Abstract
Biochar is a carbonized biomass that can be used as a soil amendment. However, the exclusive use of biochar may present some limitations, such as the lack of nutrients. Thus, biochar enrichment techniques have made it possible to obtain biochar-based fertilizers (BCFs), with great potential to improve soil fertility. Nevertheless, there is still a lack of information about the description, advantages, and limitations of the methods used for biochar enrichment. This review provides a comprehensive overview of the production methods of enriched biochar and its performance in agriculture as a soil amendment. Studies demonstrate that the application of BCF is more effective in improving soil properties and crop yields than the exclusive application of pure biochar or other fertilizers. The post-pyrolysis method is the most used technique for enriching biochar. Future studies should focus on understanding the mechanisms of the long-term application of BCFs.
Keywords: Enriched biochar, Organomineral fertilizer, Agriculture, Soil fertility
Enriched biochar; Organomineral fertilizer; Agriculture; Soil fertility.
1. Introduction
Biochars are solid materials, rich in carbon, obtained from the thermochemical decomposition of organic biomasses in an oxygen-limited environment (Lehmann and Joseph, 2015; Kumar et al., 2021). Research on the use of biochar in agriculture has progressed considerably in the last two decades due to its beneficial actions for sustainable agriculture, as biochar improves soil fertility and crop productivity. Furthermore, numerous advantages such as waste management, carbon sequestration, reduction of greenhouse gas emissions, and climate change mitigation have been achieved using biochar (Lehmann et al., 2003; Van Zwieten et al., 2014).
The characteristics of biochars are influenced by the chemical composition of the feedstock and pyrolysis conditions. Biomass with higher lignin content such as eucalyptus, pine bark, sawdust has a higher yield of biochar. Typically, these biochars have an alkaline pH, higher carbon content (C), and higher aromaticity; however, they have a lower ash content and nutrient content such as N, P, K, and Ca (Domingues et al., 2017). On the other hand, biomass with a high content of cellulose and hemicellulose (crop residues) yielded biochar with a high concentration of volatile compounds and nutrients (Tripathi et al., 2016). Biochar produced from sewage sludge (biosolids) has more hydrogen and less carbon when compared to that derived from lignocellulosic biomass. However, this type of biochar has a high nutrient (especially N and P) and heavy metal content (Liu et al., 2018). In general, pyrolysis performed at temperatures above 500 °C produces biochar with high hydrophobicity, surface area, and micropore volume, suitable for absorbing organic pollutants. On the other hand, at temperatures below 500 °C, the produced biochar tends to have more oxygenated functional groups and is more suitable for immobilizing inorganic pollutants (Enaime et al., 2020).
Despite the positive effects of biochar in agricultural systems, there is still no consensus on the "absolute benefits" of biochar application on nutrient cycling. This is because the addition of biochar to soils is not always accompanied by consistent increases in crop productivity, as plant responses may differ (Kizito et al., 2019). One reason for such behaviour is the variety of biomass used in biochar production, which varies in nutrient content and may not meet plant nutrient requirements. The mechanisms of capture and subsequent release of organic or inorganic mineral nutrients from different types of biochar are also not sufficiently understood. In many cases, the high sorption capacity of most biochars can also limit the supply of nutrients to plants as the essential elements bind strongly to the biochar surface, making them unavailable (Kasozi et al., 2010; Bruun et al., 2012). Furthermore, the indiscriminate use of biochars can increase soil toxicity because they may contain heavy metals and negatively interfere with plant growth, primarily by causing nutritional imbalance (Verheijen et al., 2009).
Biochar is considered one of the best organic fertilizers due to its stability and ability to retain nutrients that reduce leaching (Lehmann and Joseph, 2009). However, to properly perform its functions, biochar must possess some specific properties such as: (i) a high mechanical strength that allows greater longevity in soil and resistance to cleavage during handling and storage; (ii) a large surface area that stimulates an increase in the specific surface area of the soil, thereby promoting better water and nutrient storage in sandy soils and better aeration in clay loam soils; (iii) a high amount of macro and micropores that increase aeration and water retention and, finally, (iv) a high nutrient content (Downie et al., 2009; Troeh and Thompson, 2005). Achieving these properties requires special attention in selecting the type of feedstocks, as well as the appropriate temperature for pyrolysis (Bagreev et al., 2001).
Moreover, applying a large amount of biochar is typically necessary to obtain high yields (between 2.5 and 20 tons ha−1) (Jeffery et al., 2011). Considering the high cost of collecting and obtaining the feedstock, as well as the high capital, operating, and maintenance costs of the pyrolysis plant, the cost of biochar in developed countries ranges from US$300 to US$700 per ton (Clare et al., 2014). Consequently, when applied at high rates, the investment may not be profitable for the farmer.
Several studies have been conducted in the last decade to develop techniques for enriching biochar, to overcome the financial and nutritional obstacles of using biochar in agriculture. Several technologies are employed for this purpose. The production of biochar-based fertilizers (BCFs), for example, has been studied as an alternative to enhance biochar's properties and make the final product more complete from the nutritional standpoint. BCFs can be produced through various techniques including direct pyrolysis of nutrient-rich feedstocks, and pre and post-pyrolysis treatments. Despite these advances in research, there are still doubts about the enrichment techniques, the properties, and the effects of enriched biochars as fertilizers and soil amendment.
The objective of this review is to present the most recent studies on the various biochar enrichment processes and the effects of enriched biochars on soil properties and crop productivity.
2. Methodology
A literature search was conducted in the Web of Science database using the keywords "biochar-based" and "fertilizer" for articles published between 1990 and October 2021. The indexing terms were used to search in "title", "abstract", and "keywords" of the articles. The first search performed resulted in 122 papers published. The search was further limited to articles in scientific journals, resulting in 105 papers. From this total, only the articles related to the use of enriched biochar in agriculture were selected. The following criteria were used for the selection: (i) studies that provided information on the methods used for the production of biochar-based fertilizers; (ii) studies that showed the effect of these fertilizers on soil properties; and (iii) studies that showed the effect on plant productivity. Thus, at the end of this search, a total of 45 articles were selected to compose the bibliographic review. The data extracted from each study included: (i) feedstocks; (ii) pyrolysis temperature; (iii) method of production (direct, pre and post-pyrolysis, and re-pyrolysis); (iv) enrichment material (including organic and chemical fertilizers, rock powder, chemicals etc.); and (v) benefits (soil and plant). Regarding the effects of biochar-based fertilizers on soil and plant properties, the following information was extracted from each study: (i) type of experiment (pot, field, laboratory scale and incubation); (ii) type of crop; (iii) soil type; (iv) biochar dose; (v) soil and plant changes.
3. Production of nutrient-enriched biochar-based fertilizer
Several techniques are studied to increase the efficiency of biochar in agriculture, improve the nutrient content and thus reduce the application rates. One of the alternatives is producing nutrient-enriched biochar-based fertilizer. Currently, biochar enrichment methods can be classified into three categories: i) direct treatment method; ii) pre-treatment method; and, iii) post-treatment method. In some cases, after the post-pyrolysis method, other treatments such as re-pyrolysis occur at low temperatures (Chia et al., 2014; Joseph et al., 2015). A scheme showing the sequence of operations in each method is presented in Figure 1.
Figure 1.
Methods of production of biochar-based fertilizers.
3.1. Direct treatment
The direct treatment method involves the pyrolysis of nutrient-rich feedstocks exclusively. In this method, the originally nutrient-rich feedstock undergoes slow pyrolysis, promoting the enrichment of nutrients in the biochar. Besides the quality of the feedstock, the most critical factor in this method is the pyrolysis temperature, which determines the rate of volatilization or concentration of the nutrients. Low temperatures (300–400 °C) are suitable for N enrichment in biochar, while higher temperatures, around 700 °C, are more suitable for P and K enrichment (Biederman and Harpole, 2013).
Various feedstocks can be used to produce nutrient-rich biochar, such as manures, sludge, composts, algae, etc as shown in Table 1. N–P–K enriched biochars were produced from human faecal remains (300–700 °C), swine manure (400–800 °C), and poultry litter (400–600 °C). The percentages of nutrients for the three biochars produced were as follows: N (2.4–4.8, 1.6–3.2, and 2.0–2.8); P (5.4–8.1, 6.1–7.7, and 4.0–5.8); and K (1.9–2.6, 2.7–3.1, and 3.8–5.8), respectively (Tsai et al., 2012; Liu et al., 2014; Subedi et al., 2016). Biochar produced from chlorella-based microalgae residue (generated from cell disruption and dehydration process) contained a high amount of N content (>10%) when processed at 400 °C, and its value decreased with increasing temperature (Chang et al., 2015). Lou et al. (2017) studied biochars derived from mushroom substrate compost rich in Ca (15–18%), K (3–4%), N (4–5%), and P (1–3%). Kannari et al. (2020) produce Ca-enriched biochar from digested sludge derived from food waste. According to this study, Ca concentration of biochar was highest (410 mg g−1) at the pyrolysis temperature of 800 °C.
Table 1.
Different production methods of biochar-based fertilizers.
| Feedstock | Pyrolysis temperature | Method of production | Enrichment material | Benefits | References |
|---|---|---|---|---|---|
| Ca rich digested sludge | 600–900 °C | Direct | Improved P recovery Increase in Ca Increase in plant growth |
Kannari et al. (2020) | |
| Banana peduncle | Thermal plasma processing | Direct | Increase in the available K content Transformation of water-soluble K fraction to exchangeable form |
Karim et al. (2017) | |
| Bacterial biomass waste of Escherichia coli | 600 °C | Direct | Improved the growth of lettuce (Lactuca sativa) in hydroculture Lower concentration of toxic metals |
Kim et al. (2018) | |
| Water hyacinth (Eichhornia crassipes) | 450 °C | Direct | Improved Phosphate recovery on low water Improved nutrient supply potentials of sandy soil Improved fresh biomass yield and nutritional status of maize seedlings |
Mosa et al. (2018) | |
| Maize stalk | 300 °C 450 °C 600 °C |
Pre | Triple superphosphate, diatomite and Urea | Higher amounts of N, Si, P, Ca and higher CEC in the BCF Increased in maize growth and yield Decrease in Cd toxicity |
Chen et al. (2021) |
| Poultry litter (PLB) and coffee husk (CHB) | 500 °C | Pre | Phosphoric acid, magnesium oxide, triple superphosphate (TSP) | Slower P release Increase soil P Increase plant growth |
Carneiro et al. (2021) |
| Dry wheat straw | 400 °C | Pre | Urea, bentonite clay, rock phosphate, Fe2O3 and FeSO4.7H2O | Increase in soil Eh (potential difference) Increase the Eh between the rhizosphere and the root membrane Increase of plant growth and microbial population |
Chew et al. (2020) |
| Wastes of peanut shell and sugarcane bagasse | 450–800 °C | Pre | MgCl2 and CaCl2 | Increase P adsorption Increase in plant nutrients and reduced metal (loid)s. |
Fang et al. (2019) |
| Pelletized Poultry manure | 300 °C | Pre | 5% triple superphosphate | Reduced Fe, Zn, Cu and Mn in soils. Increased N, P and K uptake Lower Fe, Zn, Cu and Mn concentrations in plants Increase plant growth reduced Ca and Mg concentrations in leaf |
Gunes et al. (2015) |
| Corn straw | 500 °C | Pre | MgCl2 solution | Slow release of nitrate and ammonium Improve soil moisture retention Improve maize growth and nitrogen use efficiency |
Khajavi-Shojaei et al. (2020) |
| Poultry litter | 500 °C | Pre | Triple superphosphate (TSP), phosphoric acid (H3PO4) magnesium oxide (MgO) | Promoted the accumulation of both labile and moderately labile P Higher biomass yield of Marandu grass |
Lustosa Filho et al. (2019) |
| Poutry litter, pig manure and sewage sludge | 500 °C | Pre | Magnesium chloride (MgCl2) | Promoted P adsorption from aqueous solution increase plant yields | Nardis et al. (2020) |
| Maize residue | 450 °C | Pre | Dorowa phosphate rock (DPR) | Increase in P and N and C content | Tumbure et al. (2020) |
| Cotton Straw (CS) | 500 °C | Co-pyrolysis under N2 | Mg3(PO4)2 and bentonite Coating with sodium alginate (NaAlg), starch, cellulose, chitosan and maltodextrin |
Slower release of nutrients superior degradability | An et al. (2020) |
| Cotton straw | 700 °C | Co pyrolysis under microwave irradiation | K3PO4 and bentonite | Better P and K slow-release | An at al. (2020) |
| Palm leaf waste | 600 °C | Post | Solution containing 200 mg of P per L | Immobilization of heavy metals Increase in soil P Improve maize growth |
Ahmad et al. (2018) |
| Rice husk | 500 °C | Post | Urea–hydrogen peroxide (UHP) | Increase Cd adsorption Slower release of N |
Chen et al. (2018) |
| Wheat | 500 °C | Post | Iron chloride (FeCl3) and iron sulphate (FeSO4) | Reduced Cd toxicity in plants Immobilization of Cd from polluted soils |
Dad et al. (2021) |
| Rice straw | 500 °C | Post | Bentonite, humic acid and fertilizer (15% N, 15% P2O5, 15% K2O) Coating with starch |
Decreased N leaching Slower release of N Improve rice nutrients uptake |
Dong et al. (2020) |
| Poultry litter Bamboo |
450 600 and 450 °C |
Post | Organic fertilizers, oxides and iron sulphate Organic fertilzers, clay material, barley straw ash and magnetite |
Increased soil ORP, P, K and Ca availability Increase total plant uptake of P, K, Ca, Al and Cu. Increased ginger plant growth |
Farrar et al. (2019) |
| Sawdust | 500 °C | Post | NPK nutrient solution (NH4NO3, KH2PO4, SSP) | Lower NO3-, PO43-, and K release Improved water retention of soils |
Gwenzi et al. (2018) |
| Straw and wheat shell | Post | Struvite (MgNH4PO4·6H2O) | Longer nutrients release cycle | Hu et al. (2019) | |
|
Acacia saligna feedstock Jarrah sawdust |
380 °C 600 °C |
Post | Clay, chicken manure and minerals (Iron bearing kaolinite, calcium carbonate, rock phosphate, manganese sulfate and ilmenite) | Improved growth, nutrients uptake and mycorrhizal colonization | Joseph et al. (2015) |
| Corn cob Wood (fig trees) |
600 °C | Post | Anaerobic digestate | Increase in soil nutrients and soil organic matter Increase plant growth |
Kizito et al. (2019) |
| Biochar based compound fertilizer | Post | EM -bokashi | Increase photosynthetic characteristics and chlorophyll content of tobacco plant | Li et al. (2020) | |
| fluecured tobacco stems |
Post | Compound fertilizer | Decrease in N and K leaching loss Increased the immobilization of NH4+-N, available P, and available K in the soil profile |
Li et al. (2019) | |
| Oilseed rape straws | 400 °C | Post | Polyvinyl alcohol, bentonite, | Bacterial community groups with higher nutrient metabolic cycling ability during harvest stage Increased nitrification and reduced denitrification |
Liao et al. (2020) |
| Oilseed rape straws | 400 °C | Post | Urea, bentonite, polyvinyl alcohol | Improved nutrient release characteristic Improved soil moisture retention |
Liu et al. (2019) |
| Jarrah sawdust | 600 °C | Post | Chicken litter, clays and minerals | High concentrations of plant available P Reduced N loss |
Lin et al. (2013) |
| Grape pruning | 400 °C | Post | Rock phosphate and organic cow manure | Reduced the sodium concentration of the soil increased the nitrifying bacteria frequency, basal respiration, total nitrogen, organic carbon, phosphorous, potassium, iron, zinc, and copper concentrations | Moradi et al. (2019) |
| Orange peel, residual wood, water-treatment sludge | 300–700 °C | Post | Anaerobically digested slurry | Slow release of K, Ca, and Mg | Oh et al. (2014) |
| Eucalyptus wood | 400 °C | Post | Additives (22% bentonite and 5% pregelatinized maize flour), and Urea | Higher nitrogen use efficiency, Promoted soil C sequestration that led to lower gas emission, higher maize yield |
Puga et al. (2020) |
| Eupatorium adenophorum and crop residues | 650–720 °C | Post | Cow urine, NPK fertilizer and compost | Higher crop yields | Schmidt et al. (2017) |
| Urban Green waste | 450–550 °C | Post | Urea, clay minerals of bentonite and sepiolite | Slower release of N and increase in maize growth Reduced N leaching |
Shi et al. (2020) |
| Agricultural waste | 600 °C | Post | Burkholderia sp. strain L2 and Bacillus megaterium strain A30 | Higher cfu count and maximum viability for strain L2 (107 cfu g−1) at 240 days of storage Enhanced the productivity of tomato plant and increased soil fertility |
Tripti et al. (2017) |
| Wheat straw | 350–400 °C | Post | Di-ammonium phosphate (DAP) | Improved soil properties and plants yield | Wali et al. (2020) |
Some feedstocks stand out because of their high concentration of a particular nutrient. Zwetsloot et al. (2016) produced P-rich biochars from bone waste, with P contents ranging from 12.7% when processed at 350 °C to 15.3% in biochar obtained at 750 °C Ma and Matsunaka (2013) obtained biochar (450 °C) with a total P of 10% from dairy cattle carcasses (mixture of skin, meat, and bone). P-enriched biochar was also produced by pyrolysis of bacterial biomass waste from Escherichia coli, and P concentration was 84.7 mg g−1, approximately 11 times higher than that of the original biomass (Kim et al., 2018). According to the authors, the large amount of P may be due to the K2HPO4 and KH2PO4 used in the culture medium and P released from the decomposition of the biomass cells.
Karim et al. (2017) evaluated the enrichment of K in biochars produced through the thermal processing of banana peduncle biomass with different gases (oxygen and argon) and at different processing times (3, 5, 7, and 9 min). They concluded that the available K content of banana peduncle (66.3 g/kg) increased to 86.2, 163.5, and 258.5 g/kg in biochar produced by argon plasma processing with residence times of 3, 5, and 7 min, respectively. Furthermore, Mosa et al. (2018) produced functionalized biochars with high phosphate recovery potential from hyacinth plants (Eichhornia crassipes) grown in synthetic contaminated water spike either with Fe2+ (Fe–B), Mn2+ (Mn–B), Zn2+ (Zn–B) or Cu2+ (Cu–B). The in-situ functionalization of the biochars was confirmed by physicochemical analyses which showed the formation of organo-mineral complexes on the biochar matrix. This led to an increase in their specific surface area, the number of positive functional groups, and consequently an increase in the anion exchange capacity (AEC). As such, the functionalized biochar showed a better nutrient supply than the unfunctionalized forms (Mosa et al., 2018).
3.2. Pre-treatment method
In the pre-treatment method, the feedstock is treated with nutrient-rich materials (e.g., nutrient-rich minerals, soluble mineral fertilizers, waste from the fertilizer industry, animal waste, etc.) before undergoing pyrolysis. This procedure enables the addition of one or more nutrients in biochar using concentrated mineral and nutrient sources. Moreover, these materials also help improve biochar properties such as heavy metal stabilization capacity and moisture retention, making them more functional (Table 1).
P-enriched biochar was produced by the slow pyrolysis (600 °C) of a mixture of sawdust and grass biomass (Panicum virgatum) with phosphate fertilizer (bone meal and triple superphosphate). Besides the high P concentration and high carbon retention, the biochar enhanced heavy metal stabilization in soils (Zhao et al., 2016). Biochar rich in K and S was obtained through thermal plasma processing of a mixture of banana peduncle and phosphogypsum for 7 min. The biochar contained high amounts of K (4.2–12.7%) and S (13.3–17.8%) in the form of potassium sulfate, which is highly soluble in water, indicating the rapid release of K and S from the biochars for further uptake by plants (Karim et al., 2019). This biochar also showed reduced bioavailable fractions of toxic contaminants such as fluoride, cadmium, lead, etc.
A biochar-based fertilizer was produced by the pyrolysis of a mixture of wheat straw with urea, bentonite clay, phosphate rock, Fe2O3, and FeSO4.7H2O. The resulting biochar exhibited the following elemental composition: C, 43%; N, 27%, K, 2%; P, 2.5% (Chew et al., 2020). Carneiro et al. (2021) produced P-enriched biochar from chicken litter and coffee husk enriched with phosphoric acid and magnesium oxide combined with triple superphosphate. The kinetic study showed that the biochar-based fertilizer acted as a slow-release fertilizer, releasing 10% of its total P content in the first hour, whereas the triple superphosphate released up to 90%. Fang et al. (2019) produced Mg/Ca modified biochar by the pyrolysis (450, 700, and 800 °C) of peanut shell and sugarcane bagasse pre-treated with MgCl2 and CaCl2 solutions. The results indicated that Mg-modified sugarcane bagasse biochar, pyrolyzed at 700 °C, showed a rapid P absorption due to its positively charged surface, high specific surface area (1440 m2/g), and porous structure.
Nardis et al. (2020) produced biochars enriched with Mg from poultry litter (PLB), pig manure (PMB), and sewage sludge (SSB) impregnated with a MgCl2 solution, to reach approximately 10% of Mg in the biochars. The biochars showed a high P adsorption capacity of 34.5, 68.0, and 28.1 mg g−1 for PLB-Mg, PMB-Mg, and SSB-Mg, respectively, and the P-loaded forms promoted plant growth and accumulation of P in maize. Similarly, a slow-release fertilizer was produced by the pyrolysis at 500 °C of maize straw impregnated with a solution of MgCl2. The addition of MgCl2 to the biomass improved biochar properties with higher specific surface area, higher CEC and AEC, higher alkalinity, and higher water absorption (Khajavi-Shojaei et al., 2020).
Tumbure et al. (2020) added samples of non-reactive Dorowa phosphate rock (DPR) from Zimbabwe to maize residues (stems and leaves) in the ratios of 1:2, 1:4, 1:6, and 1:8, which was later pyrolyzed at 450 °C for 30 or 60 min to produce a set of biochar-based fertilizers (BBFs). At a residence time of 60 min, the 1:4, 1:6, and 1:8 mixtures produced biochars with higher yield (25%), higher carbon retention (43%), and higher N content (26%), as well as higher alkalinity compared to pyrolysis of maize residue alone. The biochar-based fertilizers also showed a higher soluble P content and consequently higher P supply to the maize plants. A novel biochar-based compound fertilizer (BCF) was synthetized with maize straw biomass, diatomite, triple superphosphate and urea at different temperatures (300 °C, 400 °C and 600 °C) and mixture proportions (Chen et al., 2021). The BCF contained higher amounts of N, Si, P, Ca and a higher CEC than the biochar alone. In addition, the BCF had more acidic functional groups than the biochar at the same pyrolysis temperature. Moreover, the authors observed an increase in pH and a decrease in yield of the BCF with an increase of pyrolysis temperature. As well as the increase in maize growth and productivity, the BCF reduced Cd toxicity in both the soil and maize plant.
3.3. Post-treatment method
In the post-treatment method, biochars are treated with a nutrient-rich source, such as soluble mineral fertilizers, clays, ground rock, composts, wastewater, etc., after the pyrolysis process either room temperature or at temperature-regulated conditions to prepare nutrient-enriched biochar-based fertilizers. Overall, about 60% of the studies on biochar enrichment used the post-pyrolysis technique (Table 1). Several biochar-based slow-release fertilizers have been produced by mixing biochars from different feedstocks with clays, mineral rocks, bentonite, chicken manure, and other nutrient sources (Lin et al., 2013; Joseph et al., 2015; Yao et al., 2015; Ye et al., 2016; Farrar et al., 2019). The resulting products enhanced properties such as higher nutrient contents, porosity, and CEC; increase in the number of functional groups, pH, surface area, and labile carbon. This results in the improvement of soil physical, chemical, and biological properties and, accordingly, increased plant growth and productivity.
Several techniques have been used to produce enriched biochars, with emphasis on the production of granules and pellets. Shi et al. (2020) obtained a granular biochar-mineral urea composite (Bio-MUC) by blending urea, bentonite, and sepiolite with green waste biochar. Microscopic analysis of the composite revealed the binding of N particles from the urea to the surface of the biochar and clay minerals. The Bio-MUC showed a slower release of N and dissolved organic carbon (DOC) of 70% and 8%, respectively, compared to urea. Sulphur-enriched biochar (Sulfachar) was prepared by passing hydrogen sulfide gas emitted from a landfill through a packed column containing biochar derived from anaerobically digested solid dairy manure (Zhang et al., 2017). Sulfachar contains a large amount (36.5%) of sulphur and a higher concentration of Ca, K, Mg, Mn, Cu, Fe, and P than unfertilized biochar. The nutrients were in the plant-available form; and uptake of S and other nutrients by the maize crop increased with sulfachar-amended soils.
Enriched biochars can be produced for specific purposes, such as the retention of heavy metals from the soil. Dad et al. (2021) produced iron-enriched biochar by treating wheat biochar with FeCl3 and FeSO4. This biochar decreased cadmium (Cd) toxicity in radish plants and Cd-contaminated soils. Similar results were obtained by Chen et al. (2018), who developed a novel biochar-based fertilizer that not only immobilized cadmium in soils but also showed a slower release of nitrogen. The fertilizer was produced by treating rice husk biochar with urea hydrogen peroxide (UHP) solution. The C/N ratio of this fertilizer (10.2) was significantly lower than that of the untreated biochar (81.2), indicating that the N content in the UHP adhered to the porous structure of the biochar and helped reduce nitrogen loss from the soil. The mixture of NPK fertilizer, biochar, and other substances, such as bentonite and humic acid, resulted in biochar-based fertilizers with a longer nutrient release cycle and therefore reduced nutrient loss through leaching (Gwenzi et al., 2018; Dong et al., 2020).
Another advantage of post-treatment methods is the possibility of controlling the type and amount of specific nutrients which will enrich the fertilizer. Ahmad et al. (2018) developed a P-enriched biochar from date palm leaf waste biochar treated with a solution containing 200 mg P L−1. Although there was a reduction in the pH value (from 10.23 in biochar to 7.04 in P-enriched biochar) and CEC (from 39.86 cmol kg−1 in biochar to 31.29 cmol kg−1 in P-enriched biochar), the P-enriched biochar had almost four times higher total P than non-enriched biochar. Hu et al. (2019) produced a biochar/struvite composite from the mixture of wheat straw biochar and a struvite solution, containing Na3PO4 (0.10 mol), MgCl2 6H2O (0.10 mol), and NH4Cl (0.10 mol). The recovery rates of N, P, and Mg in the solution were 99.02%, 97.23%, and 95.22%, respectively, forming the compound with the following element contents: C (8.32%); O (40.23%); N (8.30%); Mg (13.40%) and P (15.23%). Besides the high nutrient content, the biochar/struvite composite showed a longer cycle of nutrient release into the soil than struvite. Biochar-based fertilizers were produced by mixing biochar with NPK fertilizer (N: P2O5:K2O 9:11:18) at the ratios of 0%, 3%, 9%, and 15%. The nutrient contents of these fertilizers were: N (95.29, 92.1, 92.76 and 92.99 g kg−1); P (116.1, 117.1, 105.6 and 108.6 g kg−1) and K (224.4, 221.3, 219.8 and 220.1 g kg−1), respectively (Li et al., 2019). Upon application to the soil, there was a reduction in the leaching of N and K and, with that, an increase in the concentrations of these nutrients, and also P in soil.
To investigate the potential use of agricultural and industrial wastes as carrier materials, Tripti et al. (2017) prepared biofertilizers by inoculating two plant growth promoting (PGP) bacteria, Burkholderia sp. strain L2 and Bacillus megaterium strain A30, on agricultural waste biochar and Flyash (SiO2 + Al2O3 + Fe2O3 > 70% with low Sulphur content). The biochar-based biofertilizer showed a high cfu count (107 cfu g−1) and maximum viability for strain L2 at 240 days of storage, indicating that biochar is a better carrier for PGP bacteria. In addition, the application of the biofertilizer prepared from biochar and Burkholderia sp. strain L2 increased seed germination, promoted tomato growth and yield, and improved soil properties and dehydrogenase activity.
4. Effects of biochar-based fertilizers on soil chemical properties
Plant growth and productivity are closely associated with soil chemical properties, such as pH, cation exchange capacity (CEC), organic matter content, and nutrient availability. Based on this premise, it is believed that organo-mineral fertilizers derived from biochars can improve soil chemical properties. They may contain a high concentration of nutrients, a high amount of organic carbon present in various aromatic structures, and a large number of functional groups on their surface. Overall, 100% of the studies evaluated here showed some benefit to the soil, plant, or both (Table 2). In the following sections, the effects of the application of enriched biochar fertilizer on different soil chemical properties and the mechanisms involved in nutrient availability will be discussed.
Table 2.
Effects of biochar-based fertilizers on soil and plant properties.
| Feedstock | Method | Type of study | Crop | Soil type | Biochar dose | Soil properties | Plant properties | References |
|---|---|---|---|---|---|---|---|---|
| Maize straw biochar | Post | Microwell bioessay Pot experiment |
Tomato (Solanum lycopersicum) | Sandy loam | 10 g kg−1 | Superior control of M. incognita Significant increase in plant growth |
Abdelnabby et al. (2018) | |
| Palm leaf waste biochar | Post | Pot experiment | Maize (Zea mays L.) | 5, 10, 20, 30 g kg−1 | Increase soil available P Decrease soil labile heavy metal heavy metals in exchangeable and reducible fractions were transformed to more stable fraction |
enhanced plant growth parameters (shoot and root lengths and dry matter) and uptake of P |
Ahmad et al. (2018) | |
| Cotton straw (CS) | Co-pyrolysis | Pot experiment | Pepper (Capiscum spp.) | Grey desert soil | 3g of CSRFs per 200g of pepper seeds | Leaching loss of P reduced | Promoted pepper seedling growth (root length, fresh weight and dry Weight, height) |
An et al. (2020) |
| Acacia saligna (AS) biochar | Post | Glasshouse experiment Field experiment |
Wheat (Triticum aestivum) Sorghum (Sorghum bicolor) |
Tenosol | 5 t ha−1 300 kg ha−1 |
Increased mycorrhizal colinisation | Increased plant growth and nutrient uptake | Blackwell et al. (2015) |
| Poultry litter (PLB) and coffee husk (CHB) | Pre | Pot experiment | Maize (Zea mays L.) Grass Beans (Phaseolus vulgaris) |
Oxisols | Equivalence of 240 mg kg−1 of P | Increase soil P content | Increase in crop yields Better plant P uptake |
Carneiro et al. (2021) |
| Rice husk biochar | Post | Leaching and pot experiment | Cabbage (Brassica oleracea var capitata) | 2% w/w of soil dry biomass | Increase in plant growth | Chen et al. (2018) | ||
| Maize straw biochar | Pre | Incubation experiment Field experiment |
Maize (Zea mays L.) | Eutyic Cambisols | 450 kg ha−1 900 kg ha−1 1800 kg ha−1 |
Reduction of up to 44.13% of Cd toxicity Improvement of soil fertility |
Increase in maize growth and yield Reduction of Cd contents in maize grains |
Chen et al. (2021) |
| Dry wheat straw | Pre | Bags experiment | Rice (Oryza sativa L.) | Clay loam soil | 0.25% (g·g−1 soil) | Increased soil Eh by 85 mV and Increased the potential difference between the rhizosphere soil and the root membrane by 65mV increased abundance of plant-growth promoting bacteria and fungi in the rhizosphere | Increase of plant biomass (by 67%), herbage N (by 40%) and P (by 46%) uptake | Chew et al. (2020) |
| Wheat biochar iron chloride (FeCl3) and iron sulphate (FeSO4) |
Post | Pot experiment | Raddish (Raphanus sativus L.) | Clay loamy soil | Increased Cd immobilization | Increased plant biomass, photosynthetic pigments, nutrient uptake, osmolyte concentration and antioxidant defense system Decreased cell membrane permeability |
Dad et al. (2021) | |
| Rice straw biochar | Post | Leaching and field scale experiment | Rice (Oryza sativa L.) | 164 kg ha−1 | Reduced N losses | No significant change in rice yield | Dong et al. (2020) | |
| Rice straw biochar | Post | Field experiment | Rice (Oryza sativa L.) | Clay loam Ultisol | 750 kg ha−1 1500 kg ha−1 2700 kg ha−1 |
Reduced CH4 emission by 33.4% | Dong et al. (2021) | |
| Poultry litter and bamboo biochar | Post | Pot experiment | Ginger (Zingiber officinale Canton) | Black dermosols | 7.5 t ha−1 | increased soil P, K, Mg and Ca. increased soil ORP (oxidation reduction potential) |
Increased yields at high application rate Increased foliar nutrients concentrations Increased total plant uptake of P, K, Ca, Al and Cu Produced more wet and dry plant biomass |
Farrar et al. (2019) |
| Maize residues | Post | Field experiment | Peanut (Arachis hypogaea) | Brown earth soils (Cambisols) | 750 kg ha− 1 | Change in soil pH, resulting in a significant increase in bacterial abundance Increase in relative abundance of Acidobacteria phyla which promoted the dissolution of some insoluble phosphorus compounds and improved the availability of P |
Gao et al. (2021) | |
| Pelletized Poultry manure | Pre | Pot Experiment | Lettuce (Lactuca sativa L. cv. Yedikule) | 10 g kg−1 | Increased N, P and K uptake Lower Fe, Zn, Cu, Ca, Mg and Mn concentrations in leaves Increase plant growth |
Gunes et al. (2015) | ||
| Sawdust biochar | Post | Laboratory (leaching) experiment | Higher water retention capacity Reduce nutrient leaching |
Gwenzi et al. (2018) | ||||
| Vinasse biochar | Post | Pot experimente | Oilseed rape (Brassica napus) | Reduced nitrate leaching Increased N concentration Increased NH3 volatilization |
Increased Nitrogen use efficiency (NUE) by oilseed rape | Jia et al. (2021) | ||
| Acacia saligna and Jarrah sawdust biochar | Post | Pot experiment | Wheat (Triticum aestivumL. var. 'Wyalkatchem') | Clay Loam soil | 100 g ha−1 200 g ha−1 |
Increased Mycorrhizal colonization at low rates Increased available P |
Increased plant growth Increased P and N uptake |
Joseph et al. (2015) |
| Ca rich digested sludge | Direct | Pot experiment | Japanese mustard spinarch (Brassica rapa var. perviridis) | Alluvial soils | Improved plant growth | Kannari et al. (2020) | ||
| Corn straw | Pre | Laboratory scale and pot experiment | Maize (Zea mays L.) | Haplocalcids (calcareous soils) | 250 kg N ha−1 | Increased soil N and water retention Reduced nutrient loss |
Improved growth | Khajavi-Shojaei et al. (2020) |
| Corn cob Wood (fig trees) |
Post | Pot experiment | Maize (Zea mays L.) | Clay loam soil | Higher soil organic matter (232%–514%) and macronutrients (110%–230%) and micronutrients Increased soil pH, EC and CEC Increase content of metalloids in soils |
Higher biomass yield of maize when compared to non-enriched biochar slightly lower than yields from chemical fertilizer reduced uptake of heavy metals |
Kizito et al. (2019) | |
| Biochar based compound fertilizer and EM bokashi | Post | Pot experiment | Tobacco (Nicotian tobacco ME.) | Yellow brown soil | 0, 100, 300, and 600 g pot−1 | Increase in net photosynthetic rate (Pn), stomatal conductance (gs), intercellular CO2 concentration (Ci), transpiration rate (Tr), and soil plant analysis development (SPAD) | Li et al. (2020) | |
| Oilseed rape straws | Post | Pot experiment | Oilseed rape (Brassica napus L.) | 200 kg ha−1 of N | Improved soil NO3−Improved microbial activity shifts bacterial community composition toward groups with high nutrient metabolic cycling ability | Increase yield (∼16.6%) and nitrogen-use efficiency NUE (∼58.79%) of rape Increase N uptake Increased plant biomass during the bolting, flowering, and harvest stages |
Liao et al. (2020) | |
| Corn stalk | Pre | Leaching experiment Pot experiment |
Maize (Zea mays L.) | Release rates of N and P 7 and 6 times lower than that of chemical fertilizer | Promoted corn growth and enhanced nutrient uptake | Luo et al. (2021) | ||
| Poultry litter | Pre | Pot experiment | Marandu grass (Urochloa brizantha cv. Marandu) | Oxisols | 25, 50, 100, 150 and 200 mg kg−1 | Increase in the labile and moderately labile P fractions | Promoted higher biomass yields in subsequent cycles | Lustosa Filho et al. (2020) |
| Poultry litter | Pre | Pot experiment | Maize (Zea mays L.) | Oxisols | 25, 50, 100, 150 and 200 mg kg−1 | Decreased water-soluble P, thereby causing a slow-release of P Improved soil pH and Mg |
Dry biomass yields equivalent to TSP treatments | Lustosa Filho et al. (2019) |
| Water hyacinth (Eichhornia crassipes) | Direct | Bioassay of early-growth seedlings | Maize (Zea mays L.) | Sandy soil | Increase in water holding capacity, anion exchange capacity (AEC) and cation exchange capacity (CEC) from the P laden biochar, increase in soil nutrient supply | Increase in biomass yield Excessive micronutrient content in maize seedlings |
Mosa et al. (2018) | |
| Poutry litter, pig manure and sewage sludge | Pre | Pot experiment | Maize (Zea mays L.) | Oxisol | 200 mg kg−1 of P, | Promoted plant growth and accumulation of P in maize Increase in P and Mg uptake |
Nardis et al. (2020) | |
| Orange peel, residual wood, water-treatment sludge | Post | Pot experiment | Lettuce (Lactuca satuva L.) | 34.2 t ha−1 | Improve water retention capacity | Lower yields than commercial fertilizer | Oh et al. (2014) | |
| Eucalyptus wood | Post | Field experiment | Maize (Zea mays L.) | Oxisol | 80 kg N ha−1 | Lower greenhouse gas emission (14%) | Average maize yield 26% higher than Urea Higher nitrogen use efficiency (12%), |
Puga et al. (2020) |
| Eupatorium adenophorum and crop residues | Post | Field experiment | 13 different crops | Silt loam soils | 0·5 and 2 t ha−1 | Higher plant yields (20%–123%) | Schmidt et al. (2017) | |
| Corn-stover derived biochar | Post | Greenhouse experiment | Spinach (Spinacia oleracea) | Entisol | Increased soil P by ∼72% | Increased P, N, K, protein, absorbic acid and yield by ∼29, 52, 33, 20, 21 and 25 respectively. | Sepulveda-Cadavid et al. (2021) | |
| Urban Green waste | Post | Pot experiment | Maize (Zea mays L.) | 2.85 g per 5 kg of soil | Improved carbon retention | Improved maize growth and increased N in the maize plant Increase in N use efficiency |
Shi et al. (2020) | |
| Wheat straw | Post | Incubation and pot experiment | Chickpea (Cicer arietinum L.) | Calciagrid | (0%, 25%, 50%, and 100%) of recommended P (60 kg ha-1) | Increase in soil extractable P, total N and soil organic matter. Increase in soil electrical conductivity, reduction in pH. |
Improved crop growth, yield, nodulation, plant physiological and chemical parameters | Wali et al. (2020) |
| Rice straw | Pre | Pot experiment | Perennial ryegrass (Lolium perene) | Silt loam | 5 t ha−1 22.5 t ha−1 |
Increased tissue Si content Reduced gray leaf spot (M. oryzae) Improved plant growth |
Wang et al. (2019) | |
| Residue of spent mushroom substrate biochar | post | Field experiment | Tea (Camellia sinensis L.) | Ultisols | 2590 kg ha−1 | Increased soil bacterial and fungal diversity Increased soil pH by 0.27 Increased soil organic matter (OM), total nitrogen (TN), Dissolved organic carbon (DOC), dissolved organic nitrogen (DON), exchangeable K, Ca, Mg and Mn. |
Increased the yield, 100-sproutweight and sprout density of tea by 39.2%, 26.6% and 10.7%, respectively Increased amino acid content of tea by 28.1% |
Yang et al. (2021) |
4.1. Effect on nutrient availability
Biochar-based fertilizers contain macro and micronutrients in concentrations that vary depending on the feedstock, the minerals, the materials used for enrichment, and the pyrolysis conditions (Table 2). As such, their application to soils increases the concentrations and availability of nutrients for plants. For example, a significant increase in the fractions of labile P in the soil was observed after applying P-enriched biochars (Ahmad et al., 2018; Lustosa Filho et al., 2020; Carneiro et al., 2021). The addition of wood and maize cob biochar enriched with anaerobic digestate increased the macronutrient contents (110%–230%) compared with the unenriched biochars and control treatments (Kizito et al., 2019).
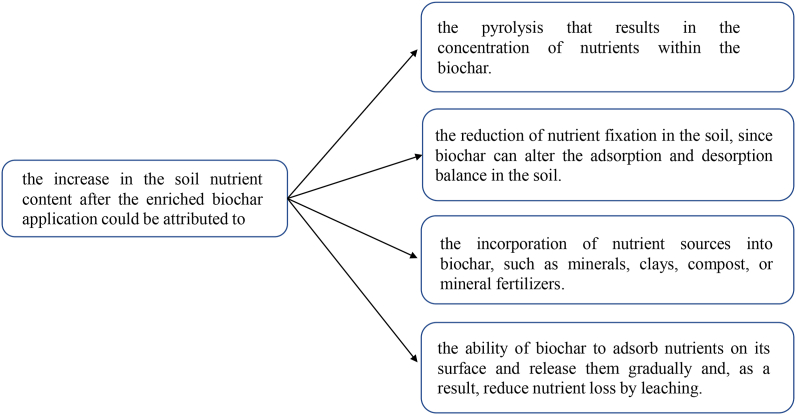
Farrar et al. (2019) also found an increase in soil Mg and Ca concentrations in the initial period (week 14) upon applying an organomineral biochar fertilizer at a dose of 7.5 t ha−1. However, soil P and K concentrations increased throughout the experiment, being highest at week 30. In the study conducted by Mosa et al. (2018), an increase in soil P and K contents were noted when treated with a functionalized biochar derived from water hyacinth (Eichhornia crassipes), grown in synthetically contaminated water spike with heavy metals. Wali et al. (2020) found that the applying a P-enriched biochar increases soil P and N content. Following these studies, the increase in the soil nutrient content could be attributed to multiple aspects, as shown in Figure 2.
Figure 2.
Enriched biochar characteristics responsible for the increase in the soil nutrient contents.
The increase in the soil's nutrient content could be attributed to: (i) the pyrolysis that results in the concentration of nutrients within the biochar; (ii) the reduction of nutrient fixation in the soil, since biochar can alter the adsorption and desorption balance in the soil (Soinne et al., 2014; Gao et al., 2016); (iii) the incorporation of nutrient sources into biochar, such as minerals, clays, compost, or mineral fertilizers; (iv) the ability of biochar to adsorb nutrients on its surface and release them gradually and, as a result, reduce nutrient loss by leaching (Kizito et al., 2019; Wali et al., 2020).
4.2. Effect on soil organic carbon
Besides the high nutrient content, biochar fertilizers are composed of a significant fraction of carbon that is highly resistant to decomposition due to the formation of condensed aromatic compounds during the pyrolysis of biomass. When added to the soil, unlike soluble mineral fertilizers, biochar-based fertilizers promote the incorporation of carbon into the soil and thus increase carbon sequestration (Puga et al., 2020; Zhang et al., 2021). Furthermore, because of their highly recalcitrant nature, biochar-based fertilizers can remain in the soil for an extended period and thus promotes the accumulation of soil organic matter (SOM). This accumulation results from the absorption of SOM on the biochar surface, which acts as a protection against microbial decomposition (Cross and Sohi, 2011). Another factor contributing to the increase in soil organic carbon (SOC) is the increased mineralization of SOM (priming effect) due to the stimulation of microbial activity.
Studies have shown that biochar-based organomineral fertilizers maintained higher labile carbon content in soil than compost and compost combined with biochar in a short-term incubation trial (Darby et al., 2016; Nguyen et al., 2017). Zhou et al. (2019) observed an increase in SOM and nutrient contents in a karst region when using biochar enriched with NPK fertilizer. Similar results were reported by Wali et al. (2020), whereby they noticed an increase in soil organic matter after using P enriched biochar. In the study conducted by Kizito et al. (2019), an increase in the soil organic carbon (231.9% and 370%) was observed in the treatments with maize cob and wood digestate-enriched biochars, respectively, compared to treatments with unenriched biochars. The variation in carbon content between the distinct biochar types could be attributed to the different carbon mineralization rates of the feedstocks, while the increase in carbon observed in the enriched biochar treatments could be attributed to the sorption of labile organic matter in the biodigester sludge that is subsequently released into the soil (Fu et al., 2012).
4.3. Effect on pH, EC, and CEC
Cation exchange capacity, pH, and soil electrical conductivity (EC) are important soil parameters directly associated with plant development, and they can be improved with the application of enriched biochars. Mosa et al. (2018) reported an increase in the CEC of sandy soil after applying functionalized biochar. This change was mainly attributed to the high content of organic matter and the formation of oxide and/or hydroxide minerals after the thermochemical functionalization process. Kizito et al. (2019) noted an increase in soil pH, CEC, and EC. According to this study, the increase in soil pH could be caused by the accumulation of ash and the subsequent dissolution of hydroxides and carbonates. On the other hand, the increase in EC was attributed to the release of basic cations in the soil (Butnan et al., 2015), while the observed rise in CEC could be explained by the existence of several chemical functional groups that leave the biochar with an active chemical exchange surface (Zornoza et al., 2016).
Furthermore, biochars derived from slow pyrolysis tend to have more functional groups such as carboxylic that retain cations on their surface, thus increasing the CEC (Zornoza et al., 2016). Therefore, biochar-based fertilizers maintain this property after the enrichment process. Wali et al. (2020) noted an increase in soil EC when treated with a biochar-based phosphorous fertilizer after 15 days of incubation. Lustosa Filho et al. (2019) reported an increase in soil pH with P-enriched biochar-based fertilizers. According to the study, the alkaline nature of the fertilizers directly contributed to the rise in soil pH around the application site.
4.4. Effect on heavy metals
The modification of biochars with different materials such as compost, synthetic fertilizers, manure, and rocks increases the potential of biochar to immobilize and stabilize heavy metals in contaminated soils. For example, in addition to increasing the content of P, the application of phosphorous loaded biochar significantly decreased the concentration of labile heavy metals in the soil. Moreover, the exchangeable and reducible fractions of these metals were transformed into a more stable fraction (Ahmad et al., 2018). The application of a fertilizer composed of rice husk biochar and urea-hydrogen peroxide (UHP) promoted the immobilization of cadmium (Cd) simultaneously with the gradual release of nitrogen (Chen et al., 2018). Similar results were obtained by Dad et al. (2021), where the amount of Cd in contaminated soils was significantly reduced after applying iron-enriched biochar. Another study showed that co-pyrolysis of biomass with phosphate fertilizer improved carbon retention in the biochar and increased the ability of biochar to stabilize heavy metals in soil (Zhao et al., 2016).
The stabilization rates of Pb, Cu, and Cd were about 4, 2, and 1 times higher than the unenriched biochars (Zhao et al., 2016). Several mechanisms could be responsible for reducing the concentration of heavy metals in the soil, such as electrostatic interaction, adsorption, surface precipitation, ionic metal complexation and exchange with alkali cations, and physical sorption phenomena (Farooq et al., 2010; Park et al., 2011; Tong et al., 2011; Usman et al., 2013). Some specific properties of biochar, such as its porous structure, high specific surface area, a large number of functional groups, and abundant mineral elements, also contribute to the immobilization of heavy metals and nutrient loading in soil.
The immobilization effects of BCF on heavy metals can be divided in three stages: nonspecific adsorption stage, transformation stage and specific adsorption stage (Zhang et al., 2019). The first stage relies mainly on electrostatic force and surface adsorption of the porous surface of biochar. The transformation stage is characterized by the gradual diffusion and penetration of the heavy metal ion in the micropores of the BCF until a balance between solid and liquid is reached. Finally, in the last stage (specific adsorption), chemical adsorption plays a key role, where in the heavy metal ion would precipitate or co-precipitate with iron-manganese oxides or combine with organic functional groups to form stable complexes.
4.5. Effect on nutrient release
Biochar-based fertilizers differ from other fertilizers in their ability to gradually release nutrients into the soil; thus, resulting in a significant reduction in the loss of these nutrients by leaching or volatilization and, therefore, increase the efficiency of nutrient utilization (Gwenzi et al., 2018; Liu et al., 2019; Lustosa Filho et al., 2019; Khajavi-Shojaei et al., 2020). Lustosa Filho et al. (2019) reported that the pyrolysis of poultry litter mixed with triple superphosphate, H3PO4, and MgO eliminated the acidity from the phosphate sources and generated P-low release phosphate fertilizers with high potential to increase P absorption and maize growth. Moreover, there was a change in the soil pH, creating microsites where P adsorption by the soil particles was reduced (Lustosa Filho et al., 2019). Dong et al. (2020) developed a slow-release fertilizer by mixing rice straw biochar with bentonite, humic acid, NPK fertilizer, and coating material. This fertilizer reduced N losses by leaching and runoff in the sowing and tillering phases of rice plants, and also provided more nutrients during the maturation stage. An et al. (2020) developed a new biochar slow-release fertilizer (BSRF) with a slower release of P and K, by the co-pyrolysis of biomass, nutrients, and bentonite under microwave irradiation. Kim et al. (2018) also showed that switchgrass-derived biochar (Panicum virgatum) enriched with fertilizers had a slower release of K and P, due to nutrient retention by small pores in the biochar.
Several mechanisms could be attributed to this gradual release of nutrients. According to An et al. (2020), the smaller and more regular the size of the biochar fertilizer pores and channels, the slower the release of nutrients. Wen et al. (2017) attributed the gradual release of N to hydrogen bonding and electrostatic interactions between functional groups that contain oxygen and NH4+. Furthermore, interactions between biochar, clays, and minerals help to control the diffusion and penetration of moisture into the BCF structure, leading to nutrient retention (Liu et al., 2019). Studies performed by Khajavi-Shojaei et al. (2020) revealed that the coating material and structure of biochar-based slow-release nitrogen fertilizer improve the slow release of nitrate and ammonia located in biochar pores or interacted with surface functional groups. Luo et al. (2021) attributed the slow release of P of a Mg-enriched biochar fertilizer to the low solubility of Mg–P precipitates formed on the biochar surface and the enhanced ‘P-trap’ effect of MgO through reprecipitation process of PO43-. On the other hand, the slow release of N was dominated by the multiple effects of biochar carrier which included: the confinement effect and electrostatic attraction for NH4+, the hydrogen bonds and pore filling effect of N-containing organic matter. Finally, the higher CEC of biochar-based fertilizers provide stronger adsorption of cations on its surface and consequently lead to a slower release of nutrients.
5. Effects on soil physical properties
The application of biochar-based fertilizers can increase soil water retention (Oh et al., 2014; Yeboah et al., 2017; Gwenzi et al., 2018; Mosa et al., 2018; Khajavi-Shojaei et al., 2020). The mechanisms leading to these improvements can either be due to direct or indirect effects of biochar on the soil. The direct effect of biochar is mainly related to its physicochemical properties including (i) porous structure, (ii) high specific surface area, and (iii) hydrophilic functional groups, which allow water retention in the soil and inhibit loss via percolation (Glab et al., 2016). Thus, when added to the soil, these biochar fertilizers stimulate an increase in soil porosity and an expansion of the specific area of soil particles. In general, these changes result in higher water retention and improvement of other soil functions such as aeration and soil water dynamics (Troeh and Thompson, 2005; Downie et al., 2009). In contrast, the indirect effect concerns the hypothesis that biochar application improves soil structure due to its interactions with SOM and minerals and the increase in microbial activity (Karhu et al., 2011). Furthermore, biochar-based fertilizers can reduce soil bulk density due to increased soil organic carbon (Kizito et al., 2019).
6. Effects on soil biological properties
The application of enriched biochar could also affect the soil microorganisms by altering the soil microbial community's abundance, activity, and diversity. In the study conducted by Zhou et al. (2019), the addition of biochar fertilizer increased microbial biomass and bacterial biodiversity in karst calcareous soils, showing that this material had significant benefits in restoring degraded karst soils. In addition, there was an increase in the size and complexity of the microbial correlation network, which was reflected in the greater number of nodes, links, and nodules in the cladogram derived from the LEfSe analysis. Also, according to these authors, there was an increase in the fungal community after the addition of biochar-based fertilizers.
Chew et al. (2020) also reported an increase in the abundance of plant growth-promoting bacteria and fungi in rice plants' rhizosphere after applying a wheat straw-based activated biochar fertilizer. Of the 121 bacterial operational taxonomic units (OTUs) significantly affected, 75 responded positively, and 46 responded negatively. Regarding fungal functional taxonomic units, 19 responded positively, while 25 responded negatively to the addition of BCF. Furthermore, Joseph et al. (2015) observed an increase in mycorrhizal colonization of wheat roots after applying biochar containing magnetic iron nanoparticles to clayey soils, while Nielsen et al. (2014) found an improvement in soil bacterial communities using a biochar-mineral complex (BMC) compared to chemical fertilizers. Yang et al. (2021) observed an increase in the relative abundance of ten key bacterial genera and thirteen fungal genera using a biochar-based fertilizer treatment. According to this study, the occurrence of these genera was significantly positively correlated with the yield and quality of tea (Camellia sinensis L.).
The changes in microorganism communities are due to the high porosity of biochar, as the pores of biochar are colonized by these microorganisms, and they are protected from predators can multiply and increase in density and diversity (Lehmann et al., 2011; Nguyen et al., 2018). Therefore, biochar-based fertilizers are habitats for soil microorganisms. Moreover, biochar pyrolysis can transform some nutrients, making them available for the metabolic activities of microorganisms (Ameloot et al., 2013). Another aspect that influences these changes is the large specific area of these fertilizers that promotes adhesion to a greater number of functional groups on the biochar surface. The resulting effect is the increase in the adsorption of nutrients which subsequently become available for microorganisms (Hanzel et al., 2013). The addition of the enriched biochars can improve soil properties such as nutrient content, CEC, pH, and water holding capacity, and thus indirectly affect the diversity and structure of the soil microbial community (Nielsen et al., 2014; Yao et al., 2015). And finally, biochar promotes plant growth, resulting in increased plant residues in the soil that is subsequently used as a substrate for microorganisms (Zhang et al., 2014).
Because of the changes that occur in microbial communities, some fundamental soil functions also change when applying biochar-based fertilizers. Liao et al. (2020) found differences in the soil microbial community due to the application of nitrogen-enriched biochar. Genome sequencing results revealed an increase in nitrifying bacteria communities while there was a reduction in denitrifying bacteria. Thus, the biochar treatments showed higher N concentration and, consequently, higher rapeseed (Brassica napus L.) yield. Studies have shown that higher N increases the abundance of nitrifying bacteria while higher C input stimulates organic matter mineralization and enhances bacterial growth (Shen et al., 2011; Simonin et al., 2015). These could be contributing factors to the higher number of nitrifying bacteria under the nitrogen-enriched biochar treated soil. Besides affecting the growth of bacterial communities, biochar-based fertilizers alter microbial function by increasing genes related to energy production and conversion, amino acid transport, nucleotide transport and metabolism, carbohydrate transport and metabolism, translation, and other genes linked to soil functions (Gao et al., 2021). Furthermore, Moradi et al. (2019) reported an increase in basal respiration and frequency of nitrifying bacteria in saline soils treated with biochar-based fertilizer.
7. Effects on crop growth and productivity
As a result of the positive effect of biochar-based fertilizers on soil health, they have also been shown to enhance plant performances compared to unenriched biochars or conventional fertilizers. Overall, the addition of biochar-based fertilizers can increase plant growth, nutrient uptake, and other properties, as shown in Table 2. Schmidt et al. (2017) showed that the addition of nutrient-enriched biochar with either cow urine or NPK fertilizer improved yields compared to their respective controls. Biochar enriched with NPK produced an average yield increase of about 20% compared to NPK fertilizer. In comparison, the cow urine enriched biochar blended with compost produced a yield of about 123% compared to cow urine blended compost. The yield increases could be explained by biochar's nutrient carrier effect, which causes a slow nutrient release, more balanced nutrients fluxes, and reduced nutrient losses. Also, the addition of liquid manure to biochar can provide an organic coating of their aromatic surfaces, increasing nutrient adsorption and the CEC (Conte and Laudicina, 2017). Evaluating the effect of biochar-based nitrogen fertilizers on tropical soils, Puga et al. (2020) observed an increase in nitrogen use efficiency, 12% higher than that of urea. As such, an increase of about 26% in the average corn yield. The increase in the N use efficiency and maize yield was attributed to the slower release of N from the biochar-based fertilizers.
An increase in the shoot and root fresh mass, and root volume of 13.8%, 25.1%, and 37.7% were obtained upon adding a granular biochar-mineral urea composite (Bio-MUC), respectively (Shi et al., 2020). The authors highlighted an improvement in N use efficiency, as biomass production was higher in the Bio-MUC treatments. Ahmad et al. (2018) showed an increase in shoot and root length and an increase in dry mass and P uptake by maize plants after applying P-enriched biochar. This increase in growth under the P-enriched biochar was due to a higher P availability. Also, applying a mineral-enriched wheat straw biochar increased rice plant biomass by 67% and N and P uptake by 40% and 46%, respectively, compared to rice grown in soils treated with conventional fertilizer (Chew et al., 2020).
Another effect of enriched biochars is their ability to improve plant growth at different stages such as germination, seedling, flowering, and harvest. Although there was no significant change in germination, An et al. (2020) reported an increase in the growth of pepper seedlings when fertilized with a biochar-based fertilizer obtained by the pyrolysis of biomass and other nutrient-rich materials. Similarly, there was no significant difference in the biomass of rapeseed during the seedling stages between the urea and controlled-release biochar nitrogen fertilizer (BCRNF) treatments. However, the biomass accumulation of plants under the BCRNF treatment was significantly higher at the pre-flowering, flowering, and harvest stages than under the urea treatments (Liao et al., 2020). Such behaviour was attributed to the increase of N uptake by the plant in the advanced stages due to the larger root volume and the higher N concentration in the soil.
Furthermore, biochar-based fertilizers can improve plant development under stress conditions, such as in soils contaminated by heavy metals or even under salinity stress. Dad et al. (2021) showed that the application of iron-enriched biochar (Fe-Bc) reduced cadmium (Cd) toxicity in radish plants. Under Cd stress conditions, there is an increase in reactive oxygen species (ROS) responsible for lipid peroxidation, and this leads to cell membrane permeability (CMP) which damages the cell membrane. In addition to that, Cd stress also affects the defense systems of plants, reduces the photosynthetic capability, plant growth and development (Hatata and Abdel-Aal, 2008; Anjum et al., 2015). However, Fe-Bc treatments could mitigate and improve the negative impacts of Cd stress on plants by reducing CMP, increasing the antioxidant defense system and osmolyte, increasing de chlorophyll concentration, nutrient uptake, and consequently biomass accumulation (Xu et al., 2012; Kizito et al., 2019). Similar results were obtained by Chen et al. (2018), who also reported an increase in the immobilization of Cd in contaminated soils and, as a result, an increase in cabbage growth resulting from the application of biochar compost. The application of biochar-based organic fertilizer in alkaline saline soil increased the growth and yield of sugar beet. The biochar fertilizer improved the activities of N assimilation and antioxidant enzymes in the roots. As such, increased the root activities. In addition, the biochar-based organic fertilizer could improve the synthesis of photosynthetic pigments, PSII (Photosystem II) activity, stomatal opening, and photosynthesis of sugar beet under saline-alkaline conditions. These results proved the importance of biochar-based organic fertilizer to alleviate the negative effect of saline-alkali stress on sugar beet (Zhang et al., 2020).
BCF can also improve plant physiological properties. Li et al. (2019) reported an increase in net photosynthetic rate (A), stomatal conductance (gs), intercellular CO2 concentration (Ci), transpiration rate (Tr), and SPAD index of tobacco (Nicotiana tabacum L.) when soils were treated with a microorganism-based biochar fertilizer (EMBF). The increase in the physiological metabolism may be related to the water retention effect of EMBF. It has been shown that EMBF can effectively control the water in the soil, releasing it slowly, increasing soil water content around the roots, thereby reducing water pressure deficit and increase photosynthesis (Zhao et al., 2010). Both physiological and morphological properties such as growth, yield, and nodulation of chickpea were improved after the application of phosphorus-enriched biochar (Wali et al., 2020). The increase in morphological properties was a result of the increase in soil P content and also microbial activities. In contrast, the increase in physiological properties was attributed to the role of biochar and P in promoting stomatal conductance. Yeboah et al. (2017) also observed an increase of approximately 52% in stomatal conductance and 50% in the net photosynthetic rate of wheat after applying N-enriched biochar (BN50). The authors also highlighted an increase in grain yield (1,905 and 2,133 kg ha−1 in 2014 and 2015, respectively) under the BN50 treatment.
Plant nutrient content can also be influenced by BCF treatments. Wali et al. (2020) noted an increase in P and N content in the shoots of chickpea after the application of a P-enriched biochar. Similarly, Nardis et al. (2020) demonstrated that P-loaded Mg-enriched biochars promoted both growth and P accumulation in maize, with a similar or higher value than triple superphosphate (TSP). Both authors attributed this increase to the greater availability of these nutrients in the soil. In the study conducted by Nardis et al. (2020), an increase in Mg uptake was also observed. This was explained by the synergistic effect between Mg and P, as magnesium acts as an enzymatic activator in most phosphorylative enzymes, bridging ATP and/or ADP and the enzyme molecule. This leads to maximum absorption of P in the presence of Mg. Farrar et al. (2019) observed an increase in the total content of the following elements in ginger (Zingiber officinale Canton) leaves: P, K, Ca, Cu, and Al at week 30 when applying the biochar organomineral at a dose of 7.5 t ha−1 compared to commercial biochar fertilizer. Biochar induces better root system development, which in turn increases nutrient uptake (Wali et al., 2020). Yang et al. (2021) observed an increase in the amino acids, caffeine content and water extracts of tea (Camellia sinensis L.) upon application of a biochar-based fertilizer (BF) made from a blend of biochar (52%), urea (22%), diammonium phosphate (10%), potassium sulfate (14%) and solid binder (2%). This could be due to the increase in nutrient uptake by tea which consequently influences the production of secondary metabolites (Petruccelli et al., 2015). Moreover, the BF could enhance the soil water holding capacity which could lead to an increase in water uptake and stimulate the production of secondary metabolites such as amino acids (Saha et al., 2019).
However, biochar-based fertilizers could also exhibit an antagonistic effect on certain nutrients. This was demonstrated by Gunes et al. (2015) when P-enriched biochar increased the concentration of macronutrients but decreased the concentration of micronutrients such as Fe, Zn, Mn, and Cu in lettuce plants. The reduction in the concentration of these elements was presumably caused by a dilution effect arising from increased plant growth or due to the highly porous structures of biochars that contain several functional groups known to be effective in immobilizing heavy metals and thus significantly reducing their concentration in the soil (Liu and Zhang, 2009; Park et al., 2011).
Besides increasing plant growth and productivity, biochar-based fertilizers have also been shown to control and suppress plant diseases and pathogens. Abdelnabby et al. (2018) developed a biochar-based fertilizer by pairing maize straw biochar and urea at different rates. The new formulations showed superior control of M. incognita in tomato plants compared to the individual effect of biochar. Studies have shown that urea is converted to ammonia in the soil which has a nematocidal activity (Tenuta and Lazarovits, 2002). In a study to evaluate the effect of alkali-enhanced biochar on silicon uptake and suppression of gray leaf (Magnaporthe oryzae) spot development in perennial ryegrass, Wang et al. (2019) observed a reduction of the disease by 65% when applying 0 KB (biochar without alkaline enhancement) or 10 CB (alkali-enhanced biochar with CaO), and by 77% when applying 10KB (alkaki-enhanced biochar with KOH) or 10 K2B (alkali-enhanced biochar with K2CO3) at a rate of 0.22% (5 t ha−1). At 1% (22.5 t ha−1) application rate, a reduction of 58 and 67% was observed for the treatments of 10 KB and 10 CB. The suppression of the gray leaf spot maybe due to the increased Si content in the plant, as plant diseases are negatively correlated with tissue Si content (Nanayakkara et al., 2009). Also, there are multiple benefits of applying biochar to soil, such as improving soil water holding capacity, enhancing microbial activity, immobilizing heavy metals, which could enhance plant growth and disease resistance.
Despite the positive responses of plants to enriched biochar, some studies have reported negative responses after their application. For example, there were no differences in the yield of rice grains and shoot biomass after the application of a biochar fertilizer (Dong et al., 2020). Although maize grown on soil fertilized with biochar enriched with anaerobically digestate showed significantly higher biomass than the unenriched biochar treatments, it was slightly lower than those in the chemical fertilizer treatments (Kizito et al., 2019). This response was attributed to slower mineralization of nutrients by biochar and, consequently, more restricted short-term uptake. Oh et al. (2014) also demonstrated lower lettuce yield under biochar-SRF applications with doses ranging from 3.7 to 34.2 t ha−1 compared to commercial fertilizer at SRF dose of 51.4 t ha−1. According to the authors, this response occurred due to an excessive increase in soil pH after applying the biochar-based fertilizer.
8. Effects on greenhouse gas (GHG) emissions
The application of BCFs has been proposed as an effective approach to reduce GHG emissions. Dong et al. (2021) showed that a slow-release fertilizer produced from rice straw biochar, urea, NH4H2PO4 and K2SO4 significantly decreased methane (CH4) emission by 33.4% during the whole rice growing season when compared to other fertilizers. The authors also observed a decrease in the soil methanogenic activities by 22.2% in the tillering stage, and an increase in CH4 oxidation by 14.1%, 52.2% and 27.8% from the regreening to filling stages. These results were due to the NO3- content of the BCF which led to an increase in the abundance and activity of nitrate reducing bacteria and a reduction of methanogenic bacteria. Puga et al. (2020) also demonstrated that biochar-based nitrogen fertilizers (BNs) promoted soil C sequestration due to their predominantly stable C, which is highly resistant to decomposition. When C sequestration by biochar (BC) was not considered, BN51/10 (51% BC, 10% N) showed the lowest GHG emission intensity with a value that was 14% lower than that of urea treatments. Investigating the response of seasonal variations in soil N2O emissions and environmental factors in subtropical Moso bamboo plantation, Zhou et al. (2021) observed a decrease in N2O emission after the application of a biochar-based fertilizer produced by combining wheat straw biochar and chemical fertilizer. The decrease in N2O emission was a result of the reduction of water-soluble organic N concentration and N-cycling enzyme activity in soil as the BCF may adsorb labile N forms in soils due to its surface area and high CEC (Li et al., 2018). As such, the limitation of mineral N supply to denitrifiers could be a key mechanism in reducing soil N2O effluxes (Van Zwieten et al., 2014).
9. Future perspectives
The incorporation of biochar-based fertilizers into soils is a novel approach for improving soil health. A large number of studies have been conducted, about 86%, were carried out in the laboratory and greenhouses over a short period. Therefore, more long-term field studies are necessary to validate the findings about the detailed mechanisms and possible negative impacts of biochar fertilizer application to soils. Furthermore, more studies need to be conducted to understand the effects of BCFs on different soil types, climate, plant species, and application methods (rates, broadcasted in the soil surface or incorporated application, incorporation depth, in-furrow application), as well as the effects in the soil-plant-atmosphere system.
Most current studies involving biochar-based fertilizers aim at enrichment with a single macronutrient, specifically N, P, or K. To obtain high performance, it may be necessary to apply chemical fertilizer with the enriched biochar. As such, research regarding producing a biochar complex rich in various macro and micronutrients is imperative to minimize the need for additional chemical fertilizer for plant growth. The production of biologically active biochar by inoculation with beneficial microorganisms could also be of great interest. Similarly, further research regarding the incorporation of organic and/or mineral nanophase compounds in biochar is required, as these materials can increase the surface area and functionality of biochars, and as a result, leads to greater nutrient retention in soil for a longer period, thus allowing for slower nutrient release and minimizing leaching loss.
The indiscriminate application of enriched biochar in soils can lead to antagonistic effects such as soil contamination, contamination of water bodies and groundwater, due to the high concentrations of some elements (mainly N and P), and the presence of heavy metals. Therefore, it is crucial to make a prior study of the biochar feedstock and the enrichment material used and to investigate the optimization of the biochar/enrichment material ratio, based on its chemical composition, to avoid the excess of nutrients. Although many studies have proven the positive impacts of enriched biochar on plant growth and productivity, there is still a lack of information on the use of these fertilizers to promote plant growth under environmental stress, such as water stress, temperature stress, salinity, contamination by heavy metals, etc. In addition, more studies should be carried out to assess the impacts of biochar-based fertilizers on the physical properties of soils, such as particle size, aggregate formation, soil bulk density, aeration, porosity, compaction, among others.
Finally, more research is needed to assess the sustainability of the use of BCFs in agriculture, the cost of production, the economic viability, the technical limitations that arise from their use, different ways to improve the efficiency of production and the social and environmental impacts.
10. Conclusion
In this review, the methods of producing biochar-based fertilizers enriched biochars and their effects on soil and plant properties were discussed. The enrichment of biochar has shown to be an effective way to overcome the limitations regarding the use of biochar, such as the lack of some nutrients, the high application rates, and the high cost of production. The production of biochar-based fertilizers can be achieved by three main methods: direct pyrolysis, pre-pyrolysis, and post-pyrolysis. Enriched biochars with enhanced physicochemical properties have proven to improve soil biological, physical, and chemical properties and plant growth and yield compared to unenriched biochars. Future researches would be required to study the long-term effect of biochar-based fertilizers in agriculture, their impact on different soil types and climate, different application technologies, and their optimization.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
To the Brazilian National Council for Scientific and Technological Development (CNPq), for the scientific productivity fellowships granted to Cícero Célio de Figueiredo and Maria Lucrecia Gerosa Ramos. We also acknowledge Professor Concepta Margaret McManus Pimentel for revising the paper.
References
- Abdelnabby H., Hu Z., Wang H., Zhang X. Furfural–biochar-based formulations show synergistic and potentiating effects against Meloidogyne incognita in tomato. J. Pest. Sci. 2018;91:203–218. [Google Scholar]
- Ahmad M., Usman A.R.A., Al-Faraj A.S., Ahmad M., Sallam A., Al-Wabel M.I. Phosphorus-loaded biochar changes soil heavy metals availability and uptake potential of maize (Zea mays L.) plants. Chemosphere. 2018;194:327–339. doi: 10.1016/j.chemosphere.2017.11.156. [DOI] [PubMed] [Google Scholar]
- Ameloot N., De Neve S., Jegajeevagan K., Yildiz G., Buchan D., Funkuin Y.N., Prins W., Bouckaert L., Sleutel S. Short-term CO2 and N2O emissions and microbial properties of biochar amended sandy loam soils. Soil Biol. Biochem. 2013;57:401–410. [Google Scholar]
- An X., Wu Z., Yu J., Cravotto G., Liu X., Li Q., Yu B. Copyrolysis of biomass, bentonite, and nutrients as a new strategy for the synthesis of improved biochar-based slow-release fertilizers. ACS Sustain. Chem. Eng. 2020;8(8):3181–3190. [Google Scholar]
- Anjum S.A., Tanveer M., Hussain S., Bao M., Wang L., Khan I., Ullah E., Tung S.A., Samad R.A., Shahzad B. Cadmium toxicity in Maize (Zea mays L.): consequences on antioxidative systems, reactive oxygen species and cadmium accumulation. Environ. Sci. Pollut. Res. Int. 2015;22:17022–17030. doi: 10.1007/s11356-015-4882-z. [DOI] [PubMed] [Google Scholar]
- Bagreev A., Bandosz T.J., Locke D.C. Pore structure and surface chemistry of adsorbents obtained by pyrolysis of sewage derived fertilizer. Carbon. 2001;39(13):1971–1979. [Google Scholar]
- Biederman L.A., Harpole W.S. Biochar and its effects on plant productivity and nutrient cycling: a meta-analysis. GCB Bioenergy. 2013;5(2):202–214. [Google Scholar]
- Blackwell P., Joseph S., Munroe P., Anawar H.M., Sorrer P., Gilkes R.J., Solaiman Z.M. Influences of biochar and biochar-mineral complex on mycorrhizal colonisation and nutrition of wheat and sorghum. Pedosphere. 2015;25:686–695. [Google Scholar]
- Bruun E.W., Ambus P., Egsgaard H., Hauggaard-Nielsen H. Effects of slow and fast pyrolysis biochar on soil C and N turnover dynamics. Soil Biol. Biochem. 2012;46:73–79. [Google Scholar]
- Butnan S., Deenik J.L., Toomsan B., Antal M.J., Vityakon P. Biochar characteristics and application rates affecting corn growth and properties of soils contrasting in texture and mineralogy. Geoderma. 2015;237–238:105–116. [Google Scholar]
- Carneiro J.S.S., Ribeiro I.C.A., Nardis B.O., Barbosa C.F., Lustosa Filho J.F., Melo L.C.A. Long-term effect of biochar-based fertilizers application in tropical soil: agronomic efficiency and phosphorus availability. Sci. Total Environ. 2021;760:143955. doi: 10.1016/j.scitotenv.2020.143955. [DOI] [PubMed] [Google Scholar]
- Chang Y.M., Tsai W.T., Li M.H. Chemical characterization of char derived from slow pyrolysis of microalgal residue. J. Anal. Appl. Pyrol. 2015;111:88–93. [Google Scholar]
- Chen L., Chen Q., Rao P., Yan L., Shakib A., Shen G. Formulating and optimizing a novel biochar-based fertilizer for simultaneous slow-release of nitrogen and immobilization of cadmium. Sustainability. 2018;10:2740. [Google Scholar]
- Chen Z., Pei J., Wei Z., Ruan X., Hua Y., Xu W., Zhang C., Liu T., Guo Y. A novel maize biochar-based compound fertilizer for immobilizing cadmium and improving soil quality and maize growth. Environ. Pollut. 2021;277:116455. doi: 10.1016/j.envpol.2021.116455. [DOI] [PubMed] [Google Scholar]
- Chew J., Zhu L., Nielsen S., Graber E., Mitchell D.R.G., Horvat J., Mohammed M., Liu M., van Zwieten L., Donne S., Munroe P., Taherymoosavi S., Pace B., Rawal A., Hook J., Marjo C., Thomas D.S., Pan G., Li L., Bian R., McBeath A., Bird M., Thomas T., Husson O., Solaiman Z., Joseph S., Fan X. Biochar-based fertilizer: supercharging root membrane potential and biomass yield of rice. Sci. Total Environ. 2020;713:136431. doi: 10.1016/j.scitotenv.2019.136431. [DOI] [PubMed] [Google Scholar]
- Chia C.H., Singh B.P., Joseph S., Graber E.R., Munroe P. Characterization of an enriched biochar. J. Anal. Appl. Pyrol. 2014;108:26–34. [Google Scholar]
- Clare A., Shackley S., Joseph S., Hammond J., Pan G.X., Bloom A. Competing uses for China’s straw: the economic and carbon abatement potential of biochar. GCB Bioenergy. 2014;7:1272–1282. [Google Scholar]
- Conte P., Laudicina V.A. Mechanisms of organic coating on the surface of a poplar biochar. Curr. Org. Chem. 2017;21(6):559–565. [Google Scholar]
- Cross A., Sohi S.P. The priming potential of biochar products in relation to labile carbon contents and soil organic matter status. Soil Biol. Biochem. 2011;43:2127–2134. [Google Scholar]
- Dad F.P., Khan W., Tanveer M., Ramzani P.M.A., Shaukat R., Muktadir A. Influence of iron-enriched biochar on Cd Sorption, its ionic concentration and redox regulation of radish under cadmium toxicity. Agriculture. 2021;11:1. [Google Scholar]
- Darby I., Xu C.-Y., Wallace H.M., Joseph S., Pace B., Bai S.H. Short term dynamics of carbon and nitrogen using compost, compost biochar mixture and organo-mineral biochar. Environ. Sci. Pollut. Res. 2016;23:11267–11278. doi: 10.1007/s11356-016-6336-7. [DOI] [PubMed] [Google Scholar]
- Domingues R.R., Trugilho P.F., Silva C.A., de Melo I.C.N., Melo L.C., Magriotis Z.M., Sanchez-Monedero M.A. Properties of biochar derived from wood and high-nutrient biomasses with the aim of agronomic and environmental benefits. PloS One. 2017;12(5) doi: 10.1371/journal.pone.0176884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong D., Wang C., Van Zwieten L., Wang H., Jiang P., Zhou M., Wu W. An effective biochar-based slow-release fertilizer for reducing nitrogen loss in paddy fields. J. Soils Sediments. 2020;20:3027–3040. [Google Scholar]
- Dong D., Li J., Ying S., Wu J., Han X., Teng Y., Zhou M., Ren Y., Jiang P. Mitigation of methane emission in a rice paddy field amended with biochar-based slow-release fertilizer. Sci. Total Environ. 2021;792:148460. doi: 10.1016/j.scitotenv.2021.148460. [DOI] [PubMed] [Google Scholar]
- Downie A., Crosky A., Munroe P. In: Biochar for Environmental Management: Science and Technology. Lehmann J., Joseph S., editors. Earthscan; London: 2009. Physical properties of biochar; pp. 13–32. [Google Scholar]
- Enaime G., Baçaoui A., Yaacoubi A., Lübken M. Biochar for wastewater treatment—conversion technologies and applications. Appl. Sci. 2020;10:3492. [Google Scholar]
- Fang L., Li J-s., Donatello S., Cheeseman C.R., Poon C.S., Tsang D.C.W. Use of Mg/Ca modified biochars to take up phosphorus from acid-extract of incinerated sewage sludge ash (ISSA) for fertilizer application. J. Clean. Prod. 2019:118853. [Google Scholar]
- Farooq U., Kozinski J.A., Khan M.A., Athar M. Biosorption of heavy metal ions using wheat based biosorbents e a review of the recent literature. Bioresour. Technol. 2010;101:5043–5053. doi: 10.1016/j.biortech.2010.02.030. [DOI] [PubMed] [Google Scholar]
- Farrar M.B., Wallace H.M., Xu C.Y., Nguyen T.T.N., Tavakkoli E., Joseph S., Bai S.H. Short-term effects of organo-mineral enriched biochar fertiliser on ginger yield and nutrient cycling. J. Soils Sediments. 2019;19:668–682. [Google Scholar]
- Fu P., Hu S., Xiang J., Sun L., Su S., Wang J. Evaluation of the porous structure development of chars from pyrolysis of rice straw: effects of pyrolysis temperature and heating rate. J. Anal. Appl. Pyrolysis. 2012;98:177–183. [Google Scholar]
- Gao S., Homan-Krull K., Bidwell A.L., DeLuca T.H. Locally produced wood biochar increases nutrient retention and availability in agricultural soils of the San Juan Islands, USA. Agric. Ecosyst. Environ. 2016;233:43–54. [Google Scholar]
- Gao M., Yang J., Liu C., Gu B., Han M., Li J., Li N., Liu N., An N., Dai J., Liu X., Han X. Effects of long-term biochar and biochar-based fertilizer application on brown earth soil bacterial communities. Agric. Ecosyst. Environ. 2021;309:107285. [Google Scholar]
- Głab T., Palmowska J., Zaleski T., Gondek K. Effect of biochar application on soil hydrological properties and physical quality of sandy soil. Geoderma. 2016;281:11–20. [Google Scholar]
- Gunes A., Inal A., Sahin O., Taskin M.B., Atakol O., Yilmaz N. Variations in mineral element concentrations of poultry manure biochar obtained at different pyrolysis temperatures, and their effects on crop growth and mineral nutrition. Soil Use Manag. 2015;31(4):429–437. [Google Scholar]
- Gwenzi W., Nyambishi T.J., Chaukura N., Mapope N. Synthesis and nutrient release patterns of a biochar-based N–P–K slow-release fertilizer. Int. J. Environ. Sci. Technol. 2018;15:405–414. [Google Scholar]
- Hanzel J., Myrold D., Sessitsch A., Smalla K., Tebbe C.C., Totsche K.U. Microbial ecology of biogeochemical interfaces–diversity, structure, and function of microhabitats in soil. FEMS Microbiol Ecol. 2013;86(1):1–2. doi: 10.1111/1574-6941.12194. [DOI] [PubMed] [Google Scholar]
- Hatata M.M., Abdel-Aal E.A. Oxidative stress and antioxidant defense mechanisms in response to cadmium treatments. J. Environ. Agric. Sci. 2008;4:655–669. [Google Scholar]
- Hu P., Zhang Y., Liu L., Wang X., Luan X., Ma X., Chu P.K., Zhou J., Zhao P. Biochar/struvite composite as a novel potential material for slow release of N and P. Environ. Sci. Pollut. R. 2019;26:17152–17162. doi: 10.1007/s11356-019-04458-x. [DOI] [PubMed] [Google Scholar]
- Jeffery S., Verheijen F.G.A., van der Velde M., Bastos A.C. A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agric. Ecosyst. Environ. 2011;144:175–187. [Google Scholar]
- Jia Y., Hu Z., Ba Y., Qi W. Application of biochar-coated urea controlled loss of fertilizer nitrogen and increased nitrogen use efficiency. Chem. Biol. Technol. Agric. 2021;8:3. [Google Scholar]
- Joseph S., Anawar H.M., Storer P., Blackwell P., Chia C., Lin Y., Munroe P., Donne S., Horvat J., Wang J., Solaiman Z.M. Effects of enriched biochars containing magnetic iron nanoparticles on mycorrhizal colonisation, plant growth, nutrient uptake and soil quality improvement. Pedosphere. 2015;25:749–760. [Google Scholar]
- Kannari N., Uesugi T., Kubota K., Masuda D., Sato K., Takarada T., Watanabe T. Calcium-enriched biochar: pyrolysis of digested sludge, phosphorus recovery, and fertilizer properties. J. Japan Inst. Energy. 2020;99:236–242. [Google Scholar]
- Karhu K., Mattila T., Bergström I., Regina K. Biochar addition to agricultural soil increased CH4 uptake and water holding capacity – results from a short-term pilot field study. Agric. Ecosyst. Environ. 2011;140:309–313. [Google Scholar]
- Karim A.A., Kumar M., Singh S.K., Panda C.R., Mishra B.K. Potassium enriched biochar production by thermal plasma processing of banana peduncle for soil application. J. Anal. Appl. Pyrolysis. 2017;123:165–172. [Google Scholar]
- Karim A.A., Kumar M., Mohapatra S., Singh S.K., Panda C.R. Co-plasma processing of banana peduncle with phosphogypsum waste for production of lesser toxic potassium–sulfur rich biochar. J. Mater. Cycles Waste Manag. 2019;21(1):107–115. [Google Scholar]
- Kasozi G.N., Zimmerman A.R., Nkedi-Kizza P., Gao M.B. Catechol and humic acid sorption onto a range of laboratory-produced black carbons (biochars) Environ. Sci. Technol. 2010;44:6189–6195. doi: 10.1021/es1014423. [DOI] [PubMed] [Google Scholar]
- Khajavi-Shojaei S., Moezzi A., Masir M.N., Taghavi M. Biomass Convers. Biorefin; 2020. Synthesis Modified Biochar-Based Slow-Release Nitrogen Fertilizer Increases Nitrogen Use Efficiency and Corn (Zea mays L.) Growth. [Google Scholar]
- Kim J.A., Vijayaraghavan K., Reddy D.H.K., Yun Y.S. A phosphorus enriched biochar fertilizer from bio-fermentation waste: a potential alternative source for phosphorus fertilizers. J. Clean. Prod. 2018;196:163–171. [Google Scholar]
- Kizito S., Luo H., Lu J., Bah H., Dong R., Wu S. Role of nutrient-enriched biochar as a soil amendment during maize growth: exploring practical alternatives to recycle agricultural residuals and to reduce chemical fertilizer demand. Sustainability. 2019;11:3211. [Google Scholar]
- Kumar A., Singh E., Singh L., Kumar S., Kumar R. Carbon material as a sustainable alternative towards boosting properties of urban soil and foster plant growth. Sci. Total Environ. 2021;751:141659. doi: 10.1016/j.scitotenv.2020.141659. [DOI] [PubMed] [Google Scholar]
- Lehmann J., Joseph S. In: Biochar for Environmental Management Science and Technology. Lehmann J., Joseph S., editors. Earthscans; London: 2009. Biochar for environmental management: an introduction; pp. 1–12. [Google Scholar]
- Lehmann J., Joseph S. second ed. Routledge; London: 2015. Biochar for Environmental Management: Science, Technology and Implementation. [Google Scholar]
- Lehmann J., da Silva J.P., Steiner C., Nehls T., Zech W., Glaser B. Nutrient availability and leaching in an archaeological Anthrosol and a Ferralsol of the Central Amazon basin: fertilizer, manure and charcoal amendments. Plant Soil. 2003;249:343–357. [Google Scholar]
- Lehmann J., Rillig M.C., Thies J., Masiello C.A., Hockaday W.C., Crowley D. Biochar effects on soil biota—a review. Soil Biol. Biochem. 2011;43(9):1812–1836. [Google Scholar]
- Li Y.F., Hu S.D., Chen J.H., Müller K., Li Y.C., Fu W.J., Lin Z.W., Wang H.L. Effects of biochar application in forest ecosystems on soil properties and greenhouse gas emissions: a review. J. Soil. Sediment. 2018;18:546–563. [Google Scholar]
- Li Y., Cheng J., Lee X., Chen Y., Gao W., Pan W., Tang Y. Effects of biochar-based fertilizers on nutrient leaching in a tobacco-planting soil. Acta Geochim. 2019;38(1):1–7. [Google Scholar]
- Li X., Shao X., Ding F., Yuan Y., Li R., Yang X., Gao C., Miao Q. Effects of effective microorganisms biochar-based fertilizer on photosynthetic characteristics and chlorophyll content of flue-cured tobacco under water-saving irrigation strategies. Chil. J. Agric. Res. 2020;80(3):422–432. [Google Scholar]
- Liao J., Liu X., Hu A., Song H., Chen X., Zhang Z. Effects of biochar-based controlled release nitrogen fertilizer on nitrogen-use efficiency of oilseed rape (Brassica napus L.) Sci. Rep. 2020;10(1):1–14. doi: 10.1038/s41598-020-67528-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y., Munroe P., Joseph S., Ziolkowski A., Zwieten L.V., Kimber S., Rust J. Chemical and structural analysis of enhanced biochars: thermally treated mixtures of biochar, chicken litter, clay and minerals. Chemosphere. 2013;91:35–40. doi: 10.1016/j.chemosphere.2012.11.063. [DOI] [PubMed] [Google Scholar]
- Liu Z., Zhang F.S. Removal of lead from water using biochars prepared from hydrothermal liquefaction of biomass. J. Hazard Mat. 2009;167:933–939. doi: 10.1016/j.jhazmat.2009.01.085. [DOI] [PubMed] [Google Scholar]
- Liu X., Li Z., Zhang Y., Feng R., Mahmood I.B. Characterization of human manure-derived biochar and energy-balance analysis of slow pyrolysis process. Waste Manage. 2014;34:1619–1626. doi: 10.1016/j.wasman.2014.05.027. [DOI] [PubMed] [Google Scholar]
- Liu Z., Singer S., Tong Y., Kimbell L., Anderson E., Hughes M., Zitomer D., McNamara P. Characteristics and applications of biochars derived from wastewater solids. Renew. Sustain. Energy Rev. 2018;90:650–664. [Google Scholar]
- Liu X., Liao J., Song H., Yang Y., Guan C., Zhang Z. A Biochar-based route for environmentally friendly controlled release of nitrogen: urea-loaded biochar and bentonite composite. Sci. Rep. 2019;9:9548. doi: 10.1038/s41598-019-46065-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou Z., Sun Y., Bian S., Baig S.A., Hu B., Xu X. Nutrient conservation during spent mushroom compost application using spent mushroom substrate derived biochar. Chemosphere. 2017;169:23–31. doi: 10.1016/j.chemosphere.2016.11.044. [DOI] [PubMed] [Google Scholar]
- Luo W., Qian L., Liu W., Zhang X., Wang Q., Jiang H., Cheng B., Ma H., Wu Z. A potential Mg-enriched biochar fertilizer: excellent slow-release performance and release mechanism of nutrients. Sci. Total Environ. 2021;768:144454. doi: 10.1016/j.scitotenv.2020.144454. [DOI] [PubMed] [Google Scholar]
- Lustosa Filho J.F., Carneiro J.S.C., Barbosa C.F., Leite A.A., Melo L.C.A. Diffusion and phosphorus solubility of biochar-based fertilizer: visualization, chemical assessment and availability to plants. Soil Tillage Res. 2019;194:104298. [Google Scholar]
- Lustosa Filho J.F., Carneiro J.S.C., Barbosa C.F., Lima K.P., Leite A.A., Melo L.C.A. Aging of biochar-based fertilizers in soil: effects on phosphorus pools and availability to Urochloa brizantha grass. Sci. Total Environ. 2020;709:136028. doi: 10.1016/j.scitotenv.2019.136028. [DOI] [PubMed] [Google Scholar]
- Ma Y.L., Matsunaka T. Biochar derived from dairy cattle carcasses as an alternative source of phosphorus and amendment for soil acidity. J. Soil Sci. Plant Nutr. 2013;59:628–641. [Google Scholar]
- Moradi S., Rasouli-Sadaghiani M.H., Sepehr E., Khodaverdiloo H., Barin M. Soil nutrients status affected by simple and enriched biochar application under salinity conditions. Environ. Monit. Assess. 2019;191:257. doi: 10.1007/s10661-019-7393-4. [DOI] [PubMed] [Google Scholar]
- Mosa A., El-Ghamry A., Tolba M. Functionalized biochar derived from heavy metal rich feedstock: phosphate recovery and reusing the exhausted biochar as an enriched soil amendment. Chemosphere. 2018;198:351–363. doi: 10.1016/j.chemosphere.2018.01.113. [DOI] [PubMed] [Google Scholar]
- Nanayakkara U.N., Uddin W., Datnoff L.E. Soil silicon amendment for managing gray leaf spot of perennial ryegrass turf on golf courses in Pennsylvania. J. Indian Dent. Assoc. 2009;31:415–426. [Google Scholar]
- Nardis B.O., Carneiro J.S.S., Souza I.M.G., Barros R.G., Melo L.C.A. Phosphorus recovery using magnesium-enriched biochar and its potential use as fertilizer. Arch. Agron. Soil Sci. 2020;67:1017–1030. [Google Scholar]
- Nguyen T.T.N., Wallace H.M., Xu C.-Y., Xu Z., Farrar M.B., Joseph S., Van Zwieten L., Bai S.H. Short-term effects of organo-mineral biochar and organic fertilisers on nitrogen cycling, plant photosynthesis, and nitrogen use efficiency. J. Soils Sediments. 2017;17:2763–2774. [Google Scholar]
- Nguyen T.T.N., Wallace H.M., Xu C.-Y., Van Zwieten L., Weng Z.H., Xu Z., Che R., Tahmasbian I., Hu H.-W., Bai S.H. The effects of short term, long term and reapplication of biochar on soil bacteria. Sci. Total Environ. 2018;636:142–151. doi: 10.1016/j.scitotenv.2018.04.278. [DOI] [PubMed] [Google Scholar]
- Nielsen S., Minchin T., Kimber S., van Zwieten L., Gilbert J., Munroe P., Joseph S., Thomas T. Comparative analysis of the microbial communities in agricultural soil amended with enhanced biochars or traditional fertilisers. Agr. Ecosyst. Environ. 2014;191:73–82. [Google Scholar]
- Oh T.-K., Shinogi Y., Lee S.-J., Chol B. Utilization of biochar impregnated with anaerobically digested slurry as slow-release fertilizer. J. Plant Nutr. Soil Sci. 2014;177:97–103. [Google Scholar]
- Park J.H., Choppala G.H., Bolan N.S., Chung J.W., Chuasavathi T. Biochar reduces the bioavailability and phytotoxicity of heavy metals. Plant Soil. 2011;348:439–451. [Google Scholar]
- Petruccelli R., Bonetti A., Traversi M.L., Faraloni C., Valagussa M., Pozzi A. Influence of biochar application on nutritional quality of tomato (Lycopersicon esculentum) Crop Pasture Sci. 2015;66:747–755. [Google Scholar]
- Puga A.P., Grutzmacher P., Cerri C.E.P., Ribeirinho V.S., Andrade C.A. Biochar-based nitrogen fertilizers: greenhouse gas emissions, use efficiency, and maize yield in tropical soils. Sci. Total Environ. 2020;704:135375. doi: 10.1016/j.scitotenv.2019.135375. [DOI] [PubMed] [Google Scholar]
- Saha A., Basaka B.B., Gajbhiyea N.A., Kalariyaa K.A., Manivela P. Sustainable fertilization through co-application of biochar and chemical fertilizers improves yield, quality of Andrographis paniculata and soil health. Ind. Crop. Prod. 2019;140:111607. [Google Scholar]
- Schmidt H.-P., Pandit B.H., Cornelissen G., Kammann C.I. Biochar-based fertilization with liquid enrichment: 21 field trials covering 13 crop species in Nepal. Land Degrad. Develop. 2017;28:2324–2342. [Google Scholar]
- Sepulveda-Cadavid C., Romero1 J.H., Torres M., Becerra-Agudelo E., López J.E. Evaluation of a biochar-based slow-release P fertilizer to improve Spinacia oleracea P use, yield, and nutritional quality. J. Soil Sci. Plant Nutr. 2021 [Google Scholar]
- Shen X.-Y., Zhang L.-M., Shen P.-J., Li L.-H., Yuan C.-L., He J.-Z. Nitrogen loading levels affect abundance and composition of soil ammonia oxidizing prokaryotes in semiarid temperate grassland. J. Soils Sediments. 2011;11:1243. [Google Scholar]
- Shi W., Ju Y., Bian R., Li L., Joseph S., Mitchell D.R.G., Munroe P., Taherymoosavi S., Pan G. Biochar bound urea boosts plant growth and reduces nitrogen leaching. Sci. Total Environ. 2020;701:134424. doi: 10.1016/j.scitotenv.2019.134424. [DOI] [PubMed] [Google Scholar]
- Simonin M., Le Roux X., Poly F., Lerondelle C., Hungate B.A., Nunan N., Niboyet A. Coupling between and among ammonia oxidizers and nitrite oxidizers in grassland mesocosms submitted to elevated CO2 and nitrogen supply. Microb. Ecol. 2015;70:809–818. doi: 10.1007/s00248-015-0604-9. [DOI] [PubMed] [Google Scholar]
- Soinne H., Hovi J., Tammeorg P., Turtola E. Effect of biochar on phosphorus sorption and clay soil aggregate stability. Geoderma. 2014;219:162–167. [Google Scholar]
- Subedi R., Taupe N., Ikoyi I., Bertora C., Zavattaro L., Schmalenberger A., Leahy J.J., Grignani C. Chemically and biologically-mediated fertilizing value of manure derived biochar. Sci. Total Environ. 2016;550:924–933. doi: 10.1016/j.scitotenv.2016.01.160. [DOI] [PubMed] [Google Scholar]
- Tenuta M., Lazarovits G. Ammonia and nitrous acid from nitrogenous amendments kill the micro sclerotia of Verticillium dahliae. Phytopathology. 2002;92:255–264. doi: 10.1094/PHYTO.2002.92.3.255. [DOI] [PubMed] [Google Scholar]
- Tong X.J., Li J.Y., Yuan J.H., Xu R.K. Adsorption of Cu (II) by biochars generated from three crop straws. Chem. Eng. J. 2011;172(2):828–834. [Google Scholar]
- Tripathi M., Sahu J.N., Ganesan P. Effect of process parameters on production of biochar from biomass waste through pyrolysis: a review. Renew. Sustain. Energy Rev. 2016;55:467–481. [Google Scholar]
- Tripti, Kumar A., Usmani Z., Kumar V., Anshumali Biochar and Flyash Inoculated with Plant Growth Promoting Rhizobacteria Act as Potential Biofertilizer for Luxuriant Growth and yield of tomato plant. J. Environ. Manage. 2017;190:20–27. doi: 10.1016/j.jenvman.2016.11.060. [DOI] [PubMed] [Google Scholar]
- Troeh F.R., Thompson L.M. sixth ed. Blackwell Publishing; Ames, Iowa: 2005. Soils and Soil Fertility. [Google Scholar]
- Tsai W.T., Liu S.C., Chen H.R., Chang Y.M., Tsai Y.L. Textural and chemical properties of swine-manure-derived biochar pertinent to its potential use as a soil amendment. Chemosphere. 2012;89(2):198–203. doi: 10.1016/j.chemosphere.2012.05.085. [DOI] [PubMed] [Google Scholar]
- Tumbure A., Bishop P., Bretherton M., Hedley M. Co-Pyrolysis of maize stover and igneous phosphate rock to produce potential biochar-based phosphate fertilizer with improved carbon retention and liming value. ACS Sustainable Chem. Eng. 2020;8:4178–4184. [Google Scholar]
- Usman A.R.A., Sallam A.S., Al-Omran A., El-Naggar A.H., Alenazi K.K.H., Nadeem M., Al-Wabel M.I. Chemically modified biochar produced from Conocarpus wastes: an efficient sorbent for Fe (II) removal from acidic aqueous solutions. Adsorpt. Sci. Technol. 2013;31:625–640. [Google Scholar]
- Van Zwieten L., Singh B., Kimber S., Murphy D., Macdonald L., Rust J., Morris S. An incubation study investigating the mechanisms that impact N2O flux from soil following biochar application. Agric. Ecosyst. Environ. 2014;191:53–62. [Google Scholar]
- Verheijen F.G.A., Jeffery S., Bastos A.C., van der Velde M., Diafas I. European Commission; Luxembourg: 2009. Biochar Application to Soils - A Critical Scientific Review of Effects on Soil Properties, Processes and Functions; p. 149. EUR 24099 EN. [Google Scholar]
- Wali F., Naveed M., Bashir M.A., Asif M., Ahmad Z., Alkahtani J., Alwahibi M.S., Elshikh M.S. Formulation of biochar-based phosphorus fertilizer and its impact on both soil properties and chickpea growth performance. Sustainability. 2020;12:9528. [Google Scholar]
- Wang M., Wang J.J., Tafti N.D., Hollier C.A., Myers G., Wang X. Effect of alkali-enhanced biochar on silicon uptake and suppression of gray leaf spot development in perennial ryegrass. Crop Prot. 2019;119:9–16. [Google Scholar]
- Wen P., Wu Z., Han Y., Cravotto G., Wang J., Ye B.-C. Microwave-assisted synthesis of a novel biochar-based slow- release nitrogen fertilizer with enhanced water-retention capacity. ACS Sustainable Chem. Eng. 2017;5:7374–7382. [Google Scholar]
- Xu G., Lv Y., Sun J., Shao H., Wei L. Recent advances in biochar applications in agricultural soils: benefits and environmental implications. Clean. 2012;40:1093–1098. [Google Scholar]
- Yang W., Li C., Wang S., Zhou B., Mao Y., Rensing C., Xing S. Influence of biochar and biochar-based fertilizer on yield, quality of tea and microbial community in an acid tea orchard soil. Appl. Soil Ecol. 2021;166:104005. [Google Scholar]
- Yao C., Joseph S., Li L., Pan G., Lin Y., Munroe P., Pace B., Taherymoosavi S., Van Zwieten L., Thomas T., Nielsen S., Ye J., Donne S. Developing more effective enhanced biochar fertilisers for improvement of pepper yield and quality. Pedosphere. 2015;25(5):703–712. [Google Scholar]
- Ye J., Zhang R., Nielsen S., Joseph S., Huang D., Thomas T. A Combination of biochar–mineral complexes and compost improves soil bacterial processes, soil quality, and plant properties. Front. Microbiol. 2016;7:372. doi: 10.3389/fmicb.2016.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeboah S., Zhang R., Cai L., Li L., Xie J., Luo Z., Wu J., Antille D.L. Soil water content and photosynthetic capacity of spring wheat as affected by soil application of nitrogen-enriched biochar in a semiarid environment. Photosynthetica. 2017;55(3):532–542. [Google Scholar]
- Zhang Y., Cong J., Lu H., Li G., Qu Y., Su X., Zhou J., Li D. Community structure and elevational diversity patterns of soil Acidobacteria. J. Environ. Sci. 2014;26:1717–1724. doi: 10.1016/j.jes.2014.06.012. [DOI] [PubMed] [Google Scholar]
- Zhang H., Voroney R.P., Price G.W., White A.J. Sulfur-enriched biochar as a potential soil amendment and fertilizer. Soil Research. 2017;55:93–99. [Google Scholar]
- Zhang L.W., Shang Z.B., Guo K.X., Chang Z.X., Liu H.L., Li D.L. Speciation analysis and speciation transformation of heavy metal ions in passivation process with thiol-functionalized nano-silica. Chem. Eng. J. 2019;369:979–987. [Google Scholar]
- Zhang P., Yang F., Zhang H., Liu X., Chen J., Wang X., Wang Y., Li C. Beneficial effects of Biochar-based organic fertilizer on nitrogen assimilation, antioxidant capacities, and photosynthesis of sugar beet (Beta vulgaris L.) under saline-alkaline stress. Agronomy. 2020;10:1562. [Google Scholar]
- Zhang S., Lia Y., Singhd B.P., Caia H.W.X., Chena J., Qina H., Lia Y., Changa S.X. Contrasting short-term responses of soil heterotrophic and autotrophic respiration to biochar-based and chemical fertilizers in a subtropical Moso bamboo plantation. Appl. Soil Ecol. 2021;157:103758. [Google Scholar]
- Zhao M.Q., Zhao J.H., Zhang D., Han F.G., Zhang G.F., Li Y.S., Jin H.S. Effects of water retention agent on diurnal changes of photosynthetic characteristics in flue-cured tobacco. Sci. Agric. Sin. 2010;43(6):1265–1273. [Google Scholar]
- Zhao L., Cao X., Zheng W., Scott J.W., Sharma B.K., Chen X. Co-pyrolysis of biomass with phosphate fertilizers to improve biochar carbon retention, slow nutrient release, and stabilize heavy metals in soil. ACS Sustain. Chem. Eng. 2016;4(3):1630–1636. [Google Scholar]
- Zhou Z., Gao T., Van Zwieten L., Zhu Q., Yan T., Xue J., Wu Y. Soil microbial community structure shifts induced by biochar and biochar-based fertilizer amendment to Karst Calcareous soil. Soil Sci. Soc. Am. J. 2019;83:398–408. [Google Scholar]
- Zhou J., Qu T., Li Y., Van Zwieten L., Wang H., Chen J., Song X., Lin Z., Zhang X., Luo Y., Cai Y., Zhong Z. Biochar-based fertilizer decreased while chemical fertilizer increased soil N2O emissions in a subtropical Moso bamboo plantation. Catena. 2021;202:105257. [Google Scholar]
- Zornoza R., Moreno-Barriga E., Acosta J.A., Munoz M.A., Faz A. Stability, nutrient availability and hydrophobicity of biochars derived from manure, crop residues, and municipal solid waste for their use as soil amendments. Chemosphere. 2016;144:122–130. doi: 10.1016/j.chemosphere.2015.08.046. [DOI] [PubMed] [Google Scholar]
- Zwetsloot M.J., Lehmann J., Bauerle T., Vanek S., Hestrin R., Nigussie A. Phosphorus availability from bone char in a P-fixing soil influenced by root-mycorrhizae- biochar interactions. Plant Soil. 2016;408:95–105. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.