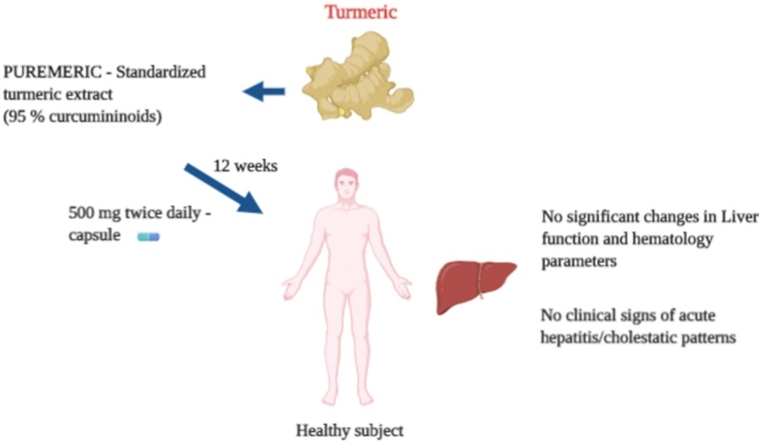
Graphical abstract
Keywords: Turmeric, Curcumin, Safety, Cholestatic hepatitis, Healthy subjects
Highlights
-
•
PUREMERIC™ is a standardized turmeric extract containing not less than 95 % curcuminoids.
-
•
12-week supplementation of 1g/day of PUREMERIC did not alter the liver function parameters of healthy human volunteers.
-
•
The safety of the extract was further confirmed by the insignificant changes in the hematological parameters and vital signs.
-
•
No serious adverse events were recorded during the study.
Abstract
Objective
Turmeric is a culinary spice valued since ancient time for its medicinal properties, mostly attributed to curcumin, the major polyphenol present. The safety of curcumin is well established in humans. However, the tolerability of curcumin is largely determined either in subjects with existing health problems, or in healthy individuals at low doses. More recently the safety of turmeric supplementation is opposed following some case reports on the occurrence of acute hepatitis due to its consumption.
Method
Here we have investigated the safety and tolerability of a standardized turmeric extract containing 95 % curcuminoids (PUREMERIC™) in an open label, single arm, prospective clinical study. Twelve healthy subjects aged 18–50 years received 500 mg PUREMERIC capsules twice daily for 90 days.
Results
After PUREMERIC supplementation, the liver function parameters such as aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), total and direct bilirubin, gamma-glutamyl transpeptidase (GGT), and lactate dehydrogenase (LDH) were not significantly altered in the serum compared to baseline. The hematological parameters were within the normal range.
Conclusion
Collectively, these data contradict the turmeric- induced liver damage and establishes the safety of the extract in healthy individuals.
1. Introduction
Turmeric is a spice derived from the rhizomes of Curcuma longa L., (Zingiberaceae). Herbal preparations from turmeric have been used extensively in Indian traditional medicine in various forms and routes [1]. Curcuminoids are the major bioactive components of turmeric, which include curcumin (diferuloyl methane), demethoxycurcumin, and bisdemethoxycurcmin. Curcumin is the main active component responsible for the nutritional and pharmacological activities of turmeric [2]. Curcumin, the major polyphenol in turmeric, has been attributed to oxidative stress management and anti-inflammation and arthritis [[3], [4], [5]].
The dietary consumption of turmeric in India accounts for 2–2.5 g/day in a 60-kg individual, corresponding to an ingestion of approximately 60−100 mg of curcumin daily and no toxicities or adverse events have been reported at the population level [6]. Several systematic studies have been performed in animals to assess the safety of curcumin. In the animal safety studies, an oral administration of curcumin up to 3500 mg/kg-BW for up to 90 days did not induce any adverse effects [7]. Administration of up to 1000 mg/kg B.W. curcumin to rats showed no reproductive toxicity as noticed in two successive generations. However, turmeric ingestion is also reported to be associated with liver toxicity in some animal species such as mice, and long-term intake in rats [8].
The safety of curcumin has been established through several clinical studies. In a study performed on Indian population, daily oral administration of 1200−2100 mg of curcumin for 2–6 weeks did not cause any toxicity [9]. In another study performed on patients with preinvasive malignant or high-risk premalignant conditions, administration of curcumin up to 8000 mg/kg daily for three months did not induce any toxic signs or adverse effects [10]. In a Phase I clinical trial by Sharma et al., daily oral administration of curcumin was well tolerated up to 3600 mg for up to 4 months in colorectal cancer patients [11]. Majority of clinical studies on curcumin were conducted in populations with existing health problems [12]. There are only a few reports evaluating the effects of curcumin consumption in healthy human volunteers. DiSilvestro et al. reported that a low dose supplementation of curcumin (80 mg/day for 4 weeks) could exert various health benefits in middle age subjects [13]. In another double-blind placebo-controlled study, 4-week treatment with 80 mg/day significantly improved the cognitive functions in healthy individuals [14]. Despite the undisputable benefits of consumption of curcumin, there are some adverse effects reported. In a dose escalation study from Lao et al., 30 % of the subjects administered with a single dose of 500−12000 mg experienced minor side effects like diarrhea, yellow stool, headache, and rashes [15]. More recently a single case was reported wherein the turmeric supplementation led to the occurrence of autoimmune hepatitis in a 71-year-old individual [16]. These findings necessitate the safety assessment of a higher dose of turmeric extract/curcumin over a longer duration in healthy subjects.
PUREMERIC™ is a Curcuma longa L. rhizome extract standardized to 95 % curcuminoids (HPLC method). PUREMERIC was previously evaluated for acute and sub-chronic toxicity using Wistar rats (data not published). Single dose oral toxicity of PUREMERIC in female Wistar rats showed that the LD50 of PUREMERIC was more than 2000 mg/kg B.W. The no-observed-adverse-effect level (NOAEL) of PUREMERIC as determined by repeated dose 90-day toxicity study was 1000 mg/kg BW. Further, to confirm the safety of PUREMERIC, the current study was accomplished in an open label, single arm, prospective clinical trial on healthy human volunteers.
2. Methods
2.1. Study design
An open label, single arm, prospective clinical study was conducted on 12 healthy human volunteers to evaluate the safety of PUREMERIC. The study was carried out at Anand Multi Specialty Hospital, Vadodara, Gujarat, India. A written informed consent signed by all subjects was obtained before the study initiation. After passing the eligibility criteria, subjects received PUREMERIC (standardized extract from C. longa containing 95 % curcuminoids, capsule form, self-administration), 500 mg orally, twice a day for 90 days. The study included 2 visits over a period of 90 days.
2.2. Ethical approval and consent
Study protocol along with informed consent form, and other appropriate study-related information were approved by Institutional Ethics Committee, Anand Multi Specialty Hospital, Vadodara, India. The aspects concerned with the study medications met the requirements of Good Manufacturing Practices. Each subject provided the written informed consent before the initiation of the clinical study. This trial was prospectively registered in Clinical Trials Registry – India (CTRI/2019/10/021611 dated 14/10/2019).
2.3. Subjects
Healthy male and female subjects aged 18–50 years with weight >50 kg and no evidence of any underlying disease were enrolled for this study. Subjects suffering from any chronic health conditions (e.g., diabetes, hypertension, chronic renal failure, heart, thyroid, and liver disease) requiring medical treatment were not included in the study. Other exclusion criteria were drug abusers, smokers, subjects with eating disorder and endocrine abnormalities, subjects undergoing cardiovascular surgery and subjects allergic to herbal products. Subjects not willing and able to give informed consent and not comply with the study procedures were also excluded from the study.
2.4. Intervention
The present study was conducted to evaluate the safety and tolerability of PUREMERIC, a proprietary extract prepared from the rhizomes of C. longa. PUREMERIC is standardized to 95 % curcuminoids as determined by HPLC analysis. Identification of the plant material was done at Vidya Herbs Pvt. Ltd., Bangalore, India. Subjects self-administered 500 mg PUREMERIC capsules orally, in morning and night after food. The total treatment period was for 90 days. The subjects had to record the date, time and amount taken for each administration of IP in a subject diary.
2.5. Study outcomes
The safety of PUREMERIC was assessed by the clinically significant changes from baseline (V1) to end of treatment (V2) in laboratory parameters (liver function, renal function and complete blood count), incidences of adverse effects and serious adverse effects (AE/SAE), physical examination parameters and vital signs.
The primary endpoint of the study was to assess the changes in liver function parameters [Aspartate aminotransferase (AST), Alanine aminotransferase (ALT), alkaline phosphatase (ALP), Gamma-glutamyl transpeptidase (GGT), Lactate Dehydrogenase (LDH), Bilirubin (Direct and Total)] from baseline to end of treatment.
The secondary endpoint of the study was to assess the safety of PUREMERIC (incidence of AEs/SAEs, Changes in laboratory parameters (Complete Blood Count, Serum creatinine), physical examination and incidence of abnormal vital signs.
2.6. Safety measurements
The safety evaluation was based on physical examination, vital signs, clinical laboratory tests and incidence of AEs. Physical examinations were performed by a physician and included the examination of the following: general appearance, eyes, ears, nose, throat, chest/respiratory, heart/cardiovascular, gastrointestinal/liver, musculoskeletal/extremities, dermatological/skin, thyroid/neck, lymph nodes, and neurological/psychiatric systems. All clinically significant treatment-emergent findings that were not present at baseline or described in the medical history were recorded as AEs.
Vital signs observed during the study included systolic blood pressure (SBP), diastolic blood pressure (DBP), pulse rate, and body temperature at baseline and end of treatment. Fasting blood samples were collected at visits V1 and V2 to evaluate the efficacy and safety analytical variables. Haematology parameter analysis included white blood cell count, red blood cell count, haemoglobin, mean cell volume and platelet count. Liver function tests included AST, ALT, ALP, GGT, LDH and Bilirubin (direct and total). Serum creatinine level was measured as a function of kidney. All the subjects were monitored throughout the study for any adverse (AEs) or serious adverse events (SAEs).
2.7. Statistical analysis
The statistical analysis was performed by SPSS 22.0 (SPSS Statistics for Windows Version 22.0, Armonk, NY, IBM Corp) and are two-sided at a significant level of 0.05.
3. Results
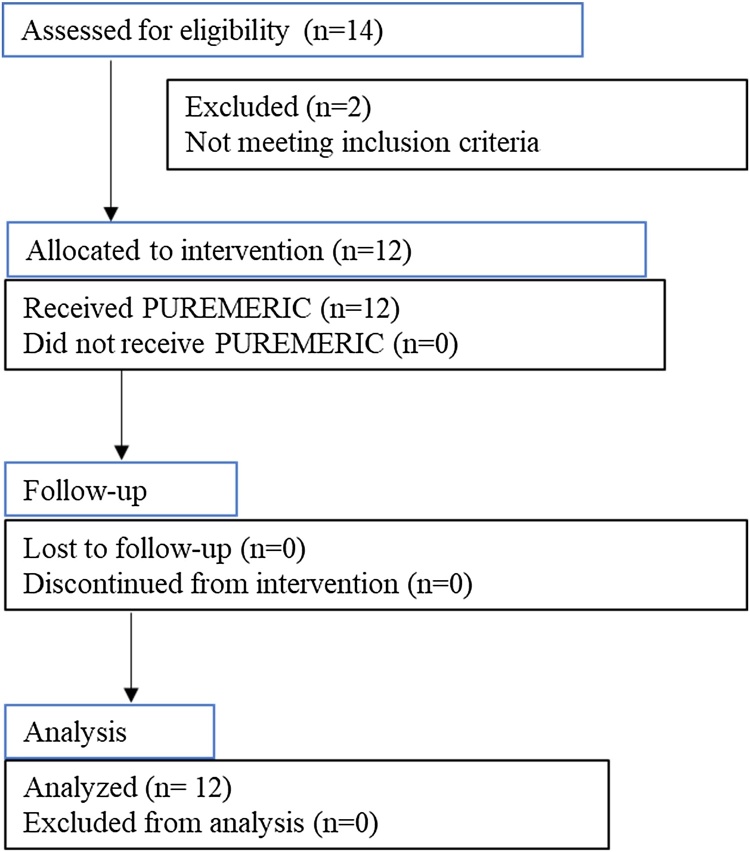
Fig. 1 shows the participant flow chart from subject selection to the analysis. Fourteen individuals were screened of which 12 subjects meeting the inclusion criteria enrolled for the study. 2 subjects were failed to meet the eligibility criteria and excluded from the study. All the 12 subjects completed the study. The mean age of subjects enrolled in the study was 31.17 ± 5.86 years. This study included all the subjects of Indian origin. There were no significant differences found in the anthropometric measurements (height, weight and BMI) from baseline to the end of study (Table 1).
Fig. 1.
Study participant flowchart.
Table 1.
Anthropometric assessments of subjects.
| Variable (Unit) | Visit 1 (Baseline) | Visit 2 (EOS) | p-value # (Visit 1 vs. Visit 2) |
|---|---|---|---|
| Weight (kg) | 59.72 ± 4.96 | 59.93 ± 4.90 | 0.4526 |
| Height (cm) | 161.67 ± 6.93 | 161.67 ± 6.93 | – |
| BMI (kg/m2) | 22.83 ± 1.02 | 22.91 ± 1.09 | 0.4789 |
The data were represented as mean ± SD; # p values were compared from baseline using Paired sample t-test. EOS: End of study.
The primary end point analysis included the assessment of liver function parameters (Table 2). There were no statistically significant variations observed in the levels of AST, ALT, ALP, GGT, LDH and bilirubin from baseline to the end of treatment. Further, the serum creatinine appeared to be in the normal range throughout the study. Summary of hematological analyses is presented in Table 3. All the evaluated parameters were within the normal range and no significant changes observed from baseline to the end of study. There was no considerable change in the vital signs measured at baseline and the end of study (Table 4).
Table 2.
Summary of changes in blood biochemical parameters (N = 12 subjects).
| Parameters (Units) | Reference range | Visits |
p-value # (Baseline vs. EOS) | |
|---|---|---|---|---|
| Baseline | EOS | |||
| Aspartate aminotransferase (U/L) | 5−50 | 28.03 ± 5.70 | 27.85 ± 5.75 | 0.8577 |
| Alanine aminotransferase (U/L) | 5−45 | 31.49 ± 6.17 | 31.05 ± 6.02 | 0.6294 |
| Alkaline phosphatase (U/L) | 42−141 | 91.56 ± 21.61 | 91.48 ± 26.78 | 0.9819 |
| Gamma-glutamyl transpeptidase (U/L) | 10−50 | 28.12 ± 7.54 | 27.50 ± 6.27 | 0.6474 |
| Lactate dehydrogenase (U/L) | 225−450 | 319.53 ± 50.45 | 314.31 ± 56.04 | 0.5259 |
| Bilirubin (total) (mg/dl) | 0−1.4 | 0.93 ± 0.16 | 0.92 ± 0.13 | 0.5028 |
| Bilirubin (direct) (mg/dl) | 0−0.6 | 0.48 ± 0.10 | 0.44 ± 0.04 | 0.2691 |
| Serum creatinine (mg/dl) | 0.6−1.4 | 1.09 ± 0.19 | 1.05 ± 0.17 | 0.1298 |
The data were represented as mean ± SD. # p values were compared from baseline using Paired Sample t-test. EOS: End of study.
Table 3.
Haematology parameter analysis (N = 12 subjects).
| Parameters (Units) | Reference range | Visits |
p-value # (Baseline vs. EOS) | |
|---|---|---|---|---|
| Baseline | EOS | |||
| Total Leukocyte Count (mm3) | 4000−10500 | 7158.33 ± 1552.39 | 7183.33 ± 1657.95 | 0.9039 |
| Red Blood Cell count (million/cmm) | 4.6−6.5 | 4.91 ± 0.19 | 4.90 ± 0.23 | 0.8578 |
| Haemoglobin (g/dL) | 12−17 | 13.82 ± 0.66 | 13.71 ± 0.75 | 0.3034 |
| Haematocrit (%) | 35−54 % | 41.19 ± 1.70 | 41.12 ± 2.15 | 0.8512 |
| Mean Cell Volume (fL) | 80−96 | 83.93 ± 1.55 | 83.98 ± 1.96 | 0.9086 |
| Mean Cell Haemoglobin (MCH) (pg) | 27−33 | 28.15 ± 0.89 | 28.00 ± 0.88 | 0.3159 |
| MCH Concentration (%) | 32−36 % | 33.54 ± 0.82 | 33.33 ± 0.65 | 0.1356 |
| Platelet Count (mm3) | 150000−450000 | 291666.70 ± 53720.38 | 288916.70 ± 44397.91 | 0.7496 |
The data were represented as mean ± SD. # p values were compared from baseline using Paired Sample t-test. EOS: End of study; mm3: Cubic millimetre; g/dl: Gram/decilitre; fL: femtolitres, 10−15 L; pg: picograms.
Table 4.
Vital signs observed during the study (N = 12 subjects).
| Parameters (Units) | Visits |
p-value # (Baseline vs. EOS) | |
|---|---|---|---|
| Baseline | EOS | ||
| Temperature (°F) | 97.74 ± 0.69 | 98.05 ± 0.30 | 0.0883 |
| Pulse rate (beats/minute) | 73.08 ± 5.25 | 74.92 ± 3.45 | 0.2966 |
| Systolic blood pressure (mmHg) | 115.83 ± 9.00 | 118.33 ± 7.18 | 0.3388 |
| Diastolic blood pressure (mmHg) | 74.17 ± 6.69 | 75.00 ± 6.74 | 0.5862 |
The data were represented as mean ± SD. EOS: End of study; #: Paired t-test (Baseline vs End of study).
The study participants were monitored for the occurrence of AEs and/or SAEs throughout the study. Three subjects reported 3 AEs which included fever and diarrhea, during the study period (Table 5). The outcomes of all the AEs was noted as resolved before the end of the study. All the 3 subjects used at least one parallel medication during the study. The medication included paracetamol and oral rehydration solution. None of the subject reported any SAE or was withdrawn from the study due to AE/SAEs (Table 6).
Table 5.
Adverse events experienced by subjects during the study.
| Adverse events | Overall (N = 12) n (%) |
|---|---|
| Total number of AEs reported | 3 (25) |
| Subjects reporting at least one AE | 3 (25) |
| Fever | 2 (16.67) |
| Diarrhoea | 1 (8.33) |
| Total Number of SAEs Reported | 0 |
| Subjects Reporting drug-related AEs | 0 |
| Subjects Reporting AEs leading to early discontinuation | 0 |
| Number of Deaths | 0 |
Data presented as n (%): Number of subjects (%).
Table 6.
Summary of adverse events by severity, causality treatment and concomitant medication used by subjects during the study.
| Adverse event term | Overall (N = 12) n (%) |
|---|---|
| Subjects with atleast one AE | 3 (25 %) |
| Severity | |
| Mild | 3 (25 %) |
| Moderate | 0 (0) |
| Severe | 0 (0) |
| Casuality | |
| Related | 0 (0) |
| Not related | 3 (25 %) |
| Outcome | |
| Resolved | 3 (25 %) |
| Ongoing | 0 (0) |
| Total number subjects with at least one concomitant medication | 3 (25 %) |
| Paracetamol | 2 (16.67) |
| Oral rehydration solution (ORS) | 1 (8.33) |
Data presented as n (%): Number of subjects (%).
4. Discussion
The present clinical trial was conducted to evaluate the safety of a turmeric extract containing 95 % curcuminoids in healthy subjects. Nevertheless, turmeric is a regularly consumed spice, content of curcumin ingested in such form is lesser than the extract itself [17]. Hence, there is an absolute requirement of determining the safety of turmeric extract at higher doses. The available literature document the safety of turmeric extract and curcumin mostly in patients having health issues [[18], [19], [20]].
In an open label, single arm pilot study we have demonstrated the safety of turmeric extract, PUREMERIC having 95 % total curcuminoids (curcumin, bisdemethoxycurcumin and demethoxycurcumin). There are several reports on the benefits of curcumin in healthy population. In a double-blind clinical study from Oliver et al. curcumin at 200 mg dose administered for 8 weeks significantly improved the endothelial function in healthy subjects [21]. In another study on healthy individuals aged 40–60 years, supplementation of low dose of lipidated form of curcumin (80 mg/day) showed several health benefits [13]. Despite having physiological functions there are some adverse effects reported with curcumin supplementation. More recently, Luber et al., reported two case studies indicating liver toxicity due to the consumption of turmeric supplement [22]. Here we have rationalized the safety of turmeric supplementation in healthy subjects, specifically measuring the liver function parameters.
The blood biochemical and hematological parameters were measured of twelve subjects who received 500 mg of turmeric extract supplementation. AST, ALT and ALP are the predominant blood markers of liver injury. In this study, there was no significant change in the levels of these serum non-specific markers from baseline to the end of study. The mean AST and ALT levels were found to be not exceeding 40 U/L following a 12-week consumption of PUREMERIC which clearly indicated the normal liver function of the participants [23]. Our results contradict the previous case reports on turmeric-induced acute hepatitis [22].
Acute hepatitis results from etiologies including alcohol, drugs, or conditions such as biliary tract dysfunction (cholestatic hepatitis) [24]. Cholestasis (acute and chronic) in general is presented by elevated AST, ALT, ALP, and total bilirubin or hyperbilirubinemia associated with elevated ALP [[25], [26], [27]]. In our study, there was no clinically significant changes in the serum levels of ALP and bilirubin indicating no signs of cholestatic liver damage. These data further confirm the safety of turmeric extract/curcumin consumption. In this study, we have measured the serum GGT level at baseline and the end of treatment. GGT is a non-specific serum marker of drug-induced damage to vital organs such as liver, brain, pancreas, kidney, heart, and seminal vesicles [28]. A 12-week administration of PUREMERIC did not cause significant elevation in the serum GGT level.
Examination of hematological parameters provides valuable information on the potential harmful effect of any foreign substances in the body [29]. Administration of PUREMERIC resulted in marginal changes of blood parameters.
Overall, the findings from our study confirms the safety of orally ingested turmeric extract (95 % curcuminoids) in healthy adult subjects and opposes the possibility of any hepatic damage caused by turmeric/curcumin supplementation. However, the limitations of this clinical study include the small sample size and the shorter duration of treatment.
Ethics approval and consent to participate
This clinical trial was approved by Institutional Ethics Committee, Anand Multi Specialty Hospital, Vadodara, India. Subjects meeting all inclusion and no exclusion criteria signed a written informed consent and enrolled in the study.
Conflict of Interest
The authors declare no conflict of interest.
Funding
Not applicable
Authors’ contributions
All the authors have read and approved the manuscript. Conceptualization: KS; Study design: HVS and TVJ; Study monitoring and coordination: HVS; writing – original draft preparation: HSV, TVJ and HVS; review and editing, HVS and KS.
Declaration of Competing Interest
The authors report no declarations of interest.
Handling Editor: Dr. Aristidis Tsatsakis
References
- 1.Prasad S., Aggarwal B.B. In: Herbal Medicine: Biomolecular and Clinical Aspects. 2nd ed. Benzie I.F.F., Wachtel-Galor S., editors. CRC Press/Taylor & Francis; Boca Raton (FL): 2011. Turmeric, the Golden spice: from traditional medicine to modern medicine. [PubMed] [Google Scholar]
- 2.Ruby A.J., Kuttan G., Babu K.D., et al. Anti-tumour and antioxidant activity of natural curcuminoids. Cancer Lett. 1995;94(1):79–83. doi: 10.1016/0304-3835(95)03827-j. [DOI] [PubMed] [Google Scholar]
- 3.Jurenka J.S. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern. Med. Rev. 2009;14(2):141–153. [PubMed] [Google Scholar]
- 4.Aggarwal B.B., Harikumar K.B. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int. J. Biochem. Cell Biol. 2009;41(1):40–59. doi: 10.1016/j.biocel.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chainani-Wu N. Safety and anti-inflammatory activity of curcumin: a component of turmeric (Curcuma longa) J. Altern. Complement. Med. 2003;9:161–168. doi: 10.1089/107555303321223035. [DOI] [PubMed] [Google Scholar]
- 6.Shah B.H., Nawaz Z., Pertani S.A., et al. Inhibitory effect of curcumin, a food spice from turmeric, on platelet-activating factor and arachidonic acid-mediated platelet aggregation through inhibition of thromboxane formation and Ca2+ signaling. Biochem. Pharmacol. 1999;58:1167–1172. doi: 10.1016/s0006-2952(99)00206-3. [DOI] [PubMed] [Google Scholar]
- 7.National Cancer Institute Clinical development plan: curcumin. J. Cell. Biochem. Suppl. 1996;26:72–85. [PubMed] [Google Scholar]
- 8.Ganiger S., Malleshappa H.N., Krishnappa H., et al. A two generation reproductive toxicity study with curcumin, turmeric yellow, in Wistar rats. Food Chem. Toxicol. 2007;45:64–69. doi: 10.1016/j.fct.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 9.Deodhar S.D., Sethi R., Srimal R.C. Preliminary study on anti-rheumatic activity of curcumin (Diferuloyl methane) Indian J. Med. Res. 1980;71:632–634. [PubMed] [Google Scholar]
- 10.Cheng A.L., Hsu C.H., Lin J.K., et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21:2895–2900. [PubMed] [Google Scholar]
- 11.Sharma R.A., Euden S.A., Platton S.L., et al. Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin. Cancer Res. 2004;10:6847–6854. doi: 10.1158/1078-0432.CCR-04-0744. [DOI] [PubMed] [Google Scholar]
- 12.Hewlings S.J., Kalman D.S. Curcumin: a review of its’ effects on human health. Foods. 2017;6(10):92. doi: 10.3390/foods6100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DiSilvestro R.A., Joseph E., Zhao S., et al. Diverse effects of a low dose supplement of lipidated curcumin in healthy middle-aged people. Nutr. J. 2012;11:79. doi: 10.1186/1475-2891-11-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cox K.H., Pipingas A., Scholey A.B. Investigation of the effects of solid lipid curcumin on cognition and mood in a healthy older population. J. Psychopharmacol. (Oxford) 2015;29:642–651. doi: 10.1177/0269881114552744. [DOI] [PubMed] [Google Scholar]
- 15.Lao C.D., Ruffin M.T., Normolle D., et al. Dose escalation of a curcuminoid formulation. BMC Complement. Altern. Med. 2006;6:10. doi: 10.1186/1472-6882-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lukefahr A.L., McEvoy S., Alfafara C., et al. Drug-induced autoimmune hepatitis associated with turmeric dietary supplement use. BMJ Case Rep. 2018;2018 doi: 10.1136/bcr-2018-224611. bcr2018224611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sahebkar A., Mohammadi A., Atabati A., et al. Curcuminoids modulate pro-oxidant-antioxidant balance but not the immune response to heat shock protein 27 and oxidized LDL in obese individuals. Phytother. Res. 2013;27(12):1883–1888. doi: 10.1002/ptr.4952. [DOI] [PubMed] [Google Scholar]
- 18.Shep D., Khanwelkar C., Gade P., et al. Safety and efficacy of curcumin versus diclofenac in knee osteoarthritis: a randomized open-label parallel-arm study. Trials. 2019;20:214. doi: 10.1186/s13063-019-3327-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saghatelyan T., Tananyan A., Janoyan N., et al. Efficacy and safety of curcumin in combination with paclitaxel in patients with advanced, metastatic breast cancer: a comparative, randomized, double-blind, placebo-controlled clinical trial. Phytomedicine. 2020;2020(70) doi: 10.1016/j.phymed.2020.153218. [DOI] [PubMed] [Google Scholar]
- 20.Kanai M., Otsuka Y., Otsuka K., et al. A phase I study investigating the safety and pharmacokinetics of highly bioavailable curcumin (Theracurmin) in cancer patients. Cancer Chemother. Pharmacol. 2013;71:1521–1530. doi: 10.1007/s00280-013-2151-8. [DOI] [PubMed] [Google Scholar]
- 21.Oliver J.M., Stoner L., Rowlands D.S., et al. Novel form of curcumin improves endothelial function in young, healthy individuals: a double-blind placebo controlled study. Foods. 2016;2016:1–6. doi: 10.1155/2016/1089653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luber R.P., Rentsch C., Lontos S., et al. Turmeric induced liver injury: a report of two cases. Case Reports Hepatol. 2019;2019:1–4. doi: 10.1155/2019/6741213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neuschwander-Tetri B.A., Unalp A., Creer M.H., et al. Influence of local reference populations on upper limits of normal for serum alanine aminotransferase levels. Arch. Intern. Med. 2008;168(6):663–666. doi: 10.1001/archinternmed.2007.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ryder S.D., Beckingham I.J. ABC of diseases of liver, pancreas, and biliary system: acute hepatitis. BMJ. 2001;322(7279):151–153. doi: 10.1136/bmj.322.7279.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lala V., Goyal A., Bansal P., et al. Liver function tests. Updated 2020 Jul 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482489/.
- 26.Roche S.P., Kobos R. Jaundice in the adult patient. Am. Fam. Physician. 2004;69:299–304. [PubMed] [Google Scholar]
- 27.Green R.M., Flamm S. AGA technical review on the evaluation of liver chemistry tests. Gastroenterology. 2002;123:1367–1384. doi: 10.1053/gast.2002.36061. [DOI] [PubMed] [Google Scholar]
- 28.Koenig G., Seneff S. Gamma-glutamyltransferase: a predictive biomarker of cellular antioxidant inadequacy and disease risk. Dis. Markers. 2015;2015:818570. doi: 10.1155/2015/818570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yakubu A., Adua M.M., Adamude H. Welfare and hematological indices of weaner rabbits as affected by stocking density. Proceedings of the 9th World Rabbit Congress; Verona, Italy; 2008. [Google Scholar]