Summary
Cells with mesenchymal stem cell properties have been identified in menstrual blood and termed menstrual blood-derived stem/stromal cells (MenSCs). MenSCs have been proposed as ideal candidates for cell-based therapy in regenerative medicine and immune-related diseases. However, MenSCs identity has been loosely defined so far and there is controversy regarding their cell markers and differentiation potential. In this review, we outline the origin of MenSCs in the context of regenerating human endometrium, with attention to endometrial eMSCs as reference cells to understand MenSCs. We summarize the cell identity markers analyzed and the immunomodulatory and reparative properties reported. We also address the recent use of MenSCs in cell reprogramming. The main goal of this review is to contribute to the understanding of the identity and properties of MenSCs as well as to identify potential caveats and new venues that deserve to be explored to strengthen their potential applications.
Subject areas: Reproductive medicine, Cell biology, Stem cells research
Graphical abstract
Reproductive medicine; Cell biology; Stem cells research
Origin: the human endometrium and endometrial stromal cells
Endometrial regeneration stem cell hypothesis
Human endometrium is the mucosal lining of the uterus and consists of luminal epithelium, glandular epithelium, and an extensively vascularized stroma. It is divided into two regions with different structure and function: the functionalis and the basalis. The functionalis consists of the upper two-thirds of the glands lined with pseudostratified columnar epithelium and is surrounded by loose vascularized stroma, and the lower basalis consists of the lower third of glands, dense stroma, and large vessels. Every month, the functionalis is shed during menses and regenerated while the basalis remains. This level of new tissue growth is similar to the cellular turnover in the highly regenerative bone marrow hematopoietic tissue, the epidermis, and intestinal epithelium. The fact that adult stem cells are responsible for cellular production in these continuously regenerating tissues inspired the endometrial regeneration stem cell hypothesis, in which adult stem or progenitor cells are responsible for the cyclical regeneration of the endometrial functionalis every month and in which these adult stem cells would reside in the basalis (reviewed in Gargett et al., 2007).
This hypothesis was supported by numerous data using endometrial biopsy tissue that showed the presence of small populations of adult endometrial stem/stromal cells with classic stem-cell properties of clonogenicity, self-renewal, and differentiation (Schwab et al., 2005; Schwab and Gargett, 2007; Chan et al., 2004; Gargett et al., 2009). Specific stem/progenitor cells include epithelial stem/progenitor cells, endometrial mesenchymal stem/stromal cells (eMSCs), and endothelial progenitor cells, based on the expression of specific surface markers by the different cell populations in culture and on their differentiation potential (reviewed in Gargett et al., 2016). Some authors also include the side population (SP) cells to identify or to enrich the stem cell fraction within the aforementioned endometrial stem cell populations, according to their cellular identity and differentiation potential (Kato et al., 2007; Masuda et al., 2015). SP cells are defined as a small population of cells capable of effluxing the vital DNA-binding dye, as this assay has been used to identify potential stem/progenitor cells within a cell population (Challen and Little, 2006).
It has been hypothesized that human endometrial epithelial stem/progenitor cells are located in the basalis layer (Prianishnikov, 1978; Padykula, 1991; Padykula et al., 1984; Gargett, 2007) (Figure 1). They were first identified as clonogenic cells, comprising 0.22% of single cell suspensions of EpCAM + epithelial cells obtained from hysterectomy tissue, which includes the basalis layer (Chan et al., 2004; Schwab et al., 2005), and up to 27% of clonogenic EpCAM + cells when only the SP cells are analyzed (Miyazaki et al., 2012; Gargett et al., 2016). Clonally derived EpCAM + epithelial cells differentiate into cytokeratin + gland-like structures in three dimensional culture (Gargett et al., 2009) and SP EpCAM + cells can reconstitute epithelial glands when transplanted into immunocompromised mice, although at very low efficiency (0.02–8%) (Masuda et al., 2010; Cervello et al., 2011). Specific markers identifying the human endometrial epithelial stem cell population are still under investigation and N-Cadherin and SSEA-1 have been suggested as two promising candidates (Valentijn et al., 2013; Nguyen et al., 2017), as recently reviewed by Cousins et al. (Cousins et al., 2021).
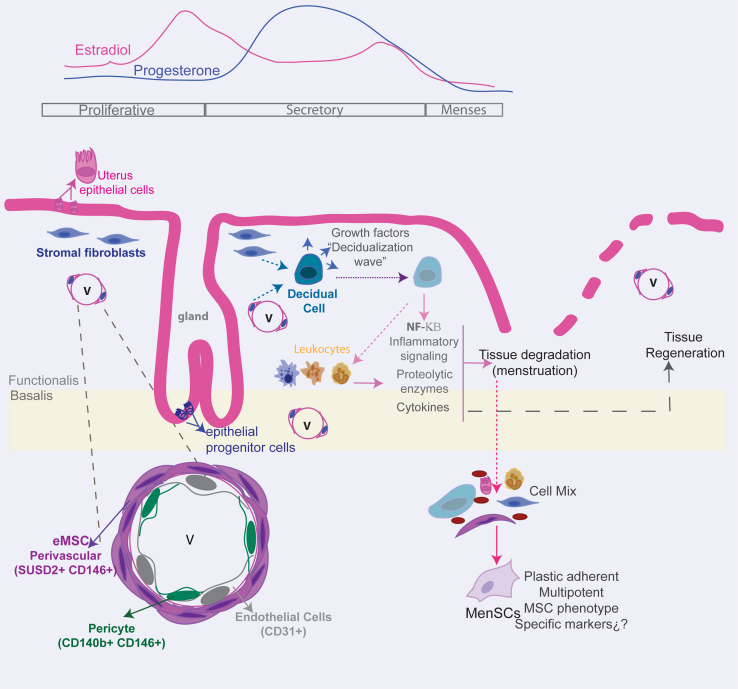
Figure 1.
Schematic showing ovarian hormonal changes during proliferative, secretory, and menses phases affecting cellular and tissue processes in human endometrium
Endometrial MSCs (eMSCs) are located around the vessels (v). A schematic enlargement of blood vessels shows the different vascular cell types according to their identified surface markers (pericytes, perivascular eMSCs, and endothelial cells). During the secretory phase, increased progesterone levels and other paracrine molecules induce the differentiation of endometrial stromal/stem cells into secretory decidualized cells that contribute to amplify the decidualization process within the endometrium stroma. In the absence of conception, hormone withdrawal is sensed by stromal cells that upregulate intracellular inflammatory signaling, recruit leukocytes, and secrete growth factors, cytokines, and proteolytic enzymes, provoking and propagating the tissue shedding of menstruation. This signaling also participates in tissue repair after menstruation. Menstrual blood contains a variety of cells whose identity has not been deeply analyzed, but when they are subjected to adherent culture conditions, a cell type called menstrual-blood derived stromal cell (MenSC) can be identified by its ISCT surface MSC-markers and multipotent phenotype, although its specific identity markers need further study.
It has been postulated that cells expressing CD31 and CD34 (classical endothelial markers) detected among the SP endometrial cells are endothelial stem cells that are located in the basalis and vascular endothelium (Tsuji et al., 2008). When endometrial SP cells are transplanted under the kidney capsule of immunodeficient mice, they generate endothelial cells that migrate into the mouse kidney parenchyma and form mature blood vessels, thus showing potential for in vivo angiogenesis (Masuda et al., 2010). More current data would be desirable to confirm the results of the relative presence of endothelial progenitor cells in the endometrium, as well as the efficiency of their differentiation potential in vitro and in vivo.
The term MSCs describes a cell population of multipotential stem/progenitor cells commonly referred to as mesenchymal stem cells, multipotential stromal cells, mesenchymal stromal cells, and mesenchymal progenitor cells (Pittenger et al., 2019). Almost all human tissues contain a small resident population of perivascular mesenchymal stem/stromal cells (MSC) (Bozorgmehr et al., 2020). MSCs were originally identified in bone marrow cultures and the defining features of bone marrow MSCs (bmMSCs), according to International Society for Cellular Therapies (ISCT), are: plastic adherence, multilineage differentiation into bone and marrow lineages (osteocytes, chondrocytes, adipocytes) in vitro, and a surface phenotype (CD29+, CD44+, CD73+, CD90+, CD105+, CD146+, CD31-, CD34-and CD45-) distinguishing them from hematopoietic stem cells (HSCs), also resident in marrow (Dominici et al., 2006; Caplan, 2007).
However, cultured fibroblasts from the endometrium (stromal cells), bone marrow, and many organs also display these classic bmMSC properties in vitro, encouraging MSC researchers to question the utility of these defining features (Hematti, 2012; Bianco et al., 2013; Phinney and Sensebe, 2013) and the necessity for using the appropriate definition of the cells under study (as perivascular MSC versus fibroblasts).
Human endometrium also contains a small population of mesenchymal stem/stromal cells called endometrial MSCs (eMSCs) (Gargett et al., 2016). Specific surface markers of clonogenic eMSCs demonstrate their perivascular localization in the endometrial functionalis and basalis and show that these markers enrich for clonogenic cells (Schwab and Gargett, 2007; Masuda et al., 2012).
The menses phase
Part of the complexity of studying the identity of eMSCs is that the human endometrium is not a tissue with a single steady state, but its structure, biochemical environment, and function change, not only from birth to old age, but during each menstrual cycle under the influence of steroid hormones from the ovary, in an orderly sequence of cellular and tissue processes.
Decidualization is the process of spontaneous terminal differentiation of endometrial stromal cells into specialized secretory decidual cells, which occurs in the mid-to-late secretory phase of each menstrual cycle driven by the postovulatory increase in circulating progesterone levels, increasing local cAMP production and local paracrine factors (Murakami et al., 2014; Evans et al., 2016). In human and other menstruating species, this process is spontaneous and occurs in response to endocrine signaling; whereas in nonmenstruating species, it is initiated during pregnancy because of embryonic cues (Emera et al., 2012). In humans, decidualization is initiated by progesterone signaling in stromal cells adjacent to blood vessels. Decidual regulation is a complex process with many molecules and signaling pathways involved, with estrogen or glucocorticoid receptors being included, as reviewed elsewhere (Gellersen and Brosens, 2014). Whether the transformation is initiated in perivascular eMSCs and stromal fibroblast surrounding spiral arterioles simultaneously or it is a sequential process has not been studied. These cells undergo mesenchymal to epithelial transition (MET) to become rounded secretory cells expressing the progesterone receptor and decidual markers as prolactin and insulin-like growth factor-binding protein 1 (Gellersen and Brosens, 2014). Decidual cells secrete factors to create a wave of decidualization that spreads to cover the whole superficial endometrial layer (including blood vessels surrounding and under the luminal epithelium) as the cycle progresses.
If pregnancy occurs, decidual cells promote the invasion of fetal extravillous trophoblasts (Grewal et al., 2008; Gonzalez et al., 2011) that, along with the uterine natural killer (uNK) cells, facilitate spiral-artery remodeling (Lockwood et al., 2007, 2009) and protect the embryo by conferring maternal immunotolerance of the fetal allograft (Nancy et al., 2012; Croxatto et al., 2014). The decidual cells also shelter the embryo from environmental stress signals (Kajihara et al., 2006; Leitao et al., 2010) and sense embryo viability to enable maternal rejection of developmentally incapable embryos (Teklenburg et al., 2010).
However, in the absence of conception, the withdrawal of estrogen and progesterone support leads to the shedding of terminally differentiated cells during menstruation by removal of the functionalis layer through the menstrual blood, whereas the basalis remains (Figure 1).
Menstruating endometrium is a complex environment that is not fully understood and in which decidualized cells, non-progesterone receptor expressing stromal cells, and leukocytes release inflammatory mediators, growth factors, and proteolytic enzymes that result in tissue breakdown (reviewed in Evans and Salamonsen, 2012). Intriguingly, endometrial tissue destruction simultaneously produces cues for repair and re-epithelialization. Similar inflammatory, growth factors, proteolytic enzymes, and recruited leukocytes contribute to epithelial repair together with mesenchymal to epithelial transition of stromal cells and Wnt signaling activation (reviewed in Evans et al., 2016).
The precise mechanisms by which the various endometrial cell populations control extracellular matrix (ECM) degradation in the functionalis are clear, whereas preserving the basalis and the respective contribution of basalis and functionalis in endometrium regeneration, are still unclear. Nevertheless, the elevated expression of proteases and gene products involved in extracellular matrix synthesis in stromal cells of lysed endometrium areas (Gaide Chevronnay et al., 2009; Evans et al., 2011) supports the hypothesis that fragments of the functionalis participate in endometrial regeneration during late menstruation (Evans et al., 2016).
The endometrial stem cell hypothesis for the regeneration of the endometrium suggests a major role of activation of basalis endometrial epithelial progenitor cells and both basalis and functionalis perivascular mesenchymal stem cells, possibly involving Wnt and Notch signaling in the process (Gargett and Masuda, 2010).
eMSCs identity markers
eMSCs were identified by the co-expression of two perivascular cell markers, CD146 and platelet-derived growth factor receptor beta (PDGFRβ or CD140b) (Schwab and Gargett, 2007), as pericytes (Figure 1). The CD146 + CD140b + subpopulation comprises 1.5% of endometrial clonogenic stromal cells and fulfills the ISCT MSC criteria (Dominici et al., 2006). These endometrial perivascular cells are distinct from the endometrial stromal CD140b + CD146-fibroblasts showing 762 differentially expressed genes (Spitzer et al., 2012) and from the endothelial (CD140b-CD146+) population.
SUSD2 was the only marker identified from a panel of perivascular markers assayed by flow cytometry, capable of isolating clonogenic eMSC from human endometrium (Masuda et al., 2012). This marker, sometimes referred to as W5C5, recognizes the sushi domain containing-2 (SUSD2) antigen (Sivasubramaniyan et al., 2013). SUSD2+ cells constituted 4.1% of endometrial clonogenic stromal cells and they fulfilled the ISCT criteria of surface marker expression and differentiation capacity (Masuda et al., 2012).
Most SUSD2+ cells expressed CD140b, whereas all SUSD2+CD146 + cells were positive for CD140b. These SUSD2+CD146 + cells generated more CFUs than the CD146 + CD140b + subpopulation, suggesting that the combination of these three markers may enrich the perivascular eMSC population (Masuda et al., 2012). In addition, gene profiling of both freshly isolated CD146 + CD140b + cells (Spitzer et al., 2012) and cultured SUSD2+ cells (Murakami et al., 2014) confirmed that eMSCs show pericytic, perivascular signature, which suggest that eMSCs may have and additional role in angiogenesis during stromal regeneration and placentation (Gargett et al., 2016).
The expression of other bmMSCs markers by SUSD2+ cells was also explored by Masuda et al. using flow cytometry (Masuda et al., 2012). They found that 60% of the cells express STRO-1 and that 84% express CD117 (c-KIT). However, the expression of these markers by eMSCs has been controversial, as they are not routinely analyzed (Rajaraman et al., 2013; Gurung et al., 2015). These results have not been reproduced in vitro for either STRO-1 (Gargett et al., 2009) or CD117 (Zlatska et al., 2017). Endometrial CD146 + PDGFRb + cells were found to be negative for STRO-1 (Schwab and Gargett, 2007; Gargett et al., 2009) and STRO-1 does not enrich for human endometrial stromal CFU when fresh endometrial stromal cells are analyzed (Schwab et al., 2008) (Table 1), although differences between freshly isolated and cultured cells can be due to the effect of cell culturing on marker expression.
Table 1.
Cell markers on eMSCs and MenSCs
| Reference cell type/lineage for the different set of markers | Marker | Endometrial mesenchymal stromal cells |
Menstrual blood-derived stromal cells |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| eMSC | SUSD2+ eMSCs | CD117 + MenSCs | ERC | MenSCs | MMCs | ||||||||||||||
| Mesenchymal (bm-MSC) | CD29 | + | + | + | + | + | + | + | + | + | + | + | + | ||||||
| CD44 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ||||
| CD73 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | |||||
| CD90 | + | + | + | + | + | + | + | + | + | + | + | + | + | ||||||
| CD105 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ||||
| STRO1 | – | – | + | – | – | – | |||||||||||||
| CD117 | + | + | – | – | |||||||||||||||
| CD10 | |||||||||||||||||||
| Endothelial | CD31 | – | – | – | – | – | |||||||||||||
| Hematopoietic | CD34 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | ||||
| CD45 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | |||||
| HLA-DR | – | – | – | – | – | ||||||||||||||
| Pericyte | CD146 | + | + | + | + | + | + | + | + | + | |||||||||
| CD140b | + | + | + | + | + | ||||||||||||||
| SUSD2 | + | + | |||||||||||||||||
| Pluripotency | OCT4 | + | + | + | + | + | – | ||||||||||||
| NANOG | – | – | – | ||||||||||||||||
| SSEA4 | + | – | + | + | |||||||||||||||
| hTERT | + | ||||||||||||||||||
| Other markers analyzed | Negative expression | CD106- CD184- CD271- CD325- | MHCII - CD38- | CD14- CD38- | CD14 - CD50 - CD133 - HLA-DR - | CD133- CD38- | CD38- CD133- | CD38- | CD14- CD106- CD133- | HLADR- | CD14- CD133- | CD14- CD16- CD19- CD133- | |||||||
| Positive expression | CD166+ | MHCI + CD166 + CD9+ | CD9+ CD59 + CD41a+ | CD13 + CD54 + CD55 + CD59+ HLA-ABC | CD10+ | CD9+ CD10+ | CD166+ | CD10+ | CD13+ | CD10 + CD55 + CD59 + CD166+ | CD13 + CD54 + CD166 + CD59+ HLA-ABC+ | ||||||||
| References | (Schwab and Gargett, 2007) | (Gargett et al., 2009) | (Rajaraman et al., 2013) | (Zlatska et al., 2017) | (Masuda et al., 2012) | (Gurung et al., 2015) | (Patel et al., 2008) | (Meng et al., 2007) (∗) | (Cui et al., 2007) | (Musina et al., 2008) | (Darzi et al., 2012) | (Kazemnejad et al., 2012) | (Li et al., 2013) | (Khanjani et al., 2014) | (Khanmohammadi et al., 2014) | (Lopez-Caraballo et al., 2020) | (Hida et al., 2008) (∗) | (Sugawara et al., 2014) | |
Summary of phenotypic marker expression by endometrial mesenchymal stem cells (eMSCs) or menstrual blood-derived stromal/stem cells (MenSCs) isolated by plastic adherence or by specific marker (SUSD2+ in eMSCs and CD117+ in MenSCs). Different designations of MenSCs are also included according to the publication where they were described (ERC, endometrial regenerative cells; MMCs, menstrual blood-derived mesenchymal cells; eMSCs, endometrial mesenchymal stem/stromal cells) (∗) No flow cytometry histogram or dot plots were shown.
Despite the incorporation of SUSD2 as a cell surface marker that enriches for endometrial MSCs, the specific cell definition and discriminatory markers for eMSCs identity is still ongoing. Some authors consider that the in vivo functional capacity of MSCs is crucial for their cell definition. Using bmMSCs, a single cell could generate heterotopic bone or bone marrow organs (ossicles) in vivo (Sacchetti et al., 2007; Bianco et al., 2013). The equivalent definition for eMSCs would be the generation of a vascularized stroma with the capacity to differentiate into decidualized stroma when transplanted into an animal at the single-cell level. Although this has not been achieved yet, in xenografts, side-population cells regenerate human endometrium-like tissue consisting mainly of stromal and vascular tissue, with occasional epithelial gland-like structures (Masuda et al., 2010; Cervello et al., 2011; Miyazaki et al., 2012). Similarly, SUSD2+ eMSCs generate stromal tissue in xenografts (Masuda et al., 2012). However, in human endometrium in vivo, whether one or more stem/progenitor cell type regenerates endometrial tissue, or a stem/progenitor cell hierarchy exists (including slow dividing stem cells, and different highly proliferative transit amplifying cells as described to potentially participate in glandular epithelium regeneration (Nguyen et al., 2017; Cousins et al., 2021) needs further investigation.
Menstrual-blood derived stromal cells: identity markers and inconsistencies
As mentioned, the presence of perivascular eMSCs in both the basalis and functionalis endometrial layers suggested that they would appear in the cellular fraction of menstrual blood.
Unlike endometrial tissue, stromal cells derived from menstrual blood have been characterized more loosely and it was assumed that, besides peripheral blood, menstrual stromal cells (MenSCs) would comprise endometrial stromal fibroblasts and perivascular eMSCs, anticipating a similar identity to eMSCs obtained from endometrial biopsies.
First evidence suggesting that endometrial stem/stromal cells are shed in menstrual blood came from culture onto plastic (similar to bmMSC) of menstrual blood (Cui et al., 2007; Meng et al., 2007). Adherent cells termed as menstrual blood mesenchymal stem cells (mbMSCs) (Hida et al., 2008; Musina et al., 2008), endometrial regenerative cells (ERCs) (Meng et al., 2007), menstrual blood-derived mesenchymal cells (MMCs) (Hida et al., 2008; Sugawara et al., 2014), or menstrual blood-derived stromal/stem cells (MenSCs or MnSCs) (Patel et al., 2008; Borlongan et al., 2010; Darzi et al., 2012; Lopez-Caraballo et al., 2020) expand with a doubling time of 18-36 h (Cui et al., 2007; Meng et al., 2007; Lopez-Caraballo et al., 2020) and have a high proliferation capacity undergoing 25–40 population doublings (PD) before senescence (Cui et al., 2007; Hida et al., 2008).
MenSCs express the classic ISCT bmMSC markers (Table 1) and are negative for HLA-DR and for hematopoietic lineage markers (Cui et al., 2007; Meng et al., 2007; Darzi et al., 2012; Kazemnejad et al., 2012; Khanjani et al., 2014; Khanmohammadi et al., 2014; Lopez-Caraballo et al., 2020); besides, similarly to eMSCs, they express CD146, but not STRO-1 nor CD133 stem markers. However, controversy arises when other markers are investigated, such as CD117 (c-KIT), which has been used as a marker to purify MenSCs (Patel et al., 2008; Borlongan et al., 2010) and has been found to be negative when analyzed by other different groups (Cui et al., 2007; Hida et al., 2008; Musina et al., 2008; Darzi et al., 2012; Wu et al., 2014; Khanjani et al., 2015). Several inconsistencies in MenSC phenotype have been observed for the pluripotency markers Oct4, SSEA4, and NANOG. The mentioned data showed that CD117 positive MenSC support Oct4 expression, although at low expression by nonquantitative PCR (Patel et al., 2008). Cytoplasmic Oct4 has also been shown in MenSC by cytoplasmic immunofluorescence labeling (Khanjani et al., 2014) and it has been detected by flow cytometry (Kazemnejad et al., 2012; Khanjani et al., 2014). However, Oct4 has not been widely analyzed (Hida et al., 2008; Musina et al., 2008; Rossignoli et al., 2013; Sugawara et al., 2014); therefore, confirmation of Oct4 expression level needs further validation, as quantitative PCR or flow cytometry analysis indicate low or absent expression compared to pluripotent cells (Lopez-Caraballo et al., 2020). Similarly, SSEA4 and NANOG expression has been analyzed, with results showing flow cytometry and immunofluorescence positive labeling in CD117 purified MenSCs (Patel et al., 2008; Borlongan et al., 2010); although in these studies no pluripotent control was shown to both ensure the specificity of the labeling and compare the level of expression, and other studies show either low (Li et al., 2013; Lopez-Caraballo et al., 2020) or negligible expression (Cui et al., 2007; Meng et al., 2007; Kazemnejad et al., 2012; Khanmohammadi et al., 2014) of the mentioned markers.
The use of enrichment protocols makes it difficult to compare results and, before using them, it would be necessary to properly define MenSC cell identity markers as well as the effect of the collection techniques or menstrual blood sampling day. So far, bmMSC markers are routinely used after MenSC collection. However, taking into account their endometrial origin, it would probably be more informative to add eMSCs markers such as SUSD2, CD146, and CD140b, as recently performed by Gurung et al. with postmenopausal endometrium, menstrual blood, placenta decidua basalis, and bone marrow MSCs, showing differential expression of these markers among cell types in culture (Gurung et al., 2020). In this study an average of 60% of MenSCs express SUSD2. The percentage of SUSD2+ cells has also been analyzed in freshly isolated menstrual fluid-derived endometrial stem/progenitor cells, where they constitute 1–18% of the total population depending on the cell donor (Wyatt et al., 2021). Again, differences between freshly isolated and cultured cells can be due to the effect of cell culturing on marker expression and further studies and replication are needed for robust conclusions.
As mentioned, MenSCs are shed from the endometrium and it has been assumed that a mixture of stromal fibroblasts and eMSCs should be present. However, their actual presence and/or their cell identity modification due to the effect of menstruation signaling (such as inflammatory NFKB-mediated cascade, Wnt, and Notch signaling) and to cell culture effects should be analyzed in depth.
Single-cell transcriptomic of both menstrual-derived freshly isolated cells and in culture from different donors will shed light into MenSC cell identity and into the selection of specific markers for MenSCs identification and/or purification. The transcriptomic transformation of human endometrium at single-cell resolution across the menstrual cycle (excluding menses days) has been characterized recently, resolving cellular heterogeneity in multiple dimensions with characteristic signatures for seven endometrial cell types (Wang et al., 2020), including stromal fibroblast, endothelium, macrophage, lymphocyte, ciliated-epithelium, and nonciliated-epithelium. Interestingly, they also identified a population with characteristic smooth muscle cells closely related to the stromal fibroblast population that express SUSD2, CD140b, and CD146, suggesting that this identified cell type contains previously identified endometrial cells with mesenchymal stem cell characteristics. This study confirms the informative and valuable role of this type of approach to study the cellular composition of tissues/samples and potential cellular hierarchies.
Cellular properties of MenSCs: reparative, regenerative, and protective effects
The MSC field has been criticized due to the limited characterization of MSC, which has resulted in outcomes of many clinical trials that were not up to the expectations (Sipp et al., 2018). Basic science experts reiterate the importance of an exhaustive and undirected characterization of the cells before their application (Galipeau and Sensebe, 2018). However, several studies have already shown the regenerative, reparative, and protective properties of eMSCs and recently also of MenSCs (Chen et al., 2019; Kong et al., 2021).
The therapeutic potential of MenSCs has been studied based on their immunomodulation properties and their paracrine effect promoting endogenous cellular repair or regeneration without substantial cell-tissue integration or differentiation, similarly to MSCs (Caplan, 2016; Galipeau and Sensebe, 2018).
The immunomodulatory properties of MenSC
The immunomodulatory properties of MenSC have been analyzed as, although it is not properly a stem cell function, both MSC and stromal cells have these properties, and, in some way, the results are not surprising since, as mentioned, they come from an environment subjected to cyclic menstruation and inflammatory signaling. Endometrial stromal cells upregulate this intracellular inflammatory activation as well as the release of inflammatory factors that contribute to uterine blood vessel vasoconstriction and leukocyte recruitment among menstrual cascade phenomena (reviewed in Evans and Salamonsen, 2012). MenSC participation in the adaptive and innate immunity has been investigated, often in comparison with bmMSC (Figure 2).
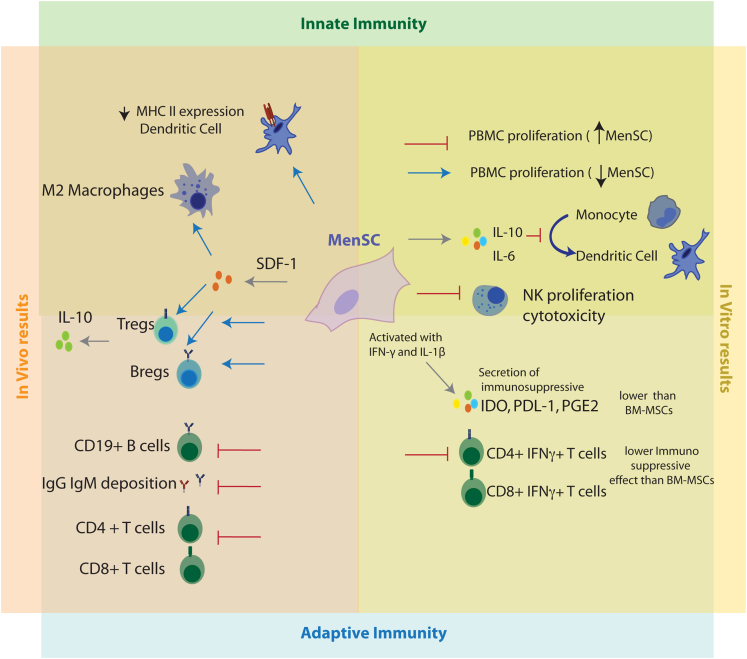
Figure 2.
Immunomodulatory effects of MenSC. Both in vitro and in vivo data support the MenSCs impact on the adaptive and innate immune system
In vivo data support an effect of MenSCs decreasing CD4+ and CD8+ activated T-cells, inhibiting IgG and IgM deposition while upregulating the presence of Tregs and Bregs and decreasing the expression of MHC II by dendritic cells. This effect can be mediated in part by the secretion of SDF-1 (also called CXCL12), which also increases the percentage of macrophages with M2 (anti-inflammatory) profile. In vitro, MenSC affect the proliferation of peripheral mononuclear cells in a dose-dependent manner and have a weaker immunosuppressive effect on activated T-cells than bmMSCs. MenSCs secrete IL-6 and IL-10, which inhibit optimal maturation of human monocyte-derived dendritic cells and, when stimulated with the pro inflammatory cytokines IFN-g and IL-1b, inhibit NK proliferation and cytotoxicity and secrete immunosuppressive cytokines, although at lower level than bmMSCs.
In vitro, MenSC’s effect on T cell proliferation is complex and still needs further characterization. These cells affect differently the proliferation of peripheral blood monocytes (PBMCs) depending of the cell ratio, with a high MenSC:PBMC ratio suppressing proliferation while a low ratio stimulates it (Nikoo et al., 2012); they also have a weaker immunosuppressive effect on PBMCs, CD4+IFNg+, and CD8+IFNg + T-cells than bmMSCs (Luz-Crawford et al., 2016; Aleahmad et al., 2018) (Figure 2, right panel).
MenSC’s secrete IL-6 and IL-10, which inhibit optimal maturation of human monocyte-derived dendritic cells (Bozorgmehr et al., 2014) and, although they induce Natural Killer (NK) cell proliferation, MnSCs inhibit both NK proliferation and cytotoxicity when stimulated with the pro inflammatory cytokines IFN-γ and IL-1β (Shokri et al., 2019) (Figure 2, right panel). Uterine NK (uNK) cells constitute the main component of the endometrial innate immune system, and these results support the importance of MenSC in tight cell-cell regulation for tissue homeostasis.
MenSCs have also been shown to exert immunomodulatory effects in vivo (Figure 2, left panel). MenSCs influence the antibody-mediated allograft rejection in mouse models of cardiac transplantation attributed in part to the cellular immunity regulation via SDF-1 (also known as CXCL12) secretion (Lan et al., 2017) and to the humoral immunity suppression that decreases IgM and IgG deposition, as well as antibody secretion of CD19 + B cells (Xu et al., 2017). In an inflammatory murine model of colitis, intravenous treatment with MenSCs decreased the presence of immature plasma cells in spleen and IgG deposition in colon while it increased the secretion of IL-10 and the level of Bregs and Tregs (Xu et al., 2018). Some of these effects were shared in a mice model with acute injury to the liver in which intravenous cell administration increased IL-10 level in serum and spleen levels of Tregs and decreased CD4+ and CD8+ T cells concentration and DC expression of MHCII (Lu et al., 2016). However, in a murine model of arthritis, MenSCs failed to establish an anti-inflammatory immune response in T cells (Luz-Crawford et al., 2016).
Taken together, MenSCs have a variety of effects on both the innate and specific immune responses that have been extensively reviewed (Bozorgmehr et al., 2020; Kong et al., 2021) and are not the focus of this study. They show inconsistencies between in vitro and in vivo findings and a considerable variation among experimental models, needing further investigation to identify the underlying mechanisms that organize the dialogue that MenSC use to modulate the immune system.
Regenerative effects of MenSCs
As mentioned, the paracrine effect of MenSCs in regenerative or reparative medicine has been analyzed recently as an attractive source with potential clinical applications. Several studies have shown that MenSCs repair damaged tissues and promote functional recovery through paracrine effects rather than cell differentiation (Bozorgmehr et al., 2020; Kong et al., 2021). Their reparative potential has been shown in several diseases such as myocardial infarction rat models (Hida et al., 2008; Jiang et al., 2013; Liu et al., 2019) where intracardiac MenSC transplantation improved left ventricular systolic function and fibrotic areas (Hida et al., 2008), reduced cell apoptosis, and increased cell proliferation primarily by the effect of secreted cytokines including PDGF, EGF, nitric oxide (NO), and TGF-β2 on AKT and STAT3 signaling activation. Intravenous MenSC injection in acute liver failure mouse model (Fathi-Kazerooni et al., 2017) and liver fibrosis (Chen et al., 2017b) promoted liver structure regeneration and liver function, indicated by the reduction in serum levels of liver enzymes and metabolites, although no mechanism or involved molecules were analyzed in this study (Hida et al., 2008), Improvement in liver fibrosis with reduced collagen fiber deposition was shown to be driven via inhibition of activated stellate cells mainly through the secretion of paracrine cytokines such as monocyte chemoattractant protein-1 (MCP-1), growth-related oncogene (GRO), IL-6, HGF, osteoprotegerin (OPG) and IL-8 (Chen et al., 2017b). The effect of MenSC transplantation on lung disease has also been tested in acute lung injury (Xiang et al., 2017) and bleomycin-induced pulmonary fibrosis mouse models (Zhao et al., 2018b). LPS-injured lungs showed improved clinical symptoms and tissue-structure repair after cell administration. MenSCs attenuated the anti-inflammatory response after lung-injury according to the reduced number of lung inflammatory cells and IL-1β levels, whereas increased IL-10 levels were observed. The repairing effect was also attributed to both an increased microvascular permeability and stimulation of cell proliferation effect (increased proliferating cell nuclear antigen (PCNA) expression in lung cells) and to the inhibition of cell apoptosis gathered from the decrease in the levels of caspase-3 detected (Xiang et al., 2017). Analysis of GSK3β, Src, and β-catenin proteins in lung cells showed a decrease in their phosphorylation after MenSC treatment, indicating a potential role of PI3K/β-catenin and gsk3β/β-catenin pathways. MenSC injection also reduced collagen deposition and inflammation in a mouse model of bleomycin-induced pulmonary fibrosis (Zhao et al., 2018b) indicated by the lower levels of TGF-β, IL-1β, and TNF-α and increased IL-10 detected in serum and lung tissue. The authors also state an antiapoptotic effect of MenSC supported by the reduced expression of the proapopototic gene Bax, while increased expression of the antiapoptotic Bcl-2 gene was identified in lung cells. In addition, the antifibrosis factors HGF and MMP-9 were also markedly elevated after MenSC treatment with a potential effect attenuating pulmonary fibrosis. Decreased muscle necrosis was observed after MenSC injection into the hindlimb muscle of a mouse model of critical limb ischemia in mice where IL-4, hypoxia inducible factor-1 alpha (HIF-a), MMP-3, and MMP-10 paracrine secreted factors were detected (Murphy et al., 2008). Moreover, skin wound repair was observed after intradermal injection of MenSC in a surgery-induced mouse model. The authors reasoned that this effect could be in part because of the upregulation of genes involved in wound repair (growth factors, cytokines, chemokines, AMPs, and MMPs) as they observed increased expression of PDGFA, PDGFB, MMP3, ELN, and MMP10 and the pro-angiogenic IL-8 and VEGF, in MenSC in vitro under pro-inflammatory stimulation (Cuenca et al., 2018). Intravenous injection of MenSCs has a therapeutic effect in a mouse model of type 1 diabetes mellitus (T1DM), in which MenSCs treatment partially reverses hyperglycemia and weight loss, prolongs lifespan, and increases insulin production, recovering islet structures and increasing the β-cell number. MenSCs did not differentiate into insulin-producing cells, but enhanced neurogenin3 (ngn3) expression, which represented endocrine progenitors that were activated, thus suggesting that β-cell regeneration occurs through promoting differentiation of endogenous progenitor cells (Wu et al., 2014). MenSC effect has also been evaluated in neural-related diseases such as stroke in a rat model where MenSC transplantation decreased neuronal cell death and improved motor symptoms by secreting brain-derived neurotrophic factor (BDNF), vascular endothelial growth factor (VEGF), and neurotrophic 3 (NT-3) (Borlongan et al., 2010). MenSC injection in the injured site of a rat spinal cord (SC) hemisection model alleviated the hindlimb motor function, increased the number of neurons, and reduced serum levels of the inflammatory factors TNF-α and IL-1β potentially because of the observed upregulation of BDNF at the lesion site (Wu et al., 2018). MenSC effect has also been analyzed in Alzheimer's disease, in which MenSC transplantation caused a change in the activated microglia that showed a greater capacity for the degradation of Aβ plaques and less production of TNF and IL-1β in APP/PS1 transgenic mice (Zhao et al., 2018a) (Table 2). Such groups of data suggest the promising therapeutic properties of MenSCs for future clinical applications; however, although reparative and regenerative effects are observed in a number of diseases as described here, there is still much information needed regarding the specific molecules and mechanism of action involved. Such mechanisms have so far been roughly described and, although potential pathways have been suggested to participate, they deserve deeper analysis in order to validate and strengthen their significance in the reparative effects observed.
Table 2.
MenSCs and regenerative effects
| Organ/Disease | Animal model | Transplantation method | Reparative/Regenerative effect | Pathway/Paracrine molecules involved | References | |
|---|---|---|---|---|---|---|
| Myocardial infarction (MI) | MI | Rat model of myocardial infarction (MI) | Intracardial MenSC injection | Improve cardiac function. Minor trans-differentiation to cardiomyocytes | Secreted cytokines (PDGF, EGF, NO and TGF-β2. Activation of Akt/Stat3 pathway. Decrease apoptosis | (Hida et al., 2008; Jiang et al., 2013) |
| MI | Rat model of myocardial infarction (MI) | Intracardial MenSC exosomes injection | Enhance myocardial cell survival and microvessel density | Secreted exosomal miR-21 enhances cell survival through the PTEN/Akt pathway | (Wang et al., 2017) | |
| Liver | Acute liver failure | BALB/c mouse (CCl444 induction) | Intravenous MenSC injection | Liver regeneration. No study of molecules involved | (Fathi-Kazerooni et al., 2017) | |
| Liver fibrosis | BALB/c mouse (CCl4 induction) | Intravenous MenSC injection | Liver regeneration. Dicreased collagen deposition. | Secretion of paracrine cytokines: MCP-1, GRO, IL-6, HGF, OPG and IL-8 | (Chen et al., 2017b) | |
| Fulminant hepatic failure (FHF) | D-GalN/LPS-induced FHF mice | Intravenous MenSC exosomes injection | Reduced hepatocyte apoptosis, proliferation of liver macrophages and pro-inflammatory cytokines improving liver function | Potential role of exosome cytokines: ICAM-1, Ang2, Axl, ANG, IGFBP-6, OPG, IL-6 and IL-8 on the reduction of the number of liver MNCs and the amount of the active apoptotic protein caspase-3 in injured liver | (Chen et al., 2017a) | |
| Lung | Acute lung injury | C57BL6 mouse (LPS-injury) | Intravenous MenSC injection | Attenuate inflammation (decrease IL-1β and increase IL-10) increase microvascular permeability and tissue repair (increase PCNA and decrease caspase-3). No study of molecules involved | Potential molecules involved in PI3K/β-catenin cross-talked with the gsk3β/β-catenin | (Xiang et al., 2017) |
| Pulmonary fibrosis | C57BL6 mouse (bleomycin) | Intravenous MenSC injection | - Decrease collagen production and wet/dry lung weight - Anti-inflammatory effect: Lower TGF-β, IL-1β and TNF-α and incrased IL-10 in serum and lung | Potential antiapoptotic effect via suppression of Bax expression, while increasing the antiapoptotic gene Bcl-2 in lung cells and antifibrosis effect via up regulation of HGF and MMP-9 | (Zhao et al., 2018b) | |
| Critical limb ischemia | BALB/c mouse (surgery induced) | MenSC injection into the hindlimb muscle | Decreased muscle necrosis | Detection of paracrine secreted factors: IL-4, HIF-a, MMP-3 an MMP-10 | (Murphy et al., 2008) | |
| Skin wound repair | C57BL6 mouse (surgery induced injury) | Intradermal injection of MenSC | Improve wound closure and vascularization | Increase MenSC expression of ANGPT1, PDGFA; PDGFB; MMP3; ELN; and MMP10, IL-8 and VEGF | (Cuenca et al., 2018) | |
| Diabetes mellitus | C57BL/6 mouse model of diabetes (STZ induced) | Intradermal injection of MenSC exosomes | Faster re-epithelialization and less scar formation | Secreted exosomes enhance neoangiogenesis through VEGFA upregulation and Re-epithelialization activation of the NF-κB signaling pathway | (Dalirfardouei et al., 2019) | |
| BALB/c mouse type 1 diabetes (STZ induced) | Intravenous MenSC injection | Reverse hyperglycemia and weight loss, prolong lifespan, and increase insulin production | β- cell regeneration by facilitating endogenous progenitor cell differentiation (increase of Ngn3+ progenitors) | (Wu et al., 2014) | ||
| Rat model of diabetes (STZ induced) | Intravenous injection of MenSC exosomes | Enhance the regeneration β- cell number and increased insulin production | Potential induction of islet regeneration through pancreatic and duodenal homeobox 1 (Pdx-1) pathway | (Mahdipour et al., 2019) | ||
| Neural related | OGD stroke | Rat oxygen glucose deprivation (OGD) stroke model | Intracerebral and intravenous MenSC injection | Decreased neuronal cell death and improved motor symptoms | MenSC secretion of BDNF, VEGF and NT-3 | (Borlongan et al., 2010) |
| SCI | Rat SC hemisection model | MenSC injection into spinal cord injured site | Improved the hindlimb motor function. Increase number of neurons, axon regeneration. Decrease inflammatory factors TNF-α and IL-1β | Regeneration mediated via the upregulation of BDNF in injured area | (Wu et al., 2018) | |
| Alzheimer's disease | APP/PS1 transgenic mice | Intracerebral MenSC injection | Improved the spatial learning and memory decrease number amyloid plaques and reduced tau hyperphosphorylation | Potential role of MenSC inducing the conversion of activated microglia to an alternative phenotype that secrete Aβ-degrading enzymes, including insulin-degrading enzyme (IDE) and neprilysin (NEP) | (Zhao et al., 2018a) | |
| Oral squamous cell carcinoma | Hamster buccal pouch carcinoma (DMBA induced) | MenSCs exosome intratumoral injection | Decrease tumor growth and a loss of tumor vasculature | Induction of apoptosis in endothelial cells and of their secretion of VEGF to increase angiogenesis | (Rosenberger et al., 2019) | |
| Premature ovarian failure (POF) | Rat busulfan model | Intravenous MenSC injection | Increase the number of follicles and restored the ovarian hormones estrogen and progesterone in plasma | MenSC localize in granulosa cells layer of immature follicles. Potential effect on follicle maturation. | (Manshadi et al., 2019) | |
| C57BL/6 mouse model (CTX induced) | Intravenous MenSC injection | Regulation of normal follicle development and estrous cycle and restoration of ovarian hormones (FSH, E2 and AMH) increase number of live births | Potential activation of ovarian transcriptional expression in ECM-dependent FAK/AKT signaling pathway | (Feng et al., 2019) | ||
| Endometrial injury and intrauterine adhesion (IUA) | ICR mouse (electrocoagulation) | Intravenous MenSC injection | Endometrium restoration with increased endometrial thickness and microvessel density. Increases embryo number | Secreted molecules (not identified) with potential activation of AKT and ERK pathways that induce the overexpression of eNOS, VEGFA, VEGFR1, VEGFR2 and TIE2 in endothelial cells (pro-angiogenic) | (Zhang et al., 2016) | |
| Rat IUA (mechanical injured) | Intravenous MenSC injection | Improved endometrial proliferation, angiogenesis, and morphology recovery and decreased collagen fibrosis and inflammation in the uterus | Detection of secretory protein IGF-1, SDF-1, and TSP-1 in the uterus. Potential involvement of Hippo signaling pathway (CTGF, Wnt5a, and Gdf5) | (Zhang et al., 2019) | ||
Summary of regenerative effects of menstrual blood-derived stromal/stem cells (MenSCs).
Most of the reparative capacities of MenSCs have been explored to treat infertility due to either premature ovarian failure (POF) or endometrial injury and intrauterine adhesion (IUA). Intraovarian or intravenous injection of MenSCs in a rat model of POF reduced follicle apoptosis, increasing their number at all developmental stages. Furthermore, it restored the estrogen and progesterone ovarian hormones in plasma (Manshadi et al., 2019) and increased the number of live births when grafted into POF mice (Feng et al., 2019). In vitro studies confirmed the role of MenSCs in the improvement of follicle growth and maturation (Feng et al., 2019). In a mouse model of IUA, intravascular injection of MenSCs increased microvessel density and endometrial thickness, improving the rate of conception and implanted embryos (Zhang et al., 2016). Similarly, intrauterine transplantation in an IUA rat model increased uterine fertility (Zhang et al., 2019). In vitro, MenSCs co-cultured with endometrial stromal cells promote their proliferation and down-regulate the expression of genes related to fibrotic generation as ⍺SMA and collagen I (Zhu et al., 2019).
MenSCs and clinical trials
As mentioned, the therapeutic potential of MenSCs has been studied recently, although the mechanisms involved are still largely unknown and a complete cell characterization is necessary. However, few trials and clinical studies in humans have already been launched. The study of Tan and collaborators (Tan et al., 2016) registered in the Chinese Clinical Trial Registry (ChiCTR-ONB-15007464), which used human autologous MenSCs transplantation in women with severe Asherman's syndrome (severe IUA), showed reduced fibrosis, endometrial morphology and functional recovery with a positive effect on pregnancy rates. A recent trial with women suffering POF registered in the Iranian Registry of Clinical Trials (IRCT20180619040147N2) showed that intraovarian injection of autologous MenSCs also improves fertility (Zafardoust et al., 2020).
Other clinical trials were registered in the NIH clinical studies database for the use of MenSCs in patients with liver cirrhosis (NCT01483248), type 1 diabetes (NCT01496339), and acute lung injury caused by H7N9 bird flu virus infection (NCT02095444), although no results have been posted and their completion status is currently unknown. It would be necessary to consider whether MenSCs are being rushed into the clinic before important issues are resolved, including their mechanism of action and cell identity.
Insights into the paracrine effect of MenSCs exosomes
Increasing studies attribute the therapeutic effects of MenSCs to the paracrine action of the extracellular vesicles (EVs) they secrete (Dalirfardouei et al., 2018). EVs include microvesicles, exosomes, and apoptotic bodies that behave as carriers of bioactive molecules such as proteins, microRNAs (miRNAs), and lipids, although most studies are focused specifically on exosomes with homogeneous size (30–170 nm) and CD81, CD63, and TSG101 expression (Doyle and Wang, 2019). Marinaro et al. identified 895 proteins in MenSC secreted exosomes related to complement activation, antigen processing and presentation, regulation of immune response, apoptosis control, and signaling pathways according to the gene ontology and biological function analysis of identified proteins (Marinaro et al., 2019a). They observed that IFNgamma-primed cells change the content of their exosomes into a wide range of proteins and miRNAs with different biological functions,.including immunomodulatory proteins such as CSF-1 (also called M-CSF). In a mouse model of acute liver function, MenSC-derived exosomes reduced hepatocyte apoptosis, proliferation of liver macrophages and pro-inflammatory cytokines improving liver function (Chen et al., 2017a). MicroRNA (miR)-21 of MenSC exosomes exerts a protective effect in a rat model of myocardial infarction (MI), enhancing myocardial cell survival through the PTEN/Akt pathway (Wang et al., 2017) (Table 3 for a complete list of abbreviations used in the text).
Table 3.
List of abbreviations used in the text
| Abbreviation | Full term | Abbreviation | Full term |
|---|---|---|---|
| AMH | Anti-Mullerian hormone | MCP-1 | Monocyte chemoattractant protein-1 |
| ANG | Angiogenin | MenSCs | Menstrual-blood derived stem/stromal cells |
| Ang2 | Angiopoietin-2 | MET | Mesenchymal to epithelial transition |
| ANGPT1 | Angiopoietin 1 | MHCII | Major histocompatibility complex II |
| APP | Amyloid precursor protein | MI | Myocardial infarction |
| BDNF | Brain-derived neurotrophic factor | MMP-9 | Matrix metallopeptidase 9 |
| bmMSCs | Bone marrow MSC | MMP10 | Matrix metallopeptidase 10 |
| Bregs | Regulatory B cells | MMP3 | Matrix metallopeptidase 3 |
| CCl4 | Carbon tetrachloride | MNC | Mononuclear cell |
| CFU | Colony forming unit | MSC | Mesenchymal stem/stromal cells |
| CTGF (CCN2) | Connective tissue growth factor | NEP | Neprilysin |
| CTX | Dyclophosphamide | NF-κΒ | Nuclear factor kappa B |
| DMBA | Dimethylbenzanthracene | Ngn3 | Neurogenin 3 |
| E2 | Estradiol | NK | Natural killer |
| ECM | Extracellular matrix | NO | Nitric oxide |
| EGF | Epidermal growth factor | NT-3 | Neurotrophin-3 |
| ELN | Elastin | OGD | Oxygen glucose deprivation |
| eMSC | Endometrial mesenchymal stem/stromal cells | OPG | Osteoprotegerin |
| eNOS | Endothelial nitric oxide synthase | PBMC | Peripheral blood monocytes |
| EpCAM | Epithelial Cell Adhesion Molecule | PCNA | Proliferating cell nuclear antigen |
| ERC | Endometrial regenerative cells | PDGF | Platelet-derived growth factor |
| FHF | Fulminant hepatic failure | Pdx-1 | Pancreatic and duodenal homeobox 1 |
| FSH | Follicle-stimulating hormone | POF | Premature ovarian failure |
| Gdf5 | Growth Differentiation Factor 5 | PS1 | Presenilin 1 |
| GRO | Growth regulated oncogene | SCI | Spinal cord injury |
| hADFs | Human adult dermal fibroblasts | SDF-1 | Stromal cell-derived factor 1 |
| HGF | Hepatocyte growth factor | SP | Side population |
| HIF-⍺ | Hypoxia inducible factor-1 alpha | STZ | Streptozotocin |
| ICAM-1 | Intercellular adhesion molecule-1 | SUSD2 | Sushi domain containing-2 |
| IDE | Insulin-degrading enzyme | TGF-β2 | Transforming growth factor β 2 |
| IFN-γ | Interferon gamma | TIE2 | Angiopoietin-1 receptor |
| IGF-1 | Insulin-like growth factor 1 | TNF-α | Tumor necrosis factor alpha |
| IGFBP-6 | Insulin-like growth factor-binding protein 6 | Tregs | Regulatory T cells |
| IL-6 | Interleukin 6 | TSP-1 | Thrombospondin-1 |
| IL-8 | Interleukin 8 | VEGF | Vascular endothelial growth factor |
| ISCT | International Society for Cellular Therapies | VEGFA | Vascular endothelial growth factor A |
| IUA | Intrauterine adhesion | VEGFR1 | Vascular endothelial growth factor receptor 1 |
| LPS/D-GalN | Lipopolysaccharide/D-galactosamine | VEGFR2 | Vascular endothelial growth factor receptor 2 |
| mbMSC | Menstrual blood mesenchymal stem cells | Wnt5a | Wnt Family Member 5A |
The intratumoral injection of exosomes in a hamster buccal pouch carcinoma (as a preclinical model for human oral squamous cell carcinoma), altered endothelial cells behavior, impeding angiogenesis and growth of tumor cells (Rosenberger et al., 2019). Exosomes of MenSCs improved non-healing wounds in a diabetic mouse model through M2 macrophage polarization, increasing neoangiogenesis and activating re-epithelialization via NF-KB signaling (Dalirfardouei et al., 2019). Intravenous administration of MenSCs' exosomes on a rat model of streptozotocin-induced diabetes enhanced the regeneration beta-cell number and increased insulin production (Mahdipour et al., 2019). As MenSCs, their secreted exosomes have also been used as coadjuvants to improve the in vitro fertilization outcomes in murine models (Blazquez et al., 2018), and the proteomic analysis of their cargo revealed an abundant expression of proteins involved in embryo development (Marinaro et al., 2019b) (Table 2).
Exosomes are considered as ideal therapeutic agents because they are non-viable and non-replicating agents that protect the contents from degradation and preserve their potency and functional integrity during handling, storage, and administration (Sun et al., 2016). Although their size facilitates their rapid clearance from the body and thus it would need either repeated administration or tissue-specific targeting, this constitutes a promising therapeutic approach that needs further research.
Interest of MenSC in cell reprogramming
The great majority of iPSC lines have been generated from dermal fibroblasts. Nonetheless, a multitude of other cell types have been used (Patel and Yang, 2010) as bone marrow mesenchymal stromal cells (bmMSCs) (Ohnishi et al., 2011). MenSCs have appeared recently in the reprogramming field, with clear advantages in accessibility, and also in reprogramming efficiency (de Carvalho Rodrigues et al., 2012; Li et al., 2013; Lopez-Caraballo et al., 2020). Strikingly they present higher reprogramming capacity than adult human dermal fibroblasts (hADFs) (Li et al., 2013; Lopez-Caraballo et al., 2020) and then bone marrow mesenchymal stromal cells (bmMSCs) (Lopez-Caraballo et al., 2020). Their higher reprogramming efficiency has been initially attributed either to their expression of pluripotency markers (specifically Oct4 and SSEA4), although as mentioned before, their expression analysis has been controversial and discordant among publications, or to their multipotent state based on MenSC transdifferentiation capacity. MenSCs can differentiate into mesodermal lineage (including chondrogenic, osteogenic, adipogenic, and cardiomyogenic fate), endodermal lineage (hepatocyte), and ectodermal lineage (neural and glial) as it has been extensively reviewed elsewhere (Bozorgmehr et al., 2020). Overall, most studies agree that MenSCs can be differentiated toward osteocytes, chondrocytes, and adipocytes. Nonetheless, the efficiency of this process varies considerably between them and either they do not compare with other cell types such as bmMSCs (Meng et al., 2007; Patel et al., 2008; Asensi et al., 2014) or they show variable efficiency percentages according to the analyzed markers.
Despite this, collectively, it has been described that the differentiation capacity of MenSCs toward osteoblasts and adipocytes is lower than that for bmMSCs. Studies are not conclusive regarding the degree of differentiation efficiency toward chondrocytes relative to bmMSC (Kazemnejad et al., 2012; Khanmohammadi et al., 2012) and recent data even show a markedly lower efficiency (Lopez-Caraballo et al., 2020). MenSCs have also been transdifferentiated to cardiomyocytes by studying the presence of troponin T (Hida et al., 2008), and, although there are few comparative studies, Rahimi et al. showed that MenSCs present increased capacity for differentiation than bmMSCs, although this difference was very reduced (Rahimi et al., 2014).
The studies by Khanjani et al. show that the ability of MenSCs to transdifferentiate toward hepatocytes is comparable to bmMSCs' (Khanjani et al., 2014). Similarly, for transdifferentiation to the neuroectodermal lineage, although neural and glial cells have been obtained according to the expression of specific markers (Azedi et al., 2014; Kozhukharova et al., 2018; Liu et al., 2018), their efficiency is again either similar to the one of bmMSCs (Azedi et al., 2014; Kozhukharova et al., 2018) or clearly lower (Lopez-Caraballo et al., 2020) depending of the differentiation protocol and identity markers used. It is important to note that the number of transdifferentiation studies is still very limited and that this field requires more replicates and the use of several analysis techniques, as well as identity markers, in order to obtain robust conclusions. Moreover, we cannot discard that differentiation protocols for MenSCs are different to those set up for bmMSCs and efficiencies may suffer substantial changes. These data suggest that the markedly different reprogramming capacity of MenSCs over bmMSCs (and hADFs) is not due to their multipotent state, referring to their potential to differentiate to cell types from the same or different germ layer. Of note, MenSCs have also been iPSC-reprogrammed with the highest efficiency using not only the canonical reprogramming cocktail (Oct4, Sox2, and Klf4) (de Carvalho Rodrigues et al., 2012; Li et al., 2013; Lopez-Caraballo et al., 2020) but also an oocyte-based combination through the over expression of the oocyte-enriched factors ASF1A, SOX15, and the pluripotency master Oct4 (AOX15 combination) (Lopez-Caraballo et al., 2020). Interestingly, a number of genes that are significantly overexpressed in MenSCs, compared to bmMSCs and hADF, are also enriched in the metaphase II human oocyte. Of interest, two of these genes are ASF1A and SOX15.
Differentiation of human germ-like, and in particular oocyte-like cells, is an inefficient and challenging process that has been achieved only from specific cell types using appropriate media (Bharti et al., 2020). Although the source for the differentiation process is mainly embryonic or induced by pluripotent cells (Kee et al., 2009; Lin et al., 2014; Sasaki et al., 2015; Jung et al., 2017), or even neonatal Wharton's jelly cells (Asgari et al., 2015), MenSCs have also been shown to differentiate into oocyte-like cells expressing characteristic markers such as the luteinizing hormone receptor and follicle-stimulating hormone receptor (Lai et al., 2016), suggesting again a potential tight connection between these cell types.
MenSCs transcriptomic and methylome profiles have been analyzed, showing that they have the most distinct expression and epigenetic signatures compared to hADFs and BM-MSCs (Lopez-Caraballo et al., 2020). Further studies are needed to uncover the molecular mechanisms underlying the reprogramming prone profile of MenSCs, but it is provocative to hypothesize that their oocyte-related signature plays a crucial role in it.
Conclusion
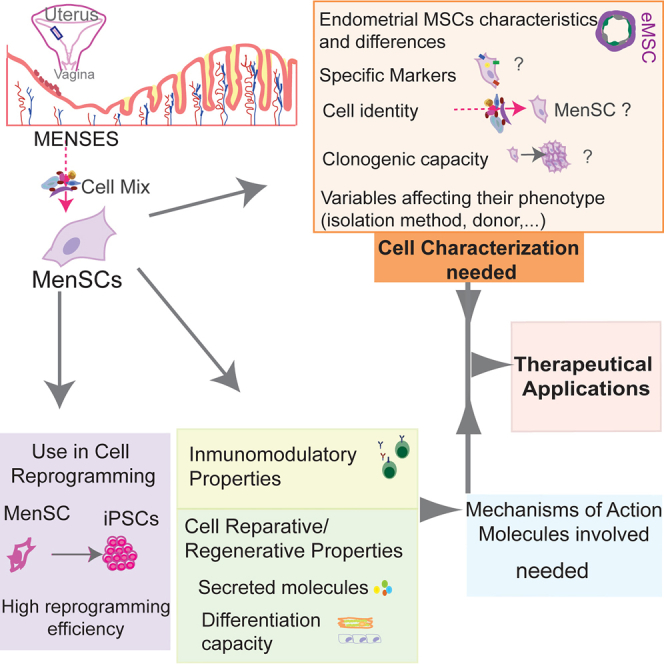
The endometrial stem cell hypothesis establishes that the cyclic regeneration of the endometrium is supported by a subpopulation of stromal cells called eMSCs with stemness properties of proliferation and differentiation similarly to other adult stem cells as bmMSCs. During menstruation, fragments of shedding endometrial tissue are released in menstrual-blood, and a population of mononuclear stromal cells can be isolated and cultured on plastic, called Menstrual-blood derived stem/stromal cells (MenSCs). However, although eMSCs and MenSCs share similar tissue origin and fulfill the ISCT criteria for MSC, they have been characterized separately. Comparative studies are still very limited to define their possible connection and they should be considered different cell types, although related. Specific markers for MenSCs identity have not been robustly delineated, with certain controversy regarding the expression of pluripotency markers and most studies still using ISCT bmMSC markers for their characterization, even if they show different proliferating, cloning, differentiation, and therapeutic effects. MenSCs are considered multipotent as they have been differentiated in vitro toward mesodermal, endodermal, and ectodermal cell fates, although more evidence and better cell characterization are needed to infer robust efficiency data. In vivo reparative properties of these cells have been attributed to their paracrine effect rather than stem cell function of differentiation and integration into the tissue, and their secretome has recently been postulated as the major component for this effect. MenSCs exert immunomodulatory and even angiogenic effects when transplanted and a thorough analysis of their mechanisms is still needed, as they show differences from bmMSCs. Moreover, we may be missing unique properties of these cells by focusing on the bmMSC as the golden standard.
MenSCs also have a noteworthy high reprogramming efficiency toward pluripotent iPSCs, and their similarities or connection to the oocyte constitute an inspiring beginning to analyze the molecular network underlying it.
Among other variables, lack of proper characterization of MSCs, their mechanism of action, and the heterogeneity of MSC products, led to the overall underwhelming results of their clinical trials. Although extensive and growing research on MSC field is very valuable for MenSCs understanding and application, altogether, there is still much research needed concerning MenSCs cell identity definition, clonogenicity characterization, specific markers expression, effect of cell culture, isolation method, donor origin or marker-based cell enrichment methods that can affect their reparative/regenerative outputs and must be first defined to avoid rushing them into the clinic.
Acknowledgments
This work was funded from Ministerio de Economía y Competitividad Gobierno de España (grant number MINECO-SAF2015-66105-R and RYC-2014-15410) and from Consejería Economía y Conocimiento Junta de Andalucía-FEDER (grant number UMA18-FEDERJA-107). Universidad de Málaga/CBUA funded for open access charge.
Author contributions
Conceptualization, A.S-M., E.G-M.; writing—original draft preparation, E.G-M.; writing—review and editing, E.G-M.; project administration, E.G-M.; funding acquisition, E.G-M. All authors have read and agreed to the published version of the manuscript.
Declaration of interests
The authors declare no conflict of interest. The funders had no role in the design of the study or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Aleahmad M., Ghanavatinejad A., Bozorgmehr M., Shokri M.R., Nikoo S., Tavakoli M., Kazemnejad S., Shokri F., Zarnani A.H. Menstrual blood-derived stromal stem cells augment CD4+ T cells proliferation. Avicenna J. Med. Biotechnol. 2018;10:183–191. [PMC free article] [PubMed] [Google Scholar]
- Asensi K.D., Fortunato R.S., dos Santos D.S., Pacheco T.S., de Rezende D.F., Rodrigues D.C., Mesquita F.C., Kasai-Brunswick T.H., de Carvalho A.C., Carvalho D.P., et al. Reprogramming to a pluripotent state modifies mesenchymal stem cell resistance to oxidative stress. J. Cell. Mol. Med. 2014;18:824–831. doi: 10.1111/jcmm.12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asgari H.R., Akbari M., Abbasi M., Ai J., Korouji M., Aliakbari F., Babatunde K.A., Aval F.S., Joghataei M.T. Human Wharton's jelly-derived mesenchymal stem cells express oocyte developmental genes during co-culture with placental cells. Iran J. Basic Med. Sci. 2015;18:22–29. [PMC free article] [PubMed] [Google Scholar]
- Azedi F., Kazemnejad S., Zarnani A.H., Behzadi G., Vasei M., Khanmohammadi M., Khanjani S., Edalatkhah H., Lakpour N. Differentiation potential of menstrual blood- versus bone marrow-stem cells into glial-like cells. Cell Biol. Int. 2014;38:615–624. doi: 10.1002/cbin.10245. [DOI] [PubMed] [Google Scholar]
- Bharti D., Jang S.J., Lee S.Y., Lee S.L., Rho G.J. In vitro generation of oocyte like cells and their in vivo efficacy: how far we have been succeeded. Cells. 2020;9:557. doi: 10.3390/cells9030557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco P., Cao X., Frenette P.S., Mao J.J., Robey P.G., Simmons P.J., Wang C.Y. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat. Med. 2013;19:35–42. doi: 10.1038/nm.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazquez R., Sanchez-Margallo F.M., Alvarez V., Matilla E., Hernandez N., Marinaro F., Gomez-Serrano M., Jorge I., Casado J.G., Macias-Garcia B. Murine embryos exposed to human endometrial MSCs-derived extracellular vesicles exhibit higher VEGF/PDGF AA release, increased blastomere count and hatching rates. PLoS One. 2018;13:e0196080. doi: 10.1371/journal.pone.0196080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borlongan C.V., Kaneko Y., Maki M., Yu S.J., Ali M., Allickson J.G., Sanberg C.D., Kuzmin-Nichols N., Sanberg P.R. Menstrual blood cells display stem cell-like phenotypic markers and exert neuroprotection following transplantation in experimental stroke. Stem Cells Dev. 2010;19:439–452. doi: 10.1089/scd.2009.0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozorgmehr M., Gurung S., Darzi S., Nikoo S., Kazemnejad S., Zarnani A.H., Gargett C.E. Endometrial and menstrual blood mesenchymal stem/stromal cells: biological properties and clinical application. Front. Cell Dev. Biol. 2020;8:497. doi: 10.3389/fcell.2020.00497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozorgmehr M., Moazzeni S.M., Salehnia M., Sheikhian A., Nikoo S., Zarnani A.H. Menstrual blood-derived stromal stem cells inhibit optimal generation and maturation of human monocyte-derived dendritic cells. Immunol. Lett. 2014;162:239–246. doi: 10.1016/j.imlet.2014.10.005. [DOI] [PubMed] [Google Scholar]
- Caplan A.I. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J. Cell. Physiol. 2007;213:341–347. doi: 10.1002/jcp.21200. [DOI] [PubMed] [Google Scholar]
- Caplan A.I. MSCs: the sentinel and safe-guards of injury. J. Cell. Physiol. 2016;231:1413–1416. doi: 10.1002/jcp.25255. [DOI] [PubMed] [Google Scholar]
- Cervello I., Mas A., Gil-Sanchis C., Peris L., Faus A., Saunders P.T., Critchley H.O., Simon C. Reconstruction of endometrium from human endometrial side population cell lines. PLoS One. 2011;6:e21221. doi: 10.1371/journal.pone.0021221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challen G.A., Little M.H. A side order of stem cells: the SP phenotype. Stem Cells. 2006;24:3–12. doi: 10.1634/stemcells.2005-0116. [DOI] [PubMed] [Google Scholar]
- Chan R.W., Schwab K.E., Gargett C.E. Clonogenicity of human endometrial epithelial and stromal cells. Biol. Reprod. 2004;70:1738–1750. doi: 10.1095/biolreprod.103.024109. [DOI] [PubMed] [Google Scholar]
- Chen L., Qu J., Cheng T., Chen X., Xiang C. Menstrual blood-derived stem cells: toward therapeutic mechanisms, novel strategies, and future perspectives in the treatment of diseases. Stem Cell Res. Ther. 2019;10:406. doi: 10.1186/s13287-019-1503-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Xiang B., Wang X., Xiang C. Exosomes derived from human menstrual blood-derived stem cells alleviate fulminant hepatic failure. Stem Cell Res. Ther. 2017;8:9. doi: 10.1186/s13287-016-0453-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Zhang C., Chen L., Wang X., Xiang B., Wu X., Guo Y., Mou X., Yuan L., Chen B., et al. Human menstrual blood-derived stem cells ameliorate liver fibrosis in mice by targeting hepatic stellate cells via paracrine mediators. Stem Cells Transl. Med. 2017;6:272–284. doi: 10.5966/sctm.2015-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins F.L., Pandoy R., Jin S., Gargett C.E. The elusive endometrial epithelial stem/progenitor cells. Front. Cell Dev. Biol. 2021;9:640319. doi: 10.3389/fcell.2021.640319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croxatto D., Vacca P., Canegallo F., Conte R., Venturini P.L., Moretta L., Mingari M.C. Stromal cells from human decidua exert a strong inhibitory effect on NK cell function and dendritic cell differentiation. PLoS One. 2014;9:e89006. doi: 10.1371/journal.pone.0089006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuenca J., Le-Gatt A., Castillo V., Belletti J., Diaz M., Kurte G.M., Gonzalez P.L., Alcayaga-Miranda F., Schuh C., Ezquer F., et al. The reparative abilities of menstrual stem cells modulate the wound matrix signals and improve cutaneous regeneration. Front. Physiol. 2018;9:464. doi: 10.3389/fphys.2018.00464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui C.H., Uyama T., Miyado K., Terai M., Kyo S., Kiyono T., Umezawa A. Menstrual blood-derived cells confer human dystrophin expression in the murine model of Duchenne muscular dystrophy via cell fusion and myogenic transdifferentiation. Mol. Biol. Cell. 2007;18:1586–1594. doi: 10.1091/mbc.E06-09-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalirfardouei R., Jamialahmadi K., Jafarian A.H., Mahdipour E. Promising effects of exosomes isolated from menstrual blood-derived mesenchymal stem cell on wound-healing process in diabetic mouse model. J. Tissue Eng. Regen. Med. 2019;13:555–568. doi: 10.1002/term.2799. [DOI] [PubMed] [Google Scholar]
- Dalirfardouei R., Jamialahmadi K., Mahdipour E. A feasible method for the isolation of mesenchymal stem cells from menstrual blood and their exosomes. Tissue Cell. 2018;55:53–62. doi: 10.1016/j.tice.2018.09.010. [DOI] [PubMed] [Google Scholar]
- Darzi S., Zarnani A.H., Jeddi-Tehrani M., Entezami K., Mirzadegan E., Akhondi M.M., Talebi S., Khanmohammadi M., Kazemnejad S. Osteogenic differentiation of stem cells derived from menstrual blood versus bone marrow in the presence of human platelet releasate. Tissue Eng. Part A. 2012;18:1720–1728. doi: 10.1089/ten.tea.2011.0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Carvalho Rodrigues D., Asensi K.D., Vairo L., Azevedo-Pereira R.L., Silva R., Rondinelli E., Goldenberg R.C., Campos de Carvalho A.C., Urmenyi T.P. Human menstrual blood-derived mesenchymal cells as a cell source of rapid and efficient nuclear reprogramming. Cell Transpl. 2012;21:2215–2224. doi: 10.3727/096368912X653048. [DOI] [PubMed] [Google Scholar]
- Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D., Deans R., Keating A., Prockop D., Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Doyle L.M., Wang M.Z. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells. 2019;8 doi: 10.3390/cells8070727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emera D., Romero R., Wagner G. The evolution of menstruation: a new model for genetic assimilation: explaining molecular origins of maternal responses to fetal invasiveness. Bioessays. 2012;34:26–35. doi: 10.1002/bies.201100099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J., Kaitu'u-Lino T., Salamonsen L.A. Extracellular matrix dynamics in scar-free endometrial repair: perspectives from mouse in vivo and human in vitro studies. Biol. Reprod. 2011;85:511–523. doi: 10.1095/biolreprod.111.090993. [DOI] [PubMed] [Google Scholar]
- Evans J., Salamonsen L.A. Inflammation, leukocytes and menstruation. Rev. Endocr. Metab. Disord. 2012;13:277–288. doi: 10.1007/s11154-012-9223-7. [DOI] [PubMed] [Google Scholar]
- Evans J., Salamonsen L.A., Winship A., Menkhorst E., Nie G., Gargett C.E., Dimitriadis E. Fertile ground: human endometrial programming and lessons in health and disease. Nat. Rev. Endocrinol. 2016;12:654–667. doi: 10.1038/nrendo.2016.116. [DOI] [PubMed] [Google Scholar]
- Fathi-Kazerooni M., Tavoosidana G., Taghizadeh-Jahed M., Khanjani S., Golshahi H., Gargett C.E., Edalatkhah H., Kazemnejad S. Comparative restoration of acute liver failure by menstrual blood stem cells compared with bone marrow stem cells in mice model. Cytotherapy. 2017;19:1474–1490. doi: 10.1016/j.jcyt.2017.08.022. [DOI] [PubMed] [Google Scholar]
- Feng P., Li P., Tan J. Human menstrual blood-derived stromal cells promote recovery of premature ovarian insufficiency via regulating the ECM-dependent FAK/AKT signaling. Stem Cell Rev. Rep. 2019;15:241–255. doi: 10.1007/s12015-018-9867-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaide Chevronnay H.P., Galant C., Lemoine P., Courtoy P.J., Marbaix E., Henriet P. Spatiotemporal coupling of focal extracellular matrix degradation and reconstruction in the menstrual human endometrium. Endocrinology. 2009;150:5094–5105. doi: 10.1210/en.2009-0750. [DOI] [PubMed] [Google Scholar]
- Galipeau J., Sensebe L. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell. 2018;22:824–833. doi: 10.1016/j.stem.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargett C.E. Review article: stem cells in human reproduction. Reprod. Sci. 2007;14:405–424. doi: 10.1177/1933719107306231. [DOI] [PubMed] [Google Scholar]
- Gargett C.E., Chan R.W., Schwab K.E. Endometrial stem cells. Curr. Opin. Obstet. Gynecol. 2007;19:377–383. doi: 10.1097/GCO.0b013e328235a5c6. [DOI] [PubMed] [Google Scholar]
- Gargett C.E., Masuda H. Adult stem cells in the endometrium. Mol. Hum. Reprod. 2010;16:818–834. doi: 10.1093/molehr/gaq061. [DOI] [PubMed] [Google Scholar]
- Gargett C.E., Schwab K.E., Deane J.A. Endometrial stem/progenitor cells: the first 10 years. Hum. Reprod. Update. 2016;22:137–163. doi: 10.1093/humupd/dmv051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargett C.E., Schwab K.E., Zillwood R.M., Nguyen H.P., Wu D. Isolation and culture of epithelial progenitors and mesenchymal stem cells from human endometrium. Biol. Reprod. 2009;80:1136–1145. doi: 10.1095/biolreprod.108.075226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellersen B., Brosens J.J. Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr. Rev. 2014;35:851–905. doi: 10.1210/er.2014-1045. [DOI] [PubMed] [Google Scholar]
- Gonzalez M., Neufeld J., Reimann K., Wittmann S., Samalecos A., Wolf A., Bamberger A.M., Gellersen B. Expansion of human trophoblastic spheroids is promoted by decidualized endometrial stromal cells and enhanced by heparin-binding epidermal growth factor-like growth factor and interleukin-1 beta. Mol. Hum. Reprod. 2011;17:421–433. doi: 10.1093/molehr/gar015. [DOI] [PubMed] [Google Scholar]
- Grewal S., Carver J.G., Ridley A.J., Mardon H.J. Implantation of the human embryo requires Rac1-dependent endometrial stromal cell migration. Proc. Natl. Acad. Sci. U S A. 2008;105:16189–16194. doi: 10.1073/pnas.0806219105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurung S., Ulrich D., Sturm M., Rosamilia A., Werkmeister J.A., Gargett C.E. Comparing the effect of TGF-beta receptor inhibition on human perivascular mesenchymal stromal cells derived from endometrium, bone marrow and adipose tissues. J. Pers. Med. 2020;10:261. doi: 10.3390/jpm10040261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurung S., Werkmeister J.A., Gargett C.E. Inhibition of transforming growth factor-beta receptor signaling promotes culture expansion of undifferentiated human endometrial mesenchymal stem/stromal cells. Sci. Rep. 2015;5:15042. doi: 10.1038/srep15042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hematti P. Mesenchymal stromal cells and fibroblasts: a case of mistaken identity? Cytotherapy. 2012;14:516–521. doi: 10.3109/14653249.2012.677822. [DOI] [PubMed] [Google Scholar]
- Hida N., Nishiyama N., Miyoshi S., Kira S., Segawa K., Uyama T., Mori T., Miyado K., Ikegami Y., Cui C., et al. Novel cardiac precursor-like cells from human menstrual blood-derived mesenchymal cells. Stem Cells. 2008;26:1695–1704. doi: 10.1634/stemcells.2007-0826. [DOI] [PubMed] [Google Scholar]
- Jiang Z., Hu X., Yu H., Xu Y., Wang L., Chen H., Chen H., Wu R., Zhang Z., Xiang C., et al. Human endometrial stem cells confer enhanced myocardial salvage and regeneration by paracrine mechanisms. J. Cell. Mol. Med. 2013;17:1247–1260. doi: 10.1111/jcmm.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung D., Xiong J., Ye M., Qin X., Li L., Cheng S., Luo M., Peng J., Dong J., Tang F., et al. In vitro differentiation of human embryonic stem cells into ovarian follicle-like cells. Nat. Commun. 2017;8:15680. doi: 10.1038/ncomms15680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajihara T., Jones M., Fusi L., Takano M., Feroze-Zaidi F., Pirianov G., Mehmet H., Ishihara O., Higham J.M., Lam E.W., Brosens J.J. Differential expression of FOXO1 and FOXO3a confers resistance to oxidative cell death upon endometrial decidualization. Mol. Endocrinol. 2006;20:2444–2455. doi: 10.1210/me.2006-0118. [DOI] [PubMed] [Google Scholar]
- Kato K., Yoshimoto M., Kato K., Adachi S., Yamayoshi A., Arima T., Asanoma K., Kyo S., Nakahata T., Wake N. Characterization of side-population cells in human normal endometrium. Hum. Reprod. 2007;22:1214–1223. doi: 10.1093/humrep/del514. [DOI] [PubMed] [Google Scholar]
- Kazemnejad S., Akhondi M.M., Soleimani M., Zarnani A.H., Khanmohammadi M., Darzi S., Alimoghadam K. Characterization and chondrogenic differentiation of menstrual blood-derived stem cells on a nanofibrous scaffold. Int. J. Artif. Organs. 2012;35:55–66. doi: 10.5301/ijao.5000019. [DOI] [PubMed] [Google Scholar]
- Kee K., Angeles V.T., Flores M., Nguyen H.N., Reijo Pera R.A. Human DAZL, DAZ and BOULE genes modulate primordial germ-cell and haploid gamete formation. Nature. 2009;462:222–225. doi: 10.1038/nature08562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanjani S., Khanmohammadi M., Zarnani A.H., Akhondi M.M., Ahani A., Ghaempanah Z., Naderi M.M., Eghtesad S., Kazemnejad S. Comparative evaluation of differentiation potential of menstrual blood- versus bone marrow-derived stem cells into hepatocyte-like cells. PLoS One. 2014;9:e86075. doi: 10.1371/journal.pone.0086075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanjani S., Khanmohammadi M., Zarnani A.H., Talebi S., Edalatkhah H., Eghtesad S., Nikokar I., Kazemnejad S. Efficient generation of functional hepatocyte-like cells from menstrual blood-derived stem cells. J. Tissue Eng. Regen. Med. 2015;9:E124–E134. doi: 10.1002/term.1715. [DOI] [PubMed] [Google Scholar]
- Khanmohammadi M., Khanjani S., Bakhtyari M.S., Zarnani A.H., Edalatkhah H., Akhondi M.M., Mirzadegan E., Kamali K., Alimoghadam K., Kazemnejad S. Proliferation and chondrogenic differentiation potential of menstrual blood- and bone marrow-derived stem cells in two-dimensional culture. Int. J. Hematol. 2012;95:484–493. doi: 10.1007/s12185-012-1067-0. [DOI] [PubMed] [Google Scholar]
- Khanmohammadi M., Khanjani S., Edalatkhah H., Zarnani A.H., Heidari-Vala H., Soleimani M., Alimoghaddam K., Kazemnejad S. Modified protocol for improvement of differentiation potential of menstrual blood-derived stem cells into adipogenic lineage. Cell Prolif. 2014;47:615–623. doi: 10.1111/cpr.12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Y., Shao Y., Ren C., Yang G. Endometrial stem/progenitor cells and their roles in immunity, clinical application, and endometriosis. Stem Cell Res. Ther. 2021;12:474. doi: 10.1186/s13287-021-02526-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozhukharova I., Zemelko V., Kovaleva Z., Alekseenko L., Lyublinskaya O., Nikolsky N. Therapeutic doses of doxorubicin induce premature senescence of human mesenchymal stem cells derived from menstrual blood, bone marrow and adipose tissue. Int. J. Hematol. 2018;107:286–296. doi: 10.1007/s12185-017-2346-6. [DOI] [PubMed] [Google Scholar]
- Lai D., Guo Y., Zhang Q., Chen Y., Xiang C. Differentiation of human menstrual blood-derived endometrial mesenchymal stem cells into oocyte-like cells. Acta Biochim. Biophys. Sin. (Shanghai) 2016;48:998–1005. doi: 10.1093/abbs/gmw090. [DOI] [PubMed] [Google Scholar]
- Lan X., Wang G., Xu X., Lu S., Li X., Zhang B., Shi G., Zhao Y., Du C., Wang H. Stromal cell-derived factor-1 mediates cardiac allograft tolerance induced by human endometrial regenerative cell-based therapy. Stem Cells Transl. Med. 2017;6:1997–2008. doi: 10.1002/sctm.17-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitao B., Jones M.C., Fusi L., Higham J., Lee Y., Takano M., Goto T., Christian M., Lam E.W., Brosens J.J. Silencing of the JNK pathway maintains progesterone receptor activity in decidualizing human endometrial stromal cells exposed to oxidative stress signals. FASEB J. 2010;24:1541–1551. doi: 10.1096/fj.09-149153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Li X., Zhao H., Feng R., Zhang X., Tai D., An G., Wen J., Tan J. Efficient induction of pluripotent stem cells from menstrual blood. Stem Cells Dev. 2013;22:1147–1158. doi: 10.1089/scd.2012.0428. [DOI] [PubMed] [Google Scholar]
- Lin I.Y., Chiu F.L., Yeang C.H., Chen H.F., Chuang C.Y., Yang S.Y., Hou P.S., Sintupisut N., Ho H.N., Kuo H.C., Lin K.I. Suppression of the SOX2 neural effector gene by PRDM1 promotes human germ cell fate in embryonic stem cells. Stem Cell Rep. 2014;2:189–204. doi: 10.1016/j.stemcr.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Niu R., Li W., Lin J., Stamm C., Steinhoff G., Ma N. Therapeutic potential of menstrual blood-derived endometrial stem cells in cardiac diseases. Cell Mol. Life Sci. 2019;76:1681–1695. doi: 10.1007/s00018-019-03019-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Yang F., Liang S., Liu Q., Fu S., Wang Z., Yang C., Lin J. N-cadherin upregulation promotes the neurogenic differentiation of menstrual blood-derived endometrial stem cells. Stem Cells Int. 2018;2018:3250379. doi: 10.1155/2018/3250379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood C.J., Krikun G., Rahman M., Caze R., Buchwalder L., Schatz F. The role of decidualization in regulating endometrial hemostasis during the menstrual cycle, gestation, and in pathological states. Semin. Thromb. Hemost. 2007;33:111–117. doi: 10.1055/s-2006-958469. [DOI] [PubMed] [Google Scholar]
- Lockwood C.J., Paidas M., Murk W.K., Kayisli U.A., Gopinath A., Huang S.J., Krikun G., Schatz F. Involvement of human decidual cell-expressed tissue factor in uterine hemostasis and abruption. Thromb. Res. 2009;124:516–520. doi: 10.1016/j.thromres.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Caraballo L., Martorell-Marugan J., Carmona-Saez P., Gonzalez-Munoz E. Analysis of menstrual blood stromal cells reveals SOX15 triggers oocyte-based human cell reprogramming. iScience. 2020;23:101376. doi: 10.1016/j.isci.2020.101376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S., Shi G., Xu X., Wang G., Lan X., Sun P., Li X., Zhang B., Gu X., Ichim T.E., Wang H. Human endometrial regenerative cells alleviate carbon tetrachloride-induced acute liver injury in mice. J. Transl. Med. 2016;14:300. doi: 10.1186/s12967-016-1051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luz-Crawford P., Torres M.J., Noel D., Fernandez A., Toupet K., Alcayaga-Miranda F., Tejedor G., Jorgensen C., Illanes S.E., Figueroa F.E., et al. The immunosuppressive signature of menstrual blood mesenchymal stem cells entails opposite effects on experimental arthritis and graft versus host diseases. Stem Cells. 2016;34:456–469. doi: 10.1002/stem.2244. [DOI] [PubMed] [Google Scholar]
- Mahdipour E., Salmasi Z., Sabeti N. Potential of stem cell-derived exosomes to regenerate beta islets through Pdx-1 dependent mechanism in a rat model of type 1 diabetes. J. Cell. Physiol. 2019;234:20310–20321. doi: 10.1002/jcp.28631. [DOI] [PubMed] [Google Scholar]
- Manshadi M.D., Navid S., Hoshino Y., Daneshi E., Noory P., Abbasi M. The effects of human menstrual blood stem cells-derived granulosa cells on ovarian follicle formation in a rat model of premature ovarian failure. Microsc. Res. Tech. 2019;82:635–642. doi: 10.1002/jemt.23120. [DOI] [PubMed] [Google Scholar]
- Marinaro F., Gomez-Serrano M., Jorge I., Silla-Castro J.C., Vazquez J., Sanchez-Margallo F.M., Blazquez R., Lopez E., Alvarez V., Casado J.G. Unraveling the molecular signature of extracellular vesicles from endometrial-derived mesenchymal stem cells: potential modulatory effects and therapeutic applications. Front. Bioeng. Biotechnol. 2019;7:431. doi: 10.3389/fbioe.2019.00431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinaro F., Macias-Garcia B., Sanchez-Margallo F.M., Blazquez R., Alvarez V., Matilla E., Hernandez N., Gomez-Serrano M., Jorge I., Vazquez J., et al. Extracellular vesicles derived from endometrial human mesenchymal stem cells enhance embryo yield and quality in an aged murine modeldagger. Biol. Reprod. 2019;100:1180–1192. doi: 10.1093/biolre/ioy263. [DOI] [PubMed] [Google Scholar]
- Masuda H., Anwar S.S., Buhring H.J., Rao J.R., Gargett C.E. A novel marker of human endometrial mesenchymal stem-like cells. Cell Transpl. 2012;21:2201–2214. doi: 10.3727/096368911X637362. [DOI] [PubMed] [Google Scholar]
- Masuda H., Maruyama T., Gargett C.E., Miyazaki K., Matsuzaki Y., Okano H., Tanaka M. Endometrial side population cells: potential adult stem/progenitor cells in endometrium. Biol. Reprod. 2015;93:84. doi: 10.1095/biolreprod.115.131490. [DOI] [PubMed] [Google Scholar]
- Masuda H., Matsuzaki Y., Hiratsu E., Ono M., Nagashima T., Kajitani T., Arase T., Oda H., Uchida H., Asada H., et al. Stem cell-like properties of the endometrial side population: implication in endometrial regeneration. PLoS One. 2010;5:e10387. doi: 10.1371/journal.pone.0010387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X., Ichim T.E., Zhong J., Rogers A., Yin Z., Jackson J., Wang H., Ge W., Bogin V., Chan K.W., et al. Endometrial regenerative cells: a novel stem cell population. J. Transl. Med. 2007;5:57. doi: 10.1186/1479-5876-5-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki K., Maruyama T., Masuda H., Yamasaki A., Uchida S., Oda H., Uchida H., Yoshimura Y. Stem cell-like differentiation potentials of endometrial side population cells as revealed by a newly developed in vivo endometrial stem cell assay. PLoS One. 2012;7:e50749. doi: 10.1371/journal.pone.0050749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami K., Lee Y.H., Lucas E.S., Chan Y.W., Durairaj R.P., Takeda S., Moore J.D., Tan B.K., Quenby S., Chan J.K., et al. Decidualization induces a secretome switch in perivascular niche cells of the human endometrium. Endocrinology. 2014;155:4542–4553. doi: 10.1210/en.2014-1370. [DOI] [PubMed] [Google Scholar]
- Murphy M.P., Wang H., Patel A.N., Kambhampati S., Angle N., Chan K., Marleau A.M., Pyszniak A., Carrier E., Ichim T.E., Riordan N.H. Allogeneic endometrial regenerative cells: an "Off the shelf solution" for critical limb ischemia? J. Transl. Med. 2008;6:45. doi: 10.1186/1479-5876-6-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musina R.A., Belyavski A.V., Tarusova O.V., Solovyova E.V., Sukhikh G.T. Endometrial mesenchymal stem cells isolated from the menstrual blood. Bull. Exp. Biol. Med. 2008;145:539–543. doi: 10.1007/s10517-008-0136-0. [DOI] [PubMed] [Google Scholar]
- Nancy P., Tagliani E., Tay C.S., Asp P., Levy D.E., Erlebacher A. Chemokine gene silencing in decidual stromal cells limits T cell access to the maternal-fetal interface. Science. 2012;336:1317–1321. doi: 10.1126/science.1220030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen H.P.T., Xiao L., Deane J.A., Tan K.S., Cousins F.L., Masuda H., Sprung C.N., Rosamilia A., Gargett C.E. N-cadherin identifies human endometrial epithelial progenitor cells by in vitro stem cell assays. Hum. Reprod. 2017;32:2254–2268. doi: 10.1093/humrep/dex289. [DOI] [PubMed] [Google Scholar]
- Nikoo S., Ebtekar M., Jeddi-Tehrani M., Shervin A., Bozorgmehr M., Kazemnejad S., Zarnani A.H. Effect of menstrual blood-derived stromal stem cells on proliferative capacity of peripheral blood mononuclear cells in allogeneic mixed lymphocyte reaction. J. Obstet. Gynaecol. Res. 2012;38:804–809. doi: 10.1111/j.1447-0756.2011.01800.x. [DOI] [PubMed] [Google Scholar]
- Ohnishi H., Oda Y., Aoki T., Tadokoro M., Katsube Y., Ohgushi H., Hattori K., Yuba S. A comparative study of induced pluripotent stem cells generated from frozen, stocked bone marrow- and adipose tissue-derived mesenchymal stem cells. J. Tissue Eng. Regen. Med. 2011;6:261–271. doi: 10.1002/term.428. [DOI] [PubMed] [Google Scholar]
- Padykula H.A. Regeneration in the primate uterus: the role of stem cells. Ann. N. Y. Acad. Sci. 1991;622:47–56. doi: 10.1111/j.1749-6632.1991.tb37849.x. [DOI] [PubMed] [Google Scholar]
- Padykula H.A., Coles L.G., Mccracken J.A., King N.W., Jr., Longcope C., Kaiserman-Abramof I.R. A zonal pattern of cell proliferation and differentiation in the rhesus endometrium during the estrogen surge. Biol. Reprod. 1984;31:1103–1118. doi: 10.1095/biolreprod31.5.1103. [DOI] [PubMed] [Google Scholar]
- Patel A.N., Park E., Kuzman M., Benetti F., Silva F.J., Allickson J.G. Multipotent menstrual blood stromal stem cells: isolation, characterization, and differentiation. Cell Transpl. 2008;17:303–311. doi: 10.3727/096368908784153922. [DOI] [PubMed] [Google Scholar]
- Patel M., Yang S. Advances in reprogramming somatic cells to induced pluripotent stem cells. Stem Cell Rev. Rep. 2010;6:367–380. doi: 10.1007/s12015-010-9123-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phinney D.G., Sensebe L. Mesenchymal stromal cells: misconceptions and evolving concepts. Cytotherapy. 2013;15:140–145. doi: 10.1016/j.jcyt.2012.11.005. [DOI] [PubMed] [Google Scholar]
- Pittenger M.F., Discher D.E., Peault B.M., Phinney D.G., Hare J.M., Caplan A.I. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen. Med. 2019;4:22. doi: 10.1038/s41536-019-0083-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prianishnikov V.A. On the concept of stem cell and a model of functional-morphological structure of the endometrium. Contraception. 1978;18:213–223. doi: 10.1016/s0010-7824(78)80015-8. [DOI] [PubMed] [Google Scholar]
- Rahimi M., Zarnani A.H., Mohseni-Kouchesfehani H., Soltanghoraei H., Akhondi M.M., Kazemnejad S. Comparative evaluation of cardiac markers in differentiated cells from menstrual blood and bone marrow-derived stem cells in vitro. Mol. Biotechnol. 2014;56:1151–1162. doi: 10.1007/s12033-014-9795-4. [DOI] [PubMed] [Google Scholar]
- Rajaraman G., White J., Tan K.S., Ulrich D., Rosamilia A., Werkmeister J., Gargett C.E. Optimization and scale-up culture of human endometrial multipotent mesenchymal stromal cells: potential for clinical application. Tissue Eng. Part C Methods. 2013;19:80–92. doi: 10.1089/ten.tec.2011.0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberger L., Ezquer M., Lillo-Vera F., Pedraza P.L., Ortuzar M.I., Gonzalez P.L., Figueroa-Valdes A.I., Cuenca J., Ezquer F., Khoury M., Alcayaga-Miranda F. Stem cell exosomes inhibit angiogenesis and tumor growth of oral squamous cell carcinoma. Sci. Rep. 2019;9:663. doi: 10.1038/s41598-018-36855-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignoli F., Caselli A., Grisendi G., Piccinno S., Burns J.S., Murgia A., Veronesi E., Loschi P., Masini C., Conte P., et al. Isolation, characterization, and transduction of endometrial decidual tissue multipotent mesenchymal stromal/stem cells from menstrual blood. Biomed. Res. Int. 2013;2013:901821. doi: 10.1155/2013/901821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchetti B., Funari A., Michienzi S., Di Cesare S., Piersanti S., Saggio I., Tagliafico E., Ferrari S., Robey P.G., Riminucci M., Bianco P. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- Sasaki K., Yokobayashi S., Nakamura T., Okamoto I., Yabuta Y., Kurimoto K., Ohta H., Moritoki Y., Iwatani C., Tsuchiya H., et al. Robust in vitro induction of human germ cell fate from pluripotent stem cells. Cell Stem Cell. 2015;17:178–194. doi: 10.1016/j.stem.2015.06.014. [DOI] [PubMed] [Google Scholar]
- Schwab K.E., Chan R.W., Gargett C.E. Putative stem cell activity of human endometrial epithelial and stromal cells during the menstrual cycle. Fertil. Steril. 2005;84:1124–1130. doi: 10.1016/j.fertnstert.2005.02.056. [DOI] [PubMed] [Google Scholar]
- Schwab K.E., Gargett C.E. Co-expression of two perivascular cell markers isolates mesenchymal stem-like cells from human endometrium. Hum. Reprod. 2007;22:2903–2911. doi: 10.1093/humrep/dem265. [DOI] [PubMed] [Google Scholar]
- Schwab K.E., Hutchinson P., Gargett C.E. Identification of surface markers for prospective isolation of human endometrial stromal colony-forming cells. Hum. Reprod. 2008;23:934–943. doi: 10.1093/humrep/den051. [DOI] [PubMed] [Google Scholar]
- Shokri M.R., Bozorgmehr M., Ghanavatinejad A., Falak R., Aleahmad M., Kazemnejad S., Shokri F., Zarnani A.H. Human menstrual blood-derived stromal/stem cells modulate functional features of natural killer cells. Sci. Rep. 2019;9:10007. doi: 10.1038/s41598-019-46316-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipp D., Robey P.G., Turner L. Clear up this stem-cell mess. Nature. 2018;561:455–457. doi: 10.1038/d41586-018-06756-9. [DOI] [PubMed] [Google Scholar]
- Sivasubramaniyan K., Harichandan A., Schumann S., Sobiesiak M., Lengerke C., Maurer A., Kalbacher H., Buhring H.J. Prospective isolation of mesenchymal stem cells from human bone marrow using novel antibodies directed against Sushi domain containing 2. Stem Cells Dev. 2013;22:1944–1954. doi: 10.1089/scd.2012.0584. [DOI] [PubMed] [Google Scholar]
- Spitzer T.L., Rojas A., Zelenko Z., Aghajanova L., Erikson D.W., Barragan F., Meyer M., Tamaresis J.S., Hamilton A.E., Irwin J.C., Giudice L.C. Perivascular human endometrial mesenchymal stem cells express pathways relevant to self-renewal, lineage specification, and functional phenotype. Biol. Reprod. 2012;86:58. doi: 10.1095/biolreprod.111.095885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara K., Hamatani T., Yamada M., Ogawa S., Kamijo S., Kuji N., Akutsu H., Miyado K., Yoshimura Y., Umezawa A. Derivation of human decidua-like cells from amnion and menstrual blood. Sci. Rep. 2014;4:4599. doi: 10.1038/srep04599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L., Xu R., Sun X., Duan Y., Han Y., Zhao Y., Qian H., Zhu W., Xu W. Safety evaluation of exosomes derived from human umbilical cord mesenchymal stromal cell. Cytotherapy. 2016;18:413–422. doi: 10.1016/j.jcyt.2015.11.018. [DOI] [PubMed] [Google Scholar]
- Tan J., Li P., Wang Q., Li Y., Li X., Zhao D., Xu X., Kong L. Autologous menstrual blood-derived stromal cells transplantation for severe Asherman's syndrome. Hum. Reprod. 2016;31:2723–2729. doi: 10.1093/humrep/dew235. [DOI] [PubMed] [Google Scholar]
- Teklenburg G., Salker M., Molokhia M., Lavery S., Trew G., Aojanepong T., Mardon H.J., Lokugamage A.U., Rai R., Landles C., et al. Natural selection of human embryos: decidualizing endometrial stromal cells serve as sensors of embryo quality upon implantation. PLoS One. 2010;5:e10258. doi: 10.1371/journal.pone.0010258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji S., Yoshimoto M., Takahashi K., Noda Y., Nakahata T., Heike T. Side population cells contribute to the genesis of human endometrium. Fertil. Steril. 2008;90:1528–1537. doi: 10.1016/j.fertnstert.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Valentijn A.J., Palial K., Al-Lamee H., Tempest N., Drury J., Von Zglinicki T., Saretzki G., Murray P., Gargett C.E., Hapangama D.K. SSEA-1 isolates human endometrial basal glandular epithelial cells: phenotypic and functional characterization and implications in the pathogenesis of endometriosis. Hum. Reprod. 2013;28:2695–2708. doi: 10.1093/humrep/det285. [DOI] [PubMed] [Google Scholar]
- Wang K., Jiang Z., Webster K.A., Chen J., Hu H., Zhou Y., Zhao J., Wang L., Wang Y., Zhong Z., et al. Enhanced cardioprotection by human endometrium mesenchymal stem cells driven by exosomal MicroRNA-21. Stem Cells Transl. Med. 2017;6:209–222. doi: 10.5966/sctm.2015-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Vilella F., Alama P., Moreno I., Mignardi M., Isakova A., Pan W., Simon C., Quake S.R. Single-cell transcriptomic atlas of the human endometrium during the menstrual cycle. Nat. Med. 2020;26:1644–1653. doi: 10.1038/s41591-020-1040-z. [DOI] [PubMed] [Google Scholar]
- Wu Q., Wang Q., Li Z., Li X., Zang J., Wang Z., Xu C., Gong Y., Cheng J., Li H., et al. Human menstrual blood-derived stem cells promote functional recovery in a rat spinal cord hemisection model. Cell Death Dis. 2018;9:882. doi: 10.1038/s41419-018-0847-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Luo Y., Chen J., Pan R., Xiang B., Du X., Xiang L., Shao J., Xiang C. Transplantation of human menstrual blood progenitor cells improves hyperglycemia by promoting endogenous progenitor differentiation in type 1 diabetic mice. Stem Cells Dev. 2014;23:1245–1257. doi: 10.1089/scd.2013.0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt K.A., Filby C.E., Davies-Tuck M.L., Suke S.G., Evans J., Gargett C.E. Menstrual fluid endometrial stem/progenitor cell and supernatant protein content: cyclical variation and indicative range. Hum. Reprod. 2021;36:2215–2229. doi: 10.1093/humrep/deab156. [DOI] [PubMed] [Google Scholar]
- Xiang B., Chen L., Wang X., Zhao Y., Wang Y., Xiang C. Transplantation of menstrual blood-derived mesenchymal stem cells promotes the repair of LPS-induced acute lung injury. Int. J. Mol. Sci. 2017;18:689. doi: 10.3390/ijms18040689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Li X., Gu X., Zhang B., Tian W., Han H., Sun P., Du C., Wang H. Prolongation of cardiac allograft survival by endometrial regenerative cells: focusing on B-cell responses. Stem Cells Transl. Med. 2017;6:778–787. doi: 10.5966/sctm.2016-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Wang Y., Zhang B., Lan X., Lu S., Sun P., Li X., Shi G., Zhao Y., Han H., et al. Treatment of experimental colitis by endometrial regenerative cells through regulation of B lymphocytes in mice. Stem Cell Res. Ther. 2018;9:146. doi: 10.1186/s13287-018-0874-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafardoust S., Kazemnejad S., Darzi M., Fathi-Kazerooni M., Rastegari H., Mohammadzadeh A. Improvement of pregnancy rate and live birth rate in poor ovarian responders by intraovarian administration of autologous menstrual blood derived- mesenchymal stromal cells: phase I/II clinical trial. Stem Cell Rev. Rep. 2020;16:755–763. doi: 10.1007/s12015-020-09969-6. [DOI] [PubMed] [Google Scholar]
- Zhang S., Li P., Yuan Z., Tan J. Platelet-rich plasma improves therapeutic effects of menstrual blood-derived stromal cells in rat model of intrauterine adhesion. Stem Cell Res. Ther. 2019;10:61. doi: 10.1186/s13287-019-1155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Lin X., Dai Y., Hu X., Zhu H., Jiang Y., Zhang S. Endometrial stem cells repair injured endometrium and induce angiogenesis via AKT and ERK pathways. Reproduction. 2016;152:389–402. doi: 10.1530/REP-16-0286. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Chen X., Wu Y., Wang Y., Li Y., Xiang C. Transplantation of human menstrual blood-derived mesenchymal stem cells alleviates alzheimer's disease-like pathology in APP/PS1 transgenic mice. Front. Mol. Neurosci. 2018;11:140. doi: 10.3389/fnmol.2018.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Lan X., Wang Y., Xu X., Lu S., Li X., Zhang B., Shi G., Gu X., Du C., Wang H. Human endometrial regenerative cells attenuate bleomycin-induced pulmonary fibrosis in mice. Stem Cells Int. 2018;2018:3475137. doi: 10.1155/2018/3475137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H., Pan Y., Jiang Y., Li J., Zhang Y., Zhang S. Activation of the Hippo/TAZ pathway is required for menstrual stem cells to suppress myofibroblast and inhibit transforming growth factor beta signaling in human endometrial stromal cells. Hum. Reprod. 2019;34:635–645. doi: 10.1093/humrep/dez001. [DOI] [PubMed] [Google Scholar]
- Zlatska A.V., Rodnichenko A.E., Gubar O.S., Zubov D.O., Novikova S.N., Vasyliev R.G. Endometrial stromal cells: isolation, expansion, morphological and functional properties. Exp. Oncol. 2017;39:197–202. [PubMed] [Google Scholar]