G3 (44%). The prevalence of LVI according to tumor subtypes and AC was also analyzed (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2021.100316). Binary logistic regression according to ER status revealed that LVI was significantly associated with all variables except time period (Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2021.100316), where the difference before/after 2005 was not significant, with increases alongside both grade and tumor size. Multivariate analysis of LVI alongside other significant characteristics provided results that were consistent with the literature (Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2021.100316).
Table 2.
Association of LVI with clinical and pathological characteristics according to ER status
| ER >0 patients |
ER <0 patients |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Negative |
Positive |
χ2 |
Negative |
Positive |
χ2 |
|||||
| n | % | n | % | P | n | % | n | % | P | |
| 11 376 | 77.6 | 3279 | 22.4 | 1741 | 65.3 | 926 | 34.7 | |||
| Age, years | <0.0001 | |||||||||
| ≤40 | 481 | 4.2 | 340 | 10.4 | <0.0001 | 192 | 11.0 | 155 | 16.8 | |
| 40.1-50 | 2305 | 20.3 | 882 | 28.9 | 419 | 24.1 | 241 | 26.1 | ||
| 50.1-74.9 | 7604 | 66.9 | 1810 | 55.2 | 1017 | 58.4 | 485 | 52.4 | ||
| ≥75 | 982 | 8.6 | 247 | 7.5 | 112 | 6.4 | 44 | 4.8 | ||
| T size, mm | <0.0001 | |||||||||
| ≤5 | 854 | 7.5 | 42 | 1.3 | <0.0001 | 162 | 9.3 | 19 | 2.1 | |
| 5.1-10 | 3671 | 32.3 | 389 | 11.9 | 434 | 24.9 | 116 | 12.5 | ||
| 10.1-19.9 | 4340 | 38.2 | 1274 | 38.9 | 569 | 32.7 | 295 | 31.9 | ||
| 20-50 | 2511 | 22.1 | 1574 | 48.0 | 576 | 33.1 | 496 | 53.6 | ||
| T histology | <0.0001 | |||||||||
| Ductal | 8545 | 75.1 | 2904 | 88.6 | <0.0001 | 1504 | 86.4 | 853 | 92.1 | |
| Lobular | 1780 | 15.6 | 201 | 6.1 | 116 | 6.7 | 43 | 4.6 | ||
| Mixed | 216 | 1.9 | 72 | 2.2 | 13 | 0.7 | 11 | 1.2 | ||
| Others | 835 | 7.3 | 102 | 3.1 | 108 | 6.2 | 19 | 2.1 | ||
| Grade | <0.0001 | |||||||||
| 1 | 5024 | 44.2 | 623 | 19.0 | <0.0001 | 293 | 16.8 | 58 | 6.3 | |
| 2 | 5307 | 46.7 | 1749 | 53.3 | 586 | 33.7 | 342 | 36.9 | ||
| 3 | 1045 | 9.2 | 907 | 27.7 | 862 | 49.5 | 526 | 56.8 | ||
| pN status | <0.0001 | |||||||||
| pN0 | 8385 | 73.7 | 1245 | 38.0 | <0.0001 | 1400 | 80.4 | 441 | 47.6 | |
| pN0 (i+) | 353 | 3.1 | 177 | 5.4 | 23 | 1.3 | 16 | 1.7 | ||
| pN1mi | 938 | 8.2 | 379 | 11.6 | 56 | 3.2 | 36 | 3.9 | ||
| pN1macro | 1700 | 14.9 | 1478 | 45.1 | 262 | 15.0 | 433 | 46.8 | ||
| Breast surgery | <0.0001 | |||||||||
| Conservative | 9642 | 84.8 | 2426 | 74.0 | <0.0001 | 1496 | 85.9 | 745 | 80.5 | |
| Mastectomy | 1489 | 13.1 | 786 | 24.0 | 221 | 12.7 | 172 | 18.6 | ||
| Unknown | 245 | 2.2 | 67 | 2.0 | 24 | 1.4 | 9 | 1.0 | ||
| Axillary surgery | <0.0001 | |||||||||
| SLNB | 7125 | 62.6 | 938 | 28.6 | <0.0001 | 763 | 43.8 | 154 | 16.6 | |
| SLNB + ALND | 2786 | 24.5 | 1293 | 39.4 | 260 | 14.9 | 183 | 19.8 | ||
| ALND | 1463 | 12.9 | 1048 | 32.0 | 718 | 41.2 | 589 | 63.6 | ||
| Periods | <0.0001 | |||||||||
| <2005 | 5221 | 45.9 | 1803 | 55.0 | <0.0001 | 1125 | 64.6 | 696 | 75.2 | |
| ≥2005 | 6155 | 54.1 | 1475 | 45.0 | 616 | 35.4 | 230 | 24.8 | ||
| T location | <0.0001 | |||||||||
| Outer/equatorial | 5416 | 47.6 | 1716 | 52.3 | <0.0001 | 866 | 49.7 | 525 | 56.7 | |
| Inner | 2488 | 21.9 | 749 | 22.8 | 418 | 24.0 | 234 | 25.3 | ||
| Unknown | 3472 | 30.5 | 815 | 24.9 | 457 | 26.2 | 167 | 18.0 | ||
| Endocrine therapy | 0.007 | |||||||||
| No | 1428 | 12.6 | 311 | 9.5 | <0.0001 | 1654 | 95.0 | 857 | 92.5 | |
| Yes | 9942 | 87.4 | 2965 | 90.5 | 87 | 5.0 | 69 | 7.5 | ||
| Chemotherapy | <0.0001 | |||||||||
| No | 8189 | 72.0 | 1158 | 35.3 | <0.0001 | 728 | 41.8 | 269 | 29.0 | |
| Yes | 3187 | 28.0 | 2121 | 64.7 | 1013 | 58.2 | 657 | 71.0 | ||
| RNI | <0.0001 | |||||||||
| No | 6471 | 69.7 | 1133 | 40.4 | <0.0001 | 814 | 58.9 | 234 | 32.7 | |
| Yes | 2810 | 30.3 | 1671 | 59.6 | 569 | 41.1 | 482 | 67.3 | ||
| Postmastectomy Radiotherapy | <0.0001 | |||||||||
| No | 607 | 40.8 | 123 | 15.7 | <0.0001 | 92 | 41.6 | 13 | 7.6 | |
| Yes | 880 | 59.2 | 662 | 84.3 | 129 | 58.4 | 159 | 92.4 | ||
| Subtypes | ||||||||||
| Luminal A | 7127 | 87.1 | 1382 | 66.1 | <0.0001 | |||||
| Luminal B HER2− G3 | 611 | 7.5 | 473 | 23.0 | ||||||
| Luminal B HER2+ | 448 | 5.5 | 224 | 10.9 | ||||||
| HER2 | 231 | 23.9 | 129 | 33.7 | <0.0001 | |||||
| Triple negative | 737 | 76.1 | 254 | 66.3 | ||||||
ALND, axillary lymph node dissection; ER, endocrine receptor; HER2, human epidermal growth factor receptor 2; LVI, lymphovascular invasion; RNI, regional node irradiation; SLNB, sentinel lymph node biopsy; T, tumor.
Prognostic impact of LVI on OS, DFS, and MFS in the entire population: univariate analysis
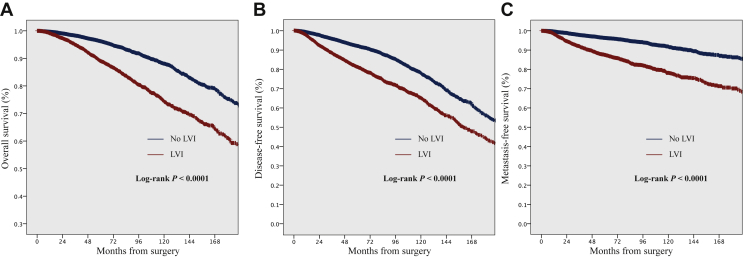
Univariate analyses were carried out on 17 292 patients. Median follow-up for all patients was 63.9 months [range: 0.1-436 months, 95% confidence interval (CI) 70.5-71.8 months]. In total, there were 727, 1086, and 628 events and 808, 1516, and 577 events for OS, DFS, and MFS in LVI-positive and LVI-negative populations, respectively. The presence of LVI was significantly associated with decreased survival: the 5-year OS, DFS, and MFS rates were 89%, 81%, and 87% in LVI-positive versus 96%, 92%, and 96% in LVI-negative populations, respectively (Figure 1A-C).
Figure 1.
Kaplan–Meier curves demonstrating (A) disease-free survival, (B) metastasis-free survival, and (C) overall survival, according to the presence of lymphovascular invasion (LVI).
Prognostic impact of LVI on OS, DFS, and MFS: multivariate analyses
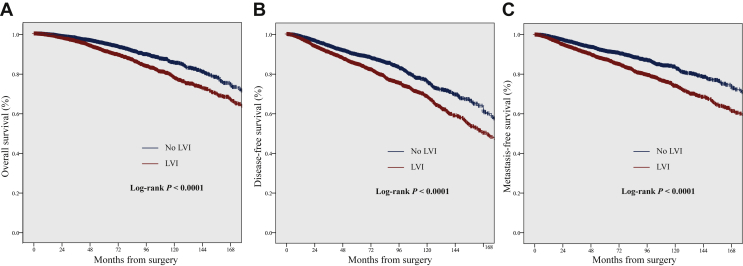
Multivariate analyses were conducted on the entire population (all patients) and the following subgroups of interest: patients with ER-positive, ER-positive/G1 or 2, ER-positive/G3, luminal A-like (ER+/HER2−/G1 or 2), ER-negative tumors, or pN0. The impact of LVI was further analyzed according to exposure to AC in the subgroups with available data (Table 3). The presence of LVI was significantly associated with a negative impact on OS, DFS, and MFS in all patients [hazard ratio (HR) = 1.345, 95% CI 1.202-1.505; HR = 1.312, 95% CI 1.201-1.432; and HR = 1.415, 95% CI 1.247-1.605, respectively; P < 0.0001], both with AC (HR = 1.439, 95% CI 1.227-1.687; HR = 1.321, 95% CI 1.170-1.491; and HR = 1.480, 95% CI 1.259-1.740, respectively; P < 0.0001) and without (HR = 1.230, 95% CI 1.043-1.450; P = 0.014; HR = 1.293, 95% CI 1.136-1.471; P < 0.0001; HR = 1.271, 95% CI 1.033-1.563; P = 0.024, respectively). This adverse prognostic impact was also observed in a propensity score-based analysis. In the matched cohort (Supplementary Figure S1 and Table S4, available at https://doi.org/10.1016/j.esmoop.2021.100316), tumors associated with LVI had significantly reduced OS, DFS, and MFS (HR = 1.574, 95% CI 1.164-1.816; HR = 1.488, 95% CI 1.330-1.666; and HR = 1.609, 95% CI 1.415-1.831, respectively; P < 0.0001) (Figure 2).
Table 3.
Association of LVI with overall, disease-free, and metastasis-free survival according to pN0 status and tumor subtypes with or without adjuvant chemotherapy
| Patients | OS |
DFS |
MFS |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | ||||
| All patients | <0.0001 | 1.345 | 1.202 | 1.505 | <0.0001 | 1.312 | 1.201 | 1.432 | <0.0001 | 1.415 | 1.247 | 1.605 |
| Without AC | 0.014 | 1.230 | 1.043 | 1.450 | <0.0001 | 1.293 | 1.136 | 1.471 | 0.024 | 1.271 | 1.033 | 1.563 |
| With AC | <0.0001 | 1.439 | 1.227 | 1.687 | <0.0001 | 1.321 | 1.170 | 1.491 | <0.0001 | 1.480 | 1.259 | 1.740 |
| ER > 0 | <0.0001 | 1.301 | 1.131 | 1.498 | <0.0001 | 1.309 | 1.176 | 1.457 | <0.0001 | 1.322 | 1.130 | 1.547 |
| ER > 0 G1-2 | 0.002 | 1.305 | 1.106 | 1.54 | <0.0001 | 1.32 | 1.165 | 1.494 | 0.002 | 1.362 | 1.125 | 1.649 |
| Without AC | 0.017 | 1.307 | 1.048 | 1.629 | 0.001 | 1.331 | 1.124 | 1.576 | 0.026 | 1.385 | 1.040 | 1.844 |
| With AC | 0.06 | 1.279 | 0.99 | 1.651 | 0.008 | 1.288 | 1.07 | 1.55 | 0.029 | 1.331 | 1.029 | 1.721 |
| ER > 0 G3 | 0.104 | 1.249 | 0.956 | 1.631 | 0.062 | 1.223 | 0.99 | 1.511 | 0.175 | 1.206 | 0.920 | 1.581 |
| HER2− | 0.854 | 0.964 | 0.652 | 1.426 | 0.281 | 1.175 | 0.876 | 1.576 | 0.878 | 1.030 | 0.710 | 1.493 |
| HER2+ | 0.506 | 1.647 | 0.378 | 7.168 | 0.939 | 1.039 | 0.392 | 2.751 | 0.515 | 0.677 | 0.209 | 2.195 |
| Luminal A | 0.134 | 1.227 | 0.939 | 1.602 | 0.030 | 1.230 | 1.020 | 1.483 | 0.460 | 1.117 | 0.833 | 1.497 |
| Without AC | 0.026 | 1.501 | 1.050 | 2.146 | 0.003 | 1.464 | 1.134 | 1.891 | 0.163 | 1.379 | 0.878 | 2.165 |
| With AC | 0.967 | 1.008 | 0.684 | 1.485 | 0.736 | 1.047 | 0.801 | 1.369 | 0.894 | 1.026 | 0.703 | 1.498 |
| ER < 0 | <0.0001 | 1.548 | 1.28 | 1.871 | <0.0001 | 1.425 | 1.221 | 1.663 | <0.0001 | 1.543 | 1.244 | 1.915 |
| ER < 0 | ||||||||||||
| TN | <0.0001 | 1.849 | 1.322 | 2.587 | <0.0001 | 1.646 | 1.26 | 2.149 | 0.001 | 1.853 | 1.294 | 2.653 |
| ER < 0 | ||||||||||||
| HER2+ | 0.011 | 2.406 | 1.227 | 4.718 | 0.018 | 1.791 | 1.103 | 2.907 | 0.019 | 2.239 | 1.140 | 4.390 |
| >2005 | 0.007 | 0.383 | 0.191 | 0.767 | 0.001 | 0.433 | 0.265 | 0.708 | 0.018 | 0.447 | 0.229 | 0.273 |
| pN0 | 0.019 | 1.225 | 1.034 | 1.452 | 0.004 | 1.210 | 1.064 | 1.375 | 0.021 | 1.269 | 1.037 | 1.553 |
Significant values are indicated in bold.
AC, adjuvant chemotherapy; CI, confidence interval; DFS, disease-free survival; ER, estrogen receptor; G, grade; HER2, human epidermal growth factor receptor 2; HR, hazard ratio; LVI, lymphovascular invasion; MFS, metastasis-free survival; OS, overall survival; TN, triple negative.
Figure 2.
Kaplan–Meier curves demonstrating (A) overall survival, (B) disease-free survival, and (C) metastasis-free survival, according to the presence of lymphovascular invasion (LVI), in the matched cohort.
The negative impact of LVI on OS, DFS, and MFS was also significant in all patients with ER-positive tumors (14 627) and pN0 (11 470). Similar results were observed for those with ER-positive/G1-2 tumors, either overall (12 703) or without AC (8809). However, in patients with AC (3876), only DFS (HR = 1.288, 95% CI 1.070-1.550; P = 0.008) and MFS (HR = 1.331, 95% CI 1.029-1.721; P = 0.029) were associated with a negative impact of LVI, while OS did not reach statistical significance (HR = 1.279, 95% CI 0.990-1.651; P = 0.06). Furthermore, there was no significant difference concerning the presence of LVI or not regarding the survival of patients with ER-positive/G3 tumors (1948), regardless of HER2 expression.
In all patients presenting the luminal A-like subtype (8471), the presence of LVI was only significantly associated with a negative impact on DFS (HR = 1.230, 95% CI 1.020-1.483; P = 0.030). In patients without AC (5946), LVI was negatively associated with both OS (HR = 1.501, 95% CI 1.050-2.146; P = 0.026) and DFS (HR = 1.464, 95% CI 1.134-1.891; P = 0.003), but not MFS (HR = 1.379, 95% CI 0.878-2.165; P = 0.163). There was no significant association between LVI and any survival outcome in patients with luminal A-like tumors who underwent AC. Furthermore, in the propensity score-matched cohort of luminal A-like tumors (Supplementary Table S5 and Figure S2, available at https://doi.org/10.1016/j.esmoop.2021.100316), AC was associated with a significant increase in both DFS and OS (HR = 0.580, 95% CI 0.363-0.927; P = 0.021 and HR = 0.469, 95% CI 0.232-0.947; P = 0.03, respectively), but statistical significance was not reached for MFS (HR = 0.689, 95% CI 0.383-1.241; P = 0.215) (Supplementary Figure S3, available at https://doi.org/10.1016/j.esmoop.2021.100316).
Cox regression analysis further demonstrated the significant association of the presence of LVI with a negative impact on OS, DFS, and MFS in patients with ER-negative tumors (2665; HR = 1.548, 95% CI 1.280-1.871; HR = 1.425, 95% CI 1.221-1.663; HR = 1.543, 95% CI 1.244-1.915, respectively; P < 0.0001). In all patients with ER-negative tumors (2667), as well as those with HER2-positive/ER-negative (360), triple-negative (1351), and pN0 tumors (11 471), LVI had an independent adverse prognostic impact on OS, DFS, and MFS (Table 3).
In patients with LVI-positive/HER2-positive/ER-negative tumors, those treated after 2005 (n = 85) (date of introduction of adjuvant trastuzumab in France) had significantly fewer events than those treated before 2005 (n = 44) (OS: HR = 0.313, 95% CI 0.127-0.775, P = 0.012; DFS: HR = 0.371, 95% CI 0.187-0.736, P = 0.005; and MFS: HR = 0.422, 95% CI 0.198-0.897, P = 0.025). More specifically, OS, DFS, and MFS at 5 years versus 7 years before 2005 were 71.9% ± 6.9% (standard error) versus 66.3% ± 7.4%, 56.5% ± 7.5% versus 53.5% ± 7.7%, and 64.1% ± 7.5% versus 64.1% ± 7.5%, respectively, whereas OS, DFS, and MFS at 5 years versus 7 years after 2005 were 90.4% ± 3.8% versus 90.4% ± 3.8%, 79.2% ± 5.0% versus 79.2% ± 5.0%, and 82.2% ± 4.7% versus 82.2% ± 4.7%, respectively.
Discussion
The decision to offer BC patients adjuvant systemic treatment is most often made for those with node-positive diseases, while prognostic factors including LVI, tumor size, tumor grade, proliferation factors, and HER2 and ER status are primarily used to identify a subset of node-negative patients with a reduced risk of recurrence without additional therapy.23 However, the exact extent of LVI’s prognostic significance remains unclear. LVI’s clinical utility in adjuvant decision making is either unrecognized or limited in major guidelines.3,9, 10, 11, 12 Indeed, the panels of both the 14th and 15th St. Gallen International Breast Cancer Conferences advocated for the consideration of LVI regarding the implementation of either AC or RNI, respectively, only in patients with other high-risk features/molecular subtypes such as triple-negative or grade 3 cancers or with >3 positive nodes, while the 16th Conference only included LVI as part of individualized chemotherapy decisions based on numerous factors.3,24,25 Yet, our results from a large population of patients with early BC have demonstrated that the presence of LVI may also be an important independent prognostic factor in patients without other high-risk features, and to our knowledge, no other study of this size has investigated the association of LVI in BC patients with treatment according to molecular subtypes. We found that LVI was significantly associated with not only every clinical and pathological characteristic that we examined, regardless of ER status and including both node-negative and node-positive early BC patients, but also with a negative impact on OS, DFS, and MFS in almost every subtype, with the notable exceptions of ER-positive G3 tumors and luminal A-like tumors with AC. These findings suggest that the analysis of LVI may hold greater prognostic significance than what was previously thought both in general and more particularly in patients with luminal A-like tumors, with subsequently greater relevance in the decision for adjuvant therapy. This contention is further supported by the results of our previously published studies on both the strong interaction between the presence of LVI and axillary lymph node involvement,26 and the significance of LVI as an independent prognostic factor in patients with triple-negative tumors.27,28
More specifically, in our present study, Cox regression analysis indicated the significant association of the presence of LVI with a negative impact on OS, DFS, and MFS in all patients with and without AC, those with ER-positive/grade 1-2 tumors without AC, and those with pN0 status. This was also the case for DFS and MFS, but not OS, in patients with ER-positive/grade 1-2 tumors treated with AC. Interestingly, no independent significant prognostic impact was detected in the ER-positive/grade 3 subgroup, regardless of HER2 expression, with most patients having received AC (73.0%). This may indicate either that the high grade already contains adverse prognostic information associated with LVI or that AC eliminates the adverse prognostic impact of LVI in these patients.
Similarly, but of greater interest, the analysis of patients within the luminal A-like subgroup did not reveal any significant negative impact of the presence of LVI on survival in those treated with AC, whereas DFS in all patients and both DFS and OS in patients without AC were negatively affected in this subgroup. We obtained similar results in an additional survival analysis of a chemotherapy propensity score-matched cohort of patients with luminal A-like cancers treated either with or without AC, though no other study exists with which we could compare these results. Our findings therefore indicate that LVI independently identifies a subgroup of patients with poor prognosis within the luminal A-like subtype, generally considered to be rather indolent, for whom chemotherapy may reduce the adverse prognostic impact of LVI. Further study is required in order to determine the extent of LVI’s association with luminal A-like cancers.
Our findings are consistent with those of other recent studies that investigated the prognostic significance of LVI,16,17,29, 30, 31, 32, 33, 34, 35, 36, 37, 38 even within the modern era of personalized medicine with its emphasis on genomic assays. However, very few of these studies have analyzed LVI in the context of molecular BC subtypes. For instance, while investigating genes associated with LVI but neither subtypes nor treatment, Kurozumi et al. found that LVI positivity was an independent poor prognostic factor in multivariate analysis with 10-year OS significantly worse in LVI-positive versus LVI-negative BC patients in two different cohorts (Molecular Taxonomy of Breast Cancer International Consortium (METABRIC): LVI+ n = 635, HR = 1.70; P < 0.0001 and The Cancer Genome Atlas (TCGA): LVI+ n = 295, HR = 2.2; P = 0.00019).37 In a study that did analyze the prognostic impact of LVI according to subtype, Ryu et al. demonstrated that LVI had greater prognostic significance than pathologic complete response in 187 BC patients treated with neoadjuvant chemotherapy, with LVI associated with worse RFS and OS in all patients while patients with both LVI and hormone receptor-negative cancers experienced the worst RFS and OS (hormone receptor status alone had no impact).30
In regard to the modern era of genomic assays, Mutai et al. analyzed the association between LVI status and the Oncotype DX RS in 657 patients with ER-positive BC. They found that the presence of LVI was significantly associated with worse DFS in all patients and worse OS in patients in an Oncotype DX intermediate-risk (RS 18-30) subgroup. Notably, they also observed that more LVI-positive patients than LVI-negative patients (42% versus 23%, respectively; P = 0.009) received AC regardless of the Oncotype DX risk category, further highlighting the perceived clinical significance of LVI. However, these results should be interpreted with caution as only 38 patients were LVI positive and the number of DFS events was too low for multivariate analysis.16 In another larger study of the relationship between LVI and the 21-gene RS, Makower et al. reported that LVI was associated with poor OS in their entire cohort of 77 425 ER-positive early BC patients with an RS (9856 LVI positive, HR = 1.24; P < 0.0001) and with N0 (HR = 1.37; P < 0.0001) but not N+ status, as well as in all 119 321 patients without an RS and those with N0 and N+ (HR = 1.21; P < 0.0001, HR = 1.15; P = 0.008, and HR = 1.22; P < 0.00001, respectively). Among patients with both an RS and N0 status, LVI was associated with worse OS in those with RS 11-25 (HR = 1.31, 95% CI 1.09-1.57) and 26-100 (HR = 1.58, 95% CI 1.30-1.93), but not RS 0-10 (HR = 1.1, 95% CI 0.77-1.53), although there was no statistically significant interaction between LVI and RS. The authors concluded that while LVI did not predict chemotherapy benefit in those with intermediate RS (11-25), it did add prognostic information in ER-positive, HER2-negative, N0 BC with RS (11-100).17 Both of these studies’ findings further support those of the Trial Assigning Individualized Options for Treatment (TAILORX) study which, though did not evaluate LVI, similarly demonstrated that while patients with an intermediate-risk Oncotype DX score (11-25) did not receive an overall benefit from AC, certain patients (pre-menopausal with RS 16-25) could still derive some advantages, especially when high-risk clinical features such as tumor size and/or grade were present.39,40 Taken together, the results of our own study and those of these studies evaluating RS suggest the existence of subgroups of patients with intermediate RS that may not normally receive AC but for whom other prognostic factors, LVI in particular, may indicate clinical benefit.
It should also be noted that while the morphological assessment of LVI status in our study relied heavily on the assessments of individual pathologists across 13 centers, the prevalence of LVI in our patient population (24.3%) corresponded to the average rate (24%) of LVI detected via hematoxylin–eosin-stained slides across 32 studies in Gujam et al.’s systematic review of data published from 1964 to 2012 regarding LVI in BC. Similarly, of the 34 studies included in the review, 32 reported the association of LVI with an unfavorable outcome (reduced survival in 19), while Gujam et al. themselves concluded that LVI was a powerful prognostic factor of poorer survival whose impact was mainly seen in patients with node-negative BC.41 However, the majority of these studies were only able to include a small number of LVI-positive low-risk or luminal A-like patients, which left the prognostic significance of LVI undecided. Even the study conducted by Ejlertsen et al., one of the largest multicenter studies to date with the inclusion of 16 172 (2453 LVI positive) patients with operable BC, only included 54 low-risk LVI-positive patients, though they reported that while LVI was consistently significantly associated with reduced OS and DFS in patients with other high-risk features, this was not the case for low-risk disease. Additionally, the authors found that the negative prognostic influence of LVI was not statistically significantly different between patients with or without adjuvant therapy, though HER2 status was not assessed, aromatase inhibitors were not fully implemented at the time of the study, and only 38 patients received adjuvant trastuzumab while none received taxanes.13 In our study, in LVI-positive/HER2-positive/ER-negative tumors, patients treated after 2005 (date of introduction of adjuvant trastuzumab in France) had fewer events than those treated before 2005, suggesting that trastuzumab was effective at improving survival outcomes in this subset of patients. However, our binary logistic regression analysis according to ER status found that the difference before/after 2005 was not significantly associated with the presence of LVI. Of note, and as previously reported,27 we found that LVI had also an independent adverse prognostic impact in triple-negative BC. In this subtype, recent data indicate that tumor-infiltrating lymphocytes (TILs) rather than tumor tissue/stromal features such as LVI42 or grade may have an important prognostic impact. Yet, TILs were not available in this large retrospective cohort, and we could not assess the impact of LVI with regard to this variable. Our study has several other limitations. Most notably, LVI was mainly assessed via HES, and while this has been shown to be less accurate than IHC for lymphatic endothelial markers, such as D2-40,5,41,43 IHC more often identifies invasive emboli in lymphatic rather than blood vessels.37,44 The limited use of IHC was mostly due to the inherent difficulty in applying an identification method such as IHC to a large and multi-institutional cohort. In addition, as mentioned previously, the analyses were carried out by individual pathologists and could allow for the misclassification of LVI if said analyses were not consistent between centers. However, our multicenter study does provide the advantages of limiting biases inherent in single-center studies while also reflecting real-world practice. Additional strengths of our study include the large sample size of >17 000 patients in multivariate and propensity score-matched analyses of the associations between LVI, numerous clinical characteristics, and OS, DFS, and MFS.
In conclusion, our study had the notable advantage of analyzing LVI in a large cohort of BC patients from 13 cancer institutions, which consequently allowed for more powerful statistical analyses and provided more applicable findings. Our results demonstrated a significant association of the presence of LVI with patient age; tumor size, histology, location, and grade; pN status; breast or axillary surgery; and adjuvant therapy; all regardless of ER status; as well as multiple subtypes of BC. Furthermore, the strong negative association between LVI and the length of OS, DFS, and MFS in all patients regardless of AC and in both ER-positive and ER-negative patients, with the notable exceptions of patients with luminal A-like tumors treated with AC, grants additional support to the argument that LVI is a more important prognostic factor than previously thought concerning the decision for adjuvant therapy. Indeed, the fact that only patients with luminal A-like tumors with AC had no association with a negative impact of LVI on OS, DFS, and MFS indicates that LVI could be especially important regarding adjuvant treatment decisions for a subset of patients with a supposed reduced risk of recurrence that may still benefit from adjuvant therapy. Accordingly, the respective role of genomic assays versus the prognostic significance of LVI should be discussed when making adjuvant decisions, while the integration of LVI into the calculation of risk provided by such assays (similarly to tumor size and node status) for luminal A-like cancers (G1-2) could improve prognostication in this subset of patients.
Another important application of our results may be to contribute to better define clinically high-risk tumors. Since genomic signature in the clinical routine may be predominantly indicated in these latter forms, the presence of LVI might identify tumors in which these tests have a high clinical utility. In addition, once a genomic signature is carried out, LVI might also help to orient adjuvant therapeutic decision, especially in case of intermediate-risk classes. We proposed that patients with luminal A-like tumors associated with LVI should be considered as clinically high risk, while there is room for considering that patients with intermediate genomic score (Oncotype DX or Prosigna) might also be considered as high risk. Nevertheless, more data are needed to elucidate the prognostic value of LVI in the context of current genomic signatures.
Acknowledgements
We would like to thank JRS Translations for their assistance in writing this manuscript.
Funding
None declared.
Disclosure
The authors have declared no conflicts of interest.
Supplementary data
References
- 1.Sung H., Ferlay J., Siegel R.L., et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 3.Burstein H.J., Curigliano G., Loibl S., et al. Estimating the benefits of therapy for early-stage breast cancer: the St. Gallen International Consensus Guidelines for the primary therapy of early breast cancer 2019. Ann Oncol. 2019;30:1541–1557. doi: 10.1093/annonc/mdz235. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz A.M., Henson D.E., Chen D., Rajamarthandan S. Histologic grade remains a prognostic factor for breast cancer regardless of the number of positive lymph nodes and tumor size: a study of 161708cases of breast cancer From the SEER Program. Arch Pathol Lab Med. 2014;138(8):1048–1052. doi: 10.5858/arpa.2013-0435-OA. [DOI] [PubMed] [Google Scholar]
- 5.Mohammed R.A.A., Martin S.G., Gill M.S., Green A.R., Paish E.C., Ellis I.O. Improved methods of detection of lymphovascular invasion demonstrate that it is the predominant method of vascular invasion in breast cancer and has important clinical consequences. Am J Surg Pathol. 2007;31(12):1825–1833. doi: 10.1097/PAS.0b013e31806841f6. [DOI] [PubMed] [Google Scholar]
- 6.Carr I. Lymphatic metastasis. Cancer Metastasis Rev. 1983;2(3):307–317. doi: 10.1007/BF00048483. [DOI] [PubMed] [Google Scholar]
- 7.Nathanson S.D., Kwon D., Kapke A., Alford S.H., Chitale D. The role of lymph node metastasis in the systemic dissemination of breast cancer. Ann Surg Oncol. 2009;16(12):3396–3405. doi: 10.1245/s10434-009-0659-2. [DOI] [PubMed] [Google Scholar]
- 8.Aleskandarany M.A., Sonbul S.N., Mukherjee A., Rakha E.A. Molecular mechanisms underlying lymphovascular invasion in invasive breast cancer. PAT. 2015;82(3-4):113–123. doi: 10.1159/000433583. [DOI] [PubMed] [Google Scholar]
- 9.Gradishar W.J., Anderson B.O., Abraham J. NCCN Guidelines Index table of contents discussion. Breast Cancer. 2019:215. [Google Scholar]
- 10.Denduluri N., Chavez-MacGregor M., Telli M.L., et al. Selection of optimal adjuvant chemotherapy and targeted therapy for early breast cancer: ASCO Clinical Practice Guideline Focused Update. J Clin Oncol. 2018;36(23):2433–2443. doi: 10.1200/JCO.2018.78.8604. [DOI] [PubMed] [Google Scholar]
- 11.Cardoso F., Kyriakides S., Ohno S., et al. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(8):1194–1220. doi: 10.1093/annonc/mdz173. [DOI] [PubMed] [Google Scholar]
- 12.Henry N.L., Somerfield M.R., Abramson V.G., et al. Role of patient and disease factors in adjuvant systemic therapy decision making for early-stage, operable breast cancer: update of the ASCO endorsement of the cancer care Ontario guideline. J Clin Oncol. 2019;37(22):1965–1977. doi: 10.1200/JCO.19.00948. [DOI] [PubMed] [Google Scholar]
- 13.Ejlertsen B., Jensen M.-B., Rank F., et al. Population-based study of peritumoral lymphovascular invasion and outcome among patients with operable breast cancer. J Natl Cancer Inst. 2009;101(10):729–735. doi: 10.1093/jnci/djp090. [DOI] [PubMed] [Google Scholar]
- 14.Colleoni M., Rotmensz N., Maisonneuve P., et al. Prognostic role of the extent of peritumoral vascular invasion in operable breast cancer. Ann Oncol. 2007;18(10):1632–1640. doi: 10.1093/annonc/mdm268. [DOI] [PubMed] [Google Scholar]
- 15.Yildirim E., Berberoglu U. Lymph node ratio is more valuable than level III involvement for prediction of outcome in node-positive breast carcinoma patients. World J Surg. 2007;31(2):276–289. doi: 10.1007/s00268-006-0487-5. [DOI] [PubMed] [Google Scholar]
- 16.Mutai R., Goldvaser H., Shochat T., Peretz I., Sulkes A., Yerushalmi R. Prognostic value of the detection of lymphovascular invasion in hormone receptor-positive early breast cancer in the era of molecular profiling. Oncology. 2019;96(1):14–24. doi: 10.1159/000492429. [DOI] [PubMed] [Google Scholar]
- 17.Makower D., Lin J., Xue X., Sparano J.A. Lymphovascular invasion, race, and the 21-gene recurrence score in early estrogen receptor-positive breast cancer. NPJ Breast Cancer. 2021;7(1):20. doi: 10.1038/s41523-021-00231-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.von Minckwitz G., Untch M., Blohmer J.-U., et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30(15):1796–1804. doi: 10.1200/JCO.2011.38.8595. [DOI] [PubMed] [Google Scholar]
- 19.Rakha E.A., Abbas A., Ahumada P.P., et al. Diagnostic concordance of reporting lymphovascular invasion in breast cancer. J Clin Pathol. 2018;71(9):802–805. doi: 10.1136/jclinpath-2017-204981. [DOI] [PubMed] [Google Scholar]
- 20.Rosenbaum P.R., Rubin D.B. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985;39(1):33–38. [Google Scholar]
- 21.Rosenbaum P.R. Modern algorithms for matching in observational studies. Ann Rev Stat Appl. 2020;7(1):143–176. [Google Scholar]
- 22.Rosenbaum P.R., Rubin D.B. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55. [Google Scholar]
- 23.Cianfrocca M. Prognostic and predictive factors in early-stage breast cancer. Oncologist. 2004;9(6):606–616. doi: 10.1634/theoncologist.9-6-606. [DOI] [PubMed] [Google Scholar]
- 24.Coates A.S., Winer E.P., Goldhirsch A., et al. Tailoring therapies—improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol. 2015;26(8):1533–1546. doi: 10.1093/annonc/mdv221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Curigliano G., Burstein H.J., Winer E.P., et al. De-escalating and escalating treatments for early-stage breast cancer: the St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann Oncol. 2017;28(8):1700–1712. doi: 10.1093/annonc/mdx308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Houvenaeghel G., Lambaudie E., Classe J.-M., et al. Lymph node positivity in different early breast carcinoma phenotypes: a predictive model. BMC Cancer. 2019;19(1):45. doi: 10.1186/s12885-018-5227-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sabatier R., Jacquemier J., Bertucci F., et al. Peritumoural vascular invasion: a major determinant of triple-negative breast cancer outcome. Eur J Cancer. 2011;47(10):1537–1545. doi: 10.1016/j.ejca.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 28.Houvenaeghel G., Sabatier R., Reyal F., et al. Axillary lymph node micrometastases decrease triple-negative early breast cancer survival. Br J Cancer. 2016;115(9):1024–1031. doi: 10.1038/bjc.2016.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morkavuk Ş.B., Güner M., Çulcu S., Eroğlu A., Bayar S., Ünal A.E. Relationship between lymphovascular invasion and molecular subtypes in invasive breast cancer. Int J Clin Pract. 2021;75(4):e13897. doi: 10.1111/ijcp.13897. [DOI] [PubMed] [Google Scholar]
- 30.Ryu Y.J., Kang S.J., Cho J.S., Yoon J.H., Park M.H. Lymphovascular invasion can be better than pathologic complete response to predict prognosis in breast cancer treated with neoadjuvant chemotherapy. Medicine (Baltimore) 2018;97(30):e11647. doi: 10.1097/MD.0000000000011647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liao G.-S., Hsu H.-M., Chu C.-H., et al. Prognostic role of lymphovascular invasion and lymph node status among breast cancer subtypes. J Med Sci. 2018;38:54–61. [Google Scholar]
- 32.Invernizzi M., Corti C., Lopez G., et al. Lymphovascular invasion and extranodal tumour extension are risk indicators of breast cancer related lymphoedema: an observational retrospective study with long-term follow-up. BMC Cancer. 2018;18(1):935. doi: 10.1186/s12885-018-4851-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chas M., Boivin L., Arbion F., Jourdan M.-L., Body G., Ouldamer L. Clinicopathologic predictors of lymph node metastasis in breast cancer patients according to molecular subtype. J Gynecol Obstet Hum Reprod. 2018;47(1):9–15. doi: 10.1016/j.jogoh.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 34.Klingen T.A., Chen Y., Stefansson I.M., et al. Tumour cell invasion into blood vessels is significantly related to breast cancer subtypes and decreased survival. J Clin Pathol. 2017;70(4):313–319. doi: 10.1136/jclinpath-2016-203861. [DOI] [PubMed] [Google Scholar]
- 35.Hwang K.-T., Kim Y.A., Kim J., et al. The influences of peritumoral lymphatic invasion and vascular invasion on the survival and recurrence according to the molecular subtypes of breast cancer. Breast Cancer Res Treat. 2017;163(1):71–82. doi: 10.1007/s10549-017-4153-4. [DOI] [PubMed] [Google Scholar]
- 36.He K.-W., Sun J.-J., Liu Z.-B., et al. Prognostic significance of lymphatic vessel invasion diagnosed by D2-40 in Chinese invasive breast cancers. Medicine (Baltimore) 2017;96(44):e8490. doi: 10.1097/MD.0000000000008490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kurozumi S., Joseph C., Sonbul S., et al. A key genomic subtype associated with lymphovascular invasion in invasive breast cancer. Br J Cancer. 2019;120(12):1129–1136. doi: 10.1038/s41416-019-0486-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Y.L., Saraf A., Lee S.M., et al. Lymphovascular invasion is an independent predictor of survival in breast cancer after neoadjuvant chemotherapy. Breast Cancer Res Treat. 2016;157(3):555–564. doi: 10.1007/s10549-016-3837-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sparano J.A., Gray R.J., Makower D.F., et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med. 2018;379(2):111–121. doi: 10.1056/NEJMoa1804710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sparano J.A., Gray R.J., Ravdin P.M., et al. Clinical and genomic risk to guide the use of adjuvant therapy for breast cancer. N Engl J Med. 2019;380(25):2395–2405. doi: 10.1056/NEJMoa1904819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gujam F.J.A., Going J.J., Edwards J., Mohammed Z.M.A., McMillan D.C. The role of lymphatic and blood vessel invasion in predicting survival and methods of detection in patients with primary operable breast cancer. Crit Rev Oncol Hematol. 2014;89(2):231–241. doi: 10.1016/j.critrevonc.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 42.Savas P., Salgado R., Denkert C., et al. Clinical relevance of host immunity in breast cancer: from TILs to the clinic. Nat Rev Clin Oncol. 2016;13(4):228–241. doi: 10.1038/nrclinonc.2015.215. [DOI] [PubMed] [Google Scholar]
- 43.Gujam F.J.A., Going J.J., Mohammed Z.M.A., Orange C., Edwards J., McMillan D.C. Immunohistochemical detection improves the prognostic value of lymphatic and blood vessel invasion in primary ductal breast cancer. BMC Cancer. 2014;14(1):676. doi: 10.1186/1471-2407-14-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mohammed R.A., Martin S.G., Mahmmod A.M., et al. Objective assessment of lymphatic and blood vascular invasion in lymph node- negative breast carcinoma: findings from a large case series with long-term follow-up. J Pathol. 2011;223(3):358–365. doi: 10.1002/path.2810. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.