Abstract
Echinococcus spp. tapeworms can cause serious diseases in mammals, including humans. Within the E. granulosus species complex, metacestodes produce unilocular cysts that are responsible for cystic echinococcosis in animal intermediate hosts. Canids are definitive hosts, harbouring adult cestodes in their intestines. Adult E. canadensis were recovered from the small intestine of 1 of 262 coyotes (Canis latrans) from Nova Scotia, Canada. Subsequently, we found unilocular cysts in lungs and livers of 4 of 8 sympatric moose (Alces alces) from Cape Breton Island. DNA was extracted from three cysts using the Qiagen DNeasy Blood and Tissue kit and assayed by polymerase chain reaction (PCR) with primers (cest4 and cest5) for a 117-bp region of the small subunit of ribosomal RNA of E. granulosus sensu lato, and further validated as E. canadensis G8 using primers targeting nicotinamide adenosine dinucleotide dehydrogenase subunit 1 (ND1) and cytochrome c oxidase subunit 1 (CO1) mitochondrial genes. These are the first records of E. canadensis in any of the three Maritime provinces, which include Nova Scotia, New Brunswick, and Prince Edward Island. The parasite was thought to be absent in this region due to extirpation of wolves (Canis spp.) in the 1800s. These findings suggest that further wildlife surveillance and risk assessment is warranted.
Keywords: Cyclophyllidean taeniid tapeworms, Echinococcosis, Metacestodes, Unilocular cysts
Highlights
-
•
Adult Echinococcus canadensis were recovered from small intestines of a coyote from Nova Scotia.
-
•
We also found cysts in lungs and livers of sympatric moose from Cape Breton Island.
-
•
DNA in cysts was confirmed as belonging to E. canadensis.
-
•
These are the 1st records of E. canadensis in the Maritimes, where it was presumed absent due to extirpation of wolves.
1. Introduction
Animals can be reservoir hosts for parasites that cause zoonotic infections. Echinococcus spp., cyclophyllidean taeniid tapeworms, are one of the most important cestodes causing disease in mammals, including humans (Eckert and Deplazes, 2004; Craig et al., 2017). As adults, these cestodes reside in the small intestine of their definitive hosts, commonly canids such as wolves (Canis spp.), coyotes (C. latrans), and domestic dogs (C. familiaris) (Torgerson and Heath, 2003; Eckert and Deplazes, 2004; Joly and Messier, 2004; Somily et al., 2005; Lichtenwalner et al., 2014; WHO, 2021). Eggs and/or gravid proglottids are shed in feces of definitive hosts and deposited onto soil and vegetation. Depending on the Echinococcus species or strain, various wild ungulates, livestock, and rodents are intermediate hosts, and become infected via the faecal-oral route. Within intermediate hosts, eggs hatch to produce oncospheres that migrate from the intestinal lumen to the lungs, liver, and/or other organs where they develop into metacestode larval stages. Unilocular cysts formed by members of the E. granulosus species complex are fluid-filled and characterized by a thick wall and contain thousands of protoscolices. Canid definitive hosts become infected by ingesting cysts in tissues of intermediate hosts. Humans can also become infected by accidently ingesting eggs from soil, vegetation, or fur of an infected canid; however, humans are seldom involved in transmission and are usually dead-end hosts (Torgerson and Heath, 2003; Eckert and Deplazes, 2004; Joly and Messier, 2004; Somily et al., 2005; Lichtenwalner et al., 2014; WHO, 2021).
Echinococcosis is a chronic illness in humans caused by zoonotic species of Echinococcus (Torgerson and Heath, 2003; Eckert and Deplazes, 2004; Moro and Schantz, 2009; Schurer et al., 2014; Conraths and Deplazes, 2015; WHO, 2021). Of these species, members of the E. granulosus complex produce unilocular cysts responsible for cystic echinococcosis (CE), a less dangerous condition than alveolar echinococcosis (AE) associated with multilocular cysts produced by E. multilocularis (Moore et al., 1994; Conraths and Deplazes, 2015; Schurer et al., 2015). Adult cestodes within the E. granulosus complex are ∼ 7-mm long taeniid tapeworms (Eckert, 2000; Eckert and Deplazes, 2004). They occur on most continents within different host assemblages, but areas of high prevalence in humans are generally associated with livestock species/strains in Australia, China, Russia, South America, and northern and eastern Africa (Eckert, 2000; McManus et al., 2003). In Canada, livestock-associated species (E. granulosus sensu lato, genotypes 1, 3, 5, 6 and 7) are absent, but E. canadensis, or cervid-associated genotypes (G8 and G10) of E. granulosus sensu lato, are present wherever cervid/canid assemblages, primarily wolf and moose (Alces alces), are present (Deplazes et al., 2017). Cases of locally acquired human CE occur in Canada, primarily in the Northwest Territories and the Canadian Prairie provinces (Somily et al., 2005; Schurer et al., 2015). To date, E. canadensis has been found in all Canadian provinces west of the Maritimes and south of the high Arctic (Schurer et al., 2013). The absence of E. canadensis from these eastern provinces is thought to be due to historical extirpation of wolves, because moose and other cervids are abundant in some locations (Sweatman, 1952; Deplazes et al., 2017).
Coyotes are now widely distributed across North America, having expanded their historic range from central North America and Mexico (Peterson and Thurber, 1993; Parker, 1995). In the past 30–40 years, they have expanded their range from the Great Lakes region of southern Canada and northcentral United States into the northeastern US and eastern Canada (Thurber and Peterson, 1991). Coyotes were first recorded in Nova Scotia in 1977 (O'Brien, 1983; Moore and Millar, 1986), and now span the entire mainland and Cape Breton Island (Patterson and Messier, 2001). Several nematodes and platyhelminths have previously been observed in coyotes in Nova Scotia (Power et al., 2015; Priest et al., 2018; Priest, 2021). As interactions among humans, their domestic animals, and coyotes become more frequent, there is an increased risk of parasite transmission (Aguirre, 2009). As part of an on-going study on coyotes in Nova Scotia (details in Shutler et al., 2021), we recovered helminths from a subset of animals (Priest, 2021) to determine risks of parasite transmission to dogs and humans. On detection of Echinococcus spp. in a coyote, we subsequently examined hunter-harvested moose for unilocular cysts.
2. Methods
Organs (heart, lungs, and gastrointestinal tracts) were taken from 262 coyotes harvested by hunters and trappers between September 2016 and April 2020 after being stored at −20 °C until processing (map of all harvested coyotes in Shutler et al., 2021). Following Jeffery et al. (2004), and detailed elsewhere (Priest et al., 2018; Priest, 2021; Shutler et al., 2021), intestines were flushed with high-pressure water into buckets, and contents were sorted for helminths. Sieve contents were examined under a light microscope, and initial genus-level Echinococcus identification was determined based on body length (<1 cm), three body segments, and presence of an armed scolex. Helminths were initially identified on site and preserved in 70% ethanol. Sampling equipment was cleaned with hot water and soap in between each sample.
We also necropsied eight Mi'kmaq-harvested moose (Alces alces andersoni) from Cape Breton Highlands National Park. Moose hearts, lungs, and livers were visually inspected for cysts on the surface and internally through full-thickness slices created in parallel approximately every 2 cm. Additional internal organs were left where the animal was harvested and not available to inspect. Samples of cysts were preserved in 95% ethanol and shipped to the Zoonotic Parasite Research Unit, Western College of Veterinary Medicine, Saskatoon, Saskatchewan, for molecular analysis. DNA was extracted from cysts using the Qiagen DNeasy Blood and Tissue kit and assayed by polymerase chain reaction (PCR) with primers (cest4 and cest5) for a 117-bp region of the small subunit of ribosomal RNA of E. granulosus sensu lato (Trachsel et al., 2007). To enable differentiation of genotypes, additional PCR assays were done using primers to amplify a ∼470-bp region of nicotinamide adenosine dinucleotide dehydrogenase subunit 1 (ND1) and a ∼446-bp region of cytochrome c oxidase subunit 1 (CO1) mitochondrial genes (Bowles et al., 1992; Bowles and McManus, 1993). PCR products were resolved by electrophoresis on a 1.5% agarose gel, viewed under UV light, purified (QIAquick PCR Purification Kit, Qiagen Inc., Valencia, CA), and sequenced in both directions (Macrogen Inc., Seoul, Korea; National Research Council Biotechnology Institute Saskatoon, SK) using PCR primers. Sequences were compared to previously reported sequences by BLAST alignment in GenBank.
3. Results
Coyotes were sampled from across the province, 222 from the mainland and 40 from Cape Breton Island. One juvenile female coyote caught (via necksnare) inland from St. Joseph du Moine and South of Squirrel Mountain (46.527616, −61.028549), on Cape Breton Island in Nova Scotia was positive for adult Echinococcus spp., identified based on size (<1 cm long), presence of a scolex with an armed rostellum and four muscular suckers, segments with a single lateral genital pore, and gravid segments containing taeniid eggs (Fig. 1). The placement of the genital pore in the posterior half of the mature segment distinguished these cestodes as E. canadensis versus E. multilocularis, the only other species of Echinococcus present in wild canids in North America, in which the genital pore is present in the anterior half of the mature segment (Fig. 1). There were approximately 30 adult cestodes in the intestine of the positive coyote.
Fig. 1.
Echinococcus canadensis recovered from a gastrointestinal flush of a coyote at necropsy from Cape Breton, Nova Scotia. Note that the genital pore (circled) is located in the posterior half of the segment.
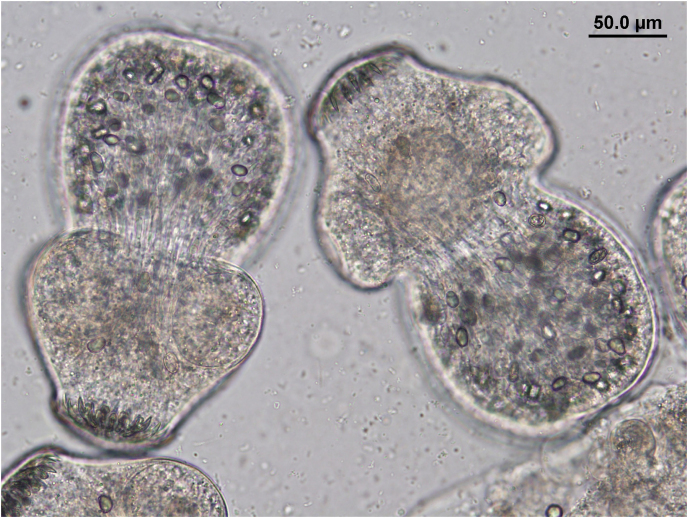
Following this discovery, we obtained organs from eight moose harvested within a 20-km2 area on North Mountain within Cape Breton Highlands National Park; the center of the block was approximately 46.800813, −60.683727. Unilocular cysts were present in lungs and livers of four individuals, ranging from 3 to 40 cysts per individual (Fig. 2). Cysts were 18–40 mm in diameter, had a thick outer cyst wall, and sand containing protoscolices was released upon dissection of cysts. The moose host and small size of the unilocular cysts were consistent with E. canadensis; moreover, livestock species of Echinococcus do not successfully infect cervids (Sweatman and Williams, 1963). Precise genotyping of three of the four infected moose samples revealed E. canadensis (G8; Laurimäe et al., 2018), the more common genotype in eastern Canada (Schurer et al., 2013). Sample sequences were most similar to complete genome sequences of Echinococcus from moose from the USA (Accession number AB235848). High quality, representative sequences for both loci were trimmed and submitted to Genbank (Accession numbers OK662608 and OK662609).
Fig. 2.
Protoscolices contained in cyst fluid released upon dissection of a unilocular cyst from the lungs of a moose from Cape Breton Island, Nova Scotia.
4. Discussion
These are the first recorded instances of E. canadensis, or indeed any species of Echinococcus, in free-ranging wildlife in a Maritime province. Introduction and establishment of parasites to new geographic regions depends on multiple factors including ease and occurrence of host movement between endemic and nonendemic areas, presence and sufficient density of susceptible hosts, and suitable climate for species with free-living stages. We hypothesize that natural range expansion and higher trophic roles for coyotes (in the absence of wolves) have newly enabled the life cycle of E. canadensis in Nova Scotia, raising the possibility that the parasite is present in New Brunswick, Prince Edward Island, and even the island of Newfoundland (McNeill et al., 1984; Lichtenwalner et al., 2014). Because of the lack of physical specimens from the Maritime provinces, it is not possible to determine if the gray wolf, C. lupus, or the eastern wolf, C. lycaon was historically native to this region (McAlpine et al., 2015; Whitaker and Beazley, 2017). It is possible that E. canadensis may have been introduced to Cape Breton Island as early as the 1940s, but in the absence of wild canids at the time, the parasite was unable to maintain transmission. Eastern moose (Alces alces americana) were native to Cape Breton, but were extirpated in the 1930s likely due to habitat destruction and excessive hunting (Parks Canada, 2018). Parks Canada introduced 18 western moose (Alces alces andersoni) from Elk Island National Park, Alberta, Canada to Cape Breton Highlands National Park in the 1940s, and the population stabilized over time (Parks Canada, 2018). Cysts similar to those we observed were apparently detected in moose lungs from Cape Breton Island in 2012 (Galvez, pers. comm., 2021), but there was no follow-up to identify the aetiological agent.
CE continues to be a public health concern in many countries (Eckert, 2000; Craig et al., 2017; WHO, 2021). The public at high risk of exposure (i.e., hunters and trappers, wildlife researchers, dog owners) should be made aware of its establishment in non-endemic areas, how to reduce its spread, and how to reduce the potential for becoming infected (Torgerson and Heath, 2003). It is important to note that people cannot be infected by consuming cysts in cervid carcasses, but instead become infected through accidental ingestion of eggs shed by coyotes or dogs that have consumed cervid carcasses (Eckert, 2000). Preventative measures include treating high risk domestic dogs (i.e., hunting dogs with access to cervid carcasses) with prophylactic cestocides (e.g., praziquantel) at minimum four times per year, disposal of organs of hunted cervids where wild and domestic canids cannot consume them, and practicing caution when consuming berries, unfiltered surface water, or produce that could be contaminated by eggs from wild or domestic canid feces (Lichtenwalner and Kantar, 2013; WHO 2021). The seriousness of this new potential health risk in a Maritime province should motivate increased wildlife, domestic dog, and human surveillance and risk assessment (Eckert, 2000; Craig et al., 2017; WHO, 2021).
Ethics statement
All tissues were sourced from animals harvested for non-research purposes, exempting animal care approval (Canadian Council on Animal Care, 2003).
Data accessibility
All data are contained within the body of the paper.
Author contributions
All authors provided editorial input. JMP recovered helminths from coyotes and wrote the first draft of the MS. DM recovered hydatid cysts from moose. DTS co-supervised JMP and provided funding. MB co-supervised JMP, initiated and oversaw coyote collection, and provided lab assistants. JWBP provided guidance on coyote helminth extraction. GC provided expertise on Echinococcus morphological identification. EJJ and TUK funded and conducted genetic analyses. DS co-supervised JMP and provided funding.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank the Trappers Association of Nova Scotia for promoting carcass collection, hunters and trappers for submitting carcasses, and the Department of Natural Resources and Renewables regional staff for voluntarily transporting samples. JMP thanks the Department of Natural Resources and Renewables - Wildlife Division for sharing laboratory facilities, expertise, and funding through the Acadia Student Skills Program. We thank research assistants in the Acadia Student Skills Program who helped with parasite collection, and students in DS's and DTS's labs for assisting with parasite recovery, feedback, and lab work. We thank Michelle Sniatynski in EJJ's lab for molecular confirmation. We thank I. V. Galvez, Department of Natural Resources and Renewables – Wildlife Technician 2021, for his historical observations. We thank NSERC, Canada for funding via an Undergraduate Research Award to JMP and Discovery Awards to EJJ, DS, and DTS. Finally, we thank two expert reviewers for their suggestions that improved the manuscript.
References
- Aguirre A.A. Wild canids as sentinels of ecological health: a conservation medicine perspective. Parasites Vectors. 2009;2:S7. doi: 10.1186/1756-3305-2-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles J., Blair D., McManus D. Genetic variants within the genus Echinococcus identified by mitochondrial DNA sequencing. Mol. Biochem. Parasitol. 1992;54:165–174. doi: 10.1016/0166-6851(92)90109-w. [DOI] [PubMed] [Google Scholar]
- Bowles J., McManus D. NADH dehydrogenase 1 gene sequences compared for species and strains of the genus Echinococcus. Int. J. Parasitol. 1993;23:969–972. doi: 10.1016/0020-7519(93)90065-7. [DOI] [PubMed] [Google Scholar]
- Canadian Council on Animal Care . Canadian Council on Animal Care; Ottawa, Ont: 2003. Guidelines on the Care and Use of Wildlife. [Google Scholar]
- Craig P.S., Hegglin D., Lightowlers M.W., Torgerson P., Wang Q. Echinococcosis: control and prevention. Adv. Parasitol. 2017;96:3–85. doi: 10.1016/bs.apar.2016.09.002. [DOI] [PubMed] [Google Scholar]
- Conraths F.J., Deplazes P. Echinococcus multilocularis: epidemiology, surveillance and state-of-the-art diagnosis from veterinary public health perspectives. Vet. Parasitol. 2015;213:149–161. doi: 10.1016/j.vetpar.2015.07.027. [DOI] [PubMed] [Google Scholar]
- Deplazes P., Rinaldi L., Alvarez Rojas C.A., Torgerson P.R., Harandi M.F., Romig T., Antolova D., Schurer J.M., Lahmar S., Cringoli G., Magambo J., Thompson R.C.A., Jenkins E.J. Chapter six – global distribution of alveolar and cystic echinococcosis. Adv. Parasitol. 2017;95:315–493. doi: 10.1016/bs.apar.2016.11.001. [DOI] [PubMed] [Google Scholar]
- Eckert J., editor. WHO/OIE Manual on Echinococcosis in Humans and Animals: a Zoonosis of Global Concern. World Organization for Animal Health; Paris: 2000. [Google Scholar]
- Eckert J., Deplazes P. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin. Microbiol. Rev. 2004;17:107–135. doi: 10.1128/CMR.17.1.107-135.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery R.A., Lankester M.W., McGrath M.J., Whitney H.G. Angiostrongylus vasorum and Crenosoma vulpis in red foxes (Vulpes vulpes) in Newfoundland, Canada. Can. J. Zool. 2004;82:66–74. [Google Scholar]
- Joly D.O., Messier F. The distribution of Echinococcus granulosus in moose: evidence for parasite-induced vulnerability to predation by wolves? Oecologia. 2004;140:586–590. doi: 10.1007/s00442-004-1633-0. [DOI] [PubMed] [Google Scholar]
- Laurimäe T., Kinkar L., Moks E., Romig T., Omer R.A., Casulli A., Umhang G., Bagrade G., Irshadullah M., Sharbatkhori M., Mirhendi H., Ponce-Gordo F., Soriano S.V., Varcasia A., Rostami-Nejad M., Andresiuk V., Saarma U. Molecular phylogeny based on six nuclear genes suggests that Echinococcus granulosus sensu lato genotypes G6/G7 and G8/G10 can be regarded as two distinct species. Parasitology. 2018;145:1929–1937. doi: 10.1017/S0031182018000719. [DOI] [PubMed] [Google Scholar]
- Lichtenwalner A., Adhikari N., Kantar L., Jenkins E., Schurer J. Echinococcus granulosus genotype G8 in Maine moose (Alces alces) Alces. 2014;50:27–33. [Google Scholar]
- Lichtenwalner A., Kantar L. University of Maine: Cooperative Extension Publications; 2013. Bulletin #1002, Echinococcus Granulosus Canadensis (EG) in Maine Moose: Suggestions for Dog Owners.https://extension.umaine.edu/publications/1002e/ Available from. [Google Scholar]
- McAlpine D.F., Soto D.X., Rutledge L.Y., Wheeldon T.J., White B.N., Goltz J.P., Kennedy J. Recent occurrences of wild-origin wolves (Canis spp.) in Canada south of the St. Lawrence River revealed by stable isotope and genetic analysis. Can. Field Nat. 2015;129:386–394. [Google Scholar]
- McManus D.P., Zhang W., Li J., Bartley P.B. Echinococcosis. Lancet. 2003;362:1295–1304. doi: 10.1016/S0140-6736(03)14573-4. [DOI] [PubMed] [Google Scholar]
- McNeill M., Rau M., Messier F. Helminths of wolves (Canis lupus L.) from southwestern Quebec. Can. J. Zool. 1984;62:1659–1660. [Google Scholar]
- Moore G.C., Millar J.S. Food habits and average weights of a fall-winter sample of eastern coyotes, Canis latrans. Can. Field Nat. 1986;100:105–106. [Google Scholar]
- Moore R.D., Urschel J.D., Fraser R.E., Nakai S.S., Geeraert A.J. Cystic hydatid lung disease in northwest Canada. Can. J. Surg. 1994;37:20–22. [PubMed] [Google Scholar]
- Moro P., Schantz P.M. Echinococcosis: a review. Int. J. Infect. Dis. 2009;13:125–133. doi: 10.1016/j.ijid.2008.03.037. [DOI] [PubMed] [Google Scholar]
- O'Brien M. Department of Natural Resources; Kentville, Nova Scotia: 1983. The Coyote - A New Mammal, New Challenges. [Google Scholar]
- Parker G.R. Nimbus Publishing; Halifax, Nova Scotia: 1995. Eastern Coyote: the Story of its Success. [Google Scholar]
- Parks Canada Cape Breton Highlands national Park – moose. 2018. https://www.pc.gc.ca/en/pn-np/ns/cbreton/decouvrir-discover/faune-animals/mammiferes-mammals/orignal-moose Available from.
- Patterson B.R., Messier F. Social organization and space use of coyotes in eastern Canada relative to prey distribution and abundance. J. Mammal. 2001;82:463–477. [Google Scholar]
- Peterson R.O., Thurber J.M. The size of eastern coyotes (Canis latrans): a rebuttal. J. Mammal. 1993;74:1075–1076. [Google Scholar]
- Power J., Weatherbee-Martin N., Boudreau M., O'Brien M., Conboy G., Smith T. Diversity and ecology of pulmonary metastrongyloidosis in coyotes (Canis latrans) of Nova Scotia, Canada. Comp. Parasitol. 2015;82:85–93. [Google Scholar]
- Priest J.M. MSc Thesis, Acadia University; Wolfville, Nova Scotia, Canada: 2021. Associations of, and Interactions Among, Helminths in Coyotes (Canis latrans) [Google Scholar]
- Priest J.M., Stewart D.T., Boudreau M., Power J., Shutler D. First report of Angiostrongylus vasorum in coyotes in mainland North America. Vet. Rec. 2018;183 doi: 10.1136/vr.105097. 747-747. [DOI] [PubMed] [Google Scholar]
- Schurer J., Shury T., Leighton F., Jenkins E. Surveillance for Echinococcus canadensis genotypes in Canadian ungulates. Int. J. Parasitol.: Parasites Wildl. 2013;2:97–101. doi: 10.1016/j.ijppaw.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurer J.M., Gesy K.M., Elkin B.T., Jenkins E.J. Echinococcus multilocularis and Echinococcus canadensis in wolves from western Canada. Parasitology. 2014;141:159–163. doi: 10.1017/S0031182013001716. [DOI] [PubMed] [Google Scholar]
- Schurer J.M., Rafferty E., Farag M., Zeng W., Jenkins E.J. Echinococcosis: an economic evaluation of a veterinary public health intervention in rural Canada. PLoS Neglected Trop. Dis. 2015;9 doi: 10.1371/journal.pntd.0003883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shutler D., Priest J.M., Stewart D.T., Boudreau M. Demographical and morphological differences among coyotes (Canis latrans) relative to sampling method. Can. J. Zool. 2021;99:197–204. [Google Scholar]
- Somily A., Robinson J.L., Miedzinski L.J., Bhargava R., Marrie T.J. Echinococcal disease in Alberta, Canada: more than a calcified opacity. BMC Infect. Dis. 2005;5:34. doi: 10.1186/1471-2334-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweatman G.K. Distribution and incidence of Echinococcus granulosus in man and other animals with special reference to Canada. Can. J. Public Health. 1952;43:480–486. [PubMed] [Google Scholar]
- Sweatman G.K., Williams R.J. Survival of Echinococcus granulosus and Taenia hydatigena eggs in two extreme climatic regions of New Zealand. Res. Vet. Sci. 1963;4:199–216. [Google Scholar]
- Thurber J.M., Peterson R.O. Changes in body size associated with range expansion in the coyote (Canis latrans) J. Mammal. 1991;72:750–755. [Google Scholar]
- Torgerson P.R., Heath D.D. Transmission dynamics and control options for Echinococcus granulosus. Parasitology. 2003;127:S143–S158. doi: 10.1017/s0031182003003810. [DOI] [PubMed] [Google Scholar]
- Trachsel D., Deplazes P., Mathis A. Identification of taeniid eggs in the faeces from carnivores based on multiplex PCR using targets in mitochondrial DNA. Parasitology. 2007;134:911–920. doi: 10.1017/S0031182007002235. [DOI] [PubMed] [Google Scholar]
- Whitaker A.N., Beazley K.F. Evidence for the historical occurrence of wolves (Canis spp.) in Nova Scotia, Canada. Can. Field Nat. 2017;131:32–36. [Google Scholar]
- WHO Echinococcosis. Fact sheet (updated may 2021) world health organization. 2021. https://www.who.int/news-room/fact-sheets/detail/echinococcosis Available at.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are contained within the body of the paper.