Abstract
A rapid enzymatic fluorometric assay for measuring d-arabinitol in serum was developed using recombinant d-arabinitol dehydrogenase from Candida albicans (rArDH). rArDH was produced in Escherichia coli and purified by dye-ligand affinity chromatography. rArDH was highly specific for d-arabinitol, cross-reacting only with xylitol (4.9%) among all polyols tested. A Cobas Fara II centrifugal autoanalyzer (Roche) was used to measure NADH fluorometrically when rArDH and NAD were added to serum extracts, and d-arabinitol concentrations were calculated from standard curves derived from pooled human serum containing known amounts of d-arabinitol. The method was precise (mean intra-assay coefficients of variation [CVs], 0.8%, and mean interassay CVs, 1.6%) and rapid (3.5 min per assay) and showed excellent recovery of added d-arabinitol in serum (mean recovery rate, 101%). The mean and median d-arabinitol/creatinine ratios were 2.74 and 2.23 μM/mg/dl, respectively, for the 11 patients with candidemia compared to 1.14 and 1.23 μM/mg/dl, respectively, for 10 healthy controls (P < 0.01). These results confirm earlier studies showing that serum d-arabinitol measurement may help to promptly diagnose invasive candidiasis. The technique shows a significant improvement in terms of accuracy, cost, simplicity, specificity, and speed compared with gas chromatography, mass spectrometry, and earlier enzymatic assays.
As the incidence of invasive candidiasis has increased dramatically in recent years, the accurate and early detection of this infection has become of major importance. Unfortunately, conventional culture-based clinical methods, which may take several days to become positive, are not very sensitive for detecting invasive disease (1, 7, 14, 15). Alternative approaches such as PCR assays and immunodiagnostic methods have been described, but these methods are not yet sufficiently sensitive and specific to have been widely adopted in clinical practice (15).
d-Arabinitol is a metabolite of several pathogenic Candida species, and several studies have shown that serum d-arabinitol concentrations and serum d-arabinitol/creatinine ratios are higher in humans and animals with invasive candidiasis than in uninfected or colonized controls (4, 9, 18, 19). Early studies used gas chromatography (GC) or GC-mass spectrometry to detect and quantify d-arabinitol in serum (2, 4, 5, 9). However, these methods require expensive equipment, and specimen processing and analysis require considerable time and effort. Enzymatic assays that used d-arabinitol dehydrogenase from Klebsiella pneumoniae (10, 11) to quantify d-arabinitol are less cumbersome, but these enzymes also react with d-mannitol, which is sometimes present in human serum (8). In 1994, Switchenko et al. (13) described a colorimetric endpoint enzymatic assay that used a clinical chemistry autoanalyzer and d-arabinitol dehydrogenase (ArDH) from Candida tropicalis to quantify d-arabinitol in serum (13). The ArDH utilized in this study was extracted and purified from C. tropicalis. A method for overproducing recombinant C. tropicalis ArDH in Escherichia coli has since been described (6), but recombinant ArDH (rArDH) has not yet been used in automated d-arabinitol assays.
In this study, we describe a sensitive, specific, and rapid enzymatic fluorometric method for measuring d-arabinitol in serum utilizing an automated analyzer. This new assay is faster and simpler than methods employing GC, GC-mass spectrometry, or the colorimetric endpoint enzymatic assay. Moreover, fewer reagents are required, and the automated equipment is available in many clinical laboratories. The key reagent is rArDH from Candida albicans, which was overproduced in E. coli and purified to homogeneity by dye-ligand affinity chromatography. The assay is based on oxidation of d-arabinitol to d-ribulose by rArDH, with the concomitant reduction of NAD to NADH. The initial rate of NADH production, which is proportional to the amount of d-arabinitol in serum, is measured fluorometrically. The d-arabinitol concentration was determined by comparing the initial rate of NADH production to that for d-arabinitol calibration curves.
MATERIALS AND METHODS
Expression, purification, and properties of rArDH. (i) Production and purification of rArDH.
In order to produce recombinant C. albicans ArDH (rArDH) in E. coli, we used PCR to amplify the C. albicans ARD1 coding sequence from plasmid pJB4 (20) and to change the CATAATGGAT sequence at the start codon (underlined) to CACCATGGAT, thereby introducing an NcoI restriction site. The PCR product was digested with NcoI and XbaI and ligated into NcoI- and XbaI-digested pET19b (Novagen), which yielded pET19b/ArDH. Next, the portion of the ARD1 coding sequence 3′ to the SpeI restriction site was excised from pET19b/ArDH with SpeI and XbaI and replaced with the SpeI-XbaI fragment from pJB4, which yielded pET19b/ArDH81. Lastly, all of the ARD1 coding sequence 5′ to the SpeI restriction site in pET19b/ArDH81 was sequenced to verify that no errors had been introduced by PCR.
Next, E. coli BL21(DE3) (Novagen) was transformed with pET19b/ArDH81, and the transformants were grown to an optical density value at 600 nm of 0.6 in Circlegrow broth (Bio 101, Vista, Calif.) supplemented with 50 μg of ampicillin (Sigma, St. Louis, Mo.) per ml at 37°C with shaking. Isopropyl-β-d-thiogalactopyranoside (American Bioanalytical, Natick, Mass.) was added to 1 mM, and the cells were shaken at 37°C for 3 more h. The cells were harvested by centrifugation and suspended in 100 mM sodium phosphate buffer (pH 7.0) (2 ml/100-ml cultures). The cells were broken by sonication for four periods of 10 s each, and cellular debris was pelleted by centrifugation at 30,000 × g for 30 min. The supernatant was cleared by ultracentrifugation at 100,000 × g for 45 min, and it was loaded onto a reactive Yellow 86 column (2.5 by 20 cm) (Sigma) and washed with 350 ml of 100 mM sodium phosphate buffer (pH 7.0) and with 350 ml of 100 mM sodium phosphate buffer (pH 7.0) plus 0.5 M NaCl. rArDH was eluted with 50 mM sodium phosphate buffer (pH 7.0)–250 mM NaCl–5 mM MgSO4–1 mM NADH. Active fractions were pooled and concentrated by ultrafiltration (Centriprep-30; Amicon, Danvers, Mass.). NADH was removed with a desalting column (Econo-Pac DG; Bio-Rad, Richmond, Calif.), and the purified rArDH was stored at −80°C in 0.1 M sodium phosphate buffer (pH 7.0)–200 mM NaCl–5 mM MgSO4.
(ii) Characterization of rArDH.
The purity of rArDH was assessed by denaturing sodium dodecyl sulfate-polyacrylamide gel electrophoresis, using a 12% polyacrylamide gel. rArDH catalytic activity was monitored using a UV2401 PC Spectrophotometer (Shimadzu Scientific, Inc., Braintree, Mass.). The 1-ml reaction mixture contained 50 mM glycine (pH 9.0), 0.1 U of rArDH, and 10 μl of 50 mM NAD; the reaction was initiated by the addition of 50 μl of 15% (wt/vol) d-arabinitol. Specific activity is reported as units per milligram of protein, where 1 U is defined as the amount required to generate 1 μmol of NADH/min. For substrate and cofactor specificity studies, the reaction mixture contained 50 mM glycine (pH 9.0), 0.5 M NAD, 0.1 U of enzyme, and 100 mM substrates. The reverse reaction was assayed in 50 mM glycine (pH 7.0), containing 0.34 mM NADH, 0.1 U of rArDH, and 100 mM substrates.
To test the effect of pH on the enzyme activity, buffers containing 50 mM sodium citrate, 50 mM sodium phosphate, and 100 mM Tris with pH values ranging from 4.0 to 10.5 were used.
Fluorometric enzymatic assay for measuring serum d-arabinitol concentration. (i) Serum treatment and analysis.
Serum was diluted 1:1 (vol/vol) with 10 mM sodium citrate (pH 4.0), boiled for 10 min, cooled on ice, and then centrifuged at 10,000 × g for 10 min. The supernatant was analyzed immediately or stored at −20°C for later analysis.
A Cobas Fara II centrifugal autoanalyzer (Roche Diagnostics) was used to detect and quantify d-arabinitol in human serum. This instrument is equipped with a sensitive fluorometer that can accurately measure fluorescence changes of 0.0001 ΔF/min.
The Cobas Fara II was programmed to mix 85 μl of pretreated sample and 135 μl of reagent containing 50 mM glycine (pH 9.5), 0.03 mg of bovine serum albumin per ml, 5 mM MgSO4, 100 mM NaCl, and 0.5 U of rArDH. After incubation at 25°C for 30 s, 10 μl of reagent containing 0.5 mM NAD was added. Measurement began after 5 s. The increase of fluorescence was recorded at 30-s intervals for 150 s (excitation wavelength, 345 nm; emission wavelength, 450 nm). The slope during the first 30 s was used to calculate the rate of NAD reduction.
(ii) Calibration curves.
Six calibrators were prepared by supplementing a normal pooled human serum with different concentrations of d-arabinitol prior to sample pretreatment. An endogenous d-arabinitol concentration of approximately 1.1 μM was determined in this normal pooled human serum by the method of Switchenko et al. (13). The final concentrations of d-arabinitol in six calibrators were 1.1, 2.1, 6.1, 11.1, 16.1, and 21.1 μM, respectively. Calibration curves were obtained by plotting the initial rate of NADH produced by standards versus the d-arabinitol concentration of the standards. Concentrations of d-arabinitol in unknown samples were determined by reference to the linear least-squares fit to the calibration curves.
(iii) Human samples.
Single serum samples were obtained from 10 healthy controls and from 11 non-human immunodeficiency virus-infected patients with Candida fungemia and no known immunodeficiency states. The serum specimen from each fungemic patient was obtained on the day where the first positive blood culture was drawn. Seven patients had C. albicans fungemia, and four had Candida parapsilosis fungemia. d-Arabinitol was measured by the automated enzyme fluorometric method, creatinine was measured by an alkaline picrate (modified Jaffe) reaction on a Hitachi 717 chemistry instrument, and d-arabinitol/creatinine ratios were calculated as previously described (16).
(iv) Statistical analysis.
The significance of differences between the d-arabinitol/creatinine ratios for Candida fungemic patients and uninfected controls was assessed by the Mann-Whitney test, and a P value of <0.05 was considered significant.
RESULTS
Catalytic and physical properties of the rArDH.
The overall yield of rArDH from the purification procedure was 80%, and its specific activity was 123.5 U/mg. Analysis of the NADH elute by sodium dodecyl sulfate-polyacrylamide gel electrophoresis showed a single band at a subunit molecular mass of 31 kDa.
The enzyme was highly specific for d-arabinitol, exhibiting 4.9% cross-reactivity with xylitol and no detectable reactivity with l-arabinitol, galactitol, glycerol, mannitol, d-ribitol, or d-ribitol-5-phosphate. For the reverse activity, the enzyme oxidized NADH in the presence of d-ribulose (137.2 U/mg), with 5% cross-reactivity with d-xylulose. No cross-reactivity was seen with glucose, d-arabinose-5-phosphate, d-ribulose-5-phosphate, or d-xylulose-5-phosphate. The apparent Km for d-arabinitol was 46.6 mM in the presence of NAD, and the Km for d-ribulose was 42.6 mM in the presence of NADH.
The effects of metal ions, pH, and temperature on the enzyme activity were determined. Addition of 5 mM MgCl2 and 5 mM MgSO4 increased the catalytic activity of rArDH by ≤33%, but 5 mM ZnSO4 and 5 mM CaCl2 decreased its activity by 66 and 30%, respectively. Also, EDTA at ≥10 μM decreased the enzyme activity by 77.5%. However, 100 mM NaCl and 0.02% (wt/vol) bovine serum albumin did not alter the catalytic activity of rArDH. Thus, NaCl and bovine serum albumin were included in the automated assay as stabilizers. The optimum pH for d-arabinitol oxidation is 9.0, and catalytic activity decreased by 50% at a pH value of ≥10.0. rArDH was stable at 4°C for 1 week, at −20°C for 3 weeks, and at −80°C for at least 3 months.
Automated enzymatic fluorometric assay of d-arabinitol in serum. (i) Calibration curves.
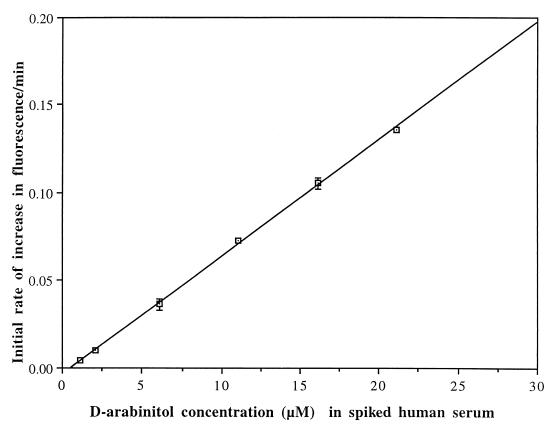
Calibration curves generated on six different days were linear with correlation coefficients of 0.998 ± 0.001 (Fig. 1). Assay precision was high, with a mean interassay variance of 2.5%.
FIG. 1.
Calibration curve for the assay of d-arabinitol in spiked human serum. A pooled human serum containing 1.1 μM endogenous d-arabinitol was supplemented with six different concentrations of d-arabinitol. The x axis gives the total concentrations of endogenous plus added d-arabinitol, whereas the y axis gives the mean ± SD of initial rates of change in fluorescence per minute assayed on six different days (R = 0.9995).
(ii) Accuracy and precision.
The interassay precision was determined by analyzing human sera spiked with 5 and 15 μM d-arabinitol and tested on six different days. The coefficients of variation were 1.7% at 5 μM and 1.4% at 15 μM (mean, 1.6%). Intra-assay precision was evaluated by determining the concentration of human sera spiked with 0.1, 0.5, 1, 5, 10, 11, 15, and 20 μM d-arabinitol. Coefficients of variation ranged from 0.1 to 1.5% (mean, 0.8%).
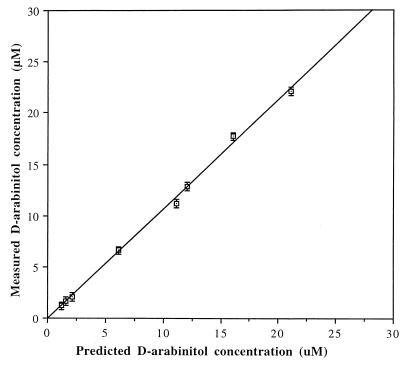
Accuracy was evaluated by determining the d-arabinitol concentrations of eight sera spiked with 0.1 to 20 μM d-arabinitol. The d-arabinitol concentrations of each spiked serum were predicted by the total concentrations of endogenous and supplemented d-arabinitol. A comparison between predicted d-arabinitol values and values measured by enzymatic fluorometric assay showed no significant difference. The linear calibration curve was obtained with correlation coefficients of 0.999 (Fig. 2). Recovery of d-arabinitol was studied by adding 2 μM d-arabinitol as a supplement to nine different serum specimens. Recoveries were measured by the difference in measured d-arabinitol concentration between supplemented and unsupplemented samples and divided by d-arabinitol concentration added. The average recovery ranged from 96 to 106% with a mean of 101% (Table 1).
FIG. 2.
Accuracy of d-arabinitol measurement determined by automated fluorometric assay. Eight serum samples that were spiked with different concentrations of d-arabinitol were assayed by the automated fluorometric method, and d-arabinitol values are shown on the y axis (mean ± SD). The d-arabinitol concentration on the x axis is predicted from the total concentrations of endogenous and added d-arabinitol (R = 0.9990).
TABLE 1.
Recoveries of 2 μM d-arabinitol following its addition to nine healthy sera
| Serum no. | Measured d-arabinitol concn (μM)
|
% Recoverya | |
|---|---|---|---|
| Unsupplemented sample | Sample supplemented with 2 μM d-arabinitol | ||
| 1 | 1.25 ± 0.06 | 3.26 ± 0.14 | 101 |
| 2 | 1.20 ± 0.01 | 3.20 ± 0.10 | 100 |
| 3 | 1.44 ± 0.02 | 3.48 ± 0.17 | 102 |
| 4 | 1.42 ± 0.06 | 3.38 ± 0.11 | 98 |
| 5 | 1.41 ± 0.03 | 3.35 ± 0.34 | 97 |
| 6 | 1.20 ± 0.14 | 3.11 ± 0.18 | 96 |
| 7 | 1.22 ± 0.01 | 3.21 ± 0.11 | 100 |
| 8 | 1.24 ± 0.10 | 3.34 ± 0.10 | 105 |
| 9 | 1.19 ± 0.04 | 3.31 ± 0.10 | 106 |
Difference between measured supplemented and unsupplemented d-arabinitol concentration divided by d-arabinitol concentration added.
(iii) Interference with assay by sugars and therapeutic drugs.
l-Arabinitol (50 μM), ribitol (50 μM), xylitol (50 μM), d-sorbitol (50 μM), galactitol (50 μM), d-galactose (500 μM), d-fructose (500 μM), d-mannose (750 μM), and glucose (5,000 μM) were tested for their ability to interfere with the assay. Only 50 μM xylitol produced a measurable response, and this represented 5.0% of the response observed with an equimolar amount of d-arabinitol.
Several drugs were also tested for possible interference with the d-arabinitol assay. These included acetaminophen (300 μg/ml), methylprednisone (120 μg/ml), amphotericin B (20 μg/ml), itraconazole (16 μg/ml), ketoconazole (16 μg/ml), cefaclor (230 μg/ml), ciprofloxacin (43 μg/ml), erythromycin (200 μg/ml), gentamicin (120 μg/ml), chloramphenicol (250 μg/ml), vancomycin (630 μg/ml), and heparin (8 U/ml). None of these drugs produced a measurable response.
(iv) d-Arabinitol concentrations and d-arabinitol/creatinine ratios in patients with candidemia.
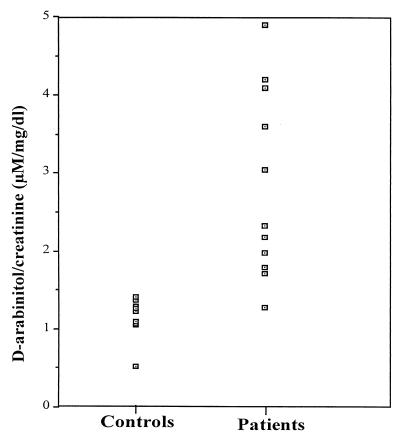
Serum d-arabinitol concentrations ranged from 1.10 to 1.58 μM in 10 healthy controls and 1.64 to 19.11 μM in the 11 patients with Candida fungemia. The mean and median d-arabinitol/creatinine ratios were 1.14 and 1.23 μM/mg/dl, respectively, for the healthy subjects, and they were 2.74 and 2.23 μM/mg/dl, respectively, for the patients with candidemia (Fig. 3). The infected patients' d-arabinitol/creatinine ratios were significantly higher than the values for the healthy subjects (P < 0.01 by the Mann-Whitney test).
FIG. 3.
d-Arabinitol/creatinine ratios (micromolar concentrations per milligram per deciliter) for 10 healthy subjects and 11 non-human immunodeficiency virus-infected patients with Candida fungemia and no known immunodeficiency state. The infected patients' d-arabinitol/creatinine ratios were significantly higher than the values for the controls (P < 0.01 by the Mann-Whitney test).
DISCUSSION
Many medically important species of Candida (including C. albicans, C. tropicalis, and C. parapsilosis) produce measurable amounts of d-arabinitol in culture as well as in infected animals and humans (3, 4, 5, 18). It has been shown elsewhere that patients with invasive candidiasis had higher serum d-arabinitol levels than did uninfected controls and that these levels declined with effective therapy (2, 4, 19). Based on these results, d-arabinitol has been recognized as a potentially useful diagnostic marker for invasive candidiasis. Since d-arabinitol is cleared primarily by glomerular filtration, the level of d-arabinitol in serum increases in proportion to creatinine when renal function is impaired. To correct for this effect, we and others have used serum d-arabinitol/creatinine ratios (4, 16, 17, 18).
Despite the usefulness of d-arabinitol as a diagnostic marker, its routine use in the clinical laboratory has been limited due to the unavailability of rapid and simple analytical methods. GC and GC-mass spectrometry were used originally to measure d-arabinitol. However, these methods are complicated, technically demanding, and time-consuming and may allow only a small number of samples to be tested each day (8, 9). The necessary equipment is also very expensive and is not routinely available in hospital diagnostic laboratories. Hence, an enzymatic method that used ArDH from K. pneumoniae was developed (10, 11, 12). However, this enzyme showed 20% cross-reactivity with d-mannitol, a sugar that may be present in normal human serum (8, 10). Thereafter, an automated enzymatic assay, based on colorimetric endpoint, for measuring d-arabinitol in human serum using a highly specific ArDH from C. tropicalis was developed (13). A large prospective clinical trial carried out by Walsh et al. (16) showed that the automated d-arabinitol testing was useful for diagnosing invasive candidiasis initially and for assessing response to therapy. However, no further investigations using this method have been reported since 1995.
If an automated d-arabinitol test is to be used widely in clinical practice, a reliable source of ArDH is needed. Although a method for overproducing recombinant C. tropicalis ArDH has been described previously (6), rArDH has not yet been used in an automated d-arabinitol assay. To ensure a steady source of rArDH, we cloned the C. albicans structural gene, overproduced rArDH in E. coli, and purified rArDH to homogeneity by dye-ligand affinity chromatography. Thus, it is now possible to generate a highly purified rArDH that is highly specific for d-arabinitol and has no cross-reactivity with sugars commonly found in human serum such as l-arabinitol, mannitol, and sorbitol. With a readily obtainable recombinant enzyme, it is technically feasible to detect and quantify d-arabinitol using clinical chemistry equipment.
The automated fluorometric enzymatic assay described here is much faster and simpler to use than either GC or GC-mass spectrometry. The assay has been set to perform automatically on a Cobas Fara II centrifugal autoanalyzer, thereby simplifying the procedure. The automated enzymatic d-arabinitol assay developed by Switchenko et al. (13) was based on two reactions: the first reaction is the oxidation of d-arabinitol and the concomitant reduction of NAD to NADH. In the second reaction, the NADH reduces p-iodonitrotetrazolium violet to iodonitrotetrazolium-formazan, which is measured spectrophotometrically. In the fluorometric assay described here, the coupling reagent and dye reagent are eliminated, saving both time and cost in reagent preparation and reducing potential sources of error. Our reaction system, which is based on initial reaction rate, is approximately five times faster than the dye-coupling method using the endpoint reaction. The dye-coupling method requires 16 min for one assay, whereas the fluorometric assay requires only 3.5 min, thereby increasing sample throughput substantially.
We have used the enzymatic fluorometric assay to monitor the levels of d-arabinitol in the serum of healthy controls and patients with candidemia. Although the numbers of studied subjects were small, significantly elevated d-arabinitol/creatinine ratios were found in the serum from patients with invasive candidiasis compared to the ratios for uninfected controls. These data, in conjunction with other clinical indicators, may provide earlier detection of invasive candidiasis, which in turn may facilitate the earlier treatment of invasive candidiasis.
The enzymatic fluorometric assay that we developed is simple, highly specific, and sensitive for measuring d-arabinitol in serum and permits analysis of many samples within a working day. Currently, we have an ongoing prospective project in the State of Connecticut for retrieving serum specimens from patients diagnosed with candidemia. The study will allow us to explore the potential role of monitoring serum d-arabinitol concentration by the fluorometric method described here. Although blood culture is unlikely to be replaced by other diagnostic tools, we anticipate that fluorometric detection of d-arabinitol will ultimately lead to improved diagnosis of invasive candidiasis.
ACKNOWLEDGMENTS
This work was supported by grants from the U.S. Department of Veterans' Affairs and from Pfizer Pharmaceuticals, Inc.
REFERENCES
- 1.Berenguer J, Buck M, Witebsky F, Stock F, Pizzo P A, Walsh T J. Lysis-centrifugation blood cultures in the detection of tissue-proven invasive candidiasis: disseminated versus single organ infection. Diagn Microbiol Infect Dis. 1993;17:103–109. doi: 10.1016/0732-8893(93)90020-8. [DOI] [PubMed] [Google Scholar]
- 2.Bernard E M, Christiansen L J, Tsang S F, Kiehn T E, Armstrong D. Rate of arabinitol production by pathogenic yeast species. J Clin Microbiol. 1981;14:189–194. doi: 10.1128/jcm.14.2.189-194.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christensson B, Wiebe T, Pehrson C, Larsson L. Diagnosis of invasive candidiasis in neutropenic children with cancer by determination of d-arabinitol/l-arabinitol ratios in urine. J Clin Microbiol. 1997;35:636–640. doi: 10.1128/jcm.35.3.636-640.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gold J W, Wong B, Bernard E M, Kiehn T E, Armstrong D. Serum arabinitol concentrations and arabinitol/creatinine ratios in invasive candidiasis. J Infect Dis. 1983;147:504–513. doi: 10.1093/infdis/147.3.504. [DOI] [PubMed] [Google Scholar]
- 5.Kiehn T E, Bernard E M, Gold J W M, Armstrong D. Candidiasis: detection by gas-liquid chromatography of D-arabinitol, a fungal metabolite, in human serum. Science. 1979;206:577–580. doi: 10.1126/science.493963. [DOI] [PubMed] [Google Scholar]
- 6.Murray J S, Wong M L, Miyada C G, Switchenko A C, Goodman T C, Wong B. Isolation, characterization and expression of the gene that encodes D-arabinitol dehydrogenase in Candida tropicalis. Gene. 1995;155:123–128. doi: 10.1016/0378-1119(94)00900-d. [DOI] [PubMed] [Google Scholar]
- 7.Murray P R. Comparison of lysis-centrifugation and agitated biphasic blood culture systems for detection of fungemia. J Clin Microbiol. 1991;29:96–98. doi: 10.1128/jcm.29.1.96-98.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roboz J, Kappatos D C, Greaves J, Holland H F. Determination of polyols in serum by selected ion monitoring. Clin Chem. 1984;30/10:1611–1615. [PubMed] [Google Scholar]
- 9.Roboz J, Suzuki R, Holland J F. Quantification of arabinitol in serum by selected ion monitoring as diagnostic technique in invasive candidiasis. J Clin Microbiol. 1980;12:594–602. doi: 10.1128/jcm.12.4.594-601.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soyama K, Ono E. Enzymatic fluorometric method for the determination of D-arabinitol in serum by initial analysis. Clin Chim Acta. 1985;149:149–154. doi: 10.1016/0009-8981(85)90328-6. [DOI] [PubMed] [Google Scholar]
- 11.Soyama K, Ono E. Improved procedure for determining serum D-arabinitol by reazurin-coupled method. Clin Chim Acta. 1987;168:259–260. doi: 10.1016/0009-8981(87)90297-x. [DOI] [PubMed] [Google Scholar]
- 12.Soyama K, Ono E. Enzymatic and gas-liquid chromatographic measurement of D-arabinitol compared. Clin Chem. 1988;34:432. [PubMed] [Google Scholar]
- 13.Switchenko A C, Miyada C G, Goodman T C, Walsh T J, Wong B, Becker M J, Ullman E F. An automated enzymatic method for measurement of d-arabinitol, a metabolite of pathogenic Candida species. J Clin Microbiol. 1994;32:92–97. doi: 10.1128/jcm.32.1.92-97.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Telenti A, Steckelberg J M, Stockman L, Edison R S, Robert G D. Quantitative blood cultures in candidemia. Mayo Clin Proc. 1991;66:1120–1123. doi: 10.1016/s0025-6196(12)65791-7. [DOI] [PubMed] [Google Scholar]
- 15.Walsh T J, Chanock S J. Diagnosis of invasive fungal infections: advances in nonculture systems. Curr Clin Top Infect Dis. 1998;18:101–153. [PubMed] [Google Scholar]
- 16.Walsh T J, Merz W G, Lee J W, Schaufele R, Sein T, Whitcomb P O, Ruddel M, Burns W, Wingard J R, Switchenko A C, Goodman T, Pizzo P A. Diagnosis and therapeutic monitoring of invasive candidiasis by rapid enzymatic detection of serum D-arabinitol. Am J Med. 1995;99:164–172. doi: 10.1016/s0002-9343(99)80136-3. [DOI] [PubMed] [Google Scholar]
- 17.Wong B, Bernard E M, Gold J W M, Fong D, Armstrong D. The arabinitol appearance rate in laboratory animals and humans: estimation from the arabinitol/creatinine ratio and relevance to the diagnosis of candidiasis. J Infect Dis. 1982;146:353–359. doi: 10.1093/infdis/146.3.353. [DOI] [PubMed] [Google Scholar]
- 18.Wong B, Bernard E M, Gold J W M, Fong D, Silber A, Armstrong D. Increased arabinitol levels in experimental candidiasis in rats: arabinitol appearance rates, arabinitol/creatinine ratios, and severity of infection. J Infect Dis. 1982;146:346–352. doi: 10.1093/infdis/146.3.346. [DOI] [PubMed] [Google Scholar]
- 19.Wong B, Brauer K L. Enantioselective measurement of fungal d-arabinitol in the sera of normal adults and patients with candidiasis. J Clin Microbiol. 1988;26:1670–1674. doi: 10.1128/jcm.26.9.1670-1674.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong B, Murray J S, Castellanos M, Croen K D. d-Arabinitol metabolism in Candida albicans: studies of the biosynthesis pathway and the gene that encodes NAD-dependent d-arabitol dehydrogenase. J Bacteriol. 1993;175:6314–6320. doi: 10.1128/jb.175.19.6314-6320.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]