Abstract
The objective of this study was to investigate the spatial variation of volatile organic compounds and antioxidant activity of turmeric essential oils (TEOs) harvested from four provinces of China. The major chemical components of these TEOs were analyzed using headspace solid-phase micro-extraction gas chromatography-mass spectrometry. More than forty volatile organic compounds in TEOs were identified, which accounted for 82.09–93.64% of the oil components. The relative abundances of the main volatile organic compounds in TEOs at the genus level were visualized by a heat map. The antioxidant activity of the TEOs of five different origins was characterized by the DPPH free radical scavenging activity, in which the antioxidant activity of the TEOs from Guangxi was superior to those of other sources. Furthermore, the IC50 values of the antioxidants TEOs collected from Guangxi, Sichuan, Yunnan, Changting, and Liancheng were 33.30, 42.5, 35.22, 63.27, and 39.96 mg/mL, respectively, which indicated the excellent free radical scavenging activity of those TEOs. Therefore, the TEOs might be considered as a natural antioxidant with potential applications in food and pharmaceutical industries.
Keywords: Turmeric essential oils (TEOs), HS-SPME-GC-MS, Antioxidant activity, DPPH, Curcuma longa L
Graphical abstract
Highlights
-
•
Turmeric essential oils stemmed from four provinces of China were investigated.
-
•
Multivariate analysis of volatile organic compounds in TEOs was performed.
-
•
The major components of volatile organic compounds exhibited a spatial variation.
-
•
Antioxidant activity of turmeric essential oils demonstrated a spatial variation.
-
•
TEOs of Guangxi had a superior antioxidant activity to those of other origins.
1. Introduction
Curcuma longa Linn. (syn. C. domestica Valeton and C. brog Valeton), family Zingiberaceae, is known as "turmeric" worldwide, "Jianghuang" in Chinese, "kurkum" in Arabic, and "haldi" in Hindi and Urdu (Dosoky and Setzer, 2018; G. Singh et al., 2010). It is assumed that turmeric originated in China, and Buddhist monks or Chinese migration brought it to the Indian subcontinent (Stanojevic et al., 2015). Currently, turmeric is cultivated in Asian countries (i.e., China, Bangladesh, Thailand, Cambodia, Malaysia, Indonesia, and Philippines) and some parts of South America (Peru and Bolivia) (Sharma et al., 2021; Stanojevic et al., 2015). There are approximately 93–100 accepted Curcuma species, however, the exact number of species is still controversial, particularly considering the turmeric rhizomes harvested from different regions.
In this regard, turmeric rhizome contains two major classes of secondary metabolites: phenolic curcuminoids and essential oils (EOs) (Stanojevic et al., 2015; Tosati et al., 2018). In addition to the curcuminoids, EOs from turmeric mainly consists of aromatic compounds and aliphatic terpenes, which are considered to have significant biological activities including antioxidant (Avanço et al., 2017), antibacterial (Avanço et al., 2017; Hu et al., 2017; Li et al., 2019), anti-inflammatory (Akinyemi et al., 2018; Toden et al., 2017), anticancer (Cheng et al., 2012; Joshi et al., 2016; Kim et al., 2013), anti-hyperlipidemic (Ling et al., 2012; V. Singh et al., 2013) and antidiabetic role (Lekshmi et al., 2012; Shinichi et al., 2006; Tozo et al., 2005). In this case, hundreds of compounds have been identified from the turmeric essential oils (TEOs); however, the major components are ar-Turmerone, α-Turmerone, β-Turmerone, ar-Curcumene, and Curlone, followed by notable amounts of α-Zingiberene, α-Bisabolene, ar-Turmerol, β-Phellandrene, α-Phellandrene, α-Terpinene, r-Terpinene, Terpinolene, α-Sesquiphellandrene, β-Sesquiphellandrene, 1,8-Cineole, Caryophyllene oxide, and β-Bisabolene (Akinyemi et al., 2018; Avanço et al., 2017; B, Kottarapat, & Ramadasan, 2011; Hwang et al., 2016; Kutti Gounder and Lingamallu, 2012; Naveen Kumar et al., 2016; Oyemitan et al., 2017; Stanojevic et al., 2015). Zhang et al. (2017) (Zhang et al., 2017) analyzed 81 components of TEO using gas chromatography-mass (GC–MS) from 20 different habitats in China, and a total of 81 chromatographic peaks were obtained, among which the main components were are ar-Turmerone, β-Turmerone, α-Zingiberene, ar-Curcumene, and β-Sesquiphellandrene. Dosoky, Satyal, and Setzer (2019) (Dosoky et al., 2019) reported that TEO was obtained and analyzed by GC-MS. TEO volatiles were dominated by α-Turmerone, Curlone, ar-Turmerone, β-Sesquiphellandrene, α-Zingiberene, Germacrone, Terpinolene, ar-Curcumene, and α-Phellandrene. Xu et al. (2020) (Xu et al., 2020) analyzed the volatile components in turmeric samples from five major production areas of China using GC-MS. A total of the chemical components including ar-Turmerone, α-Turmerone, β-Turmerone, (E)-Atlantone, Caryophyllene, ar-Curcumene, (−)-Zingiberene, β-Bisabolene, β-Sesquiphellandrene and (6R,7R)-Bisabolene were identified. However, the comparative data on the chemical compositions of volatile organic compounds of TEOs stemmed from different regions are scarce, particularly lack of assessing tools with a high efficacy, such as headspace solid-phase micro-extraction gas chromatography-mass spectrometry (HS-SPME-GC-MS). Moreover, considering the extraction of the different terpenoids and other compounds from EOs, HS-SPME-GC-MS would be a powerful tool that requires a minimal amount of sample. HS-SPME-GC-MS was chosen in this study because it can determine accurately, conveniently, and rapidly the chemical compositions of those TEOs. (Durant et al., 2014). Furthermore, HS sampling is a fundamental technique to characterize the volatile fraction of aromatic plants (Da Porto and Decorti, 2008).
In recent years, the evaluation of antioxidant potential of foods has received much attention. The considerable research efforts have been attached to the antioxidant and related antimicrobial, insecticidal, antifungal, and antioxidation properties. For example, the essential oil of turmeric rhizome showed major radical scavenging activity against DPPH free radical (Tsai et al., 2011). Furthermore, an obvious variation was demonstrated by the antioxidant activities of TEOs extracted from Chongqing and Guangdong of C. longa: high DPPH radical-scavenging activity was exhibited by the TEOs from Guangdong than Chongqing (Zhang et al., 2017). Since TEOs have been discovered to be toxic to fungi engaged in the deterioration of agricultural products, TEOs could serve as an alternative to synthetic pesticides for the control of food fungi and pests. The interest in their use has been increasing because they demonstrate lower risks for human health and the environment. TEOs would not leave residues in food, another growing concern of the population. Furthermore, very recently, the price of turmeric has been increased continuously with the increase in demand, and the quality variations of turmeric of geographic locations have become dramatically important. In this case, since the turmeric essential oil (TEO) is one of the main active components of turmeric, the studies on the spatial variation of the chemical composition and antioxidant activity of such a component would provide insights into the evaluation of turmeric quality.
The objective of this study was to evaluate the spatial variation of the chemical compositions and antioxidant activity of the TEOs harvested from four different provinces of China. The major chemical compositions of these TEOs were analyzed using headspace solid-phase micro-extraction gas chromatography-mass spectrometry HS-SPME-GC-MS. The multivariate analysis of the chemical compositions in these TEOs were performed using the hierarchical cluster analysis and principal component analysis (PCA). The antioxidant activity of the TEOs of four provinces was characterized by the DPPH-radical-scavenging activity to provide a certain scientific basis for the quality evaluation of this plant.
2. Materials and methods
2.1. Chemicals and reagents
1,1-Diphenyl-2-picrylhydrazyl (DPPH) was purchased from Shanghai Macklin Biochemical Co., Ltd. The ultrapure water (18.2 MΩ cm−1) was used in this study, provided by a Simplicity-UV water purification system (Millipore, Bedford, USA).
2.2. Plant materials and TEOs extraction
The C. longa rhizomes used in this study were harvested from five different origins in Guangxi, Sichuan, Yunnan, and Fujian provinces of China. The geographic information of these origins was shown in Table 1. TEOs of Curcuma species could be obtained by hydro- or steam-distillation of the fresh or dry rhizome. Alternatively, Curcuma volatiles have also been attained by solvent extraction or supercritical fluid extraction of the powdered rhizome. In this study, the fresh rhizomes of turmeric (C. longa) harvested from Liancheng and Changting, Fujian Province were washed in running water, dried in the air, and thinly grated for further utilization; while the dry rhizomes of C. longa obtained from Sichuan Province, Guangxi Province, and Yunnan Province were pulverized into fine powders. Then, all these treated dry and fresh rhizomes were passed through an 80-mesh sieve to obtain a uniform powder, respectively. TEOs were extracted by the hydrodistillation of 200 g of rhizomes for 5 h using a Clevenger apparatus (Commission, 2015). The TEOs were collected, and the water remaining after extraction was removed by adding anhydrous sodium sulphate (Na2SO4), followed by filtration. The TEOs were stored at 4 °C in sealed glass vials and protected from light prior to chemical analysis and further use. The TEOs were denoted as LC, CT, SC, GX, and YN for the rhizomes of C. longa harvested from Liancheng and Changting, Fujian Province, Sichuan Province, Guangxi Province, and Yunnan Province accordingly.
Table 1.
Geographic and climatic information on the collected turmeric for the study.
| Province | District | Locality | Longitude | Latitude | Accessions collected | Climate | Elevation |
|---|---|---|---|---|---|---|---|
| Guangxi | Baise | Napo | 105°84′ | 23°41′ | GX | subtropical monsoon climate | 1681 m |
| Yunnan | Wenshan | Maguan | 103°52′ | 22°42′ | YN | subtropical monsoon climate | 1447 m |
| Sichuan | Yibin | Yibin | 104°53′ | 28°69′ | SC | subtropical monsoon humid climate | 422 m |
| Fujian | Liancheng | Chixi | 116°32′ | 25°13′ | LC | subtropical monsoon climate | 375 m |
| Fujian | Changting | Tongfang | 116°57′ | 25°88′ | CT | subtropical monsoon climate | 658 m |
2.3. Chemical analysis and identification of the major components of TEOs
HS-SPME-GC-MS was used for identifying thermally labile volatile compounds from TEOs. The PDMS/DVB fibers (57329-U, Supelco, USA) were used for the extraction of the volatile organic compounds of TEOs of five different origins. The fibers were conditioned for 12 min at 250 °C in the GC/MS injector (GCMS-TQ8040, SHIMADZU, Japan) before SPME-GC/MS analysis. For TEOs of each origin, a small amount of TEOs was put in a 15 mL vial. The fiber coatings were embedded into the headspace to determine the values of the temperature and time set in the experiments. The temperature was set at 50 °C while the incubation and extraction time was set at 10 and 30 min, respectively. The fibers containing the extracted volatile organic compounds of TEOs were injected into the GC/MS injector. Separation of TEOs was used in a Rxi-5Sil MS column (30 m × 0.25 mm, 0.25 μm). The measurement of each sample using GC/MS equipped with an auto-sampler was set for about 40 min. The temperature of the injector and detector was 250 and 280 °C, respectively. The initial temperature was kept at 50 °C for 2 min, and then was gradually increased to 180 °C at a temperature ramp rate of 5 °C/min and was dwelled at 180 °C for 0 min; then the temperature was increased to 280 °C at a rate of 10 °C/min and was held at 280 °C for 2 min.
The gas chromatography and mass spectrometry (GC-MS) analyses were performed under the following conditions: the mass spectrometer was operated at an ionization voltage of 70 eV; the temperature of an ion source was 200 °C; the full mode range was conducted with a scan mass range of 35–550 amu; the carrier gas was helium, at a flow rate of 1.0 mL/min, and the split ratio was 100:1.
2.4. Identification of components
The components were identified according to the search and match of gas chromatographic retention indices, mass spectra with FFNSC 1.2., NIST14. and NIST14s. libraries, and the literature (Qin et al., 2007; Zhang et al., 2017). The peak area normalization method was used to calculate the relative amount of an individual component of the essential oil. The retention indices were calculated using a homologous series of n-alkanes C10–C25.
2.5. DPPH-radical-scavenging activity
The free-radical-scavenging activity of the TEOs was determined based on the scavenging activity of the stabilized DPPH radical according to the previous method of Cuendet (1997) with some modifications (Cuendet et al., 1997). Initially, 0.5 mL of TEOs dilutions in absolute ethanol at concentrations of 8, 12, 16, 20, and 24 mg/mL was added 2.5 mL of 24 μg/mL DPPH. 24 μg/mL DPPH was obtained by dissolving 12 mg of DPPH in absolute ethanol and diluting to a final volume of a 500 mL volumetric flask. After vigorously shaken, the mixing solution of TEOs and DPPH was allowed standing for 30 min in the dark at room temperature, following which the absorbance was recorded at 517 nm using an Ultraviolet–visible (UV–Vis) spectrophotometer (T6 New Century, Beijing Puxi General Instrument Co., Ltd.) (Zhou et al., 2017). The same amount of the absolute ethanol was added instead of the TEOs solution as a negative control. Each sample was tested three times for comparison. The DPPH radical scavenging capacity was calculated using the following equation:
where Ai is the sample absorbance, and A0 is the negative control absorbance at 517 nm, respectively. The IC50 is the concentration of an antioxidant EOs at which 50% inhibition of DPPH free radical activity is observed. The lower IC50 value indicates the greater overall effectiveness of the antioxidant.
2.6. Statistical analysis
Principal component analysis (PCA) was carried out with the substances presented in the TEOs using SIMCA 13.0. Heat map representing an unsupervised, hierarchical cluster analysis of the TEOs of five different origins by Origin 2019b 32Bit. The IC50 values of the DPPH assay were calculated from GraphPad Prism 8.0 software.
3. Results and discussion
3.1. The total ion chromatograms of the turmeric essential oils of five different origins
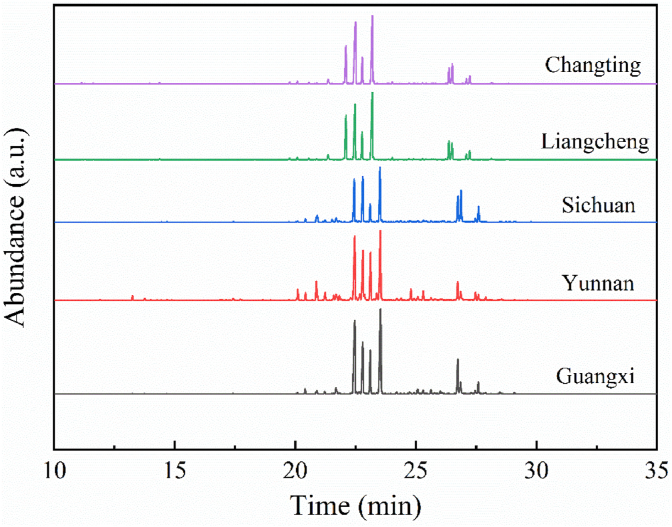
The TEOs of five different origins were analyzed by the HS-SPME-GC-MS method. The total ion chromatograms (TICs) of TEOs collected from five different origins were shown in Fig. 1. The TICs represent the summed intensity across the entire range of masses being detected at every point in the analysis. From these chromatograms in Fig. 1, the main constituents demonstrated by the position of the peaks of the TEOs of the five different origins were quite similar although there were considerable differences in the chemical compositions of these TEOs, as evidenced by the responses of detected peaks, i.e., the intensity of the peaks.
Fig. 1.
The total ion chromatograms (TICs) of TEOs collected from different origins.
3.2. Chemical compositions of the turmeric essential oils of five different origins
The major chemical compositions of the TEOs of five different origins were analyzed using HS-SPME-GC-MS, which were compiled in Table 2, respectively. A total of 40 peaks were identified, accounting for 82.08–93.66% composition of the volatile organic constituents. Sesquiterpenes (i.e., hydrocarbons and oxygenated) consisted of the highest composition (79.50–93.06%) of the TEOs of all five origins whereas monoterpenes (i.e., hydrocarbons and oxygenated) consisted of a comparatively lower composition (0.16–3.31%). Moreover, sesquiterpene hydrocarbons accounted for the highest composition in the TEOs of all five origins. The natural bicyclic sesquiterpenes demonstrated a high abundance in these TEOs and the potential anticancer activity of the natural bicyclic sesquiterpenes has been extensively studied in the literature (Abu-Izneid et al., 2020; Afoulous et al., 2013; Dahham et al., 2015; Pant et al., 2019).
Table 2.
The chemical compositions of TEOs of five different origins in China.
|
Number |
Compounds | Formula | RTb | RIc | Relative Content% (mean ± SD)a |
||||
|---|---|---|---|---|---|---|---|---|---|
| Guangxi | Yunnan | Sichuan | Liancheng | Changting | |||||
| 1 | β-Pinene | C10H16 | 8.22 | 976 | −d | 0.45 ± 0.25a | 8.22 | 0.03 ± 0.01b | 0.06 ± 0.02b |
| 2 | Eucalyptol | C10H18O | 9.808 | 1032 | 0.05 ± 0.02b | 0.47 ± 0.23b | 0.24 ± 0.05b | 0.42 ± 0.07b | 3.25 ± 0.85a |
| 3 | Camphor | C10H16O | 13.245 | 1139 | 0.06 ± 0.01b | 0.64 ± 0.17a | 0.02 ± 0.01b | – | – |
| 4 | DL-Isoborneol | C10H18O | 13.753 | 1155 | 0.05 ± 0.01b | 0.37 ± 0.03a | 0.02 ± 0.01b | – | – |
| 5 | 2-Undecanone | C11H22O | 17.422 | 1279 | 0.09 ± 0.02b | 0.36 ± 0.12a | 0.08 ± 0.03b | 0.03 ± 0.01b | 0.03 ± 0.01b |
| 6 | 2-Tridecanol | C13H28O | 17.715 | 1289 | 0.01 ± 0.01b | 0.23 ± 0.13a | – | 0.02 ± 0.01b | 0.02 ± 0.01b |
| 7 | β-Elemene | C15H24 | 20.096 | 1375 | 0.34 ± 0.05b | 1.38 ± 0.62a | 0.25 ± 0.05b | 0.54 ± 0.08b | 0.47 ± 0.09b |
| 8 | 7-epi-Sesquithujene | C15H24 | 20.413 | 1387 | 0.97 ± 0.08ab | 1.04 ± 0.28a | 0.72 ± 0.18b | 0.69 ± 0.05b | 0.69 ± 0.09b |
| 9 | cis-α-Bergamotene | C15H24 | 21.218 | 1414 | 0.45 ± 0.02b | 1.01 ± 0.50a | 0.61 ± 0.07ab | 0.25 ± 0.02b | 0.24 ± 0.02b |
| 10 | β-Sesquiphellandrene | C15H24 | 21.397 | 1419 | 0.50 ± 0.03a | 0.46 ± 0.15a | 0.55 ± 0.06a | 0.31 ± 0.02b | 0.27 ± 0.02b |
| 11 | (E)-β-Farnesene | C15H24 | 21.683 | 1428 | 1.70 ± 0.15a | 1.42 ± 0.31a | 1.37 ± 0.18a | 1.68 ± 0.02a | 1.50 ± 0.19a |
| 12 | α-Humulene | C15H24 | 21.813 | 1432 | 0.34 ± 0.03a | 0.57 ± 0.36a | 0.59 ± 0.04a | – | – |
| 13 | ar-Curcumene | C15H22 | 22.465 | 1452 | 22.80 ± 0.98a | 14.50 ± 1.83bc | 11.40 ± 1.98d | 15.45 ± 1.91b | 12.01 ± 0.79cd |
| 14 | β-Eudesmene | C15H24 | 22.678 | 1458 | – | 1.12 ± 0.44 | – | – | – |
| 15 | α-Zingiberene | C15H24 | 22.794 | 1462 | 12.33 ± 0.59c | 14.07 ± 5.58c | 15.57 ± 0.60bc | 20.94 ± 1.96ab | 24.41 ± 3.96a |
| 16 | β-Bisabolene | C15H24 | 23.109 | 1472 | 8.77 ± 0.24a | 8.50 ± 1.39a | 5.18 ± 0.55c | 8.35 ± 0.59ab | 7.07 ± 0.46b |
| 17 | δ-Cadinene | C15H24 | 23.372 | 1480 | 0.32 ± 0.07a | – | – | 0.08 ± 0.03b | 0.06 ± 0.03b |
| 18 | β-Cedrene | C15H24 | 23.537 | 1484 | 25.37 ± 0.43a | 17.04 ± 0.56b | 15.75 ± 1.23b | 25.89 ± 1.02a | 24.37 ± 1.22a |
| 19 | cis-Sesquisabinene hydrate | C15H26O | 24.202 | 1504 | 0.42 ± 0.01b | 0.47 ± 0.05a | – | 0.18 ± 0.01c | 0.14 ± 0.02c |
| 20 | Humulene epoxide II | C15H24O | 24.214 | 1504 | – | – | 0.37 ± 0.02 | – | – |
| 21 | Germacrene B | C15H24 | 24.372 | 1508 | 0.14 ± 0.02c | 0.49 ± 0.03b | 0.63 ± 0.05a | 0.61 ± 0.02a | 0.50 ± 0.05b |
| 22 | 3,3,5,5-Tetramethylcyclopentene | C9H16 | 24.515 | 1511 | 0.08 ± 0.01bc | 0.07 ± 0.01c | 0.13 ± 0.01a | 0.09 ± 0.01b | 0.07 ± 0.01c |
| 23 | ar-Turmerol | C15H22O | 24.742 | 1517 | 0.48 ± 0.07b | – | 0.75 ± 0.09a | 0.14 ± 0.04c | 0.09 ± 0.03c |
| 24 | Caryophyllene oxide | C15H24O | 24.917 | 1521 | 0.25 ± 0.01b | 0.34 ± 0.08a | 0.22 ± 0.01b | 0.04 ± 0.01c | 0.02 ± 0.01c |
| 25 | Cryptomeridiol | C15H28O2 | 25.2 | 1527 | – | 0.17 ± 0.06 | – | – | – |
| 26 | Epicurzerenone | C15H18O2 | 25.295 | 1530 | 0.88 ± 0.08 | 2.08 ± 0.30 | – | – | – |
| 27 | Zingiberenol | C15H26O | 25.622 | 1537 | 0.92 ± 0.05a | 0.66 ± 0.27b | 0.45 ± 0.05bc | 0.39 ± 0.04c | 0.31 ± 0.12c |
| 28 | trans-Nuciferol | C15H22O | 25.772 | 1541 | 0.33 ± 0.04a | – | – | 0.23 ± 0.02b | 0.18 ± 0.07b |
| 29 | α-Acorenol | C15H26O | 26.09 | 1548 | – | 0.31 ± 0.06a | – | 0.15 ± 0.02b | 0.12 ± 0.03b |
| 30 | β-Ylangene | C15H24 | 26.086 | 1548 | 0.30 ± 0.02 | – | – | – | – |
| 31 | (E)-γ-Atlantone | C15H22O | 26.35 | 1554 | 0.14 ± 0.02b | – | 0.58 ± 0.04a | 0.10 ± 0.03b | 0.09 ± 0.03b |
| 32 | ar-Turmerone | C15H20O | 26.737 | 1563 | 7.94 ± 0.57ab | 5.47 ± 1.43c | 9.90 ± 1.22a | 6.15 ± 0.56bc | 5.52 ± 1.72c |
| 33 | Turmerone | C15H22O | 26.853 | 1566 | 3.03 ± 0.37c | 3.12 ± 0.62c | 13.31 ± 1.70a | 5.87 ± 0.82b | 5.87 ± 0.57b |
| 34 | (E)-α-Santalal | C15H22O | 27.113 | 1572 | – | 0.22 ± 0.14 | – | – | – |
| 35 | (E, E)-Germacra-3,7(11),9-trien-6-one | C15H22O | 27.46 | 1580 | 0.75 ± 0.10b | 1.84 ± 0.32a | 1.39 ± 0.24a | 1.79 ± 0.10a | 1.62 ± 0.39a |
| 36 | Curlone | C15H22O | 27.588 | 1583 | 2.60 ± 0.26b | 1.69 ± 0.42b | 6.02 ± 0.80a | 2.64 ± 0.25b | 2.56 ± 0.81b |
| 37 | Curdione | C15H24O2 | 27.889 | 1591 | 0.22 ± 0.06 | 0.90 ± 0.25 | – | – | – |
| 38 | Curcumenol | C15H22O2 | 28.116 | 1596 | 0.05 ± 0.02a | 0.26 ± 0.12a | – | 0.05 ± 0.02a | 0.17 ± 0.27a |
| 39 | Bisabolone | C15H24O | 28.48 | 1604 | 0.39 ± 0.04a | 0.21 ± 0.08a | 0.24 ± 0.06a | 0.39 ± 0.02a | 0.46 ± 0.33a |
| 40 |
(E)-Atlantone |
C15H22O |
29.078 |
1616 |
0.25 ± 0.04b |
0.15 ± 0.05c |
0.41 ± 0.05a |
0.16 ± 0.02c |
0.14 ± 0.05c |
| Total identified (%) | 93.34 ± 0.45 | 82.09 ± 1.78 | 86.74 ± 0.58 | 93.64 ± 0.62 | 92.28 ± 1.55 | ||||
| Sesquiterpene hydrocarbons | 74.33 ± 2.08 | 61.61 ± 2.45 | 52.62 ± 4.74 | 74.78 ± 0.87 | 71.57 ± 5.55 | ||||
| Oxygenated sesquiterpenes | 18.67 ± 1.64 | 17.89 ± 3.36 | 33.62 ± 4.19 | 18.28 ± 1.00 | 17.28 ± 4.27 | ||||
| Monoterpene hydrocarbons | – | 0.45 ± 0.25 | – | 0.03 ± 0.01 | 0.06 ± 0.02 | ||||
| Oxygenated Monoterpenes | 0.16 ± 0.04 | 1.48 ± 0.09 | 0.28 ± 0.09 | 0.42 ± 0.07 | 3.25 ± 0.85 | ||||
Relative content (%) is given as means ± SDs (n = 3); The different labelled letters (a, b, c, d) in a row indicted the values are significantly different among the TEOs of different origins (P < 0.05) by Duncan's Multiple Range Test.
RT is the abbreviation for retention time.
Retention indices (RI) represents the retention index obtained using the C10–C25 n-alkane series as the reference in the Rxi-5Sil MS column.
- represents that the component was not retrieved in the corresponding sample.
The major components of the volatile organic compounds in the TEOs of five different origins were quite similar, as demonstrated in Fig. 1 β-Cedrene, ar-Curcumene, and α-Zingiberene represented the main components. However, the corresponding components in the TEOs of five different origins showed significantly different. β-Cedrene, the largest component identified in all five origins of the TEOs except Changting, is a member of the class of the compounds known as sesquiterpenoids. α-Zingiberene, the largest component identified in the TEOs from Changting, is a monocyclic sesquiterpene that is a generally predominant compound of the TEOs from many cultivars of ginger. Moreover, α-Zingiberene has a warm, woody-spicy, and very tenacious odor (Yeh et al., 2014). α-Zingiberene has been reported as a bioactive compound that is efficacious for anticancer (Bou et al., 2013). Furthermore, ar-Curcumene, a type of sesquiterpene, was the second-largest component identified in the TEOs from Guangxi and Yunnan. These components were concentrated in the period after the GC-MS peak, which was tentatively related to the relative amount of components volatility and extraction equilibrium of the extraction head. In addition, other chemical compounds identified in the lower abundance in the TEOs of five different origins were β-Bisabolene, ar-Turmerone, Turmerone, Curlone, and (E)-β-Farnesene.
The results obtained in the present study were different from those of Hwang et al. (2016) (Hwang et al., 2016), in which the major constituents of TEO by GC-MS from Korea were α-Zingiberene (27.70–36.75%), ar-Turmerone (19.54–32.24%), β-Sesquiphellandrene (13.14–18.23%), α-Turmerone (3.72–6.50%), β-Turmerone (2.86–5.60%), and β-Bisabolene (2.50–3.46%). In contrast, Mustapha et al. (2019) (Mustapha et al., 2019) reported that the major compounds in the TEO by GC-MS from Klang, Malaysia were Turmerone (35.46%), Cumene (20.61%), ar-Turmerone (13.82%), Cymene (0.90%) and Curcumene (0.43%). Furthermore, Naveen Kumar, Venkataramana, Allen, Chandranayaka, Murali, and Batra (2019) (Naveen Kumar et al., 2016) reported that ar-Turmerone (53.1%), β-Turmerone (6.42%), α-Turmerone (6.15%), ar-Curcumene (4.81%), β-Phellandrene (4.39%), α-Terpinene (3.28%), and Limonene (3.15%) were as major compounds in the TEO by GC-MS from the Ooty, Tamil Nadu, India. The distinct results could be caused by the TEO harvested from different origins and different measurement methods as well.
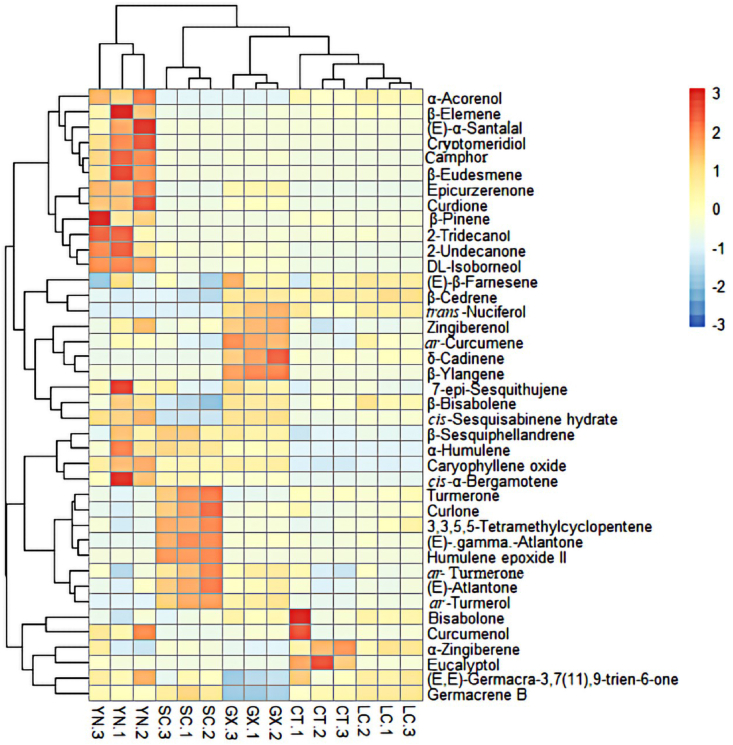
Heat map representing an unsupervised, hierarchical cluster analysis of the TEOs of five different origins. The heat map of volatile organic compounds in the TEOs at the genus level based on relative abundance was shown in Fig. 2. In the comparison of different origins of TEOs, the relative abundance of volatile organic compounds, especially Turmerone, Curlone, 3,3,5,5-Tetramethylcyclopentene, (E)-γ-Atlantone, Humulene epoxide II, ar-Turmerone, (E)-Atlantone and ar-Turmerol in SC were much higher than those in GX, YN, LC, and CT. In addition, β-Eudesmene, Cryptomeridiol, and (E)-α-Santalal were only detected in YN. In GX, the relative amount of volatile organic compounds, trans-Nuciferol, Zingiberenol, ar-Curcumene, δ-Cadinene, and β-Ylangene were higher, whereas, in CT, Bisabolone, α-Zingiberene, and Eucalyptol were higher. The differences in these results could be attributed to the TEOs harvested from different regions since the longitude, latitude, climate, and elevation of these regions are different. The cluster heat map clearly demonstrated the spatial variation of the relative abundance of volatile organic compounds collected from five different origins in the TEOs at the genus level.
Fig. 2.
HCA dendrogram associated with the heat map of the components of TEOs from Yunnan(YN), Sichuan(SC), Guangxi(GX), Changting(CT) and Liangcheng(LC).
To evaluate the quality variation and differentiate the volatile organic compounds in the TEOs of five different origins, the principal component analysis (PCA) was performed based on the normalized relative peak areas of 40 components (Fig. 3). PC1 could separate CT and LC from SC, YN, and GX. The volatile organic compounds Humulene epoxide II, Eucalyptol, α-Zingiberene, and Bisabolone in the TEOs were contributed to the separation of the CT and LC samples. The SC samples were clustered in the positive PC2 cluster, which was contributed by the volatile organic compounds in the following sequence: Curlone, (E)-Atlantone, ar-Turmerone, and Curcumenol (Fig. 3A). PC1 could separate CT and LC from SC, YN, and GX whereas PC3 could allow YN, CT, and LC to be differentiated from SC and GX. Moreover, the YN samples were clustered in the positive PC3 cluster, which was contributed by the volatile organic compounds with the following order: α-Acorenol, cis-α-Bergamotene, 2-Undecanone, and β-Elemene (Fig. 3B). These findings indicated that the volatile organic compounds of TEOs were closely correlated with the five geographic distributions among populations, and the harvest time as well as modes of processing. Additionally, the results in the previous studies have indicated that the volatile organic compounds in the TEOs are related to the employed peak areas and multivariate statistics methods.
Fig. 3.
Principal component analysis (PCA) score plot of the components in the TEOs of five different origins GX, YN, SC, LC, and CT. (A) The PCA score plot of the first two principal components (PC1 and PC2); (B) The PCA score plot of the first and third principal components (PC1 and PC3). GX, YN, SC, LC, and CT represented the TEOs from Guangxi, Yunnan, Sichuan, Liancheng, and Changting.
Furthermore, the taxon-based analysis could reveal the specific key phylotypes of the volatile organic compounds in the TEOs responding to the CT and LC. (Fig. 4). Compared with the CT group, the relative abundances of ar-Curcumene and β-Bisabolene were markedly increased in the GX group, whereas the relative abundances of Turmerone, Eucalyptol, and α-Zingiberene were significantly decreased (Fig. 4A). Compared with the CT group, the relative abundances of Curlone, Turmerone, and ar-Turmerone were notably increased in the SC group, whereas the relative abundances of β-Cedrene, β-Bisabolene, and Eucalyptol were significantly decreased (Fig. 4B). Compared with the CT group, the relative abundance of Epicurzerenone was dramatically increased in the YN group, whereas the relative abundances of β-Cedrene, Turmerone, and Eucalyptol were significantly decreased (Fig. 4C). Compared with the CT group, the relative abundances of cis-Sesquisabinene hydrate and Germacrene B were remarkably increased in the LC group, whereas the relative abundances of Eucalyptol were significantly reduced (Fig. 4D). Compared with the LC group, the relative abundances of ar-Curcumene and ar-Turmerone were strikingly increased in the GX group, whereas the relative abundances of α-Zingiberene and Turmerone were significantly diminished (Fig. 4E). Compared with the LC group, the relative abundance of Turmerone, Curlone, and ar-Turmerone were markedly increased in the SC group, whereas the relative abundances of β-Cedrene, β-Bisabolene, and α-Zingiberene were significantly decreased (Fig. 4F). Compared with the LC group, the relative abundance of Epicurzerenone was particularly increased in the YN group, whereas the relative abundances of β-Cedrene and Turmerone were significantly decreased (Fig. 4G).
Fig. 4.
The extended error bar plot identified the differences of volatile organic compounds in the TEOs among the mean proportions of bacterial taxa. The differences between groups were determined using a Welsh’s t-test, while the Benjamini–Hochberg procedure was used to control the false discovery rate due to multiple tests. The corresponding p values were shown on the right sides. (A) CT (green) versus GX (red); (B) CT (green) versus SC (black); (C) CT (green) versus YN (purple pink); (D) LC (blue) versus CT (green); (E) LC (blue) versus GX (red); (F) LC (blue) versus SC (black); (G)LC (blue) versus YN (purple pink). The confidence intervals were provided to allow for the critical assessment of the biological relevance of the test results. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.3. Antioxidant activity of the TEOs of five different origins
The antioxidant activity of EOs from aromatic plants is mainly attributed to the active compounds present in them. This may be due to the high proportion of the main ingredients, or the presence of small amounts of other ingredients, or the synergy between them(Politeo et al., 2006). In this regard, DPPH has been widely used to evaluate the antioxidant capacity, which would change color from purple to yellow upon the acceptance of electrons/hydrogens, thus indicating the scavenging activity (Feng et al., 2014; H. Singh, Mittal, Kaur, Batish and Kohli, 2009). Avanço et al. (2017) (Avanço et al., 2017) have reported that TEOs demonstrated dose-dependent DPPH-radical-scavenging activity, indicating that the oils could serve as a hydrogen donor antioxidant. As shown in Fig. 5, the scavenging activity was 41.41%, 32.56%, 37.39%, 36.58%, and 24.31% at the concentration of 24 mg/mL for the TEOs of Guangxi, Sichuan, Yunnan, Changting, and Liancheng, respectively. Moreover, the IC50 value was 33.30, 42.5, 35.22, 39.96, and 63.27 mg/mL of TEOs from Guangxi, Sichuan, Yunnan, Liancheng, and Changting, respectively to quench DPPH free radicals (50% inhibition of DPPH free radical activity),which was different from the IC50 value of 10.03 mg/mL reported by Avanço et al. (2017) (Avanço et al., 2017). The scavenging activity of DPPH free radical was the highest in the TEO of Guangxi. The results indicated the significant scavenging activities of DPPH free radical at different concentrations for the TEOs of Guangxi, Sichuan, Yunnan, Changting, and Liancheng. In this case, the TEOs could reduce the concentration of DPPH free radical. The antioxidant activity indicated the curcumin-free TEOs could act as a proton donor and an antioxidant. The results reported here could demonstrate that the TEOs might be considered as the potential natural antioxidants, which could be applied as a part of daily supplements or additives to prevent oxidative stress that causes many degenerative diseases.
Fig. 5.
The DPPH-radical-scavenging activity (%) of the TEOs of Guangxi, Yunnan, Sichuan, Liancheng, and Changting.
4. Conclusions
In summary, the spatial variations of the chemical compositions and antioxidant activity of turmeric (Curcuma longa L.) essential oils (TEOs) harvested from four provinces (i.e., five different origins) of China were investigated. The results indicated that the turmeric growing in China exhibited considerable differences in the chemical compositions of TEOs among different populations, thus indicating spatial variation. Moreover, the TEOs of five different origins demonstrated notably different antioxidant activities, in which the antioxidant activity of the TEOs from Guangxi was superior to that of other sources. Furthermore, TEOs might be considered as a natural antioxidant with potential applications in food and pharmaceutical industries.
CRediT authorship contribution statement
Yueyue Qiang: Conceptualization, Methodology, Investigation, Data curation, Writing – original draft. Ruiru Si: Conceptualization, Validation, Formal analysis, Visualization, Writing – review & editing. Suo Tan: Investigation, Formal analysis. Hang Wei: Investigation, Resources. Biao Huang: Writing – review & editing. Miaohong Wu: Resources. Mengzhu Shi: Resources. Ling Fang: Resources. Jianwei Fu: Conceptualization, Supervision, Project administration, Funding acquisition. Shaoxiao Zeng: Visualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the Public Welfare Project Program of Fujian Province (Grant No. 2019R1002-5 and 2021R1022005), China; the Science and Technology Innovation Team Construction Project of FAAS (Grant No. STIT2017-1-12); the Team Project of Quanzhou City for High-level Talents (Grant No. 2019CT008), Fujian Province, China.
References
- Abu-Izneid T., Rauf A., Shariati M.A., Khalil A.A., Imran M., Rebezov M., Rengasamy K.R.R. Sesquiterpenes and their derivatives-natural anticancer compounds: an update. Pharmacol Res. 2020;161:105165. doi: 10.1016/j.phrs.2020.105165. [DOI] [PubMed] [Google Scholar]
- Afoulous S., Ferhout H., Raoelison E.G., Valentin A., Moukarzel B., Couderc F., Bouajila J. Chemical composition and anticancer, antiinflammatory, antioxidant and antimalarial activities of leaves essential oil of Cedrelopsis grevei. Food Chem Toxicol. 2013;56:352–362. doi: 10.1016/j.fct.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Akinyemi A.J., Faboya O.L., Paul A.A., Olayide I., Faboya O.A., Oluwasola T.A. Nephroprotective effect of essential oils from ginger (Zingiber officinale) and turmeric (Curcuma longa) Rhizomes against cadmium-induced nephrotoxicity in Rats. J Oleo Sci. 2018;67(10):1339–1345. doi: 10.5650/jos.ess18115. [DOI] [PubMed] [Google Scholar]
- Avanço G.B., Ferreira F.D., Bomfim N.S., Santos P.A., Peralta R.M., Brugnari T., Machinski M., Jr. Curcuma longa L. essential oil composition, antioxidant effect, and effect on Fusarium verticillioides and fumonisin production. Food Control. 2017;73:806–813. doi: 10.1016/j.foodcont.2016.09.032. [DOI] [Google Scholar]
- B L.V., Kottarapat J., Ramadasan K. An evaluation of antioxidant, anti-inflammatory, and antinociceptive activities of essential oil from Curcuma longa L. Indian J pharma. 2011;43(5):526–532. doi: 10.4103/0253-7613.84961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bou D.D., Lago J.H., Figueiredo C.R., Matsuo A.L., Guadagnin R.C., Soares M.G., Sartorelli P. Chemical composition and cytotoxicity evaluation of essential oil from leaves of Casearia sylvestris, its main compound alpha-zingiberene and derivatives. Molecules. 2013;18(8):9477–9487. doi: 10.3390/molecules18089477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S.B., Wu L.C., Hsieh Y.C., Wu C.H., Chan Y.J., Chang L.H., Wu C.C. Supercritical carbon dioxide extraction of aromatic turmerone from Curcuma longa Linn. induces apoptosis through reactive oxygen species-triggered intrinsic and extrinsic pathways in human hepatocellular carcinoma HepG2 cells. J Agric Food Chem. 2012;60(38):9620–9630. doi: 10.1021/jf301882b. [DOI] [PubMed] [Google Scholar]
- Commission N.P., National Pharmacopoeia Commission . Volatile Oil Determination Method. China Medical Science Press; Beijing: 2015. Chinese pharmacopoeia; pp. 2300–2301. [Google Scholar]
- Cuendet M., Hostettmann K., Potterat O. Iridoid glucosides with free Radical scavenging properties from fagraea blumei. Helvetica Chimica Acta. 1997;80(4):1144–1152. [Google Scholar]
- Da Porto C., Decorti D. Analysis of the volatile compounds of flowers and essential oils from Lavandula angustifolia cultivated in Northeastern Italy by headspace solid-phase microextraction coupled to gas chromatography-mass spectrometry. Planta Med. 2008;74(2):182–187. doi: 10.1055/s-2008-1034295. [DOI] [PubMed] [Google Scholar]
- Dahham S.S., Tabana Y.M., Iqbal M.A., Ahamed M.B., Ezzat M.O., Majid A.S., Majid A.M. The anticancer, antioxidant and antimicrobial properties of the sesquiterpene beta-caryophyllene from the essential oil of Aquilaria crassna. Molecules. 2015;20(7):11808–11829. doi: 10.3390/molecules200711808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosoky N.S., Satyal P., Setzer W.N. Variations in the volatile compositions of curcuma species. Foods. 2019;8(2) doi: 10.3390/foods8020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosoky N.S., Setzer W.N. Chemical composition and biological activities of essential oils of curcuma species. Nutrients. 2018;10(9) doi: 10.3390/nu10091196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durant A.A., Rodríguez C., Herrera L., Almanza A., Santana A.I., Spadadora C., Gupta M.P. Anti-malarial activity and HS-SPME-GC-MS chemical profiling of Plinia cerrocampanensis leaf essential oil. BioMed Central. 2014;13(1) doi: 10.1186/1475-2875-13-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S., Luo Z., Zhang Y., Zhong Z., Lu B. Phytochemical contents and antioxidant capacities of different parts of two sugarcane (Saccharum officinarum L.) cultivars. Food Chem. 2014;151:452–458. doi: 10.1016/j.foodchem.2013.11.057. [DOI] [PubMed] [Google Scholar]
- Hu Y., Zhang J., Kong W., Zhao G., Yang M. Mechanisms of antifungal and anti-aflatoxigenic properties of essential oil derived from turmeric (Curcuma longa L.) on Aspergillus flavus. Food Chem. 2017;220:1–8. doi: 10.1016/j.foodchem.2016.09.179. [DOI] [PubMed] [Google Scholar]
- Hwang K.-W., Son D., Jo H.-W., Kim C.H., Seong K.C., Moon J.-K. Levels of curcuminoid and essential oil compositions in turmerics (Curcuma longa L.) grown in Korea. Appl. Biol. Chem. 2016;59(2):209–215. doi: 10.1007/s13765-016-0156-9. [DOI] [Google Scholar]
- Joshi J.V., Jagtap S.S., Paradkar P.H., Walwatkar P., Paradkar H.S., Affandi Z.M., Vaidya A.D. Cytologic follow up of Low-grade Squamous Intraepithelial Lesions in Pap smears after integrated treatment with antimicrobials followed by oral turmeric oil extract. J Ayurveda Integr Med. 2016;7(2):109–112. doi: 10.1016/j.jaim.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D., Suh Y., Lee H., Lee Y. Immune activation and antitumor response of ar-turmerone on P388D1 lymphoblast cell implanted tumors. Int J Mol Med. 2013;31(2):386–392. doi: 10.3892/ijmm.2012.1196. [DOI] [PubMed] [Google Scholar]
- Kutti Gounder D., Lingamallu J. Comparison of chemical composition and antioxidant potential of volatile oil from fresh, dried and cured turmeric (Curcuma longa) rhizomes. Industrial Crops and Products. 2012;38:124–131. doi: 10.1016/j.indcrop.2012.01.014. [DOI] [Google Scholar]
- Lekshmi P.C., Arimboor R., Indulekha P.S., Menon A.N. Turmeric (Curcuma longa L.) volatile oil inhibits key enzymes linked to type 2 diabetes. Int J Food Sci Nutr. 2012;63(7):832–834. doi: 10.3109/09637486.2011.607156. [DOI] [PubMed] [Google Scholar]
- Li Z., Lin S., An S., Liu L., Hu Y., Wan L. Preparation, characterization and anti-aflatoxigenic activity of chitosan packaging films incorporated with turmeric essential oil. Int J Biol Macromol. 2019;131:420–434. doi: 10.1016/j.ijbiomac.2019.02.169. [DOI] [PubMed] [Google Scholar]
- Ling J., Wei B., Lv G., Ji H., Li S. Anti-hyperlipidaemic and antioxidant effects of turmeric oil in hyperlipidaemic rats. Food Chemistry. 2012;130(2):229–235. doi: 10.1016/j.foodchem.2011.07.039. [DOI] [Google Scholar]
- Mustapha F.A., Jai J., Nik Raikhan N.H., Sharif Z.I.M., Yusof N.M. Response surface methodology analysis towards biodegradability and antimicrobial activity of biopolymer film containing turmeric oil against Aspergillus Niger. Food Control. 2019;99:106–113. doi: 10.1016/j.foodcont.2018.12.042. [DOI] [Google Scholar]
- Naveen Kumar K., Venkataramana M., Allen J.A., Chandranayaka S., Murali H.S., Batra H.V. Role of Curcuma longa L. essential oil in controlling the growth and zearalenone production of Fusarium graminearum. LWT - Food Sci Technol. 2016;69:522–528. doi: 10.1016/j.lwt.2016.02.005. [DOI] [Google Scholar]
- Oyemitan I.A., Elusiyan C.A., Onifade A.O., Akanmu M.A., Oyedeji A.O., McDonald A.G. Neuropharmacological profile and chemical analysis of fresh rhizome essential oil of Curcuma longa (turmeric) cultivated in Southwest Nigeria. Toxicol Rep. 2017;4:391–398. doi: 10.1016/j.toxrep.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pant P., Sut S., Castagliuolo I., Gandin V., Maggi F., Gyawali R., Dall'Acqua S. Sesquiterpene rich essential oil from Nepalese Bael tree (Aegle marmelos (L.) Correa) as potential antiproliferative agent. Fitoterapia. 2019;138:104266. doi: 10.1016/j.fitote.2019.104266. [DOI] [PubMed] [Google Scholar]
- Politeo O., Jukić M., Miloš M. Chemical composition and antioxidant activity of essential oils of twelve spice plants. Croatica Chemica Acta. 2006;79(4):545–552. [Google Scholar]
- Qin N.Y., Yang F.Q., Wang Y.T., Li S.P. Quantitative determination of eight components in rhizome (Jianghuang) and tuberous root (Yujin) of Curcuma longa using pressurized liquid extraction and gas chromatography-mass spectrometry. J Pharm Biomed Anal. 2007;43(2):486–492. doi: 10.1016/j.jpba.2006.07.034. [DOI] [PubMed] [Google Scholar]
- Sharma S., Dhalsamant K., Tripathy P.P., Manepally R.K. Quality analysis and drying characteristics of turmeric (Curcuma longa L.) dried by hot air and direct solar dryers. Lwt. 2021;138 doi: 10.1016/j.lwt.2020.110687. [DOI] [Google Scholar]
- Shinichi H., Fumiki A., Hozumi T., Hideyuki K., Tozo N., Shinji O., Tatsumasa M. Effects of ingested turmeric oleoresin on glucose and lipid metabolisms in obese diabetic mice: a DNA microarray study. J. Agric. Food Chem. 2006;54(24) doi: 10.1021/jf061788t. [DOI] [PubMed] [Google Scholar]
- Singh G., Kapoor I.P., Singh P., de Heluani C.S., de Lampasona M.P., Catalan C.A. Comparative study of chemical composition and antioxidant activity of fresh and dry rhizomes of turmeric (Curcuma longa Linn.) Food Chem Toxicol. 2010;48(4):1026–1031. doi: 10.1016/j.fct.2010.01.015. [DOI] [PubMed] [Google Scholar]
- Singh H., Mittal S., Kaur S., Batish D., Kohli R. Chemical composition and antioxidant activity of essential oil from residues of Artemisia scoparia. Food Chemistry. 2009;114(2):642–645. doi: 10.1016/j.foodchem.2008.09.101. [DOI] [Google Scholar]
- Singh V., Jain M., Misra A., Khanna V., Rana M., Prakash P., Barthwal M.K. Curcuma oil ameliorates hyperlipidaemia and associated deleterious effects in golden Syrian hamsters. Br J Nutr. 2013;110(3):437–446. doi: 10.1017/S0007114512005363. [DOI] [PubMed] [Google Scholar]
- Stanojevic J., Stanojevic L., Cvetkovic D., Danilovic B. Chemical composition, antioxidant and antimicrobial activity of the turmeric essential oil (curcuma longa L.) Advanced Technologies. 2015;4(2):19–25. doi: 10.5937/savteh1502019S. [DOI] [Google Scholar]
- Toden S., Theiss A.L., Wang X., Goel A. Essential turmeric oils enhance anti-inflammatory efficacy of curcumin in dextran sulfate sodium-induced colitis. Sci Rep. 2017;7(1):814. doi: 10.1038/s41598-017-00812-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosati J.V., Oliveira E.F.d., Oliveira J.V., Nitin N., Monteiro A.R. Light-activated antimicrobial activity of turmeric Residue edible coatings against cross-contamination of Listeria innocua on sausages. Food Control. 2018;80(4) doi: 10.1016/j.foodcont.2017.07.026. [DOI] [Google Scholar]
- Tozo N., Tatsumasa M., Hideyuki K., Misuzu T., Yoshihiro M., Minpei K., Mikio K. Curcuminoids and sesquiterpenoids in turmeric (curcuma longa l.) suppress an increase in blood glucose level in type 2 diabetic kk-ay mice. Journal of Agricultural and Food Chemistry. 2005;53(4):959–963. doi: 10.1021/jf0483873. [DOI] [PubMed] [Google Scholar]
- Tsai S.-Y., Huang S.-J., Chyau C.-C., Tsai C.-H., Weng C.-C., Mau J.-L. Composition and antioxidant properties of essential oils from curcuma Rhizome. Asian J.Arts.Sci. 2011;2(1):57–66. [Google Scholar]
- Xu L.L., Shang Z.P., Lu Y.Y., Li P., Sun L., Guo Q.L., Ye M. Analysis of curcuminoids and volatile components in 160 batches of turmeric samples in China by high-performance liquid chromatography and gas chromatography mass spectrometry. J Pharm Biomed Anal. 2020;188:113465. doi: 10.1016/j.jpba.2020.113465. [DOI] [PubMed] [Google Scholar]
- Yeh H.-y., Chuang C.-h., Chen H.-c., Wan C.-j., Chen T.-l., Lin L.-y. Bioactive components analysis of two various gingers (Zingiber officinale Roscoe) and antioxidant effect of ginger extracts. LWT - Food.Sci.Technol. 2014;55(1):329–334. doi: 10.1016/j.lwt.2013.08.003. [DOI] [Google Scholar]
- Zhang L., Yang Z., Chen F., Su P., Chen D., Pan W., Du Z. Composition and bioactivity assessment of essential oils of Curcuma longa L. collected in China. Industrial Crops and Products. 2017;109:60–73. doi: 10.1016/j.indcrop.2017.08.009. [DOI] [Google Scholar]
- Zhou F., Peng J., Zhao Y., Huang W., Jiang Y., Li M., Lu B. Varietal classification and antioxidant activity prediction of Osmanthus fragrans Lour. flowers using UPLC-PDA/QTOF-MS and multivariable analysis. Food Chem. 2017;217:490–497. doi: 10.1016/j.foodchem.2016.08.125. [DOI] [PubMed] [Google Scholar]