Abstract
Background
The prevalences of obstructive and restrictive spirometric phenotypes, and their relation to early-life risk factors from childhood to young adulthood remain poorly understood. The aim was to explore these phenotypes and associations with well-known respiratory risk factors across ages and populations in European cohorts.
Methods
We studied 49 334 participants from 14 population-based cohorts in different age groups (≤10, >10–15, >15–20, >20–25 years, and overall, 5–25 years). The obstructive phenotype was defined as forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) z-score less than the lower limit of normal (LLN), whereas the restrictive phenotype was defined as FEV1/FVC z-score ≥LLN, and FVC z-score <LLN.
Results
The prevalence of obstructive and restrictive phenotypes varied from 3.2–10.9% and 1.8–7.7%, respectively, without clear age trends. A diagnosis of asthma (adjusted odds ratio (aOR=2.55, 95% CI 2.14–3.04), preterm birth (aOR=1.84, 1.27–2.66), maternal smoking during pregnancy (aOR=1.16, 95% CI 1.01–1.35) and family history of asthma (aOR=1.44, 95% CI 1.25–1.66) were associated with a higher prevalence of obstructive, but not restrictive, phenotype across ages (5–25 years). A higher current body mass index (BMI was more often observed in those with the obstructive phenotype but less in those with the restrictive phenotype (aOR=1.05, 95% CI 1.03–1.06 and aOR=0.81, 95% CI 0.78–0.85, per kg·m−2 increase in BMI, respectively). Current smoking was associated with the obstructive phenotype in participants older than 10 years (aOR=1.24, 95% CI 1.05–1.46).
Conclusion
Obstructive and restrictive phenotypes were found to be relatively prevalent during childhood, which supports the early origins concept. Several well-known respiratory risk factors were associated with the obstructive phenotype, whereas only low BMI was associated with the restrictive phenotype, suggesting different underlying pathobiology of these two phenotypes.
Short abstract
Obstructive and restrictive phenotypes are present from childhood to adulthood but without age trends. Established risk factors for airway disease are associated with the obstructive phenotype, whereas low BMI is associated with the restrictive. https://bit.ly/3BMoMtI
Introduction
Low peak lung function detected by spirometry in early adulthood relates to the increased incidence of respiratory, cardiovascular and metabolic abnormalities, as well as premature death [1, 2]. Spirometry allows the identification and quantification of the severity of a ventilatory impairment, as well as the first step of classification into two main phenotypes, obstructive and restrictive patterns. The obstructive phenotype is defined by a lower than expected forced expiratory volume in 1 s/forced vital capacity (FEV1/FVC) ratio, and the restrictive pattern by an abnormally low FVC with a normal FEV1/FVC ratio (yet acknowledging that body plethysmography is needed to diagnose restrictive lung disease) [3]. Although both phenotypes are relatively common [4–10] and well-studied in adult cohorts [3, 9, 11, 12], to date, no large study has investigated their respective prevalence, age dependency or associated risk factors in the period from childhood to young adulthood. The CADSET (Chronic Airway Disease Early Stratification) clinical research collaboration launched by the European Respiratory Society (ERS) in 2018 [13] offers a unique opportunity to combine individual data from multiple cohorts to increase the sample size required to explore these questions. In the current study, we collected data from almost 50 000 subjects across 14 population-based cohorts in Europe in order to report the age-specific prevalence, characteristics and risk factors for spirometric phenotypes from early childhood to young adulthood (5 to 25 years of age), using the Global Lung Function Initiative (GLI) reference values [14].
Methods
Study design and subjects
14 European population-based cohorts from seven countries were included in the current meta-analysis. A total of 49 334 participants were included in the current study, and 18 430 of them (from seven cohorts) had repeated lung function measurements from 4 to 25 years of age. Details about each cohort and the definition of covariates are provided in the online supplementary material.
Measurements and definitions of outcomes
Pre-bronchodilator lung function was tested in each cohort according to the American Thoracic Society (ATS/ERS spirometry criteria [15]. FEV1, FVC and FEV1/FVC were converted into z-scores according to the equations from the GLI [14] for each cohort separately. Although no disease selective cohorts were included, a high heterogeneity in GLI fit between age groups and between cohorts (see Results, “The fit of GLI z-scores” and table 1) was observed, and we therefore applied a centring approach to make the cohorts more comparable. Centring was performed separately for each cohort and age group by subtracting the mean z-score (of FEV1, FVC and FEV1/FVC, respectively) of non-smoking individuals without asthma (where a perfect fit would give a mean of 0 z-scores) from each individual z-score lung function variable.
TABLE 1.
Characteristics and forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC) and FEV1/FVC ratio z-scores of each cohort included in the age groups
| Cohorts | Nations | Age groups | n | Age # | FEV1 | FVC | FEV1/FVC ratio | |||
| GLI fit # | Mean-centred ¶ | GLI fit # | Mean-centred ¶ | GLI fit # | Mean-centred ¶ | |||||
| ALSPAC+ | UK | >5–10 | 6804 | 8.7±0.3 | 0.04±1.00 | −0.09±1.02 | −0.06±1.02 | −0.02±1.04 | 0.15±1.02 | −0.10±1.07 |
| >15–20 | 4519 | 15.5±0.3 | −0.68±1.29 | −0.04±1.26 | −1.02±1.29 | 0.04±1.25 | 0.61±1.16 | −0.14±1.21 | ||
| >20–25 | 3731 | 24.5±0.8 | −0.52±1.01 | 0.06±1.00 | −0.37±1.02 | 0.13±0.98 | −0.27±0.94 | −0.1±0.98 | ||
| BAMSE+ | Sweden | >5–10 | 1832 | 8.3±0.5 | 0.47±0.94 | −0.06±0.94 | 0.59±0.90 | −0.00±0.91 | −0.27±0.87 | −0.07±0.89 |
| >15–20 | 2052 | 16.7±0.4 | 0.06±0.92 | −0.11±0.94 | 0.16±0.92 | −0.01±0.92 | −0.19±0.93 | −0.15±0.96 | ||
| >20–25 | 2032 | 22.5±0.5 | −0.23±0.85 | −0.03±0.86 | −0.12±0.85 | 0.07±0.85 | −0.20±0.88 | −0.15±0.90 | ||
| Generation R+ | Netherlands | >5–10 | 4738 | 9.8±0.3 | 0.19±0.93 | −0.04±0.93 | 0.21±0.89 | 0.02±0.92 | −0.08±0.90 | −0.01±0.96 |
| >10–15 | 3869 | 13.6±0.4 | −0.15±1.01 | −0.06±0.93 | −0.09±0.98 | 0.04±0.99 | −0.13±0.92 | 0.00±1.02 | ||
| HUNT1 | Norway | >10–15 | 2705 | 14.1±0.6 | −0.23±1.11 | −0.02±1.11 | −0.19±1.10 | 0.01±1.10 | −0.07±1.03 | −0.05±1.03 |
| >15–20 | 5256 | 17.1±1.3 | −0.12±1.04 | −0.03±1.04 | −0.09±1.01 | 0.02±1.01 | −0.08±0.98 | −0.08±0.98 | ||
| HUNT3 | Norway | >10–15 | 2792 | 14.1±0.6 | 0.01±1.03 | −0.01±1.03 | 0.10±1.05 | 0.04±1.05 | −0.15±1.02 | −0.22±1.02 |
| >15–20 | 4363 | 16.9±1.2 | 0.09±0.99 | −0.04±0.99 | 0.19±1.01 | 0.03±1.01 | −0.19±0.98 | −0.09±0.97 | ||
| INMA+ | Spain | 1–5 | 704 | 4.5±0.2 | −0.59±1.20 | −0.01±1.21 | −0.53±1.23 | 0.01±1.25 | −0.03±0.95 | −0.03±0.97 |
| >5–10 | 1277 | 7.4±0.6 | 0.21±0.98 | −0.01±0.99 | 0.40±0.95 | 0.01±0.96 | −0.34±0.99 | −0.03±1.00 | ||
| >5–10 | 476 | 9.3±0.9 | −0.02±1.07 | −0.03±1.05 | 0.17±1.04 | −0.00±1.01 | −0.21±0.98 | −0.06±0.95 | ||
| >10–15 | 988 | 11.2±0.6 | −0.20±1.00 | −0.01±1.03 | −0.06±1.03 | 0.02±1.04 | −0.25±0.95 | −0.04±1.01 | ||
| >10–15 | 266 | 14.6±0.2 | 0.04±0.94 | −0.04±0.95 | −0.04±0.98 | −0.02±0.95 | 0.13±0.87 | −0.03±0.90 | ||
| >15–20 | 120 | 17.7±0.3 | −0.34±0.92 | −0.03±0.89 | −0.32±0.98 | 0.07±0.94 | −0.04±0.99 | −0.15±1.02 | ||
| IoW | UK | >5–10 | 980 | 9.9±0.3 | 0.37±0.97 | −0.04±0.99 | 0.16±0.88 | 0.02±0.88 | 0.33±0.96 | −0.09±1.01 |
| >15–20 | 836 | 17.8±0.6 | 0.30±0.92 | −0.15±0.99 | 0.14±0.85 | 0.01±0.93 | 0.22±1.07 | −0.21±1.11 | ||
| LEAD | Austria | >5–10 | 451 | 8.4±1.0 | 0.46±1.27 | −0.07±0.99 | 0.31±1.26 | 0.10±0.92 | 0.35±1.17 | −0.31±1.13 |
| >10–15 | 526 | 12.3±1.5 | 0.01±1.03 | −0.35±1.02 | −0.19±0.97 | −0.14±0.98 | 0.36±0.95 | −0.39±1.04 | ||
| >15–20 | 540 | 17.4±1.4 | −0.05±1.07 | −0.36±1.01 | −0.22±1.08 | −0.11±0.97 | 0.30±1.08 | −0.44±1.12 | ||
| >20–25 | 703 | 22.5±1.4 | −0.13±1.04 | −0.42±0.92 | −0.31±1.09 | −0.16±0.92 | 0.22±0.99 | −0.46±1.02 | ||
| Lifelines | Netherlands | >15–20 | 2556 | 18.9±0.8 | −0.42±0.91 | −0.06±0.92 | −0.34±0.90 | 0.03±0.89 | −0.19±0.98 | −0.14±1.01 |
| >20–25 | 5028 | 23.3±1.5 | −0.42±0.90 | −0.06±0.91 | −0.29±0.89 | 0.05±0.87 | −0.23±0.96 | −0.16±0.96 | ||
| MAAS+ | UK | >5–10 | 778 | 8.0±0.2 | 0.15±0.93 | −0.07±0.10 | 0.26±0.90 | 0.00±0.94 | −0.24±0.89 | −0.11±0.93 |
| >10–15 | 778 | 11.5±0.5 | −0.12±0.95 | −0.05±1.00 | −0.18±1.12 | 0.05±1.12 | 0.14±1.03 | −0.15±1.10 | ||
| >15–20 | 566 | 16.0±0.6 | −0.26±0.95 | −0.06±1.05 | −0.43±0.90 | 0.03±0.96 | 0.31±1.02 | −0.16±1.11 | ||
| >15–20 | 504 | 19.4±0.8 | −0.24±0.87 | −0.04±0.95 | −0.40±0.90 | 0.10±0.90 | 0.26±0.97 | −0.24±1.04 | ||
| OLIN | Sweden | >15–20 | 1470 | 18.2±0.5 | −0.06±1.01 | −0.04±1.01 | 0.08±1.06 | 0.01±1.03 | −0.28±0.93 | −0.07±0.96 |
| PIAMA+ | Netherlands | >5–10 | 1058 | 8.1±0.3 | 0.54±0.88 | −0.05±0.91 | 0.29±0.88 | 0.00±0.90 | 0.42±1.06 | −0.09±1.09 |
| >10–15 | 1267 | 12.7±0.4 | −0.56±0.84 | −0.04±0.86 | −0.37±0.85 | −0.01±0.86 | −0.37±0.87 | −0.04±0.89 | ||
| >15–20 | 720 | 16.4±0.2 | −0.19±0.86 | −0.07±0.88 | 0.02±0.86 | −0.00±0.82 | −0.40±0.92 | −0.10±0.94 | ||
| SEATON+ | UK | 1–5 | 446 | 4.9±0.2 | 0.17±0.96 | −0.03±0.95 | −0.13±1.05 | −0.00±1.05 | 0.08±0.93 | −0.04±0.95 |
| >10–15 | 430 | 10.3±0.2 | −0.12±1.10 | −0.06±1.09 | −0.24±1.04 | −0.01±1.04 | 0.15±0.93 | −0.08±0.96 | ||
| >15–20 | 534 | 15.1±0.3 | −0.36±1.00 | −0.04±1.00 | −0.77±1.02 | 0.01±1.00 | 0.83±1.09 | −0.10±1.13 | ||
| SUS | Denmark | >15–20 | 2294 | 18.9±1.3 | −0.29±0.90 | −0.01±0.90 | −0.33±0.88 | 0.05±0.88 | 0.01±1.00 | −0.09±1.00 |
Data are expressed as mean±sd. GLI: Global Lung Function Initiative. #: based on the whole group of participants; ¶: based on non-asthmatic, asymptomatic lifelong nonsmokers; +: they are longitudinal cohorts with repeated measurement at different age.
The diagnostic algorithm of our spirometry phenotypes was based on the lower limit of normal (LLN) as the lower fifth percentile of distribution that corresponds to a z-score −1.645 (rounded to −1.65 if two decimals were used) and used as follows (supplementary Table E1): normal lung function was defined as the FEV1/FVC ratio and FVC z-scores equal to or higher than LLN. The obstructive phenotype was defined as FEV1/FVC ratio z-score lower than LLN, and severity defined according to Quanjer et al. [16] as mild, moderate and severe according to thresholds of FEV1: z-score less than LLN but ≥−2, <−2 but ≥−3 and <−3, respectively. In addition to these, a very mild group was added, defined as a FEV1/FVC ratio z-score lower than LLN and FEV1 z-score greater or equal to LLN. The restrictive phenotype (“Low FVC, non-obstructive”) was defined as FEV1/FVC ratio z-score equal to or higher than LLN, and FVC z-score lower than LLN. Severity was evaluated as mild, moderate and severe according to two thresholds of FVC: z-score less than LLN but ≥−2, <−2 but ≥−3 and <−3, respectively [16].
Statistical analysis
We performed cohort-specific analyses followed by meta-analysis. The associations between the cohort-specific prevalence of obstructive and restrictive phenotypes and age were tested by Pearson correlation. Comparisons of the prevalence of wheezing and asthma between obstructive and restrictive phenotypes and normal lung function groups were performed using the Wilcoxon Rank Sum test. Multivariable regression models were conducted to identify risk factors of obstructive and restrictive phenotypes, and FEV1, FVC and FEV1/FVC ratio z-scores, respectively. We used two models to explore selected potential risk factors. In the first model, three well-known early-life respiratory risk factors (asthma family history, maternal smoking during pregnancy and preterm birth (delivery before 37 completed weeks of gestation)), and two lifestyle factors (body mass index (BMI) and smoking status) were evaluated using logistic (for obstructive and restrictive phenotypes) and linear regressions (for z-scores). In the second model, current asthma was added as predictor to the models to specifically evaluate the influence of asthma, since this is typically classified as an obstructive disease. In the meta-analysis of cohort-specific results, we combined data from each cohort in age groups separately (≤10, >10–15, >15–20, >20–25 years), as well as overall across ages (5–25 years). For those cohorts that had repeated lung function measurements from multiple time points (i.e., data from several age groups), regression analysis for each time point was performed and included in the relevant age group. Where multiple time points existed for a cohort in an age group, only the largest age group was used in the meta-analyses (both age-bin specific and overall) to provide estimates from truly independent datasets. Heterogeneity was assessed with the Q and the I2 statistic. The Q statistic was calculated according to the weighted sum of squared differences between individual study effects and the pooled effect across studies and is distributed as a chi-square statistic with k (number of studies) minus 1 degrees of freedom [17]. The I2 statistic describes the percentage of variation across studies that is due to heterogeneity rather than chance [18, 19]. A random-effects model was used to pool data if substantial heterogeneity was observed (I2>50% or p<0.1 for Q statistic), otherwise we used a fixed-effects model. Meta-analyses were performed using the R software (version 4.0.4) with “meta” package (version 4.18.1).
Results
Basic characteristics
Table 1 illustrates the basic characteristics of the cohorts. Of the 14 included cohorts, seven (ALSPAC, BAMSE, Generation R, INMA, MAAS, PIAMA, SEATON) contributed repeated data from early childhood to young adulthood (age 4 to 25 years). Two, eight, eight, 13 and four cohorts contributed results in the 1–5, >5–10, >10–15, >15–20, >20–25 years age groups, respectively. As the 1–5 age bin only contained two cohorts (INMA and SEATON), the 1–5 and >5–10 age groups were combined into the <10 age bin in the meta-analysis. Although INMA contributed data at 4, 7 and 9 years, only the largest dataset (7 years) was included in the meta-analysis in the <10 age bin.
The fit of GLI z-scores
The fit of GLI z-scores is described for each cohort in non-asthmatic, asymptomatic lifelong nonsmokers as mean and sd in table 1. While the overall GLI fit was good in many age groups in the cohorts, a high heterogeneity was observed. For six, four and three cohorts, GLI fit estimates for FEV1, FVC and FEV1/FVC z-scores, respectively, were outside the suggested range using 0.4 as a cut-off [20] (supplementary Figure E1). Therefore, we proceeded with mean-centred z-scores (as described in the Methods) for comparisons of prevalences across cohorts, and for the meta-analyses. While mean GLI z-score values varied by cohort and age, the sd was close to 1 in all groups.
Prevalence of spirometric phenotypes and association with respiratory symptoms
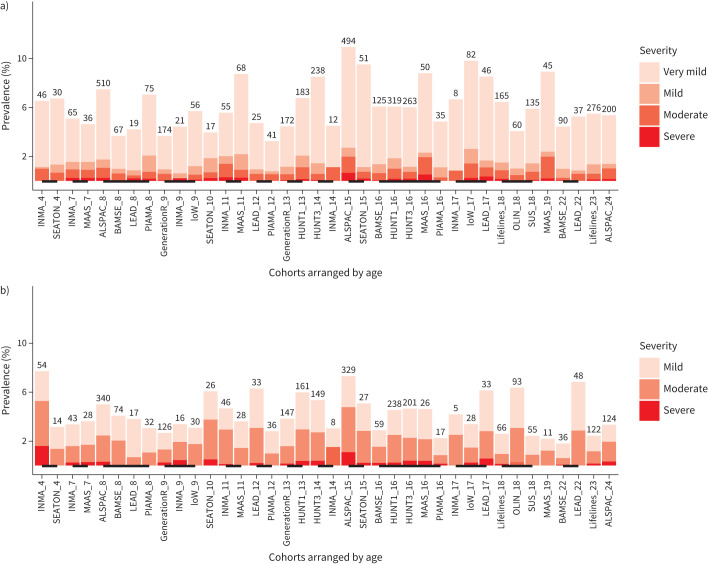
We used the mean-centred z-scores to explore the prevalence of spirometry impairment phenotypes across ages and cohorts. The prevalence of obstructive and restrictive phenotypes during early childhood and young adulthood ranged from 3.2 to 10.9% and 1.8 to 7.7%, respectively (figure 1a and b). There was no overall association between age and the prevalence of obstructive and restrictive phenotypes (r=0.14, p=0.39 and r=−0.14, p=0.41, respectively, supplementary Figure E2A and E2B). Most participants with the obstructive phenotype were classified as having a (very) mild impairment, while most participants with the restrictive phenotype were classified as having a mild to moderate impairment (figure 1a and b).
FIGURE 1.
Prevalence of the a) obstructive and b) restrictive phenotypes from early childhood to young adulthood. The numbers above each bar represent the number of cases in the respective study. Cohorts linked by lines had the same mean age (in years).
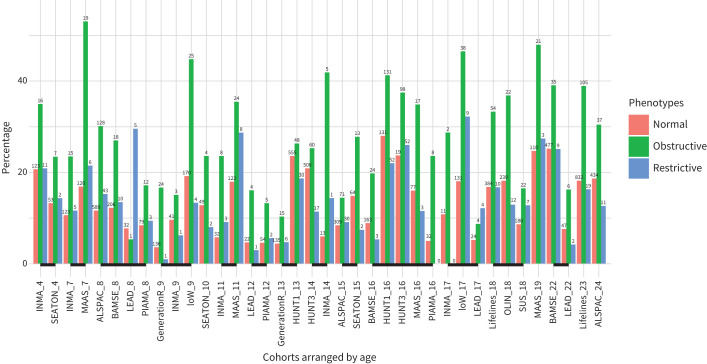
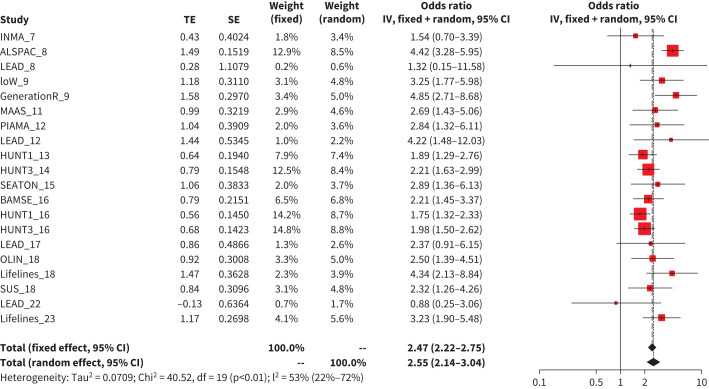
Participants with the obstructive phenotype more frequently reported wheezing in the previous 12 months and having been diagnosed with asthma when compared to participants with normal lung function (median=26.6%, interquartile range (IQR)=16.7 to 36.4% versus 12.5%, IQR=7.9 to 18.1%, p<0.001, and 29.8%, IQR= 21.9 to 42.3% versus 12.3%, IQR=7.1 to 22.5%, p<0.001, respectively, figure 2 and supplementary Figure E3). However, no significant difference in respiratory symptoms between participants with the restrictive phenotype and normal lung function was observed (12.7%, IQR=7.6 to 18.1% versus 12.5%, IQR=7.9 to 18.1%, p=0.95, and 10.1%, IQR=7.1 to 20.9% versus 12.3%, IQR=7.1 to 22.5%, p=0.57, respectively, figure 2 and supplementary Figure E1). In the adjusted regression models, a current diagnosis of asthma was strongly associated with the obstructive phenotype (5–25 years age group aOR=2.55, 95% CI 2.14–3.04, figure 3, for other subgroups see supplementary Figure E4). No association between a current diagnosis of asthma and the restrictive phenotype (supplementary Figure E5) was observed.
FIGURE 2.
Prevalence of any wheezing in participants with obstructive or restrictive phenotypes, or normal lung function. The numbers above each bar represent the number of cases in the respective study. Cohorts linked by lines had the same mean age (in years).
FIGURE 3.
Meta-analysis results of association between asthma and obstructive phenotype in 5–25 years subgroup. TE: the estimated treatment effect; IV: inverse variance.
Risk factors associated with impaired lung function
We explored the mutually adjusted associations of three well-known early-life respiratory risk factors (preterm birth, maternal smoking during pregnancy and asthma family history), as well as the lifestyle factors at the time of lung function testing, BMI and smoking status (former and current smoker) and obstructive and restrictive phenotypes as well as FEV1, FVC and FEV1/FVC ratio z-scores. The combined meta-analysis results are illustrated in table 2 (for spirometry phenotypes) and supplementary Table E2 (for z-scores) and results additionally adjusted for asthma in table 3 and supplementary Table E3.
TABLE 2.
Meta-analysis results of obstructive and restrictive phenotypes in Model 1# in different age groups
| Variables | Age groups | Number of cohorts | I2 | p-value for Q statistic | OR (95% CI) |
| Obstructive phenotype | |||||
| Preterm birth | <10 | 9 | 60.8 | 0.0089 | 1.82 (1.08–3.06) 2.73 (1.67–4.46) 1.61 (1.13–2.29) 1.37 (0.88–2.12) 1.84 (1.27–2.66) |
| >10–15 | 5 | 17.6 | 0.30 | ||
| >15–20 | 8 | 6.7 | 0.38 | ||
| >20–25 | 4 | 0.0 | 0.63 | ||
| 5–25 | 10 | 51.6 | 0.016 | ||
| Maternal smoking during pregnancy | <10 | 9 | 0.0 | 0.61 | 1.11 (0.94–1.30) 1.20 (0.90–1.59) 1.43 (1.14–1.78) 1.43 (1.07–1.93) 1.16 (1.01–1.35) |
| >10–15 | 6 | 0.0 | 0.98 | ||
| >15–20 | 10 | 9.1 | 0.36 | ||
| >20–25 | 3 | 0.0 | 0.41 | ||
| 5–25 | 11 | 0.0 | 0.53 | ||
| Asthma family history | <10 | 9 | 26.9 | 0.20 | 1.34 (1.14–1.58) 1.39 (1.01–1.93) 1.46 (1.24–1.72) 1.59 (1.22–2.07) 1.44 (1.25–1.66) |
| >10–15 | 6 | 0.0 | 0.55 | ||
| >15–20 | 11 | 0.0 | 0.52 | ||
| >20–25 | 3 | 29.6 | 0.24 | ||
| 5–25 | 12 | 0.0 | 0.83 | ||
| BMI | <10 | 9 | 40.6 | 0.097 | 1.06 (1.02–1.10) |
| >10–15 | 8 | 55.3 | 0.022 | 1.04 (1.00–1.09) 1.05 (1.04–1.07) 1.03 (1.01–1.05) 1.05 (1.03–1.06) |
|
| >15–20 | 12 | 17.7 | 0.27 | ||
| >20–25 | 4 | 0.0 | 0.47 | ||
| 5–25 | 13 | 44.7 | 0.019 | ||
| Former smoker | >10–15 | 2 | 0.0 | 0.61 | 0.67 (0.45–1.01) 0.83 (0.71–0.98) 1.37 (1.04–1.82) 0.93 (0.75–1.16) |
| >15–20 | 10 | 28.9 | 0.18 | ||
| >20–25 | 4 | 0.0 | 0.63 | ||
| 10–25 | 11 | 41.3 | 0.048 | ||
| Current smoker | >10–15 | 2 | 51.0 | 0.15 | 0.88 (0.38–2.05) 1.21 (1.01–1.44) 1.34 (1.01–1.78) 1.24 (1.05–1.46) |
| >15–20 | 11 | 35.5 | 0.11 | ||
| >20–25 | 4 | 0.0 | 0.70 | ||
| 10–25 | 11 | 29.8 | 0.13 | ||
| Restrictive phenotype | |||||
| Preterm birth | <10 | 8 | 0.0 | 0.79 | 1.17 (0.78–1.75) 1.46 (0.72–2.97) 1.16 (0.72–1.87) 0.88 (0.41–1.92) 1.20 (0.84–1.70) |
| >10–15 | 5 | 0.0 | 0.92 | ||
| >15–20 | 6 | 0.0 | 0.79 | ||
| >20–25 | 3 | 0.0 | 0.87 | ||
| 5–25 | 9 | 0.0 | 0.98 | ||
| Maternal smoking during pregnancy | <10 | 9 | 0.0 | 0.92 | 0.91 (0.73–1.14) 1.16 (0.79–1.70) 0.98 (0.61–1.58) 0.84 (0.52–1.36) 1.00 (0.82–1.22) |
| >10–15 | 6 | 0.0 | 0.95 | ||
| >15–20 | 9 | 40.9 | 0.095 | ||
| >20–25 | 4 | 0.0 | 0.73 | ||
| 5–25 | 11 | 0.0 | 0.66 | ||
| Asthma family history | <10 | 9 | 3.8 | 0.40 | 0.92 (0.74–1.15) 0.91 (0.58–1.44) 0.89 (0.71–1.12) 0.97 (0.66–1.42) 0.96 (0.79–1.16) |
| >10–15 | 6 | 0.0 | 0.97 | ||
| >15–20 | 10 | 13.4 | 0.32 | ||
| >20–25 | 4 | 0.0 | 0.64 | ||
| 5–25 | 12 | 0.0 | 0.84 | ||
| BMI | <10 | 9 | 69.3 | 0.001 | 0.88 (0.80–0.96) 0.80 (0.75–0.85) 0.80 (0.75–0.85) 0.84 (0.70–1.00) 0.81 (0.78–0.85) |
| >10–15 | 8 | 51.6 | 0.035 | ||
| >15–20 | 12 | 78.8 | <0.001 | ||
| >20–25 | 4 | 92.6 | <0.001 | ||
| 5–25 | 13 | 75.1 | <0.001 | ||
| Former smoker | >10–15 | 2 | 0.0 | 0.35 | 0.65 (0.40–1.04) 0.98 (0.79–1.21) 0.57 (0.27–1.23) 0.93 (0.76–1.12) |
| >15–20 | 9 | 25.3 | 0.24 | ||
| >20–25 | 4 | 56.6 | 0.075 | ||
| 10–25 | 11 | 35.9 | 0.10 | ||
| Current smoker | >10–15 | 2 | 62.2 | 0.10 | 0.89 (0.27–2.97) 0.80 (0.60–1.05) 0.72 (0.48–1.06) 0.85 (0.67–1.08) |
| >15–20 | 11 | 27.3 | 0.18 | ||
| >20–25 | 4 | 0.0 | 0.54 | ||
| 10–25 | 11 | 19.6 | 0.23 | ||
#: Model 1 was adjusted for asthma family history, maternal smoking during pregnancy, preterm birth, body mass index (BMI) and smoking status.
TABLE 3.
Meta-analysis results of obstructive and restrictive phenotypes in Model 2# in different age groups
| Variables | Age groups | Number of cohorts | I2 | p-value for Q statistic | OR (95% CI) |
| Obstructive phenotype | |||||
| Preterm birth | <10 | 8 | 53.1 | 0.037 | 2.04 (1.24–3.34) 2.83 (1.73–4.46) 1.53 (1.06–2.22) 1.49 (0.94–2.36) 1.83 (1.31–2.57) |
| >10–15 | 6 | 21.0 | 0.28 | ||
| >15–20 | 8 | 7.1 | 0.38 | ||
| >20–25 | 4 | 0.0 | 0.47 | ||
| 5–25 | 10 | 40.1 | 0.06 | ||
| Maternal smoking during pregnancy | <10 | 9 | 0.0 | 0.70 | 1.06 (0.89–1.28) 1.28 (0.95–1.73) 1.48 (1.20–1.82) 1.23 (0.86–1.75) 1.13 (0.97–1.33) |
| >10–15 | 6 | 0.0 | 0.97 | ||
| >15–20 | 10 | 17.0 | 0.29 | ||
| >20–25 | 3 | 0.0 | 0.79 | ||
| 5–25 | 11 | 0.0 | 0.58 | ||
| Asthma family history | <10 | 9 | 21.9 | 0.25 | 1.09 (0.90–1.32) 1.17 (0.82–1.67) 1.28 (1.07–1.52) 1.33 (0.74–2.40) 1.21 (1.04–1.41) |
| >10–15 | 6 | 0.0 | 0.63 | ||
| >15–20 | 11 | 0.0 | 0.84 | ||
| >20–25 | 3 | 69.6 | 0.037 | ||
| 5–25 | 12 | 0.0 | 0.67 | ||
| BMI | <10 | 9 | 53.7 | 0.027 | 1.05 (1.00–1.11) 1.06 (1.04–1.09) 1.04 (1.03–1.06) 1.04 (1.01–1.07) 1.04 (1.03–1.06) |
| >10–15 | 8 | 0.0 | 0.50 | ||
| >15–20 | 12 | 16.5 | 0.28 | ||
| >20–25 | 4 | 0.0 | 0.88 | ||
| 5–25 | 13 | 34.4 | 0.071 | ||
| Former smoker | >10–15 | 2 | 0.0 | 0.72 | 0.67 (0.45–1.01) 0.83 (0.70–0.97) 1.55 (1.14–2.11) 0.96 (0.76–1.22) 0.89 (0.37–2.15) |
| >15–20 | 10 | 20.8 | 0.25 | ||
| >20–25 | 4 | 0.0 | 0.59 | ||
| 10–25 | 11 | 47.0 | 0.023 | ||
| Current smoker | >10–15 | 2 | 54.1 | 0.14 | |
| >15–20 | 11 | 45.1 | 0.052 | 1.18 (0.90–1.54) 1.28 (0.92–1.79) 1.18 (0.93–1.49) |
|
| >20–25 | 4 | 0.0 | 0.67 | ||
| 10–25 | 11 | 37.6 | 0.07 | ||
| Restrictive phenotype | |||||
| Preterm birth | <10 | 8 | 0.0 | 0.70 | 1.08 (0.68–1.73) 1.80 (0.88–3.71) 1.17 (0.72–1.92) 0.76 (0.30–1.92) 1.13 (0.77–1.68) |
| >10–15 | 5 | 0.0 | 0.85 | ||
| >15–20 | 6 | 0.0 | 0.83 | ||
| >20–25 | 3 | 0.0 | 0.95 | ||
| 5–25 | 9 | 0.0 | 0.97 | ||
| Maternal smoking during pregnancy | <10 | 9 | 0.0 | 0.71 | 0.95 (0.75–1.22 1.19 (0.78–1.79) 0.98 (0.59–1.62 1.01 (0.61–1.69) 1.06 (0.86–1.32) |
| >10–15 | 6 | 0.0 | 0.98 | ||
| >15–20 | 9 | 43.8 | 0.076 | ||
| >20–25 | 4 | 0.0 | 0.99 | ||
| 5–25 | 11 | 0.0 | 0.59 | ||
| Asthma family history | <10 | 9 | 3.8 | 0.40 | 0.99 (0.77–1.27) 0.89 (0.54–1.46) 0.88 (0.69–1.12) 0.99 (0.63–1.56) 1.02 (0.83–1.25) |
| >10–15 | 5 | 0.0 | 0.88 | ||
| >15–20 | 10 | 25.9 | 0.21 | ||
| >20–25 | 4 | 0.0 | 0.61 | ||
| 5–25 | 12 | 0.0 | 0.84 | ||
| BMI | <10 | 9 | 75.0 | <0.001 | 0.86 (0.77–0.95) 0.79 (0.74–0.84) 0.80 (0.75–0.85) 0.84 (0.70–1.01) 0.81 (0.77–0.85) |
| >10–15 | 8 | 50.8 | 0.039 | ||
| >15–20 | 12 | 75.9 | <0.001 | ||
| >20–25 | 4 | 92.2 | <0.001 | ||
| 5–25 | 13 | 76.9 | <0.001 | ||
| Former smoker | >10–15 | 2 | 0.0 | 0.35 | 0.64 (0.40–1.04) 0.96 (0.77–1.20) 0.69 (0.43–1.08) 0.91 (0.75–1.11) |
| >15–20 | 9 | 40.3 | 0.12 | ||
| >20–25 | 4 | 46.7 | 0.13 | ||
| 10–25 | 11 | 35.2 | 0.11 | ||
| Current smoker | >10–15 | 2 | 62.8 | 0.10 | 0.89 (0.26–3.07) 0.74 (0.49–1.13) 0.73 (0.47–1.15) 0.87 (0.68–1.13) |
| >15–20 | 11 | 41.8 | 0.07 | ||
| >20–25 | 4 | 0.0 | 0.56 | ||
| 10–25 | 11 | 19.2 | 0.24 | ||
#: Model 2 was adjusted for asthma family history, maternal smoking during pregnancy, preterm birth, body mass index (BMI) and smoking status, as well as for current asthma.
Preterm birth was associated with a higher likelihood of having the obstructive phenotype in the <10, >10–15, >15–20 and the overall 5–25 years age groups (aOR=1.82, 95% CI 1.08–3.06, aOR=2.73, 95% CI 1.67–4.46; aOR=1.61, 95% CI 1.13–2.29 and aOR=1.84, 95% CI 1.27–2.66, table 2), and effect estimates remained similar when current asthma was included in the model (table 3). Maternal smoking during pregnancy was also associated with a higher risk of the obstructive phenotype in several age groups, including the overall 5–25 years group (aOR=1.16, 95% CI 1.01–1.35), but the effect estimates somewhat attenuated when current asthma was adjusted for in the model (table 3). Asthma family history was associated with the obstructive phenotype in Model 1, but the effect estimates attenuated with additional adjustment for current asthma in Model 2 (aOR decreased from 1.44 to 1.21). No association between these risk factors and the restrictive phenotype was observed. Using spirometry indices as continuous trait outcomes, preterm birth was negatively associated with FEV1, FVC and FEV1/FVC ratio z-scores (supplementary Table E2). Maternal smoking during pregnancy was negatively associated with FEV1 and FEV1/FVC ratio. Asthma family history was negatively associated with FEV1 and FEV1/FVC ratio z-scores, but not with FVC z-scores.
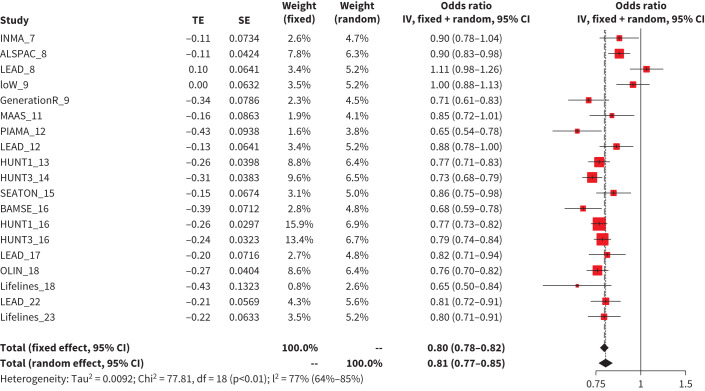
BMI was positively associated with the obstructive phenotype in all age groups in both models (from aOR=1.03, 95% CI 1.01–1.05 to aOR=1.06, 95% CI 1.04–1.09 per kg·m−2 increase, tables 2 and 3). In contrast, BMI was negatively associated with the restrictive phenotype in all age groups in both models (from aOR=0.79, 95% CI 0.74–0.84 to aOR=0.88, 95% CI 0.80–0.96 per kg·m−2 increase, tables 2 and 3 and figure 4) except in the >20–25 age bin (supplementary Figure E6). In addition, a higher BMI was associated with higher FEV1 and FVC but lower FEV1/FVC ratio z-scores (supplementary Table E2).
FIGURE 4.
Meta-analysis results of association between body mass index and restrictive phenotype in Model 2 in 5–25 years age group. TE: the estimated treatment effect; IV: inverse variance.
Current smoking was positively associated with the obstructive phenotype in the >15–20, >20–25 and >10–25 years age groups in Model 1, but the association somewhat attenuated when current asthma was adjusted for in the model (table 3). No clear associations between former smoking and an obstructive phenotype were observed in the current study because former smoking was both positively and negatively associated with obstructive phenotype. No association between participants’ smoking status and the restrictive phenotype was observed. In addition, both former and current smoking were associated with higher FVC but lower FEV1/FVC ratio z-scores (supplementary Table E2).
Discussion
The main observations in the current study using data from 14 population-based cohorts are that: 1) the obstructive and restrictive phenotypes are present at any age from childhood to early adulthood without an apparent age trend; and 2) a diagnosis of asthma, family history of asthma, maternal smoking during pregnancy, preterm birth, a higher BMI and current smoking were risk factors for the obstructive phenotype, while a lower BMI was the only factor associated with a restrictive phenotype in this age range.
Previous studies
Previous studies have reported that the prevalence of an obstructive spirometric phenotype in young adults is between 5 and 7% [21–23]. Our current results extend these previous findings by demonstrating that an obstructive phenotype widely exists in the general population from childhood to early adulthood. The prevalence of the restrictive phenotype during early childhood to young adulthood ranged from 1.8 to 7.7% in our study. These figures are lower than in population-based studies of adults 40 years or older, where prevalence ranges from around 7 to 20% [5–10]. Most participants with obstructive and restrictive phenotypes in our study were classified as having mild impairments, and interestingly, no difference in respiratory symptoms between participants with the restrictive phenotype and normal lung function was observed. As such, they may be at the early stage of the impairment and indicate a potential window of opportunity for early interventions to conserve or improve their lung function [24].
Interpretation of key findings
Several early-life potential risk factors, including asthma family history, maternal smoking during pregnancy and preterm birth, were associated with the obstructive phenotype. Preterm birth is associated with several respiratory sequelae during early childhood, such as bronchopulmonary dysplasia and higher risk of lower respiratory tract/respiratory syncytial virus infections [25]. Further, preterm birth has been associated with substantial impairments in airflow later during childhood and adolescence [26, 27]. In the current study, preterm birth was related to a higher risk of having an obstructive phenotype and a lower FEV1 z-score up to early adulthood. Despite recent substantial advances in neonatal care, with more babies surviving after preterm birth (including extreme prematurity, defined as <28 weeks’ gestation), the underlying pathophysiological mechanisms related to future respiratory health in these patients need further investigation [28].
Maternal smoking during pregnancy is a well-known in utero exposure that is negatively associated with fetal lung development and respiratory function in new-born infants [29] and with a higher risk of recurrent wheezing throughout childhood [30, 31]. Our results support these findings by demonstrating that maternal smoking during pregnancy is associated with a higher risk of the obstructive phenotype and impaired lung function development assessed as FEV1 and FEV1/FVC ratio z-scores in offspring.
Further, asthma and smoking are other well-known factors associated with airway obstruction [32], and children with persistent asthma are at higher risk for fixed airflow obstruction and possibly COPD in early adulthood [33, 34]. As expected, current asthma was strongly associated with the obstructive phenotype (>2-fold) and lung function impairment (lower FEV1 and FEV1/FVC z-scores) in our study. Current smoking of the participants was also associated with a higher likelihood of the obstructive phenotype (almost 20%). However, the association between current smoking and the obstructive phenotype was attenuated somewhat when a current diagnosis of asthma was taken into consideration, and the same trend was also observed for asthma family history and maternal smoking during pregnancy, suggesting that involved mechanisms partly overlap with asthma pathophysiology. It should be noted that we did not consider asthma as a confounder in the regression model (Model 2), since asthma and airway obstruction may represent the same disease entity, but rather to explore shared risk factors between asthma and our spirometry outcomes. However, we acknowledge that not all individuals classified as having asthma necessarily have clinical asthma, as the criteria for asthma in young children are typically based on symptoms only and not any objective tests. Future studies may explore more specific characteristics of asthma, such as airway hyperresponsiveness or airway inflammation, in relation to lower lung function development.
We did not identify any association between explored risk factors and the restrictive phenotype, except for BMI, where we found a lower BMI to be associated with the restrictive phenotype, indicating different underlying metabolic pathobiology between the obstructive and restrictive phenotypes. Although restrictive spirometry outcomes and lung disease are receiving increased attention in respiratory research lately, most studies to date were designed to explore health consequences of restrictive disease [9, 10, 12], while origins and risk factors remain poorly studied. In adults, early-life circumstances, such as low birthweight and intrauterine growth restriction [35, 36], pneumonia before school age [37], smoking [38], abdominal adiposity [38] and dust exposure [39] have been associated with lower FVC levels. In our study, smoking status was not associated with the restrictive phenotype, possibly because of the low cigarette load among adolescents and young adults. However, we observed a two-way relationship between BMI and lung function in our study. On the one hand, a lower BMI was associated with increased likelihood of restrictive phenotype and BMI correlated negatively with FVC z-scores from childhood to adolescence. On the other hand, a higher BMI was associated with increased likelihood of obstructive phenotype and correlated negatively with FEV1/FVC ratio z-scores from childhood to young adulthood. In children, faster weight growth is associated with higher FVC and FEV1 values [40]. BMI gain during early childhood has, however, greater influence on lung volume than airway growth, which may lead to airway dysanapsis [41, 42]. This is a phenomenon where the growth of the lung parenchyma is beyond the calibre of the airways leading to a higher FVC than FEV1, and a lower than expected FEV1/FVC ratio [43]. Of high clinical relevance is the observation that among obese children with asthma, dysanapsis has been associated with severe disease exacerbations [41].
Lower BMI during early childhood has in other studies been associated with lower FVC [42] and restrictive spirometric phenotype [44]. Those results indicate that maintenance of normal BMI during childhood to early adulthood may lead to improved respiratory health. In addition, the influence of BMI on lung function could differ depending on the proportion of different body components (i.e., fat mass and lean mass) [45, 46]. Owing to lack of body composition data in our study, we cannot explore these mechanisms further.
Strengths and limitations
Using data from 14 population-based cohorts in Europe, we provide robust estimates on the prevalence of obstructive and restrictive phenotypes from childhood to young adulthood. However, some limitations of the current study should be noted. Firstly, our current study is exploratory. Although three well-known early-life risk factors and lifestyle factors were taken into account, residual confounding, by e.g., diet or physical activity, and unexplored risk factors may still be an issue. Besides, the definition of restrictive phenotype in the current study was based on spirometry, which is commonly used in population-based studies [9, 10] but was not confirmed by residual volume and/or total lung capacity data. While the obstructive disease has received much attention in recent years, less is known about factors associated with restrictive outcomes. Future studies may explore the association between a restrictive phenotype and other early-life factors, such as additional perinatal factors (including extreme prematurity), growth trajectories, air pollution exposure, respiratory insults and diet [47, 48]. In addition, we did not have the possibility to explore potential influence of allergic comorbidities such as atopy, atopic dermatitis or allergic rhinitis, as earlier studies have indicated [49, 50]. Secondly, our study included almost 50 000 participants from 14 population-based cohorts from Europe, which provides high study power and external validity of the results, but also introduces some heterogeneity according to the fit of the GLI equation. In order to make the results comparable between cohorts, we centred the z-scores in each cohort according to the mean values of non-asthmatic, asymptomatic lifelong nonsmokers [14]. Thirdly, not all cohorts contributed data on all the risk factors, but we included all available variables in the regression analysis. In addition, definitions in some risk factors slightly differed between cohorts as appears in the online supplementary material.
Conclusions
Both obstructive and restrictive phenotypes do indeed occur during childhood and early adulthood but without a clear age trend. Participants with the obstructive phenotype more often reported asthma and wheezing symptoms. In addition, several well-known risk factors for airway disease in adults were associated with the obstructive phenotype across ages, including asthma family history, preterm birth, smoking and higher BMI, while the only identified factor related to the restrictive phenotype was lower BMI, pointing to other reasons for this phenotype in children compared to adults. Further studies on the mechanisms of these functional abnormalities are warranted.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00457-2021.SUPPLEMENT (1.7MB, pdf)
Acknowledgements
Cohort-specific acknowledgements are presented in the supplementary material. We also acknowledge collaboration with the EXPANSE consortium (funded by the EU H2020 programme, grant number 874627). We thank Elise Heuvelin, European Respiratory Society, Lausanne, Switzerland, for her assistance on the current project.
Footnotes
Provenance: Submitted article, peer reviewed.
This article has supplementary material available from openres.ersjournals.com
Author contributions: E. Melén, A.H. Maitland van der Zee and J. Hallberg conceived and designed the study with input from the CADSET management group (A. Agusti, R. Faner, G.C. Donaldson, J.A. Wedzicha and E. Heuvelin). R. Granell (ALSPAC), G. Wang (BAMSE), A. Mian (Generation R), A. Langhammer (HUNT), M. Casas Sanahuja (INMA together with G. Wang), D. Charalampopoulos (IoW, MAAS and SEATON), R. Breye-Kohansal (LEAD), N. Olvera (Lifelines), H.M. Boezen (OLIN), J.M. Vonk (PIAMA) and L.M. Laustsen (SUS) conducted the cohort-specific analyses. G. Wang meta-analysed all results. G. Wang, J. Hallberg, A.H. Maitland van der Zee and E. Melén wrote the first draft of the manuscript. All authors read and critically revised subsequent drafts, and approved the final version.
Support statement: This CADSET study was supported by the Clinical Research Collaborations programme funded by the European Respiratory Society (ERS). The CADSET ERS Clinical Research Collaboration has been supported by financial and other contributions from the following consortium partners: the ERS, AstraZeneca UK Ltd, Chiesi Farmaceutici, GlaxoSmithKline LLC, Menarini and Sanofi-Genzyme. Cohort-specific funding is presented in the supplementary material. Funding information for this article has been deposited with the Crossref Funder Registry.
Conflict of interest: G. Wang has nothing to disclose.
Conflict of interest: J. Hallberg reports receiving payment or honoraria for lectures, presentations, speaker bureaus, manuscript writing or educational events from AstraZeneca AB, outside the submitted work.
Conflict of interest: D. Charalampopoulos has nothing to disclose.
Conflict of interest: M. Casas Sanahuja has nothing to disclose.
Conflict of interest: R. Breyer-Kohansal has nothing to disclose.
Conflict of interest: A. Langhammer has nothing to disclose.
Conflict of interest: R. Granell has nothing to disclose.
Conflict of interest: J.M. Vonk has nothing to disclose.
Conflict of interest: A. Mian reports that this study was funded by the Erasmus Medical Centre, Rotterdam; the Erasmus University Rotterdam; and the Netherlands Organization for Health Research and Development. The project received funding from the European Union's Horizon 2020 research and innovation programme (LIFECYCLE, grant agreement number 733206, 2016; EUCAN-Connect grant agreement number 824989; and ATHLETE, grant agreement number 874583). The researchers are independent from the funders. The study sponsors had no role in the study design, data analysis, interpretation of data, or writing of this report. Consulting fees received for an opponent PhD defence committee at Copenhagen University, outside the submitted work.
Conflict of interest: N. Olvera reports funding for salary payment for the current manuscript from Centro de Investigación Biomédica en Red (Instituto de Salud Carlos III).
Conflict of interest: L.M. Laustsen has nothing to disclose.
Conflict of interest: E. Rönmark has nothing to disclose.
Conflict of interest: A. Abellan has nothing to disclose.
Conflict of interest: A. Agusti has nothing to disclose.
Conflict of interest: S.H. Arshad has nothing to disclose.
Conflict of interest: A. Bergström has nothing to disclose.
Conflict of interest: H.M. Boezen has nothing to disclose.
Conflict of interest: M-K. Breyer has nothing to disclose.
Conflict of interest: O. Burghuber has nothing to disclose.
Conflict of interest: A.C. Bolund has nothing to disclose.
Conflict of interest: A. Custovic reports grants or contracts from the Medical Research Council and Wellcome Trust, outside the submitted work; personal consulting fees from Philips and Stallergenes Greer, outside the submitted work; speaker's fees from Thermo Fisher Scientific, Sanofi, Stallergenes Greer, AstraZeneca and GSK, outside the submitted work; and is on the Board of Directors of the WAO and BSACI council, outside the submitted work.
Conflict of interest: G. Devereux has nothing to disclose.
Conflict of interest: G.C. Donaldson reports payment or honoraria for serving as a statistical editor for the American Journal of Respiratory and Critical Care Medicine from the American Thoracic Society), for reviewing grants for FWO and EOS (Belgium); and for participation on data safety monitoring or advisory boards for Novartis and AstraZeneca, outside the submitted work.
Conflict of interest: L. Duijts reports this study was funded by The Erasmus Medical Centre, Rotterdam; the Erasmus University Rotterdam; and the Netherlands Organization for Health Research and Development. The project received funding from the European Union's Horizon 2020 research and innovation programme (LIFECYCLE, grant agreement number 733206, 2016; EUCAN-Connect grant agreement number 824989; and ATHLETE, grant agreement number 874583. The researchers are independent from the funders. The study sponsors had no role in the study design, data analysis, interpretation of data, or writing of this report. She also reports consulting fees for an opponent PhD defence committee at Copenhagen University, outside the submitted work.
Conflict of interest: A. Esplugues has nothing to disclose.
Conflict of interest: R. Faner reports that during the planning of this work, she was supported by the Spanish National Health System Research Contrat Miguel Sevet (CP16/00039); grants from Menarini, GSK and AZ, outside this work; and consulting fees as well as payment or honoraria for lectures, presentations, speaker bureaus, manuscript writing or educational events from GSK and Chiesi, outside the submitted work.
Conflict of interest: F. Ballester has nothing to disclose.
Conflict of interest: J. Garcia-Aymerich has nothing to disclose.
Conflict of interest: U. Gehring has nothing to disclose.
Conflict of interest: S. Haider has nothing to disclose.
Conflict of interest: S. Hartl reports grants from GSK, Böhringer, Menarini, Chiesi, AstraZeneca, MSD, Novartis, Air Liquide, Vivisol, Pfizer and TEVA; and personal fees from GSK, Böhringer, Menarini, Chiesi, AstraZeneca, MSD, Novartis, Roche, Abbvie, TEVA and Takeda, outside the submitted work.
Conflict of interest: H. Backman has nothing to disclose.
Conflict of interest: J.W. Holloway has nothing to disclose.
Conflict of interest: G.H. Koppelman reports support for the current manuscript from the Lung Foundation of the Netherlands, payment made to his institution; grants or contracts paid to institution from H2020, Vertex, the Ubbo Emmius Foundation, TEVA the Netherlands and GSK, outside the submitted work; consulting fees paid to his institution from PURE IMS and GSK, outside the submitted work; and unpaid membership of the board of the Stichting Astma Bestrijding, outside the submitted work.
Conflict of interest: A. Lertxundi has nothing to disclose.
Conflict of interest: T.L. Holmen has nothing to disclose.
Conflict of interest: L. Lowe has nothing to disclose.
Conflict of interest: S.M. Mensink-Bout reports that this study was funded by The Erasmus Medical Centre, Rotterdam; the Erasmus University Rotterdam; and the Netherlands Organization for Health Research and Development. The project received funding from the European Union's Horizon 2020 research and innovation programme (LIFECYCLE, grant agreement number 733206, 2016; EUCAN-Connect grant agreement number 824989; ATHLETE, grant agreement number 874583). The researchers are independent from the funders. The study sponsors had no role in the study design, data analysis, interpretation of data, or writing of this report. She also reports consulting fees received for an opponent PhD defence committee at Copenhagen University, outside the submitted work.
Conflict of interest: C.S. Murray reports grants or contracts from Asthma UK, MRC, the Moulton Charitable Foundation and the NIHR, outside the submitted work; payment or honoraria for lectures, presentations, speaker bureaus, manuscript writing or educational events from GlaxoSmithKline, Novartis, AstraZeneca and ThermoFisher, outside the submitted work; and participation on a data safety monitoring or advisory board for Boehringer Ingelheim, outside the submitted work.
Conflict of interest: G. Roberts has nothing to disclose.
Conflict of interest: L. Hedman has nothing to disclose.
Conflict of interest: V. Schlünssen has nothing to disclose.
Conflict of interest: T. Sigsgaard has nothing to disclose.
Conflict of interest: A. Simpson reports a grant from UK Medical Research Council, outside the submitted work.
Conflict of interest: J. Sunyer has nothing to disclose.
Conflict of interest: M. Torrent has nothing to disclose.
Conflict of interest: S. Turner has nothing to disclose.
Conflict of interest: M. van den Berge reports grants from GlaxoSmithKline, AstraZeneca, Sanofi and Novartis, outside the submitted work.
Conflict of interest: R.C.H. Vermeulen has nothing to disclose.
Conflict of interest: S.A.A. Vikjord reports payment or honoraria for lectures, presentations, speaker bureaus, manuscript writing or educational events from AstraZeneca and Boehringer Ingelheim, outside the submitted work.
Conflict of interest: J.A Wedzicha reports grants or contracts from GSK, AstraZeneca, Boehringer, Chiesi, Novartis and Greentech, outside the submitted work.
Conflict of interest: A.H. Maitland-van der Zee reports grants or contracts from Vertex, Boehringer Ingelheim and Health Holland, outside the submitted work; consulting fees paid to her institution received from AstraZeneca and Boehringer Ingelheim, outside the submitted work; payment or honoraria for lectures, presentations, speaker bureaus, manuscript writing or educational events from GSK, outside the submitted work; and leadership or fiduciary roles in FIGON and EASYM, outside the submitted work.
Conflict of interest: E. Melén has nothing to disclose.
References
- 1.Vasquez MM, Zhou M, Hu C, et al. Low lung function in young adult life is associated with early mortality. Am J Respir Crit Care Med 2017; 195: 1399–1401. doi: 10.1164/rccm.201608-1561LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agustí A, Noell G, Brugada J, et al. Lung function in early adulthood and health in later life: a transgenerational cohort analysis. Lancet Respir Med 2017; 5: 935–945. doi: 10.1016/S2213-2600(1730434-4 [DOI] [PubMed] [Google Scholar]
- 3.Vaz Fragoso CA, McAvay G, Van Ness PH, et al. Phenotype of spirometric impairment in an aging population. Am J Respir Crit Care Med 2016; 193: 727–735. doi: 10.1164/rccm.201508-1603OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adeloye D, Chua S, Lee C, et al. Global and regional estimates of COPD prevalence: systematic review and meta-analysis. J Glob Health 2015; 5: 020415. doi: 10.7189/jogh.05.020415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jankowich M, Elston B, Liu Q, et al. Restrictive spirometry pattern, cardiac structure and function, and incident heart failure in African Americans. The Jackson Heart Study. Ann Am Thorac Soc 2018; 15: 1186–1196. doi: 10.1513/AnnalsATS.201803-184OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Honda Y, Watanabe T, Shibata Y, et al. Impact of restrictive lung disorder on cardiovascular mortality in a general population: The Yamagata (Takahata) study. Int J Cardiol 2017; 241: 395–400. doi: 10.1016/j.ijcard.2017.04.049 [DOI] [PubMed] [Google Scholar]
- 7.Mannino DM, Ford ES, Redd SC. Obstructive and restrictive lung disease and functional limitation: data from the Third National Health and Nutrition Examination. J Intern Med 2003; 254: 540–547. doi: 10.1111/j.1365-2796.2003.01211.x [DOI] [PubMed] [Google Scholar]
- 8.Mannino DM, Buist AS, Petty TL, et al. Lung function and mortality in the United States: data from the First National Health and Nutrition Examination Survey follow up study. Thorax 2003; 58: 388–393. doi: 10.1136/thorax.58.5.388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wijnant SRA, De Roos E, Kavousi M, et al. Trajectory and mortality of preserved ratio impaired spirometry: the Rotterdam Study. Eur Respir J 2020; 55: 1901217. doi: 10.1183/13993003.01217-2019 [DOI] [PubMed] [Google Scholar]
- 10.Guerra S, Sherrill DL, Venker C, et al. Morbidity and mortality associated with the restrictive spirometric pattern: a longitudinal study. Thorax 2010; 65: 499–504. doi: 10.1136/thx.2009.126052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agustí A, Hogg JC. Update on the pathogenesis of chronic obstructive pulmonary disease. N Engl J Med 2019; 381: 1248–1256. doi: 10.1056/NEJMra1900475 [DOI] [PubMed] [Google Scholar]
- 12.Wan ES, Fortis S, Regan EA, et al. Longitudinal phenotypes and mortality in preserved ratio impaired spirometry in the COPDGene Study. Am J Respir Crit Care Med 2018; 198: 1397–1405. doi: 10.1164/rccm.201804-0663OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agusti A, Faner R, Donaldson G, et al. Chronic Airway Diseases Early Stratification (CADSET): a new ERS Clinical Research Collaboration. Eur Respir J 2019; 53: 1900217. doi: 10.1183/13993003.00217-2019 [DOI] [PubMed] [Google Scholar]
- 14.Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J 2012; 40: 1324–1343. doi: 10.1183/09031936.00080312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005; 26: 319–338. doi: 10.1183/09031936.05.00034805 [DOI] [PubMed] [Google Scholar]
- 16.Quanjer PH, Pretto JJ, Brazzale DJ, et al. Grading the severity of airways obstruction: new wine in new bottles. Eur Respir J 2014; 43: 505–512. doi: 10.1183/09031936.00086313 [DOI] [PubMed] [Google Scholar]
- 17.Gavaghan DJ, Moore AR, McQuay HJ. An evaluation of homogeneity tests in meta-analyses in pain using simulations of individual patient data. Pain 2000; 85: 415–424. doi: 10.1016/S0304-3959(9900302-4 [DOI] [PubMed] [Google Scholar]
- 18.Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21: 1539–1558. doi: 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 20.Quanjer PH, Stocks J, Cole TJ, et al. Influence of secular trends and sample size on reference equations for lung function tests. Eur Respir J 2011; 37: 658–664. doi: 10.1183/09031936.00110010 [DOI] [PubMed] [Google Scholar]
- 21.Wang G, Kull I, Bergström A, et al. Early-life risk factors for reversible and irreversible airflow limitation in young adults: findings from the BAMSE birth cohort. Thorax 2021; 76: 503–507. doi: 10.1136/thoraxjnl-2020-215884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Çolak Y, Afzal S, Nordestgaard BG, et al. Prevalence, characteristics, and prognosis of early chronic obstructive pulmonary disease. The Copenhagen General Population Study. Am J Respir Crit Care Med 2020; 201: 671–680. doi: 10.1164/rccm.201908-1644OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Backman H, Eriksson B, Hedman L, et al. Restrictive spirometric pattern in the general adult population: methods of defining the condition and consequences on prevalence. Respir Med 2016; 120: 116–123. doi: 10.1016/j.rmed.2016.10.005 [DOI] [PubMed] [Google Scholar]
- 24.Agusti A, Breyer-Kohansal R, Faner R. Transitioning from infancy to adulthood: a black box full of opportunities. Eur Respir J 2021; 57: 2003997. doi: 10.1183/13993003.03997-2020 [DOI] [PubMed] [Google Scholar]
- 25.Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet 2008; 371: 261–269. doi: 10.1016/S0140-6736(0860136-1 [DOI] [PubMed] [Google Scholar]
- 26.den Dekker HT, Sonnenschein-van der Voort AMM, de Jongste JC, et al. Early growth characteristics and the risk of reduced lung function and asthma: a meta-analysis of 25,000 children. J Allergy Clin Immunol 2016; 137: 1026–1035. doi: 10.1016/j.jaci.2015.08.050 [DOI] [PubMed] [Google Scholar]
- 27.Um-Bergström P, Hallberg J, Thunqvist P, et al. Lung function development after preterm birth in relation to severity of Bronchopulmonary dysplasia. BMC Pulm Med 2017; 17: 97. doi: 10.1186/s12890-017-0441-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simpson SJ, Hallberg J. The PELICAN (Prematurity's Effect on the Lungs In Children and Adults Network) ERS Clinical Research Collaboration: understanding the impact of preterm birth on lung health throughout life. Eur Respir J 2021; 57: 2004387. doi: 10.1183/13993003.04387-2020 [DOI] [PubMed] [Google Scholar]
- 29.Stick SM, Burton PR, Gurrin L, et al. Effects of maternal smoking during pregnancy and a family history of asthma on respiratory function in newborn infants. Lancet 1996; 348: 1060–1064. doi: 10.1016/S0140-6736(9604446-7) [DOI] [PubMed] [Google Scholar]
- 30.Gilliland FD, Li YF, Peters JM. Effects of maternal smoking during pregnancy and environmental tobacco smoke on asthma and wheezing in children. Am J Respir Crit Care Med 2001; 163: 429–436. doi: 10.1164/ajrccm.163.2.2006009 [DOI] [PubMed] [Google Scholar]
- 31.Neuman Å, Hohmann C, Orsini N, et al. Maternal smoking in pregnancy and asthma in preschool children: a pooled analysis of eight birth cohorts. Am J Respir Crit Care Med 2012; 186: 1037–1043. doi: 10.1164/rccm.201203-0501OC [DOI] [PubMed] [Google Scholar]
- 32.Ulrik CS. Outcome of asthma: longitudinal changes in lung function. Eur Respir J 1999; 13: 904–918. doi: 10.1034/j.1399-3003.1999.13d35.x [DOI] [PubMed] [Google Scholar]
- 33.McGeachie MJ, Yates KP, Zhou X, et al. Patterns of growth and decline in lung function in persistent childhood asthma. N Engl J Med 2016; 374: 1842–1852. doi: 10.1056/NEJMoa1513737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Melén E, Guerra S, Hallberg J, et al. Linking COPD epidemiology with pediatric asthma care: implications for the patient and the physician. Pediatr Allergy Immunol 2019; 30: 589–597. doi: 10.1111/pai.13054 [DOI] [PubMed] [Google Scholar]
- 35.Hancox RJ, Poulton R, Greene JM, et al. Associations between birth weight, early childhood weight gain and adult lung function. Thorax 2009; 64: 228–232. doi: 10.1136/thx.2008.103978 [DOI] [PubMed] [Google Scholar]
- 36.Pei L, Chen G, Mi J, et al. Low birth weight and lung function in adulthood: retrospective cohort study in China, 1948-1996. Pediatrics 2010; 125: e899–e905. doi: 10.1542/peds.2008-3086 [DOI] [PubMed] [Google Scholar]
- 37.Johnston ID, Strachan DP, Anderson HR. Effect of pneumonia and whooping cough in childhood on adult lung function. N Engl J Med 1998; 338: 581–587. doi: 10.1056/NEJM199802263380904 [DOI] [PubMed] [Google Scholar]
- 38.Vatrella A, Calabrese C, Mattiello A, et al. Abdominal adiposity is an early marker of pulmonary function impairment: findings from a Mediterranean Italian female cohort. Nutr Metab Cardiovasc Dis 2016; 26: 643–648. doi: 10.1016/j.numecd.2015.12.013 [DOI] [PubMed] [Google Scholar]
- 39.Berger KI, Reibman J, Oppenheimer BW, et al. Lessons from the World Trade Center disaster: airway disease presenting as restrictive dysfunction. Chest 2013; 144: 249–257. doi: 10.1378/chest.12-1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sonnenschein-van der Voort AMM, Howe LD, Granell R, et al. Influence of childhood growth on asthma and lung function in adolescence. J Allergy Clin Immunol 2015; 135: 1435–1443.e7. doi: 10.1016/j.jaci.2014.10.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Forno E, Weiner DJ, Mullen J, et al. Obesity and airway dysanapsis in children with and without asthma. Am J Respir Crit Care Med 2017; 195: 314–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peralta GP, Abellan A, Montazeri P, et al. Early childhood growth is associated with lung function at 7 years: a prospective population-based study. Eur Respir J 2020; 56: 2000157. doi: 10.1183/13993003.00157-2020 [DOI] [PubMed] [Google Scholar]
- 43.Mead J. Dysanapsis in normal lungs assessed by the relationship between maximal flow, static recoil, and vital capacity. Am Rev Respir Dis 1980; 121: 339–342. [DOI] [PubMed] [Google Scholar]
- 44.Ali GB, Bui DS, Lodge CJ, et al. Infant body mass index trajectories, and asthma & lung function. J Allergy Clin Immunol 2021; 148: 763–770. doi: 10.1016/j.jaci.2021.02.020 [DOI] [PubMed] [Google Scholar]
- 45.Mensink-Bout SM, Santos S, van Meel ER, et al. General and organ fat assessed by magnetic resonance imaging and respiratory outcomes in childhood. Am J Respir Crit Care Med 2020; 201: 348–355. doi: 10.1164/rccm.201905-0942OC [DOI] [PubMed] [Google Scholar]
- 46.Peralta GP, Fuertes E, Granell R, et al. Childhood body composition trajectories and adolescent lung function. Findings from the ALSPAC study. Am J Respir Crit Care Med 2019; 200: 75–83. doi: 10.1164/rccm.201806-1168OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Breyer-Kohansal R, Faner R, Breyer M-K, et al. Factors associated with low lung function in different age bins in the general population. Am J Respir Crit Care Med 2020; 202: 292–296. doi: 10.1164/rccm.202001-0172LE [DOI] [PubMed] [Google Scholar]
- 48.Agier L, Basagaña X, Maitre L, et al. Early-life exposome and lung function in children in Europe: an analysis of data from the longitudinal, population-based HELIX cohort. Lancet Planet Health 2019; 3: e81–e92. doi: 10.1016/S2542-5196(1930010-5) [DOI] [PubMed] [Google Scholar]
- 49.Hallberg J, Ballardini N, Almqvist C, et al. Impact of IgE sensitization and rhinitis on inflammatory biomarkers and lung function in adolescents with and without asthma. Pediatr Allergy Immunol 2019; 30: 74–80. doi: 10.1111/pai.12994 [DOI] [PubMed] [Google Scholar]
- 50.Lødrup Carlsen KC, Mowinckel P, Hovland V, et al. Lung function trajectories from birth through puberty reflect asthma phenotypes with allergic comorbidity. J Allergy Clin Immunol 2014; 134: 917–923. doi: 10.1016/j.jaci.2014.05.020 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00457-2021.SUPPLEMENT (1.7MB, pdf)