Abstract
Cerinomyces (Dacrymycetes, Basidiomycota) is a genus traditionally defined by corticioid basidiocarps, in contrast to the rest of the class, which is characterized by gelatinous ones. In the traditional circumscription the genus is polyphyletic, and the monotypic family Cerinomycetaceae is paraphyletic. Aiming for a more concise delimitation, we revise Cerinomyces s.l. with a novel phylogeny based on sequences of nrDNA (SSU, ITS, LSU) and protein-coding genes (RPB1, RPB2, TEF1-α). We establish that monophyletic Cerinomyces s.s. is best characterized not by the corticioid morphology, but by a combination of traits: hyphal clamps, predominantly aseptate thin-walled basidiospores, and low content of carotenoid pigments. In our updated definition, Cerinomyces s.s. encompasses five well-supported phylogenetic clades divided into two morphological groups: (i-iii) taxa with arid corticioid basidiocarps, including the generic type C. pallidus; and (iv-v) newly introduced members with gelatinous basidiocarps, like Dacrymyces enatus and D. tortus. The remaining corticioid species of Cerinomyces s.l. are morphologically distinct and belong to the Dacrymycetaceae: our analysis places the carotenoid-rich Cerinomyces canadensis close to Femsjonia, and we transfer the clamps-lacking C. grandinioides group to Dacrymyces. In addition, we address genera related to Cerinomyces s.l. historically and morphologically, such as Ceracea, Dacryonaema and Unilacryma. Overall, we describe twenty-four new species and propose nine new combinations in both Cerinomycetaceae and Dacrymycetaceae.
Key words: Ceracea, Cerinomycetaceae, Corticioid, New taxa, Phylogeny, Taxonomy, Type studies, Typification
Taxonomic novelties: New species: Cerinomyces aeneus A. Savchenko, Miettinen & J.C. Zamora; C. atrans A. Savchenko; C. borealis Miettinen, Spirin & A. Savchenko; C. brevisetus Chikowski, Alvarenga & A. Savchenko; C. concretus A. Savchenko; C. creber J.C. Zamora, A. Savchenko, Trichies & Olariaga; C. enterolaxus Shirouzu & A. Savchenko; C. favonius Spirin, Miettinen & A. Savchenko; C. fugax A. Savchenko; C. hesperidis A. Savchenko; C. inermis A. Savchenko; C. lipoferus J.C. Zamora & A. Savchenko; C. nepalensis A. Savchenko; C. neuhoffii J.C. Zamora & A. Savchenko; C. paulistanus A. Savchenko; C. pinguis A. Savchenko; C. ramosissimus A. Savchenko; C. tristis Miettinen & A. Savchenko; C. verecundus A. Savchenko; C. volaticus A. Savchenko, V. Malysheva & J.C. Zamora; Dacrymyces burdsallii A. Savchenko; D. grandii A. Savchenko & Miettinen; D. sobrius A. Savchenko; D. venustus A. Savchenko
New combinations: Cerinomyces cokeri (McNabb) A. Savchenko & J.C. Zamora; C. enatus (Berk. & M.A. Curtis) A. Savchenko; C. tortus (Willd.) Miettinen, J.C. Zamora & A. Savchenko; Dacrymyces ceraceus (Ginns) A. Savchenko; D. cereus (Rick) A. Savchenko; D. grandinioides (McNabb) A. Savchenko; D. lagerheimii (Pat.) A. Savchenko; D. pengii (B. Liu & L. Fan) A. Savchenko; D. pulchrus (Lowy) A. Savchenko
Typifications (basionyms): Lectotypifications: Ceracea aureofulva Bres., Ce. cerea Rick, D. confluens P. Karst., Tremella enata Berk. & M.A. Curtis
Neotypification: Tremella torta Willd
Introduction
The Dacrymycetes is a phylogenetically and morphologically well-established class of basidia-bearing fungi, inhabiting dead wood and causing brown rot (Oberwinkler 2014, Floudas et al. 2015, Nagy et al. 2016). The class is relatively small, and includes two orders (Dacrymycetales and Unilacrymales), four families (Cerinomycetaceae Jülich, Dacrymycetaceae J. Schröt., Dacryonaemataceae J.C. Zamora & Ekman, and Unilacrymaceae Shirouzu, Tokum. & Oberw.), more than 10 generally accepted genera, and ca. 400 published species names. Many of these names are taxonomic synonyms, and the true number of described species is at least 120. Members of the class can be readily distinguished from other basidiomycetes by their bisterigmate Y-shaped basidia, with the single exception of Unilacryma unispora, which has unisterigmate basidia (Wells 1994, Shirouzu et al. 2013). On an ultrastructural level, dacrymycetes are characterized by dolipore septa with imperforate parenthesomes, or rarely with a single pore (Maekawa 1987, Oberwinkler 1994, Shirouzu et al. 2013). In terms of macromorphology, the class is dominated by so-called “jelly fungi” with pustulate-pulvinate, cupulate, dendroid, and spathulate basidiocarps, coloured with carotenoids in different tints of yellow and orange (Fig. 1, Goodwin 1953, Czeczuga 1980, Zamora & Ekman 2020). In addition to the soft-gelatinous species with prominent basidiocarps, the class also includes members with corticioid basidiocarps of arid or waxy-gelatinous consistency, attached to the substrate with an applanate subiculum. These fungi are traditionally classified in the genus Cerinomyces Martin. In contrast to the gelatinous dacrymycetes, basidiocarps of the traditional Cerinomyces species do not noticeably change in shape or swell in transition between dry and moist conditions. Most important, corticioid-resupinate morphotype was thought to be a definitive character of Cerinomyces. In result of such prioritization, the genus has accumulated a large variation in microscopic features over time, and its scope has become ever broader.
Fig. 1.
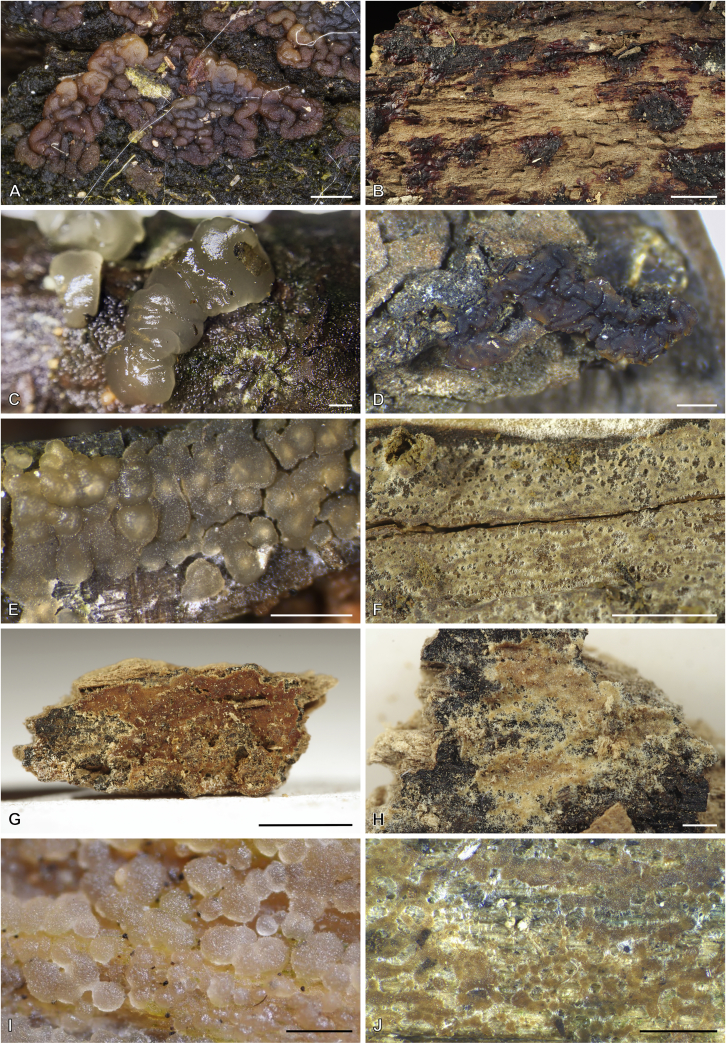
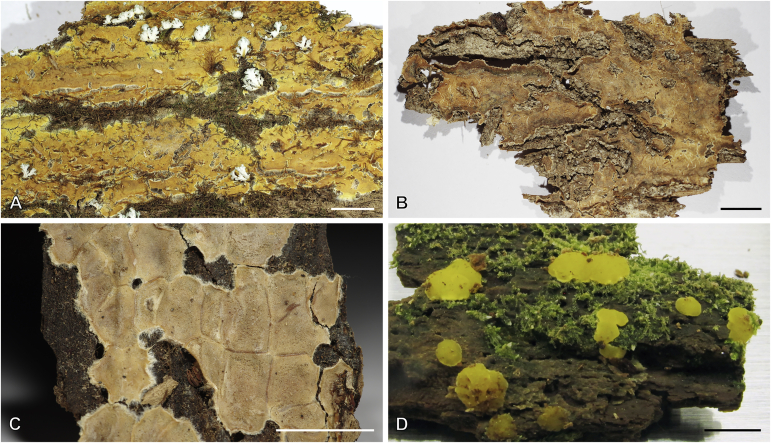
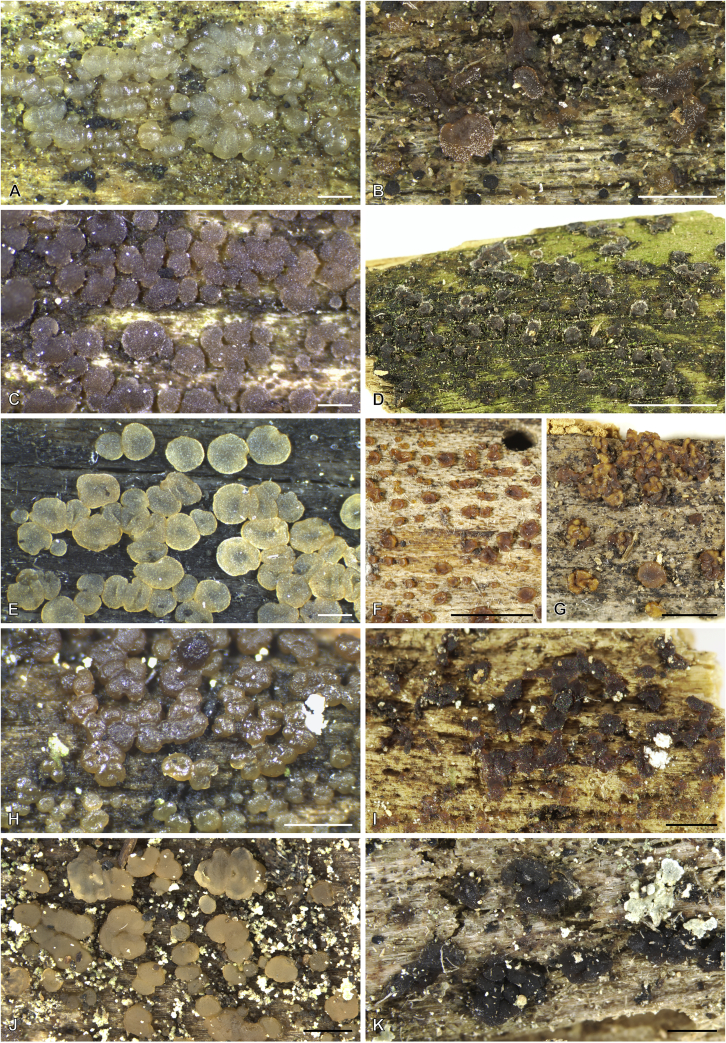
Some of the most common basidiocarp morphotypes in Dacrymycetes. A. Pustulate Dacrymyces adpressus (H6012680). B. Cerebriform D. chrysospermus (TU135035). C. Cylindrical and dendroid Calocera furcata (H6012626). D. Capitate Ditiola radicata (H6012689). E. Spathulate Dacryopinax sp. (H:Miettinen 13068). F. Corticioid Cerinomyces borealis (GB-0071203). G. Cupulate Guepiniopsis buccina (CWU(MYC)7014). H. Synnematous Dacryonaema rufum (H:Poelt, Fungi 242). Scale bars: A, C, D, F, G, H ≈ 1 mm; B, E ≈ 5 mm.
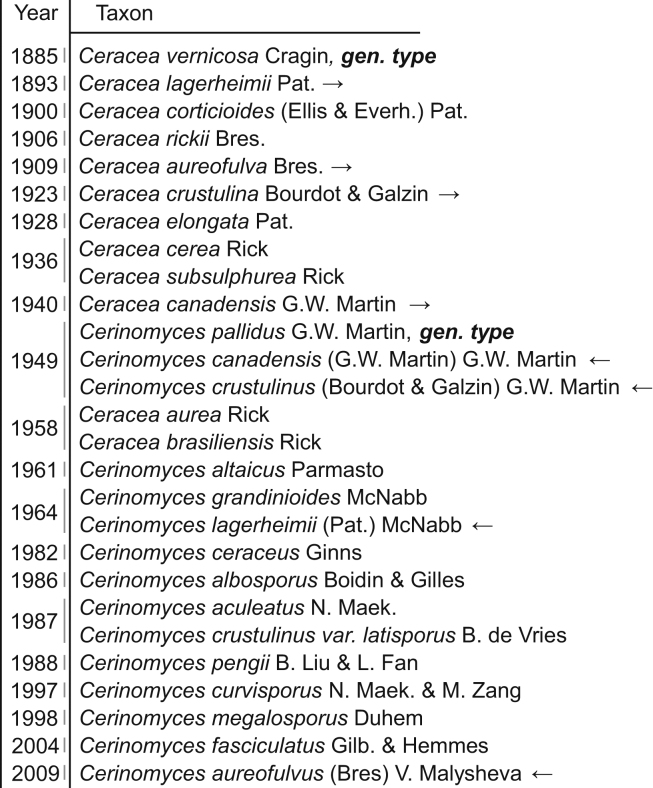
The name Cerinomyces initially appeared as a typographic error in “Ceriomyces” in the index of the 10th volume of the “Handwörterbuch der Naturwissenschaften” encyclopedia, p. 1085 (Korschelt et al. 1915). However, this misspelling had no formal nomenclatural value, and when Martin (1949) described Cerinomyces to accommodate some fungi formerly classified in Ceracea Cragin, his genus was published in a valid way. (We hereinafter abbreviate Ceracea as “Ce.” and Cerinomyces as “C.”). The nomenclature of Cerinomyces prior to Martin (1949) is tied to Ceracea. Cragin (1885) originally introduced Ceracea as a monotypic genus for an applanate brown fungus with “mostly bifurcate filaments”, believed by subsequent researchers to be a reference to the Y-shaped dacrymycete basidia, bearing elliptical aseptate spores at the apices of these basidia. The substrate of the genus type, Ce. vernicosa Cragin, was a polypore fungus, but in other details the vague description suited resupinate dacrymycetes. In the same year, the description was reproduced in a literature synopsis, which is sometimes erroneously cited as the genus’ original publication (Kellerman 1885). Soon after, Patouillard described from Ecuador a new species Ce. lagerheimii, and supplied it with imagery showing doubtlessly dacrymycete microstructures (Patouillard & Lagerheim 1893). In the beginning of the twentieth century, more species and combinations were assigned to Ceracea (Fig. 2): Ce. corticioides (Ellis & Everh.) Pat., Ce. rickii Bres., Ce. aureofulva Bres., Ce. crustulina Bourdot et Galzin, Ce. elongata Pat, Ce. cerea Rick, and Ce. subsulphurea Rick.
Fig. 2.
Ceracea and Cerinomyces species and varieties published and combined prior to the given work. Arrows indicate Ceracea species that were transferred to Cerinomyces.
Lloyd reinterpreted Ceracea with a statement that “no doubt it is the same as Arrhytidia” — a genus whose type A. flava Berk. & M.A. Curtis is strongly similar to Femsjonia Fries (Fries 1851, Lloyd & Stevenson 1921). Coker (1928) agreed with Lloyd and synonymized A. flava, Ceracea aureofulva Bres., Dacrymyces corticioides Ellis & Everh. and D. involutus Schw. as A. involuta (Schwein.) Coker (orth. var. involutus). In doing so, they started a tradition of naming effused dacrymycetes as Arrhytidia Berk. & M.A. Curtis. Wide use of the umbrella name A. involuta added further confusion, because it is a close relative if not a synonym of D. capitatus Schwein., a typical gelatinous Dacrymyces species (McNabb 1973).
Brasfield (1938) mentioned that “Ceracea auct. not Cragin” is a synonym to Arrhytidia. Later he changed his mind and purported that Ceracea is a correct name for species with resupinate basidiocarps without a distinct “root” in substrate. Since the type of Ce. vernicosa was assumed to be lost, he intended to typify Ceracea by Ce. lagerheimii (Brasfield 1940). Martin (1940) accepted Brasfield’s concept of the genus and described a new species Ce. canadensis. Luckily, the original material of Ce. vernicosa was found, and Martin (1949) revealed it to represent an anamorphic ascomycete fungus, possibly a parasite of poly-pores. Thus, the name became unavailable for corticioid dacrymycetes. To encompass the latter, Martin introduced the genus Cerinomyces consisting of C. pallidus as the type, and new combinations C. crustulinus and C. canadensis. The last species of Ceracea, Ce. aurea and Ce. brasiliensis, appeared in a post-mortem publication of Rick (1958).
In the following years, the taxonomic position of Cerinomyces was a matter of discordance. Donk (1956, 1957), Eriksson (1958) and Eriksson & Ryvarden (1973) suggested the genus is affined with corticioid fungi, while others treated it as a member of dacrymycetes (Martin 1957, Kennedy 1958a, Parmasto 1961). Within the most comprehensive revision of dacrymycetes to date, McNabb (1964) accepted the latter opinion, followed by the rest of the community. For example, Donk (1966) had already cited Cerinomyces in the Dacrymycetales. In the same work, McNabb described C. grandinioides and combined C. lagerheimii — a species that differs from the rest of the genus by its absence of clamps.
By the end of the twentieth century, the genus was expanded with a number of new taxa. Cerinomyces ceraceus Ginns joined a morphogroup of clampless Cerinomyces species. Boidin and Gilles introduced C. albosporus, which was later accompanied with macromorphologically similar C. aculeatus N. Maek., C. curvisporus N. Maek. & M. Zang, and C. fasciculatus Gilb. & Hemmes. A few more taxa with less certain relations were described as C. crustulinus var. latisporus B. de Vries (the first strictly gelatinous taxon in the genus), C. pengii Liu & Fan, and C. megalosporus Duhem.
Shirouzu and co-authors pioneered the phylogenetic studies of the class, and in an ongoing series of works demonstrated that most genera in the class are polyphyletic, and Cerinomyces is not an exception (Shirouzu et al. 2007, 2009, 2013, 2016, 2020). They showed that species with corticioid arid basidiocarps, three-septate basidiospores and clampless septa, like C. lagerheimii, C. ceraceus, and C. grandinioides, belong to the core Dacrymycetaceae as a sister clade to some typical Dacrymyces and Guepiniopsis Pat. species. On the other hand, species like Dacrymyces punctiformis Neuhoff with pustulate gelatinous basidiocarps, aseptate basidiospores and clamped hyphae clustered in the Cerinomycetaceae (Shirouzu et al. 2009, 2013). Aside from these shifts, the Cerinomycetaceae appeared to be a well-supported clade in most phylogenetic analyses. In a recent work, Zamora & Ekman (2020) confirmed the earlier results and clarified a mean stem age for the Cerinomycetaceae, estimated at 197 million years ago. They also pointed out that the studied species did not have conspicuous carotenoid contents, and young spores in the genus are binucleate, in contrast to uninucleate ones in the rest of dacrymycetes (see also Yen 1947). Finally, a continuing production of dacrymycete genomes including species of Cerinomyces s.l. in the 1 000 Fungal Genomes Project promises further refinement of the group phylogeny (Grigoriev et al. 2014).
The aims of this paper are: (i) to assess all existing species of Cerinomyces s.l. and describe new ones; (ii) to revise the Cerinomycetaceae as a family consisting of Cerinomyces s.s.; (iii) to find practical nomenclatural solutions for the species excluded from Cerinomyces s.s.; and (iv) to establish connections between phylogenetic groups and morphological characters whenever possible.
Materials and methods
Morphological study
Specimens were obtained from the herbaria ARIZ, BPI, CFMR, CWU, EA, FH, GB, H, HMAS, ILLS, K, KAS, L, LE, LSUM, NCSLG, NCU, NY, O, PC, PDD, PRM, S, TAAM, TNM, TNS, TRTC, TU, UBC, UPS, URM, and personal herbarium of R. Enzlin (see specimen index in Supplementary Table S1). Herbaria acronyms follow Index Herbariorum (http://sweetgum.nybg.org/science/ih/). Collector’s numbers are shown without a collector’s name abbreviation. Studied type specimens are accompanied with exclamation mark (!), and these specimens are not duplicated under “Specimens examined”. Descriptions are based primarily on sequenced specimens marked with an asterisk (∗), and specimens without sequences are incorporated only in the absence of sequenced ones or when their morphology agrees well with the adopted species concept. Detailed information on specimens, high-resolution macro photographs, and scanned notes and labels are available under CC BY 4.0 license via the PlutoF platform (https://plutof.ut.ee, Abarenkov et al. 2010).
Microscopic studies were performed with Leica DMLB, Leica DM1000 LED and Nikon Eclipse 80i microscopes. For slide preparation small parts of basidiocarps were moisturized with tap water, then cut with a razor blade and placed for a short time in a small drop of water; excess of water was removed with filter paper before further dying. The routine mountant used for measurements and drawings was Cotton Blue (CB): 0.1 mg aniline blue (Merck 1275) dissolved in 60 g of pure lactic acid. After applying a cover-glass, the slide was heated without reaching the boiling point, then the preparation squashed by tapping on the cover-glass, and excess of CB removed with filter paper. In cases when CB was not suitable for measurements, 1 % KOH with addition of water solution of Congo Red was used instead. Whenever spore measurements in KOH are reported, it is mentioned. Illustrations were produced from microscopic slides using either a drawing tube at ×1 000 magnification (×2 000 for spore drawings) or rarely from integrated camera photos; later vectorized with Wacom DTK-2700 in CorelDRAW 2017. Measurements were done using ×1 000 magnification, oil immersion, and phase contrast illumination; eyepiece scale bar with 1 μm grid was used, and dimensions were estimated with a subjectively defined accuracy of 0.1 μm; when working with Nikon hardware, spores were photographed and measured in NIS-Elements BR < v. 5.20.00. Spore statistics were produced in LibreOffice Calc ≤ v. 6.0.6.2. When preparing summaries, individual measured spores were omitted only when considered immature or overgrown; not more than five spores from original measurements were excluded per taxon. The following abbreviations are used in descriptions: L for mean spore length, W for mean spore width, Q for L/W ratio, Q’ for variation of length to width ratio of individual basidiospores. To show variation in basidiospore dimensions, 5 % of measurements from each end of the range are excluded and given in parentheses. In case of identical values, parts in parentheses are omitted. For the types and representative specimens at least 30 randomly selected mature basidiospores and well-developed basidia were measured when possible; a total number of measured structures against a number of studied specimens is shown as “n = 30/1”. By default, spore walls thickness measurements were obtained from outer walls, not septal, which are often thicker. Following Ingold (1983), we distinguish parts of two-lobed apiculus (or hilar apparatus) in basidiospores of dacrymycetes as: (A) hilum itself — the point of attachment to basidia; and (B) more prominent hilar appendix (Fig. 3). Herewith, length of apiculus is not added to the spore length. Terms “basidia” and “sterigmata” are used for parts below and above the bifurcation point, respectively. The widths of basidia were measured immediately below the bases of sterigmata. Sterile elements in hymenium are referred here as “hyphidia”, instead of traditional “dikaryophyses” (see Discussion). Raw morphometric data for the studied specimens are provided in the Supplementary Table S2. Taxonomic novelties were deposited in MycoBank (Crous et al. 2004).
Fig. 3.
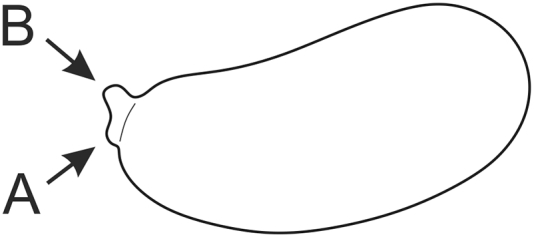
Basidiospore apiculus structure. A = hilum, B = hilar appendix.
DNA extraction, PCR, and sequencing
Protocols for most of UPS and TNS herbaria specimens follow respectively Zamora & Ekman (2020) and Shirouzu et al. (2016); most other materials were processed as indicated below. DNA from the fresh specimens was routinely extracted in 100 μL of “10× reaction buffer B” (0.8 M Tris-HCl pH 8.8–8.9, 0.2 M (NH4)2SO4, 0.2 % w/v Tween-20; Solis Biodyne, Estonia) including 2.5 μL of proteinase K (20 mg/mL; Thermo Fisher Scientific, Waltham, Massachusetts, USA), incubated at 56 °C overnight and deactivated in 98 °C for 15 min.; supernatant then extracted and stored at -80 °C. For older specimens and types the High Pure PCR template preparation kit (Roche Applied Science, Penzberg, Germany) was used following the protocol of manufacturer. Polymerase chain reaction (PCR) was performed with the following primers; forward and reverse ones are separated by slash. The whole ITS region with a part of LSU: ITSOF (ACTTGGTCATTTAGAGGAAGT, Tedersoo et al. 2008) / LR5 (TCCTGAGGGAAACTTCG, Hopple & Vilgalys 1994) or LB-W (CTTTTCATCTTTCCCTCACGG, Tedersoo et al. 2008), or ITS4 (TCCTCCGCTTATTGATATGC, White et al. 1990); ITS1: ITFOF / ITS2 (GCTGCGTTCTTCATCGATGC, White et al. 1990); ITS2: 58A1F (GCATCGATGAAGAACGC, Martin & Rygiewicz 2005) / ITS4; LSU, both by Hopple & Vilgalys (1994): LR0R (ACCCGCTGAACTTAAGC) / LR7 (TACTACCACCAAGATCT); SSU, both modified by L. Tedersoo after White et al. (1990): NS1a (TCTCAAAGAYTAAGCCATGC) / NS8a (CCTCTAAATGACCRAGTTTG); TEF1-α, both by Rehner & Buckley (2005): EF1-983F (GCYCCYGGHCAYCGTGAYTTYAT) / EF1-1567R (ATGACACCRACRGCRACRGTYTG); RPB1: RPB1-Af (GARTGYCCDGGDCAYTTYGG, Stiller & Hall 1997) / RPB1-Cr (CCNGCDATNTCRTTRTCCATRTA, Matheny et al. 2002); RPB2, both by Liu et al. (1999): fRPB2-5F (GAYGAYMGWGATCAYTTYGG) / fRPB2-7cR (CCCATRGCTTGYTTRCCCAT). Routine PCR load was: 5 μL of HOT FIREPol Blend Master Mix (with 10 mM MgCl2; Solis BioDyne), 0.5 μL of each primer and 1–3 μL of DNA extract in 0.1× concentration, filled up to the total 25 μL volume with Milli-Q water. Amount and concentration of DNA extracts were tweaked to troubleshoot PCR problems; in case of Roche kit extractions and in all RPB1 and RPB2 amplifications we used DNA extracts in 1× concentration. SSU, ITS, LSU were amplified with the following PCR cycle: pre-denaturation at 95 °C for 15 min; 35 cycles: denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s, elongation at 72 °C for 1 min; final elongation at 72 °C for 10 min. PCR cycle for TEF1α, RPB1, RPB2, designed by Zheng Wang, via the PolyPeet project: pre-denaturation at 94 °C for 15 min; 9 cycles: denaturation at 94 °C for 40 s, annealing at 60 °C −1 °C per cycle for 40 s, elongation at 72 °C for 2 min; 37 cycles: 94 °C for 45 s, 53 °C for 90 s, 72 °C for 2 min; final elongation at 72 °C for 10 min. Sequencing was performed by Macrogen Europe (Amsterdam, Netherlands) using the primers listed above except ITS5 (GGAAGTAAAAGTCGTAACAAGG, White et al. 1990) for ITSOF products and CTB6 (GCATATCAATAAGCGGAGG, Garbelotto et al. 1997) for LR0R products. The resulting sequences are available in GenBank and the accession numbers are listed in the Table 1. ITS sequences were also parsed by UNITE (https://unite.ut.ee/, Nilsson et al. 2018) and, if passed automated quality check, assigned to Species Hypotheses (SHs, Kõljalg et al. 2020). The list of taxa, numbers of sequences used in SH building and relevant SH codes at different similarity thresholds are presented in the Supplementary Table S3.
Table 1.
Specimens and corresponding sequence accession numbers used in this study. Accession numbers of newly generated sequences are indicated in bold.
| Species | Specimen ID | Country | SSU | ITS | LSU | TEF1-α | RPB1 | RPB2 | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Calocera cornea | CWU(MYC)6922 | Ukraine | MW158985 | MW191969 | MW159089 | MW130299 | MW130381 | MW130423 | this study |
| UPS:F-940774 | Sweden | MN593442 | MN595626 | MN595626 | MN580325 | MN580225 | MN580265 | Zamora & Ekman (2020) | |
| UPS:F-940775 | Sweden | MN593443 | MN595627 | MN595627 | MN580326 | MN580226 | MN580266 | Zamora & Ekman (2020) | |
| Ca. furcata | H:Spirin 10949 | Russia | – | MW191975 | MW159088 | MW130298 | – | – | this study |
| TU135016 | Estonia | MW158984 | MW191958 | MW159087 | MW130297 | MW130380 | MW130422 | this study | |
| Ca. glossoides | CWU(MYC)6247 | Ukraine | MW159005 | MW191968 | MW159084 | MW130307 | MW130388 | MW130428 | this study |
| Ca. viscosa | CWU(MYC)6937 | Ukraine | MW158986 | MW191970 | MW159090 | MW130302 | MW130382 | MW130424 | this study |
| UPS:F-940773 | Sweden | MN593444 | MN595628 | MN595628 | MN580327 | MN580227 | MN580267 | Zamora & Ekman (2020) | |
| Cerinomyces aculeatus holotype | TUMH61942 (TUFC50098) | Japan | MW158956 | MW191955 | MW159053 | MW130323 | – | – | this study |
| MAFF 247114 | Japan | – | – | LC492249 | – | – | – | Shirouzu et al. (2020) | |
| TNS-F-15706 | Japan | AB712482 | AB712440 | AB299050 | – | – | AB712524 | Shirouzu et al. (2007, 2013) | |
| TU135069 | Russia | MW158955 | MW191883 | MW159044 | – | – | – | this study | |
| TU135070 | Russia | MW158954 | MW191884 | MW159045 | MW130322 | MW130375 | MW130410 | this study | |
| C. aff. aculeatus 1 | TNM-F16565 | Taiwan | – | – | AY600248 | – | – | – | Kirschner & Yang (2005) |
| C. aeneus holotype | H7009708 | Ukraine | – | MW191920 | – | – | – | – | this study |
| H:Miettinen 15065.2 | Finland | – | MW191917 | – | – | – | – | this study | |
| O146179 | Norway | – | MW191918 | – | – | – | – | this study | |
| PRM929891 | Czech Republic | – | MW192003 | MW192003 | MW130354 | – | MW130444 | this study | |
| PRM934335 | Czech Republic | – | MW192004 | MW192004 | MW130355 | – | MW130445 | this study | |
| TU135065 | Ukraine | MW158966 | MW191919 | MW159054 | MW130337 | MW130367 | – | this study | |
| UPS:F-560919 | Sweden | MW159012 | MW191987 | MW191987 | – | – | – | this study | |
| UPS:F-946499 | Sweden | MW159013 | MW191988 | MW191988 | – | – | – | this study | |
| C. albosporus holotype | LY11692 | Réunion | – | MW191885 | – | MW130321 | – | – | this study |
| C. atrans holotype | GB-0071218 | Canada | – | MW191928 | – | MW130330 | – | – | this study |
| GB-0071217 | Canada | – | MW191929 | – | MW130331 | – | – | this study | |
| GB-0180499 | Canada | MW159009 | MW191984 | MW191984 | MW130351 | – | – | this study | |
| TUFC30545 | ? | AB712485 | AB712443 | AB712423 | – | – | AB712527 | Shirouzu et al. (2013) | |
| C. borealis holotype | O160848 | Norway | – | MW191890 | MW159042 | – | – | – | this study |
| H:Miettinen 14094 | Finland | MW158982 | MW191891 | MW159037 | MW130343 | MW130369 | MW130414 | this study | |
| H:Miettinen 21156.1 | Finland | MW158963 | MW191889 | MW159038 | MW130344 | MW130371 | MW130411 | this study | |
| H:Spirin 10443 | Russia | MW158965 | MW191888 | MW159036 | MW130345 | MW130372 | MW130413 | this study | |
| H6055125 | Finland | MW158964 | MW191892 | MW159039 | MW130346 | MW130370 | MW130412 | this study | |
| O101812 | Norway | – | MW191893 | – | – | – | – | this study | |
| C. brevisetus holotype | URM:Chikowski 1544 | Brazil | MW158957 | MW191886 | MW159046 | MW130320 | – | – | this study |
| "C." canadensis | H:Spirin 8468 | USA | MW158983 | MW191945 | MW159069 | MW130349 | MW130376 | MW130419 | this study |
| TAAM007082 | Russia | – | MW191947 | – | – | – | – | this study | |
| TAAM061880 | Russia | – | MW191946 | – | – | – | – | this study | |
| C. cokeri | TU135089 | Canada | MW159008 | MW191983 | MW191983 | MW130350 | MW130393 | – | this study |
| C. concretus holotype | O:F-919450 | Colombia | – | MW191933 | – | – | – | – | this study |
| C. creber holotype | UPS:F-946512 | Spain | MW159010 | MW191985 | MW191985 | MW130352 | MW130394 | – | this study |
| H:Trichies 07077 | France | – | MW191927 | – | – | – | – | this study | |
| UPS:F-946506 | Spain | MW159018 | MW191993 | MW191993 | – | MW130399 | MW130435 | this study | |
| UPS:F-946507 | Spain | MW159019 | MW191994 | MW191994 | – | MW130400 | MW130436 | this study | |
| UPS:F-979574 | Spain | – | MZ147629 | MZ147629 | – | – | – | this study | |
| C. aff. crustulinus 1 | UPS:F-958851 | Spain | MW159011 | MW191986 | MW191986 | MW130353 | MW130395 | MW130431 | this study |
| C. enatus | CFMR:HHB-671 | USA | – | MW191977 | – | – | – | – | this study |
| CFMR:HHB-7334 | USA | – | MW191978 | – | – | – | – | this study | |
| H:Spirin 10764 | Russia | MW158980 | MW191937 | MW159033 | MW130338 | – | – | this study | |
| H:Spirin 7774 | Russia | – | MW191939 | MW159034 | MW130339 | – | – | this study | |
| H:Spirin 7780 | Russia | MW158981 | MW191938 | MW159035 | – | – | – | this study | |
| OTU_263 | Japan | – | – | LC492284 | – | – | – | Shirouzu et al. (2020) | |
| TNS-F-21034 | Japan | AB712483 | AB712441 | AB472696 | – | – | AB712525 | Shirouzu et al. (2009, 2013) | |
| TNS-F-21035 | Japan | – | – | AB472697 | – | – | – | Shirouzu et al. (2009) | |
| TNS-F-21036 | Japan | – | – | AB472698 | – | – | – | Shirouzu et al. (2009) | |
| TNS-F-21037 | Japan | LC585259 | LC585257 | AB472699 | – | – | – | this study, Shirouzu et al. (2009) | |
| TNS-F-21064 | Japan | LC585260 | – | AB472724 | – | – | – | this study, Shirouzu et al. (2009) | |
| TNS-F-61320 | Japan | – | LC585250 | LC003923 | – | – | – | this study, Shirouzu et al. (2016) | |
| TNS-F-88754 | Japan | – | – | LC492177 | – | – | – | Shirouzu et al. (2020) | |
| TNS-F-88777 | Japan | – | – | LC492200 | – | – | – | Shirouzu et al. (2020) | |
| C. cf. enatus | OTU_466 | Japan | – | – | LC492292 | – | – | – | Shirouzu et al. (2020) |
| C. enterolaxus holotype | TNS-F-61292 | Japan | – | LC585244 | LC003895 | – | – | – | this study, Shirouzu et al. (2016) |
| MAFF 247132 | Japan | – | – | LC492267 | – | – | – | Shirouzu et al. (2020) | |
| OTU_356 | Japan | – | – | LC492290 | – | – | – | Shirouzu et al. (2020) | |
| TNS-F-15723 | Japan | AB712504 | AB712462 | AB299052 | – | – | AB712546 | Shirouzu et al. (2007, 2013) | |
| TNS-F-15724 | Japan | – | LC585256 | AB299056 | – | – | – | this study, Shirouzu et al. (2007) | |
| TNS-F-15725 | Japan | LC585261 | LC585258 | AB299071 | – | – | – | this study, Shirouzu et al. (2007) | |
| TNS-F-61296 | Japan | – | LC585245 | LC003899 | – | – | – | this study, Shirouzu et al. (2016) | |
| TNS-F-61306 | Japan | – | LC585246 | LC003909 | – | – | – | this study, Shirouzu et al. (2016) | |
| TNS-F-61316 | Japan | – | LC585247 | LC003919 | – | – | – | this study, Shirouzu et al. (2016) | |
| TNS-F-61317 | Japan | – | LC585248 | LC003920 | – | – | – | this study, Shirouzu et al. (2016) | |
| TNS-F-61319 | Japan | – | LC585249 | LC003922 | – | – | – | this study, Shirouzu et al. (2016) | |
| TNS-F-61324 | Japan | – | LC585251 | LC003927 | – | – | – | this study, Shirouzu et al. (2016) | |
| TNS-F-61327 | Japan | – | LC585252 | LC003930 | – | – | – | this study, Shirouzu et al. (2016) | |
| TNS-F-61334 | Japan | – | LC585253 | LC003937 | – | – | – | this study, Shirouzu et al. (2016) | |
| TNS-F-61335 | Japan | – | LC585254 | LC003938 | – | – | – | this study, Shirouzu et al. (2016) | |
| TNS-F-88723 | Japan | – | – | LC492146 | – | – | – | Shirouzu et al. (2020) | |
| TNS-F-88726 | Japan | – | – | LC492149 | – | – | – | Shirouzu et al. (2020) | |
| TNS-F-88728 | Japan | – | – | LC492151 | – | – | – | Shirouzu et al. (2020) | |
| TNS-F-88734 | Japan | – | – | LC492157 | – | – | – | Shirouzu et al. (2020) | |
| TNS-F-88742 | Japan | – | – | LC492165 | – | – | – | Shirouzu et al. (2020) | |
| TNS-F-88745 | Japan | – | – | LC492168 | – | – | – | Shirouzu et al. (2020) | |
| TNS-F-88753 | Japan | – | – | LC492176 | – | – | – | Shirouzu et al. (2020) | |
| TNS-F-88762 | Japan | – | – | LC492185 | – | – | – | Shirouzu et al. (2020) | |
| TNS-F-88763 | Japan | – | – | LC492186 | – | – | – | Shirouzu et al. (2020) | |
| TNS-F-88767 | Japan | – | – | LC492190 | – | – | – | Shirouzu et al. (2020) | |
| TNS-F-88768 | Japan | – | – | LC492191 | – | – | – | Shirouzu et al. (2020) | |
| TNS-F-88781 | Japan | – | – | LC492204 | – | – | – | Shirouzu et al. (2020) | |
| C. favonius holotype | H7008893 | USA | MW158962 | MW191895 | MW159041 | MW130347 | MW130374 | MW130416 | this study |
| H7008894 | USA | MW158961 | MW191894 | MW159040 | – | MW130373 | MW130415 | this study | |
| C. fugax holotype | CFMR:HHB-8856 | USA | – | MW191905 | MW159051 | – | – | – | this study |
| C. hesperidis holoype | NY01782362 | USA | – | MW191921 | MW159065 | – | – | – | this study |
| C. inermis holotype | PDD87816 | New Zealand | – | MW191887 | – | MW130324 | – | – | this study |
| C. lipoferus holotype | UPS:F-940777 | Sweden | MN593436 | MN595620 | MN595620 | MN580319 | MN580219 | MN580259 | Zamora & Ekman (2020) |
| ENZ20001 | The Netherlands | – | MZ147626 | MZ147626 | MZ152908 | – | – | this study | |
| GB-0161225 | Sweden | – | MW192002 | MW192002 | – | – | – | this study | |
| UPS:F-940778 | Sweden | – | MW192001 | MW192001 | – | – | – | this study | |
| C. nepalensis holotype | O:F-904088 | Nepal | – | MW191896 | – | – | – | – | this study |
| C. neuhoffii holotype | UPS:F-941020 | Sweden | MN593441 | MN595625 | MN595625 | MN580324 | MN580224 | MN580264 | Zamora & Ekman (2020) |
| CWU(MYC)6281 | Ukraine | MW158979 | MW191925 | MW159031 | MW130335 | MW130366 | – | this study | |
| CWU(MYC)6342 | Ukraine | MW158977 | MW191923 | MW159030 | MW130334 | – | – | this study | |
| H:Miettinen 15893 | Finland | – | MW191924 | MW159028 | – | – | – | this study | |
| H:Miettinen 20778 | Finland | MW158978 | MW191926 | MW159032 | MW130336 | – | – | this study | |
| TU135067 | Ukraine | MW158976 | MW191922 | MW159029 | MW130333 | MW130365 | – | this study | |
| UPS:F-941019 | Spain | MN593440 | MN595624 | MN595624 | MN580323 | MN580223 | MN580263 | Zamora & Ekman (2020) | |
| UPS:F-946501 | Sweden | MW159026 | MW192000 | MW192000 | MW130364 | MW130407 | MW130443 | this study | |
| UPS:F-946503 | Sweden | MW159015 | MW191990 | MW191990 | MW130356 | MW130397 | MW130433 | this study | |
| UPS:F-946505 | Cyprus | MW159017 | MW191992 | MW191992 | MW130358 | MW130398 | MW130434 | this study | |
| UPS:F-946510 | Sweden | MW159022 | MW191997 | MW191997 | – | MW130403 | MW130439 | this study | |
| C. pallidus | ARIZ-M-AN09245 | USA | – | MZ147624 | – | – | – | – | this study |
| CFMR:WBC-39924 | USA | – | MW191932 | – | – | – | – | this study | |
| GB-0071214 | USA | – | MW191936 | – | – | – | – | this study | |
| NY:”C. pallidus №1” | USA | – | MW191931 | – | MW130332 | – | – | this study | |
| C. paulistanus holotype | O:Ryvarden 24759 | Brazil | – | MW191935 | – | – | – | – | this study |
| TAAM192120 | Brazil | – | MW191934 | – | – | – | – | this study | |
| C. pinguis holotype | O:F-904085 | Nepal | – | MW191907 | MW159043 | MW130348 | – | – | this study |
| C. ramosissimus holotype | CFMR:FP-150848 | Belize | AB712488 | AB712446 | AB712426 | – | – | AB712530 | Shirouzu et al. (2013) |
| C. tortus neotype | UPS:F-946515 | Sweden | MW159025 | MW191999 | MW191999 | MW130363 | MW130406 | MW130442 | this study |
| H:Miettinen 12740.1 | Finland | – | MW191909 | – | – | – | – | this study | |
| H:Miettinen 14095 | Finland | – | MW191914 | – | – | – | – | this study | |
| H:Miettinen 20996 | Finland | MW158969 | MW191910 | MW159060 | – | – | – | this study | |
| H:Miettinen 21034 | Finland | MW158974 | – | MW159057 | – | – | – | this study | |
| H:Miettinen 21058 | Finland | MW158971 | MW191908 | MW159062 | – | – | – | this study | |
| H:Miettinen 21288 | Finland | MW158970 | – | MW159058 | MW130342 | – | – | this study | |
| H:Miettinen 21292 | Finland | MW158973 | – | MW159059 | – | – | – | this study | |
| H:Savchenko 181108-1431 | Finland | MW158968 | – | MW159056 | MW130341 | – | – | this study | |
| O160046 | Norway | – | MW191916 | MW159061 | – | – | – | this study | |
| TU135048 | Estonia | MW158975 | MW191911 | MW159063 | – | – | – | this study | |
| TU135049 | Estonia | MW158972 | MW191915 | MW159064 | – | – | – | this study | |
| TU135066 | Ukraine | MW158967 | MW191913 | MW159055 | MW130340 | MW130368 | – | this study | |
| UPS:F-015301 | Sweden | – | MW191912 | – | – | – | – | this study | |
| UPS:F-941016 | Sweden | MN593438 | MN595622 | MN595622 | MN580321 | MN580221 | MN580261 | Zamora & Ekman (2020) | |
| UPS:F-941017 | Sweden | MN593439 | MN595623 | MN595623 | MN580322 | MN580222 | MN580262 | Zamora & Ekman (2020) | |
| UPS:F-941018 | Sweden | MW159014 | MW191989 | MW191989 | – | MW130396 | MW130432 | this study | |
| UPS:F-946508 | Sweden | MW159020 | MW191995 | MW191995 | MW130359 | MW130401 | MW130437 | this study | |
| UPS:F-946511 | Sweden | MW159023 | MW191979 | – | MW130361 | MW130404 | MW130440 | this study | |
| UPS:F-946514 | Sweden | MW159024 | MW191998 | MW191998 | MW130362 | MW130405 | MW130441 | this study | |
| C. aff. tortus 1 | UPS:F-946504 | Sweden | MW159016 | MW191991 | MW191991 | MW130357 | – | – | this study |
| UPS:F-946509 | Finland | MW159021 | MW191996 | MW191996 | MW130360 | MW130402 | MW130438 | this study | |
| C. aff. tortus 2 | UPS:F-940948 | Norway | MN593437 | MN595621 | MN595621 | MN580320 | MN580220 | MN580260 | Zamora & Ekman (2020) |
| C. aff. tortus 3 | TNS-F-88757 | Japan | – | – | LC492180 | – | – | – | Shirouzu et al. (2020) |
| TNS-F-88780 | Japan | – | – | LC492203 | – | – | – | Shirouzu et al. (2020) | |
| C. tristis holotype | H7009711 | USA | MW158958 | MW191906 | MW159050 | MW130325 | – | MW130409 | this study |
| CFMR:FP-133094 | USA | – | MW191897 | – | – | – | – | this study | |
| GB-0071225 | Canada | – | MW191898 | – | – | – | – | this study | |
| H:OM19013 | USA | – | MZ147625 | – | – | – | – | this study | |
| C. verecundus holotype | PDD93708 | New Zealand | – | MW191930 | MW159052 | MW130329 | – | – | this study |
| C. volaticus holotype | S:F250344 | Sweden | MW159027 | MW191982 | – | – | – | – | this study |
| GB-0071193 | Sweden | – | MW191903 | – | – | – | – | this study | |
| GB-0071206 | Sweden | – | MW191900 | – | – | – | – | this study | |
| LE242249 | Russia | MW158960 | MW191902 | MW159048 | MW130327 | – | – | this study | |
| LE295748 | Russia | MW158959 | MW191901 | MW159047 | MW130326 | – | MW130408 | this study | |
| O:F-247959 | Norway | MN593435 | MN595619 | MN595619 | MN580318 | MN580218 | MN580258 | Zamora & Ekman (2020) | |
| O189348 | Norway | – | MW191899 | MW159049 | – | – | – | this study | |
| PC0706779 | France | – | MW191904 | – | MW130328 | – | – | this study | |
| Cerinomyces sp. | 05151-1B2 | Japan | – | LC585255 | LC003874 | – | – | – | Shirouzu et al. (2016) |
| 1611_131A1 | Japan | – | – | LC492227 | – | – | – | Shirouzu et al. (2020) | |
| NBRC110591 | Japan | LC004021 | LC004000 | LC003883 | – | – | – | Shirouzu et al. (2016) | |
| OTU_258 | Japan | – | – | LC492283 | – | – | – | Shirouzu et al. (2020) | |
| Dacrymyces burdsallii holotype | CFMR:HHB-6908 | USA | AB712486 | AB712444 | AB712424 | – | – | AB712528 | Shirouzu et al. (2013) |
| D. cf. capitatus | TU135101 | Estonia | MW158995 | MW191962 | MW159081 | – | – | – | this study |
| D. ceraceus holotype | CFMR:HHB-8969 | USA | AB712484 | AB712442 | AB712422 | – | – | AB712526 | Shirouzu et al. (2013) |
| D. aff. ceraceus | CFMR:HHB-6817 | USA | – | MW191951 | – | MW130310 | – | – | this study |
| D. cereus | URM:Chikowski 1167 | Brazil | – | MW191954 | – | – | – | – | this study |
| D. chrysocomus | UPS:F-940134 | Sweden | MN593446 | MN595630 | MN595630 | MN580329 | MN580229 | MN580269 | Zamora & Ekman (2020) |
| UPS:F-940136 | Spain | MN593445 | MN595629 | MN595629 | MN580328 | MN580228 | MN580268 | Zamora & Ekman (2020) | |
| D. chrysospermus | H:Miettinen 14818 | USA | MW159000 | MW191961 | MW159077 | MW130305 | – | – | this study |
| H:Spirin 10795 | Russia | MW159001 | MW191974 | MW159078 | MW130306 | MW130387 | – | this study | |
| D. aff. chrysospermus | UPS:F-593536 | Japan | MN593447 | MN595631 | MN595631 | MN580330 | MN580230 | MN580270 | Zamora & Ekman (2020) |
| D. corticioides | NY:"C. canadensis №1" | USA | – | MW191940 | MW159067 | MW130315 | – | – | this study |
| NY02686162 | USA | MW159006 | MW191944 | MW159068 | MW130314 | – | – | this study | |
| TAAM102301 | – | MW191941 | – | – | – | – | this study | ||
| TAAM126607 | – | MW191943 | MW159066 | – | – | – | this study | ||
| TAAM150056 | – | MW191942 | – | – | – | – | this study | ||
| D. estonicus | UPS:F-940137 | Sweden | MN593448 | MN595632 | MN595632 | MN580331 | MN580231 | MN580271 | Zamora & Ekman (2020) |
| UPS:F-940138 | Sweden | MN593449 | MN595633 | MN595633 | MN580332 | MN580232 | MN580272 | Zamora & Ekman (2020) | |
| D. fennicus | H:Miettinen 20574 | Finland | MW158990 | – | MW159072 | MW130303 | MW130379 | MW130430 | this study |
| H:Miettinen 21174 | Finland | MW158989 | MW191957 | MW159071 | – | MW130378 | MW130421 | this study | |
| UPS:F-946596 | Sweden | MZ130256 | MZ147627 | MZ147627 | – | MZ152906 | – | this study | |
| UPS:F-946597 | Sweden | MZ130257 | MZ147628 | MZ147628 | MZ152909 | MZ152907 | – | this study | |
| D. grandii holotype | NCSLG21158 | USA | – | MW191953 | – | MW130309 | – | – | this study |
| D. grandinioides holotype | K(M):237139 | Kenya | – | MW191980 | – | – | – | – | this study |
| H7008841 | Kenya | MW158994 | MW191950 | MW159076 | MW130312 | MW130390 | MW130418 | this study | |
| LY11615 | Réunion | – | MW191981 | – | – | – | – | this study | |
| D. cf. minor | H:Miettinen 19137 | Finland | MW158998 | MW191967 | MW159080 | MW130301 | MW130385 | MW130427 | this study |
| H:Miettinen 20591 | Finland | MW158997 | MW191965 | MW159079 | MW130300 | MW130384 | MW130426 | this study | |
| D. minutus | UPS:F-940776 | Finland | MN593450 | MN595634 | MN595634 | MN580333 | MN580233 | MN580273 | Zamora & Ekman (2020) |
| D. ovisporus | H:Miettinen 20787 | Finland | MW159004 | MW191964 | MW159074 | MW130318 | MW130392 | – | this study |
| H:Spirin 11145 | Norway | MW159003 | MW191960 | MW159073 | MW130317 | MW130391 | – | this study | |
| UPS:F-940139 | Sweden | MN593451 | MN595635 | MN595635 | MN580334 | MN580234 | MN580274 | Zamora & Ekman (2020) | |
| UPS:F-940140 | Sweden | MN593452 | MN595636 | MN595636 | MN580335 | MN580235 | MN580275 | Zamora & Ekman (2020) | |
| D. pinacearum | UPS:F-593533 | Japan | MN593453 | MN595637 | MN595637 | MN580336 | MN580236 | MN580276 | Zamora & Ekman (2020) |
| UPS:F-593535 | Japan | MN593454 | MN595638 | MN595638 | MN580337 | MN580237 | MN580277 | Zamora & Ekman (2020) | |
| D. sobrius holotype | CFMR:RLG-13487 | USA | AB712487 | AB712445 | AB712425 | – | – | AB712529 | Shirouzu et al. (2013) |
| CFMR:FP-102085 | USA | – | MW191952 | – | – | – | – | this study | |
| D. stillatus (anamorph) | UPS:F-939814 | Sweden | MN593455 | MN595676 | MN595676 | MN580338 | MN580238 | MN580278 | Zamora & Ekman (2020) |
| UPS:F-939816 | Sweden | MN593457 | – | MN593494 | MN580340 | MN580240 | MN580280 | this study | |
| D. stillatus (teleomorph) | UPS:F-939814 | Sweden | MN593456 | MN595677 | MN595677 | MN580339 | MN580239 | MN580279 | Zamora & Ekman (2020) |
| UPS:F-939816 | Sweden | MN593458 | – | MN593495 | MN580341 | MN580241 | MN580281 | this study | |
| D. cf. stillatus | H:Miettinen 20608 | Finland | MW158996 | MW191963 | MW159082 | MW130304 | MW130383 | MW130425 | this study |
| D. venustus holotype | O:Adane 150 | Ethiopia | MW158993 | MW191949 | MW159075 | MW130311 | – | MW130417 | this study |
| D. aff. venustus 1 | LY7839 | Gabon | – | MW191948 | – | – | – | – | this study |
| Dacryonaema macnabbii | UPS:F-940949 | Sweden | MN593472 | MN595650 | MN595650 | MN580353 | – | MN580292 | Zamora & Ekman (2020) |
| UPS:F-940992 | Sweden | MN593475 | MN595653 | MN595653 | MN580356 | MN580211 | MN580295 | Zamora & Ekman (2020) | |
| D. macrosporum | UPS:F-940998 | Finland | MN593480 | MN595660 | MN595660 | MN580360 | MN580215 | MN580302 | Zamora & Ekman (2020) |
| UPS:F-941001 | Finland | MN593481 | MN595661 | MN595661 | MN580361 | MN580216 | MN580303 | Zamora & Ekman (2020) | |
| D. rufum | UPS:F-941005 | Sweden | MN593469 | MN595646 | MN595646 | MN580349 | MN580209 | MN580288 | Zamora & Ekman (2020) |
| UPS:F-941012 | Finland | MN593470 | MN595649 | MN595649 | MN580351 | – | MN580290 | Zamora & Ekman (2020) | |
| Dacryopinax elegans | TENN 066927 | USA | MN593460 | MN595640 | MN595640 | MN580342 | MN580242 | MN580282 | Zamora & Ekman (2020) |
| Dacryopinax sp. | H7008759 | Kenya | MW158992 | MW191959 | MW159091 | – | – | – | this study |
| D. spathularia | H:Miettinen 16740.1 | USA | MW158999 | MW191973 | MW159085 | MW130308 | MW130389 | MW130429 | this study |
| H:Miettinen 20559 | Indonesia | MW159007 | MW191976 | MW159092 | – | – | – | this study | |
| Ditiola radicata | H:Miettinen 20590.2 | Finland | MW158987 | MW191966 | MW159083 | MW130313 | – | – | this study |
| UPS:F-939957 | Sweden | MN593461 | MN595641 | MN595641 | MN580343 | MN580243 | MN580283 | Zamora & Ekman (2020) | |
| UPS:F-939961 | Finland | MN593462 | – | – | MN580344 | MN580244 | MN580284 | this study | |
| Femsjonia peziziformis | H:Haikonen 24269 | Finland | MW158991 | MW191972 | MW159070 | MW130316 | MW130377 | MW130420 | this study |
| H:Haikonen 30097 | Finland | MN593463 | MN595642 | MN595642 | MN580345 | MN580245 | MN580285 | Zamora & Ekman (2020) | |
| Guepiniopsis buccina | CWU(MYC)7014 | Ukraine | MW159002 | MW191971 | MW159086 | MW130319 | MW130386 | – | this study |
| UPS:F-940947 | Spain | MN593464 | MN595643 | MN595643 | MN580346 | MN580246 | MN580286 | Zamora & Ekman (2020) | |
| Heterotextus alpinus | H:Spirin 8744 | USA | MW158988 | – | – | – | – | – | this study |
| H. miltinus | TENN 42208 | New Zealand | MN593465 | MN595644 | MN595644 | MN580347 | MN580247 | – | Zamora & Ekman (2020) |
| Unilacryma bispora | UPS:F-941254 | Sweden | MN593488 | MN595670 | MN595670 | MN580367 | MN580253 | MN580312 | Zamora & Ekman (2020) |
| UPS:F-941268 | Sweden | MN593490 | MN595672 | MN595672 | MN580369 | MN580255 | MN580314 | Zamora & Ekman (2020) | |
| U. unispora | UPS:F-941277 | Sweden | MN593483 | MN595665 | MN593500 | MN580362 | MN580248 | MN580307 | Zamora & Ekman (2020) |
| UPS:F-941278 | Sweden | MN593484 | MN595666 | MN595666 | MN580363 | MN580249 | MN580308 | Zamora & Ekman (2020) |
Phylogenetic analysis
General sequence management and contig assembly were done in Geneious v. 7.0.6 and 9.1.8 (https://www.geneious.com). Alignments were performed in MAFFT v. 7 online with E-INS-i method (https://mafft.cbrc.jp/alignment/server/, Katoh et al. 2019). Borders of ITS1, 5.8S, ITS2 and LSU were identified with ITSx (Bengtsson-Palme et al. 2013) as implemented at PlutoF (https://plutof.ut.ee/#/analysis) or using ITS2 database (http://its2.bioapps.biozentrum.uni-wuerzburg.de/, Ankenbrand et al. 2015). Parts of the alignments were excluded by hand (poorly aligned and heterogenous regions of nrDNA, most of introns in protein-coding genes). Final alignments contain positions with > 60 % of gaps; more stringent trimming (50 % gaps allowed) resulted in trees with identical topologies and similar supports (not presented here), and therefore more relaxed alignments were retained. Trimming was performed in BMGE (Criscuolo & Gribaldo 2010) as implemented at NGPhylogeny.fr (Lemoine et al. 2019); test trees were built in RAxML similar to described below. Manual adjustments to alignments were done with AliView v. 1.26 (Larsson 2014). Eight concatenated datasets were built with different genes (Table 2) using package evobiR (Adams 2015) in R environment (R Core Team 2019). Regions of nrDNA were treated as five separate partitions (SSU, ITS1, 5.8S, ITS2, LSU), and protein coding genes were divided into 1–2 vs 3 codon positions, yielding six partitions for TEF1-α, RPB1 and RPB2. To check incongruence between partitions in Dacrymycetes set, we compared maximum likelihood trees (built as explained below) based on separate genes (SSU, 5.8S, LSU, and not partitioned into codons TEF1-α, RPB1, RPB2). We considered lack of conflict among single-region trees when no samples were included in different supported clades across trees (≥ 70 % bootstrap support). The only discordance occurred in RPB2 tree were Dacrymyces grandinioides and D. venustus fell outside of the core Dacrymycetaceae clade, separated from D. burdsallii, D. ceraceus, and D. sobrius.
Table 2.
List of concatenated sequence datasets and gene partitions included in them. Numbers of characters (total and parsimony-informative) are separated with slash (/).
| Dataset name | Rows | Length | SSU | ITS1 | 5.8S | ITS2 | LSU | TEF1-α, 1–2 pos. | TEF1-α, 3 pos. | RPB1, 1–2 pos. | RPB1, 3 pos. | RPB2, 1–2 pos. | RPB2, 3 pos. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cerinomyces albosporus clade, Fig. 5 C | 10 | 1 407/180 | — | 87/33 | 149/5 | 132/41 | 526/23 | 342/39 | 171/39 | — | — | — | — |
| C. borealis clade, Fig. 7 A | 11 | 3 621/254 | 1 454/44 | 96/31 | 149/4 | 136/36 | 1 227/94 | 373/23 | 186/22 | — | — | — | — |
| C. enatus clade, Fig. 6 | 55 | 2 879/436 | 1 636/144 | 101/50 | 151/14 | 157/87 | 834/141 | — | — | — | — | — | — |
| C. pallidus clade (C. atrans subclade), Fig. 5 A | 12 | 382/109 | — | 90/36 | 149/9 | 143/64 | — | — | — | — | — | — | — |
| C. pallidus clade (C. volaticus subclade), Fig. 5 B | 13 | 1 598/143 | — | 76/34 | 149/8 | 147/53 | 1 226/48 | — | — | — | — | — | — |
| C. tortus clade, Fig. 7 B | 37 | 2 731/304 | 1 582/96 | 88/43 | 151/33 | 119/64 | 791/68 | — | — | — | — | — | — |
| Dacrymycetes, Fig. 4 | 110 | 6 471/2 679 | 1 511/340 | — | 136/38 | — | 1 152/343 | 644/178 | 322/288 | 472/221 | 237/233 | 1 332/398 | 665/640 |
| Dacrymyces grandinioides clade, Fig. 8 | 10 | 2 500/171 | 1 585/24 | 107/32 | 142/12 | 169/71 | 497/32 | — | — | — | — | — | — |
Bayesian inference was performed with MrBayes v. 3.2.7a (Ronquist et al. 2012) as implemented at CIPRES portal (Miller et al. 2010). Default priors were used, nucleotide substitution models were estimated with model jumping method (nst = mixed) with gamma-distributed rate variation across sites and proportion of invariable sites not estimated. Analyses were carried out in four parallel runs with four MCMC chains each, for 10 M generations, sampling trees every 5 000 generations, with temperature constant 0.1. A burn-in was set to a fraction 25 %. The analyses were automatically stopped if the average standard deviation of split frequencies dropped below 0.01. Effective sample sizes (ESS) were assumed sufficient with values reaching above 200, and potential scale reduction factor (PSRF) approximating to 1. Tracer v. 1.7.1 (Rambaut et al. 2018) and RWTY (Warren et al. 2017) were used to observe convergence of model parameters and tree topologies. The consensus tree was built using 50 % majority rule.
Maximum likelihood (ML) analyses were performed with RAxML v. 8.2.12 (Stamatakis 2014), implemented at CIPRES portal as “RAxML-HPC2 Workflow”, using the same partitioning as above, GTRGAMMA model for all datasets, with 10 randomized maximum-likelihood initial trees and 1 000 iterations of standard non-parametric bootstrap. The trees were plotted in FigTree v. 1.4.3 (http://tree.bio.ed.ac.uk/software/figtree/). The alignments and phylograms have been deposited in TreeBASE, study number S28188 (https://www.treebase.org/). Data related to the page are also available at https://plutof.ut.ee/#/doi/10.15156/BIO/1420800.
Results
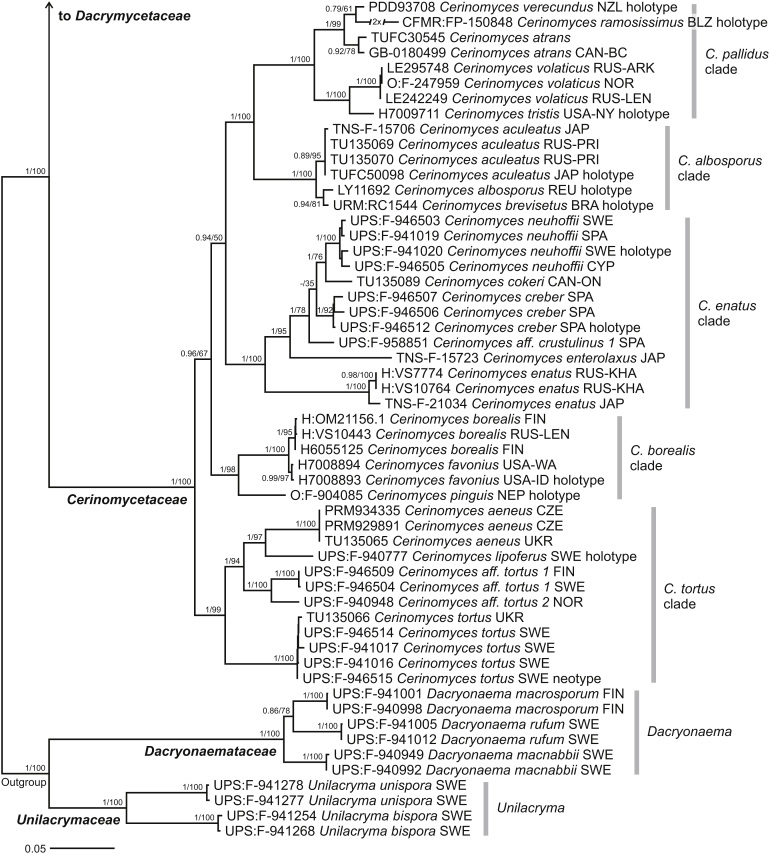
A dataset of SSU, 5.8S, LSU, TEF1-α, RPB1, and RPB2 genes was used to infer the class phylogeny and resolve the positions of Cerinomyces s.l. clades. Our analysis confirmed the earlier reported family arrangement of the Dacrymycetes, with the robustly supported Cerinomycetaceae as a sister clade to the Dacrymycetaceae (Fig. 4). From a morphological perspective, the combination of characters that unites the Cerinomycetaceae when compared to other families is: (i) presence of simple clamps on all hyphal septa; (ii) curved-cylindrical thin-walled basidiospores that only rarely and tardily develop up to three transverse septa; (iii) low amount of carotenoid pigments in hyphae and basidiospores; and (iv) corticioid, resupinate, pustulate, pulvinate and only slightly cerebriform basidiocarps. In addition, young basidiospores of the family members appear to be binucleate, while in the rest of the class uninucleate state is usual. The character was observed in fresh material of ten Cerinomyces taxa and reported in descriptions.
Fig. 4.
Phylogeny of the Dacrymycetes. Maximum-likelihood consensus tree based on SSU, 5.8S, LSU. RPB1, RPB2, and TEF1-α sequences. Numbers before and after slash (/) indicate posterior probabilities of Bayesian analysis and ML bootstrap support values. Codes after the species names denote country and admin. division of origin (ISO 3166).
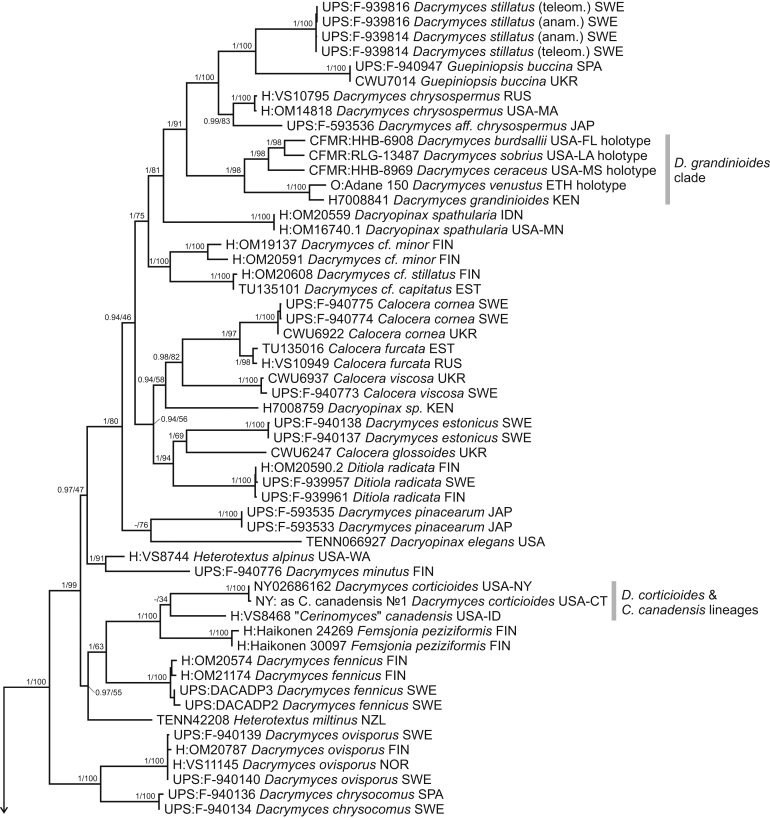
In contrast to the traditional view, corticioid morphotype alone does not define the family: while most corticioid dacrymycetes do belong to the Cerinomycetaceae, a number of Cerinomyces s.l. are found in the Dacrymycetaceae. In agreement with earlier studies, one of the excluded groups — a clade containing D. grandinioides — is resolved as a sister to Dacrymyces stillatus, D. chrysospermus and Guepiniopsis buccina. Here we formally transfer all taxa related to D. grandinioides to Dacrymyces. Two other corticioid species, “Cerinomyces” canadensis and D. corticioides, are recovered in proximity to Femsjonia peziziformis. We refrain from nomenclatural rearrangements of these until a dedicated study of Femsjonia is undertaken.
Based on the same dataset, we empirically designated five clades within the Cerinomycetaceae to highlight connections between phylogenetic and morphological groups. We treat all clades as part of Cerinomyces: in our opinion, division of the family into several genera is impractical. Even though morphology is generally uniform within the clades, it is still not possible to identify characters that would unambiguously define every putative genus. In addition, establishing generic boundaries is not justified given the low support values in some of the deeper nodes in the family phylogeny.
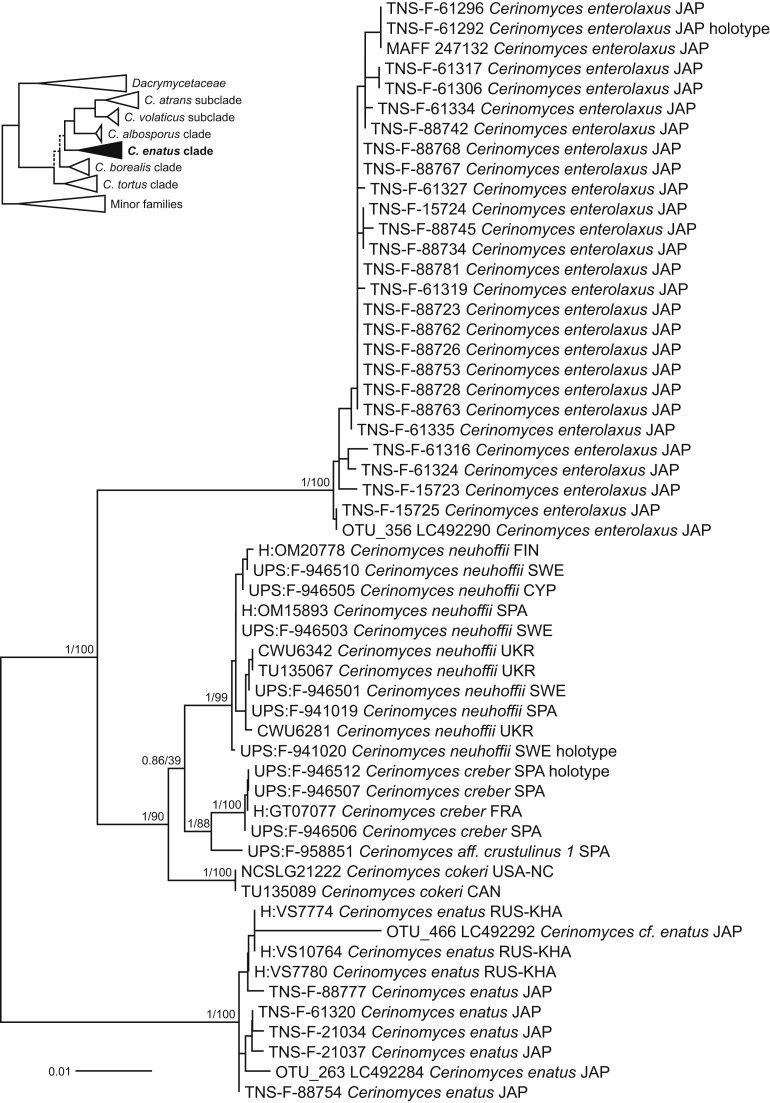
Two main types of basidiocarps, arid corticioid and gelatinous pustulate, are found in the Cerinomycetaceae. As shown in the Fig. 4, corticioid species are scattered across three groups (labelled here as C. pallidus, C. albosporus and C. borealis clades), while the gelatinous ones form two (C. enatus and C. tortus clades). To increase the resolution within these clades, we utilized ITS and TEF1-α sequences. ITS was too variable for family-wide alignments, so we divided the data into clade- or even subclade sets, presented in separate trees (Fig. 5, Fig. 6, Fig. 7). SSU and LSU were also incorporated in the concatenated sets, but predictably showed little parsimony-informative signals at the species level. For the species delimitation, we primarily used ITS as a barcoding marker available for most of the taxa. In total, the genus Cerinomyces includes 29 species, of which 20 are newly described here. In addition, we propose three new combinations, designate four informal taxa and exclude seven species from the genus. The clades are detailed below.
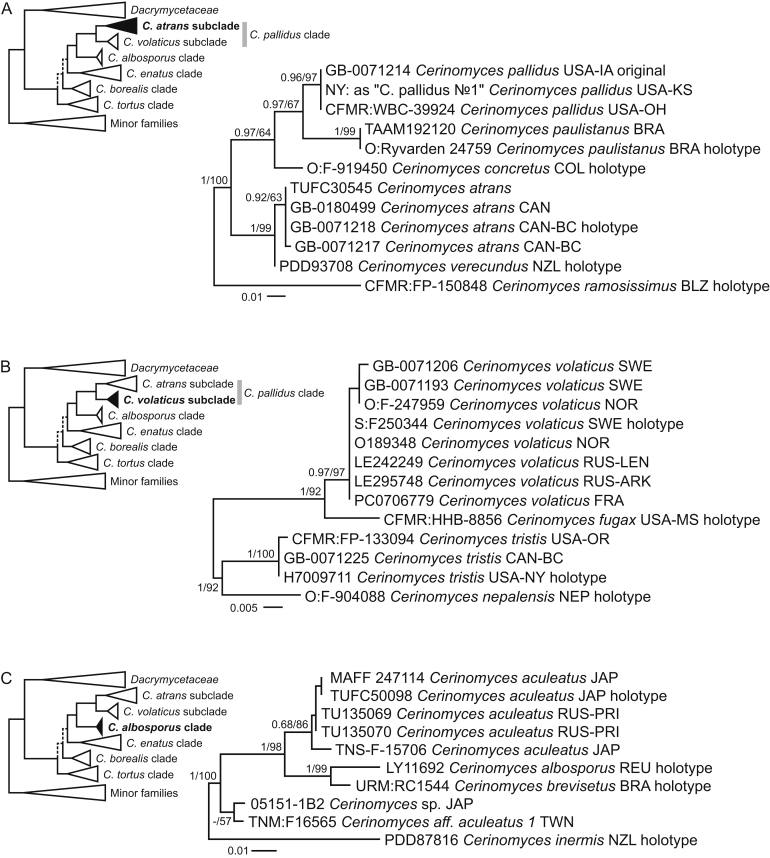
Fig. 5.
Phylogenies of Cerinomyces clades. A. The C. atrans subclade from the C. pallidus clade, based on ITS. B. The C. volaticus subclade from the C. pallidus clade, based on ITS and LSU. C. The C. albosporus clade, based on ITS, LSU, and TEF1-α. Mid-rooted maximum-likelihood consensus trees. Numbers before and after slash (/) indicate posterior probabilities of Bayesian analysis and ML bootstrap support values. Codes after the species names denote country and admin. division of origin (ISO 3166).
Fig. 6.
Phylogeny of the Cerinomyces enatus clade. Mid-rooted maximum-likelihood consensus tree based on SSU, ITS, and LSU sequences. Numbers before and after slash (/) indicate posterior probabilities of Bayesian analysis and ML bootstrap support values. Codes after the species names denote country and admin. division of origin (ISO 3166).
Fig. 7.
Phylogenies of Cerinomyces clades. A. The C. borealis clade, based on SSU, ITS, LSU, and TEF1-α. B. The C. tortus clade, based on SSU, ITS, and LSU. Mid-rooted maximum-likelihood consensus trees. Numbers before and after slash (/) indicate posterior probabilities of Bayesian analysis and ML bootstrap support values. Codes after the species names denote country and admin. division of origin (ISO 3166).
The C. pallidus clade encompasses the generic type C. pallidus and its nine corticioid relatives. These inhabit angiosperm and gymnosperm wood mostly in the temperate zones of the Northern and Southern hemispheres. Basidiocarps are pale ochraceous, varying from arachnoid to crustose with a thin cottony subiculum. Cerinomyces pallidus and C. paulistanus develop hyphal pegs, and several related species demonstrate microscopic peg-like hyphal constructions (like Fig. 39 C), but never as abundant and regular as in the C. albosporus clade members. Microscopic features often blend between species, requiring comparison of multiple characters for non-molecular identification. Because of the differences in ITS, we prepared separate phylogenies for the C. atrans and C. volaticus subclades (Fig. 5 A, B). Basidiocarps in the first subclade are more likely to become crustose and darken to different extents. This character was observed in all members, namely in C. atrans, C. concretus, C. pallidus, C. paulistanus, C. ramosissimus, and C. verecundus. Species in the second subclade (C. fugax, C. nepalensis, C. tristis, and C. volaticus) usually remain arachnoid or solid, but still cottony, and light-coloured.
Fig. 39.
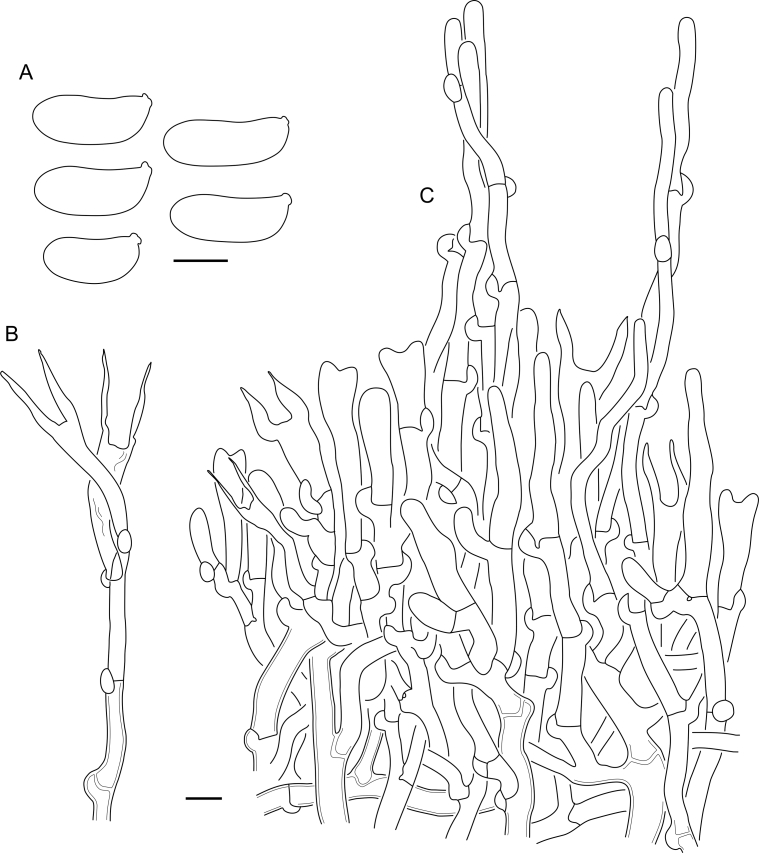
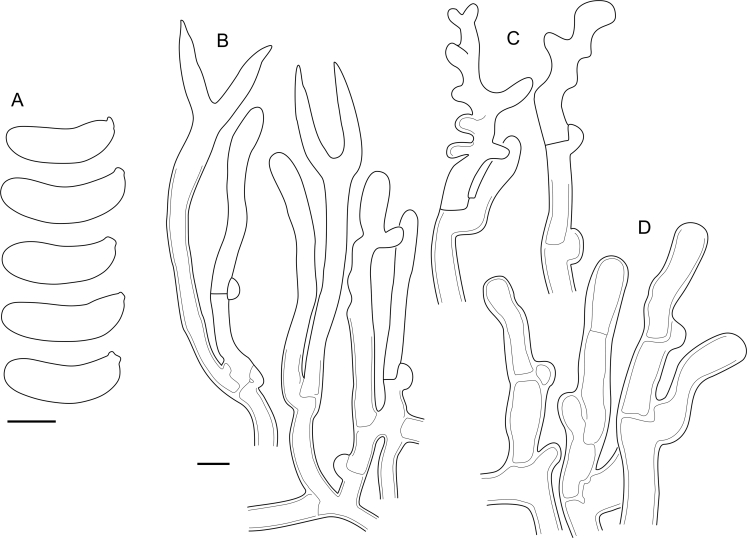
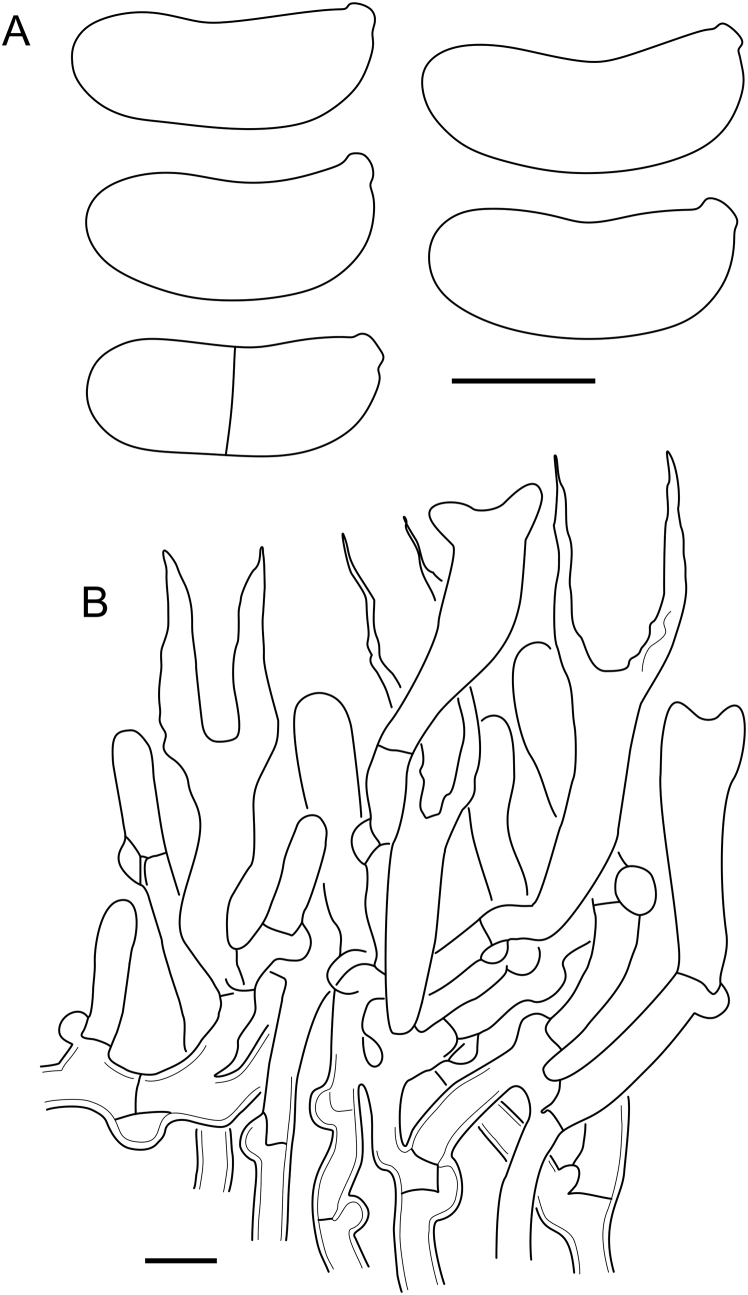
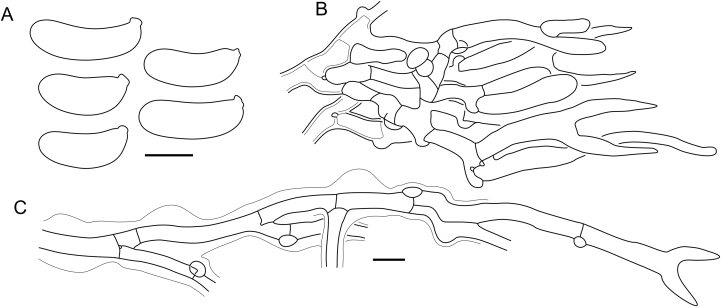
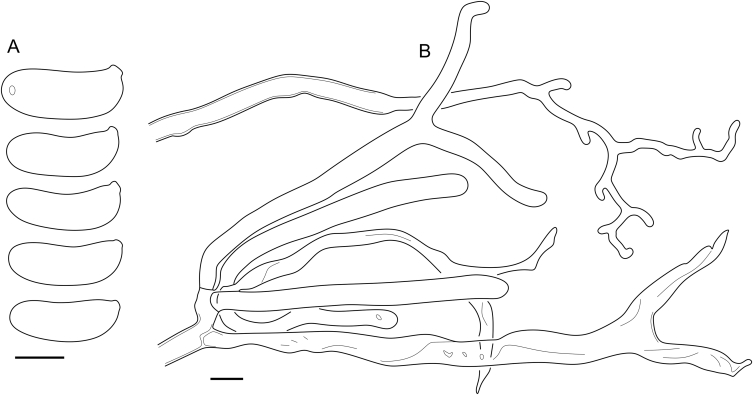
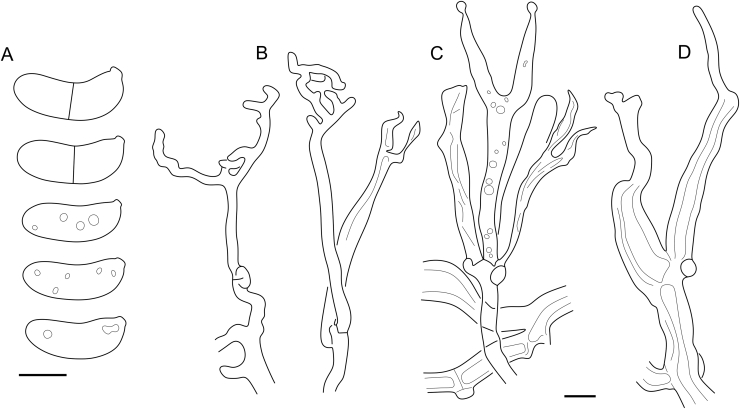
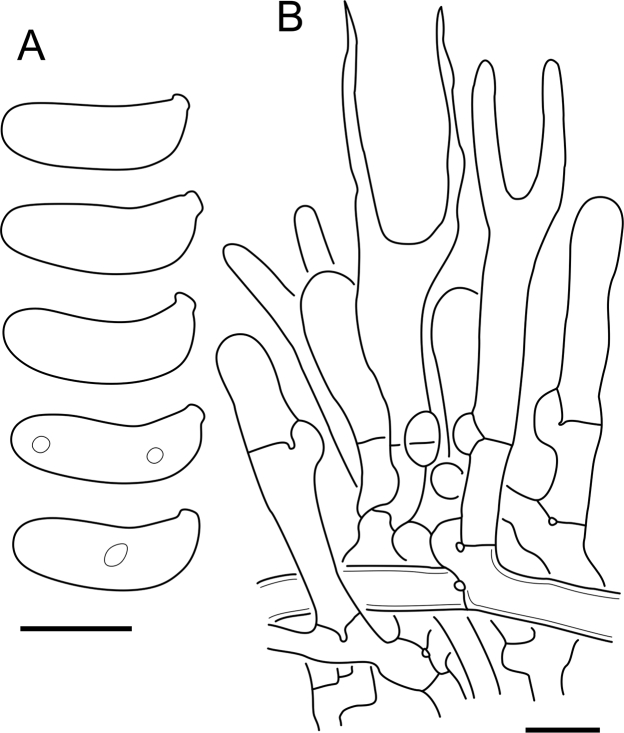
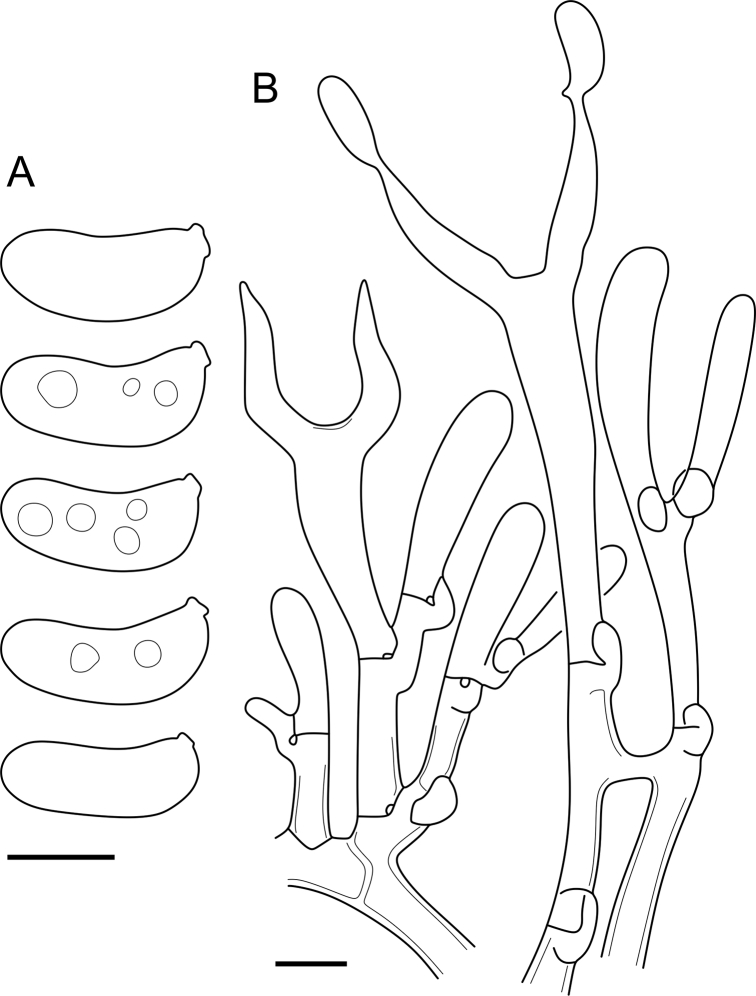
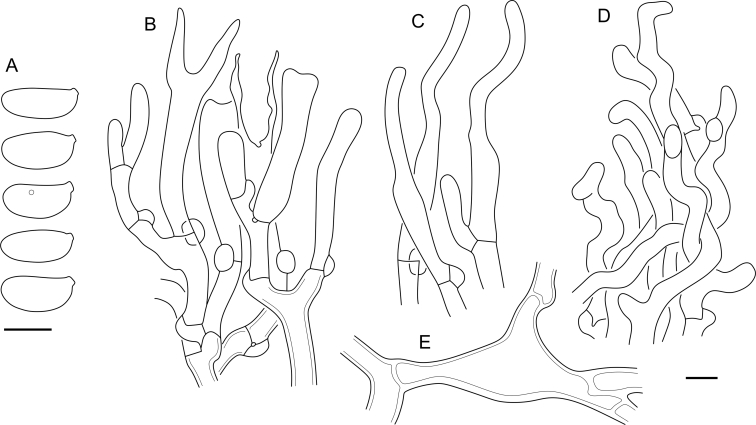
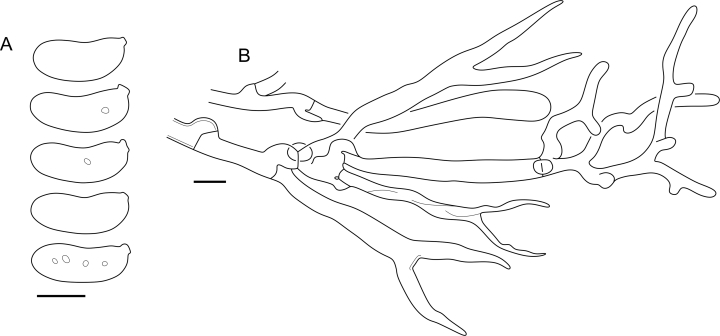
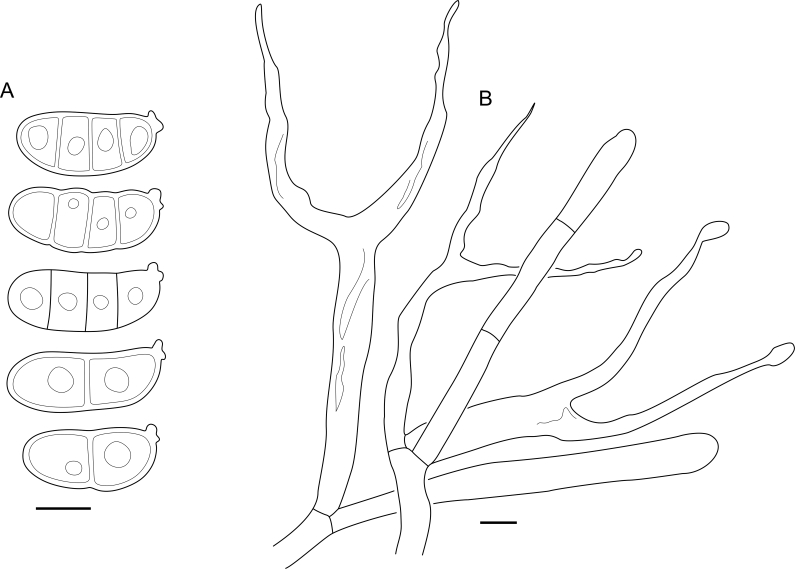
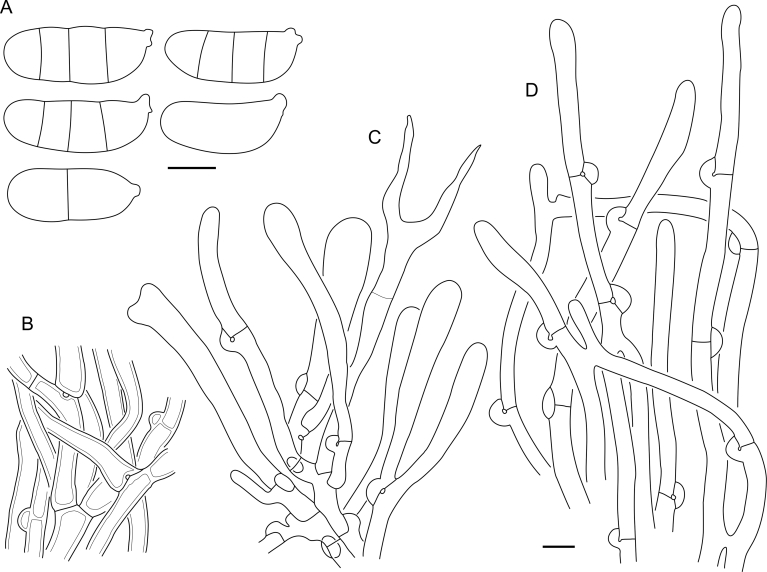
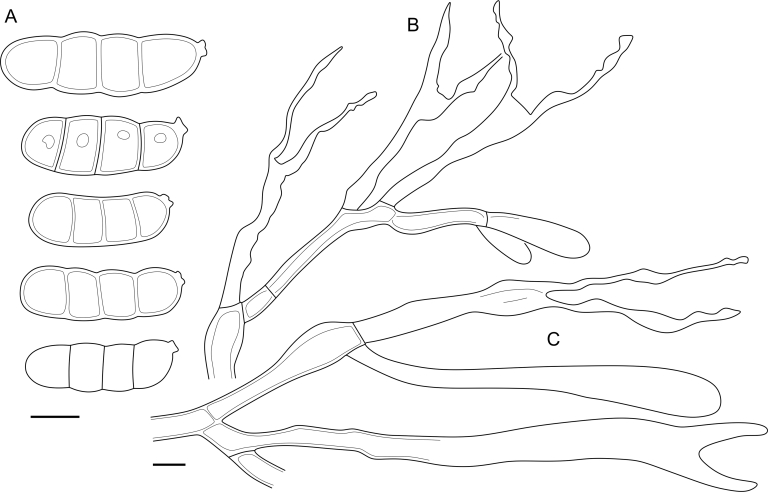
Cerinomyces pallidus micromorphology. A. Spores. B. Single hypha followed from subiculum to hymenium. C. Hymenium, subhymenium, subiculum and two developing hyphal pegs. All drawn from BPI726052. Scale bars = 5 μm.
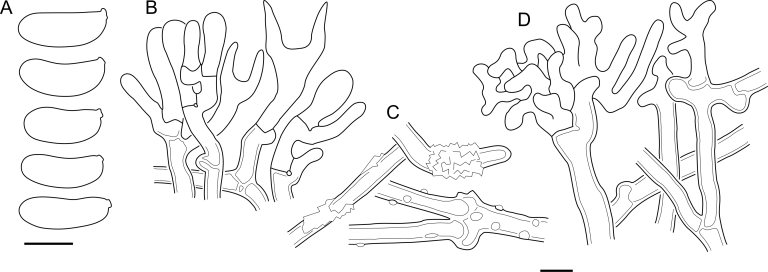
The C. albosporus clade (Fig. 5 C), sister to the C. pallidus clade, includes four corticioid species confined to climates ranging from humid temperate to tropical. Three of these species (C. aculeatus, C. albosporus, and C. brevisetus) have abundant, regularly distributed hyphal pegs and larger microstructures than in other arid Cerinomyces. The fourth species, C. inermis, is distinguished by the absence of hyphal pegs and basidiospores that conform better to some members of the C. borealis and C. pallidus clades. In the absence of sequence data, our judgement from morphology indicates that further peg-bearing species are also allied with the clade: C. fasciculatus collected from Hawaiʻi and C. curvisporus from Southwest China, known for its strongly bent basidiospores.
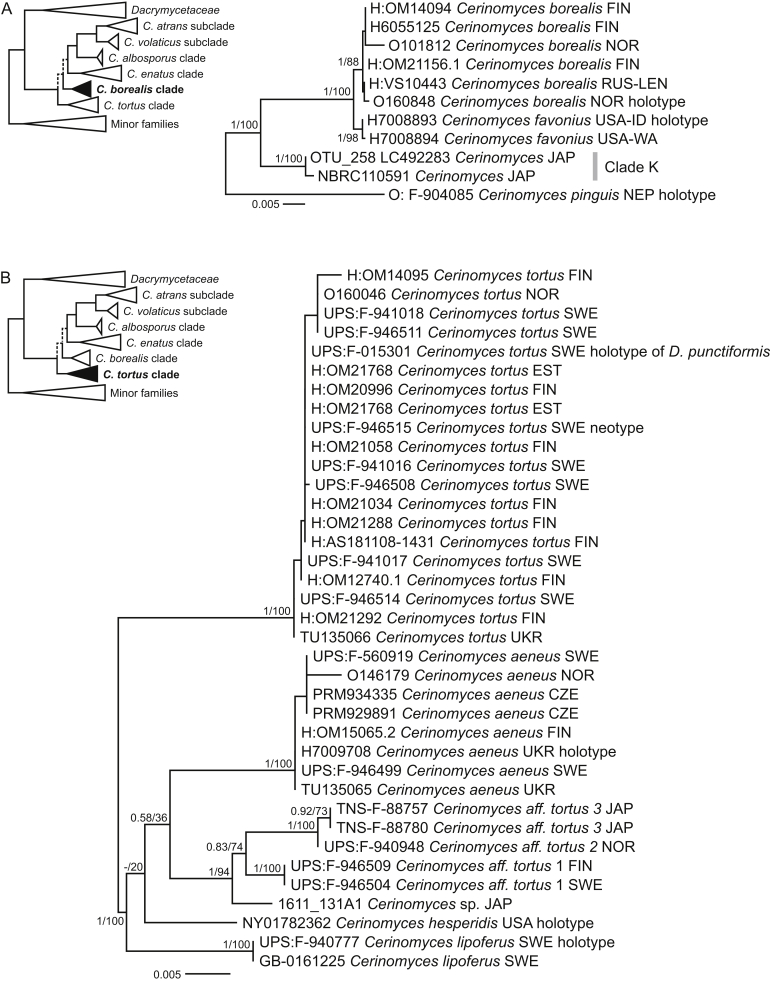
The C. borealis clade includes three corticioid species growing on coniferous wood. Two closely related species from temperate Europe and North America, C. borealis and C. favonius, have the narrowest basidiospores in the genus. The third species, C. pinguis, was collected from mountains of Nepal, and possesses much larger basidiospores. Macromorphologically the group is difficult to distinguish from other arid Cerinomyces members, though it develops more delicate, often arachnoid basidiocarps without pegs. Clade K from Shirouzu et al. (2016, 2020) corresponds to a part of the C. borealis clade and represents Japanese environmental samples and strains. Their sequences differ from the specimen-based ones, suggesting undescribed diversity in the clade (Fig. 7 A).
The C. enatus clade members (Fig. 6) look like gelatinous Dacrymyces species with pustulate basidiocarps, though they lack the hallmark bright yellow tints of Dacrymycetaceae, being instead pale yellow, ochraceous, dark brown and reddish brown. Microscopically, the clade is characterized by heavily gelatinized hyphae and the presence of dendroid hyphidia in the hymenium of all the species. The clade consists of C. cokeri, C. creber, C. enatus, C. enterolaxus, and C. neuhoffii, all of which grow in moderate to highly humid conditions in the biogeographic Northern temperate zone. We found that widely recognized C. enatus (≡ D. enatus [Berk. & M.A. Curtis] Massee) occurs only in North America and East Asia. For European material formerly identified as D. enatus, we introduce a new species, C. aeneus, that belongs to the C. tortus clade. Cerinomyces crustulinus, whose name was massively misapplied to corticioid members, is likely to be related to the C. enatus clade. However, in the absence of fresh collections and sequence data, its position is difficult to resolve with confidence.
The C. tortus clade (Fig. 7 B) comprises four species with gelatinous basidiocarps, including C. tortus, one of the earliest described Dacrymyces species (as D. tortus [Willd.] Fr). The distribution and morphological characters of the clade often overlap with the C. enatus clade, but in the phylogeny their relation is not well supported (Fig. 4). The clade members develop mostly pustulate basidiocarps, with the exception of C. aeneus, that also can demonstrate coalescing to resupinate morphology. Cerinomyces tortus itself has a number of morphologically similar relatives, from which we formally describe only C. lipoferus. At least two more Nordic taxa are represented by scarce specimens that are not suitable as types, and whose intraspecific variation is poorly known. The main character that helps to effectively distinguish C. tortus, C. hesperidis and C. lipoferus from the other gelatinous species is the lack of finely branched hyphidia. In addition, C. lipoferus demonstrates a high amount of lipid droplets in hyphae, which is unusual for the family, and scattered 3-septate basidiospores, unique among the gelatinous species studied and very infrequent in the genus. Morphology-based identification in C. enatus and C. tortus clades is possible, though differentiating European species can be problematic if basidiocarps are young or weathered. Identification by ITS marker can also be difficult due to high levels of intragenomic polymorphism hampering Sanger sequencing and contig assembly.
All other known corticioid species of Cerinomyces s.l. belong to the Dacrymycetaceae and are considerably different from the Cerinomycetaceae. They have more robust, richly coloured corticioid basidiocarps with hymenial surfaces that become waxy- or firmly-gelatinous if moisturized, thick light-coloured subiculum, and fimbriate margins. In these groups we describe four new species, propose six combinations and one informal taxon.
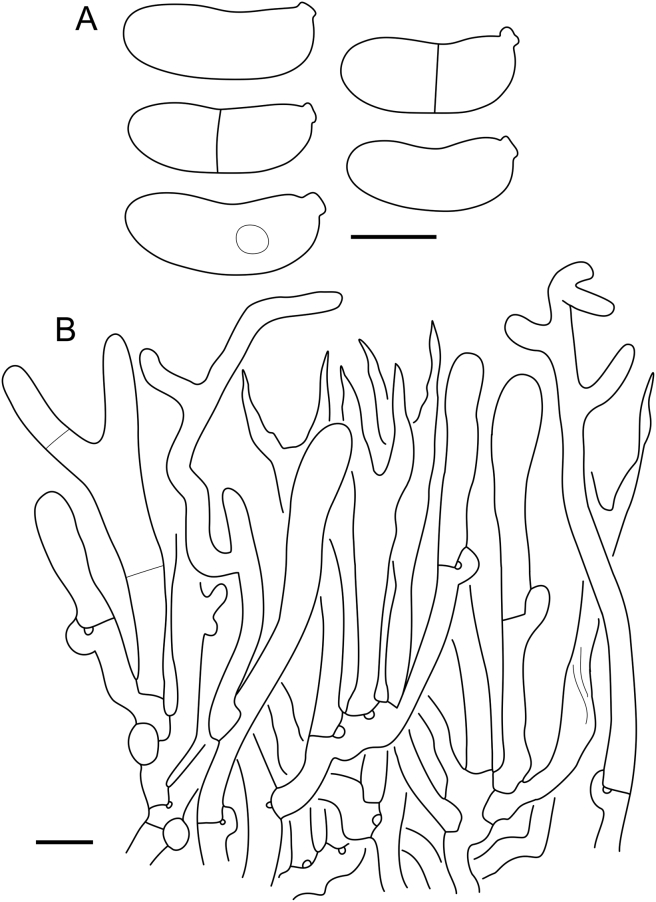
The C. canadensis lineage consists of a single species collected in East Asia and North America. It has corticioid pale to dark orange basidiocarps of various shapes, sometimes effused over several centimeters. The species sometimes possesses so-called pseudoclamps that are rare among dacrymycetes (see a note under the species and Fig. 23 D). “Cerinomyces” canadensis together with D. corticioides are related to Femsjonia (Fig. 4), which is well reflected in their morphological similarity to Femsjonia species.
Fig. 23.
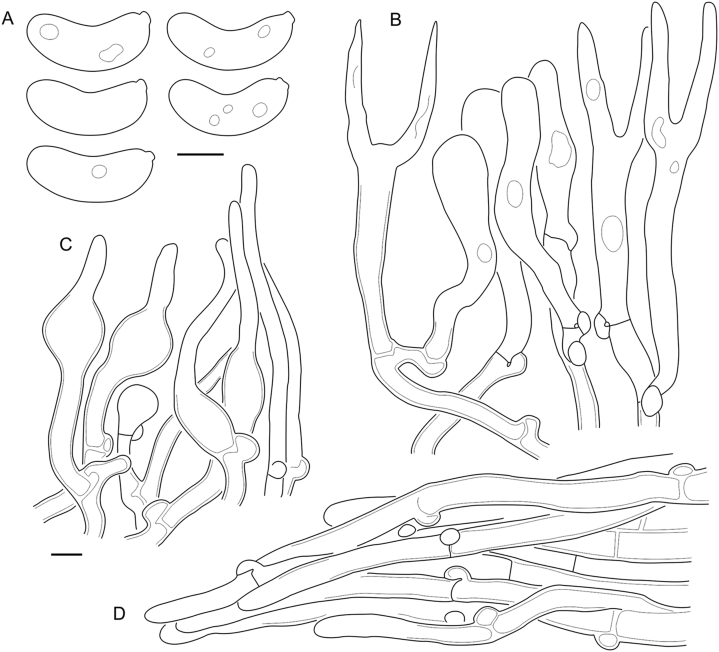
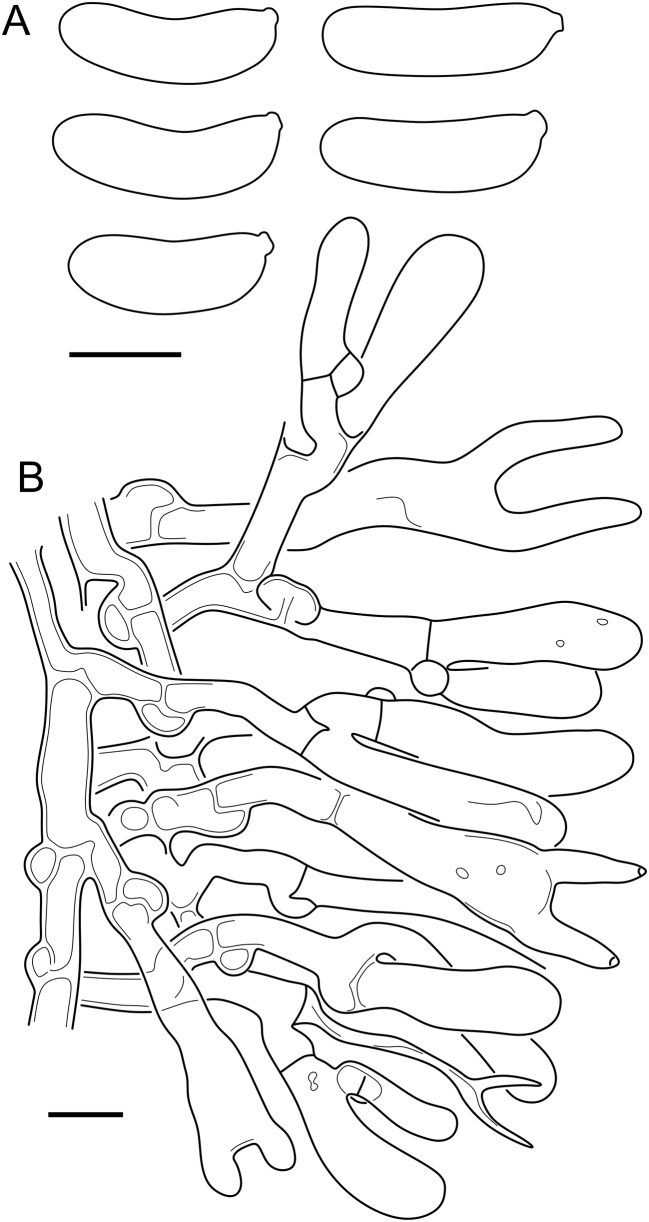
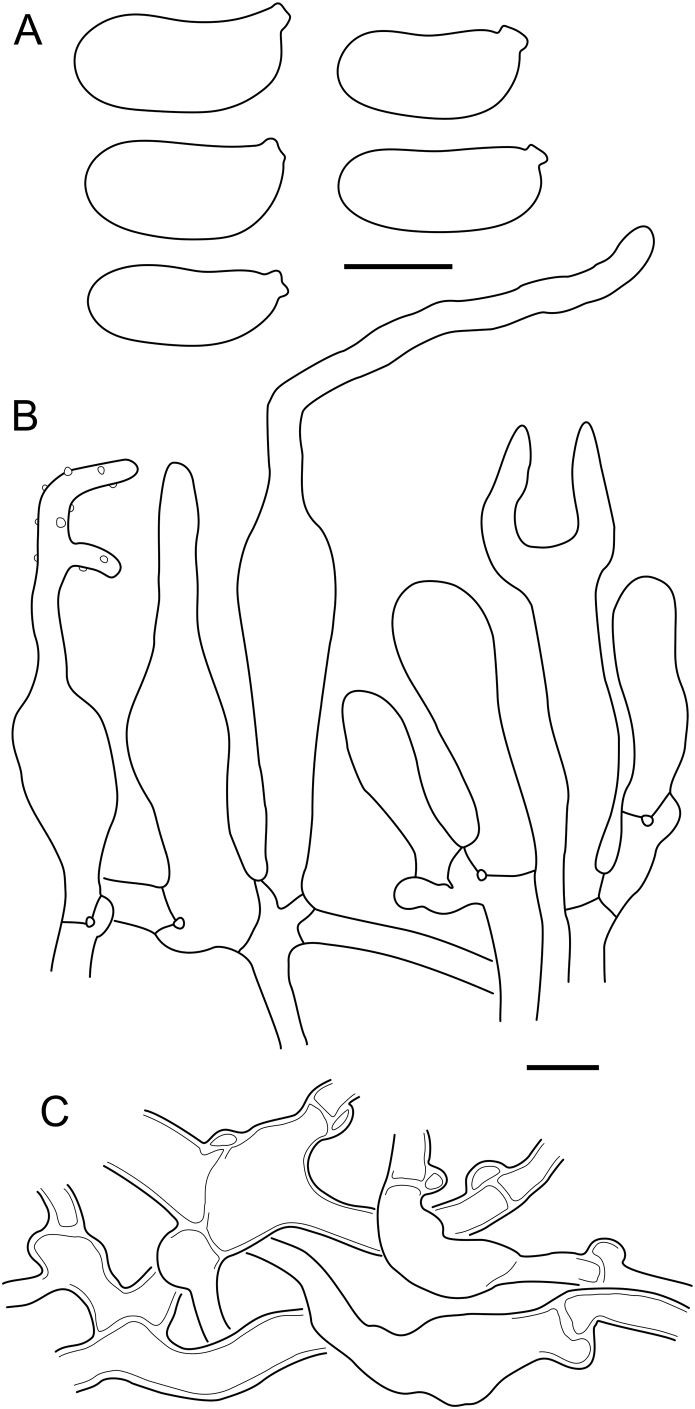
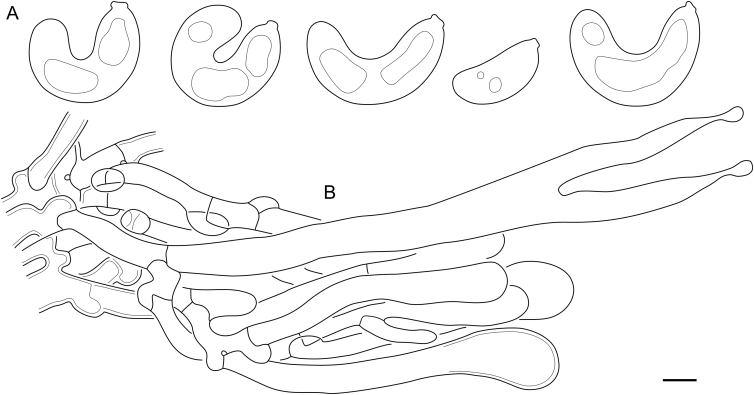
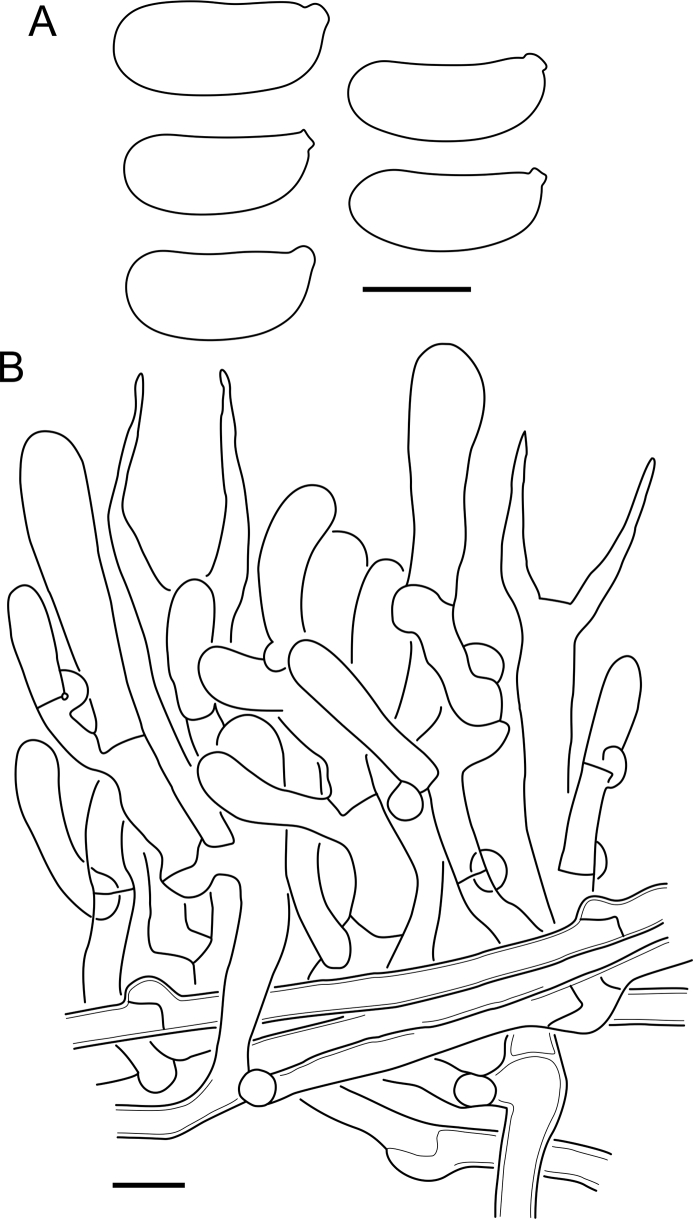
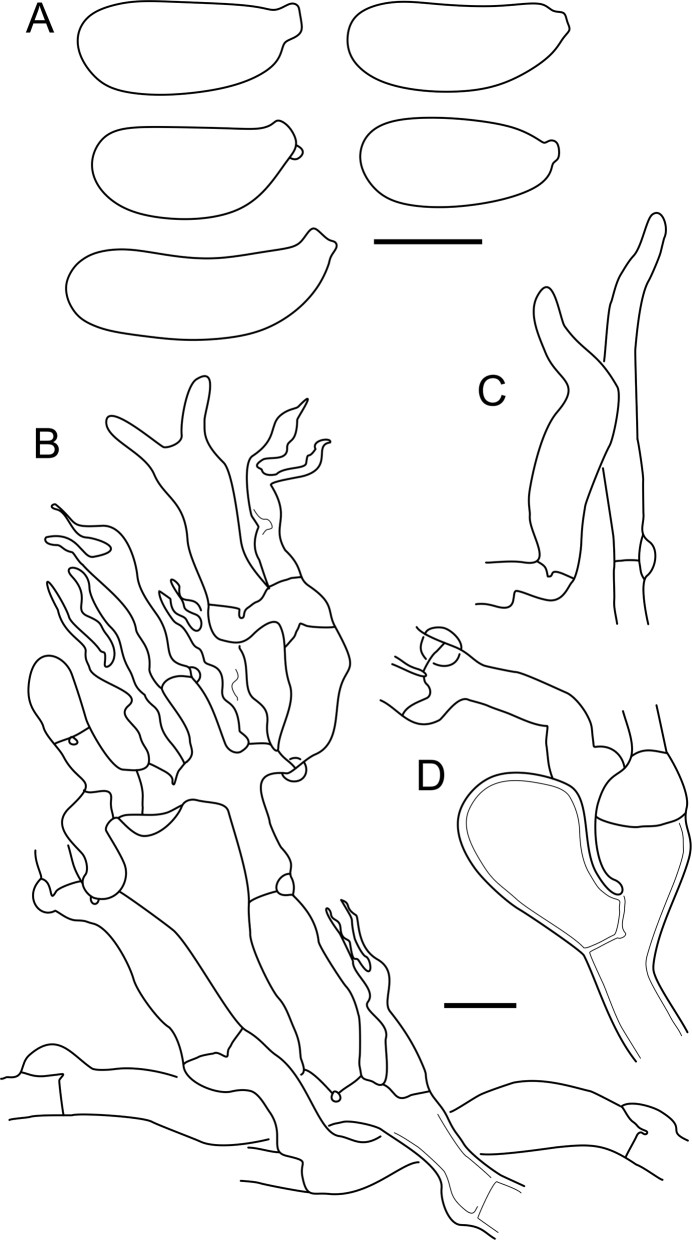
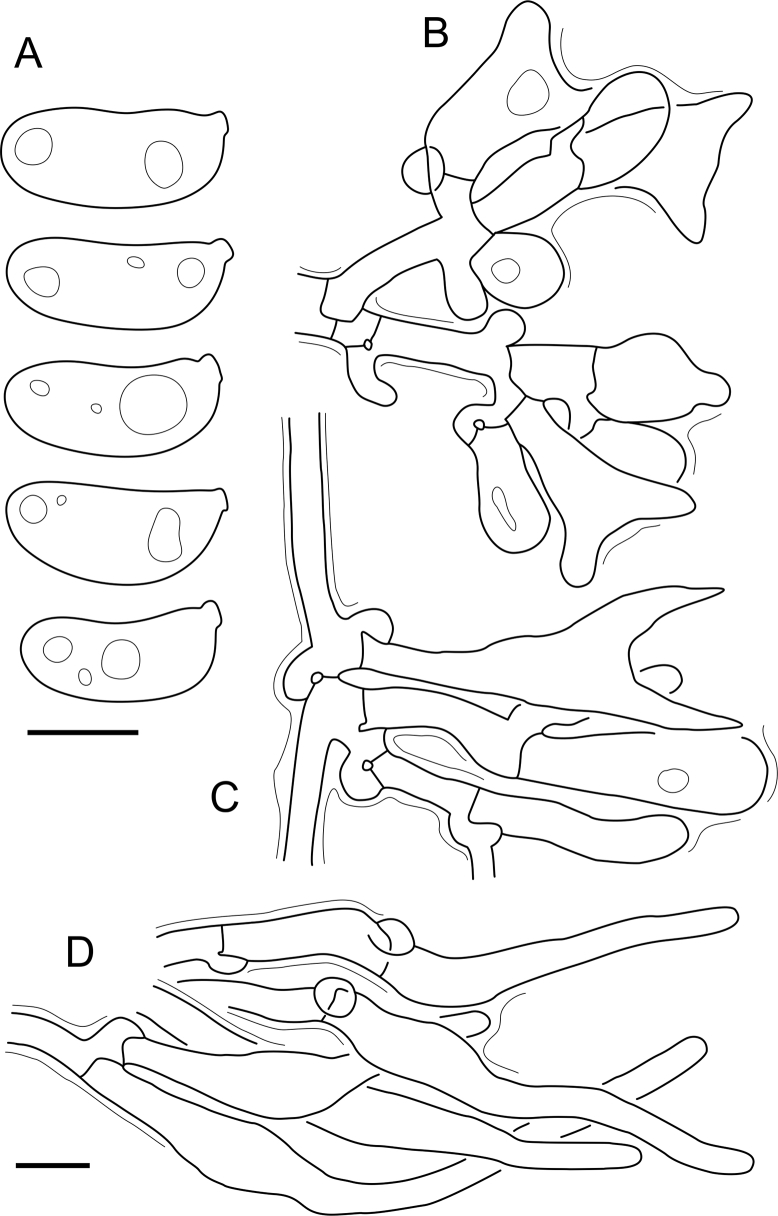
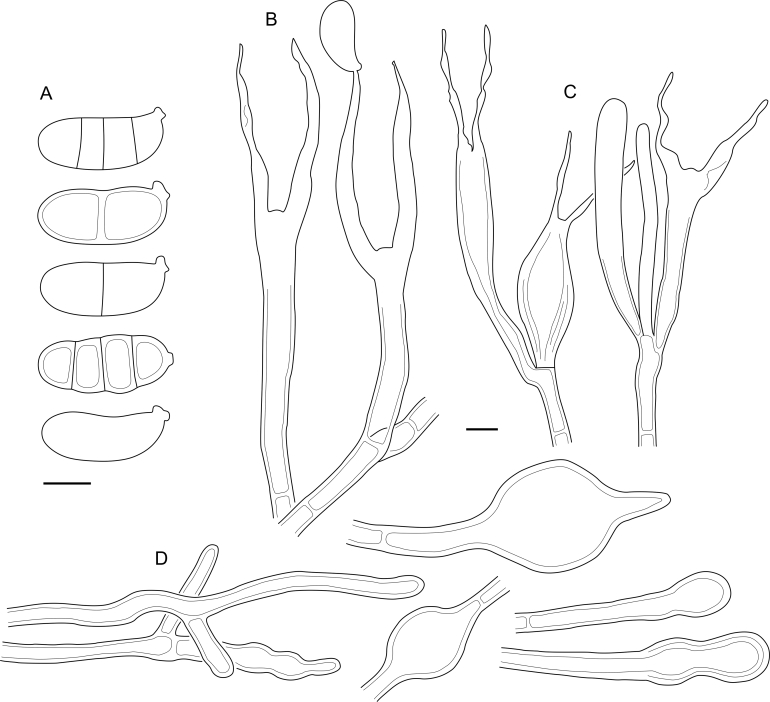
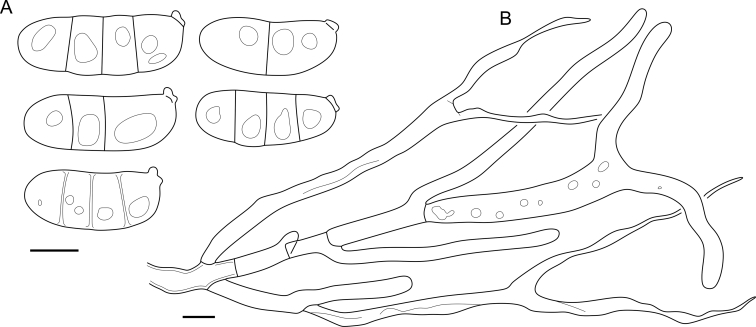
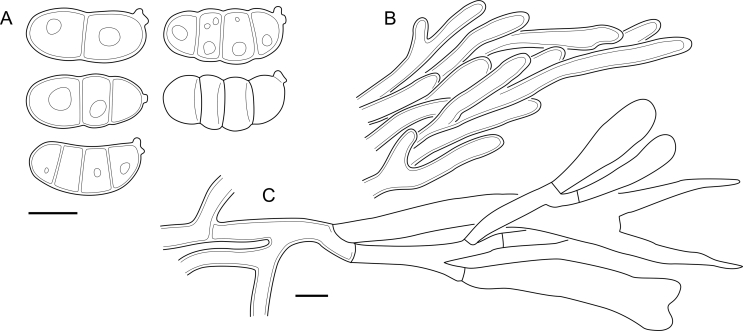
“Cerinomyces” canadensis micromorphology. A. Spores. B. Hymenium and subhymenium. C. Marginal hyphae. D. Different types of clamps. All drawn from H:Spirin 8468. Scale bars = 5 μm.
The D. corticioides lineage encompasses one species with circular, separate, later coalescing basidiocarps of yellow to orange colour, hyphae with clamp connections and tardily septate basidiospores. Here we show that D. corticioides, traditionally recognized as a North American species, has an amphi-Pacific distribution in the Northern Hemisphere and is identical to C. altaicus described from the Russian Far East. Femsjonia uniseptata with brightly yellow firm-gelatinous basidiocarps appears to belong to D. corticioides. European D. confluens is also very similar morphologically to D. corticioides, but due to the lack of sequence data, we treat it separately.
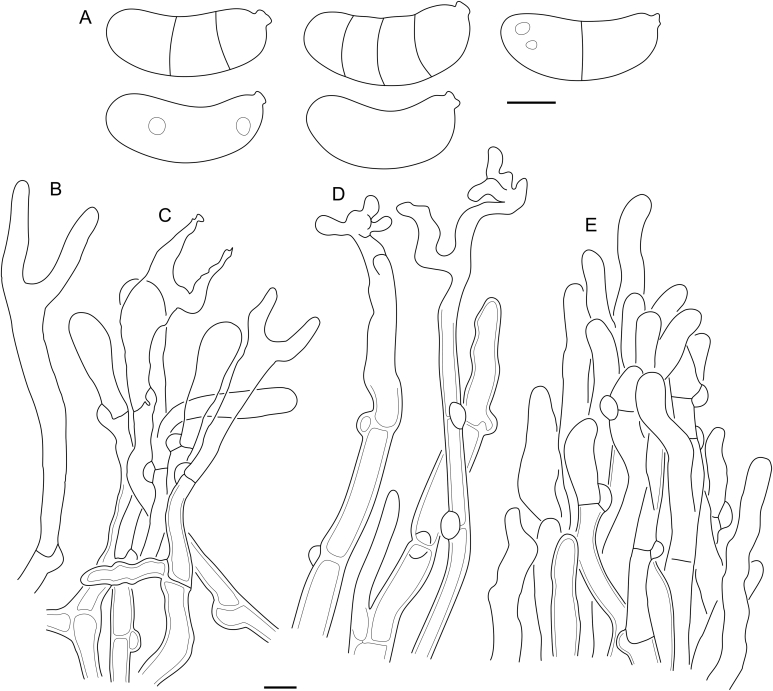
The seven species of the D. grandinioides clade tend to have relatively thick basidiocarps, a waxy-gelatinous yellow hymenial layer, clampless hyphae and three-septate, usually thick-walled basidiospores. Members of the clade occur in Africa and in the Americas, and are accordingly divided into two subclades (Fig. 8). On the grounds of morphology, we associate two non-sequenced species with this group: D. pulchrus and D. lagerheimii. We suppose “Cerinomyces bambusicola” nom. prov. belongs here as well because of its colour and prominently odontioid basidiocarps (Gminder 2016). In this group, microscopical characters vary even within a single specimen, which makes species delimitation particularly difficult.
Fig. 8.
Phylogeny of the Dacrymyces grandinioides clade. Maximum-likelihood consensus tree based on SSU, ITS, and LSU sequences, rooted between subclades. Numbers before and after slash (/) indicate posterior probabilities of Bayesian analysis and ML bootstrap support values. Codes after the species names denote country and admin. division of origin (ISO 3166).
Taxonomy
Key and identification tables
The key covers all dacrymycetes with corticioid basidiocarps regardless of colour, as well as small gelatinous taxa without long stalks, of bleak yellow, ochraceous, light to dark brown and reddish brown colour. Some of them can be confused with members of the Cerinomycetaceae: certain Dacrymyces species and members of the families Unilacrymaceae and Dacryonaemaceae, excluding Dacryonaema rufum that has unique, easily recognizable synnematous basidiocarps (Nannfeldt 1947, Zamora & Ekman 2020). We render difficult groups as separate character tables outside of the main key: clamped, arid, pale-coloured corticioids from the C. pallidus, C. borealis and C. albosporus clades (Table 3), Cerinomyces species with gelatinous basidiocarps from the C. enatus and C. tortus clades (Table 4), and clampless, slightly gelatinous, yellow corticioid species with three-septate basidiospores — Dacrymyces species from the D. grandinioides clade (Table 5).
-
1.
Basidiocarps pustulate, shallow-cupulate, less often cerebriform or resupinate. Always fully gelatinous when fresh…………………………………………………………2
-
1.
Basidiocarps corticioid, either always arid or with a waxy-gelatinous hymenial surface when fresh………………19
-
2.
Most hyphal septa clampless. Elongated, medallion-like clamps occur in subhymenium. Dacryonaemataceae. The following choice #3 fide Zamora & Ekman (2020)…………3
-
2.
Clamps present on all or almost all hyphal septa, of typical semi-circular form……………..…………………………….4
-
3.
Mature basidiospores on average < 14.0 × 5.3 μm…….….….….….….….…….…Dacryonaema macnabbii
-
3.
Mature basidiospores on average > 14.5 × 5.3 μm….…….……………………Dacryonaema macrosporum
-
4.
Basidiospores subglobose or ellipsoid (Q < 2.2), basidia ≥ 60 μm in length. In internal hyphae clamps sometimes absent or appear as pseudoclamps. Unilacrymaceae…………………………………………….5
-
4.
Basidiospores cylindrical to slightly curved-cylindrical (Q ≥ 2.2), basidia < 60 μm in length. Clamps present on all septa...………………………………………………………6
-
5.
Basidia unisterigmate, basidiospores subglobose (Q = 1.4, Q’ = 0.9–1.9)……………Unilacryma unispora
-
5.
Basidia bisterigmate, basidiospores ellipsoid (Q = 2, Q’ = 1.6–2.4)……………………...…Unilacryma bispora
-
6.
Basidiospores regularly 3-septate…………………...…...7
-
6.
Basidiospores aseptate or tardily septate…….9 and Table 4
-
7.
Branched hyphidia present…………………………………………………………Dacrymyces paraphysatus s.l.
-
7.
Branched hyphidia absent…………………………………8
-
8.
Angiosperm substrates. Central European species……….…….……………..…Dacrymyces adpressus s.l.
-
8.
Gymnosperm substrates. Northern European species….……….…….…………………...Dacrymyces fennicus
-
9.
Angiosperm substrates…………………………………..10
-
9.
Gymnosperm substrates…………………………………12
-
10.
Basidiocarps often protrude through cracks or lenticels in bark. North American and Asian species…………………........................Cerinomyces enatus
-
10.
Basidiocarps usually grow on decorticated wood. European species………………………………………………11
-
11.
Basidiocarps yellowish brown to reddish brown and dark brown, in mature state usually resupinate-cerebriform…………………………………Cerinomyces aeneus
-
11.
Basidiocarps light yellow to light brown even when dry, not cerebriform……………………..................................Cerinomyces crustulinus and C. aff. crustulinus 1
-
12.
Hyphidia absent or rare, weakly branched and robust (e.g., Fig. 43 C)……………………………………………13
-
12.
Hyphidia always present, abundant, finely branched (e.g., Fig. 38 B)……………………………………………15
-
13.
Mature basidiocarps often short stalked and centrally depressed, ≤ 1 mm in diam. North American species………………………..……Cerinomyces hesperidis
-
13.
Basidiocarps sessile, rooted in substrate, but usually without visible stalk. Mature basidiocarps > 1 mm in diam. European species…………………………………14
-
14.
Basidiospores 0–1(–3)-septate. Hyphae with large amount of lipid droplets. Fresh basidiocarps typically whitish to yellowish or cream coloured…………………………………………………….Cerinomyces lipoferus
-
14.
Basidiospores aseptate or extremely rarely 1-septate. Hyphae with low amount of lipid droplets. Fresh basidiocarps light yellowish to brown…………………………………………………………......Cerinomyces tortus
-
15.
Basidiocarps pulvinate, also resupinate and cerebriform, often > 1.5 mm in the longest dimension, commonly protruding through bark…………………………………..16
-
15.
Basidiocarps pustulate, flattened, cupulate or slightly cerebriform, normally < 1.5 mm in diam, usually growing on decorticated wood…………………………………….17
-
16.
Basidiocarps light yellow or light brown when fresh, dark brown when dry. No swollen cells in subiculum………………………………..Cerinomyces cokeri
-
16.
Basidiocarps dark brown when fresh, almost black when dry. Swollen cells abundant in subiculum of mature basidiocarps………………………..Cerinomyces enatus
-
17.
Subicular hyphae loosely arranged, hyphal walls in subiculum ≤ 0.5 μm in width, without a substantial gelatinous layer. Asian species……………………………………………………......Cerinomyces enterolaxus
-
17.
Subicular hyphae densely arranged, hyphal walls in subiculum with a conspicuous gelatinous layer, together 0.5–1 μm in width. European species……………………………………….18 and Cerinomyces aff. tortus 1 & 2
-
18.
Basidiocarps frequently dark brown to greyish brown when fresh, often but not always coalescing. Basidiospores on average 11.6 × 3.8 μm. Occurs on Pinaceae wood…...…………………………Cerinomyces neuhoffii
-
18.
Basidiocarps frequently light brown when fresh, readily coalescing. Basidiospores on average 9.4 × 3.3 μm. Occurs on Cupressaceae wood…Cerinomyces creber
-
19.
Clamps absent on all septa……………………….Table 5
-
19.
Clamps present on all septa………………………..……20
-
20.
Hyphal pegs visible to naked eye…………………….…21
-
20.
Hyphal pegs absent or microscopic…………………..…27
-
21.
Basidiospores strongly curved………………………........………………..………………Cerinomyces curvisporus
-
21.
Basidiospores only slightly curved………………………22
-
22.
Macroscopic pegs irregular, scattered, can be absent. Microscopic pegs always present. Basidiospores L < 9 μm………………………………………………....…23
-
22.
Macroscopic pegs regular, frequent, always present. Basidiospores L > 9 μm………………………………..…24
-
23.
Macroscopic pegs present on most of well-developed basidiocarps. Hyphal swellings in subiculum absent or rare. North American species…………………………………………….................Cerinomyces pallidus
23. Well-developed basidiocarps often have no macroscopic pegs. Hyphal swellings present in subiculum. South American species……………Cerinomyces paulistanus
-
24.
Basidiospores 0–1(–3)-septate, L > 12 μm. Hyphal pegs > 150 μm in length………………………………….25
-
24.
Basidiospores aseptate, L < 12 μm. Hyphal pegs < 150 μm in length………………………………….26
-
25.
Basidiocarps are the most robust among peg-bearing taxa. Found on woody Asteraceae shrub. African species…………………………….Cerinomyces albosporus
-
25.
Basidiocarps more subtle. Grows mostly on gymnosperm wood. Asian species………..…Cerinomyces aculeatus
-
26.
The smallest basidiospores among the related species, 9.3–11.1(–11.7) × 3.5–4.4(–4.6) μm. South American species…………………………Cerinomyces brevisetus
-
26.
Basidiospores slightly larger, 10–12.5(–13) × 4.5–5.5 μm. The species known only from Hawaiʻi…………………….…Cerinomyces fasciculatus
-
27.
Basidiocarps thin, white to ochraceous, subiculum and margins delicate or lacking. Hymenial surface typically arid when fresh. Mature basidiospores aseptate……………………………………………………Table 3
-
27.
Basidiocarps thick, yellow to orange when fresh, with coarse subiculum and fimbriate margins. Hymenial surface firm waxy-gelatinous when fresh. Mature basidiospores 3-septate……………………………………….…28
-
28.
Basidiocarps orange to dark orange when fresh, of irregular shapes. Basidiospores < 11 × 5 μm, 0–1-septate………………………….“Cerinomyces” canadensis
-
28.
Basidiocarps yellow when fresh, growing as circular patches that easily coalesce. Basidiospores > 11 × 5 μm, 0–3-septate………..Dacrymyces confluens & D. corticioides
Table 3.
Cerinomyces species with pale-coloured corticioid basidiocarps, arid or rarely slightly gelatinous when wet; C. fasciculatus fideGilbertson & Hemmes (2004). Ster. — maximal sterigmata length. The table is sorted by region and substrate.
| Name | Spores | L | W | Q | Basidia | Ster. | Pegs | Substrate | Region |
|---|---|---|---|---|---|---|---|---|---|
| C. albosporus | (11.8–)12.0–17.8(–18.0) × (5.0–)5.2–7.0(–7.1) | 15.3 | 6.0 | 2.6 | 25–62 × 3–6.5 | 20 | + | Angiosperm | Africa |
| C. aculeatus | (9.7–)10.5–17.2(–17.7) × 4.0–6.5(–7.4) | 13.8 | 5.0 | 2.8 | 12–46 × 3–7 | 29 | + | Gymnosperm, angiosperm? | Asia |
| C. aff. aculeatus 1 | 9.5–11.3(–11.5) × (3.2–)3.3–4.4(–4.5) | 10.5 | 3.7 | 2.8 | 11–20 × 3–4.5 | 23 | − | Gymnosperm | Asia |
| C. curvisporus | (16–)16.3–20.0(–20.4) × 5.9–7.1(–7.5) | 18.4 | 6.5 | n/d | 41–77 × 6–10 | 34 | + | Gymnosperm | Asia |
| C. nepalensis | (5.0–)5.2–7.7(–7.9) × (2.1–)2.5–3.4(–3.5) | 6.6 | 3.0 | 2.2 | 7–17 × 2.5–4.5 | 10 | − | Gymnosperm? | Asia |
| C. pinguis | (8.7–)9.0–10.5(–12.6) × 3.5–4.5(–5.0) | 9.8 | 3.9 | 2.5 | 10–21 × 4.5–6.5 | 12 | − | Gymnosperm? | Asia |
| C. borealis | (6.2–)7.1–11.1(–12) × (2.2–)2.3–3.4(–3.6) | 9.1 | 2.9 | 3.2 | 9–21 × 3–6 | 14 | − | Gymnosperm | Europe |
| C. volaticus | (6.1–)6.5–9.3(–10.1) × (2.5–)2.6–3.7(–3.9) | 7.9 | 3.1 | 2.5 | 10–26 × 2–5 | 17 | − | Gymnosperm | Europe |
| C. inermis | (8.1–)8.2–10.2(–10.6) × (2.9–)3.0–3.9 | 9.5 | 3.3 | 2.9 | 12–25 × 3–5 | 21 | − | Gymnosperm | New Zealand |
| C. verecundus | (7.9–)8.0–10.1(–10.5) × (3.0–)3.1–4.5(–4.8) | 9.2 | 3.8 | 2.4 | 12–25 × 2.5–4.5 | 15 | − | Gymnosperm | New Zealand |
| C. favonius | (6.3–)7.1–10.3(–10.8) × 2.3–3.0(–3.1) | 8.3 | 2.7 | 3.1 | 8–18 × 3.5–5 | 16 | − | Gymnosperm | North America |
| C. fugax | (7.2–)7.3–9.3(–10.0) × (2.9–)3.0–3.8(–3.9) | 8.5 | 3.3 | 2.6 | 9–22 × 2.5–5 | 13 | − | Gymnosperm | North America |
| C. tristis | 6.0–8.9(–9.7) × (2.5–)2.7–4.0(–4.1) | 7.4 | 3.3 | 2.2 | 9–22 × 2.5–5 | 16 | − | Gymnosperm | North America |
| C. pallidus | (6.1–)6.5–10.2(–11.3) × 2.9–4.1(–4.8) | 8.0 | 3.3 | 2.4 | 10–27 × 2.5–5 | 19 | +/− | Angiosperm, rarely gymnosperm | North America |
| C. atrans | (6.7–)6.9–10.8(–11.1) × (2.3–)2.4–3.9(–4.0) | 8.8 | 3.1 | 2.9 | 13–24 × 3–4.5 | 21 | − | Angiosperm, rarely gymnosperm? | North America |
| C. fasciculatus | 10.0–12.5(–13) × 4.5–5.5 | n/d | n/d | n/d | 25–40 × 4–6 | 35 | + | Angiosperm | Oceania |
| C. ramosissimus | (7.0–)7.3–9.6 × (2.7–)2.8–3.2(–3.4) | 8.3 | 3.0 | 2.8 | 8–16 × 3–5 | 11 | − | Gymnosperm | South & Central America |
| C. brevisetus | 9.3–11.1(–11.7) × 3.5–4.4(–4.6) | 10.3 | 3.9 | 2.6 | 12–23 × 3–5.5 | 19 | + | Angiosperm? | South & Central America |
| C. concretus | (7.7–)7.8–10.5(–12.0) × (3.3–)3.4–4.5(–5.0) | 9.0 | 4.0 | 2.2 | 15–28 × 3–6 | 11 | − | Angiosperm? | South & Central America |
| C. paulistanus | (6.0–)6.5–9.6(–11.2) × (2.5–)2.6–3.9(–4.4) | 7.6 | 3.3 | 2.3 | 10–23 × 2.5–5 | 13 | +/− | Angiosperm? | South & Central America |
Table 4.
Cerinomyces species with pustulate, pulvinate, or resupinate basidiocarps, fully gelatinous in moist conditions. Sept. — number of septa in basidiospores, Ster. — maximal sterigmata length. The table is sorted by region and substrate.
| Name | Spores | L | W | Q | Sept. | Basidia | Ster. | Substrate | Region |
|---|---|---|---|---|---|---|---|---|---|
| C. enterolaxus | 9.1–13.7(–14.1) × (3.0–)3.2–4.5(–4.6) | 11.6 | 3.9 | 3.0 | 0(–1) | 14–33 × 3–6 | 19 | Gymnosperm, angiosperm | Asia |
| C. enatus | (7.0–)7.4–13.2(–14.2) × (2.7–)2.9–4.5(–5.4) | 9.5 | 3.5 | 2.7 | 0(–1) | 13–56 × 3–6.5 | 27 | Angiosperm, gymnosperm | Asia, North America |
| C. creber | (7.4–)7.7–11.0(–12.8) × (2.5–)2.8–4.0(–4.2) | 9.4 | 3.3 | 2.9 | 0 | 14–36 × 3–5 | 18 | Gymnosperm | Europe |
| C. lipoferus | (9.1–)9.5–15.2(–16.8) × (3.7–)3.9–5.2(–5.7) | 11.8 | 4.5 | 2.6 | 0(–1) | 19–62 × 3–6 | 34 | Gymnosperm | Europe |
| C. neuhoffii | (8.2–)9.6–14.0(–16.1) × (2.9–)3.0–4.8(–5.1) | 11.6 | 3.8 | 3.1 | 0 | 14–43 × 3–7 | 26 | Gymnosperm | Europe |
| C. tortus | (9.0–)9.7–14.4(–15.6) × (3.0–)3.1–4.9(–5.3) | 12.1 | 4.0 | 3.0 | 0 | 17–56 × 2.5–5 | 31 | Gymnosperm | Europe |
| C. aff. tortus 1 | (8.2–)9.0–12.0(–12.4) × 3.0–4.1(–4.4) | 10.4 | 3.6 | 2.9 | 0 | 11–32 × 2.5–4.5 | 24 | Gymnosperm | Europe |
| C. aff. tortus 2 | 7.8–10.7 × 3.3–4.2(–4.4) | 9.4 | 3.8 | 2.4 | 0(–1) | 21–40 × 2.5–4 | 31 | Gymnosperm | Europe |
| C. aeneus | (7.9–)8.1–11.2(–13.0) × (2.7–)3.0–4.5(–5.0) | 9.5 | 3.8 | 2.5 | 0(–1) | 16–38 × 2–6 | 24 | Angiosperm | Europe |
| C. crustulinus | (7.4–)7.7–13.4(–14.0) × 2.8–4.0 | 9.8 | 3.3 | 3.0 | 0 | 16–38 × 2.5–5 | 28 | Angiosperm | Europe |
| C. aff. crustulinus 1 | (8.4–)8.6–10.9(–11.6) × (3.4–)3.4–4.2(–4.2) | 9.8 | 3.7 | 2.7 | 0 | 20–49 × 3–5 | 23 | Angiosperm | Europe |
| C. cokeri | (9.6–)9.7–12.8(–13.0) × (3.4–)3.5–4.6(–5.0) | 11.2 | 4.0 | 2.8 | 0(–1) | 36–60 × 3–6 | 34 | Gymnosperm | North America |
| C. hesperidis | (9.9–)10.0–12.3(–13.4) × (3.1–)3.2–4.2(–4.5) | 11.3 | 3.7 | 3.0 | 0(–1) | 23–41 × 2.5–4.5 | 35 | Gymnosperm | North America |
Table 5.
Species with clampless septa and brightly coloured corticioid basidiocarps, waxy-gelatinous when wet (the Dacrymyces grandinioides group). Ster. — maximal sterigmata length. The table is sorted by region and substrate.
| Name | Spores | L | W | Q | Basidia | Ster. | Pegs | Substrate | Region |
|---|---|---|---|---|---|---|---|---|---|
| D. venustus | (12.8–)13.7–16.2(–16.7) × 4.9–6.7(–6.8) | 14.8 | 5.5 | 2.7 | 33–59 × 2.5–6 | 46 | + | Gymnosperm | Africa |
| D. aff. venustus 1 | (13.1–)13.9–18.0(–18.2) × (4.9–)5.0–6.2(–6.3) | 15.4 | 5.6 | 2.8 | 17–60 × 3–6 | 34 | + | n/d | Africa |
| D. grandinioides | (11.3–)12.0–16.5(–18.3) × (4.3–)4.6–6.5(–6.9) | 14.3 | 5.5 | 2.6 | 23–48 × 2.5–5 | 46 | + | Angiosperm, gymnosperm | Africa |
| D. burdsallii | (11.6–)11.9–15.0 × (4.8–)4.9–6.1(–6.3) | 13.4 | 5.3 | 2.5 | 24–48 × 2.5–6 | 48 | + | Gymnosperm | North America |
| D. grandii | (13.2–)13.6–16.3(–16.4) × (4.8–)5.0–6.2(–6.8) | 14.9 | 5.5 | 2.7 | 20–60 × 3–6 | 51 | + | Gymnosperm | North America |
| D. ceraceus | (11.9–)12.0–16.5(–16.6) × (4.3–)4.4–6.0 | 13.7 | 5.1 | 2.7 | 25–46 × 3.5–6.5 | 27 | + | Angiosperm | North America |
| D. sobrius | 11.0–17.1 × (4.7–)4.9–6.2(–6.3) | 13.5 | 5.4 | 2.5 | 21–52 × 3–7 | 38 | + | Angiosperm | North America |
| D. cereus | (9.7–)10.4–13.9(–14.0) × (4.0–)4.1–5.9(–6.1) | 12.0 | 4.9 | 2.5 | 16–45 × 3–7 | 45 | − | n/d | South America |
| D. lagerheimii | (12.6–)12.8–17.6(–18.5) × (3.5–)5.0–7.1(–7.6) | 14.5 | 6.0 | 2.4 | 15–64 × 3–8 | 67 | − | Angiosperm | South America |
| D. pulchrus | (13.8–)14.1–19.4(–20.6) × (4.5–)4.8–6.4(–6.6) | 16.3 | 5.4 | 3.0 | 14–65 × 3.5–8 | 54 | − | Angiosperm? | South America |
Fig. 43.
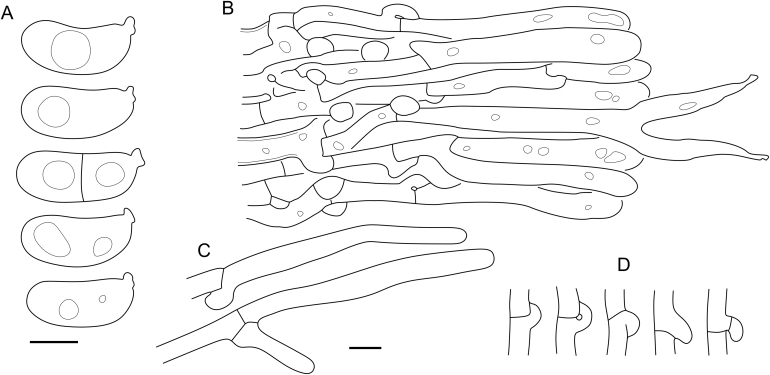
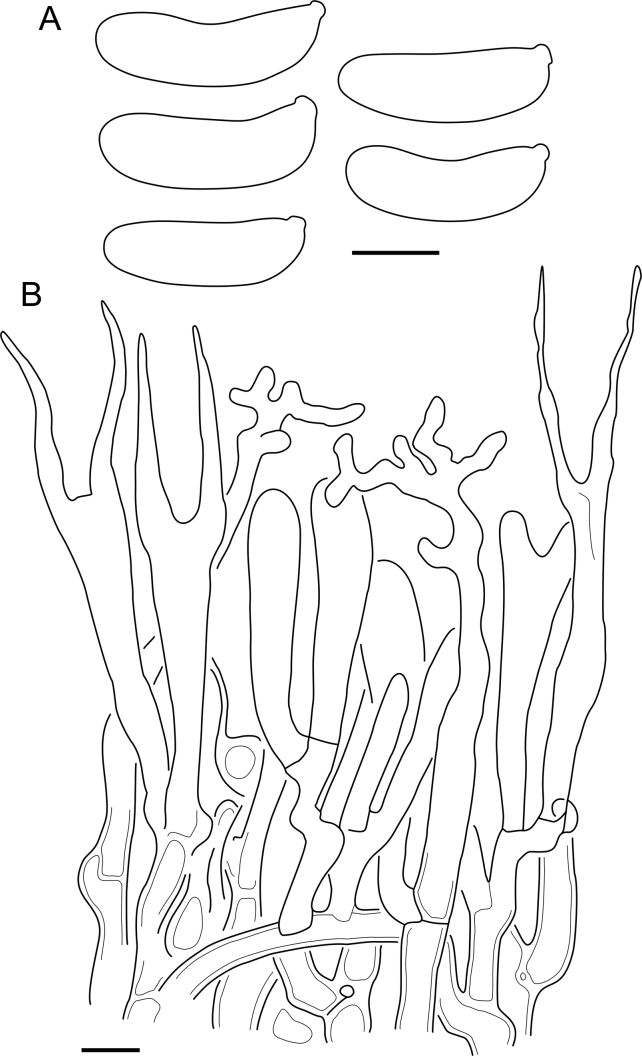
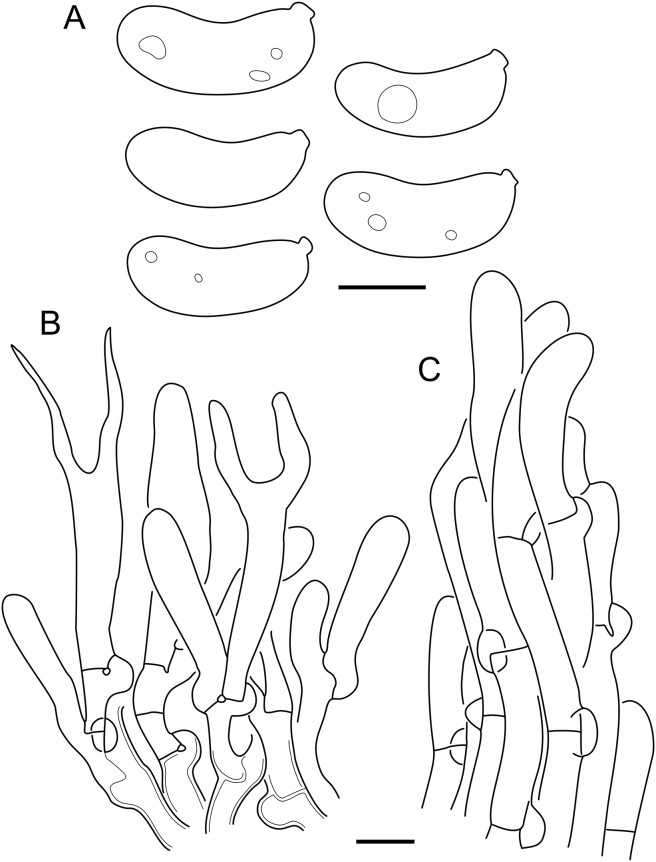
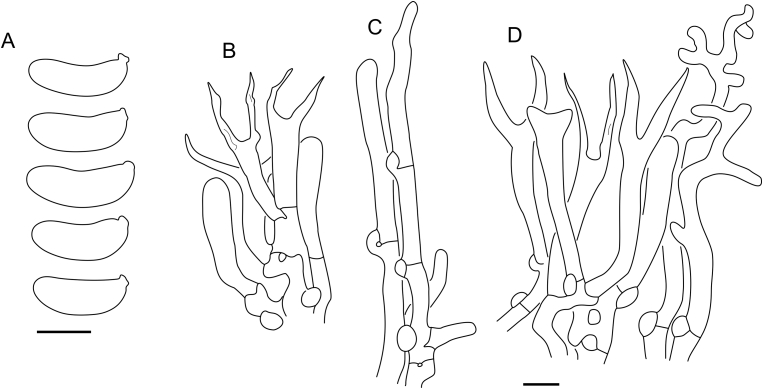
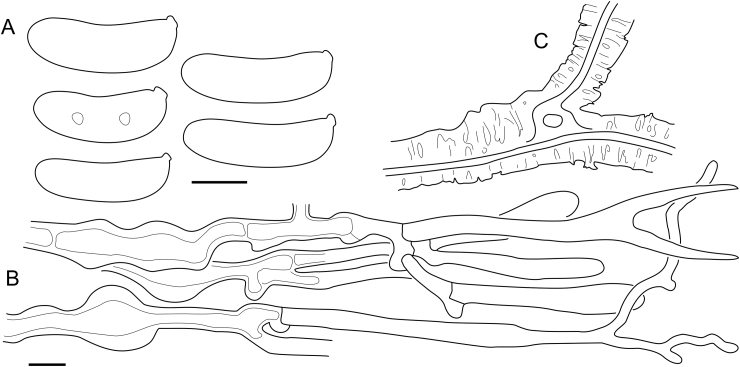
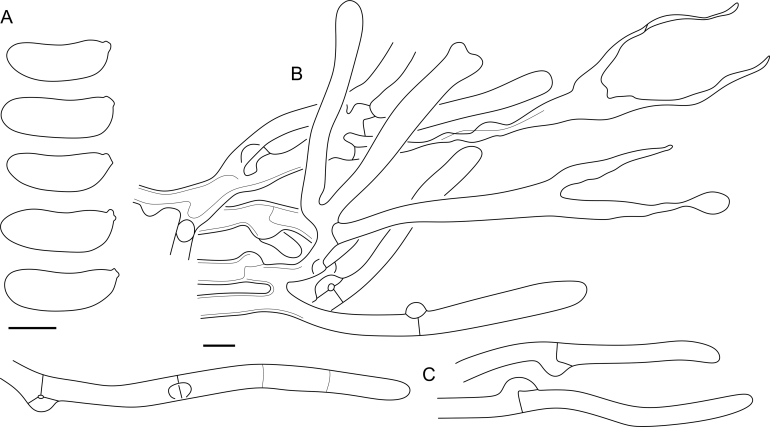
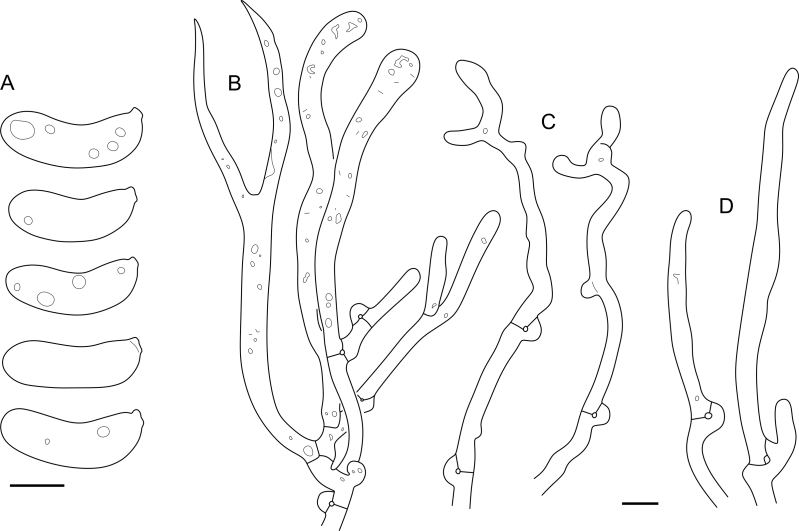
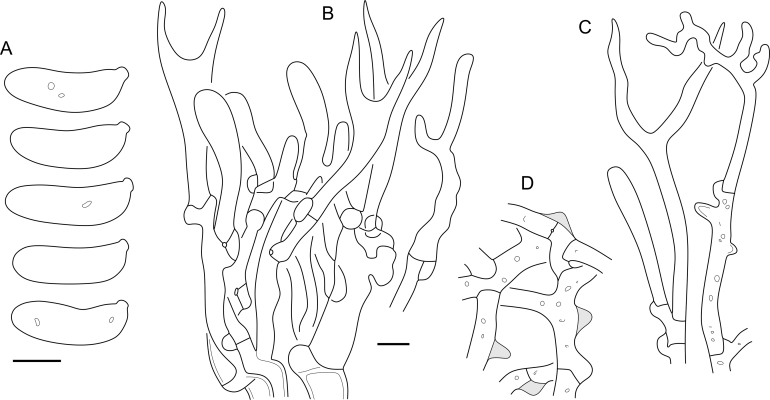
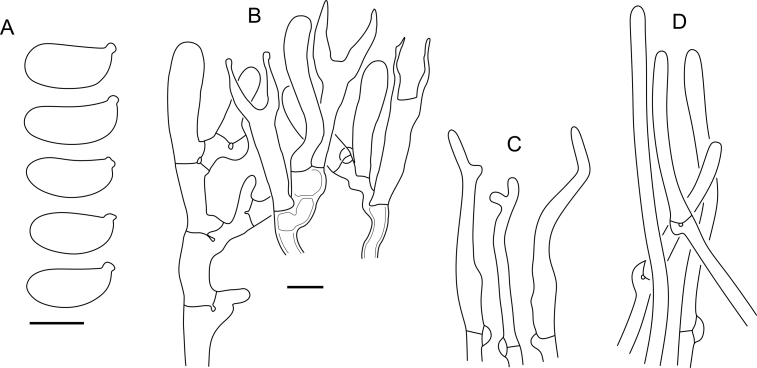
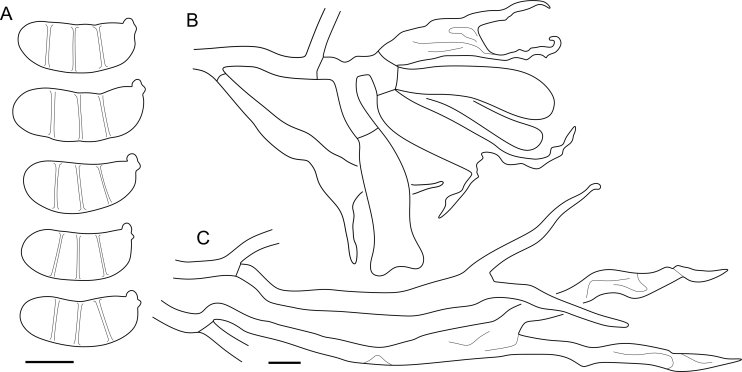
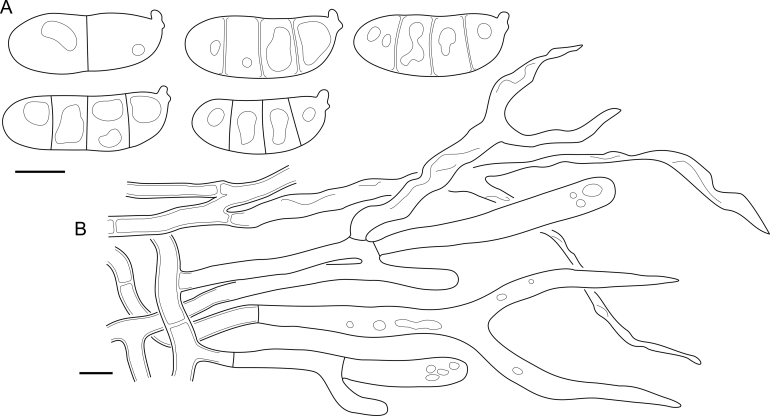
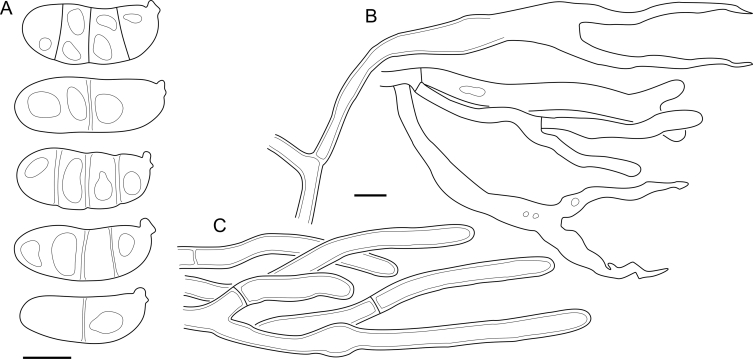
Cerinomyces tortus micromorphology. A. Spores. B. Hymenium with a single hyphidium, and subhymenium. C. Hyphidia. D. Marginal hyphae. All drawn from Dacrymyces punctiformis holotype, UPS:F-015301. Scale bars = 5 μm.
Fig. 38.
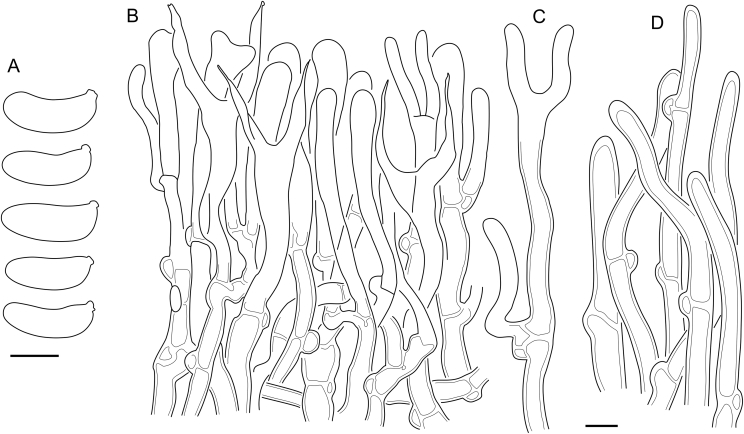
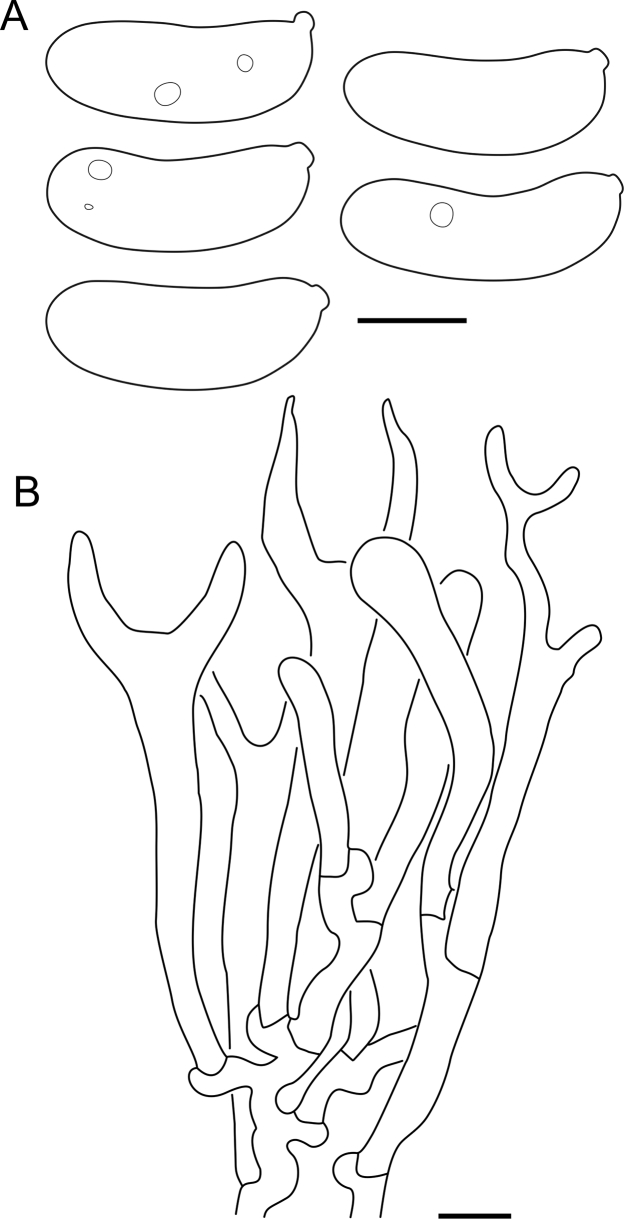
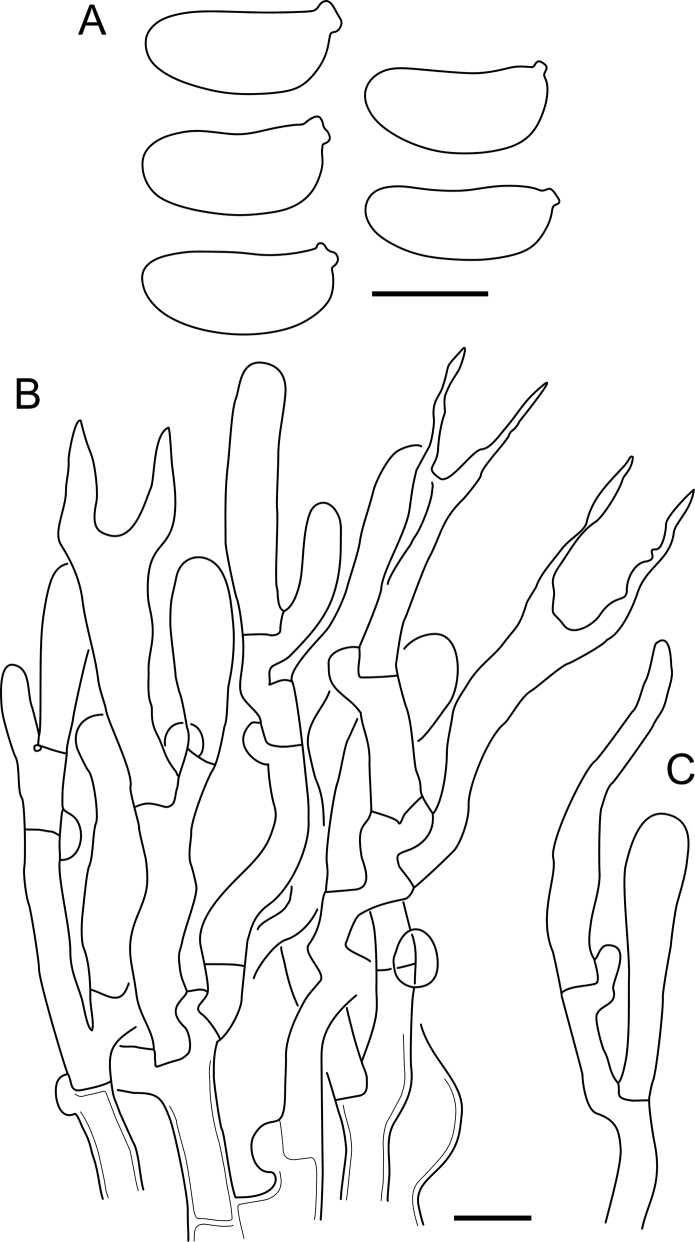
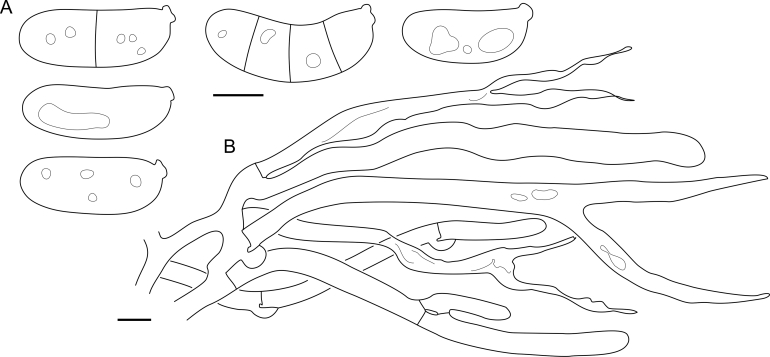
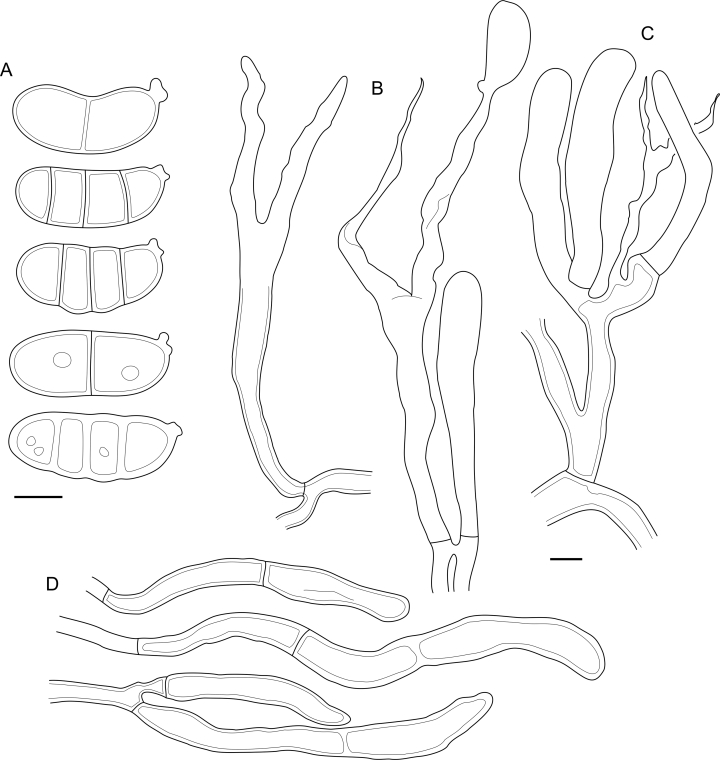
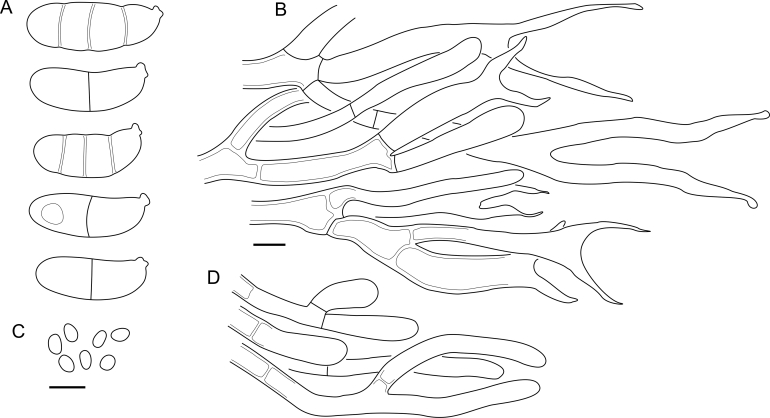
Cerinomyces neuhoffii micromorphology. A. Spores. B. Hymenium with hyphidia, and subhymenium. Drawn from TU135067 (A); CWU(MYC)6342 (B). Scale bars = 5 μm.
Taxa descriptions
Cerinomycetaceae Jülich, Bibliotheca Mycologica 85: 358 (1981).
A monotypic family in the class Dacrymycetes, consists of the genus Cerinomyces.
Cerinomyces G.W. Martin, Mycologia 41: 82 (1949).
Typus: Cerinomyces pallidus G.W. Martin, Mycologia 41: 83 (1949).
Description: Basidiocarps either arid and corticioid or gelatinous and pustulate, pulvinate, and resupinate; colour from white to ochraceous, pale yellow, brown, and dark brown. Basidiocarps monomitic, consist of hymenium and supporting structure (subiculum), some species have a distinct margin. Hymenium amphigenous, hymenial surface either smooth or in some corticioid species bears hyphal pegs. Hyphae transparent, smooth, hyphal width and wall thickness can vary inside a single basidiocarp, wall gelatinization occurs in many species, gelatinous layer sometimes roughened. Clamps present on each septum except occasional clampless secondary septa inside basidia. Clamps mostly simple, loop-like clamps can occur in subicular areas. Hymenium includes basidia and, in some species, hyphidia with thickened base and thinner cylindrical apical part, which is either branched or simple. Hyphidia of the second type tend to organize in hyphal pegs. Mature basidia long-clavate, with two subulate sterigmata. Basidia occasionally bear one or rarely three sterigmata. Basidiospores cylindrical to curved-cylindrical, aseptate or rarely with up to three transverse septa in few species, walls thin and smooth, contents usually hyaline, germination with germ tubes or subglobose to cylindrical conidia.
Habitat: decayed, usually decorticated wood of gymnosperm and angiosperm trees and shrubs. According to our observations, cause brown rot.
Distribution: Occur worldwide in forested areas, as follows (informal taxa not listed). Africa: C. albosporus. Asia: C. aculeatus, C. curvisporus, C. enatus, C. enterolaxus, C. nepalensis, C. pinguis. Australia and Oceania: C. fasciculatus, C. inermis, C. verecundus. Europe: C. aeneus, C. borealis, C. creber, C. crustulinus, C. lipoferus, C. neuhoffii, C. tortus, C. volaticus. North America: C. atrans, C. cokeri, C. enatus, C. favonius, C. fugax, C. hesperidis, C. pallidus, C. tristis. South and Central America: C. brevisetus, C. concretus, C. paulistanus, C. ramosissimus.
Cerinomyces aculeatus N. Maek., Canadian Journal of Botany 65 (3): 583 (1987). Fig. 9, Fig. 16.
Fig. 9.
Cerinomyces species with arid corticioid basidiocarps. A.C. aculeatus (holotype, TUMH61942). B.C. aff. aculeatus 1 (TNM:F16565). C.C. albosporus (holotype, LY11692). D.C. albosporus, cracks in basidiocarp showing dark hymenium and light subiculum (same specimen). E.C. atrans (holotype, GB-0071218). F.C. borealis (holotype, O160848). G. C. brevisetus (holotype, URM: Chikowski 1544). H. C. concretus (holotype, O:F-919450). I. C. curvisporus (isotype, TNS-F-11473). J. C. favonius (holotype, H7008893). Scale bars: A–C, E–J = 5 mm; D = 1 mm.
Fig. 16.
Cerinomyces aculeatus micromorphology. A. Spores. B. Hymenium and subhymenium. C. Group of hyphidia organizing in a microscopic peg. D. Apical part of a hyphal peg. Drawn from holotype, TUMH61942 (A); TU135070 (B, D); TNS-F-15706 (C). Scale bars = 5 μm.
Typus: Japan, Honshu, Chūgoku reg., Tottori pref., Kokufu-cho, Okamasu, on Pinus densiflora, 12 Jul. 1984, N. Maekawa (holotype TUMH61942! ex TMI794, ex-type culture TUFC50098∗).
Description: Basidiocarps arid, originate as patches of white mycelium, coalesce into seamless formations up to 5 cm in the longest dimension. Hymenial surface first arachnoid, then solid, light ochraceous to buff; subiculum light ochraceous, cottony; margin similar to subiculum or arachnoid if present. In older areas hymenium cracked, brownish. Basidiocarps covered with hyphal pegs up to 300 μm in length. Hyphae clamped, gelatinized, in subiculum 2–4(–5) μm in diam, with walls 0.5–1.2 μm in width. Subhymenial hyphae of the same diam, with walls 0.3–0.7 μm in width. Hyphal pegs consist of parallel hyphae, 2–5 μm in diam, walls 0.5–1.5 μm in width, thicker in the base and core of peg. Hymenium composed of basidia and hyphidia. Hyphidia with thick-walled clavate base up to 11 μm in diam and simple cylindrical thin-walled apical part; occasional or in groups developing into hyphal pegs. Basidia clavate, 12–46 × 3–7 μm, with sterigmata up to 29(–66) μm in length (n = 115/4), basidial walls sometimes thickened at the base. Basidiospores slightly curved-cylindrical, 0–1(–3)-septate, (9.7–)10.5–17.2(–17.7) × 4.0–6.5(–7.4) μm, L = 13.8 μm, W = 5.0 μm, Q = 2.8, Q’ = 1.7–3.5 (n = 121/4), walls ∼ 0.2–0.3 μm in width.
Habitat and distribution: Gymnosperm (Pinus) and perhaps angiosperm wood; East Asia.
Material examined: Japan, Honshu, Chūbu reg., Nagano pref., Sugadairakougen, on Pinus densiflora, 18 Aug. 2006, T. Shirouzu HNo.478 (TNS-F-21063); Kansai reg., Kyoto pref., Mt. Daimonji, on P. densiflora, 20 Apr. 2006, T. Shirouzu HNo.191 (TNS-F-15706∗). Russia, Primorsk reg., Kedrovaya Pad Nature Reserve, on angiosperm wood (?), Jun. 2016, I. Viner КЮН1422 (reads “KUN1422”, H7008652), КЮН2396 (TU135069∗), КЮН2417 (TU135070∗).
Notes: The species was synonymized with C. albosporus shortly after description (Maekawa & Zang 1997). The two species are related, but clearly different in the ITS sequences, basidiocarp appearance, spore size, and distribution. Maekawa (1987) provided a detailed study on the cultural characteristics of the species, showing that young basidiospores are binucleate, and when deposited on MEA, aseptate basidiospores develop one to four transverse septa before or rarely after germination. He also described curved-cylindrical conidia 3.6–5.6 × 1.2–1.6 μm produced from hyphae in monokaryotic culture and pictured basidiospores with conidiogenous scars. Shirouzu et al. (2009) reported subglobose conidia born from basidiospores, measuring 6 × 3 μm.
Cerinomyces aff. aculeatus 1. Fig. 9, Fig. 17.
Fig. 17.
Cerinomyces aff. aculeatus 1 micromorphology. A. Spores. B. Hymenium and subhymenium. All drawn from TNM:F16565. Scale bars = 5 μm.
Description: Basidiocarps at first arachnoid, then turning into smooth brownish thin film, waxy and semitransparent when wet, in better developed (or preserved) areas more arid, solid and light ochraceous. Hyphal pegs absent. Hyphae clamped, subicular hyphae 2–4 μm in diam, walls 0.3–0.5 μm in width; in subhymenium 2–3 μm in diam, walls 0.3–0.4 μm in width. Hymenium simple, basidia clavate, 11–20 × 3–4.5 μm, with sterigmata up to 23 μm in length (n = 30/1). Basidiospores cylindrical, slightly curved, 0–1-septate, 9.5–11.3(–11.5) × (3.2–)3.3–4.4(–4.5) μm, L = 10.5 μm, W = 3.7 μm, Q = 2.8, Q’ = 2.4–3.3 (n = 30/1), walls ∼ 0.2–0.3 μm in width. Basidiospores produce cylindrical conidia 2.2–2.6 × 1.2–1.3 μm.
Habitat and distribution: Gymnosperm wood (Pinus); East Asia.
Material examined: Taiwan, Taipei, Peitou dist., on Pinus luchuensis, 17 Jul. 1999, R. Kirschner 542 (TNM:F16565∗).
Notes: The taxon belongs to the C. albosporus clade by similarity in LSU sequences, but we postpone a formal description until more markers are sequenced or new material found. The most similar LSU sequence was obtained from a Japanese environmental sample 05151-1B2 (Shirouzu et al. 2016). For the same Taiwanese material, Kirschner & Yang (2005) reported sporothrix-like conidiogenesis in culture, with curved-cylindrical conidia < 5 μm in length.
Cerinomyces aeneus A. Savchenko, Miettinen & J.C. Zamora, sp. nov. MycoBank MB 839760. Fig. 11, Fig. 18.
Fig. 11.
Cerinomyces species with gelatinous basidiocarps in rewetted and dry state. A.C. aeneus, rewetted (TU135065). B.C. aeneus, dry (holotype, H7009708). C.C. cokeri, colourless morph, fresh (TU135089). D.C. cokeri, dry (holotype, NCU-F-0031543). E.C. creber, rewetted (UPS:F-946507). F.C. creber, dry (H:Trichies 07077). G. C. crustulinus, dry, well-developed basidiocarp (lectotype, PC0706688). H.C. crustulinus, dry, young basidiocarps (isolectotype, BPI726061). I.C. aff. crustulinus 1, rewetted, (UPS:F-958851). J.C. aff. crustulinus 1, dry (same specimen). Scale bars: A, C, D, E, H, I = 1 mm; B, F, G, J = 5 mm.
Fig. 18.
Cerinomyces aeneus micromorphology. A. Spores. B. Hymenium with hyphidia, and subhymenium. Drawn from holotype, H7009708 (A); TU135065 (B). Scale bars = 5 μm.
Typus: Ukraine, Transcarpathian reg., Mizhhirya dist., Synevir National Park, 300–400 m to the east from Synevir lake, on Fagus sylvatica, 9 Aug. 2010, O. Akulov & A. Ordynets (holotype H7009708∗!, isotypes CWU(MYC)7500!, UPS!).
Etymology: aeneus (Lat.) — bronze, referring to the colour of basidiocarps.
Description: Basidiocarps gelatinous, first pustulate, then coalesce into smooth to densely cerebriform resupinate formations up to several cm in the longest dimension, light brownish yellow at margins, reddish brown in the center, becoming almost black when dried. Some pustulate basidiocarps can remain isolated outside of these formations. Hyphae clamped, in subiculum (1.5–)2–3.5 μm in diam, walls 0.3–0.5 μm in width, gelatinized, sometimes roughened, often agglutinated. Subhymenial hyphae 1.5–3 μm in diam, walls ∼ 0.3 μm in width. Marginal hyphae simple cylindrical or slightly clavate up to 4 μm in diam, rarely branched, walls 0.3–0.5 μm in width. Hymenium includes abundant branched cylindrical hyphidia, in base 2–2.5 μm in diam, with apical part 1–2 μm in diam. Basidia clavate, 16–38 × 2–6 μm. Sterigmata up to 24(–35) μm in length (n = 57/3). Basidiospores cylindrical to slightly curved-cylindrical, 0(–1)-septate, at least some binucleate, (7.9–)8.1–11.2(–13.0) × (2.7–)3.0–4.5(–5.0) μm, L = 9.5 μm, W = 3.8 μm, Q = 2.5, Q’ = 1.9–3.5 (n = 176/5), walls ∼ 0.2 μm in width.
Habitat and distribution: Angiosperm wood (Alnus, Carpinus, Fagus, Quercus, and unident.); Europe.
Material examined: Czech Republic, Vysočina reg., Jihlava dist., near Třešť, Velký Špičák Nature Reserve, on Fagus sylvatica, 28 Oct. 2011, M. Brom (PRM929903), same loc., on angiosperm wood, 5 Jul. 2011, M. Brom (PRM929897), near Třešť, V Klučí Nature Reserve, loco “V Ohradě”, on angiosperm wood, 17 Nov. 2010, M. Brom (PRM929896), (PRM929907), 27 Oct. 2011 (PRM929891∗); Ústecký reg., Teplice dist., Vlčí důl Nature Reserve, Osek castle, on F. sylvatica, 27 Sep. 2014, M. Kříž (PRM934335∗). Finland, Uusimaa prov., Helsinki, Vantaanjokivarsi, Patola, on Alnus incana, 6 Nov. 2011, O. Miettinen 15065.2 (H6013355∗). Norway, Sogn og Fjordane co., Balestrand mun., Suphelledalen in Fjærland, on Alnus, 10 Sep. 2000, S. Evans (O146179∗). Sweden, Kalmar co., Öland, Mörbylånga mun., Södra Ottenby lund, 100 m SW of St. Finnslätten, on Quercus robur, 29 Aug. 2000, T. Knutsson 2000-06 (UPS:F-560919∗); Västra Götaland co., Uddevalla mun., Uddevalla, Rimnersvallen N, on Q. robur, 27 Dec. 2017, J. Olsson (UPS:F-946499∗). Ukraine, Ternopil reg., Zalishchyky dist., Dnistrovskyi Canyon National Park, between Dnister and Dzhuryn rivers, near Ustechko village, on Carpinus betulus, 5 Oct. 2016, V. Hukov 5-30, AS0077 (CWU(MYC), TU135065∗); Zakarpttia reg., Velykyi Bereznyi dist., Uzhanskyi National Park, near to Kaminy mountain, on F. sylvatica, 30 Jul. 2014, M. Kit (CWU(MYC)7035).
Notes: The species was traditionally identified as Dacrymyces enatus (≡ C. enatus). Cerinomyces aeneus develops resupinate basidiocarps and grows only on angiosperm, typically decorticated wood in Europe, while C. enatus produces mostly pulvinate or rarely resupinate basidiocarps and occurs on both angiosperm and gymnosperm wood with bark in North America and East Asia. Cerinomyces crustulinus and C. aff. crustulinus 1, similar European taxa growing on angiosperm substrates, have basidiocarps of different, lighter colour. Also, in contrast to C. enatus, in these species outlines of separate pustules remain visible even in mature basidiocarps.
Cerinomyces albosporus Boidin & Gilles, Bulletin de la Société Mycologique de France 102 (3): 318 (1986). Fig. 9, Fig. 19.
Fig. 19.
Cerinomyces albosporus micromorphology. A. Spores. B. Single full-sized basidium. C. Hymenium with short basidia, and subhymenium. D. Hyphidia. E. Apical part of a hyphal peg. All drawn from holotype, LY11692. Scale bars = 5 μm.
Typus: France, Réunion, Saint-Benoît dept., Forêt de Bébour, on Senecio ambavilla, 8 May 1985, J. Boidin (holotype LY11692∗!).
Description: Basidiocarps arid, originate as circular patches of white mycelium that develops into thick cottony subiculum. Above the subiculum, hymenial surface arachnoid to solid, light ochraceous to buff, covered with abundant hyphal pegs up to 500 μm in height; margin irregular, white, fimbriate. In well-developed areas surface cracks showing darker, brownish hymenium and subhymenium, and lighter subiculum. Hyphae clamped, densely packed, in subiculum 2–5 μm in diam, walls 0.5–1.0 μm in width; in subhymenium of the same diam, walls 0.4–0.8 μm in width. Hyphal pegs consist of parallel hyphae, 2.5–3 μm in diam, with wall width of 0.5 μm at the top and sides of peg, thicker at the base and in the core. Hymenium composed of basidia and occasional cylindrical hyphidia, simple or, rarely, branched at the apical part, walls thin, only thickened at the base; usually occurring in groups that develop into hyphal pegs. Basidia clavate, 25–62 × 3–6.5 μm, with sterigmata up to 20 μm in length (n = 30/1), basidial walls sometimes thickened at the base. Basidiospores slightly curved-cylindrical, 0–1(–3)-septate, (11.8–)12.0–17.8(–18.0) × (5.0–)5.2–7.0(–7.1) μm, L = 15.3 μm, W = 6.0 μm, Q = 2.6, Q’ = 1.8–3.3 (n = 34/1), spore walls ∼ 0.2–0.3 μm in width. Basidiospores bear cylindrical conidia 3.1–4.1 × 1.8–2.1 μm.
Habitat and distribution: Angiosperm shrub (Senecio); Africa (known only from the type locality).
Notes: The species has the most robust basidiocarps and the largest basidiospores among the peg-bearing Cerinomyces species. Three-septate basidiospores are extremely rare and most easy to find in substrate scrapes made next to basidiocarps.
Cerinomyces atrans A. Savchenko, sp. nov. MycoBank MB 839782. Fig. 9, Fig. 20.
Fig. 20.
Cerinomyces atrans micromorphology. A. Spores. B. Hymenium and subhymenium. C. A single unevenly gelatinized hypha followed from subiculum up to hymenium. Drawn from holotype, GB-0071218 (A, B); GB-0071217 (C). Scale bars = 5 μm.
Typus: Canada, British Columbia, Greater Vancouver, near the campus of University of British Columbia, on angiosperm wood, 16 Sep. 1982, N. Hallenberg 7309 (holotype GB-0071218∗!, isotype UBC:F1032!).
Etymology: atrans (Lat.) — darkening; due to a brown colour of well-developed basidiocarp areas.
Description: Basidiocarps arachnoid, arid and light ochraceous, in older areas smooth, solid, crustose, slightly waxy when wet, dark ochraceous to brown, especially in prominent or bruised areas; with thin subiculum and indistinct arachnoid margins. Hyphae clamped, subicular hyphae 2–3.5 μm in diam, walls ∼ 0.5 μm in width, gelatinous layer up to 4 μm in width; in subhymenium 2–3 μm in diam, walls ∼ 0.3 μm in width. Hymenium includes hyphidia, simple, cylindrical or with slightly thickened base, with apical part ∼ 2 μm in diam, up to 100 μm in total length; sometimes agglutinated, more abundant closer to margins. Basidia clavate, 13–24 × 3–4.5 μm, with sterigmata up to 21 μm in length (n = 40/2). Basidiospores cylindrical, slightly curved, aseptate, (6.7–)6.9–10.8(–11.1) × (2.3–)2.4–3.9(–4) μm, L = 8.8 μm, W = 3.1 μm, Q = 2.9, Q’ = 2.3–3.5 (n = 53/2), walls ∼ 0.2 μm in width.
Habitat and distribution: Angiosperm (Alnus and unident.) and possibly gymnosperm wood; Western North America.
Material examined: Canada, British Columbia, Vancouver Island, Juan de Fuca reg., Fairy lake near Port Renfrew, 12 Aug. 1988, N. Hallenberg 10674 (GB-0071217∗); British Columbia, Vancouver Island, Mesachie lake, on Alnus, 5–6 Aug. 1982, N. Hallenberg 7069 (GB-0180499∗). USA, Oregon, between Sweet Home and Cascadia, on gymnosperm wood, 20 Nov. 1937, A.M. Rogers & D.P. Rogers (NY: Herb. D.P. Rogers 396), Cascade Head Expt. Forest, Siuslaw National Forest, on Alnus, 11 Oct. 1972, M.J. Larsen (CFMR:FP-133356).
Notes: Older, prominent, and damaged areas of the studied basidiocarps have dark brown tints, but we suppose this character can be absent in young fungi. Compared to C. pallidus, another North American species inhabiting mostly angiosperm wood, C. atrans does not produce hyphal pegs visible to the naked eye, but only microscopic aggregations of hyphidia. When dealing with occurrences on gymnosperm wood, a great attention is needed to distinguish between C. atrans–C. pallidus and exclusively conifers-inhabiting North American species. However, basidiocarps of the latter group do not turn into darkened crust so easily, instead remaining arachnoid or firm-cottony.
An earlier published ITS sequence of a culture TUFC30545 aligns well with our sequences, though we did not examine its voucher material UBC6108.
Cerinomyces borealis Miettinen, Spirin & A. Savchenko, sp. nov. MycoBank MB 839783. Fig. 9, Fig. 21.
Fig. 21.
Cerinomyces borealis micromorphology. A. Spores. B. Hymenium and subhymenium. All drawn from holotype, O160848. Scale bars = 5 μm.
Typus: Norway, Hedmark co., Løten mun., near Ebru, on gymnosperm wood, 28 Aug. 1976, E. Høgholen 528/76 (holotype O160848∗!).
Etymology: borealis (Lat.) — northern.
Description: Basidiocarps appear as small patches, then coalesce into arachnoid, arid, at first white then light ochraceous or light grey mats up to 0.3 mm thick. Subiculum thin and indistinct; hymenium at first irregular, but in well-developed basidiocarps becomes smooth and solid; margin indistinct or absent. Dark brown to black underlying layers of old collapsed basidiocarps sometimes present. Hyphae clamped, in subiculum 2–4 μm in diam, walls ∼ 0.5 μm in width, with gelatinous layer up to 1 μm; in subhymenium 2–3 μm in diam, with walls ∼ 0.3 μm in width. Swollen up to 10 μm in width, thick-walled cells sometimes occur closer to substrate. Marginal hyphae simple, cylindrical, similar to subicular. Hymenium simple, basidia clavate, 9–21 × 3–6 μm, with sterigmata up to 14 μm in length (n = 46/5). Sometimes young basidioles are distinctly clavate. Basidiospores cylindrical, slightly curved, aseptate, (6.2–)7.1–11.1(–12.0) × (2.2–)2.3–3.4(–3.6) μm, L = 9.1 μm, W = 2.9 μm, Q = 3.2, Q’ = 2.2–3.9 (n = 112/5; 31 measured in 1 % KOH), walls ∼ 0.2 μm in width.
Habitat and distribution: Gymnosperm wood (Picea, Pinus, and unident.); Northern Europe.
Material examined: Finland, Etelä-Häme prov., Padasjoki mun., Vesijako Nature Reserve, on Picea abies, 2 Oct. 1984, K. Hjortstam 14504 (GB-0071224); Pohjois-Häme prov., Jyväskylä mun., Vuoritsalo, on Pinus sylvestris, 22 Jul. 2010, O. Miettinen 14094 (H6012704∗), Saarijärvi mun., Pyhä-Häkki National Park, on Pic. abies, 14 Aug. 2017, O. Miettinen 21156.1 (H∗); Kainuu prov., Kuhmo mun., Kuikkajärvi, Rytiniemi, on P. sylvestris, 14 Jun. 2015, H. Kotiranta 26863 (H6055125∗). Norway, Hedmark co., Løten mun., near Vesl-Bronken, PN 4232, 17 Sep. 1982, K. Hjortstam 13211 & E. Høgholen (GB-0071222), 13214 (GB-0071223), Stor-Elvdal mun., Fagervoll ved Atnsjøen, on P. sylvestris, 21 Aug. 1996, L. Ryvarden 39230 (O101812∗); Møre og Romsdal co., Aure mun., [N of] Lia, on P. sylvestris, 1 Jun. 2009, F. Oldervik 006.09 (O288478); Nordland co., Rana mun., Ørtfjellmoen, in Dunderlandsdalen, on Pic. abies, 1976, J. Hereng 375 (O101814), Ørtfjellmoen, on Pic. abies, 17 Aug. 1982, K.-H. Larsson 2771 (GB-0071221); Oppland co., Gausdal mun., Ormtjernkampen National Park, on Pic. abies, 3 Jul. 1975, I. Johansen 686/75 (O101811), Lunner mun., Rinilhaugen Nature Reserve, on Pic. abies, 17 Sep. 2016, V. Spirin 11136 (H), Øyer mun., Bårdsengbekken, on Pic. abies, 10 Sep. 1979, B. Bakke 1566 (O101809), 1581 (O101808); Oslo co., Sorkedalen, Svartor ved Kjellerberget, on Pic. abies, 28–29 Sep. 1977, K. Hjortstam 8929 & K.-H. Larsson (GB-0071220); Sør-Trøndelag co., Hemne mun., Gammelsetra, [up to] Rennsjøen, on P. sylvestris, 24 Oct. 2004, F. Oldervik 609.04 (O188052). Russia, Leningrad reg., Boksitogorsky dist., Kolp, on Pic. abies, 29 Jul. 2016, V. Spirin 10443 (H∗). Sweden, Dalarna co., Malung-Sälen mun., Lybergsgnupen, on Pic. abies, 7 Oct. 1982, T. Hallingbäck & K.-H. Larsson 3401b (GB-0071196); Jämtland County, Bräcke mun., between Stavre and lake Bodsjön, on gymnosperm wood, 29 Jul. 1958, J. Eriksson 8194 (GB-0071198), 30 Jul. 1958, J. Eriksson 8195 (GB-0071197); Jönköping co., Vaggeryd mun., E of Kacklesjön (NE of Marieholm), on Pic. abies, 1 Nov. 1981, T. Hallingbäck (GB-0071192); Norrbotten co., Jokkmokk mun., S of Muddus national park, near Rimojokk, on P. sylvestris, 13 Aug. 1958, J. Eriksson 8658 (GB-0071199); Värmland co., Forshaga mun., Pannkakan, on gymnosperm wood, 6 Oct. 1982, T. Hallingbäck & K.-H. Larsson 3226 (GB-0071195); Västerbotten co., Sorsele mun., Grannäs, västra Lairobäcken, on gymnosperm wood, 29 Aug. 1983, K.-H. Larsson 4194 (GB-0071200); Västernorrland co., Sollefteå mun., Junsele parish, Storhögen, S of the national forest area Ulfvik, on P. sylvestris, 22 Sep. 1970, K. Hjortstam 4408 (GB-0071213); Västra Götaland co., Alingsås mun., Gräskärr, Simmenäshalvön, on Pic. abies, 18 Oct. 1992, K. Hjortstam 17423 (GB-0087175), S of Stora Hyggesjön, on Pic. abies, 16 Jul. 1972, K.-H. Larsson 754b (GB-0071204), W side of lake Lille Trän, on Pic. abies, 14 Oct. 1971, K.-H. Larsson 545 (GB-0071203), Bengtsfors mun., Tisselskog par., W side of lake Råvarp, close to a small stream, on Pic. abies, 30 Sep. 1972, A. Hjortstam & K. Hjortstam 8553 (GB-0071194), Bollebygd mun., Töllsjö, Sjögaredsbergen, on Pic. abies, 23 Aug. 1969, K. Hjortstam 1526 (GB-0071202, GB-0071212, TAAM099151), SW from Kolsjöhatt, on P. sylvestris, 8 Jul. 1969, K. Hjortstam 1408 (GB-0071208), Borås mun., NE of Hemsjön lake, on Pic. abies, 10 Sep. 1969, K. Hjortstam 2115/a (GB-0071207), Grästorp mun., Hunneberg Nature Reserve, Jonstorpsmossen, on Pic. abies, 1 Oct. 1978, K.-H. Larsson 2414 (GB-0071211), Karlsborg mun., Undenäs par., "Trollkyrkoreservatet" (Tivedens National Park?), on Pic. abies, 19 Aug. 1977, K. Hjortstam 8553 & J. Ginns (O101815).
Notes: Cerinomyces borealis is a relatively widespread species in Northern Europe. In Nordic herbaria, the majority of the specimens labelled as C. crustulinus belong to C. borealis — e.g., GB-0071198, cited as such in “The Corticiaceae of North Europe” (Eriksson & Ryvarden 1973). The species can be distinguished from similarly looking C. volaticus by longer, thinner basidiospores (mostly ≤ 3.0 μm in width) and the more delicate, arachnoid basidiocarps. Intermediate forms with large basidiospores also occur, but even then, higher Q values of C. borealis still hold.
In three specimens identified as C. borealis chiefly by the thin basidiospores, we found hyphidia: long cylindrical with thickened bases, typical for the C. pallidus clade (GB-0071209), and branched, either only slightly (GB-0071221), or with knot-like heads, more characteristic for some gelatinous Cerinomyces species (GB-0071210). Sequencing attempts of these specimens were unsuccessful.
Cerinomyces brevisetus Chikowski, Alvarenga & A. Savchenko, sp. nov. MycoBank MB 839784. Fig. 9, Fig. 22.
Fig. 22.
Cerinomyces brevisetus micromorphology. A. Spores. B. Hymenium and subhymenium. C. Apical part of a hyphal peg. All drawn from holotype, URM:Chikowski 1544. Scale bars = 5 μm.
Typus: Brazil, Pernambuco, Igarassu mun., Refugio Ecológico Charles Darwin, 12 May 2017, R. Chikowski 1544 (holotype URM∗!, isotypes H!, TU135139!).
Etymology: brevi (Lat.) — short; seta (Lat.) — bristle-like organ, peg herein.
Description: Basidiocarps arid, at first arachnoid, later mature areas become crustose, covering thin cottony subiculum; hymenial surface solid, light ochraceous, with regular brownish hyphal pegs up to 80 μm in height. Hyphae clamped, subicular hyphae 2–3 μm in diam, walls 0.5–1.0 μm in width; in subhymenium 2–4 μm in diam, walls ∼ 0.3 μm in width. Pegs rooted in subiculum, their hyphae parallel and densely arranged, core hyphae similar to subicular, outer hyphae 3.5–4.5 μm in diam, walls 0.3–0.4 μm in width. Hymenium includes rare simple hyphidia with cylindrical or obclavate base and cylindrical long tip; scattered or organized in microscopic pegs. Basidia clavate, 12–23 × 3–5.5 μm, with sterigmata up to 19 μm in length (n = 30/1). Basidiospores cylindrical, slightly curved, aseptate, 9.3–11.1(–11.7) × 3.5–4.4(–4.6) μm, L = 10.3 μm, W = 3.9 μm, Q = 2.6, Q’ = 2.4–2.9 (n = 30/1), walls ∼ 0.2–0.3 μm in width.
Habitat and distribution: Unidentified wood; South America (known only from the type locality).
Notes: Cerinomyces brevisetus has the shortest pegs in the genus, which helps to differentiate it from the closest relatives in the C. albosporus group, as well as from the similarly looking C. pallidus and C. paulistanus. In addition, all other members of the C. albosporus clade have longer basidiospores, while the C. pallidus clade is characterized by the shorter ones.
Cerinomyces canadensis (H.S. Jacks. & G.W. Martin) G.W. Martin, Mycologia 41: 85 (1949). Fig. 14, Fig. 23.
Fig. 14.
Basidiocarps of corticioid species related to Femsjonia, in dry state unless otherwise specified. A. “Cerinomyces” canadensis (H:Spirin 8468). B.Dacrymyces confluens (lectotype of Ceracea aureofulva, H7009712). C.D. corticioides (syntype, NY00738305). D.D. corticioides, fresh (holotype of Femsjonia uniseptata, TNS-F-54019). Scale bars = 5 mm.
Basionym: Ceracea canadensis H.S. Jacks. & G.W. Martin, Mycologia 32: 693 (1940).
Typus: Canada, Ontario, Port Alexander, on gymnosperm wood, 13 Sep. 1939, H.S. Jackson (holotype TRTC14103).
Description: Basidiocarps corticioid, of variable shapes, cover up to several cm in the longest dimension, with cottony thick white subiculum and fimbriate white margins. In younger areas margins indistinct, arachnoid. Hymenial surface smooth and solid, waxy-gelatinous when fresh, crust-like when dried, from yellow to deep orange colour, in old collections bleaks to pale ochraceous. Hyphae clamped, pseudoclamps occasionally present, subicular hyphae 2–4 μm in diam, walls 0.2–0.3 μm in width; in subhymenium 2–3.5 μm in diam, walls of the same width; marginal hyphae cylindrical, similar to subicular. Hymenium consists of clavate basidia 19–43 × 3–6 μm, with sterigmata up to 31 μm in length (n = 54/3). Basidiospores cylindrical to slightly curved-cylindrical, often with conspicuous lipid droplets, 0–1-septate, (8.5–)9.2–14.7(–16.5) × (4.1–)4.3–6.2(–6.8) μm, L = 11.7 μm, W = 5.1 μm, Q = 2.3, Q’ = 1.6–3.2 (n = 126/4; 75 measured in 1 % KOH), walls ∼ 0.2 μm in width.
Habitat and distribution: Gymnosperm wood (Abies, Picea, Tsuga, and unident.); East Asia and North America.
Material examined: Russia, Krasnoyarsk reg., Yenisey dist., E of mouth of the river Kolchim, on gymnosperm wood, 14 Aug. 1958, E. Parmasto (TAAM007082∗); Primorsk reg., Chuguyev dist., Lesosetshnaya river, 9 km upstream from Bulyga-Fadejevo / Sandagou village, on Abies nephrolepis, 7 Sep. 1961, E. Parmasto (TAAM016177); Sakhalin, Tymovsky dist., Nabili Mountains, in the walley of Pilenga river, on Picea jezoensis, 22 Aug. 1970, B. Kullman & A. Raitviir (TAAM061880∗). USA, Idaho, Bonner co., Trapper Creek, on Tsuga heterophylla, 14 Oct. 2014, V. Spirin 8468 (H∗, UPS).
Notes: The species does not belong to Cerinomyces s.s., but we postpone raising a new synonym (see Discussion). The type material could not be located in TRTC hebarium (S. Margaritescu, 15 Sep. 2016, pers. comm.), but morphology of the studied specimens agrees well with the original concept of Ceracea canadensis. It can be confused with Dacrymyces corticioides, though the latter species possesses larger microstructures, basidiospores with up to three septa and basidiocarps of well-defined circular form. Maekawa (1987) reported ovoid to straight cylindrical conidia born from hyphae in a monokaryotic strain of C. canadensis (CCCM 0194), but we did not check identity of this strain.
Under pseudoclamp we understand a clamp which development was arrested before the terminal part of a clamp fused with the parental hypha (upper Fig. 23 C, three rightmost pictures on Fig. 23 D, Clémençon et al. 2004). The character is rather rare in the species but was found in all studied specimens. Sequences from TAAM007082 and TAAM061880 were not used in the analyses, but they are similar to the ITS of Spirin 8468.
Cerinomyces cokeri (McNabb) A. Savchenko & J.C. Zamora, comb. nov. MycoBank MB 839809. Fig. 11, Fig. 24.
Fig. 24.
Cerinomyces cokeri micromorphology. A. Spores. B. Hymenium and a single hyphidium. Drawn from holotype, NCU-F-0031543 (A); NCSLG21222 (B). Scale bars = 5 μm.
Basionym: Dacrymyces cokeri McNabb, New Zealand Journal of Botany 11: 475 (1973).
Synonym: Dacrymyces pallidus Coker, Journal of the Elisha Mitchell Scientific Society 35: 171 (1920) nom. illeg., not D. pallidus Lloyd, 1919.
Typus: USA, North Carolina, Orange co., Chapel Hill, on gymnosperm wood, 4 Feb. 1920, J.N. Couch 4072 (holotype NCU-F-0031543!).
Description: Basidiocarps gelatinous, of irregular shapes, pustulate or cerebriform with thin resupinate margins, up to 15 mm in the longest dimension, pale yellow when fresh, light brown at margins to brown at middle, dark brown when dried, often erumpent through bark. Hyphae clamped, in subiculum 1.5–2.5 μm in diam, walls 1.5–3.0 μm in width together with gelatinous layer; small knots of ramificated hyphae rarely occur; subhymenial hyphae of the same diam, walls 0.2–0.3(–0.5) μm in width; margins covered with simple cylindrical or slightly clavate anastomosing hyphae 1.5–2.5 μm in diam, walls 0.2–0.5 μm in width. Hymenium includes dendroid cylindrical hyphidia with base 2–3.5 μm in diam and apical part 1–2 μm in diam; up to 40 μm in total length. Basidia clavate, 36–60 × 3–6 μm. Sterigmata up to 34 μm in length (n = 30/1). Basidiospores cylindrical to slightly curved-cylindrical, 0(–1)-septate, at least some binucleate, (9.6–)9.7–12.8(–13.0) × (3.4–)3.5–4.6(–5.0) μm, L = 11.2 μm, W = 4.0 μm, Q = 2.8, Q’ = 2.3–3.2 (n = 55/2), walls ∼ 0.2 μm in width.
Habitat and distribution: Gymnosperm wood (Pinus and unident.); North America.
Material examined: Canada, Ontario, Ottawa, on Pinus resinosa, 27 Jan. 2020, I. Khomenko 2020-034 (UPS:F-979575∗, TU135089). USA, North Carolina, Wake co., Raleigh, Lake Johnson Nature Park, on Pinus taeda, 28 Oct. 1972, J.A. Menge 396 (NCSLG21222∗).
Notes: Cerinomyces cokeri can be confused with C. enatus, both occuring on coniferous wood with bark. Cerinomyces enatus is generally darker coloured, becoming almost black in dry conditions, while C. cokeri remains dark brown. Also, we did not find in C. cokeri swollen hyphal compartments and such thick gelatinous layer on hyphae as in C. enatus.
Cerinomyces concretus A. Savchenko, sp. nov. MycoBank MB 839785. Fig. 9, Fig. 25.
Fig. 25.
Cerinomyces concretus micromorphology. A. Spores. B. Hymenium with hyphidia. C. Subhymenial hyphae and swollen cells. All drawn from holotype, O:F-919450. Scale bars = 5 μm.
Typus: Colombia, Magdalena, Santa Marta, Park Sierra Nevada de Santa Marta, Reserva Forestal San Lorenzo, 17–19 Jun. 1978, L. Ryvarden (holotype O:F-919450∗!).
Etymology: concretus (Lat.) — grown together, condensed. Reference to a dense hymenium becoming crustose.
Description: Basidiocarps arid, crustose, slightly gelatinous when rewetted, with thin subiculum and indistinct margins, hymenial surface smooth and solid but cracking in older areas, of pale ochraceous to yellowish brown colour. Hyphae clamped, in subiculum loose, 1.5–3(–3.5) μm in diam, walls 0.3–0.4(–0.8) μm in width, covered with small crystals (≤ 1 μm) that can cluster in amorphic masses. Subhymenial hyphae of the similar type, densely packed, with occasional swellings. Hymenium includes simple hyphidia of total length up to 70 μm, with thickened clavate base 4–5 μm in diam, and apical cylindrical part 2 μm in diam, up to 50 μm in length, occasionally branched or incrusted with minute crystals. Basidia clavate, 15–28 × 3–6 μm. Sterigmata up to 11(–19) μm in length, widely spaced (n = 30/1). Basidiospores slightly curved-cylindrical, aseptate, (7.7–)7.8–10.5(–12.0) × (3.3–)3.4–4.5(–5.0) μm, L = 9.0 μm, W = 4.0 μm, Q = 2.2, Q’ = 1.9–3.0 (n = 30/1), wall ∼ 0.2–0.3 μm in width.
Habitat and distribution: Unidentified wood; Central America.
Material examined: Venezuela, La Silla, boundary between Distrito Federal and Estado Miranda, 22 Aug. 1975, A.E. Liberta & A.J. Navas 22-43, ex ILL1499 (BPI1106573).
Notes: Comparing to other arid Cerinomyces, C. concretus is characterized by the large basidiospores. Two species with spore W > 3.5 μm, C. pinguis and C. verecundus, do not overlap with C. concretus in distribution. The type specimen has a slight meruloid pattern under hymenium that may be an evidence of an old, overgrown basidiocarp. A lone 1-septate spore was found in the type.
Cerinomyces creber J.C. Zamora, A. Savchenko, Trichies & Olariaga, sp. nov. MycoBank MB 839786. Fig. 11, Fig. 26.
Fig. 26.
Cerinomyces creber micromorphology. A. Spores. B. Hymenium with short basidia and subhymenium. C. Marginal hyphae. D. Hymenium with full-sized basidia, hyphidia, and subhymenium. All drawn from H:Trichies 07077. Scale bars = 5 μm.
Typus: Spain, Castilla–La Mancha comm., Toledo, pinar de la Bastida, on Cupressus arizonica, date unknown, J. De Esteban (holotype UPS:F-946512∗!).
Etymology: creber (Lat.) — close, numerous; referring to masses of coalescing basidiocarps.
Description: Basidiocarps gelatinous, pustulate, up to 1 mm in diam, readily coalescing with outlines of separate basidiocarps remaining visible. Young basidiocarps pale yellow to pale cream, later light brown to greyish brown, becoming brown and dark brown when dried. Internal or marginal areas can look opalescent in well-developed basidiocarps; margin becomes fimbriate upon drying. Hyphae clamped, subicular hyphae 2.5–4.5 μm in diam, walls 0.4–0.8 μm in width; in subhymenium 2–3.5 μm in diam, walls 0.2–0.8 μm in width. Hymenium includes dendroid cylindrical hyphidia with thickened base 2–2.5 μm in diam and branching apical part 1.5–2 μm in diam; up to 50 μm in total length, from rare to abundant in different areas. Basidia clavate, rarely asymmetric, 14–36 × 3–5 μm, with sterigmata up to 18 μm in length (n = 55/2). Basidiospores cylindrical, slightly curved, aseptate, binucleate, (7.4–)7.7–11.0(–12.8) × (2.5–)2.8–4.0(–4.2) μm, L = 9.4 μm, W = 3.3 μm, Q = 2.9, Q’ = 2.4–3.6 (n = 61/2), walls ∼ 0.2 μm in width.
Habitat and distribution: Gymnosperm wood (Cupressus, Juniperus, and unident.); Western and South-Western Europe.
Material examined: France, Moselle dept., Neufchef, carreau de l'ancienne mine de fer du Conroy, on gymnosperm plywood, 7 Mar. 2007, G. Trichies 07077 (H∗). Spain, Castilla–La Mancha comm., Guadalajara prov., Tamajón, near ermita de la Virgen de los Enebrales, on Juniperus thurifera, 28 Dec. 2019, J.C. Zamora & al. (UPS:F-979574∗); Madrid comm., Hoyo de Manzanares, collado del Portacho, on J. oxycedrus, 4 Jan. 2018, I. Olariaga (UPS:F-946506∗), Hoyo de Manzanares, cerro Camorrila, on J. oxycedrus, 8 March 2018, I. Olariaga (UPS:F-946507∗).
Notes: Cerinomyces creber is similar to C. neuhoffii in the branched hyphidia and tendency to develop dense coalescing basidiocarp groups, but the former species has smaller basidiospores, slightly smaller basidia, and often more light-coloured basidiocarps that coalesce more readily. Also, it seems that C. creber is confined to Cupressaceae wood (according to the substrates we were able to identify), while all studied specimens of C. neuhoffii were found on Pinus wood. Cerinomyces aff. crustulinus 1 resembles C. creber by macro- and micromorphology but represents a distinct taxon with even paler basidiocarps growing on angiosperm wood. Sequence of UPS:F-979574 was produced during the revision of this study; it was not used in the analyses but matches other included sequences.
Cerinomyces crustulinus (Bourdot & Galzin) G.W. Martin, Mycologia 41: 85 (1949). Fig. 11, Fig. 27.
Fig. 27.
Cerinomyces crustulinus micromorphology. A. Spores. B. Hymenium and subhymenium. C. Single basidium. D. Marginal hyphae. All drawn from isotype, BPI726061. Scale bars = 5 μm.
Basionym: Ceracea crustulina Bourdot & Galzin, Bulletin de la Société Mycologique de France 39: 266 (1923).
Typus: France, Gard dept., Saint-Guiral, on Fagus, 6 May 1910, A. Galzin 5793, H. Bourdot herb. (?) n. 7352 (lectotype PC0706688!, isolectotype BPI726061!).
Description: Basidiocarps gelatinous or waxy-gelatinous, originate as small gregarious pustules, coalesce into thin resupinate conglomerate with outlines of separate basidiocarps remaining visible, ochraceous to dull orange. Margin and subiculum become fimbriate yellowish white when dry. Hyphae clamped; subicular hyphae 2.5–4 μm in diam, walls 0.5–1.0 μm in width; subhymenial hyphae 2–3(–5) μm with walls 0.6–0.8(–1.0) μm in width; marginal hyphae resembling subicular, with cylindrical or slightly clavate endings. Hymenium simple, occasional hyphidia cylindrical. Basidia clavate, 16–38 × 2.5–5 μm with two sterigmata up to 28 μm in length (n = 60/2). Basidiospores cylindrical, slightly curved, aseptate, (7.4–)7.7–13.4(–14.0) × 2.8–4.0 μm, L = 9.8 μm, W = 3.3 μm, Q = 3.0, Q’ = 2.1–3.6 (n = 58/2), walls ∼ 0.2 μm in width.
Habitat and distribution: Angiosperm wood (Fagus); Europe (known only from the type locality).
Notes: No dendroid hyphidia were observed in contrast to an earlier report (McNabb 1964). We assume hyphidia were unevenly distributed in basidiocarps and destroyed with past preparations. Among the species described in this revision, the closest morphological relative to C. crustulinus is resupinate C. aeneus that also inhabits angiosperm wood in Europe, but differs in darker cerebriform-resupinate basidiocarps with reddish tint and abundant branched hyphidia. The extremely scanty type material and absence of fresh collections that fully agree with our observations prevent resolution of the exact position of C. crustulinus (but see C. aff. crustulinus 1 below). Roberts (2006) reported the species from Jamaica, and while it does resemble C. crustulinus, the robust gelatinous discoid to coalescing brown basidiocarps with light margins, abundant branched hyphidia, larger spores (11.4–)11.8–13.8(–14.5) × 4.0–5.2(–5.7) μm place it closer to either C. enatus or even members of the Dacrymycetaceae.
Cerinomyces aff. crustulinus 1. Fig. 11, Fig. 28.
Fig. 28.
Cerinomyces aff. crustulinus 1 micromorphology. A. Spores. B. Hyphidia. C. Hymenium and subhymenium. D. Marginal hyphae. All drawn from UPS:F-958851. Scale bars = 5 μm.
Description: Basidiocarps gelatinous, pustulate and gregarious, < 1 mm in diam, readily coalescing into resupinate conglomerate with shapes of separate basidiocarps remaining visible, from yellowish white to light yellow when fresh, light brown when dry. Margin slightly opalescent when fresh and fimbriate when dry. Hyphae clamped; subicular and subhymenial hyphae similar, 2–3.5 μm in diam, walls 0.2–0.3(–0.5) μm in width with gelatinous layer up to 1.5 μm in width; marginal hyphae slightly clavate, sometimes with thinner rarely branching apical part. Hymenium includes abundant dendroid cylindrical hyphidia with base either thickened to 2–4 μm in diam or of the same width as apical branching part of 1.5–2 μm in diam; up to 60 μm in total length. Basidia clavate, 20–49 × 3–5 μm, walls often thickened towards the base, with sterigmata up to 23 μm in length (n = 40/1). Basidiospores cylindrical, slightly curved, 0(–1)-septate, at least some apparently binucleate, (8.4–)8.6–10.9(–11.6) × (3.4–)3.4–4.2(–4.2) μm, L = 9.8 μm, W = 3.7 μm, Q = 2.7, Q’ = 2.3–3.0 (n = 45/1), walls ∼ 0.2 μm in width.
Habitat and distribution: Angiosperm wood; Europe.
Material examined: Spain, Asturias comm., Somiedo, Coto de la Buena Madre, on angiosperm wood, 2 Jun. 2018, E. Rubio 7557 (UPS:F-958851∗).
Notes: The taxon is the closest match to C. crustulinus except for difference in branched hyphidia (but see commentaries on C. crustulinus above about previous report of hyphidia in this species). In absence of additional material, we are inclined to keep this specimen and the type material of C. crustulinus as two separate taxa.
Cerinomyces curvisporus N. Maek. & M. Zang, Mycotaxon 61: 344 (1997). Fig. 9, Fig. 29.
Fig. 29.
Cerinomyces curvisporus micromorphology. A. Spores. B. Hymenium and subhymenium. All drawn from isotype, TNS-F-11473. Scale bar = 5 μm.
Typus: China, Yunnan, Lijiang co., Ganhaizi, at the foot of Yulong mountain, on Pinus densata, 16 Sep. 1993, N. Maekawa 93091618 (holotype HKAS27392!, isotypes TMI-18048, TNS-F-11473!).
Description: Basidiocarps arid, arachnoid on margins and solid in well developed areas, subiculum thin, hymenial surface light ochraceous, covered with regular hyphal pegs up to 300 μm in height. Hyphae clamped, in subiculum 2–3(–5) μm in diam, walls 0.5–1.0 μm in width, with minute crystals; in subhymenium 2.5–5 μm in diam, walls 0.3–0.5 μm in width. Internal hyphae in hyphal pegs parallel, similar to subicular, 3.5–5 μm in diam, walls 0.5–1.0 μm in width; at the top 3–5 μm in diam, walls 0.3–0.5 μm in width. Hymenium consists of clavate basidia 41–77 × 6–10 μm, with sterigmata up to 34 μm in length (n = 30/1), some probasidia have thickened walls in upper parts. Basidiospores strongly curved-cylindrical, 0(–3)-septate, (16–)16.3–20.0(–20.4) × 5.9–7.1(–7.5) μm, L = 18.4 μm, W = 6.5 μm (n = 30/1), spore walls ∼ 0.2–0.3 μm in width.
Habitat and distribution: Gymnosperm wood (Pinus); Southeast Asia (known only from the type locality).
Notes: The species is easy to recognize by large, strikingly curved basidiospores. Sequencing was not attempted because the basidiocarp was difficult to separate from another, overgrown corticioid fungus. Judging from abundance of hyphal pegs, C. curvisporus belongs to the C. albosporus group. Septate basidiospores are extremely rare, 1-septate ones were reported in the original description (Maekawa & Zang 1997), and in addition we found a single collapsed 3-septate spore. The longest spore dimension was measured as length. Q values are not shown, being not comparable to other Cerinomyces species with straighter basidiospores.
Cerinomyces enatus (Berk. & M.A. Curtis) A. Savchenko, comb. nov. MycoBank MB 839812. Fig. 12, Fig. 30.
Fig. 12.
Cerinomyces species with gelatinous basidiocarps in rewetted and dry state. A.C. enatus, rewetted (H:Spirin 7774). B.C. enatus, dry (same specimen), highlight: dissected basidiocarp with white core exposed. C.C. enterolaxus, rewetted (TNS-F-15723). D.C. enterolaxus, dry (TNS-F-61319). E.C. hesperidis, rewetted (holotype, NY01782362). F.C. hesperidis, dry (same specimen). Scale bars: A, C, E = 1 mm; B, D, F = 5 mm.
Fig. 30.
Cerinomyces enatus micromorphology. A. Spores. B. Subicular hyphae with roughened gelatinous layer. C. Hymenium with hyphidium, and gelatinized subhymenium with swellings. All drawn from H:Spirin 7774. Scale bars = 5 μm.
Basionym: Tremella enata Berk. & M.A. Curtis, Grevillea 2 (14): 20 (1873).
Synonyms: Dacrymyces enatus (Berk. & M.A. Curtis) Massee, Journal of Mycology 6 (4): 182 (1891).
Dacrymyces fuscominus Coker, Journal of the Elisha Mitchell Scientific Society 35: 171 (1920).
Arrhytidia enata (Berk. & M.A. Curtis) Coker, Journal of the Elisha Mitchell Scientific Society 43: 237 (1928).
Dacrymyces gangliformis Brasf., Lloydia 3: 105 (1940).
Typus: USA, South Carolina, Darlington co., Society Hill, on Quercus, Jan. 1850, H.W. Ravenell 2456 (lectotype [designated here]: FH00596873!, isolectotypes K(M):94215!, K(M):141181!). MycoBank typification MBT10001414.
Description: Basidiocarps gelatinous, variable in shape, pulvinate when young, in maturity most often pustulate, but also slightly cerebriform or flat and resupinate, up to 3(–4) mm in diam, from reddish brown when fresh to dark brown and black when dried; margins of resupinate basidiocarps light yellow and semitransparent. Basidiocarps often grow through holes in bark; when growing on decorticated wood can develop short stalk or, inversely, become resupinate. Light subicular core visible through darker hymenium in well-developed basidiocarps. Hyphae clamped, in subiculum 2–5 μm in diam, walls 0.5–1.0 μm in width, with gelatinous layer up to 6 μm in width, agglutinated, often deeply roughened. Swollen cells abundant in upper subiculum of well-developed basidiocarps, up to 20 μm in diam, with thickened roughened walls. Subhymenial hyphae 2–3.5 μm in diam, walls 0.3–0.5 μm in width. Sterile surfaces and margins covered with simple cylindrical or attenuating hyphae. Hymenium includes abundant branched cylindrical hyphidia with base ∼3 μm in diam and apical part 1–2 μm in diam; up to 30 μm in total length. Occasional simple, not branched hyphidia up to 50(–100) μm in length present, single or in peg-like groups. Basidia clavate, 13–56 × 3–6.5 μm, with walls 0.3–0.5 μm in width. Sterigmata up to 27 μm in length (n = 208/9). Basidiospores cylindrical to slightly curved-cylindrical, 0(–1)-septate, (7.0–)7.4–13.2(–14.2) × (2.7–) 2.9–4.5(–5.4) μm, L = 9.5 μm, W = 3.5 μm, Q = 2.7, Q’ = 1.7–3.8 (n = 196/9), walls ∼ 0.2 μm in width. Basidiospores bear ellipsoid to cylindrical conidia 3.0–4.0 × 1.5–1.7 μm.
Habitat and distribution: Angiosperm (Alnus, Castanopsis, Clethra, Quercus, Rhododendron, and unident.) and gymnosperm (Pinus) wood; East Asia, North America.
Material examined: Japan, Honshu, Chūbu reg., Nagano pref., Shioda, on Pinus densiflora, 27 Sep. 2006, T. Shirouzu (same coll. for all Japanese collections) HNo.505 (TNS-F-21064∗), Sugadairakougen, on P. densiflora, 7 Nov. 2016, HNo.1169 (TNS-F-88754∗), same loc. and substrate, 3 Sep. 2018, HNo.1212 (TNS-F-88777∗); Kansai reg., Kyoto pref., Mt. Daimonji, on P. densiflora, 20 Apr. 2006, HNo.199 (TNS-F-21034∗), Takaragaike, on Clethra barbinervis, 21 Apr. 2006, HNo.208 (TNS-F-21035∗), Midorogaike, on Rhododendron macrosepalum, 21 Apr. 2006, HNo.216 (TNS-F-21036∗), Mt. Kiyomizu, on Castanopsis cuspidata, 22 Apr. 2006, HNo.219 (TNS-F-21037∗); Kantō reg., Ibaraki pref., on P. densiflora, 14 Oct. 2014, HNo.1113 (TNS-F-61320∗). Russia, Khabarovsk reg., Solnechnyi dist., Igdomi, on Alnus hirsuta, 2 Sep. 2016, V. Spirin 10764 (H∗), Verkhnebureinsky dist., Dublikan Nat. Res., on Alnus alnobetula ssp. fruticosa, 21 Aug. 2014, V. Spirin 7774 (H∗), 7780 (H∗). USA, Maryland, Prince Georges co., Greenbelt, Greenbelt park, Campgrounds, on Pinus, 17 Jun. 1968, H.H. Burdsall (CFMR:HHB-671∗); Massachusetts, Norfolk co., Canton, on P. strobus, 7 Nov. 1932, D.H. Linder, det. id.: Whelden 142 (holotype of Dacrymyces gangliformis, FH00304774); North Carolina, Chapel Hill, back of Athletic Field, on oak bark, 4 Feb. 1920, Couch 4075 (holotype of Dacrymyces fuscominus, NCU-F-0009150); South Carolina, on Alnus, date unknown, M.A. Curtis (UPS:F-116976), same loc., on unidentified wood, (UPS:F-116977); Wisconsin, Dane co., Madison, University of Wisconsin Arboretum, Leopold Pines, on P. resinosa, 16 Jul. 1973, H.H. Burdsall (CFMR:HHB-7334∗).
Notes: Cerinomyces enatus is similar to C. cokeri but demonstrates darker colouration, swollen cells and thicker gelatinous layer on internal hyphae. In all studied specimens septate basidiospores are extremely rare. The species shows high variability in morphological characters, nrDNA sequences and substrate preferences. In all publications of Shirouzu et al. Japanese materials were cited as C. canadensis or C. pallidus. For American specimens CFMR:HHB-671 and CFMR:HHB-7334 we were able to sequence only 5.8S and part of ITS2. The sequences were too short to use in the phylogeny reconstruction but sufficient to identify them as C. enatus. Interestingly, Seifert (1983) studied a culture UBC 6124 from CFMR:HHB-671 (D. punctiformis in his paper), and found in a wood block decay test that it was almost unable to degrade Pinus wood — a substrate, from which it was originally collected.
The studied types of C. enatus from K and FH are contaminated by mycoparasites, contain only few, collapsed basidiospores, and show a highly agglutinated hymenial layer, which altogether deny proper measurements. Nevertheless, characteristic macromorphology, presence of branched hyphidia, thick-walled subicular hyphae with occasional swollen compartments and simple marginal hyphae help to connect type material to the other collections cited here.
Dacrymyces gangliformis has 0–1-septate basidiospores that are slightly wider than typically in C. enatus — 9.2–12 × 4.5–6 μm. The species-defining “ganglia” were found to be altered swollen basidiospores, germinating with hyphae. The type specimen is scanty, poorly preserved and contaminated with anamorphic fungi. Considering similarities in microstructures and basidiocarp, we believe the specimen belongs to C. enatus. Dacrymyces fuscominus type is also in a rather bad condition, but observed morphology allows us to treat the species as a synonym of C. enatus, which has already been suggested by Kennedy (1958b).
Cerinomyces enterolaxus Shirouzu & A. Savchenko, sp. nov. MycoBank MB 839790. Fig. 12, Fig. 31.
Fig. 31.
Cerinomyces enterolaxus micromorphology. A. Spores. B. Hymenium with hyphidium, and subhymenium. Drawn from holotype, TNS-F-61292 (A); TNS-F-61319 (B). Scale bars = 5 μm.
Typus: Japan, Honshu, Kantō reg., Ibaraki pref., Sakuragawa, Mt. Tsukuba, on Pinus densiflora, 15 May 2013, T. Shirouzu HNo.1082 (holotype TNS-F-61292∗!).
Etymology: έντερα (Gr.) — intestines; laxus (Lat.) — loose; in reference to wide and loosely arranged subicular hyphae.
Description: Basidiocarps gelatinous, pustulate to cerebriform, up to 1(–2) mm in diam, can coalesce into slightly cerebriform film, from almost transparent and yellow to dark brown with reddish tint. Hyphae clamped, in subiculum embedded in gelatinous hyaline matrix, loosely arranged, 2–4 μm in diam, walls 0.3–0.5 μm in width. Subhymenial hyphae densely organized, 1.5–3 μm in diam, walls 0.3(–0.5) μm in width. In sterile areas terminal hyphae branched or simple, cylindrical or clavate, 2–4 μm in diam. Hymenium includes abundant branched cylindrical hyphidia 1.5–2 μm in diam. Basidia clavate, 14–33 × 3–6 μm. Sterigmata up to 19 μm in length (n = 62/8). Basidiospores cylindrical to slightly curved-cylindrical, occasionally slightly obpyriform, 0(–1)-septate, 9.1–13.7(–14.1) × (3.0–)3.2–4.5(–4.6) μm, L = 11.6 μm, W = 3.9 μm, Q = 3.0, Q’ = 2.4–3.8 (n = 92/9), walls ∼ 0.2 μm in width. Basidiospores produce cylindrical conidia 2.5–3.0 × 1.0–1.2 μm.
Habitat and distribution: Gymnosperm (Pinus) and rarely angiosperm (Clethra) wood; East Asia.
Material examined: Japan, Honshu, Chūbu reg., Nagano pref., Shioda, Ueda-shi, on Pinus densiflora, 20 May. 2006, T. Shirouzu (same coll. for all collections) HNo.285 (TNS-F-15725∗), Sugadairakougen, on P. densiflora, 11 Jul. 2016, HNo.1135 (TNS-F-88723∗), same loc. and substr., 11 Jul. 2016, HNo.1138 (TNS-F-88726∗), same loc. and substr., 11 Jul. 2016, HNo.1140 (TNS-F-88728∗), same loc. and substr., 11 Jul. 2016, HNo.1148 (TNS-F-88734∗), same loc. and substr., 1 Sep. 2016, HNo.1156 (TNS-F-88742∗), same loc. and substr., 1 Sep. 2016, HNo.1159 (TNS-F-88745∗), same loc. and substr., 7 Nov. 2016, HNo.1168 (TNS-F-88753∗), same loc. and substr., 12 Jul. 2017, HNo.1181 (TNS-F-88762∗), same loc. and substr., 12 Jul. 2017, HNo.1182 (TNS-F-88763∗), same loc. and substr., 12 Jul. 2017, HNo.1187 (TNS-F-88767∗), same loc. and substr., 12 Jul. 2017, HNo.1188 (TNS-F-88768∗), same loc. and substr., 3 Sep. 2018, HNo.1216 (TNS-F-88781∗); Kansai reg., Kyoto pref., Mt. Daimonji, on P. densiflora, 20 Apr. 2006, HNo.196 (TNS-F-15723∗), Takaraga-ike, on Clethra barbinervis, 21 Apr. 2006, HNo.213 (TNS-F-15724∗); Kantō reg., Ibaraki pref., Sakuragawa, Mt. Tsukuba, on P. densiflora, 14 Jun. 2013, HNo.1086 (TNS-F-61296∗), 15 Jul. 2013, HNo.1097 (TNS-F-61306∗), 14 Oct. 2013, HNo.1109 (TNS-F-61316∗), HNo.1110 (TNS-F-61317∗), HNo.1112 (TNS-F-61319∗), 12 Dec. 2013, HNo.1117 (TNS-F-61324∗), 14 Mar. 2014, HNo.1120 (TNS-F-61327∗), HNo.1128 (TNS-F-61334∗), HNo.1129 (TNS-F-61335∗).
Notes: Cerinomyces enterolaxus is distinct from most of European gelatinous species by septate basidiospores, thin-walled internal hyphae without heavy gelatinous layer and generally lighter basidiocarp colour. A single collapsed and deformed 3-septate spore was noted. Specimens TNS-F-61327 and TNS-F-61334 are infected by an intrahymenial Tremella species. Compared to other C. enterolaxus sequences, LSU of TNS-F-88726 contains few short indels and a 20 bp-long duplicating insertion that were cut from the final alignment. In the earlier publications of Shirouzu et al. the species was cited as Dacrymyces punctiformis.
Cerinomyces fasciculatus Gilb. & Hemmes, Memoirs of the New York Botanical Garden 89: 81 (2004).
Typus: USA, Hawaii, Island of Hawaiʻi, Kaʻu dist., Hawaiʻi Volcanoes National Park, Kīpuka Puaulu, on Pipturus albidus, 17 Nov. 1998, R.L. Gilbertson 22101 (holotype BPI, isotype ARIZ).
Description fide Gilbertson & Hemmes (2004): Basidiocarps annual, resupinate, arid, membranous, effused to 10 cm; hymenial surface light buff, smooth, with projecting sterile hyphal pegs, margin thinning out, whitish with radiating fibrils at the edge; pegs up to 40 μm diam and projecting up to 70 μm, composed of thin-walled clamped hyphae, arising in the subiculum and appearing as more compactly interwoven columns in the surrounding subicular tissue, apices with projecting rounded hyphal ends. Hyphae in subiculum hyaline, with abundant clamps, 2–4.5 μm diam. Hymenium. Some irregularly lobed or branched hymenial hyphidia present; basidia clavate at first, 25–40 × 4–6 μm, becoming bifurcate, sterigmata to 35 μm long and 3–4 μm diam, tapering to pointed apex. Basidiospores curved-cylindrical, aseptate, hyaline, smooth, thin-walled, negative in Melzer's reagent, 10–12.5(–13) × 4.5–5.5 μm.
Habitat and distribution: Angiosperm shrubs (Pipturus); known only from Hawaiʻi.
Notes: We have not seen the specimens, but according to the described morphology, C. fasciculatus is related to the C. albosporus clade. The types could not be traced in BPI (several loan requests) or ARIZ (A.E. Arnold, 7 Jul. 2019, pers. comm.).
Cerinomyces favonius Spirin, Miettinen & A. Savchenko, sp. nov. MycoBank MB 839791. Fig. 9, Fig. 32.
Fig. 32.
Cerinomyces favonius micromorphology. A. Spores. B. Hymenium and subhymenium. All drawn from holotype, H7008893. Scale bars = 5 μm.
Typus: USA, Idaho, Bonner co., Trapper Creek, on Tsuga heterophylla, 14 Oct. 2014, V. Spirin 8473 (holotype H7008893∗!).
Etymology: Favonius (Lat.) — Roman god of west wind.
Description: Basidiocarps arid, subiculum thin, filling depressions of substrate under the hymenial crust; well-developed hymenial surface smooth and solid, pale ochraceous to pale brown; margin arachnoid, indistinct, concolourous with hymenium. Substrate under old basidiocarps is often dark brown. Hyphae clamped, loosely interwoven in subiculum, 2–3 μm in diam, with walls 0.3–0.6 μm in width; tightly and vertically arranged in subhymenium, 2–2.5(–3) μm in diam, walls 0.3–0.4 μm in width. Hymenium simple, basidia clavate, 8–18 × 3.5–5 μm, with sterigmata up to 16 μm in length (n = 40/2). Basidiospores cylindrical, slightly curved, aseptate, (6.3–)7.1–10.3(–10.8) × 2.3–3.0(–3.1) μm, L = 8.3 μm, W = 2.7 μm, Q = 3.1, Q’ = 2.5–3.9 (n = 60/2), walls ∼ 0.2 μm in width.
Habitat and distribution: Gymnosperm wood (Abies, Picea, Pinus, Tsuga); North America.
Material examined: Canada, British Columbia, Fraser-Fort George reg. dist., McLeod lake co., pine forest near the McKenzie road, on Pinus contorta, 27 Jun. 1969, B. Eriksson & J. Eriksson 12222 (GB-0071216), 12223 (GB-0071215). USA, Oregon, Willamette National Forest, Frog Camp, on Abies lasiocarpa, 15 Jul. 1958, A.M. Rogers & D.P. Rogers 2811 (BPI1106574); Washington, Pend Oreille co., Gypsy Meadows, on Picea engelmannii, 17 Oct 2014, V. Spirin 8688b (H7008894∗); Wisconsin, Vilas co., on Tsuga, 16 Jul. 1964, A.E. Liberta 550 (BPI1106575).
Notes: Cerinomyces favonius can be separated from other corticioid North American species by its narrower basidiospores and often by thinner basidiocarps. Though, specimen GB-0071216, that was identified as C. favonius primarily by the spores, has relatively robust basidiocarps and cylindrical hyphidia with thickened bases that arrange into microscopic hyphal pegs, which is more characteristic to the C. pallidus clade members. The closest phylogenetic relative to C. favonius, European C. borealis, features slightly larger basidiospores and basidia.
Cerinomyces fugax A. Savchenko, sp. nov. MycoBank MB 839792. Fig. 10, Fig. 33.
Fig. 10.
Cerinomyces species with arid corticioid basidiocarps. A.C. fugax (holotype, CFMR:HHB-8856). B.C. inermis (holotype, PDD87816). C.C. nepalensis (holotype, O:F-904088). D. C. pallidus (isotype, NY02136493). E.C. paulistanus (holotype, O:Ryvarden 24759). F. C. paulistanus, hyphal pegs (same specimen). G.C. pinguis (holotype, O:F-904085). H.C. ramosissimus (holotype, CFMR:FP-150848). I.C. tristis (holotype, H7009711). J.C. verecundus (holotype, PDD93708). K.C. volaticus (holotype, S:F250344). Scale bars: A–E, H–K = 5 mm; F = 250 μm; G = 2.5 mm.
Fig. 33.
Cerinomyces fugax micromorphology. A. Spores. B. Hymenium and subhymenium. All drawn from holotype, CFMR:HHB-8856. Scale bars = 5 μm.
Typus: USA, Mississippi, Stone co., 5 miles west of Wiggins, S of Red Rock, on Pinus taeda, 30 Mar. 1976, H.H. Burdsall (holotype CFMR:HHB-8856∗!).
Etymology: fugax (Lat.) — shy, timid; reference to subtle basidiocarps.
Description: Basidiocarps arid, appear as loose cottony subiculum patches, later covered with solid smooth hymenium, light ochraceous, without distinct margin. Hyphae clamped, subicular hyphae 2–3.5 μm in diam, walls 0.5 μm in width; in subhymenium 2–3 μm in diam, walls 0.3 μm in width. Hymenium simple, basidia clavate, 9–22 × 2.5–5 μm, with sterigmata up to 13 μm in length (n = 30/1). Basidiospores cylindrical, slightly curved, aseptate, (7.2–)7.3–9.3(–10.0) × (2.9–)3.0–3.8(–3.9) μm, L = 8.5 μm, W = 3.3 μm, Q = 2.6, Q’ = 2.4–3.0 (n = 30/1), walls ∼ 0.2 μm in width.
Habitat and distribution: Gymnosperm wood (Pinus, Pseudotsuga); North America.
Material examined: Canada, British Columbia, Vancouver Island, Lake Cowichan provincial forest, on Pseudotsuga menziesii, 6 Sep. 1967, B. Eriksson & J. Eriksson 7507 (GB-0071219).
Notes: Cerinomyces fugax together with C. favonius and C. tristis form a difficult morphogroup of conifers-dwelling North American species. However, basidiospores of C. fugax are wider than of C. favonius and longer than of C. tristis. Micromorphologically, C. fugax is almost indistinguishable from its closest phylogenetic neighbor — European C. volaticus. Among the specimens that we were unable to sequence but tentatively identified as C. fugax, one has larger basidiospores 9.0–10.9 × 2.9–4.2 μm, L = 10.0 μm, W = 3.4 μm, Q = 3.0, basidia 13–20 × 2.5–4.5 μm, and sterigmata up to 18 μm in length; it can represent either a better developed C. fugax or yet another species in this complex (USA, Oregon, Cascade Head Expterimental Forest, Siuslau National Forest, on gymnosperm wood, 11 Oct. 1972, M.J. Larsen [CFMR:FP-133350]).
Cerinomyces hesperidis A. Savchenko, sp. nov. MycoBank MB 839793. Fig. 12, Fig. 34.
Fig. 34.
Cerinomyces hesperidis micromorphology. A. Spores. B. Hymenium and subhymenium. C. Marginal hyphae. All drawn from NY01782362. Scale bars = 5 μm.
Typus: USA, Arizona, Pima co., Coronado National Forest, Santa Catalina Mountains, General Hitchcock Camp, on Pinus ponderosa, 31 Jan. 1968, R.L. Gilbertson 7794 (holotype NY01782362∗!).
Etymology: hesperidis (Gr., latinized) — of evening, western.
Description: Basidiocarps gelatinous, pustulate to shallow cupulate, sometimes slightly cerebriform, often with short stalk, up to 1 mm in diam, growing in groups, but usually not coalescing, from pale yellow-brown when fresh to dark brown when dried. Rarely young basidiocarps grow directly on older ones. Hyphae clamped, internal hyphae 2.5–4 μm in diam, walls ∼ 0.3 μm in width, with gelatinous layer 0.5–1.0 μm in width, sometimes roughened. Subhymenial hyphae (2–)2.5–3 μm in diam, walls ∼ 0.3 μm in width, with gelatinous layer up to 0.5 μm in width. Marginal hyphae simple cylindrical or slightly clavate, 2.5–3.5 μm in diam, walls of the same width as in subiculum. When stalks present, they covered in palisade of clavate or rarely slightly conical terminal cells 4–8 μm in diam, walls together with gelatinous layer ∼ 1.0 μm in width. Hymenium consists of basidia and rare cylindric hyphidia, simple or slightly branched. Basidia clavate, 23–41 × 2.5–4.5 μm. Sterigmata up to 35 μm in length (n = 30/1). Basidiospores slightly curved-cylindrical, 0(–1)-septate, (9.9–)10.0–12.3(–13.4) × (3.1–)3.2–4.2(–4.5) μm, L = 11.3 μm, W = 3.7 μm, Q = 3.0, Q’ = 2.6–3.9 (n = 30/1), walls ∼ 0.2 μm in width, collapsed basidiospores often constricted in the middle and have slightly attenuated apical part.
Habitat and distribution: Gymnosperm wood (Pinus and unident.); North America.
Material examined: USA, Montana, Flathead co., North Fork Flathead River, Glacier Nature Park, on Pinus ponderosa, 3 Aug. 1964, R.L. Gilbertson 4907 (NY01782364); New York, Warren co., Picnic area, north end of Pack Forest, on gymnosperm wood (?), 17 May 1968, H.H. Burdsall (CFMR:HHB-553)
Notes: Cerinomyces hesperidis differs from European gelatinous Cerinomyces species by basidiocarps that are more regularly shaped, often with a central depression or of cupulate form, rooted in substrate with a short stalk, without a tendency to coalesce, resembling some collections of C. tortus. Cited here non-sequenced specimens fit well in the concept of C. hesperidis, but differ from the type by having thick-walled, pronouncedly clavate terminal hyphae covering basidiocarp stems.
Cerinomyces inermis A. Savchenko, sp. nov. MycoBank MB 839794. Fig. 10, Fig. 35.
Fig. 35.
Cerinomyces inermis micromorphology. A. Spores. B. Hymenium and subhymenium. All drawn from holotype, PDD87816. Scale bars = 5 μm.
Typus: New Zealand, Te Ika-a-Māui, Bay of Plenty reg., Te Urewera, Tarapounamu, UA 2 NW 16m, on gymnosperm wood, 10 Oct. 2004, P.R. Johnston & B.C. Paulus 752 (holotype PDD87816∗!).
Etymology: inermis (Lat.) — unarmored, referring to the absence of hyphal pegs.
Description: Basidiocarps originate as arid white subicular patches, turning into smooth and solid, light yellowish brown to dark brown, semitransparent, thin film, waxy when moisture applied, with thin subiculum and white fimbriate margins. Hyphal pegs absent. Hyphae clamped, gelatinized, subicular hyphae 2–3(–4) μm in diam, walls ∼ 0.3 μm in width; in subhymenium 1.5–3 μm in diam, walls 0.3–0.5 μm in width. Hymenium simple, basidia clavate, 12–25 × 3–5 μm, with sterigmata up to 21 μm in length (n = 30/1). Basidiospores cylindrical, slightly curved, often with lipid droplets within, aseptate, (8.1–)8.2–10.2(–10.6) × (2.9–)3.0–3.9 μm, L = 9.5 μm, W = 3.3 μm, Q = 2.9, Q’ = 2.3–3.4 (n = 30/1), walls ∼ 0.2–0.3 μm in width.
Habitat and distribution: Gymnosperm wood; New Zealand (known only from the type locality).
Notes: Cerinomyces inermis can be confused with the C. pallidus clade members, but it has averagely longer basidiospores. In contrast to its relatives from the C. albosporus clade, basidiocarps of C. inermis lack hyphal pegs and are prone to gelatinize when re-wetted, which puts it close to C. aff. aculeatus 1 morphology-wise.
Cerinomyces lipoferus J.C. Zamora & A. Savchenko, sp. nov. MycoBank MB 839795. Figs 13 A, B, 36.
Fig. 13.
Cerinomyces species with gelatinous basidiocarps in rewetted and dry state. A.C. lipoferus, rewetted (holotype, UPS:F-940777). B.C. lipoferus, dry (same specimen). C.C. neuhoffii, rewetted (holotype, UPS:F-941020). D.C. neuhoffii, dry (same specimen). E.C. tortus, rewetted (neotype, UPS:F-946515). F, G. C. tortus, dry, young and mature basidiocarps (holotype of Dacrymyces punctiformis, S:F-015301). H.C. aff. tortus 1, rewetted (UPS:F-946504). I.C. aff. tortus 1, dry (same specimen). J.C. aff. tortus 2, rewetted (UPS:F-940948). K. C. aff. tortus 2, dry (same specimen). Scale bars: A, C, E, H–K = 1 mm; B, D, F, G = 5 mm.
Fig. 36.
Cerinomyces lipoferus micromorphology. A. Spores. B. Hymenium and subhymenium. C. Hyphidia. D. Marginal hyphae. All drawn from GB-0161225. Scale bars = 5 μm.
Typus: Sweden, Uppsala co., Uppsala mun., Stadsskogen Nature Reserve, on Pinus sylvestris, 26 Sep. 2018, J.C. Zamora & L. Martín (holotype UPS:F-940777∗!, isotype H7009042!).
Etymology: λίπος (Gr.) — fat; φέρω (Gr.) — carry; reference to a large amount of lipid droplets.
Description: Basidiocarps gelatinous, pustulate, gregarious, can coalesce with shapes of single basidiocarps remaining visible, whitish to pale yellow or cream coloured, rarely pale brown when fresh, yellowish brown to dark brown when dried, single basidiocarps up to 1.5(–2) mm in diam. Hyphae clamped, with comparatively high content of lipid droplets; clamps often have a hole under the loop (see Fig. 36 B–D). Subicular hyphae 2–3.5(–4.5) μm in diam, walls 0.3–0.5 μm in width, gelatinized and sometimes roughened. Subhymenial hyphae densely arranged, (1.5–)2–3.5 μm in diam, walls 0.3–0.4 μm in width. Sterile areas and margins covered with simple cylindrical, slightly inflated or narrowly clavate hyphae 2–4 μm in diam, walls of the same width as in subiculum. Hymenium includes rare, weakly branched cylindrical hyphidia 2–3.5 μm in diam, and clavate basidia 19–62 × 3–6 μm. Sterigmata up to 34 μm in length (n = 54/2). Basidiospores slightly curved-cylindrical, 0–1(–3)-septate, at least some binucleate before septation (but a few uninucleate basidiospores also seen), (9.1–)9.5–15.2(–16.8) × (3.7–)3.9–5.2(–5.7) μm, L = 11.8 μm, W = 4.5 μm, Q = 2.6, Q’ = 2.1–3.6 (n = 155/2), walls ∼ 0.2 μm in width.
Habitat and distribution: Gymnosperm wood (Picea, Pinus, and unident.), Northern Europe.
Material examined: Sweden, Uppsala co., Uppsala mun., Stadsskogen Nature Reserve, on Pinus sylvestris, 24 Oct. 2018, J.C. Zamora & L. Martín (UPS:F-940778∗), same loc., 15 Oct. 2019, J.C. Zamora & M. Westberg (UPS:F-946513); Västra Götaland co., Göteborg city, Haga area, Haga Nygata nr., on vertical wood wall, 12 Nov. 1976, J. Eriksson (GB-0161225∗ — herb. GB scripsit “19548”). The Netherlands, Gelderland, Hoge Veluwe, Deelense Straal, on Pinus, 5 Jan. 2020, N. Dam ND20004 (L.3983554); Overijssel, near Enschedé, Haagse Bos, on Picea abies, 26 Jan. 2020, R. Enzlin ENZ20001∗ (herb. Enzlin).
Notes: The species resembles C. tortus in rarely branched hyphidia but differs in high number of lipid bodies in hyphae, presence of 1- or rarely 3-septate basidiospores, and paler fruitbodies. Zamora & Ekman (2020) referred to the species as to Dacrymyces tortus s. lat. 1. Sequences of ENZ20001 were produced during the revision of this study; they were not used in the analyses but match other included sequences.
Similarly large amount of lipid drops and matching spore measurements were observed in a specimen from a montane habitat (China, Jilin, Huang Song Pu, Chang Bai Shan Forest Reserve, 1 200 m a.s.l., on Larix olgensis, 11 Sep. 1983, L. Ryvarden 21458 [K(M): 34585]). We do not assign it to C. lipoferus due to the geographical distance between occurrences.
Cerinomyces nepalensis A. Savchenko, sp. nov. MycoBank MB 839796. Fig. 10, Fig. 37.
Fig. 37.
Cerinomyces nepalensis micromorphology. A. Spores. B. Hymenium, subhymenium and subiculum. C. Hyphidia. D. Subhymenial hyphae with swollen cells. All drawn from holotype, O:F-904088. Scale bars = 5 μm.
Typus: Nepal, Dhaulagiri zone, Myagdi dist., Ghorepani, 30 Oct. 1979, L. Ryvarden 18670B (holotype O:F-904088∗!).
Etymology: Reference to the type collection locality.
Description: Basidiocarps arid, solid and smooth, with crustose pale fawn hymenial surface and cottony white subiculum. Hyphae clamped, in subiculum agglutinated in strands, loose, 2–4 μm in diam, walls 0.3–0.6 μm in width. Subhymenial hyphae of the same diam, walls up to 0.8 μm in width, decreasing to 0.3 μm towards hymenium, with occasional swollen cells. Hymenium includes hyphidia with cylindrical to obclavate base up to 4 μm in diam and simple apical part ∼ 1.5 μm in diam; of total length up to 25 μm, in groups or scattered among basidia. Basidia clavate to almost cylindrical, 7–17 × 2.5–4.5 μm. Sterigmata up to 10(–15) μm in length (n = 30/1). Basidiospores ellipsoid to slightly curved-cylindrical, often with long apiculus and distinct hilar appendix, aseptate, (5.0–)5.2–7.7(–7.9) × (2.1–)2.5–3.4(–3.5) μm, L = 6.6 μm, W = 3.0 μm, Q = 2.2, Q’ = 1.8–2.8 (n = 30/1), wall ∼ 0.2 μm in width.
Habitat and distribution: Unidentified, probably gymnosperm wood; South Asia (known only from the type locality).
Notes: The species has the shortest basidiospores in the genus. In our opinion, pronounced apicular part of the basidiospores (and large space between hilum and its appendix) can indicate their immaturity, so the reported measurements may not represent the full range for the trait. Type locality of C. pinguis is also in Nepal, but this species has substantially larger microstructures than C. nepalensis. The type specimen was first reported as C. pallidus by Hjortstam & Ryvarden (1984).
Cerinomyces neuhoffii J.C. Zamora & A. Savchenko, sp. nov. MycoBank MB 839797. Fig. 13, Fig. 38.
Typus: Sweden, Stockholm co., Stockholm, Årsta, on Pinus sylvestris, 10 Sep. 2017, J.C. Zamora, P. Posio (holotype UPS:F-941020∗!, isotype H7009709!).
Etymology: In honour of the German mycologist Walther Neuhoff.
Description: Basidiocarps gelatinous, pustulate to pulvinate, rarely slightly cerebriform or centrally depressed, growing in dense groups, often coalescing with shapes of initial basidiocarps remaining visible. Rarely, basidiocarps stay clearly isolated and only gregarious. Separate basidiocarps up to 1(–1.5) mm in diam, from pale brown when fresh and young to dark brown or dark greyish brown, in maturity with or without a whitish subicular core, becoming almost black when dried. Hyphae clamped, gelatinized, densely arranged, in subiculum 2.5–4(–5) μm in diam, walls 0.4–1.0 μm in width. Subhymenial hyphae 2–3.5 μm in diam, walls 0.3–0.6 μm in width. Marginal hyphae similar to subicular, simple cylindrical or slightly clavate, gelatinized. Some branched anastomosing hyphae observed between fertile and sterile areas. Hymenium consists of basidia and well-branched cylindrical hyphidia with base 2–3.5 μm in diam and apical part 1.5–2 μm in diam; up to 60 μm in total length, from occasional to abundant in different basidiocarps. Basidia clavate, some of them slightly curved,14–43 × 3–7 μm, with walls up to 0.5 μm in width. Sterigmata up to 26 μm in length (n = 120/8). Basidiospores slightly curved-cylindrical, aseptate, binucleate, (8.2–)9.6–14.0(–16.1) × (2.9–)3.0–4.8(–5.1) μm, L = 11.6 μm, W = 3.8 μm, Q = 3.1, Q’ = 2.3–3.9 (n = 180/9), walls ∼ 0.2–0.3 μm in width. Conidia subglobose, 1.8–2.1 μm in diam.
Habitat and distribution: Gymnosperm wood (Pinus and unident.), Europe.
Material examined: Cyprus, Limassol, Mesa Potamos, picnic area, on Pinus brutia, 2 Dec. 2017, J.C. Zamora (UPS:F-946505∗). Finland, Uusimaa prov., Helsinki, Myllypuro, on gymnosperm wood, 10 Jun. 2017, O. Miettinen 20778 (H∗). Spain, Andalucía comm., Jaén, Venta de los Santos, close to the Dañador dam, on P. pinaster, 15 May 2017, B. Zamora, J. Señoret (UPS:F-941019∗), Málaga comm., Mijas village area, on Pinus, 16 Nov. 2012, O. Miettinen 15893 (H∗). Sweden, Kalmar co., Öland, Borgholm mun., Bödakusten västra Nature Reserve, Byrums Sandvik, on P. sylvestris, 2 Oct. 2017, J.C. Zamora (UPS:F-946503∗); Stockholm co., Stockholm, Masmo, on P. sylvestris, 15 Oct. 2017, J.C. Zamora & P. Posio (UPS:F-941252), Solna mun., Bergshamra, on P. sylvestris, 23 Jun. 2018, J.C. Zamora (UPS:F-941021); Uppsala co., Uppsala mun., Norra Lunsen Nature Reserve, on P. sylvestris, 20 Aug. 2017, J.C. Zamora (UPS:F-946501∗); Värmland co., Lekvattnet, on P. sylvestris, 7 Oct. 2018, J.C. Zamora (UPS:F-946510∗). Ukraine, Kharkiv reg., Krasnokutsk dist., Slobozhanskyi National Park, forest lot 42, on P. sylvestris, 2 Nov. 2013, A. Savchenko (CWU(MYC)6342∗), forest lot 58, on P. sylvestris, 1 Dec. 2012, A. Savchenko (CWU(MYC)6281∗); Ternopil reg., Zalishchyky dist., Dzhuryn river valley, on Pinus, 5 Oct. 2016, O. Akulov AS0060 (CWU(MYC), TU135067∗).
Notes: Cerinomyces neuhoffii and C. tortus are among the most common gelatinous Cerinomyces species. The former one is widespread in Europe, while the latter occurs mostly in its northern part. Basidiocarps of C. neuhoffii are generally smaller, darker, with a softer consistency, and often organized in large, closely crowded groups. On microscopic level, C. tortus very rarely forms dendroid hyphidia, in contrast to the finely branched, abundant structures of C. neuhoffii. Also, C. neuhoffii has shorter and stouter basidia, and at least some of them tend to be slightly bent or asymmetric. Cerinomyces creber is highly similar to C. neuhoffii in hyphidia and gregarious coalescing basidiocarps, but it is often more light-coloured, has smaller basidiospores, slightly smaller basidia, and seems to be associated with Cupressaceae. Two undescribed taxa designated below as C. aff. tortus 1 and 2 both have branched hyphidia, but their basidiospores are on average shorter than of C. neuhoffii. We also found that basidiospores of C. neuhoffii specimens from Southern and Eastern Europe are slightly wider compared to the Northern collections. Zamora & Ekman (2020) cited the species as Dacrymyces tortus s. lat. 4.
Neuhoff (1934) actually distinguished two putative species (D. punctiformis and D. romelii) in what had been previously considered as Dacrymyces tortus s.l. Although the original descriptions may partially cover the morphological characteristics of C. neuhoffii, they also fit within the variation of C. tortus. The type specimens of both D. punctiformis and D. romelii turned out to be conspecific to C. tortus (see note under that species), so in absence of available names we describe C. neuhoffii as a new species, while honouring Neuhoff’s clue that C. tortus has to be split.
Cerinomyces pallidus G.W. Martin, Mycologia 41: 83 (1949). Fig. 10, Fig. 39.
Typus: USA, Iowa, Johnson co., Iowa City, on Quercus, 28 Jul. 1939, G.W. Martin 4673 (holotype TRTC, isotypes BPI726049! ex US, BPI726050! ex MO186440, FH00489909!, ISC-V-0045114, K, LSU00135942 ex MO186440, NY02136493!, UC940258).
Description: Basidiocarps arid, appear as small white patches of cottony subiculum, later coalescing, covering areas up to 15 × 3 cm or more (fide Martin 1949), 0.1–0.5 mm thick. Well-developed hymenial surface solid, crust-like, light ochraceous, becoming pale fawn to pale brown in older, prominent, and bruised areas; smooth or irregularly covered with hyphal pegs up to 300 μm in height. Margin fimbriate or indistinct, concolourous with hymenium surface; subiculum relatively thick, filling depressions of substrate under the hymenial crust. Older basidiocarps can be found under new ones as collapsed dark brown to black layers. Hyphae clamped, in subiculum interwoven, 2–3 μm in diam, walls 0.5–0.6(–0.8) μm in width; in subhymenium mostly perpendicular to substrate, 2–3.5 μm in diam, walls 0.3–0.5(–0.7) μm in width. Pegs formed by parallel, compact and often agglutinated hyphae 1–2.5 μm in diam, walls 0.3–0.7 μm in width. Hymenium consists of basidia and rare hyphidia with thickened base up to 5 μm in diam and simple cylindrical apical part 2–2.5 μm in diam; hyphidia tend to form small groups, developing into microscopic hyphal pegs. Basidia clavate, 10–27 × 2.5–5(–6.5) μm, with sterigmata up to 19 μm in length (n = 116/6). Basidiospores cylindrical, slightly curved, aseptate, (6.1–)6.5–10.2(–11.3) × 2.9–4.1(–4.8) μm, L = 8.0 μm, W = 3.3 μm, Q = 2.4, Q’ = 1.7–3.5 (n = 176/6), walls ∼ 0.2 in width.
Habitat and distribution: Angiosperm (Malus, Quercus, and unident.), very rarely gymnosperm (Pinus) wood; North America.
Material examined: USA, Iowa, Johnson co., Iowa City, on Malus, 21 Aug. 1939, G.W. Martin 3916 (original material, BPI726052), on Quercus, 1 Nov. 1940, G.W. Martin 5180 (original material, GB-0071214∗); Kansas, Leavenworth co., Ravine near Piper river, on angiosperm wood, 29 Sep. 1972, T.R. Rockett (NY: as “C. pallidus №1”∗); Louisiana, East Feliciana par., [Bob R. Jones-] Idlewild Research Station of LSU, on Pinus taeda, 23 Aug. 1986, R.L. Gilbertson 15966, AN027337 (ARIZ-M-AN09245∗); Maryland, Prince Georges co., Hoffman Hill Rd., Beltsville Expt. Forest, on Quercus, 6 Nov. 1968, H.H. Burdsall (CFMR:HHB-1768); Ohio, Delaware co., Camp Lazarus Reserve, on angiosperm wood, 2 Sep. 1968, W.B. Cooke & V.G. Cooke (CFMR:WBC-39924∗).
Notes: Cerinomyces pallidus can be identified by association with angiosperm substrates and by the presence of pegs in mature basidiocarps. It shares these features with C. paulistanus, but the latter species occurs only in South America and slightly differs in morphology. In some collections, pegs can be very rare or even absent, leading to confusion with another North American angiosperm-inhabiting species, C. atrans, that has similar micromorphology, but darker basidiocarps. Both species can occasionally appear on gymnosperm wood, and then it is difficult to tell them apart from strictly conifers-dwelling taxa.
In the protologue of C. pallidus Martin assigned a “type” and, perhaps already after publication, distributed its duplicates to a number of herbaria. Maekawa (1987) cited a holotype deposited in TRTC, but the specimen could not be traced (S. Margaritescu, 14 Sep. 2016, pers. comm.). Worth noting, that under the same number 4673, Martin put another gathering from 28 July of 1948 (ISC-V-0045115), also marked as a type. Besides of the same locality (Iowa City) and substrate (oak), it is unknown how exactly the specimen is connected to the original type material from 1939. Martin (1949) reported ellipsoid conidia produced at the tops of its hyphal pegs, but we did not see any anamorphic structures in C. pallidus.
A non-sequenced specimen UBC:F873 (Canada, British Columbia) deserves a specific mention having unusually short, ellipsoid basidiospores (6.5–8 × 3–4 μm, L = 7.4 μm, W = 3.6 μm, Q = 2.1, Q’ = 1.6–2.6, n = 16/1), and can represent either an immature C. pallidus or a potential new species. Sequence of ARIZ-M-AN09245 was produced during the revision of this study; it was not used in the analyses but matches other included sequences.
Cerinomyces paulistanus A. Savchenko, sp. nov. MycoBank MB 839798. Fig. 10, Fig. 40.
Fig. 40.
Cerinomyces paulistanus micromorphology. A. Spores. B. Hymenium and subhymenium. C. Hyphidia. D. Apical part of a hyphal peg. E. Swellings on subicular hyphae. Drawn from TAAM192129 (A, B); holotype, O:Ryvarden 24759 (C–E). Scale bars = 5 μm.
Typus: Brazil, São Paulo, Cananéia, Parque Estadual Ilha do Cardoso, 2–5 Feb. 1987, D.N. Pegler, K. Hjortstam & L. Ryvarden 24759 (holotype O∗!, isotype TU135088!).
Etymology: Reference to the type collection locality.
Description: Basidiocarps arid, appear as white arachnoid patches that develop into a relatively thick whitish subiculum. Hymenial surface arachnoid to solid, from pale ochraceous to brown in older or damaged areas. Margin fimbriate, concolourous with subiculum. Well-developed basidiocarps covered with scattered hyphal pegs 300(–500) μm in height. Hyphae clamped, subicular hyphae 2–3.5(–6) μm in diam, with occasional swellings up to 10 μm in diam, walls 0.3–0.8 μm in width; in subhymenium 2–4 μm in diam, walls 0.2–0.6 μm in width. Hyphae inside of pegs 3–4 μm in diam, originating from subiculum and becoming gradually thinner and more thin-walled towards the top of peg, being 2–3(–4) μm in diam on sides and at the top. Hymenium consists of basidia and hyphidia that have slightly swollen base 2.5–3 μm in diam and simple cylindrical apical part 1.5–2 μm in diam; ≤ 50 μm in total length. Hyphidia tend to arrange in agglutinated groups, microscopic pegs. Basidia clavate, 10–23 × 2.5–5 μm, with sterigmata up to 13 μm in length (n = 49/7). Basidiospores cylindrical, slightly curved, aseptate, (6.0–)6.5–9.6(–11.2) × (2.5–)2.6–3.9(–4.4) μm, L = 7.6 μm, W = 3.3 μm, Q = 2.3, Q’ = 1.8–3.1 (n = 126/7, 91 measured in 1 % KOH), walls ∼ 0.2 μm in width.
Habitat and distribution: Unidentified, probably angiosperm wood; South America.
Material examined: Brazil, São Paulo, Parque do Estado, [near] Instituto de Botânica, 18 Mar. 1964, K. Wells (all specimens) 1380-2 (TAAM192122), 1380-3 (BPI726051), (LSU00173761), 2 Apr. 1964, 1391-3 (BPI726053), 1391-4 (TAAM192121), 6 May 1964, 1542-2 (TAAM192120∗), 1542-4 (BPI726054).
Notes: Cerinomyces paulistanus is a close relative to C. pallidus in phylogeny, being also highly similar morphologically, but having slightly shorter basidia, swellings in subicular hyphae, and occurring in South instead of North America. They are the only two species in Cerinomyces outside of the C. albosporus clade that develop macroscopic hyphal pegs, though we noted that in C. paulistanus many of otherwise well-developed basidiocarps have no such pegs.
Cerinomyces pinguis A. Savchenko, sp. nov. MycoBank MB 839799. Fig. 10, Fig. 41.
Fig. 41.
Cerinomyces pinguis micromorphology. A. Spores. B. Hymenium with short gelatinized basidia. C. Gelatinized hymenium with full-sized basidia, and subhymenium. D. Group of hyphidia. All drawn from holotype, O:F-904085. Scale bars = 5 μm.
Typus: Nepal, Gandaki zone, Kaski dist., Annapura trek, Kuldi [Khuldighar?], 7 Nov. 1979, L. Ryvarden 18922 (holotype O:F-904085∗!).
Etymology: pinguis (Lat.) — fat; reference to the shapes of some basidia.
Description: Basidiocarps arid, arachnoid, white to pale fawn, without distinct subiculum or margin, hardly visible by the naked eye. Hyphae clamped, not differentiated. Subhymenial hyphae 2–3 μm in diam, walls 0.3–0.5 μm in width, gelatinized, agglutinated in strands closer to substrate. Hymenium includes hyphidia with thickened base 3–5 μm in diam and simple cylindrical apical part 1.5–2 μm in diam; up to 50 μm in total length. Basidia clavate to broadly clavate, 10–21 × 4.5–6.5 μm. Sterigmata up to 12(–16) μm in length (n = 30/1), sometimes covered with gelatinous layer. Basidiospores slightly curved-cylindrical, aseptate, (8.7–)9.0–10.5(–12.6) × 3.5–4.5(–5.0) μm, L = 9.8 μm, W = 3.9 μm, Q = 2.5, Q’ = 2.2–2.9 (n = 30/1), walls ∼ 0.2 μm in width.
Habitat and distribution: Unidentified, probably gymnosperm wood; South Asia (known only from the type locality).
Notes: Cerinomyces pinguis has the largest basidiospores and arguably the most delicate basidiocarps among the peg-lacking corticioid Cerinomyces species. The type specimen was originally reported as C. crustulinus by Hjortstam & Ryvarden (1984).
Cerinomyces ramosissimus A. Savchenko, sp. nov. MycoBank MB 839800. Fig. 10, Fig. 42.
Fig. 42.
Cerinomyces ramosissimus micromorphology. A. Spores. B. Hymenium and subhymenium. C. Subicular hyphae with crystals. D. Dendroid hyphae covering sterile areas. All drawn from holotype, CFMR:FP-150848. Scale bars = 5 μm.
Typus: Belize, Cayo dist., Mountain Pine Ridge, Five Sisters Lodge, Lower Nature Train, on Pinus, 20 Nov. 2001, K. Nakasone (holotype CFMR:FP-150848∗!).
Etymology: ramosissimus (Lat.) — very much branched; reference to a structure of subhymenium.
Description: Basidiocarps arid, at first arachnoid, later covered with solid smooth hymenium, light ochraceous to brown in damaged areas, with cottony whitish subiculum and indistinct margin. Hyphae clamped, densely packed, in subiculum often arranged in threads, 2–4 μm in diam, walls 0.8–1.0 μm in width, bearing crystals: from scattered transparent pustulate ≤ 2 μm in height to amorphic brownish coating. In some subicular areas crystals can be completely absent. Subhymenium develops from subiculum as tangles of ramificated hyphae. Subhymenial hyphae well-branched and tightly arranged, 2–3 μm in diam, walls 0.3–0.6 μm in width. Some sterile areas covered with the same type of branched hyphae. Hymenium consists of rare robust weakly branched hyphidia and clavate basidia, 8–16 × 3–5 μm. Sterigmata up to 11 μm in length (n = 30/1). Basidiospores slightly curved-cylindrical, aseptate, (7.0–)7.3–9.6 × (2.7–)2.8–3.2(–3.4) μm, L = 8.3 μm, W = 3.0 μm, Q = 2.8, Q’ = 2.4–3.3 (n = 30/1), walls ∼ 0.2 μm in width.
Habitat and distribution: Gymnosperm wood (Pinus); Central America (known only from the type locality).
Notes: The species is characterized with highly branched subhymenial hyphae and presence of crystals.
Cerinomyces tortus (Willd.) Miettinen, J.C. Zamora & A. Savchenko, comb. nov. MycoBank MB 839813. Fig. 13, Fig. 43.
Basionym: Tremella torta Willd., Botanisches Magazin 2 (4): 18 (1788).
Synonyms: Dacrymyces tortus (Willd.) Fr., Elenchus Fungorum 2: 36 (1828), nom. sanct.
Dacrymyces deliquescens var. castaneus Bourdot, Bulletin de la Société Mycologique de France 48: 206 (1932).
Dacrymyces punctiformis Neuhoff, Schweizerische Zeitschrift für Pilzkunde: 81 (1934).
Dacrymyces romellii Neuhoff, Schweizerische Zeitschrift für Pilzkunde. 12: 82 (1934).
Dacrymyces tortus f. romellii (Neuhoff) Raitv., Живая природа Дальнего Востока: 131 (1971).
Typus: Sweden, Uppsala co., Uppsala mun., Norra Lunsen Nature Reserve, on Pinus sylvestris, 24 May 2019, J.C. Zamora & S. Ekman (neotype [designated here] UPS:F-946515∗!, isoneotypes H7009710!, TU135086!). MycoBank typification MBT10001415.
Description: Basidiocarps gelatinous, first pustulate, then flattened, often centrally depressed, discoid or slightly cerebriform, sometimes with short stalk. Gregarious, occasionally can coalesce with shapes of single basidiocarps remaining visible; separate basidiocarps up to 3 mm in diam. Colour from translucent light yellow to brown with lighter internal core of subiculum inside of mature basidiocarps, dark brown to black when dried, very rarely of orange shades. Sometimes young basidiocarps grow directly on older ones. Hyphae clamped, in subiculum heavily gelatinized, 2.5–3.5 μm in diam, rarely swollen, walls 0.3–0.8 μm in width. Subhymenial hyphae densely arranged, 2.5–4.5 μm in diam, walls 0.3–0.8 μm in width. In cupulate basidiocarps sterile areas and margins covered with terminal hyphae 3.5–5 μm in diam, walls 0.5–1.0 μm in width. On borders between fertile and sterile areas branched anastomosing hyphae are often abundant. Hymenium consists of basidia and rare cylindrical hyphidia with few short branches, 2–3.5 μm in diam, walls width varies from up to 0.8 μm in the base of hyphidium to ∼ 0.3 μm at the top. Basidia clavate 17–56 × 2.5–5 μm, with thicker walls in the bases. Sterigmata up to 31(–38) μm in length (n = 87/9). Basidiospores slightly curved-cylindrical, aseptate, binucleate, (9.0–)9.7–14.4(–15.6) × (3.0–)3.1–4.9(–5.3) μm, L = 12.1 μm, W = 4.0 μm, Q = 3.0, Q’ = 2.4–4.2 (n = 140/10), walls ∼ 0.2–0.3 μm in width.
Habitat and distribution: Gymnosperm wood (Picea, mostly Pinus, and unident.); Europe, mostly northern part.
Material examined: Estonia, Viljandi co., Põhja-Sakala par., Soomaa National Park, on gymnosperm wood, 16 Sep. 2018, O. Miettinen (OM henceforth) 21763 (TU135048∗), 21768 (TU135049∗). Finland, Pohjois-Häme prov., Jyväskylä mun., Vuoritsalo, on Pinus sylvestris, 22 Jun. 2008, OM 12740.1 (H6012436∗), on Pinus (?), 22 Aug. 2010, OM 14095 (H∗), on Pinus sylvestris, 15 Jul. 2017, OM 20996 (H∗), Kivijärvi mun., Viinakangas, on P. sylvestris, 31 Aug. 2017, OM 21288 (H∗), 21292 (H∗), Saarijärvi mun., Pyhä-Häkki National Park, on P. sylvestris, 24 Jul. 2017, OM 21034 (H∗); Etelä-Häme prov., Urjala mun., Nuutajärvi, on P. sylvestris, 6 Aug. 2017, OM 21058 (H∗). France, Aveyron dept., on Pinus, 17 May 1915, A. Galzin 17717 or Herb. H. Bourdot 19460 (lectotype of Dacrymyces deliquescens var. castaneus, PC). Norway, Innlandet co., Åsnes mun., Vermunden, on P. sylvestris, 3 Jun. 1983, A.-E. Torkelsen 27/83 (O160046∗). Sweden, Kalmar co., Nybro mun., Rismåla naturreservat, on Pinus, 5 Jul. 2017, T. Knutsson (UPS:F-946511∗), Öland, Borgholm mun., Bödakusten västra naturreservat, Byrums Sandvik, on P. sylvestris, 2 Oct. 2017, J.C. Zamora (UPS:F-946508∗); Norrbotten co., Övertorneå mun., Luppioberget, on P. sylvestris, J.C Zamora & P. Posio (UPS:F-946514∗); Stockholm co., Stockholm, Årsta, on P. sylvestris, 10 Sep. 1017, J.C. Zamora & P. Posio (UPS:F-941018∗), Stockholm, on gymnosperm wood, 1893, L. Romell 2461 (isotype of Dacrymyces romellii, UPS:F-729991); Uppsala co., Uppsala mun., Årby skog, Storvreta, gymnosperm wood, 14 May 1930, S. Lundell 862 (holotype of Dacrymyces punctiformis, UPS:F-015301∗), Uppsala mun., Vänge, close to the east limit of Fiby Urskog Nature Reserve, on gymnosperm wood, 7 Apr. 2017, J.C. Zamora, S. Ekman, M. Westberg & M. Svensson (UPS:F-941016∗), Tierp mun., forest 5 km NW of Nilsbo, on P. sylvestris, 31 Mar. 2017, S. Ryman 9264d (UPS:F-941017∗). Ukraine, Zakarpattia reg., Rakhiv dist., Carpathian Biosphere Reserve, near Lysycha alpine meadow, on Picea abies, 8 Aug. 2015, O. Akulov AS0078.1 (CWU(MYC), TU135066∗).
Notes: Basidiocarps of C. tortus are similar to the ones of C. neuhoffii, but generally larger, more light-coloured, occasionally yellowish when fresh or dull orange when dry. In addition, C. tortus has a northern distribution and does not produce such easily coalescing groups as C. neuhoffii or C. creber. The branched hyphidia of C. tortus are rare and more robust than of any other gelatinous Cerinomyces species. Cerinomyces lipoferus is different from C. tortus by its in average wider basidiospores, as well as by longer basidia and much higher content of lipid droplets in hyphae. In C. lipoferus, single-septate basidiospores are rare but consistently present, whereas in C. tortus they occur only in exceptional cases (e.g., in the specimen Miettinen 21768). Other affined taxa, C. aff. tortus 1 and 2, possess well-branched hyphidia. In Zamora & Ekman (2020) C. tortus sensu typi was referred to as Dacrymyces tortus s. lat. 3.
When describing Tremella torta, Willdenow (1788) mentioned that it is a rare fungus occurring in hedges near Berlin, but he did not elaborate much on morphology. Fries (1828) transferred the species to Dacrymyces and specified Pinus as the only substrate. He also synonymized Dacrymyces lacrymalis sensu Sommerfeld with D. tortus. It caused some confusion, since Sommerfeld’s concept of D. lacrymalis is a mix of two species: one growing on gymnosperm wood and another one on angiosperm (Sommerfelt 1826). Fries’ herbarium in UPS contains two specimens that both support the current concept of C. tortus as a conifers-dwelling species. We could not prove that this material was used for the sanctioning description, and therefore it is unavailable for lectotypification (ICN 2018, Art. 9.4).
Later Bourdot (1932) described Dacrymyces deliquescens var. castaneus characterized by discoid, short stalked, brown with orange tint basidiocarps, absence of hyphidia, and occasionally 1-septate basidiospores. We think the variety represents a well-developed C. tortus s.l. (For information on D. castaneus Rabenh. see the next section.) Neuhoff (1934) added superfluous D. punctiformis and D. romellii. Excellent condition of D. punctiformis holotype allowed us to sequence ITS region that, together with the typical morphology, confirmed the type identity to C. tortus. We have checked D. romellii isotype and found that it is very scanty, contains no spores, and is massively contaminated by intrahymenial Tremella species. Curiously enough, Neuhoff in pers. comm. to Kennedy (1957) said that D. romellii is a “luxuriant state” of D. punctiformis, which is opposite to the state of the types. Basidiocarps and hyphal structure of D. romellii are similar to C. tortus, though some microstructural differences are present, such as hyphal knots in subiculum and more thick-walled hyphidia. We consider it a synonym of C. tortus, assuming morphological peculiarities could be caused by the parasite and the old specimen age, respectively.
Cerinomyces aff. tortus 1. Fig. 13, Fig. 44.
Fig. 44.
Cerinomyces aff. tortus 1 micromorphology. A. Spores. B. Hymenium with short basidia and hyphidium, and subhymenium. C. Full-sized basidia and well-developed hyphidium. D. Internal hyphae with gelatinous “pouches”. Drawn from UPS:F-946509 (A, B); UPS:F-946504 (C, D). Scale bars = 5 μm.
Description: Basidiocarps gelatinous, pustulate, often with a central depression when mature, rooted in substrate. Colour from translucent light yellow to brown when fresh, dark brown to black when dried. Basidiocarps up to 0.5(–1) mm in diam, gregarious, close groups can coalesce with the shapes of separate basidiocarps remaining visible. Hyphae clamped, in subiculum agglutinated, 2.5–4.5 μm in diam, walls 0.3–0.5 μm in width with uneven gelatinous layer up to 1.5 μm. Subhymenial hyphae in a very thin layer, 2–3(–5) μm in diam, walls 0.3 μm in width. Sterile areas and margins covered with terminal hyphae 3–4 μm in diam, walls 0.3 μm in width, gelatinized. Hymenium consists of basidia and branched hyphidia up to 70 μm in length, with base 2–3 μm in diam, and apical part 1–2 μm in diam. Basidia clavate, 11–32 × 2.5–4.5 μm, with sterigmata up to 24 μm in length (n = 60/2). Basidiospores cylindrical, slightly curved, aseptate, binucleate, (8.2–)9.0–12.0(–12.4) × 3.0–4.1(–4.4) μm, L = 10.4 μm, W = 3.6 μm, Q = 2.9, Q’ = 2.3–3.7 (n = 60/2), walls ∼ 0.2 μm in width.
Habitat and distribution: Gymnosperm wood (Pinus); Northern Europe.
Material examined: Finland, Perä-Pohjanmaa prov., Rovaniemi, on Pinus sylvestris, 14 Jul. 2018, J.C. Zamora & P. Posio (UPS:F-946509∗, H6014413). Sweden, Kalmar co., Öland, Borgholm mun., Bödakusten västra Nature Reserve, Byrums Sandvik, on P. sylvestris, 2 Oct. 2017, J.C. Zamora (UPS:F-946504∗).
Notes: The taxon is similar in basidiocarp appearance and distribution to C. tortus, C. lipoferus, C. neuhoffii, and C. aff. tortus 2, though the first two do not have abundant, finely branched dendroid hyphidia. In turn, basidiospores of C. aff. tortus 1 are averagely smaller than of C. neuhoffii and larger than of C. aff. tortus 2, though additional collections are needed to confidently delimit the taxa. Related Japanese material is discussed under C. aff. tortus 2.
Cerinomyces aff. tortus 2. Fig. 13, Fig. 45.
Fig. 45.
Cerinomyces aff. tortus 2 micromorphology. A. Spores. B. Hymenium with hyphidium, and subhymenium. All drawn from UPS:F-940948. Scale bars = 5 μm.
Description: Basidiocarps gelatinous, pustulate, often centrally depressed, later slightly cerebriform, rooted in substrate. Fresh basidiocarps of light yellow to brown colour, becoming dark brown to black when dried. Basidiocarps up to 1.5 mm in diam, gregarious, coalescing when close, with the outlines of separate basidiocarps remaining visible. Hyphae clamped, in subiculum 2.5–4 μm in diam, walls 0.4–0.7(–1.0) μm in width, gelatinized; in subhymenium 2–3(–5) μm in diam, walls 0.3–0.5 μm in width. Margins covered with slightly swollen hyphae 2–3.5 μm in diam, apical parts slightly attenuated, and walls thickened. Hymenium includes branched hyphidia up to 70 μm in total length, with thickened base 2–3 μm in diam, and apical part 1.5–2 μm in diam. Basidia clavate 21–40 × 2.5–4 μm, with sterigmata up to 31 μm in length (n = 30/1). Basidiospores slightly curved-cylindrical, 0(–1)-septate, binucleate, 7.8–10.7 × 3.3–4.2(–4.4) μm, L = 9.4 μm, W = 3.8 μm, Q = 2.4, Q’ = 1.9–3.0 (n = 38/1), walls ∼ 0.2 μm in width.
Habitat and distribution: Gymnosperm wood (Pinus); Northern Europe.
Material examined: Norway, Hedmark co., Kongsvinger mun., Kubergskogen, on Pinus sylvestris (?), 6 Oct. 2018, J.C. Zamora & P. Posio (UPS:F-940948∗, H7009043).
Notes: The taxon highly resembles C. tortus and C. lipoferus in basidiocarp morphology, and C. neuhoffii with C. aff. tortus 1 in presence of well-branched hyphidia. Among them C. aff. tortus 2 has the on average shortest basidiospores. Zamora & Ekman (2020) cited the taxon as Dacrymyces tortus s. lat. 2.
Shirouzu et al. (2020) added two undescribed taxa related to C. aff. tortus 1 & 2. Two collections are similar in the LSU sequences to the Norwegian specimen, but they are likely to represent a separate taxon, cited here as C. aff. tortus 3 (Japan, Honshu, Chūbu reg., Nagano pref., Sugadairakougen, on Pinus densiflora, 12 Jul. 2017, T. Shirouzu HNo.1176 [TNS-F-88757∗], 3 Sep. 2018, HNo.1215 [TNS-F-88780∗]). Another, slightly different, LSU sequence was obtained from a Japanese environmental strain 1611_131A1, but we are not aware of any specimens supporting this taxon.
Cerinomyces tristis Miettinen & A. Savchenko, sp. nov. MycoBank MB 839801. Fig. 10, Fig. 46.
Fig. 46.
Cerinomyces tristis micromorphology. A. Spores. B. Hymenium and subhymenium. C. Hyphidia. D. Marginal hyphae. All drawn from holotype, H7009711. Scale bars = 5 μm.
Typus: USA, New York, Arbutus Lake, on Picea (?), 17 Sep. 2013, O. Miettinen 16934 (holotype H7009711∗!, isotype NY!).
Etymology: tristis (Lat.) — dull coloured; reference to basidiocarp colour.
Description: Basidiocarps arid, with relatively thick cottony subiculum, hymenial surface solid and smooth, light ochraceous with whitish margin; covering several cm in the longest dimension. Hyphae clamped, in subiculum loose, 2–4 μm in diam, walls 0.2–0.6 μm in width. Subhymenial hyphae of the same type, wall thickness decreases towards hymenium. Margin covered with simple or rarely branched thin-walled hyphae 1.5–2 μm in diam. Hymenium consists of basidia and rare hyphidia with thickened base up to 3 μm in diam and rarely branched cylindrical apical part up to 2 μm in diam; 15–40 μm in total length, often in groups. Basidia clavate, 9–22 × 2.5–5 μm. Sterigmata up to 16 μm in length (n = 45/3). Basidiospores long-ellipsoid to slightly curved-cylindrical, aseptate, 6.0–8.9(–9.7) × (2.5–)2.7–4.0(–4.1) μm, L = 7.4 μm, W = 3.3 μm, Q = 2.2, Q’ = 1.6–2.8 (n = 108/3), walls ∼ 0.2 μm in width.
Habitat and distribution: Gymnosperm wood (Larix, Picea [?], Pinus, Tsuga, and unident.); North America.
Material examined: Canada, British Columbia, Columbia-Shuswap dist., Bugaboo Provincial Park, on gymnosperm wood, 19 Aug. 1982, N. Hallenberg & L. Hallenberg 6649 (GB-0071225∗); Ontario, Nipissing dist., Lake Temagami, Bear Island, on Pinus, 14 Aug. 1946, H.S. Jackson (NY:ex TRTS20941). USA, Oregon, Cascade Head Expt. Forest, Siuslaw Nat. Forest, on Larix, 17 Nov. 1971, M.J. Larsen (CFMR:FP-133094∗); Washington, Callam co., Olympic National Park & Forest, Rugged Ridge, on Tsuga heterophylla, 21 Oct. 2014, O. Miettinen 19013 (H∗).
Notes: Cerinomyces trisis, together with C. atrans, C. favonius, and C. fugax, belongs to a difficult morphological group of North American conifers-dwelling species. Among them, C. tristis is characterized by the shortest basidiospores.
A possibly related to C. tristis specimen demonstrates some of the lowest Q values in Cerinomyces, having basidiospores (5–)5.7–7(–7.2) × (2.9–)3.1–3.9(–4) μm, L = 6.3 μm, W = 3.5 μm, Q = 1.8, basidia 11–19 × 3.5–4 μm, and sterigmata up to 13 μm in length (Canada, British Columbia, Vancouver island, China beach, on Picea sitchensis, 20 Sep. 1967, B. Eriksson, J. Eriksson 8401 & J. Ginns [GB-0071226]). Hilar appendix in spores of this specimen is often distanced from the hilum itself. This pattern was also observed in the holotype of C. nepalensis, potentially indicating spore immaturity. For the same Canadian specimen, authors of “The Corticiaceae of North Europe” reported 3- to 4-sterigmate basidia (Eriksson & Ryvarden 1973). We could not confirm any of these, but 3-sterigmate basidia do occasionally occur in other species of Cerinomyces (own observations). Interestingly, the specimen NY:ex TRTS20941 bears a name “Ceracea temagamensis Jacks.” — the author correctly assumed it belongs to a new species, but never published the result. Sequence of O. Miettinen 19013 was produced during the revision of this study; it was not used in the analyses but matches other included sequences.
Cerinomyces verecundus A. Savchenko, sp. nov. MycoBank MB 839802. Fig. 10, Fig. 47.
Fig. 47.
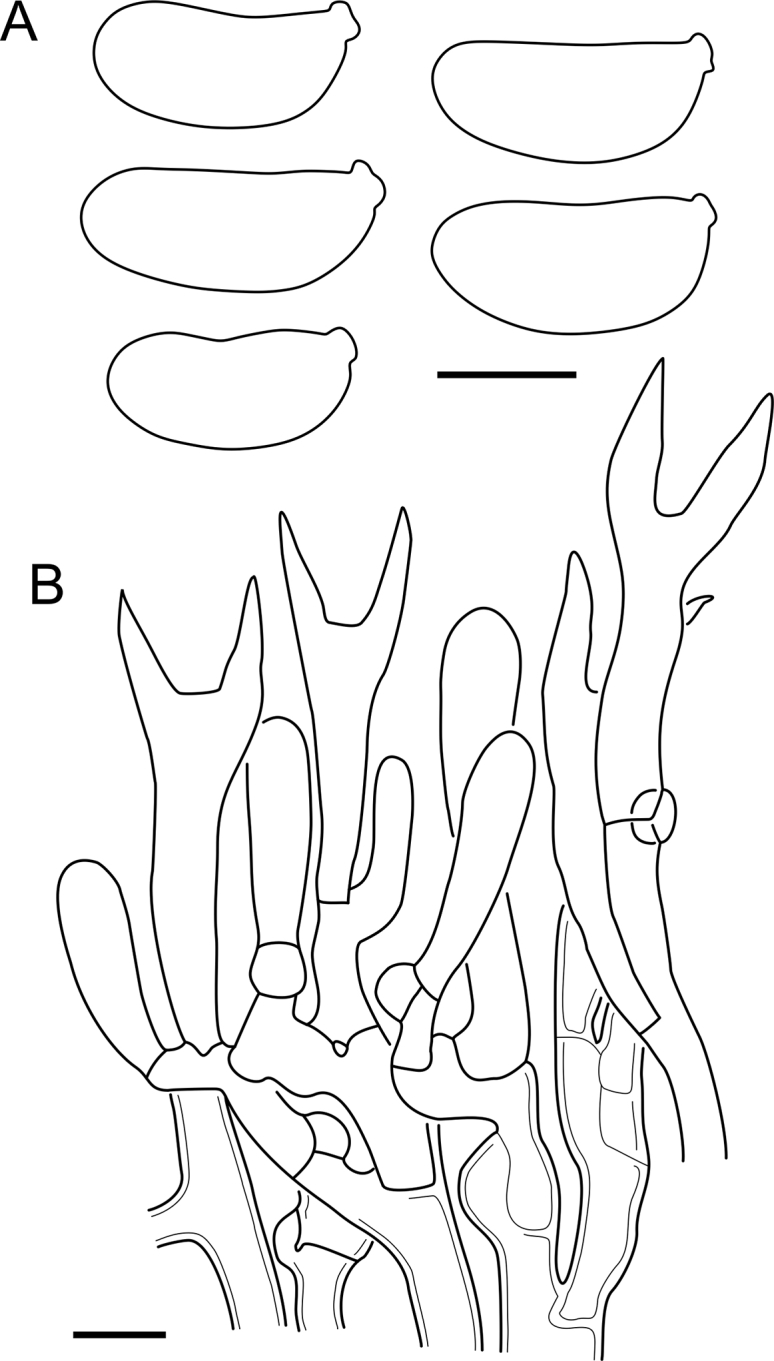
Cerinomyces verecundus micromorphology. A. Spores. B. Hymenium and subhymenium. All drawn from holotype, PDD93708. Scale bars = 5 μm.
Typus: New Zealand, Northland, near Whangarei, Glenbervie Pine Forest, on Pinus radiata, 8 Aug. 2007, A.J. O’Donnell & B.C. Paulus 4851 (holotype PDD93708∗!, isotype TU135087!).
Etymology: verecundus (Lat.) — modest, shy; as a reference to bleak basidiocarps.
Description: Basidiocarps arid, hymenial surface solid, crustose, dull grey to pale ochraceous, damaged or prominent areas brown; subiculum thin or indistinct; margin either fimbriate and white or absent. Hyphae clamped, not differentiated, 2–3 μm in diam, walls 0.3–0.5 μm in width, gelatinized. Hymenium consists of broadly clavate basidia 12–25 × 2.5–4.5 μm. Sterigmata up to 15(–17) μm in length (n = 30/1). Basidiospores cylindrical, slightly curved, aseptate, (7.9–)8.0–10.1(–10.5) × (3.0–)3.1–4.5(–4.8) μm, L = 9.2 μm, W = 3.8 μm, Q = 2.4, Q’ = 2.0–2.9 (n = 30/1), walls ∼ 0.2 μm in width.
Habitat and distribution: Gymnosperm wood (Pinus); New Zealand (known only from the type locality).
Notes: Comparing C. verecundus to its closest relative, C. atrans, the former has larger basidiospores and occurs only in New Zealand. Notably, there are more differences in TEF1-α sequences of the two species than in ITS regions that are nearly identical.
Cerinomyces volaticus A. Savchenko, V. Malysheva & J.C. Zamora, sp. nov. MycoBank MB 839803. Fig. 10, Fig. 48.
Fig. 48.
Cerinomyces volaticus micromorphology. A. Spores. B. Hymenium and subhymenium. C. Hyphidium and young probasidium. Drawn from holotype, S:F250344 (A); GB:K.-H. Larsson 8688 (B, C). Scale bars = 5 μm.
Typus: Sweden, Uppsala co., Älvkarleby mun., 6 km NNW from Gårdskär, Långsandsörarna isl., on gymnosperm wood, 30 Aug. 1991, N. Lindqvist 18723 (holotype S:F250344∗!).
Etymology: volaticus (Lat.) — unreliable, inconstant; reference to variation in basidiocarp robustness and in spore sizes among specimens.
Description: Basidiocarps arid, first arachnoid and remaining so on indistinct white margins; in the middle thicker, smooth and solid, light to rarely dark ochraceous. Subiculum thin, whitish, visible in well-developed areas through cracks. Collapsed old basidiocarps often form a dark brown underlayer. Hyphae clamped, subicular hyphae 2–4 μm in diam, walls 0.3–0.5(–1.0) μm in width, slightly gelatinized; in subhymenium 2–3.5 μm in diam, walls 0.2–0.3 μm in width. Hymenium contains occasional simple cylindrical or barely branched hyphidia with thickened base and thin apical part; up to 40 μm in total length. Basidia clavate, 10–26 × 2–5 μm (n = 108/7), with sterigmata up to 17 μm in length. Basidiospores cylindrical, slightly curved, aseptate, (6.1–)6.5–9.3(–10.1) × (2.5–)2.6–3.7(–3.9) μm, L = 7.9 μm, W = 3.1 μm, Q = 2.5, Q’ = 2.0–3.4 (n = 178/7), walls ∼ 0.2 μm in width.
Habitat and distribution: Gymnosperm wood (Picea, Pinus, and unident.); Europe, mostly northern part.
Material examined: Estonia, Saare co., Torgu par., [near] Mässa, forest div. block 208, 12 Aug. 1999, E. Larsson 23-99 (GB, TU114612). France, Haute-Savoie dept., cirque du Fer-à-Cheval, forest at a foot of "Cornes de Chamois", on Picea, 29 Aug. 1997, B. Duhem 3683 (PC0706779∗). Norway, Finnmark co., Sør-Varanger mun., Ødevann i Øvre Pasvik National Park, on Pinus sylvestris, 10 Aug. 1976, L. Ryvarden 13602 (O101810), 13605 (O160847); Møre og Romsdal co., Aure mun., Skålvassdalen, on P. sylvestris, 26 Aug. 2003, F. Oldervik 402.03 (O189348∗); Rogaland co., Hjelmeland mun., Furunesvatnet, on P. sylvestris, 15 Oct. 1998, K.-H. Larsson 8688 (GB); Sør-Trøndelag co., Orkdal mun., Grytdalen Nature Reserve, on Picea abies, 26 Aug. 1982, L. Ryvarden 20204 (O101813); Vest-Agder co., Sirdal mun., Skotet, on P. sylvestris, 17 Sep. 2012, J. T. Klepsland 12-S025 (O:F-247959∗). Russia, Arkhangelsk reg., Krasnoborsky dist., Verkhne-Vashkinskiy forest, near Sestra river, P. sylvestris, 5 Aug. 2013, V. Kotkova (LE295748∗, TU135064∗); Leningrad reg., Vyborgsky dist., Zapadnyi Beryozovyi island, on Pic. abies, 8 Jul. 2004, V. Kotkova (LE242249∗, TU135063∗). Sweden, Jönköping co., Värnamo mun., Björs, on Pic. abies, 16 Oct. 1960, J. Eriksson, Herb. Erikss. 873 (GB-0071193∗); Västra Götaland co., Alingsås mun., E of Valebråta, 8 Sep. 1969, K. Hjortstam 1926 (GB-0071206∗), Lerum mun., Östad parish, SW of Skäfthult, on Pic. abies, 11 Jun. 1970, K. Hjortstam 3277 (GB-0071201), Vårgårda mun., 400 m N of Kampetå, on Pic. abies, 19 Aug. 1972, K.-H. Larsson 890 (GB-0071205).
Notes: Cerinomyces volaticus seems to be a common species in Northern Europe, and it was also found in France and reported from Germany as C. pallidus (Huckfeldt & Hechler 2004). Many of the specimens in European herbaria labelled as C. crustulinus belong to this species. It is similar in appearance to C. borealis, but can be separated by its shorter and thicker basidiospores, presence of hyphidia, and more robust basidiocarps. Note though, that some specimens show intermediate morphologies and can be difficult in identification. Spore shape and size vary between individuals: for instance, the collection O189348 has more ellipsoid, smaller than usual, and probably immature basidiospores, measuring 5.1–7.8 × 2.4–3.5 μm, Q = 2.3. A specimen presumably belonging to C. volaticus was recorded in Italy from Tamarix gallica (Losi 2003), but otherwise C. volaticus is strictly associated with gymnosperm wood. The most closely related species to C. volaticus is the North American C. fugax, and the two are hardly distinguishable by morphology.
Dacrymyces burdsallii A. Savchenko, sp. nov. MycoBank MB 839804. Fig. 15, Fig. 49.
Fig. 15.
Basidiocarps of Dacrymyces species from the D. grandinioides group, in dry state unless otherwise specified. A.D. burdsallii (holotype, CFMR:HHB-6908). B. D. ceraceus (holotype, CFMR:HHB-8969). C.D. cereus (lectotype, FH00304801). D.D. grandii (holotype, NCSLG21158). E.D. grandinioides, in nature (H7008841). F. D. lagerheimii, partially rewetted (S:F19444). G.D. pulchrus (holotype, LSU00135939). H.D. sobrius (holotype, CFMR:RLG-13487). I. D. venustus (holotype, O:Adane 150). J.D. aff. venustus 1 (LY7839). Scale bars = 5 mm.
Fig. 49.
Dacrymyces burdsallii micromorphology. A. Spores. B. Hymenium. All drawn from holotype, CFMR:HHB-6908. Scale bars = 5 μm.
Typus: USA, Florida, Marion co., Ocklawaha river, on Taxodium distichum, 3 Aug. 1972, H.H. Burdsall (holotype CFMR:HHB-6908∗!).
Etymology: In honour of the North American mycologist Harold H. Burdsall.
Description: Basidiocarps appear as patches of cottony white subiculum, later coalescing. Subiculum thick, covered with solid, arid to waxy, light ochraceous to yellowish brown hymenium; margin wide, fimbriate, white. Hyphal pegs present in older areas, rare, irregularly scattered, up to 250 μm in height. Hyphae without clamps, subicular hyphae 2–4 μm in diam, walls ∼ 0.5 μm in width; in subhymenium of the same diam, walls ∼ 0.3 μm in width; in margin terminal hyphae cylindrical, 2–3 μm in diam, walls 0.3–0.4 μm in width. Hyphae in pegs agglutinated, densely arranged, similar to marginal. Octahedral transparent crystals abundant in subiculum, with the longest edge 15–30 μm and more. Crystal agglomerations visible by the naked eye. Small crystals also occur in margin. Hymenium simple, basidia clavate, 24–48 × 2.5–6 μm, with sterigmata up to 48 μm in length (n = 30/1). Basidiospores cylindrical, slightly curved, 0–3-septate, (11.6–)11.9–15.0 × (4.8–)4.9–6.1(–6.3) μm, L = 13.4 μm, W = 5.3 μm, Q = 2.5, Q’ = 2.1–3.0 (n = 30/1), walls ∼ 0.3–0.6(–1.0) μm in width.
Habitat and distribution: Gymnosperm wood (Taxodium); North America.
Material examined: USA, Florida, Alachua co., Austin Cory Forest, picnic area, on Taxodium distichum, 20 Jul. 1972, H.H. Burdsall (CFMR:HHB-6685).
Notes: Compared to the allied North American species, D. burdsallii has smaller microstructures and much less abundant pegs than D. grandii — another species that also inhabits gymnosperm wood. At the same time, D. ceraceus and D. sobrius apparently prefer angiosperm substrates. Aside the intron TTGAGAG present in the end 5.8S, D. burdsallii is different from relatives by changes in the ITS, SSU, LSU and RPB2 regions. A non-sequenced specimen CFMR:HHB-6685 possesses swollen up to 6.5 μm cells in subhymenial hyphae, but never as wide as in D. ceraceus.
Dacrymyces ceraceus (Ginns) A. Savchenko, comb. nov. MycoBank MB 839814. Fig. 15, Fig. 50.
Fig. 50.
Dacrymyces ceraceus micromorphology. A. Spores. B. Hymenium with full-sized basidia. C. Hymenium with short basidia. D. Marginal hyphae. All drawn from holotype, CFMR:HHB-8969. Scale bars = 5 μm.
Basionym: Cerinomyces ceraceus Ginns, Canadian Journal of Botany 60 (4): 519 (1982).
Typus: USA, Mississippi, Harrison co., Choctaw Creek Basin, Harrison Expt. Forest, Rd H5, Desoto National Forest, on Magnolia grandiflora, 5 Apr. 1976, H.H. Burdsall (holotype CFMR:HHB-8969∗!, isotype UBC 6160?).
Description: Basidiocarps appear as cottony white subicular patches, later covered with solid, ochraceous to yellowish brown hymenium, arid to waxy, with rare scattered hyphal pegs up to 200 μm in height. Margin wide, fimbriate, white. Hyphae without clamps, subicular hyphae 2–4 μm in diam, walls 0.5–1.0 μm in width; subhymenial hyphae 2–3.5 μm in diam, walls 0.3–0.7 μm in width. Swollen cells up to 20 μm, with thickened walls, occasional to abundant, occurring primarily in subhymenium. Marginal hyphae have cylindrical to clavate ends often with few constrictions, 3–5 μm in diam, with walls 0.5–1.2 μm in width. Margin contains crystal aggregations, marginal and hymenial hyphae often covered with crystal shielding. Hymenium densely arranged, basidia clavate, 25–46 × 3.5–6.5 μm, with sterigmata up to 27 μm in length (n = 30/1), basidial wall often slightly thickened towards the base. Basidiospores slightly curved-cylindrical, 0–3-septate, (11.9–)12.0–16.5(–16.6) × (4.3–)4.4–6.0 μm, L = 13.7 μm, W = 5.1 μm, Q = 2.7, Q’ = 2.2–3.3 (n = 30/1), walls ∼ 0.6–0.8(–1.0) μm in width. Many basidiospores constricted at septa and have swollen walls.
Habitat and distribution: Angiosperm wood (Magnolia); North America (known only from the type locality).
Notes: Dacrymyces ceraceus is the most similar species to D. sobrius, while related D. burdsallii and D. grandii differ in growing on coniferous substrates. Marginal cells, often with moniliform constrictions that were not found in other species, can help in identification. Dacrymyces ceraceus is conventionally characterized by the presence of wide swollen compartments in subhymenial hyphae. However, abundance of swollen cells varies highly in preparations even from the same basidiocarp, and sometimes they can be found in other species (D. burdsallii, D. grandinioides, and D. sobrius) so this character should be used with caution. Another character that was utilized in the key by Ginns (1982), thickness of hyphal wall and its level of gelatinization in KOH, have doubtful value as an identification cue. It is often difficult to tell apart the wall itself and gelatinous layer, while the extent of gelatinization varies, depending on time of exposure to water, age of specimens, and nature conditions before collection. At this point, the most reliable way to identify the species is sequencing of ITS marker. As for culture characteristics, Ginns (1982) reported obovoid to cylindrical, 3–4.5 × 2 μm conidia produced in monokaryotic culture.
The ITS region of CFMR:HHB-6817 (USA, Florida, Levy co., State Route 24, near Otter creek, on Taxodium distichum, 27 Jul. 1972, H.H. Burdsall) aligns well with the D. ceraceus ex-type sequence but differs in a few base pairs. Considering low sequence quality, we cannot conclude whether it is an indication of a separate species or intraspecific variation. Notably, the specimen also has slightly moniliform terminal cells.
Dacrymyces cereus (Rick) A. Savchenko, comb. nov. MycoBank MB 839815. Fig. 15, Fig. 51.
Fig. 51.
Dacrymyces cereus micromorphology. A. Spores. B. Hymenium with short basidia. C. Hymenium with full-sized basidia. Drawn from BPI726063 (A); lectotype, FH00304801 (B, C). Scale bars = 5 μm.
Basionym: Ceracea cerea Rick, Brotéria, Ciências Naturais 5: 75 (1936).
Typus: Brazil, Rio Grande do Sul, São Leopoldo, 1933, J.E. Rick (lectotype [designated here] FH00304801!). MycoBank typification MBT10001416.
Description: Basidiocarps corticioid, hymenial surface waxy-gelatinous, smooth and solid, mostly light yellow, becoming dark yellow and light brown in the older areas; subiculum thin, cottony; margin indistinct. Hyphae without clamps, in subiculum 2–4 μm in diam, with walls 0.4–0.8 μm in width, gelatinized. Subhymenial hyphae 2–3 μm in diam, walls 0.3–0.6 μm in width. Hymenium consists of basidia and rare cylindrical hyphidia. Basidia clavate, 16–45 × 3–7 μm, with sterigmata up to 45 μm in length (n = 70/3). Basidiospores slightly curved-cylindrical, 0–3-septate, sometimes constricted at septa, (9.7–)10.4–13.9(–14.0) × (4.0–)4.1–5.9(–6.1) μm, L = 12.0 μm, W = 4.9 μm, Q = 2.5, Q’ = 1.8–3.9 (n = 69/3), walls ∼ 0.2–0.4 μm in width.
Habitat and distribution: Unidentified wood; South America.
Material examined: Brazil, Rio Grande do Sul, São Leopoldo, 1930, J.E. Rick (original material, BPI726063); Sergipe, Areia Branca, Parna Serra de Itabaiana, 17 Nov. 2014, R. Chikowski 1167 (URM∗).
Notes: The species is similar to D. lagerheimii but has smaller basidiospores and much more delicate basidiocarps. Basidiocarp in the specimen BPI726063 is partially covered with visible by the naked eye large crystal agglomerations of unknown origin. Another South American species that does not demonstrate hyphal pegs, D. pulchrus, has substantially longer basidiospores with up to five septa.
A specimen without pegs, with similarly small basidiospores 11.7–13.6 × 4.3–5.1 μm, and abundant conidia 3.0–3.3 × 2.2–3.0 μm was collected from Tahiti (Punaauia, on Hibiscus tiliaceus, 21 May 1956, L.S. Olive T272 [BPI726045]) and reported as Arrhytidia lagerheimii (Olive 1958a), but we refrain from assigning it to D. cereus considering scanty material and geographical distance between the finds.
Dacrymyces confluens P. Karst., Fungi Europaei et Extraeuropaei Exsiccati, Centurie XXXVI, no. 3522 (1886). Fig. 14, Fig. 52.
Fig. 52.
Dacrymyces confluens micromorphology. A. Spores. B. Hymenium with hyphidia, and subhymenium. Drawn from lectotype, H6049587 (A); Ceracea aureofulva lectotype, H7009712 (B). Scale bars = 5 μm.
Synonyms: Dacrymyces paradoxus P. Karst., Hedwigia 25: 232 (1886).
Ceracea aureofulva Bres., Annales Mycologici 4 (1): 39 (1906).
Cerinomyces aureofulvus (Bres.) V. Malysheva, Acta Mycologica Warszawa 44 (1): 4 (2009), nom. illeg.
Typus: Finland, Etelä-Häme prov., Mustiala, on Pinus sylvestris, Sep. 1886, P. Karsten, Karsten Herb. No. 1344, in Rabenhorst & Winter, Fungi Eur. et Extraeur. Exsicc., no. 3522 (lectotype [designated here] H6049587!). MycoBank typification MBT10001417.
Description: Basidiocarps originate as small fimbriate white patches, becoming smooth and solid with waxy-gelatinous hymenial surface, yellow to orange when fresh, ochraceous and brown when dried, with thick white cottony subiculum and margin, up to 1 cm in the longest dimension, of relatively circular form, can coalesce with boundaries of separate basidiocarps remaining visible. Hyphae clamped, gelatinized, in subiculum 2.5–4.5(–6) μm in diam, walls ∼ 0.5 μm in width; subhymenial hyphae 2–3.5 μm, walls ∼ 0.3 μm in width; marginal hyphae simple, with cylindrical or slightly clavate endings, 2.5–4.5 μm, walls ∼ 0.3 μm in width. Hymenium consists of rare simple cylindrical hyphidia up to 80 μm in length and 2–3 μm in diam, and clavate basidia 30–71 × 2.5–6 μm, with sterigmata up to 32 μm in length (n = 41/3). Basidiospores slightly curved-cylindrical, with lipid droplets, 0–1(–3)-septate, (11.6–)12.1–17.4(–19.7) × (4.0–)4.3–6.8(–7.5) μm, L = 14.3 μm, W = 5.6 μm, Q = 2.6, Q’ = 2.0–3.6 (n = 146/5, 50 measured in 1 % KOH), walls ∼ 0.3 μm in width.
Habitat and distribution: Gymnosperm wood (Abies, Pinus and unident.); Europe.
Material examined: Finland, Etelä-Häme prov., Mustiala, Salois (Saloinen), on gymnosperm wood, Sep. 1886, P. Karsten, Karsten Herb. No. 1407 (probable original material of D. confluens H6049575), same loc., on Pinus, 1 Nov. 1886, P. Karsten, Karsten Herb. No. 1321 (original material of D. paradoxus). Germany, Saxony, Königstein, on Abies, date unknown, W. Krieger (probable original material of Ceracea aureofulva: S:F19431), Elbe Mountains, near Schrammsteine, Oct. 1905, W. Krieger, Fungi Saxon. Exs. 1909 (lectotype of Ceracea aureofulva [designated here]: H7009712, isolectotypes LE22381, S:F19424, S:F19438; MycoBank typification MBT10001418).
Notes: Three-septate basidiospores are extremely rare in specimens from both Finland and Germany; perhaps, septation occurs only immediately before germination.
We synonymize Ceracea aureofulva to Dacrymyces confluens considering their high morphological similarity and European distribution. Ceracea aureofulva types have smaller basidiospores than specimens of D. confluens, but we expect this to be a particularity of the group, drawing a parallel from Cerinomyces altaicus–D. corticioides case of similarly wide spore variation. Another synonym, D. paradoxus, was formally published in the same year as D. confluens, but the latter name has a priority: the 25th issue of Hedwigia, where D. paradoxus was first described, also contains a reference to the already published D. confluens (Winter 1886). Further synonymization of D. confluens and D. corticioides, first proposed by von Höhnel (1908), is not carried out considering lack of sequences and gap in the geographic distribution. Specimen S:F19431 of Ceracea aureofulva bears an early provisional name Ce. aurea provided by G. Bresadola and should not be confused with Ce. aurea Rick. Cerinomyces aureofulvus is a superfluous name, because in its protologue D. corticioides was cited as a synonym. Meanwhile, Dacrymyces corticioides is an older name than Ce. aureofulva, and therefore ought to have been adopted as a basionym (ICN 2018, Art. 52.1).
Dacrymyces corticioides Ellis & Everh., Journal of Mycology 1 (12): 149 (1885). Fig. 14, Fig. 53.
Fig. 53.
Dacrymyces corticioides micromorphology. A. Spores. B. Subicular hyphae. C. Hymenium with hyphidia, and subhymenium. D. Marginal hyphae. Drawn from NY00738307 (A, B); syntype, H7009713 (C, D). Scale bars = 5 μm.
Synonyms: Ceracea corticioides (Ellis & Everh.) Pat., Essai taxonomique sur les familles et les genres des Hyménomycètes: 29 (1900).
Cerinomyces altaicus Parmasto, Eesti NSV Teaduste Akadeemia toimetised. Bioloogia: 233 (1961).
Femsjonia uniseptata Shirouzu, Phytotaxa 312 (2): 273 (2017).
Typus: USA, New Jersey, Gloucester co., Newfield, on Pinus, Oct. 1885, J.B. Ellis & B.M. Everhart, Exsiccate: North American fungi. Series II, 1587 (syntypes H7009713!, K, NY00738305!, NY00738306!).
Description: Basidiocarps originate as circular white sterile patches. Mature basidiocarps waxy-gelatinous when fresh, up to 1 cm in the longest dimension, tend to coalesce, but boundaries between coalesced basidiocarps remain visible. Hymenial surface solid and smooth of yellow to orange colour that bleaks to light or dark ochraceous with time. Subiculum cottony, thick, white; margin fimbriate, white. Hyphae clamped, heavily gelatinized, in subiculum 2.5–4.5(–6) μm in diam, walls 0.5–1.0 μm in width; subhymenial hyphae 2–4(–5) μm in diam, walls 0.3–0.5 μm in width; marginal hyphae simple, with cylindrical endings 2–3 μm in diam, walls 0.3–0.5 μm in width, often anastomosing, becoming slightly clavate closer to hymenium. Hymenium consists of basidia and rare simple cylindrical hyphidia up to 70 μm in length and 2.5–3(–3.5) μm in diam. Basidia clavate, 30–56 × 3–6(–7) μm, with sterigmata up to 35 μm in length (n = 40/4). Basidiospores slightly curved, cylindrical, 0–3-septate, (11.2–)11.8–16.3(–17.0) × (4.5–)4.9–7.0(–7.9) μm, L = 13.9 μm, W = 5.6 μm, Q = 2.5, Q’ = 1.9–3.3 (n = 81/6; 30 measured in 1 % KOH), walls ∼ 0.2–0.3 μm in width.
Habitat and distribution: Gymnosperm (Abies, Picea, Pinus, and unident.) and perhaps angiosperm wood; East Asia, North America.
Material examined: Japan, Honshu, Kantō reg., Ibaraki pref., Sakuragawa, Mt. Tsukuba, on Pinus densiflora, 26 Oct. 1985, T. Hosoya (TNS-F-24345). Russia, Altai rep., Turochaksky dist., near Telezkoje lake, middle course of the river Oyor, on P. sibirica (?), 20 Aug. 1959, E. Parmasto (holotype of Cerinomyces altaicus, TAAM008610); Khabarovsk reg., Khabarovsk dist., Bolshekhekhtsirsk forest experimental area, Klyutch Levyi (?), on P. koraiensis, 1 Sep. 1979, P. Gordienko & E. Parmasto (TAAM102301∗), Nanai dist., Arsenyevo village, on P. koraiensis, 19 Sep. 1979, B. Kullman (TAAM120963); Primorsk reg., Chuguyevsky dist., Bulyga-Fadejevo / Sandagou village, Narzan, on Abies nephrolepis, 11 Sep. 1975, E. Parmasto (TAAM059772), same village, Kljuch Bolshoi Medvezhi, on P. koraiensis, 12 Sep. 1975, E. Parmasto (TAAM059890), Kavalerovsky dist., Gornorechensk / Kentsukhe, on P. koraiensis, 9 Oct. 1977, E. Parmasto (TAAM101900, H7019017), Sikhote-Alin Nature Reserve, Ust-Serebryanka river, on P. koraiensis, 14 Sep. 1987, E. Parmasto (TAAM150056∗), Terneysky dist., Kabaniy, on angiosperm wood (?), 18 Sep. 1990, I. Parmaso (TAAM126607∗), same dist., Maysa river, on P. koraiensis, 10 Sep. 1990, E. Parmasto (TAAM150903). USA, Connecticut, Litchfield co., Catlin Woods, E side of Miry Brool, woods of White Memorial Foundation, SW side of Litchfield, 30 Sep. 1978, C.T. Rogerson (NY:as “C. canadensis №1”∗); Georgia, Rabun co., Line branch of Tallulah River, 19 Oct. 1960, C.T. Rogerson 3955 (NY); New Carolina, Macon Co., Highlands Biological Station, on gymnosperm wood, 15 Oct. 1960, C.T. Rogerson 3959 (LSU00173760, NY); New Hampshire, Carroll co., Chocorua, 1906–1907, W.G. Farlow, Exsiccate: Thaxter, Reliquiae Farlowianae 304 (FH00621439, S:F250273), Grafton co., Enfield, shore of lake Mascoma, 24 Sep. 1957, J.A. Stevenson (BPI726048); New Jersey, Gloucester co., Newfield, on Pinus, Oct. 1885, J.B. Ellis & B.M. Everhart Exsiccate (?): North American fungi. Series II, 1587 (original material or syntype: NY00738307), same state, no locality, on Pinus, Oct. 1892, C.L. Shear 2082 (FH00486013); New York, Albany co., Alcove, on P. strobus, Oct. 1892, C.L. Shear, Exsiccate: Shear, New York Fungi 119 (FH00486162, FH00621438), Franklin co., Paul Smith’s, on Picea, 8 Oct. 1960, R.L. Gilbertson (CFMR:RLG-2685), Suffolk co., Shirley, Wertheim NWR, on Pinus rigida, 28 Nov. 2015, M. Horman (NY02686162∗); Maryland, Prince Georges co., Cornell Rd. at Fomes Rd., Beltsville Expt. Forest, Laurel, 5 Feb. 1968, H. Burdsall & H.H. Burdsall (CFMR:HHB-416).
Notes: Dacrymyces corticioides can be identified by conspicuous circular coalescing basidiocarps with white margins that are fimbriate in dry state. Basidiospores are mostly aseptate but become 3-septate at maturity. Majority of the specimens were reported from gymnosperm wood with a single possible exception of TAAM126607. Sequences from TAAM102301, TAAM126607, and TAAM150056 were not used in the analyses, but they are similar to the other included sequences.
Basidiocarps of Femsjonia uniseptata are much more gelatinized and brightly coloured than most of the studied specimens. However, DNA sequences and micromorphology of F. uniseptata are highly similar to D. corticioides: Shirouzu et al. (2017) reported simple hyphidia, basidia 30–40 × 4 μm, and basidiospores 14–17 × 5–6 μm that agree with our description above. The difference in basidiocarp appearance can be due to the old age of C. altaicus and D. corticioides collections that have lost characteristic pigmentation over time — a common tendency in dacrymycetes (Zamora & Ekman [2020] and personal observations). On a side note, many of the D. corticioides specimens from the 20th century bear a misapplied name Arrhytidia involuta.
Dacrymyces grandii A. Savchenko & Miettinen, sp. nov. MycoBank MB 839805. Fig. 15, Fig. 54.
Fig. 54.
Dacrymyces grandii micromorphology. A. Spores. B. Hymenium. All drawn from holotype, NCSLG21158. Scale bars = 5 μm.
Typus: USA, North Carolina, Wayne co., cliffs of the Neuse State Park, on Taxodium distichum, 16 Jul. 1999, L.F. Grand & C. Vernia (holotype NCSLG21158∗!).
Etymology: In honour of the North American mycologist Larry F. Grand.
Description: Basidiocarps first appear as patches of cottony white to light ochraceous subiculum, becoming corticioid, with slightly waxy-gelatinous, solid, ochraceous hymenial surface, thick cottony subiculum and distinct fimbriate margins, covering areas up to 5 cm in the longest dimension. Abundant light-coloured hyphal pegs cover mature areas, up to 350 μm in height, often with fringy apices. Hyphae without clamps, subicular hyphae 2.5–3.5 μm in diam, walls 0.4–0.6 μm in width, sometimes with small crystals. In subhymenium 2–3.5 μm in diam, walls ∼ 0.3 μm in width. Internal hyphae in pegs agglutinated, densely arranged. Terminal hyphae at the top of pegs and in margins simple cylindrical, of the same type as in subhymenium. Hymenium consists of clavate basidia 20–60 × 3–6 μm, with sterigmata up to 51 μm in length (n = 30/1). Basidiospores cylindrical, slightly curved, 0–3-septate, (13.2–)13.6–16.3(–16.4) × (4.8–)5.0–6.2(–6.8) μm, L = 14.9 μm, W = 5.5 μm, Q = 2.7, Q’ = 2.2–3.3 (n = 36/1), walls ∼ 0.3–0.5 μm in width.
Habitat and distribution: Gymnosperm wood (Taxodium); North America.
Notes: The species has averagely the largest basidiospores and basidia among its North American relatives, as well as the most abundant and prominent hyphal pegs.
Dacrymyces grandinioides (McNabb) A. Savchenko, comb. nov. MycoBank MB 839816. Fig. 15, Fig. 55.
Fig. 55.
Dacrymyces grandinioides micromorphology. A. Spores. B. Hymenium and subhymenium. All drawn from H7008841. Scale bars = 5 μm.
Basionym: Cerinomyces grandinioides McNabb, New Zealand Journal of Botany 2: 422 (1964).
Typus: Kenya, Bungoma co., mount Elgon, 17 Apr. 1963, P.H. Irwin 539 (holotype K(M):237139∗!, isotype PDD25551).
Description: Basidiocarps appear as small white subicular patches, later coalescing. Well-developed basidiocarps can cover up to 10 cm in the longest dimension, with thick, solid, waxy-gelatinous, yellow to orange hymenial surface, thick cottony subiculum, white fimbriate margins, and abundant light-coloured hyphal pegs or denticles up to 300 μm in height, oriented downwards. Hyphae without clamps, subicular hyphae 2.5–4.5 μm in diam, with some compartments and hyphal junctions swollen up to 5 μm in diam, walls 0.5–1.0 μm in width; subhymenial hyphae 2–3.5 μm in diam, walls 0.3–0.5 μm in width. Marginal hyphae and hyphae of pegs have cylindrical terminal parts, 2.5–3.5 μm in diam, with walls 0.2–0.4 μm in width. Scattered or aggregated crystals present. Hymenium consists of clavate basidia 22–48 × 2.5–5 μm, with sterigmata up to 46(–55) μm in length (n = 120/4). Basidiospores slightly curved-cylindrical, 0–3-septate, (11.3–)12.0–16.5(–18.3) × (4.3–)4.6–6.5(–6.9) μm, L = 14.3 μm, W = 5.5 μm, Q = 2.6, Q’ = 2.1–3.1 (n = 120/3), walls ∼ 0.2–0.4(–0.5) μm in width.
Habitat and distribution: Angiosperm (Tabernaemontana and unident.) and gymnosperm (Cryptomeria) wood; East Africa.
Material examined: France, Réunion, Saint-Benoît co., Forêt de Bébour, on Cryptomeria japonica, 5 May 1985, J. Boidin (LY11601), (LY11604), (LY11615∗, PDD48050); Saint-Paul co., La Petit France, Route de Maïdo, Ravine Baptiste, alt. ca. 1 500 m (Fundort 5), 14 Mar. 1998, G. Langer & E. Langer (KAS:GEL4761). Kenya, Taita-Taveta co., Taita Hills, Chawia forest, on angiosperm wood, 27 Nov. 2017, A. Savchenko 171127/1129F (H7008890, EA), on Tabernaemontana stapfiana, 27 Nov. 2017, A. Savchenko 171127/1559A (H7008841∗, EA).
Notes: Dacrymyces grandinioides and D. venustus are morphologically cryptic African species. However, their ITS sequences are distinct enough for easy delimitation: for example, D. grandinioides lacks insertion AACGTA in the end of 5.8S region, which is characteristic to the other species. The type specimen of D. grandinioides has smaller basidiospores and more subtle basidiocarps compared to the recent collections, which are also of much deeper orange colour. In the type we recorded cylindrical to curved-cylindrical conidia 3.7–4.1 × 1.3–1.8 μm but were unable to confirm their origin.
Dacrymyces lagerheimii (Pat.) A. Savchenko, comb. nov. MycoBank MB 839817. Fig. 15, Fig. 56.
Fig. 56.
Dacrymyces lagerheimii micromorphology. A. Spores. B. Full-sized basidia. C. Hymenium with short basidia, and subhymenium. D. Marginal hyphae. Drawn from lectotype, FH00304786 (A, C); FH00304787 (B, D). Scale bars = 5 μm.
Basionym: Ceracea lagerheimii Pat., Bulletin de la Société Mycologique de France 9: 141 (1893).
Synonyms: Arrhytidia lagerheimii (Pat.) L.S. Olive, Bulletin of the Torrey Botanical Club 85: 104 (1958).
Cerinomyces lagerheimii (Pat.) McNabb, New Zealand Journal of Botany 2: 421 (1964).
Typus: Ecuador, San Jorge, 1892, N.G. von Lagerheim (lectotype FH00304786!).
Description: Basidiocarps corticioid, pale ochraceous to yellow and orange, brown in old basidiocarps, hymenial surface smooth and solid, waxy-gelatinous when moist; subiculum thick, cottony, lighter than surface; margin indistinct, fimbriate, white. Hyphae without clamps, in subiculum 3–5(–7) μm in diam, with walls 1.0–2.0 μm in width. Subhymenial hyphae 2–4.5 μm in diam, with walls 1.0–1.5 μm in width, but sometimes swollen to 3.5 μm, gelatinized. Marginal hyphae simple cylindrical, similar to subicular. Hymenium consists of clavate basidia 15–64 × 3–8 μm, with sterigmata up to 67 μm in length (n = 80/4), basidial wall often thickened towards the base. Basidiospores slightly curved-cylindrical, 0–3-septate, sometimes constricted at septa, (12.6–)12.8–17.6(–18.5) × (3.5–)5–7.1(–7.6) μm, L = 14.5 μm, W = 6.0 μm, Q = 2.4, Q’ = 1.8–3.7 (n = 125/4), walls ∼ 0.3–0.5(–1.0) μm in width.
Habitat and distribution: Angiosperm wood (Chusquea and unident.); South America (known only from the type locality).
Material examined: Ecuador, San Jorge, Jul. 1892, N.G. von Lagerheim (syntypes FH00304787, FH00304788), same loc., on Chusquea, 1892, same collector (syntype FH00304790), same loc., no date, same coll. (original material?, S:F19444), no loc., no date, same coll. (original material?, S:F19448).
Notes: Dacrymyces cereus and D. pulchrus are morphologically related to D. lagerheimii, except D. cereus has smaller basidiospores and delicate basidiocarps, and D. pulchrus exhibit large, 1–3(–5)-septate basidiospores. Unfortunately, the lectotype of D. lagerheimii contains old basidiocarps of unrepresentative dull brown colour and deformed microstructures, while better preserved specimens like S:F19444 demonstrate yellow colouration. In the absence of sequenced material, and considering the high morphological variability in the group, we limit the scope of the species exclusively to the Ecuadorian collections.
Edward A. Burt labelled but never published “Ceracea triseptata Burt, n. sp.”, that was intended to be different from Ce. lagerheimii by 3-septate basidiospores — the protologue of Ce. lagerheimii mentions only 1-septate spores — and crystalline matter in basidiocarps (Trinidad, 1912–1913, R. Thaxter, FH00304802). It is not known if the specimen stands for a distinct taxon, but small spores and presence of crystals place it closer to D. cereus.
Ginns (1982) suggested to separate Cerinomyces lagerheimii from C. ceraceus and C. grandinioides on the basis of its smooth surface, gelatinization of hyphae in 2 % KOH, and predominantly 1-septate basidiospores. While the absence of pegs is indeed a key feature, the latter two characters can vary depending on the condition and treatment of a specimen, or development stage, respectively.
We did not see conidia in the studied specimens, but Martin (1949) presented illustrations of ellipsoid to cylindrical conidia from the lectotype. Measurements of these conidia (5–7 × 2–3 μm) provided by Ginns (1982) were apparently made from the Martin’s illustration and overestimate the real sizes. McNabb (1964) reported globose to broadly oval conidia 4.5 × 3.5 μm originating from phialide-like conidiophores, but this data most certainly originates from North American specimens that he assigned to the species.
Dacrymyces pulchrus (Lowy) A. Savchenko, comb. nov. MycoBank MB 839818. Fig. 15, Fig. 57.
Fig. 57.
Dacrymyces pulchrus micromorphology. A. Spores. B. Hymenium with short basidia. C. Hymenium with full-sized basidia, and subhymenium. All drawn from holotype, LSU00135939. Scale bars = 5 μm.
Basionym: Arrhytidia pulchra Lowy, Mycologia 64(4): 904 (1972).
Typus: Argentina, Buenos Aires prov., El Tigre, 7 Sep. 1968, J.E. Wright T110 (holotype LSU00135939!).
Description: Basidiocarps corticioid, hymenial surface waxy-gelatinous, smooth and solid, pale ochraceous to yellow and yellow brown; subiculum thin, cottony, white; margin inconstant, fimbriate, white. Hyphae without clamps, subicular hyphae loosely interwoven, 3–5.5 μm in diam, with walls 0.5–1.0 μm in width, gelatinized; subhymenial hyphae densely arranged, of the same type. Hymenium consists of clavate basidia 14–65 × 3.5–8 μm, with sterigmata up to 54 μm in length (n = 30/1), basidial wall often thickened towards the base. Basidiospores cylindrical, slightly curved, 0–3(–5)-septate, sometimes constricted at septa, (13.8–)14.1–19.4(–20.6) × (4.5–)4.8–6.4(–6.6) μm, L = 16.3 μm, W = 5.4 μm, Q = 3.0, Q’ = 2.4–3.7 (n = 29/1), walls ∼ 0.3–0.5 μm in width.
Habitat and distribution: Unidentified, probably angiosperm wood; South America (known only from the type locality).
Notes: Dacrymyces pulchrus is largely similar to D. lagerheimii but differs in presence of 5-septate basidiospores. In contradiction to the protologue, we did not find clamps on hyphae.
Dacrymyces sobrius A. Savchenko, sp. nov. MycoBank MB 839806. Fig. 15, Fig. 58.
Fig. 58.
Dacrymyces sobrius micromorphology. A. Spores. B. Apical part of a hyphal peg. C. Hymenium and subhymenium. All drawn from holotype, CFMR:RLG-13487. Scale bars = 5 μm.
Typus: USA, Louisiana, East Baton Rouge par., east bank of Mississippi river, near Profit Island Road, on Celtis, 1 Sep. 1981, R.L. Gilbertson (holotype CFMR:RLG-13487∗!).
Etymology: sobrius (Lat.) — temperate, sober; referring to morphological similarity to related species.
Description: Basidiocarps corticioid, appear as patches of subiculum, later coalescing, covering areas up to 10 cm in the longest dimension. Thick, cottony, white subiculum covered with solid, waxy-gelatinous, light ochraceous to yellowish brown hymenium; margin fimbriate, white. Hyphal pegs present in mature areas, irregularly scattered, up to 250 μm in height. Hyphae without clamps, subicular hyphae 2–3 μm in diam, walls 0.4–1.0 μm in width; in subhymenium 2.5–4.5 μm in diam, very rarely swollen, walls of the same width; in margin terminal hyphae cylindrical to clavate, 2–3.5 μm in diam, walls of the same width. Hyphae in pegs agglutinated, densely arranged, similar to marginal. Aggregations of crystals occur in subhymenium. Hymenium simple, basidia clavate, 21–52 × 3–7 μm, with sterigmata up to 38 μm in length (n = 41/2). Basidiospores cylindrical, slightly curved, 0–3-septate, 11.0–17.1 × (4.7–)4.9–6.2(–6.3) μm, L = 13.5 μm, W = 5.4 μm, Q = 2.5, Q’ = 1.9–3.2 (n = 50/2), walls ∼ 0.3–0.7 μm in width.
Habitat and distribution: Angiosperm wood (Celtis, Populus); North America.
Material examined: USA, Kentucky, Hancock co., Hawesville, Wilamette Industries, on Populus deltoides, 22 May 1986, K. Nakasone (CFMR:FP-102085∗).
Notes: The species is nearly indistinguishable from Dacrymyces ceraceus and D. burdsallii, except the latter one was collected from coniferous wood, and the rest from deciduous. We saw that swollen cells of D. ceraceus are larger and more abundant than of D. sobrius, in which they can be totally absent in some areas of basidiocarp.
Dacrymyces venustus A. Savchenko, sp. nov. MycoBank MB 839807. Fig. 15, Fig. 59.
Fig. 59.
Dacrymyces venustus micromorphology. A. Spores. B. Hymenium with basidia of different length. C. Apical part of a hyphal peg. All drawn from holotype, O:Adane 150. Scale bars = 5 μm.
Typus: Ethiopia, Oromia dist., Arsi, Munessa, on burned Podocarpus, May–Aug. 2000, A. Bitew (holotype O:Adane 150∗!).
Etymology: venustus (Lat.) — attractive; as a reference to noticeable basidiocarps.
Description: Basidiocarps start as arid, circular, coalescing patches. Hymenial surface solid, waxy-gelatinous, yellow, covered with regular hyphal pegs ≤ 170 μm in height. Subiculum thin, cottony, white; margins fimbriate, concolourous to subiculum. Hyphae without clamps, in subiculum 2.5–4 μm in diam, walls 0.6–0.8 μm in width, masses of octahedral to amorphic crystals present among hyphae. Subhymenial hyphae 1.5–3 μm in diam, walls ∼ 0.3 μm in width. Marginal hyphae simple, cylindrical, 2–3 μm in diam, with walls 0.6–0.8 μm in width. Hymenium consists of clavate basidia 33–59 × 2.5–6 μm, with sterigmata up to 46 μm in length, basidial wall often thickened towards the base. Basidiospores cylindrical, slightly curved, 0–3-septate, often constricted at septa, (12.8–)13.7–16.2(–16.7) × 4.9–6.7(–6.8) μm, L = 14.8 μm, W = 5.5 μm, Q = 2.7, Q’ = 2.1–3.1 (n = 30/1), walls ∼ 0.2–0.4 μm in width.
Habitat and distribution: Gymnosperm wood (Podocarpus); Africa (known only from the type locality).
Notes: The species is morphologically similar to D. grandinioides, and the easiest way to distinguish them is to compare ITS regions. The type was collected from deeply charred wood.
Dacrymyces aff. venustus 1. Fig. 15, Fig. 60.
Fig. 60.
Dacrymyces aff. venustus 1 micromorphology. A. Spores. B. Hymenium and subhymenium. C. Spore-born conidia. D. Marginal hyphae. All drawn from LY7839. Scale bars = 5 μm.
Description: Basidiocarps appear as patches of thick cottony white subiculum later covered with solid, arid to waxy, light ochraceous to yellow or yellowish brown hymenium. Margin fimbriate, white. Hyphal pegs light-coloured, up to 300 μm in height. Hyphae without clamps, subicular hyphae 2–4 μm in diam, walls 0.3–1.0 μm in width; in subhymenium 2–4 μm in diam, walls 0.3–1.0 μm in width; in margin terminal hyphae cylindrical to slightly clavate, 2.5–3.5 μm in diam, walls ∼ 0.3 μm in width. Hyphae in pegs agglutinated, densely arranged, similar to marginal. Octahedral transparent crystals abundant in subiculum, with the longest edge up to 20 μm. Hymenium densely arranged, consists of clavate basidia 17–60 × 3–6 μm with sterigmata up to 34 μm in length (n = 29/1), basidial wall often thickened towards the base. Basidiospores cylindrical, slightly curved, 0–3-septate, (13.1–)13.9–18.0(–18.2) × (4.9–)5.0–6.2(–6.3) μm, L = 15.4 μm, W = 5.6 μm, Q = 2.8, Q’ = 2.3–3.2 (n = 32/1), walls ∼ 0.3–0.5 μm in width. Conidia born from basidiospores, subglobose, 2.3–2.7 × 1.3–1.7 μm (n = 6/1).
Habitat and distribution: Unidentified wood; Africa.
Material examined: Gabon, Ogooue-Ivindo prov., Makoku, île aux chauve souris, 13 May 1976, J. Boidin (LY7839∗).
Notes: The taxon is almost indistinguishable from D. venustus and D. grandinioides. Considering this and also scanty molecular data available for analysis, we do not erect a formal species. The studied specimen also includes Asterostroma species (Russulales).
Notes on taxa historically or morphologically related to Cerinomyces s.l.
Ceracea aurea Rick, Iheringia, Ser. Bot. 2: 51 (1958).
Judging from the original description we suggest that this species belongs to Femsjonia s.l. or even is not related to dacrymycetes. The protologue says: “Aurea, sicca ochracea, margine albo-lansolo. Sporis grosse obliqueque apiculatis, 12–18 × 10 my. Basidiis 10 my latis, mediis, pedicellatis. Ad corticem. A C. [Arrhytidia] flava videtur diversa.” (Rick 1958). Type not traced.
Ceracea brasiliensis Rick, Iheringia, Série Botânica 2: 50 (1958).
The protologue mentions 3-septate ellipsoid basidiospores, allowing to assume that the species is not a member of the Cerinomycetaceae. “Effusa, pallida, ceraceo-mollis, sicca lutea. Sporis 6–7 × 4–5 my, in sicco triseptatis, luteolis. Ad lignum mucidum. Dacryomyces ovisporus Bref., sporis conveniens, forsan huc ducendus.” (Rick 1958). Type not traced.
Ceracea elongata Pat., Memoires de L'academie Malgache VI: 9 (1928).
The species can be related to Femsjonia, because it has effused basidiocarps and large 7-septate basidiospores 21–31 × 7–9 μm. Also cited as Ce. elongata Pat., Bull. Mus. Hist. nat., Paris: 406 (1928). Type not traced, absent in FH (H. Merchant, 04 Mar. 2020, pers. comm.) and PC (loan requests, visits).
Typus: Madagascar, Tananarive, Jan. <1925, M. Waterlot (holotype).
“Cerinomyces bambusicola”
An undescribed taxon proposed by Gminder (2016). The author mentioned brightly orange basidiocarps with meruloid to dentate surface, collected from bamboo and Hagenia abyssinica in high altitude (> 2 400 m a.s.l.) forests of Kafa Biosphere Reserve, Ethiopia. We suspect it to belong to the D. grandinioides group.
Cerinomyces crustulinus var. latisporus B. de Vries, Mycotaxon 28 (1): 86 (1987).
The taxon most probably belongs to the C. tortus clade of the Cerinomycetaceae; the type material is too scanty to connect it with the existing species. Basidiocarps resupinate, gelatinous when water applied, greyish-transparent to light brown and reddish brown; hyphae clamped, 1.4–3(–4) μm in diam; basidia 17–34 × 3.5–8 μm, sterigmata up to 20 μm in length in length (n = 30/1); basidiospores slightly curved-cylindrical, thin-walled, aseptate, (9.0–)9.1–13.0(–15.0) × (3.9–)4.0–5.9 μm, L = 11.2 μm, W = 4.7 μm, Q = 2.4, Q’ = 1.9–3.0 (n = 30/1), walls ∼ 0.2–0.3 μm in width.
Typus: France, Côtes-d'Armor dept., Forêt de Beffou, Taxus, 24 Jul. 1980, B. de Vries 4103 (holotype L0202240!).
Dacrymyces adpressus Grognot, Plantes Cryptogames–Cellulaires du department de Saone-et-Loire avec des tableaux synoptiques: 200 (1863).
The species resembles C. tortus in general but has 3-septate basidiospores and probably belongs to the Dacrymycetaceae as a relative of D. fennicus. The studied lectotype has orange basidiocarps; clamped thick-walled gelatinized hyphae 3–4 μm in diam, cylindrical to slightly clavate on margins; basidia 40–75 × 4–6 μm, sterigmata up to 35 μm in length (n = 7/1); basidiospores slightly curved-cylindrical, thin-walled, 0–3-septate, 12.2–16.0 × 4.8–5.8 μm (n = 6/1), walls ∼ 0.2–0.3 μm in width.
Specimens distributed with Fungi Selecti Gallici exsiccate most likely represent different Dacrymyces species and other fungi collected from both angiosperm and gymnosperm wood, and thus should not be automatically treated as isolectotypes.
Typus: France, Saône-et-Loire dept., Montjeu, on Quercus (?), ≤ 1886, C. Grognot, in Roumeguère, Fungi selecti Gallici exsiccati 2216 (lectotype K(M):257626!).
Dacrymyces castaneus Rabenh., Deutschlands Kryptogamenflora 1: 53 (1844).
Dacrymyces castaneus was described from Italy with a protologue mentioning pustulate gregarious brownish fruitbodies and egg-form spores (Rabenhorst 1844). Concluding from the original description, the species is probably related to D. ovisporus. Note that D. deliquescens var. castaneus Bourdot is not based on D. castaneus: Bourdot (1932) did mention the species in description, but he explicitly pointed out that the two taxa share only the colour. No original material of D. castaneus is known.
Dacrymyces corticioides var. conigenus Ellis & Everh., Journal of Mycology. 2(8):87 (1886).
The species probably belongs to the Cerinomycetaceae, but its precise position cannot be resolved, since the isotypes are extremely scanty and fresh collections are absent. It has a unique substrate among all dacrymycetes, growing on pine cones.
Typus: USA, New Jersey, Gloucester Co., Newfield, on cones of Pinus rigida, 24 May 1886, collector unknown (isotype NY00738309!), May 1886, North American Fungi Exsiccate (Second Series) 2028 (isotype NY00738308!).
Dacrymyces deliquescens var. ardosiacus Bourdot & Galzin, Hyménomycètes de France: 68 (1928).
Concluding from the description, the variety is probably a synonym of C. aeneus.
Typus: France, Aveyron dept., near St. Leruin, 6 Jan. 1910, A. Galzin 5172 (lectotype PC).
Dacrymyces deliquescens var. myriadeus Bourdot & Galzin, Bulletin Trimestriel de la Société Mycologique de France 25: 33 (1909).
Following the description, we assume it is a possible synonym of C. tortus.
Typus: France, Allier dept., Cosne, Forêt de Dreville, 26 Sep. 1906, H. Bourdot 4482 (lectotype PC).
Dacrymyces deliquescens var. nigricans Bourdot & Galzin, Bulletin Trimestriel de la Société Mycologique de France 25: 34 (1909).
The variety used to be associated with D. tortus (Kennedy 1958b), but according to the protologue and revision of McNabb (1973), it possesses at least 3-septate basidiospores and thus belongs to the Dacrymycetaceae, probably as a synonym to D. adpressus.
Typus: France, Allier dept., near St. Priest-en-Murat, 12 Sep. 1906, H. Bourdot 4499 (lectotype PC).
Dacrymyces enatus var. macrosporus L.L. Kenn., Mycologia 50: 902 (1959).
The variety has prominent branched hyphidia like many taxa in the Cerinomycetaceae, but considering regularly 3-septate thick-walled basidiospores, it probably belongs to the Dacrymycetaceae as a relative of D. paraphysatus. It was first published in a not effective way as D. enatus var. brunnescens (Kennedy 1957).
Dacrymyces enatus var. macrosporus has gelatinous basidiocarps dark brown when wet to black when dried, pustulate to pulvinate and cerebriform with short stalks; hyphae clamped, gelatinized; hyphidia finely branched, of 1–2 μm in diam constant through the length, often with several clamps; basidia 31–66 × 3.5–7 μm, sterigmata up to 42 μm in length (n = 30/1); basidiospores slightly curved-cylindrical, 0–3-septate, 13.1–15.1(–15.4) × (5.3–)5.4–6.6(–6.8) μm, L = 14.1 μm, W = 5.9 μm, Q = 2.4, Q’ = 2.2–2.6 (n = 30/1), walls ∼ 0.6 μm in width.
Typus: Panama, Canal Zone, Barro Colorado Island, 23 Aug. 1952, G.W. Martin & A.L. Welden 8662 (holotype BPI725717! ex IA, isotypes NY03684200!, LSU00135945!, TAAM192134!).
Dacrymyces fennicus Lowy, Sydowia 14: 104 (1960).
D. fennicus is morphologically similar to D. adpressus but differs in substrate and distribution; it can also be confused with C. tortus, though the latter has aseptate basidiospores. The holotype has light yellow basidiocarps; clamped thin-walled hyphae without heavy gelatinous layer; basidia 35–60 × 3–6 μm, sterigmata up to 34 μm in length (n = 30/1); basidiospores slightly curved-cylindrical, thin-walled (contrary to what is stated in the protologue), 0–3-septate, (11.0–)11.5–14.0(–14.1) × (3.3–)4.0–5.0 μm, L = 12.7 μm, W = 4.5 μm, Q = 2.8, Q’ = 2.4–3.5 (n = 21/1).
Typus: Finland, Uusimaa prov., Helsinki, Lauttasaari, on Pinus, 22 Jul. 1944, V. Kujala, 25 (holotype LSU00135946!).
Material examined: Finland, Uusimaa prov., Helsinki, Koskela, on P. sylvestris, 18 Feb. 2017, O. Miettinen 20574 (H∗), Veräjämäki, on Picea abies, 15 Aug. 2017, O. Miettinen 21174 (H∗).
Dacrymyces paraphysatus L.S. Olive, Bulletin of the Torrey Botanical Club 85: 106 (1958).
Basidiocarps of the species are resupinate and gelatinous, of reddish brown colour, highly resembling ones of C. aeneus. Basidiospores having three septa en masse suggest it belongs to the Dacrymycetaceae. Hyphae with clamps, gelatinized; hyphidia well branched, often with few clamps, diam 1–2 μm, constant through the length; basidia 40–78 × 3.5–6 μm, sterigmata up to 41 μm in length (n = 10/1); basidiospores slightly curved-cylindrical, 0–3-septate, 12.8–16.0 × 5.2–6.0 μm, L = 14.4 μm, W = 5.7 μm, Q = 2.5, Q’ = 2.3–3 (n = 10/1), walls ∼ 0.3–1 μm in width.
Typus: French Polynesia, Tahiti, Pirae dist., trail to Cascades, on Citrus limon, 8 Apr. 1956, L.S. Olive T122 (holotype NY00738304!).
Dacrymyces pengii (B. Liu & L. Fan) A. Savchenko, comb. nov. MycoBank MB 839819.
Basionym: Cerinomyces pengii B. Liu & L. Fan, Journal of Shanxi University, Natural Science Edition: 73 (1988).
This species is not a Cerinomyces and probably belongs to the D. grandinioides clade of the Dacrymycetaceae: its protologue mentions clampless hyphae, 3-septate basidiospores and resupinate dark yellow basidiocarps. No material except detailed author’s notes was found in the box of the loaned type specimen.
Typus: China, Hunan prov., Mengshan mountain, 22 Apr. 1977, Y.B. Peng 1690, ex MHSU720 (holotype HMAS85668).
Dacrymyces quercicolus Sosin, Notuae Systematicae e Sectione Cryptogamica Instituti Botanici Nomine V. I. Komarovii Academiae Scientarum URSS 13: 214 (1960).
The species might belong to Cerinomyces, being potentially a large-spored relative to C. aeneus. For the rarity of original publication, we recite the description: “Receptaculum teres, plicatum, sessile, gelatinosum, flavo-brunneum, siccum nigro-brunneum, cartilagineum, 1–3 cm diametro. Basidia clavata, 50–54 × 6–7 μ, sterigmatis elongatis. Sporae amygdaliformes contentu brunneo, episporio laevi, unicellulares, 9–15 × 6–7.5 μ; cystidia clavata 77 × 8.4 μ.”
The type was originally stored in PWU, but the herbarium could not be reached. It was not found in KW either, to where some of P.E. Sosin’s types were transferred from PWU (M. Zykova, 3 Mar. 2020, pers. comm.).
Typus: Ukraine, Vinnytsia reg., Nemyriv dist., Korzhiv, on rotten Quercus trunk, 5 Nov. 1939, Balkovsky 708 (holotype PWU).
Dacrymyces subtristis Rick, Iheringia, Série Botânica 2: 52 (1958).
McNabb (1973) suggested that D. subtristis is related to D. enatus var. macrosporus, but it has substantially larger 0–3-septate basidiospores 15–25 × 9 μm (Rick 1958). Type not traced.
Dacrymyces tristis Pat., Énumération Méthodique des Champignons Recueillis à la Guadeloupe et à la Martinique: 11 (1903).
The species has dark-coloured resupinate to cerebriform basidiocarps but, having clampless septa, should belong to the Dacrymycetaceae.
Typus: Guadeloupe, Basse-Terre, 1901 (?), A. Duss 514 (syntype FH00965285!).
Dacryonaema macnabbii (D.A. Reid) J.C. Zamora & Ekman, Persoonia 44: 192 (2020).
Basionym: Dacrymyces macnabbii D.A. Reid, Transactions of the British Mycological Society 62(3): 456 (1974).
Basidiocarps generally similar to ones of the C. tortus clade members: small, pale ochraceous to brown, pustulate to shallow-cupulate. Clamps often medallion-form, usually present in subhymenium and absent in subiculum, walls of subicular hyphae thick and gelatinized up to 1 μm. Hymenium consists of dendroid hyphidia and clavate basidia 35–56 × 3.5–8.0 μm with sterigmata up to 28 μm in length (n = 17/1). Basidiospores slightly curved-cylindrical, aseptate or exceptionally rarely 1-septate, (11.9–)12.0–16.6(–16.7) × 4.2–5.4(–5.7), L = 14.3 μm, W = 4.9 μm, Q = 2.9, Q’ = 2.4–3.5 (n = 38/2, measured in 1 % KOH), walls ∼ 0.2 μm thick.
Typus: United Kingdom, Wester Ross, Kinlochewe, Coille na Glas-Leitire, on Pinus sylvestris, 20 Aug. 1963, R.W.G. Dennis (holotype K(M):81678∗!).
Material examined: Sweden, Jämtland co., Åre mun., ca. 2 km W of Sällsjö, on P. sylvestris, 26 Jul. 2018, J.C. Zamora (UPS:F-940995), (UPS:F-940996), (H7009035); Uppsala co., Älvkarleby mun., Långsandsörarna island, on P. sylvestris, 17 Apr. 2018, J.C. Zamora, S. Ekman & M. Zuluaga (UPS:F-940954∗, H7009036). For the full list of materials see Zamora & Ekman (2020); cited here specimens were separately studied to produce the description above.
Dacryonaema macrosporum J.C. Zamora & Ekman, Persoonia 44: 194 (2020).
One of the isotypes (H6014412) shows a large number of basidiospores distinctly smaller to what was reported by Zamora & Ekman (2020), which can be a sign of spore immaturity. Here we report the data anyway, to claim for attention on this character, which suitability may need to be re-evaluated with further material.
Basidiocarps of Da. macrosporum are similar to Da. macnabbii but smaller and darker even in fresh state. Clamps often medallion-form, usually present in subhymenium and absent in subiculum, walls of subicular hyphae thick and gelatinized up to 1 μm. Hymenium includes dendroid hyphidia. Basidia 41–98 × 3.0–8.0 μm, sterigmata up to 26 μm in length (n = 40/1), young probasidia conspicuously clavate. Basidiospores slightly curved-cylindrical to narrowly ellipsoid, aseptate, (10.2–)10.3–16.9(–17.3) × (3.9–)4.0–6.2(–6.4) μm, L = 13.0 μm, W = 5.0 μm, Q = 2.6, Q’ = 2.1–4.0 (n = 33/1), walls ∼ 0.2 μm in width.
Typus: Finland, Perä-Pohjanmaa prov., Rovaniemi, on Pinus sylvestris, 14 Jul. 2018, J.C. Zamora (holotype UPS:F-941001∗!, isotype H6014412!). For the full list of materials see Zamora & Ekman (2020); cited here specimens were separately studied to produce the description above.
Femsjonia pezizoidea (Henn.) McNabb, New Zealand Journal of Botany 3: 226 (1965).
Basionym: Guepinia pezizoidea Henn., Hedwigia 43: 197 (1904).
Synonyms: Ceracea rickii Bres., Brotéria Série Botânica 5: 9 (1906).
Ditiola rickii (Bres.) Bres., Annales Mycologici 18 (1-3): 52 (1920).
The species belongs to the Dacrymycetaceae by virtue of 3-septate basidiospores. The species develops both resupinate and stipitate basidiocarps, as well as intermediate forms; firm-gelatinous, coloured from yellow to brown, ochraceous in old specimens; hyphae clamped, gelatinized; hyphidia long and widely branched, often with few clamps through the length, base ∼ 3 μm in diam, apical part 1–3 μm in diam; basidia 32–65 × 2.5–6 μm, sterigmata up to 40 μm in length (n = 45/4); basidiospores slightly curved-cylindrical, 0–3-septate, (10.0–)10.6–17.6(–18.6) × (3.9–)4.0–6.0(–6.2) μm, L = 13.6 μm, W = 4.7 μm, Q = 2.8, Q’ = 2.1–4.0 (n = 70/3, 8 measured in 1 % KOH), walls ∼ 0.3–1.0 μm in width; conidium straight-cylindrical 3.0 × 1.0.
Typus: Brazil, São Paulo, Alto da Serra, 1902, A. Puttemans 761 (isotype S:F20949!).
Material examined: Brazil, Distrito Federal, Taguatinga dist., Floresta Nacional de Brasília, 12 Jan. 2017, R.L.M. Alvarenga 425 (URM); Rio Grande do Sul, São Leopoldo, on Bambusa, 1904, J.E. Rick 9 (holotype of Ceracea rickii, S:F20231), same loc., 1930, J.E. Rick (FH00304807), same loc., no date (BPI726062), Serra Azul, 1928, J.E. Rick 413 (FH00304805), 495 (FH00304806).
Unilacryma bispora J.C. Zamora & Ekman, Persoonia 44: 198 (2020).
Basidiocarps of the species are very small, gelatinous, pustulate to shallow-cupulate, brown. Clamps present, sometimes “unfinished”, rarely absent. Branched hyphidia present. Basidia 56–102 × 5.0–9.0 μm, two sterigmata each up to 37 μm in length (n = 31/2). Basidiospores ellipsoid, with 0–1(–3) early transverse septa and multiple longitudinal and transverse septa at later stages, with long apiculum, (12.1–)12.3–17.4(–18.3) × 6.1–8.7(–8.8) μm, L = 14.7 μm, W = 7.3 μm, Q = 2.0, Q’ = 1.6–2.4 (n = 40/2), walls ∼ 0.3 μm in width. Unilacryma bispora was found only in the Northern Europe.
Typus: Sweden, Uppsala co., Uppsala mun., Norra Lunsen Nature Reserve, on Pinus sylvestris, 19 Nov. 2017, J.C. Zamora (holotype UPS:F-941257∗, isotype H7009037!).
Material examined: Sweden, Jӓmtland co., Åre mun., Mörsil par., Klukshåckren, on Pinus sylvestris, 23 Jul. 2018, J.C. Zamora (UPS:F-941264∗), (H7009038). For the full list of materials see Zamora & Ekman (2020); cited here specimens were separately studied to produce the description above.
Unilacryma unispora (L.S. Olive) Shirouzu, Tokum. & Oberw. Mycologia 105(5): 1120 (2013).
Basionym: Platygloea unispora L.S. Olive, The Journal of the Elisha Mitchell Scientific Society 74: 41. (1958).
Synonyms: Achroomyces unisporus (L.S. Olive) Wojewoda, Mala Flora Grzybów 2: 205 (1981), not validly published.
Dacrymyces unisporus (L.S. Olive) K. Wells, Mycologia 86(1): 31 (1994), not validly published.
Two of the synonyms above were not published validly (ICN 2018, Art. 6.10) because they were based on an early designation released without a Latin description (Olive 1944), which was fixed later with a valid publication (Olive 1958b). Basidiocarps of U. unispora are small, gelatinous, pustulate, of brown colour, generally resemble young basidiocarps of the C. tortus clade species, though U. unispora can be easily separated from the rest of dacrymycetes by unique unisterigmate basidia. Clamps present, rarely “unfinished” or absent. Basidiospores subglobose to ellipsoid with 0–1(–3) early transverse septa and late longitudinal and additional transverse septa, with long apiculum, 12.0–20.5(–20.8) × (9.5–)10.0–14.8(–18.9) μm, L = 16.9 μm, W = 12.3 μm, Q = 1.4, Q’ = 0.9–1.9 (n = 34/2), walls ∼ 0.5 μm in width. Identification can be problematic, since collapsed basidia sometimes are difficult to recognize. In certain cases, the species may be even confused with immature Naohidea sebacea.
Typus: USA, North Carolina, Orange co., Chapel Hill, Battle Park, 11 Dec. 1943, L.S. Olive (holotype NCU-F-0026842); Georgia, Clarke co., Athens, woods above stadium, on Juniperus, 18 Jan. 1946, L.S. Olive Ga77 (paratype TENN043282!).
Material examined: Estonia, Pärnu co., Lääneranna par., Puhtulaid, on Pinus sylvestris, 5 Jun. 2019, A. Savchenko 190605-1240 (TU135055), 190605-1244 (TU135056); Põlva co., Põlva par., vicinities of Aarna, on Picea abies, 20 Oct. 2019, A. Savchenko 191020-1400 (TU135075). Japan, Honshu, Chūbu reg., Nagano pref., Sugadairakougen, on Pinus densiflora, 16 Jun. 2006, T. Shirouzu HNo.332 (TNS-F-15731∗), 2 Jul. 2009, T. Shirouzu HNo.881 (TNS-F-38904∗). For the full list of materials see Zamora & Ekman (2020); cited here specimens were separately studied to produce the description above.
Notes on species excluded from dacrymycetes.
Ceracea subsulphurea Rick, Brotéria, Ciências Naturais 5: 74 (1936).
The species is Cerocorticium molle s.l. (Polyporales).
Material examined: Brazil, São Leopoldo, Rio Grande do Sul, 1931, J.E. Rick (original material, FH00304803).
Ceracea vernicosa Cragin, Bulletin of the Washburn Laboratory of natural History 1 (2): 82 (1885).
Synonym: Tremella vernicosa Cragin, Bulletin of the Washburn Laboratory of natural History 1 (1): 28 (1884), not validly published, nom. nud.
The species is an anamorphic sporotrichum-like fungus growing on decayed basidiocarps of a trimitic polypore, probably Trametes.
Typus: USA, Kansas, Topeka, Shunganunga Creek woods, Feb. 1884, W.F. Cragin, no. 255, 954 (lectotype NY01042800!).
Cerinomyces megalosporus Duhem, Bulletin de la Société Mycologique de France 114 (2): 2 (1998).
This species does not belong to Cerinomyces and should be placed in Dendrothele s.l. (Corticiales).
Typus: France, Haute-Savoie dept., Samoëns, Jardin alpin “La Jaÿsinia”, on Cornus mas, 4 Sep. 1995, B. Duhem (BD henceforth) 3472 (holotype PC0140726!).
Material examined: France, type locality and substrate, 4 Sep. 1995, BD 3473 (PC0706775), 3640 (PC0706776), 7 Sep. 1994, BD 3360 (PC0706777); Provence-Alpes-Côte d'Azur reg., on the side of the D563 road between Seillans and Mons, on Quercus pubescens, 17 Dec. 1997, BD 3823 (PC0706778).
Discussion
Previous phylogenetic studies have settled the boundaries of Cerinomycetaceae, separated it from neighbouring families, and shown that Cerinomyces s.l. is polyphyletic (Shirouzu et al. 2013, Zamora & Ekman 2020). In this work we took the next logical step: a formal reassessment of the family, using both molecular and morphological data on all known taxa. The number of new taxa was unexpectedly high considering how rarely the genus occurs in nature, and how rarely it is collected. Many new species, particularly arid Cerinomyces, were recovered by extensive herbarium sampling — this, once again, points to the importance of natural history repositories. Only gelatinous Cerinomyces species are sufficiently represented with the recent specimens, while the rest of the genus is covered primarily by material from 1955–1985 collected by experts of “holobasidial” corticioid fungi. In many fungal groups, specimens of this age are considered to be unfit for sequencing, but Sanger sequencing of certain DNA regions (e.g., parts of the nrDNA, such as ITS) of dacrymycetes has a relatively high rate of success.
Such DNA preservation may be facilitated by the ability of dacrymycetes to survive dry periods in a desiccated state and then restore sporulation after humidity increases (Shirouzu et al. 2013). We suppose that gelatinization of basidiocarps, a landmark character in the class, is connected to this ability, and gelatinous matter serves as a water storage and protection for hyphal structures. Concluding from the phylogenies, this character was independently lost several times and substituted by corticioid basidiocarps of different degrees of aridness. The ecological background of this change is yet to be understood, but we hypothesize that the transitions can be linked with adjustments to micro-niches. For example, we observe that the gelatinous Cerinomycetaceae species grow mostly on fine, hard branches, while corticioids prefer larger, more decayed trunks. In this context, basidiocarps appearing on small, often suspended debris are at risk of rapid drying that interrupts sporulation, and therefore they depend on gelatinization for revival. Meanwhile, species preferring stably wet habitats like half-decomposed logs do not need to prepare for several sporulation episodes. In this case the corticioid morphotype is beneficial, providing a larger hymenial surface and relief from production of voluminous gelatinous matrices.
Compared to the last revision (McNabb 1964), the main changes made to Cerinomyces s.s. are: the exclusion of the brightly pigmented femsjonia-like corticioids and species lacking hyphal clamps, and the incorporation of dacrymyces-like members with gelatinous pale ochraceous and brownish basidiocarps. Following this pattern, a specimen can be rather reliably identified as Cerinomyces without a microscope just by the visibly low carotenoid content, regardless of whether it is gelatinous or corticioid. When working with gelatinous species, one should also consider Unilacrymaceae and Dacryonaemataceae, but basidiocarps in these families are generally much smaller than in Cerinomyces. There are a few species in the Dacrymycetaceae with brownish basidiocarps (e.g., Calocera fusca Lloyd, Guepiniopsis fulva P. Delivorias), but they have unusual shapes for the Cerinomycetaceae. Lack of colour or semitransparency are not rare in gelatinous dacrymycetes, but this normally appears when the basidiocarps have developed in darkness (Bulat 1954, Vail & Lilly 1968). This is held to be a stable character in only a few species (e.g., Ditiola haasii Oberw., Dacrymyces cylindricus Shirouzu), which differ from Cerinomyces by basidiocarp morphologies. Discoloured morphs in Cerinomyces were seen in C. cokeri (Fig. 11 C) and C. tortus, but can be expected in other species growing in the shade or during the darkest part of the year.
Introducing new taxa to Cerinomyces s.s. is relatively straightforward now that the family Cerinomycetaceae consists of one genus only. At the same time, transfer of brightly coloured corticioids to the Dacrymycetaceae raises the question of how to choose generic names for them. Genus division in this family is clearly a difficult task, complicated by the poly- and paraphyly of the current genera, numerous morphological homoplasies, and lack of molecular data. These uncertainties explain the minimalistic approach to nomenclature we have adopted here. First, for the Dacrymyces corticioides and “Cerinomyces” canadensis lineages we decided to postpone new combinations until the upcoming studies of Femsjonia. Second, we included the D. grandinioides clade members to the genus Dacrymyces, judging from their close position to Dacrymyces s.s. in the core Dacrymycetaceae. The alternative solution of establishing a new genus is impractical from our viewpoint. Considering the diversity of undescribed taxa in the vicinity of the D. grandinioides clade, premature generic splitting may only multiply the problems in this part of the family. Moreover, lack of a common stark trait in Dacrymyces should not be a sole reason for splitting. Morphological plasticity even within the well-defined and monophyletic genera of dacrymycetes seems to be the norm rather than the exception. Unilacryma has members with 1- and 2-sterigmate basidia, Dacryonaema includes taxa with synnematous and pustulate basidiocarps, and Cerinomyces now unites arid and gelatinous species. In the same way, Dacrymyces can be potentially redefined as both monophyletic and morphologically inclusive genus.
For the convenience of identification, we provided detailed information on all dacrymycetous corticioids from the two families. In certain morphogroups many of the reviewed traits are not diagnostic alone, and instead a combination of characters is needed for identification. This prevented us from extending the dichotomic key to the species level in all cases. As a substitution, identification tables (Table 3, Table 4, Table 5) should be sufficient for most identification purposes. However, there are a few morphological species groups that entail difficulties in identification of young or weathered basidiocarps. These include North American corticioids C. atrans, C. favonius, C. fugax, and C. tristis; European gelatinous C. creber, C. lipoferus, C. neuhoffii, and C. tortus together with undescribed taxa; and possible, yet uncovered, complexes of European angiosperm-dwelling members related to C. crustulinus or C. aeneus. The D. grandinioides clade is particularly difficult, and in many cases morphological identification within the group seems impossible. While the South American peg-lacking species D. cereus, D. lagerheimii and D. pulchrus can be distinguished using spore size and septation, identification of their peg-bearing relatives is challenging. For both the African complex of D. grandinioides, D. venustus, and D. aff. venustus 1; and North American complex of D. burdsallii, D. grandii, D. ceraceus, and D. sobrius, we detected wide variation and overlap in trait measurements. More material is needed to bring higher morphological resolution to the group and provide new ways for identification. For example, many specimens identified in the D. grandinioides group demonstrate abundant conidia and conidiogenous structures of largely unexplored diagnostic value. Anamorphic development has a high importance in some species in dacrymycetes, up to apparent loss of teleomorphic stages (members of Dacryoscyphus), hence this area should be assessed in taxonomic studies in more detail.
We argue that biogeography is a working determination criterion even for species based on a single specimen or with exceedingly restricted known localities. Species ranges in the studied groups are likely to be confined to continents and biogeographical zones, judging from the overall low number of cosmopolite species in dacrymycetes. Only three species in this study have a multicontinental distribution, found in both East Asia and North America (“Cerinomyces” canadensis, C. enatus, D. corticioides). Notably, there are no findings of Cerinomyces from the African region, except for C. albosporus found at the island of Réunion, and no gelatinous species are recorded in South America. Conversely, corticioid species from the D. grandinioides clade were found only in Africa and the Americas, which is unlikely to be caused by sampling density alone.
Tree host specialization in Cerinomyces s.l. is generally limited to angiosperm or gymnosperm wood; association with a certain tree species cannot be confidently established. The majority of Cerinomyces s.s. prefer coniferous wood, except for C. aeneus, C. crustulinus, and C. pallidus that occur only on angiosperm trees; C. albosporus on Asteraceae shrubs; C. enatus on both angio- and gymnosperms; and a few more species with dubious preferences or unidentified hosts. Shirouzu et al. (2014) provided additional insight on host specificity with their wood block decay test, which confirmed the ability of C. enatus to decompose both Pinus densiflora and Fagus crenata (data under C. canadensis in Shirouzu et al. 2014, but also mind our note under C. enatus). At the same time, members of the D. grandinioides clade seem to occur on a high variety of substrates including shrubs, bamboos, charred wood, etc. Hence, it is difficult to judge host preferences, particularly when only few specimens are available.
Spore measurements have always been a principal component of dacrymycete identification. However, not all of the spore characteristics are equally useful. The maximal number of internal septa in basidiospores should be used with caution: this number fluctuates within one specimen and changes during the basidiocarp lifetime. It is very common that in fresh specimens basidiospores are aseptate while still attached, but after discharge they can massively generate 1–3 septa before germination (Maekawa 1987). This quality led to errors in protologues of C. albosporus, Dacrymyces confluens, and D. lagerheimii for which only one septum was reported, instead of the actual maximal three. In case of uncertainty, we strongly recommend checking the number of septa with germinating spore prints on agar. If this material is not viable, one can look for discharged basidiospores in substrate scrapes close to the basidiocarp.
On a methodological note, we observed that microscopic slide mountants affect the measurements and shapes of microstructures. In this work we used Cotton Blue (CB) in lactic acid, providing sufficient contrast and stability of microstructures. Notably, use of KOH-based solutions in concentrations conventional for studies of aphyllophoroid fungi can lead to unreproducible results, as gelatinous matter that covers hyphae can thicken or even dissolve depending on the concentration of KOH and exposure time. Spore walls easily swell, most dramatically in thick-walled basidiospores of the Dacrymycetaceae, adding several micrometers to spore dimensions. On the other hand, preparations with KOH (with or without a stain) help to reveal the organization of densely interwoven hyphae in cases where CB fails. Therefore, for measurements in dacrymycetes we recommend using CB as a primary mountant and a weak solution of KOH (e.g., 1 %) where necessary.
Aiming to reduce number of superfluous terms describing fungal morphology, we propose to replace the word “dikaryophysis” (sterile element in hymenium of dacrymycetes) with the more general term “hyphidium”. The term “dikaryoparaphysis” was introduced by Lowy (1954) as a name for sterile hymenial hyphae in all basidiomycetes — in such a manner the author wanted to counterpose it to ascomycetous monokaryotic “paraphysis”. Kennedy (1957) started a tradition to use it in a simplified form: “dikaryophysis”. The term has never been widely used and apparently survived only in dacrymycete-related works, adding unnecessary confusion. Here we report hyphidia only when they are dendroid (the C. tortus, C. enatus clades), simple with a thickened base and long thin apical part (the C. pallidus clade), or simple cylindrical of even width (the D. corticioides lineage). We would not use “hyphidia” to describe occasional subhymenium extensions or young cylindrical probasidia that can be found in most dacrymycetes.
Further sequencing of more gene regions in the least-represented groups of the Cerinomycetaceae will allow for better-supported phylogenies. Building a robust multigene phylogeny for the Dacrymycetaceae will help to refine nomenclature of former Cerinomyces species if they need to be treated as separate genera. Continuing collection efforts and herbarium searches in unidentified Dacrymyces will certainly uncover more new species. This applies not only to understudied regions, but also to Europe, where knowledge on diversity of gelatinous species is still incomplete. Higher sampling density and established distributions will facilitate recognition of endangered species as potential red lists candidates. Ecological roles and requirements of dacrymycetes are poorly understood and deserve be studied more extensively. For example, several dacrymycetes including C. tortus are abundant in the pine wood-decay communities in the Northern boreal zone, but their habitat specialization strategies, if any, are mostly unknown. Environmental DNA obtained from wood will be invaluable in expanding both the taxonomical and ecological knowledge of this group. Finally, genomic and enzymatic studies in Cerinomycetaceae and minor families of Dacrymycetes can help to reveal origins and evolution of brown wood rot mechanisms.
Acknowledgements
We are indebted to all herbaria staff involved in preparation of loans and organization of visits. Personal specimens and sequences for the study were kindly provided by Olexander Akulov, Renato Lúcio Mendes Alvarenga, Renata Chikowski, Igor Khomenko, Karl-Henrik Larsson, Alexander Ordynets, Gérard Trichies, Ilya Viner, and Leif Ryvarden. We greatly appreciate help in specimens handling by Paul Kirika of Nairobi National Museum (Kenya). Author AS thanks Iryna Koshova for aid in translations from French and German, and Iryna Yatsiuk for pieces of helpful advice. Finally, we are grateful to the editor and two anonymous reviewers for their work, and Jacquelin DeFaveri for partial proofreading. This study was supported by the following grants: Estonian Research Council projects IUT20-30 & PRG1170, CIMO Fellowship TM-15-9800, Societas Pro Fauna et Flora Fennica grant, European Regional Development Fund/The Archimedes Foundation Dora Plus T1.1 and T1.2 scholarships. Author VM was supported by the project AAAA-A19-119020890079-6 of the Komarov Botanical Institute of the Russian Academy of Sciences. Several sequences were produced in the Finnish Barcode of Life (FinBOL) project.
Footnotes
Peer review under responsibility of Westerdijk Fungal Biodiversity Institute.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.simyco.2021.100117.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Abarenkov K., Tedersoo L., Nilsson R.H., et al. PlutoF—a web based workbench for ecological and taxonomic research, with an online implementation for fungal its sequences. Evolutionary Bioinformatics Online. 2010;6:189–196. [Google Scholar]
- Adams H.B. and RH. 2015. evobiR: Comparative and Population Genetic Analyses. [Google Scholar]
- Ankenbrand M.J., Keller A., Wolf M., et al. ITS2 Database V: Twice as Much. Molecular Biology and Evolution. 2015;32(11):3030–3032. doi: 10.1093/molbev/msv174. [DOI] [PubMed] [Google Scholar]
- Bengtsson-Palme J., Ryberg M., Hartmann M., et al. Improved software detection and extraction of ITS1 and ITS2 from ribosomal ITS sequences of fungi and other eukaryotes for analysis of environmental sequencing data. Methods in Ecology and Evolution. 2013;4(10):914–919. [Google Scholar]
- Bourdot H. Hyménomycètes nouveaux ou peu connus. Bulletin trimestriel de la Société mycologique de France. 1932;48:204–232. [Google Scholar]
- Brasfield T.W. The Dacrymycetaceae of temperate North America. American Midland Naturalist. 1938;20(1):211. [Google Scholar]
- Brasfield T.W. Notes on the Dacrymycetaceae. Lloydia. 1940;3(2):105–108. [Google Scholar]
- Bulat T.J. Effect of light on color in Dacrymyces. Mycologia. 1954;46(1):32–36. [Google Scholar]
- Clémençon H., Emmett V., Emmett E.E. J. Cramer; Berlin, Stuttgart: 2004. Cytology and Plectology of the Hymenomycetes. [Google Scholar]
- Coker W.C. Notes on basidiomycetes. Journal of Elisha Mitchell Scientific Society. 1928;43:233–256. [Google Scholar]
- Cragin F.W. A new genus and species of tremelline fungus. Bulletin of the Washburn College Laboratory of Natural History. 1885;1:82. [Google Scholar]
- Criscuolo A., Gribaldo S. BMGE (Block Mapping and Gathering with Entropy): a new software for selection of phylogenetic informative regions from multiple sequence alignments. BMC Evolutionary Biology. 2010;10(1):210. doi: 10.1186/1471-2148-10-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Gams W., Stalpers J.A., et al. MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology. 2004;50(1):19–22. [Google Scholar]
- Czeczuga B. Investigations on carotenoids in fungi. IX. Dacrymycetaceae. Acta Mycologica. 1980;16(1):115–120. [Google Scholar]
- Donk M.A. Notes on resupinate Hymenomycetes. II. The tulasnelloid fungi. Reinwardtia. 1956;3:363–379. [Google Scholar]
- Donk M.A. The generic names proposed for Hymenomycetes. VII: ‘Thelephoraceae’. Taxon. 1957;6(1):17–28. [Google Scholar]
- Donk M.A. Check list of European Hymenomycetous Heterobasidiae. Persoonia. 1966;4(2):145–335. [Google Scholar]
- Eriksson J. Studies in the heterobasidiomycetes and homobasidiomycetes. Aphyllophorales of Muddus National Park in North Sweden. Symbolae Botanicae Upsaliensis. 1958;16:1–172. [Google Scholar]
- Eriksson J., Ryvarden L. The Corticiaceae of North Europe. Fungiflora; Oslo: 1973. The Corticiaceae of North Europe. Volume 2. Aleurodiscus – Confertobasidium; pp. 57–284. [Google Scholar]
- Floudas D., Held B.W., Riley R., et al. Evolution of novel wood decay mechanisms in Agaricales revealed by the genome sequences of Fistulina hepatica and Cylindrobasidium torrendii. Fungal Genetics and Biology. 2015;76:78–92. doi: 10.1016/j.fgb.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries E.M. Sumptibus Ernesti Mauritii; Greifswald: 1828. Elenchus fungorum: sistens commentarium in Systema mycologicum. [Google Scholar]
- Fries E.M. 1851. Novae symbolae mycologicae: in peregrinis terris a botanicis danicis collectae. Reg. Acad. Typographus, Upsaliae. [Google Scholar]
- Garbelotto M.M., Lee H.K., Slaughter G., et al. Heterokaryosis is not required for virulence of Heterobasidion annosum. Mycologia. 1997;89(1):92–102. [Google Scholar]
- Gilbertson R.L., Hemmes D.E. Fungi in Forest Ecosystems: Systematics, Diversity, and Ecology. The New York Botanical Garden Press; 2004. New species of lignicolous basidiomycetes from Hawaiʻi; pp. 81–92. [Google Scholar]
- Ginns J. Cerinomyces ceraceus sp. nov. and the similar C. grandinioides and C. lagerheimii. Canadian Journal of Botany. 1982;60(4):519–524. [Google Scholar]
- Gminder A. 2016. Assessment of fungi in the Kafa Biosphere Reserve. [Google Scholar]
- Goodwin T.W. Studies in carotenogenesis. 8. The carotenoids present in the basidiomycete Dacromyces stillatus. Biochemical Journal. 1953;53(4):538–540. doi: 10.1042/bj0530538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoriev I.V., Nikitin R., Haridas S., et al. MycoCosm portal: gearing up for 1000 fungal genomes. Nucleic Acids Research. 2014;42(Database issue):D699–704. doi: 10.1093/nar/gkt1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjortstam K., Ryvarden L. Some new and noteworthy Basidiomycetes (Aphyllophorales) from Nepal. Mycotaxon. 1984;20(1):133–151. [Google Scholar]
- von Höhnel F. Fragmente zur Mycologie. Sitzungsberichte der Kaiserlichen Akademie der Wissenschaften. 1908;117:985–1032. [Google Scholar]
- Hopple J., Vilgalys R. Phylogenetic relationships among coprinoid taxa and allies based on data from restriction site mapping of nuclear rDNA. Mycologia. 1994;86(1):96–107. [Google Scholar]
- Huckfeldt T., Hechler J. Cerinomyces pallidus Martin: Erstfund für Deutschland. Zeitschrift für Mykologie. 2004;70(1):97–106. [Google Scholar]
- Ingold C.T. Basidiospore germination and conidium development in Dacrymycetales. Transactions of the British Mycological Society. 1983;81(3):563–571. [Google Scholar]
- Katoh K., Rozewicki J., Yamada K.D. MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Briefings in Bioinformatics. 2019;20(4):1160–1166. doi: 10.1093/bib/bbx108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellerman W.A. New Literature. The Journal of Mycology. 1885;1:56–58. [Google Scholar]
- Kennedy L.L. University of Iowa; 1957. A taxonomic study of the genus Dacrymyces. PhD thesis. [Google Scholar]
- Kennedy L.L. The Genera of the Dacrymycetaceae. Mycologia. 1958;50(6):874–895. [Google Scholar]
- Kennedy L.L. The Genus Dacrymyces. Mycologia. 1958;50(6):896–915. [Google Scholar]
- Kirschner R., Yang Z.L. Dacryoscyphus chrysochilus, a new staurosporous anamorph with cupulate conidiomata from China and with affinities to the Dacrymycetales (Basidiomycota) Antonie van Leeuwenhoek. 2005;87(4):329–337. doi: 10.1007/s10482-004-6784-9. [DOI] [PubMed] [Google Scholar]
- Kõljalg U., Nilsson H.R., Schigel D., et al. The Taxon hypothesis paradigm—on the unambiguous detection and communication of taxa. Microorganisms. 2020;8(12):1910. doi: 10.3390/microorganisms8121910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korschelt E., Linck G.E., Oltmanns F., et al. Gustav Fischer; Jena: 1915. Handwörterbuch der naturwissenschaften. [Google Scholar]
- Larsson A. AliView: a fast and lightweight alignment viewer and editor for large datasets. Bioinformatics. 2014;30(22):3276–3278. doi: 10.1093/bioinformatics/btu531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemoine F., Correia D., Lefort V., et al. NGPhylogeny.fr: new generation phylogenetic services for non-specialists. Nucleic Acids Research. 2019;47(W1):W260–W265. doi: 10.1093/nar/gkz303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.J., Whelen S., Hall B.D. Phylogenetic relationships among ascomycetes: evidence from an RNA polymerse II subunit. Molecular Biology and Evolution. 1999;16(12):1799–1808. doi: 10.1093/oxfordjournals.molbev.a026092. [DOI] [PubMed] [Google Scholar]
- Lloyd C.G., Stevenson J.A. 1921. Mycological writings of C. G. Lloyd. Volume VI. Cincinnati. [Google Scholar]
- Losi C. Macrofungus flora of the lagoon of Venice and adjacent areas (Italy). Non-gilled basidiomycetes III. Polyporoid, hydnoid, clavarioid Aphyllophorales and Heterobasidiomycetes. Mycotaxon. 2003;88:349–358. [Google Scholar]
- Lowy B. A New Dacrymyces. Bulletin of the Torrey Botanical Club. 1954;81(4):300–303. [Google Scholar]
- Maekawa N. A new species of the genus Cerinomyces. Canadian Journal of Botany. 1987;65(3):583–588. [Google Scholar]
- Maekawa N., Zang M. Cerinomyes curvisporus sp. nov. (Dacrymycetales) from Yunnan, China. Mycotaxon. 1997;61:343–346. [Google Scholar]
- Martin G.W. Some Heterobasidiomycetes from Eastern Canada. Mycologia. 1940;32(6):683–695. [Google Scholar]
- Martin G.W. The genus Ceracea Cragin. Mycologia. 1949;41(1):77–86. [Google Scholar]
- Martin G.W. The Tulasnelloid Fungi and their bearing on basidial terminology. Brittonia. 1957;9(1):25–30. [Google Scholar]
- Martin K.J., Rygiewicz P.T. Fungal-specific PCR primers developed for analysis of the ITS region of environmental DNA extracts. BMC Microbiology. 2005;5(28):1–11. doi: 10.1186/1471-2180-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheny P.B., Liu Y.J., Ammirati J.F., et al. Using RPB1 sequences to improve phylogenetic inference among mushrooms (Inocybe, Agaricales) American Journal of Botany. 2002;89(4):688–698. doi: 10.3732/ajb.89.4.688. [DOI] [PubMed] [Google Scholar]
- McNabb R.F.R. Taxonomic studies in the Dacrymycetaceae: I. Cerinomyces Martin. New Zealand Journal of Botany. 1964;2(4):415–424. [Google Scholar]
- McNabb R.F.R. Taxonomic studies in the Dacrymycetaceae VIII. Dacrymyces Nees ex Fries. New Zealand Journal of Botany. 1973;11(3):461–524. [Google Scholar]
- Miller M.A., Pfeiffer W., Schwartz T. 2010 Gateway Computing Environments Workshop (GCE) 2010. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. [Google Scholar]
- Nagy L.G., Riley R., Tritt A., et al. Comparative genomics of early-diverging mushroom-forming fungi provides insights into the origins of lignocellulose decay capabilities. Molecular Biology and Evolution. 2016;33(4):959–970. doi: 10.1093/molbev/msv337. [DOI] [PubMed] [Google Scholar]
- Nannfeldt J.A. Sphaeronaema rufum Fr., a misunderstood member of Dacrymycetaceae. Svensk Botanisk Tidskrift. 1947;41(3):321–338. [Google Scholar]
- Neuhoff W. Gallerttränen (Zur Kenntnis der europäischen Dacrymycesarten) Schweizerische Zeitschrift für Pilzkunde. 1934;12:78–82. [Google Scholar]
- Nilsson R.H., Larsson K.-H., Taylor A.F.S., et al. The UNITE database for molecular identification of fungi: handling dark taxa and parallel taxonomic classifications. Nucleic Acids Research. 2018;47(D1):D259–D264. doi: 10.1093/nar/gky1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberwinkler F. Genera in a monophyletic group: the Dacrymycetales. Mycologia Helvetica. 1994;6(1):35–72. [Google Scholar]
- Oberwinkler F. In: Systematics and Evolution. McLaughlin D.J., Spatafora J.W., editors. Springer; Berlin, Heidelberg: 2014. 13 Dacrymycetes; pp. 357–372. [Google Scholar]
- Olive L.S. New or rare heterobasidiomycetes from North Carolina-I. The Journal of the Elisha Mitchell Scientific Society. 1944;60:17–26. [Google Scholar]
- Olive L.S. The Lower Basidiomycetes of Tahiti [Continued] Bulletin of the Torrey Botanical Club. 1958;85(2):89–110. [Google Scholar]
- Olive L.S. Latin diagnoses of earlier described species of Tremellales. The Journal of the Elisha Mitchell Scientific Society. 1958;74:41. [Google Scholar]
- Parmasto E. A preliminary review of the genus Cerinomyces Martin in the USSR. Eesti NSV Teaduste Akadeemia toimetised. 1961;10:231–235. [Google Scholar]
- Patouillard N.T., Lagerheim N.G. Champignons de l’Equateur (Pugillus III) Bulletin de la Société Mycologique de France. 1893;9:124–165. [Google Scholar]
- R Core Team . 2019. R: a language and environment for statistical computing. [Google Scholar]
- Rabenhorst L. Eduard Kummer; Leipzig: 1844. Deutschlands Kryptogamenflora. [Google Scholar]
- Rambaut A., Drummond A.J., Xie D., et al. Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Systematic Biology. 2018;67(5):901–904. doi: 10.1093/sysbio/syy032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehner S.A., Buckley E. A Beauveria phylogeny inferred from nuclear ITS and EF1-α sequences: evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia. 2005;97(1):84–98. doi: 10.3852/mycologia.97.1.84. [DOI] [PubMed] [Google Scholar]
- Rick J. Basidiomycetes Eubasidii in Rio Grande do Sul – Brasilia. 1. Auriculariaceae, Sirobasidiaceae, Tremellaceae, Dacryomycetaceae. Iheringia. 1958;2:1–56. [Google Scholar]
- Roberts P. Caribbean heterobasidiomycetes: 2. Jamaica. Mycotaxon. 2006;96:83–107. [Google Scholar]
- Ronquist F., Teslenko M., van der Mark P., et al. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology. 2012;61(3):539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert K.A. Decay of wood by the Dacrymycetales. Mycologia. 1983;75(6):1011–1018. [Google Scholar]
- Shirouzu T., Fukasawa Y., Nakamura Y. Femsjonia uniseptata (Dacrymycetes, Basidiomycota), a new species collected from Pinus densiflora forests in Japan. Phytotaxa. 2017;312(2):271–278. [Google Scholar]
- Shirouzu T., Hirose D., Oberwinkler F., et al. Combined molecular and morphological data for improving phylogenetic hypothesis in Dacrymycetes. Mycologia. 2013;105(5):1110–1125. doi: 10.3852/12-147. [DOI] [PubMed] [Google Scholar]
- Shirouzu T., Hirose D., Tokumasu S. Sequence analyses of the 28S rRNA gene D1/D2 region suggest Dacrymyces (Heterobasidiomycetes, Dacrymycetales) is polyphyletic. Mycoscience. 2007;48(6):388–394. [Google Scholar]
- Shirouzu T., Hirose D., Tokumasu S. Taxonomic study of the Japanese Dacrymycetes. Persoonia. 2009;23:16–34. doi: 10.3767/003158509X468443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirouzu T., Matsuoka S., Doi H., et al. Complementary molecular methods reveal comprehensive phylogenetic diversity integrating inconspicuous lineages of early-diverged wood-decaying mushrooms. Scientific Reports. 2020;10(1):1–12. doi: 10.1038/s41598-020-59620-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirouzu T., Osono T., Hirose D. Resource utilization of wood decomposers: mycelium nuclear phases and host tree species affect wood decomposition by Dacrymycetes. Fungal Ecology. 2014;9:11–16. [Google Scholar]
- Shirouzu T., Uno K., Hosaka K., et al. Early-diverging wood-decaying fungi detected using three complementary sampling methods. Molecular Phylogenetics and Evolution. 2016;98:11–20. doi: 10.1016/j.ympev.2016.01.015. [DOI] [PubMed] [Google Scholar]
- Sommerfelt S.C. Second Edition. Typis Borgianis et Gröndahlianis; Chrystianiae: 1826. Supplementum Florae Lapponicae. [Google Scholar]
- Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30(9):1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiller J.W., Hall B.D. The origin of red algae: Implications for plastid evolution. Proceedings of the National Academy of Sciences. 1997;94(9):4520–4525. doi: 10.1073/pnas.94.9.4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedersoo L., Jairus T., Horton B.M., et al. Strong host preference of ectomycorrhizal fungi in a Tasmanian wet sclerophyll forest as revealed by DNA barcoding and taxon-specific primers. The New Phytologist. 2008;180(2):479–490. doi: 10.1111/j.1469-8137.2008.02561.x. [DOI] [PubMed] [Google Scholar]
- Vail W.J., Lilly V.G. The location of carotenoid pigments and thickness of the cell wall In light- and dark-grown cells of Dacryopinax spathularia. Mycologia. 1968;60(4):902–907. [Google Scholar]
- Warren D.L., Geneva A.J., Lanfear R. RWTY (R We There Yet): An R Package for Examining Convergence of Bayesian Phylogenetic Analyses. Molecular Biology and Evolution. 2017;34(4):1016–1020. doi: 10.1093/molbev/msw279. [DOI] [PubMed] [Google Scholar]
- Wells K. Jelly Fungi, Then and Now! Mycologia. 1994;86(1):18–48. [Google Scholar]
- White T.J., Bruns T., Lee S., et al. PCR protocols: a guide to methods and applications. Academic Press; London: 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics; pp. 315–322. [Google Scholar]
- Willdenow C.L. Observationes botanici. Botanisches Magazin. 1788;2(4):7–19. [Google Scholar]
- Winter G. Rabenhorstii Fungi europaei et extraeuropaei exsiccati cura Dr. G. Winter. Centuria XXXV et XXXVI. Hedwigia. 1886;25:257–263. [Google Scholar]
- Yen H.C. Recherches experimentales sur la sexualite des Calocerales. Comptes rendus hebdomadaires des séances de l’Académie des sciences. 1947;225:1367–1368. [Google Scholar]
- Zamora J.C., Ekman S. Phylogeny and character evolution in the Dacrymycetes, and systematics of Unilacrymaceae and Dacryonaemataceae fam. nov. Persoonia. 2020;44:161–205. doi: 10.3767/persoonia.2020.44.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.