Abstract
Agar dilution and microdilution (both in air) and E test and disk diffusion (both in air and CO2) were used to test the activity of telithromycin against 110 erythromycin-susceptible and 106 erythromycin-resistant pneumococci. The MICs at which 50 and 90% of strains are inhibited (MIC50s and MIC90s, respectively) for erythromycin-susceptible strains varied between 0.008 and 0.016 μg/ml and 0.016 and 0.03 μg/ml when the samples were incubated in air. By comparison, telithromycin MIC50s and MIC90s for erythromycin-resistant strains were in air 0.03 to 0.125 and 0.125 to 0.5 μg/ml, respectively. When agar dilution was used as the reference method, essential agreement was found for 112 of 216 strains (51.9%) for microdilution, 168 of 216 (77.8%) for E test in air, and 132 of 216 (61.1%) for E test in CO2. With the exception of four strains tested by E test in CO2, all organisms were susceptible to a proposed telithromycin susceptibility breakpoint of ≤1 μg/ml. By disk diffusion with 15-μg telithromycin disks, all strains but one had zones of inhibition ≥19 mm in diameter when incubated in CO2, while all strains had zone diameters of ≥22 mm when incubated in air. Zone diameters in air were generally 4 to 5 mm larger than in CO2. By all methods, MICs and zones of all erythromycin-resistant strains occurred in clusters separated from those seen with erythromycin-susceptible strains. The results for macrolide-resistant strains with erm and mef resistance determinants were similar. The results show that (i) telithromycin is very active against erythromycin-susceptible and -resistant strains irrespective of macrolide resistance mechanism; (ii) susceptibility to telithromycin can be reliably tested by the agar, microdilution, E test, and disk diffusion methods; and (iii) incubation in CO2 led to smaller zones by disk diffusion and higher MICs by E test, but at a susceptible MIC breakpoint of ≤1 μg/ml and a susceptible zone diameter cutoff of ≥19 mm in CO2, 215 of 216 strains were found to be susceptible to telithromycin.
Streptococcus pneumoniae continues to be a significant cause of morbidity and mortality in humans and is the leading cause of bacterial pneumonia, sinusitis, and otitis media and an important cause of meningitis (2, 4, 10–12). The past 5 years have witnessed a dramatic worldwide increase in the incidence of pneumococcal strains which are resistant to penicillin G and other β-lactam and non-β-lactam antimicrobials, such as macrolides, clindamycin, tetracycline, chloramphenicol, and trimethoprim-sulfamethoxazole (5, 8, 13). The problem has been exacerbated by the tendency of these strains to spread from country to country and from continent to continent (16, 17).
In the United States, a recent study has shown that for 23.6% of 1,527 clinically significant pneumococci from 30 U.S. centers, penicillin MICs were ≥0.125 μg/ml, with 14.1% being intermediate and 9.5% being resistant (8). Penicillin-resistant pneumococci were more likely to be resistant to macrolides and other unrelated agents, such as chloramphenicol, tetracycline, and trimethoprim-sulfamethoxazole. Erythromycin resistance among pneumococci has increased in the United States from approximately 0.2% in the late 1980s to 5 and 15% in some areas of the country (5, 8, 22). In the survey described above (8), 20% of penicillin-intermediate pneumococcal strains and 49% of penicillin-resistant strains were erythromycin resistant. In a recently published study from our laboratory performed in 1997 (13), 49.6% of 1,476 pneumococcal strains obtained from outpatients in six geographic regions of the United States were penicillin susceptible, 17.9% were intermediate, and 32.5% were penicillin resistant. In the latter survey, 95% of penicillin-susceptible, 65% of intermediate, and 33% of penicillin-resistant strains were macrolide susceptible. Strains for which penicillin MICs were increased were also more likely to be multiresistant (13).
Pneumococcal strains that are resistant to erythromycin exhibit cross-resistance to other macrolides and azalides such as azithromycin, clarithromycin, and roxithromycin (7, 9, 24). Telithromycin (HMR 3647), a new ketolide (1), has been shown to have low MICs for both macrolide-susceptible and macrolide-resistant pneumococcal strains (6, 14, 21; D. Felmingham, M. J. Robbins, A. Leakey, R. Cooke, C. Dencer, H. Solman, G. L. Ridgway, R. N. Grüneberg, and A. Bryskier, Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-116, 1997). Although MICs are slightly higher for macrolide-resistant strains, all strains are susceptible at ≤2.0 μg/ml, irrespective of the macrolide resistance mechanism (21).
Currently, the National Committee for Clinical Laboratory Standards (NCCLS) recommends incubation in air for microdilution testing and in CO2 for disk diffusion (18, 19). The manufacturer of the E test (AB Biodisk, Solna, Sweden) also recommends incubation in CO2 for testing of S. pneumoniae because 5 to 10% of strains do not grow without CO2 on primary isolation (7, 15). Because incubation in CO2 has previously been shown to affect macrolide MICs for S. pneumoniae (7, 9, 24), we tested activity by agar dilution and microdilution in air and disk diffusion and E test in air as well as CO2 atmospheres. We are aware of no previous published data on incubation in air compared with CO2 for ketolides.
MATERIALS AND METHODS
Bacteria and antibiotics.
A total of 216 isolates of S. pneumoniae (collected between 1995 and 1997 from various laboratories in the United States) were selected from our culture collection for testing. All strains had been subcultured several times prior to use and all grew well in air. Cultures were stored at −70°C in double-strength skim milk (Difco Laboratories, Detroit, Mich.). Telithromycin and erythromycin were obtained from Hoechst Marion Roussel Anti-infectives, Romainville, France. Powders were stored at −4°C.
Macrolide resistance mechanism determination.
All strains were screened for macrolide susceptibility by the double disk method with erythromycin and clindamycin disks (18). Strains with erythromycin zone diameters of ≤20 mm were tested for the presence of the ermB and mefE genes as follows. DNA was extracted from isolated strains derived from single colonies with the Prep-A-Gene kit (Bio-Rad, Hercules, Calif.) as recommended by the manufacturer. Previously described primer sets for amplification of the ermB and mefE genes were used (20, 23). An initial denaturing step at 96°C for 3 min and a final elongation step at 72°C for 5 min were performed for each PCR run. The ermB and mefE genes were amplified by 30 cycles of three steps: 96°C for 60 s, 52°C for 60 s, and 72°C for 60 s. The PCR products for ermB genes were 640 bp, and those for mefE genes were 348 bp. Amplifications were carried out in a final volume of 50 μl in a GeneAmp PCR system 2400 (Perkin-Elmer, Foster City, Calif.). The final PCR mixture contained 10 mM Tris-HCl (pH 8.3); 50 mM KCl; 2.0 mM MgCl2; 200 μM concentrations of each of the deoxynucleotides dATP, dCTP, dGTP, and dTTP; 30 to 50 pmol of each primer set; 1 ng of template DNA; and 2.5 U of Taq polymerase (Fisher BioTech). Samples of each PCR product were then electrophoresed on 2% agarose gels (Bio-Rad) and stained in ethidium bromide (0.5 μg/ml) in 0.5× Tris-borate-EDTA buffer. PCR products were visualized under UV illumination, and their sizes were estimated with PCR markers (Promega, Madison, Wis.). Negative controls ruled out the possible influence of amplicon modification.
Agar dilution MICs.
Agar dilution MICs were determined by the methods used in our laboratory (7) on Mueller-Hinton agar supplemented with 5% sheep blood, incorporating telithromycin and erythromycin at concentrations of 0.002 to 64 μg/ml in doubling dilutions. Inocula were prepared by suspending growth from overnight cultures in sterile saline to a turbidity of a 0.5 McFarland standard. Final inocula contained 104 CFU/spot. Plates were inoculated with a Steers replicator with 3-mm inoculating pins and incubated overnight at 35°C in air. The lowest concentration of antibiotic resulting in no growth was read as the MIC. The quality control strains Staphylococcus aureus ATCC 29213 and S. pneumoniae ATCC 49619 were included in each run. Inoculum checks were performed for each strain.
Microdilution MICs.
Telithromycin MICs were determined by the method recommended by the NCCLS (19), using cation-adjusted Mueller-Hinton broth (Difco Laboratories) supplemented with 5% lysed defibrinated horse blood. Suspensions with a turbidity equivalent to that of a 0.5 McFarland standard were prepared by suspending growth from blood agar plates in 2 ml of sterile saline. Suspensions were further diluted 1:10 to obtain a final inoculum of 5 × 105 CFU/ml. Trays were incubated overnight in ambient air at 35°C. Standard quality control strains and inoculum checks (as above) were included.
E test MICs.
Mueller-Hinton plates supplemented with 5% sheep blood (BBL Microbiology Systems, Cockeysville, Md.) were inoculated with a 0.5 McFarland suspension harvested from overnight growth on plates, and telithromycin E test strips (AB Biodisk) were placed on each plate (7, 15). After overnight incubation at 35°C, the MIC was read where the ellipse of growth inhibition intersected the strip. E test MICs were determined both in air and in CO2. Standard quality control strains (see above) were used with each run.
Disk diffusion.
Disk diffusion was performed by standard NCCLS methodology (19) using Mueller-Hinton plates supplemented with 5% sheep blood (source as above), inoculated with a 0.5 McFarland suspension; 15-μg telithromycin disks (BBL Microbiology Systems) were placed on the plates. After overnight incubation in both air and 5% CO2 at 35°C, the diameters of zones of inhibition were measured with calipers. Standard quality control strains (see above) were used with each run.
Interpretation of results.
For telithromycin, provisional breakpoints of ≤1.0 μg/ml for susceptible, 2.0 μg/ml for intermediate, and ≥4.0 μg/ml for resistant results were used. Essential agreement was defined as the MIC by one method being within 1 log2 dilution of the MIC by agar dilution (taken as the reference method). Interpretative category discrepancies were defined as very major discrepancies when the reference method showed resistance and the comparative method showed susceptibility; major discrepancies occurred when the reference method showed susceptibility and the comparative method showed resistance; and minor discrepancies occurred when an intermediate result was obtained with one method and a resistant or susceptible result was obtained with the other (7).
RESULTS
Of the 216 isolates included in this study, 110 were erythromycin and clindamycin susceptible by disk diffusion testing, with erythromycin MICs being ≤0.12 μg/ml by agar and microdilution. The remaining 106 isolates were erythromycin resistant (erythromycin MICs, ≥0.5 μg/ml), with 32 being clindamycin susceptible and positive for mefE gene products. All remaining 74 macrolide-resistant strains were positive for erm gene products.
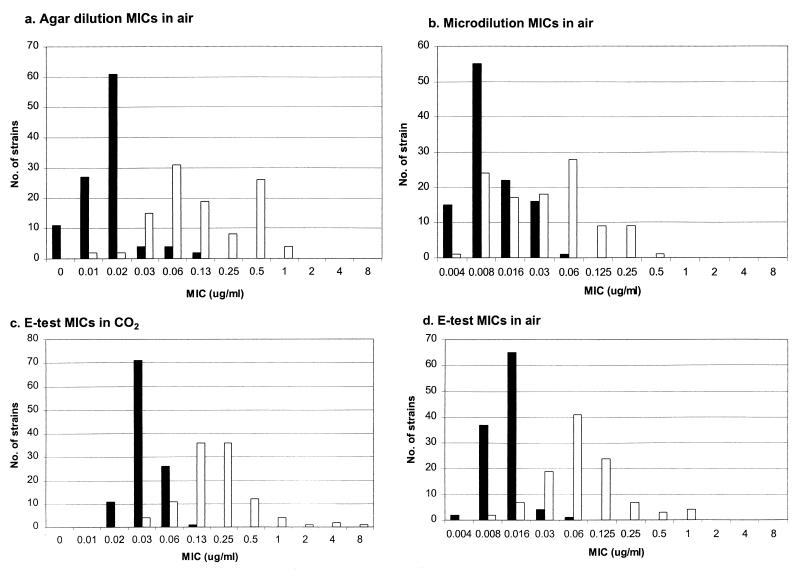
The telithromycin MIC results for the four susceptibility testing methods are presented in Table 1 and Fig. 1. The MICs at which 50% and 90% of the strains are inhibited (MIC50s and MIC90s, respectively) for the erythromycin-susceptible strains varied between 0.008 and 0.016 μg/ml and 0.016 and 0.03 μg/ml, respectively, for methods using incubation in air. By comparison, the telithromycin MIC50s and MIC90s in air of erythromycin-resistant strains were 0.03 to 0.125 and 0.125 to 0.5 μg/ml, respectively. By the E test method with incubation in CO2, telithromycin MIC50s and MIC90s were 0.03 and 0.06 and for erythromycin-susceptible strains, respectively, and 0.25 and 0.5 μg/ml for erythromycin-resistant strains, respectively. Table 2 presents agar dilution results for erythromycin-susceptible and -resistant strains. As can be seen, telithromycin MIC50s and MIC90s rose from 0.016 and 0.016 μg/ml for the erythromycin-susceptible group to 0.06 and 0.5 μg/ml for the erythromycin-resistant group, compared to erythromycin MICs of 0.03 and 0.06 μg/ml and >64 and >64 μg/ml for the erythromycin-susceptible and -resistant groups, respectively.
TABLE 1.
Telithromycin MICs with the three methods tested against 216 strains
| Method and strain typea | MIC (μg/ml)
|
||
|---|---|---|---|
| Range | 50% | 90% | |
| Agar dilution, air | |||
| S | ≤0.004–0.125 | 0.016 | 0.016 |
| R | 0.008–1.0 | 0.125 | 0.5 |
| Microdilution, air | |||
| S | ≤0.004–0.06 | 0.008 | 0.03 |
| R | ≤0.004–0.5 | 0.03 | 0.125 |
| E test, air | |||
| S | ≤0.004–0.06 | 0.016 | 0.016 |
| R | 0.008–1.0 | 0.06 | 0.25 |
| E test, CO2 | |||
| S | 0.016–0.125 | 0.03 | 0.06 |
| R | 0.03–8.0 | 0.25 | 0.5 |
Strains tested were either susceptible (S) or resistant (R) to erythromycin.
FIG. 1.
Histograms of telithromycin MICs for erythromycin-susceptible (solid bars) and -resistant (open bars) strains.
TABLE 2.
Comparison of agar dilution MIC results for erythromycin-susceptible (n = 110) and erythromycin-resistant (n = 106) strains
| Drug | Susceptible strains
|
Resistant strains
|
||||
|---|---|---|---|---|---|---|
| Range | 50% | 90% | Range | 50% | 90% | |
| Telithromycin | 0.004–0.125 | 0.016 | 0.016 | 0.008–1.0 | 0.06 | 0.5 |
| Erythromycin | 0.008–0.125 | 0.03 | 0.06 | 0.5–>64.0 | >64.0 | >64.0 |
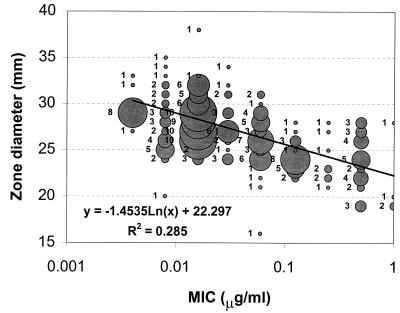
The disk diffusion results are shown in Fig. 2. Mean telithromycin disk diffusion zone diameters were 26.7 mm in CO2, compared with 32.9 mm in air. For erythromycin-susceptible and -resistant groups, mean zone diameters were 28.5 and 24.9 mm, respectively, in CO2, compared to 34.9 and 30.0 mm, respectively, in air. For incubation in CO2, 215 of 216 strains were susceptible at a zone diameter breakpoint of ≥19 mm, while all strains were susceptible in air at a zone diameter breakpoint of ≥22 mm. A scatter plot of telithromycin disk diffusion zones in CO2 versus agar dilution MICs is presented in Fig. 3.
FIG. 2.
Disk diffusion histograms with incubation in air (a) and CO2 (b) for erythromycin-susceptible (solid bars) and -resistant (open bars) strains.
FIG. 3.
Scatter plot comparison of disk diffusion zone diameters in CO2 with agar dilution MICs in air.
Categorical discrepancies with the four methods are presented in Table 3. The number of strains with essential agreement varied between 47 (21.8%) and 168 (77.8%), depending upon which methods were being compared and whether CO2 was used as opposed to air. Minor discrepancies were obtained with the E test incubated in CO2 for one strain, and major discrepancies for three strains were obtained with the E test compared with agar and microdilution.
TABLE 3.
Comparison of results of telithromycin susceptibility testing by four methods
| Comparison
|
No. of strains (n = 216) with log2 ratio of reference to test MIC (method A vs. method B) of:
|
No. (%) ± 1 log2 dilution | No. of categorical discrepancies at breakpoints of ≤1, 2, and ≥4 μg/ml (S, I, and R)a
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Method A | Method B | ≥1/8 | 1/4 | 1/2 | 0 | 2 | 4 | 8 | Major | Very major | Minor | |
| Microdilution | Agar dilution | 33 | 63 | 53 | 35 | 24 | 8 | 0 | 112 (51.9) | 0 | 0 | 0 |
| E test, CO2 | Agar dilution | 0 | 6 | 23 | 26 | 83 | 53 | 25 | 132 (61.1) | 3 | 0 | 1 |
| E test, air | Agar dilution | 17 | 21 | 41 | 94 | 33 | 10 | 0 | 168 (77.8) | 0 | 0 | 0 |
| E test, air | E test, CO2 | 12 | 87 | 107 | 10 | 0 | 0 | 0 | 117 (54.2) | 0 | 0 | 0 |
| E test, air | Microdilution | 1 | 10 | 18 | 55 | 77 | 47 | 8 | 150 (69.4) | 0 | 0 | 0 |
| E test, CO2 | Microdilution | 0 | 0 | 2 | 15 | 30 | 93 | 76 | 47 (21.8) | 3 | 0 | 1 |
S, I, and R, susceptible, intermediate, and resistant.
The effect of incubation in CO2 on the susceptibility results showed that incubation of E tests in CO2 resulted in MICs approximately 1 dilution higher and zone diameters 4 to 5 mm narrower than those obtained with incubation in air (Table 1; Fig. 1 and 2). Additionally, although all strains were susceptible to telithromycin by agar dilution, microdilution, and E test incubated in air, four strains gave telithromycin MICs between 2 and 8 μg/ml when E tests were incubated in CO2, yielding one minor and three major discrepancies with the breakpoints used, irrespective of which susceptibility method was used as the reference. The MICs and zones for erythromycin-resistant strains occurred in clusters different from those seen with erythromycin-susceptible strains. The telithromycin MICs for erythromycin-resistant strains were similar irrespective of macrolide resistance mechanism.
DISCUSSION
The results of this study confirm the excellent antipneumococcal activity of telithromycin irrespective of erythromycin susceptibility, as reported previously (1). Clustering of MICs depended on the erythromycin susceptibility of the strains (21). The agar and microdilution methods, both with incubation in air, produced similar MIC values. When E tests were incubated in air, excellent correlation with the results of the agar and microdilution methods was obtained. However, when E tests were incubated in CO2, MICs rose, generally by 1 dilution. With a preliminary susceptible breakpoint of ≤1.0 μg/ml, all strains were telithromycin susceptible by the three MIC methods in air; however, incubation of E tests in CO2 resulted in four strains for which telithromycin MICs were increased to between 2 and 4 μg/ml.
Disk diffusion tests showed that, with the exception of one strain, zone diameters in CO2 of ≥19 mm corresponded to telithromycin MICs of ≤1.0 μg/ml. In air, disk diffusion zone diameters of ≥22 mm corresponded with telithromycin MICs of ≤1.0 μg/ml in all 216 strains tested. NCCLS recommends incubation of S. pneumoniae in air for agar dilution and microdilution but in CO2 for disk diffusion, as is also the recommendation of the manufacturer of the E test. In previous studies, we have reported results similar to this CO2 effect on telithromycin susceptibilities with other macrolides (9, 24). Because of the higher MICs observed with incubation in CO2, there is a need to determine macrolide and ketolide MICs in air as well as in increased CO2 (as used in the current study) to allow reliable testing by the E test method in the clinical laboratory.
In summary, our results show that (i) telithromycin is active against erythromycin-susceptible and -resistant strains irrespective of macrolide resistance mechanism, although MICs for erythromycin-resistant strains are higher; (ii) susceptibility to telithromycin may be reliably tested by agar, microdilution, E test, and disk diffusion; and (iii) disk diffusion tests gave narrower zones and E tests gave higher MICs when plates were incubated in CO2 than when incubated in air, but at a preliminary breakpoint of ≤1 μg/ml and zone diameter of ≥19 mm (CO2), all strains but one were telithromycin susceptible irrespective of incubation atmosphere or method. More information from clinical or animal data is required before breakpoints can be firmly established and recommended.
ACKNOWLEDGMENT
This study was supported by a grant from Hoechst Marion Roussel Anti-infectives.
REFERENCES
- 1.Agouridas C, Bonnefoy A, Chantot J F. Antibacterial activity of RU 64004 (HMR 3004), a novel ketolide derivative active against respiratory pathogens. Antimicrob Agents Chemother. 1997;41:2149–2158. doi: 10.1128/aac.41.10.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Appelbaum P C. Antimicrobial resistance in Streptococcus pneumoniae—an overview. Clin Infect Dis. 1992;15:77–83. doi: 10.1093/clinids/15.1.77. [DOI] [PubMed] [Google Scholar]
- 3.Arthur M, Molinas C, Mabilat C, Courvalin P. Detection of erythromycin resistance by the polymerase chain reaction in conserved regions of erm rRNA methylase genes. Antimicrob Agents Chemother. 1990;34:2024–2026. doi: 10.1128/aac.34.10.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Block S. Causative pathogens, antibiotic resistance and therapeutic considerations in acute otitis media. Pediatr Infect Dis J. 1997;16:449–456. doi: 10.1097/00006454-199704000-00029. [DOI] [PubMed] [Google Scholar]
- 5.Breiman R F, Butler J C, Tenover F C, Elliott J A, Facklam R R. Emergence of drug-resistant pneumococcal infections in the United States. JAMA. 1994;271:1831–1835. [PubMed] [Google Scholar]
- 6.Bryskier A, Agouridas C, Chantot J-F. Ketolides: new semisynthetic 14-membered ring macrolides. In: Zinner S H, Young L S, Acar J F, Neu H C, editors. Expanding indications for the new macrolides, azalides and streptogramins. New York, N.Y: Marcel Dekker; 1996. pp. 39–50. [Google Scholar]
- 7.Clark C L, Jacobs M R, Appelbaum P C. Antipneumococcal activities of levofloxacin and clarithromycin as determined by agar dilution, microdilution, E-test, and disk diffusion methodologies. J Clin Microbiol. 1998;36:3579–3584. doi: 10.1128/jcm.36.12.3579-3584.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doern G V, Brueggemann A, Holley H P, Rauch A M. Antimicrobial resistance of Streptococcus pneumoniae recovered from outpatients in the United States during the winter months of 1994 to 1995: results of a 30-center national surveillance study. Antimicrob Agents Chemother. 1996;40:1208–1213. doi: 10.1128/aac.40.5.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fasola E L, Bajaksouzian S, Appelbaum P C, Jacobs M R. Variation in erythromycin and clindamycin susceptibilities of Streptococcus pneumoniae by four test methods. Antimicrob Agents Chemother. 1997;41:129–134. doi: 10.1128/aac.41.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedland I R, Istre G S. Management of penicillin-resistant pneumococcal infections. Pediatr Infect Dis J. 1992;11:433–435. doi: 10.1097/00006454-199206000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Friedland I R, McCracken G H., Jr Management of infections caused by antibiotic-resistant Streptococcus pneumoniae. N Engl J Med. 1994;331:377–382. doi: 10.1056/NEJM199408113310607. [DOI] [PubMed] [Google Scholar]
- 12.Jacobs M R. Treatment and diagnosis of infections caused by drug-resistant Streptococcus pneumoniae. Clin Infect Dis. 1992;15:119–127. doi: 10.1093/clinids/15.1.119. [DOI] [PubMed] [Google Scholar]
- 13.Jacobs M R, Bajaksouzian S, Zilles A, Lin G, Pankuch G A, Appelbaum P C. Susceptibilities of Streptococcus pneumoniae and Haemophilus influenzae to 10 oral antimicrobial agents based on pharmacodynamic parameters: 1997 U.S. surveillance study. Antimicrob Agents Chemother. 1999;43:1901–1908. doi: 10.1128/aac.43.8.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones R N, Biedenbach D J. Antimicrobial activity of RU-66647, a new ketolide. Diagn Microbiol Infect Dis. 1997;27:7–12. doi: 10.1016/s0732-8893(96)00181-2. [DOI] [PubMed] [Google Scholar]
- 15.Jones R N, Erwin M E, Croco J L. Critical appraisal of E test for the detection of fluoroquinolone resistance. J Antimicrob Chemother. 1996;38:21–25. doi: 10.1093/jac/38.1.21. [DOI] [PubMed] [Google Scholar]
- 16.McDougal L K, Facklam R, Reeves M, Hunter S, Swenson J M, Hill B C, Tenover F C. Analysis of multiply antimicrobial-resistant isolates of Streptococcus pneumoniae from the United States. Antimicrob Agents Chemother. 1992;36:2176–2184. doi: 10.1128/aac.36.10.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Munoz R, Musser J M, Crain M, Briles D E, Marton A, Parkinson A J, Sorensen U, Tomasz A. Geographic distribution of penicillin-resistant clones of Streptococcus pneumoniae: characterization by penicillin-binding protein profile, surface protein A typing, and multilocus enzyme analysis. Clin Infect Dis. 1992;15:112–118. doi: 10.1093/clinids/15.1.112. [DOI] [PubMed] [Google Scholar]
- 18.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. NCCLS publication no. M7-A4. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 19.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial disk susceptibility tests. NCCLS publication no. M2-A6. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 20.Pankuch G A, Jueneman S A, Davies T A, Jacobs M R, Appelbaum P C. In vitro selection of resistance to four β-lactams and azithromycin in Streptococcus pneumoniae. Antimicrob Agents Chemother. 1998;42:2914–2918. doi: 10.1128/aac.42.11.2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pankuch G A, Visalli M A, Jacobs M R, Appelbaum P C. Susceptibilities of penicillin- and erythromycin-susceptible and -resistant pneumococci to HMR 3647 (RU 66647), a new ketolide, compared with susceptibilities to 17 other agents. Antimicrob Agents Chemother. 1998;42:624–630. doi: 10.1128/aac.42.3.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spika J S, Facklam R R, Plikaytis B D, Oxtoby M J the Pneumococcal Surveillance Working Group. Antimicrobial resistance of Streptococcus pneumoniae in the United States, 1979–1987. J Infect Dis. 1991;163:1273–1278. doi: 10.1093/infdis/163.6.1273. [DOI] [PubMed] [Google Scholar]
- 23.Sutcliffe J, Grebe T, Tait-Kamradt A, Wondrack L. Detection of erythromycin-resistant determinants by PCR. Antimicrob Agents Chemother. 1996;40:2562–2566. doi: 10.1128/aac.40.11.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Visalli M A, Jacobs M R, Appelbaum P C. Susceptibility of penicillin-susceptible and -resistant pneumococci to dirithromycin compared with susceptibilities to erythromycin, azithromycin, clarithromycin, roxithromycin, and clindamycin. Antimicrob Agents Chemother. 1997;41:1867–1870. doi: 10.1128/aac.41.9.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]