Abstract
Diabetes is a metabolic disorder which is characterised by high levels of blood glucose. Most of the oral drugs available today for the treatment of diabetes are associated with various side-effects. Herbal medicines are considered relatively safer alternatives and Gymnema sylvestre (GS) is one such known traditional medicinal plant widely used for the treatment of diabetes. In our previous work, we isolated active triterpene glycosides (TG) from Gymnema sylvestre (GS) and screened for yeast α-glucosidase inhibitory activity in vitro. The present study aims to use in silico techniques to understand and predict the inhibitory role of the isolated triterpene glycosides (TG); Gymnemic acid I, IV, VII and gymnemagenin against disaccharidase enzymes. enzyme kinetic analysis using Lineweaver-Burk plot indicated that TG competitively inhibited yeast α-glucosidase at IC50 concentration with Ki 0.0028 μM. TG also exhibited significant inhibitory activity against mammalian sucrase and maltase respectively, compared to control.
Practical applications
The molecular docking simulation reveals that TG is capable of docking well with crystallographic structures of the selected enzyme targets. Inhibition of α-glucosidases could delay the absorption of glucose in the blood during post-meal digestion. Thus the current study highlights the dietary intervention of TG towards the selected enzyme targets, thus making TG a potential nutraceutical candidate towards management of blood glucose.
Keywords: Gymnema sylvestre, Antidiabetic, In silico docking, Dissacharidases, Inhibition
Gymnema sylvestre; Antidiabetic; in silico docking; Dissacharidases; Inhibition.
1. Introduction
Diabetes mellitus is characterized by an abnormal increase in plasma glucose, known as hyperglycemia, caused either by a deficiency in insulin secretion (Type1 diabetes mellitus or T1DM), resistance to insulin secretion (Type 2 diabetes mellitus or T2DM) or both (Willson et al., 2000; Shearer and Billin, 2007; Feldman et al., 2008). It has been estimated worldwide that 347 million people have diabetes with a prevalence of 8.3% (Woerle et al., 2004). Hyperglycemia and oscillating blood glucose concentrations attribute directly to the development of cardiovascular disease (Ceriello et al., 2008; Kato et al., 2008). Management of postprandial hyperglycemia is considered as a first therapeutic strategy for T2DM treatment. This can be accomplished by delaying the release of glucose through the inhibition of carbohydrate hydrolysing enzyme α-glucosidase (EC 3.2.1.20) in the digestive tract (Lee et al., 2012). Inhibiting this class of enzyme retards gastrointestinal absorption of dietary carbohydrates by restricting the breakdown of linear or branched oligosaccharide units like α-limit dextrins, maltose and maltotriose to produce glucose thereby preventing glucose absorption into blood stream (Lee et al., 2012). Commercially, α-glucosidase inhibitors are extensively used as monotherapy and in combination with other antidiabetic agents to reduce postprandial increase in blood glucose level (Fujisawa et al., 2005). But most of these are known to have certain adverse effects such as liver and gastrointestinal toxicity (Williams and Pickup, 1991; Rao et al., 1997). Thus, there is an increased demand for natural products with antidiabetic activity with no side effects. Indian traditional systems of medicine have a plethora of promising plants for treatment of diabetes, of which Gymnema sylvestre (GS) is most well established and extensively used (Shanmugasundaram et al., 1983). Several compounds have been isolated over the years from GS such as gymnemic acids, gymnemasaponins, gymnemasides, gymnemasins, deacylgymnemicacid, gymnemagenin, gymnestrogenin b and gymnemanol (Sahu et al., 1996; Saneja et al., 2010). Not much evidence is available on the mechanism of inhibition of these compounds in the treatment of diabetes. In our previous studies, we have isolated an active fraction TG from Gymnema sylvestre consisting of mixture of compounds; Gymnemic acid I, IV, VII and gymnemagenin (Rashmi et al., 2018).
We have also evaluated the efficacy of isolated TG towards in vitro inhibition of α-glucosidases. The specific role of these proteins is listed in Table 1 with their identities in the Protein Data Bank (PDB) (Berman et al., 2000).
Table 1.
Enzymes and their substrates used in the study.
| Enzymes to be Docked | Pdb Id | Substrates | Functions of Enzymes | |
|---|---|---|---|---|
| 1. | α-glucosidase from yeast (Isomaltase) | 3A47/3AXI | p-NPP | Isomaltase is an enzyme that breaks the bonds linking saccharides, which cannot be broken by amylase or maltase |
| 2. | Sucroseα-glucosidase (Sucrase) | 3CZE | Sucrose | catalyzes the hydrolysis of sucrose to fructose and glucose |
| 3. | Sucrase isomaltase (Maltase) | 3LPO/3LLP | Maltose | Catalyzes the hydrolysis of maltose to the simple sugar glucose. |
In this background, we have attempted to study the inhibitory effect of TG containing; gymnemic acid I, IV, VII and gymnemagenin from Gymnema sylvestre towards yeast α-glucosidase and mammalian α-glucosidases (sucrase and maltase). The in vitro studies have shown promising results towards the inhibition of disaccharidases as compared to positive control acarbose. The present work focuses on in silico methods to elucidate the inhibitory effect of TG on enzyme targets associated with elevated levels of blood glucose.
2. Materials and methods
2.1. Materials
α-glucosidase from Saccharomyces cerevisiae, para-nitrophenyl-glucopyranoside (pNPG), starch, sucrose, maltose was procured from SRL chemicals. (Bangalore, India). Rat intestinal acetone powder (source of sucrase and maltase) was obtained from Sigma-Aldrich, St. Louis, USA. The dried leaf powder of Gymnema Sylvestre was obtained from Nikhila Karnataka Central Ayurvedic Pharmacy (Mysore, India). The chemicals used in our research were of analytical grade and purchased from Himedia chemicals (India).
2.2. Isolation of TG, assessment of α-glucosidase and α-amylase inhibitory activities
A known amount of the dried powder Gymnema sylvestre was extracted using absolute ethanol, purified using preparative gradient HPLC method to obtain four fractions The fraction III (TG) exhibiting maximum α-glucosidase activity was chosen for further studies. The isolated fraction was freeze dried and characterized using TLC, FTIR and NMR, after which it was subjected to in vitro anti diabetic activities (Rashmi et al., 2018).
Yeast α-glucosidase inhibitory activity was performed according to the method described (Kim et al., 2005), using pNPG as the substrate. The α-glucosidase inhibitory activity was determined by measuring the yellow-coloured para-nitrophenol released from pNPG at 405 nm. Similarly the mammalian α-glucosidase (sucrase and maltase) inhibitory activities were determined using sucrose and maltose as the substrates (Adisakwattana and Chanathong, 2011). Glucose released was analysed using glucose oxidase method at a wavelength of 505 nm. Concentrations of extracts resulting in 50% inhibition of enzyme activity (IC50) were determined graphically for all the studied enzymes.
2.3. Kinetics of enzyme inhibition
Inhibitory kinetics was performed by maintaining fixed enzyme (yeast α-glucosidase) concentration 10 μg (1.12 U/mL) and varying substrate (para-nitrophenyl-glucopyranoside (pNPG)) concentrations (0–0.0025 μM). The kinetic graph was drawn using the standard Micheles-Menten curve for α-glucosidase (Data not shown). The reciprocal plot was used to obtain the Lineweaver-Burk (LB) plot. Using the LB kinetic plot as a reference, the α-glucosidase inhibitory assay was performed at one of the concentrations of TG (500 μg) (Rashmi et al., 2018).
2.4. Molecular docking analysis
To know the potential binding mode of the selected ligands at the molecular level as α-glucosidase inhibitors, all the chemical structures of the ligands used in the study like gymnemic acid I, IV, VII and gymnemagenin (Figure 1) were retrieved from PubChem database into sdf format (Momany and Rone, 1992). Similarly, the crystal structure of α-glucosidase from yeast (isomaltase), sucrose α-glucosidase (sucrase), sucrose isomaltase (maltase) were obtained from the Protein data bank and details are as shown in Table 1, Figure 2. Further, the retrieved ligands and protein structures were minimized using the conjugate gradient protocol and employing the CHARMM force field to depreciate ligands with Max Steps-2000 implemented in Discovery Studio 3.5 software (Accelrys, San Diego, USA). The initial step was carried out by preparing protein and identifying the binding sites by using define and edit binding site tool (Adisakwattana and Chanathong, 2011). To explain the activity order of gymnemic acid I, IV, VII and gymnemagenin against selected targets, ligands were then docked to the binding site of selected proteins. Docking studies were carried out using the CDOCKER protocol under the protein–ligand interaction section in Discovery Studio 3.5. In general, CDOCKER is a grid-based molecular docking method that employs CHARMM force fields (Momany and Rone, 1992). Protein was first held rigid while the ligands were allowed to flex during the refinement. Total molecules of water and ions not needed for catalytic activity were uncovered to preserve the entire protein. Docking runs were adjusted to 10 for evaluation of the accurate binding pose. For each ligand, top ten ligand binding poses were ranked according to their CDOCKER energies, and the predicted binding interactions were analyzed. Finally, the result was analysed for lowest binding energies, ligand amino acid interaction and all the values tabulated against individual targets. Ligand-bound images were considered for further analysis as per stronger binding affinity. Results were analysed and compared to in vitro results.
Figure 1.
Represents the chemical structures of Triterpene glycosides (TG) Ligands; Gymnemic acid I, IV, VII and gymnemagenin retrieved from PubChem database used in the study.
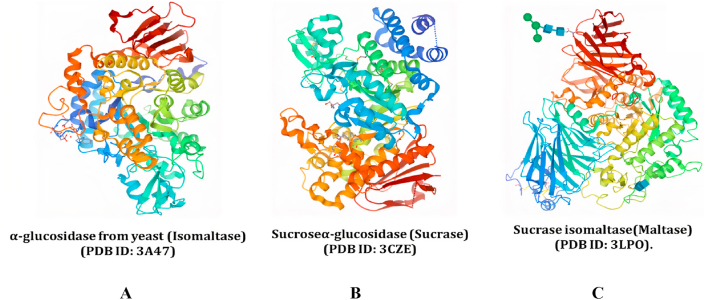
Figure 2.
Represents the crystal structure of α-glucosidase from yeast (Isomaltase), Sucrose alpha-glucosidase (Sucrase), Sucrose isomaltase (Maltase) obtained from the Protein data bank (A) represents crystal structure of isomaltase (PDB ID: 3A47) from Saccharomyces cerevisiae B) shows crystal Structure Analysis of Sucrose hydrolase (SUH) (PDB ID: 3CZE) C) represents crystal structure of the N-terminal domain of sucrase-isomaltase (PDB ID: 3LPO).
3. Results
Our in vitro results reveal that fraction III (TG) exhibited the highest inhibitory activity of 53.3% compared to standard acarbose (positive control), which exhibited 26% inhibition against α-glucosidase. When subjected to different concentrations of TG, the inhibition of α-glucosidase was observed to be concentration dependent. This activity was higher compared to acarbose at their respective similar concentrations. TG showed α-glucosidase inhibitory activity with an IC50 value of 3.16 ± 0.05 μg/mL. Similarly, sucrase and maltase enzyme activities were inhibited by TG with IC50 values of 74.07 ± 5.11 μg/mL and 5.69 ± 0.02 μg/mL respectively (Rashmi et al., 2018).
3.1. Mode of inhibition (kinetics)
The mode of inhibition of the active fraction TG on α-glucosidase was determined using the Lineweaver-Burk plot at one of the concentrations (500 μg). As seen in Figure 3 the Y-intercepts closely matched with each other which possibly displayed competitive mode of inhibition of the enzyme. The graph displayed straight lines with same intercept of 1/Vm values in the presence and absent of TG and different -1/Ki values as seen on the x-axis which is the characteristic of competitive inhibition. The Ki value was calculated as reciprocal of -1/Ki as 0.0028μM (Figure 3).
Figure 3.
Lineweaver-Burk plot displaying possible competitive mode at IC50 concentration.
3.2. Docking analysis with selected inhibitors
3.2.1. Receptor-ligand interactions of α- glucosidase from yeast (isomaltase)
Docking score and hydrogen bonding energy in each ligand bound cavity were considered for comparing the binding selectivity of the ligands. Figure 4A represents the stero view of isomaltase complexed with maltose. Fig. 4B, C represents the hydrogen bonding network after maltose and p-NPG is bound to the active site of the enzyme. The pose with minimum binding energy and inhibitory constant was selected as the best interaction ligands. The results reveal that gymnemagenin, gymnemic Acid I and gymnemic Acid VII interacted with active site amino acid residues of α-glucosidase (Fig. 5A, B, C, D). However, gymnemic acid IV did not exhibit any interaction as shown in Table 2. Gymnemic Acid I binds at the hydrophobic pocket, surrounded by the residues LYS156, ASP197, ASP233, ARG315, ASP352, ASP242, GLN239, ASP300 and LEU313, thus forming a stable hydrophobic binding and displayed strong interaction with amino acid residues respectively. Similarly, gymnemagenin exhibited interactions with ASP197, SER240, ARG315, ASP352, ASP242, ASP300 amino acids and gymnemic Acid VII interacted with ASP197, ASP300, ASP352, ARG315 across enzyme α-glucosidase, revealing its potential binding mode at the molecular level in the catalytic site (Table 2). Results are tabulated as shown in Table 2. Gymnemic Acid I showed high affinity for the enzyme α-glucosidase with lowest free binding energy of -83.2898 kcal/mol, similar to p-NPG (−22.677 kcal/mol) followed by -82.2118 kcal/mol for gymnemagenin, and -59.3684 kcal/mol for gymnemic Acid VII respectively (Table).
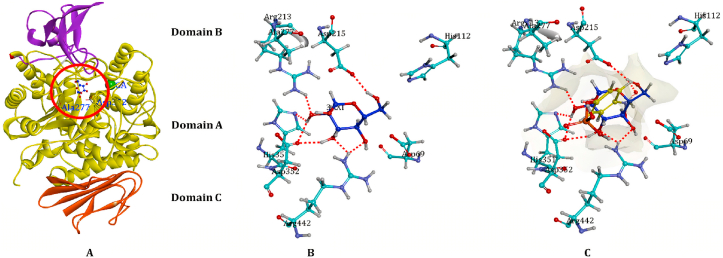
Figure 4.
Overall structure of isomaltase. (A) represents the stereo view of overall structure of isomaltase in complex with maltose. Domain A (residues 1–113 and 190–512) is shown in yellow, domain B (residues 114–189) in blue, and domain C (residues 513–589) in red. A calcium ion is shown as a magenta sphere. The reducing end of the glucose residue is displayed in green. The three catalytic acidic residues are shown as a stick model. (B) represents the view of the hydrogen bond network in the active site of isomaltase. After maltose binding to the active site. The nonproducing end of the glucose residue is displayed as a ball-and-stick model. (C) Isomaltase with substrate p-NPG.
Figure 5.
Schematic representation of the interactions between the best pose found for binding of ligands to α-glucosidase (Isomaltase) active pocket. (A) Gymnemagenin, (B) Gymnemic Acid I, and (C) Gymnemic Acid VII interacting with active site amino acid residues of α-glucosidase. (D) Codocking with all the ligands simultaneously at the active site.
Table 2.
Represents the Receptor-Ligand Interactions with selected ligands with Isomaltase and respective binding energies.
| Enzyme | Pdb Id | Pubchem Cid And Inhibitor Docked | Active Site Residues | Residues Interactions | Cdocker Energy (kcal/mol) | Common Interacting Residues |
|---|---|---|---|---|---|---|
| α glucosidase from yeast (Isomaltase) | 3AXI | CID 44144284 Gymnemic Acid I |
ASP69, HIS 112 Asp197,ARG213 ASP 215,ASP233 Asp300, HIS 351 ASP 352,ARG442 |
LYS156, ASP197, ASP233, ARG315, ASP352, ASP242, GLN239, ASP300, LEU313 | -83.2898 | Asp197, ARG315 ASP352 Asp300 |
| CID 10051937 Gymnemagenin |
ASP197, SER240, ARG315, ASP352, ASP242, ASP300 | -82.2118 | ||||
| CID 91617592 Gymnemic Acid VII |
ASP197, ASP300, ASP352, ARG315 | -59.3684 | ||||
| Gymnemic Acid IV | NO interactions found | NO interactions found | NO interactions found | NO interactions found |
3.2.2. Receptor-ligand interactions of sucrose α-glucosidase (sucrase)
Similar to α-glucosidase, docking studies with sucrose α-glucosidase (sucrase) exhibited interesting results. The figure shows the structure of sucrose hydrolase (sucrase) SUH in the E322Q–sucrose complex (Fig. 6A, B). Figure 6A represents the stereo view of sucrase complexed with sucrose at the active site, in ball and stick model. Figure 6B represents the hydrogen bonding network of sucrase when complexed with sucrose. Gymnemic acid I, gymnemagenin, and gymnemic acid IV revealed strong binding interactions with sucrase (Fig 7A, B, C). Interestingly the ligand gymnemic Acid VII did not show any interaction. However, gymnemic acid I could bind to amino acid residues like HIS443, GLU322, ALA323, ILE324, VAL325, ASP392, ASP393 and GLY395 displaying strong interactions at the catalytic site with binding energy of -95.8172 kcal/mol. Similarly gymnemagenin, showed interactions with the amino acid residues GLU322, SER281, HIS443, PHE345, SER241, with binding energy of -73.8771 kcal/mol. Gymnemic Acid IV displayed strong interactions towards SER281, TYR284, ALA323, ILE324, HIS443 with highest binding energies -101.119 kcal/mol revealing their potential binding mode at molecular level in the catalytic site of sucrase (Table3).
Figure 6.
Structure of Sucrose hydrolase (sucrase) SUH in the E322Q–sucrose complex. (A)Sucrose bound in the active site is shown as a ball-and-stick model. (B) Represents hydrogen bonding network of sucrase complexed with sucrose at the active site of the enzyme.
Figure 7.
Schematic representation of the interactions between the best pose found for binding of ligands to Sucrose α-glucosidase (sucrase) active pocket. (A) Gymnemagenin, (B) Gymnemic Acid I, and (C) Gymnemic Acid IV interacting with active site amino acid residues of α-glucosidase. (D) Codocking with all the ligands simultaneously at the active site.
Table 3.
Represents the Receptor-Ligand Interactions with selected ligands with Sucrose α-glucosidase (sucrase) and respective binding energies.
| Enzyme | Pdb Id | Pubchem Cid And Inhibitor Docked | Active Site Residues | Cdocker Energy (kcal/mol) | Residues Interactions |
|---|---|---|---|---|---|
|
Sucrose α-glucosidase Sucrose hydrolase (SUH) |
3CZE/3CZK | CID 44144284 Gymnemic Acid I |
PHE 140,PHE244 ARG278,ASP280 SER281,TYR284 GLU322,ILE324 VAL325, ASP392, HIS443, ARG515. |
-95.8172 | HIS443, GLU322, ALA323 |
| CID 10051937 Gymnemagenin |
-73.8771 | ILE324, VAL325, ASP392 | |||
| CID 14264063 Gymnemic acid IV |
-101.119 | ASP393, GLY395 | |||
| CID 91617592 Gymnemic Acid VII (NO interactions) |
NO interactions | GLU322, SER281, HIS443, PHE345, SER241, |
3.2.3. Receptor-ligand interactions sucrose isomaltase (maltase)
The crystal structure for N-terminal human sucrose-isomaltase (ntSI) was selected for docking studies (PDB ID-3LLP). The structural analysis of enzyme depicted that the active site is composed of a shallow-substrate binding pocket including -1 and +1 sub sites. In order to predict the binding mode of compounds, docking analysis with human N-terminal sucrose isomaltase (3LPP) was performed. The active site residues include ASP231, ASP571, LEU233, TRP327, TRP435, PHE479, VAL605, AND TYR63 (Table 4). The simulations were performed based on the ligand interactions with enzyme and were looked into best conformation changes. Outcome of docking studies confirm that gymnemic Acid I, gymnemagenin, and gymnemic Acid IV binds to sucrose-isomaltase and exhibit strong binding with ligand at the active site and nearby residues indicating the inhibitory effect of these ligands. Details of ligand interactions and binding energies are presented in Table 4.
Table 4.
Represents the Receptor-Ligand Interactions with selected ligands with Sucrose α-glucosidase (sucrase) and respective binding energies.
| Enzyme | Pdb Id | Pubchem Cid And Inhibitor Docked | Active Site Residues | Cdocker Energy (kcal/mol) | Residues Interactions |
|---|---|---|---|---|---|
| Human N-terminal sucrase isomaltase (Maltase) | 3LPO/3LLP | CID 44144284 Gymnemic Acid I |
Asp231, Asp571,Leu233, Trp327, Trp435, Phe479, Val605, Tyr634, | -83.2898 | LEU311, D443, D542, H600, D327, Y299, W406, F450, K480, T204, T205, D542, A576, D443 |
| CID 10051937 Gymnemagenin |
-82.2118 | ARG563, GLU538, HIS569, LYS805, MET314, ARG549, ARG 563. HIS674, GLN809, VAL675, ASP806 | |||
| CID 91617592 Gymnemic Acid VII |
-59.3684 | ILE808, ARG563, LEU311 LYS805, GLU538, HIS569, LYS805, MET314, ARG549, HIS674, GLN809, VAL675, ASP806 and 563. | |||
| CID 91617592 Gymnemic Acid VII (NO interactions) |
(NO interactions) | (NO interactions) | (NO interactions) |
4. Discussion
Gymnema sylvestre, a traditional medicinal plant in Asia has been widely used as a remedy for diabetes mellitus. It has been reported that the extract of Gymnema sylvestre (GS) inhibits the sweet taste sensitivity, glucose absorption in the gastrointestinal tract and increase plasma insulin level by repairing or regenerating the pancreatic islet (Berman et al., 2000; The UniProt Consortium, 2010). This folk medicine also possesses the properties for the treatment of snake bite, eye complaints, stomachic and diuretic problems, and asthma (Izutani et al., 2005). The leaves of GS contain triterpenoidal saponins belonging to oleanane and dammarene classes. Twenty different saponins and glycosides have been reported in Gymnema sylvestre (Ahmed et al., 2010; Praveen et al., 2014). Previously, we isolated and chatacterised active fraction Gymnema sylvestre aned termed as TG (Saneja et al., 2010); containing mixture of triterpene glycosides namely gymnemic acid I, IV, VII and gymnemagenin. TG has shown remarkable in vitro antidiabetic activities (Saneja et al., 2010). In another study researchers have shown that gymnemoside b, gymnemic acids III, V, and VII exhibited weak inhibitory activity on plasma glucose in rats during the oral glucose tolerance test (Khramov et al., 2004). We have observed that isolated active fraction TG inhibited the activity of yeast α-glucosidase and intestinal α-glucosidases (sucrase and maltase) in a concentration-dependent manner.
We found IC50 value for TG (3.16 ± 0.05 μg/mL) against yeast α-glucosidase higher than compared to acarbose. In a similar study, GS showed remarkable inhibitory effect on α -glucosidase with IC50 at 68.70 ± 1.22 μg/mL, compared with the positive control acarbose at 59.03 ± 2.30 μg/mL (Trivedi and Pundarikakshudu, 2008).
Up to now, more than 30,000 terpenes have been isolated from Gymnema sylvestre. Due to their relatively complex structures, triterpenoids in free form (sapogenins), linked to glycosides (saponins) or acetylated are vital and widely exist in plants, which have exerted important pharmacological functions both in vitro and in vivo researches (Yoshikawa et al., 1999). In our study, we were unable to isolate the individual compounds from the active fraction, due to the complexity involved (Saneja et al., 2010). In this regard, docking studies offer some insight into the interaction between ligand and selected enzymes which can substantiate the experimental results. As binding pocket/cavity plays a key role in the binding interaction between target receptor and ligand of any drug design process, binding pocket analysis were carried out using define and edit binding site option in DS 3.5 (Adisakwattana and Chanathong, 2011). The ligands used in the study were analyzed among the selected target enzymes. Molecular docking poses thus obtained were saved and ranked according to their dock score function (Adisakwattana and Chanathong, 2011).
Our studies revealed that the individual compounds in the mixture dock well with the crystallographic structures of the disaccharidases. When TG was docked with yeast α-glucosidase, we observed that all the docked ligands displayed common interacting residues like ASP197, ARG315, ASP352, ASP300 indicating the binding affinity and inhibition of enzyme with selected ligands against α-glucosidase. Of the four compounds studied, gymnemic Acid IV did not show any interaction. But the rest of the active molecules (gymnemic acid I, VII and gymnemagenin) facilitated ligands to anchor in the binding site of the α-glucosidase. The interaction between docked ligands and α-glucosidase were almost similar. This was consistent with the results of the in vitro anti-α-glucosidase assay where TG competitively inhibited yeast α-glucosidase with Ki 0.0028 μM (Figure 3). The binding of the ligand complexes with the enzyme is reported in Figure 5A, B, C, D and interactions of the residues are as depicted in Table 3. All these residues were preferably involved in positioning of ligand molecules within the active site pocket of α-glucosidase. Three highly conserved and catalytic residues such as ASP197, GLU233 and ASP300 from glucosidase family were reported using crystallographic studies and all these residues are found to be part of critical ligand interaction. Hence, the above molecular docking studies give us rational explanation of the interactions between gymnemic acid I, gymnemagenin, gymnemic acid VII with α-glucosidase, which provided valuable information for further development of α-glucosidase inhibitors.
Similar results were observed with sucrose α-glucosidase (sucrase) and sucrose-isomaltase (matlase). It was exciting to note that gymnemic acid VII failed to show interactions with sucrose α-glucosidase. While it has shown interesting interactions with yeast α-glucosidase (isomaltase). All the ligands bound to sucrase exhibited significantly common pharmacophoric residues like HIS443, GLU322, ALA323 ILE324, ASP392, and SER281, with the actual active site located at the region composed of the C-terminal ends of the parallel β-strands in the barrel fold and is covered by the B and B′-domains receptor interface, thus revealing the collective inhibitory action of the enzyme. Another disaccharidase sucrose-isomaltase is composed of duplicated catalytic domains, N- and C-terminal. Each domain displayed overlapping specificities. We observed similar results to that of maltase, where Gymnemic Acid VII failed to show interactions with sucrase-isomaltase.
Hence, to appreciate the difference in binding affinities of Gymnemic Acid VII and Gymnemic Acid IV inhibitors, they were analyzed for the number of hydrogen bond donors, acceptors as shown in Table 5 and RMSD analysis as shown in Table 6. As evident from Table 2 for Gymnemic acid I ligand, inhibitor was found to interact with ASP197, ASP233 and ASP300. Similarly, ligand Gymnemagenin interacted with ASP197, ASP352, ASP300 and Gymnemic Acid VII with ASP197 and ASP300 amino acid residues. The reason behind the low affinity of Gymnemic Acid IV across α-glucosidase from yeast (Isomaltase) was that, there was no hydrogen bond forming residues at all across RMSD value of 5 Å as compared to other inhibitors. However, Gymnemic Acid IV inhibitor was still at a distance away for interaction with active site residues from this region. Likewise, Gymnemic acid VII exhibited a similar trend with sucrose α-glucosidase, sucrose hydrolase (SUH) and human N-terminal sucrose isomaltase (Maltase).
Table 5.
Shows the details of ligands with number of hydrogen bond donors/acceptors and rotatable bond count.
| Sl NO | Ligand name | Pubchem CID And Inhibitor Docked | Molecular Formula | Molecular Weight | No of hydrogen bond donors | Rotatable Bond Count | No of hydrogen bond acceptors |
|---|---|---|---|---|---|---|---|
| 1. | Gymnemic Acid I | CID11953919 | C43H66O14 | 807 g/mol | 7 | 10 | 14 |
| 2. | Gymnemic Acid IV | CID 14264063 | C41H64O13 | 764.95 g/mol | 8 | 8 | 13 |
| 3. | Gymnemagenin | CID 10051937 | C30H50O6 | 506.7 g/mol | 6 | 2 | 6 |
| 4. | Gymnemic Acid VII | CID 91617592 | C36H58O11 | 666.8 g/mol | 8 | 5 | 11 |
Table 6.
Shows the Rmsd Values of overlaid Ligands.
| Gymnemic Acid I | Gymnemic Acid IV | Gymnemagenin | Gymnemic Acid VII | |
|---|---|---|---|---|
| Gymnemic Acid I CID11953919 |
- | - | - | - |
| Gymnemic Acid IV CID 14264063 |
0.513322 | - | - | - |
| Gymnemagenin CID 10051937 |
0.577903 | 0.662436 | - | - |
| Gymnemic Acid VII CID 91617592 |
0.652622 | 0.596591 | 0.55979 | - |
The above results thus provide an insight into the mechanism of individual active components present in the active triterpene glycoside fraction. In our previous in vitro studies conducted, we have shown that the active fraction TG showed α-glucosidase inhibitory activity with an IC50 value of 3.16 ± 0.05 μg/mL. Similarly, sucrase and maltase enzyme activities were inhibited by TG with IC50 values of 74.07 ± 5.11 μg/mL and 5.69 ± 0.02 μg/mL respectively. We have also shown that TG has the potential to increase insulin secretion in pancreatic beta cell lines when glucose concentrations were increased (Rashmi et al., 2018). The docking studies are in agreement with our in vitro results, where it proves that the active principles gymnemic acid I, IV, VII and gymnemagenin in combination is responsible for the overall antidiabetic activity. We can thus suggest TG (An active triterpene glycoside fraction), having multifunctional attributes can be used as a natural remedy for the treatment of diabetes mellitus. In future, we intent to incorporate TG into a food formulation so that it could be used as a complementary therapy by diabetic patients.
5. Conclusions
In summary, a series of active molecules gymnemic Acid I, gymnemic acid IV, gymnemagenin and gymnemic Acid VII termed together as triterpene glycosides (TG) have been isolated and evaluated for their α-glucosidase inhibitory activities. The majority of ligands compounds exhibited superior α-glucosidase inhibitory activity. Molecular docking studies were carried out to appreciate the molecular interaction of compounds with the active site of α-glucosidase, sucrose α-glucosidase (sucrase) and sucrase isomaltase (maltase). This study has identified lead compounds with good potential which can provide valuable insights towards diabetes treatment.
Declarations
Author contribution statement
Rashmi SS: Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Jagadeesh Kumar D: Performed the experiments; Wrote the paper.
Nagendra HG: Performed the experiments.
HK Manonmani: Conceived and designed the experiments.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors (Rashmi S Shenoy, Jagadeesh Kumar, Nagendra HG, HK Manonmani) thank Director(Dr Raghav Rao) CSIR-Central Food Technological Research Institute, Mysore for providing the necessary facilities to carry out this work.
References
- Adisakwattana S., Chanathong B. α-glucosidase inhibitory activity and lipid-lowering mechanisms of Moringa oleifera leaf extract. Eur. Rev. Med. Pharmacol. Sci. 2011;15:803–808. [PubMed] [Google Scholar]
- Ahmed A.B.A., Rao A.S., Rao M.V. In vitro callus and in vivo leaf extract of Gymnema sylvestre stimulate –cells regeneration and anti-diabetic activity in Wistar rats. Phytomedicine. 2010;17:1033–1039. doi: 10.1016/j.phymed.2010.03.019. [DOI] [PubMed] [Google Scholar]
- Berman H.M.1., Westbrook J., Feng Z., Gilliland G., Bhat T.N., Weissig H., Shindyalov I.N., Bourne P.E. The “protein data bank”. Nucleic Acids Res. 2000;28(1):235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceriello A., Esposito K., Piconi L., Ihnat M.A., Thorpe J.E., Testa R., Boemi M., Giugliano D. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes. 2008:1349–1354. doi: 10.2337/db08-0063. [DOI] [PubMed] [Google Scholar]
- Feldman P.L., Lambert M.H., Henke B.R. PPAR modulators and PPAR pan agonists for metabolic diseases: the next generation of drugs targeting per-oxisome proliferator-activated receptors? Curr. Top. Med. Chem. 2008;8:728–749. doi: 10.2174/156802608784535084. [DOI] [PubMed] [Google Scholar]
- Fujisawa T., Ikegami H., Inoue K., Kawabata Y., Ogihara T. Effect of two alpha- glucosidase inhibitors, voglibose and acarbose, on postprandial hyperglycemia correlates with subjective abdominal symptoms. Metabolism. 2005;54:387–390. doi: 10.1016/j.metabol.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Izutani Y., Murai T., Imoto T., Ohnishi M., Oda M., Ishijima S. Interaction of gymnemic acid with cyclodextrins analyzed by isothermal titration calorimetry, NMR and dynamic light scattering. FEBS Lett. 2005;579:4333–4336. doi: 10.1111/j.1742-4658.2005.05014.x. [DOI] [PubMed] [Google Scholar]
- Kato A., Minoshima Y., Yamamoto J., Adachi I., Watson A.A., Nash R.J. Protective effects of dietary chamomile tea on diabetic complications. J. Agric. Food Chem. 2008;56:8206–8211. doi: 10.1021/jf8014365. [DOI] [PubMed] [Google Scholar]
- Khramov V.A., Spasov A.A., Samokhina M.P. Chemical composition of dry extracts of Gymnema sylvestre leaves. Pharm. Chem. 2004;2(1):29–31. [Google Scholar]
- Kim Y.M., Jeong Y.K.M., Wang H.W., Lee Y., Rhee H.I. Inhibitory effect of pine extract on α-glucosidase activity and postprandial hyperglycemia. J. Nutr. 2005;21(6):756–761. doi: 10.1016/j.nut.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Lee B.H., Eskandari R., Jones K., Reddy K.R., Quezada-Calvillo R., Nichols B.L., Rose D.R., Hamaker B.R., Pinto B.M. Modulation of starch digestion for slow glucose release through ‘‘toggling’’ of activities of mucosal a-glucosidases. J. Biol. Chem. 2012;287:31929–31938. doi: 10.1074/jbc.M112.351858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momany F.A., Rone R.J. Validation of the general purpose QUANTA 3.2/CHARMm force field. J. Comput. Chem. 1992;13:888–900. [Google Scholar]
- Praveen N., Thiruvengadam M., Yang Y.S., Kim S.H., Murthy H.N., Chung I.M. Production of gymnemic acid from hairy root cultures of Gymnema sylvestre R. Br.. as influenced by polyunsaturated fatty acids (PUFAs) and their antioxidant activity. Ind. Crop. Prod. 2014;54:54–61. [Google Scholar]
- Rao B.K., Giri R., Kesavulu M.M. Herbal medicines:in the treatment of diabetes mellitus. Manohar Vaidya Patrika. 1997;4:33–35. [Google Scholar]
- Rashmi S.S., Harish Prashanth K.V., Manonmani H.K. In vitro antidiabetic effects of isolated triterpene glycoside fraction from Gymnema sylvestre. Evid. Based Complement. Alternat. Med. 2018 doi: 10.1155/2018/7154702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu N.P., Mahato S.B., Sarkar S.K., Poddar G. Triterpenoid saponins from Gymnema sylvestre. Phytochem. 1996;41(4):1181–1185. doi: 10.1016/0031-9422(95)00782-2. [DOI] [PubMed] [Google Scholar]
- Saneja A., Sharma C., Aneja K.R., Pahwa R. Gymnema sylvestre (gurmar):a review. Der Pharm. Lett. 2010;2(1):275–284. [Google Scholar]
- Shanmugasundaram K.R., Panneerselvam C., Samudram C.P., Shanmugasundaram E.R. Enzyme changes and glucose utilization in diabetic rabbits: the effect of Gymnema sylvestre, R.Br. J. Ethnopharmacol. 1983;7:205–234. doi: 10.1016/0378-8741(83)90021-1. [DOI] [PubMed] [Google Scholar]
- Shearer B.G., Billin A.N. The next generation of PPAR drugs: do we have the tools to find them? Biochim. Biophys. Acta. 2007;1771:1082–1093. doi: 10.1016/j.bbalip.2007.05.005. [DOI] [PubMed] [Google Scholar]
- The UniProt Consortium. Nucleic Acids Res. 2010;38:D142–D148. doi: 10.1093/nar/gkp846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi P.D., Pundarikakshudu K. A validated high performance thin-layer method for the chromatographic estimation of gymnemic acids through gymnemagenin in Gymnema sylvestre, materials, extracts and formulations, Int. J. Appl. Sci. 2008;6:1–19. [Google Scholar]
- Williams G., Pickup J. Blackwell; Oxford: 1991. New Drugs in the Management of Diabetes Mellitus. Text Book of Diabetes II; pp. 977–993. [Google Scholar]
- Willson T.M., Brown P.J., Sternbach D.D., Henke B.R. The PPARs: from orphan receptors to drug discovery. J. Med. Chem. 2000;43:527–550. doi: 10.1021/jm990554g. [DOI] [PubMed] [Google Scholar]
- Woerle H.J., Pimenta W.P., Meyer C., Gosmanov N.R., Szoke E., Szombathy T., Mitrakou A., Gerich J.E. Diagnostic and therapeutic implications of relationships between fasting, 2-h post challenge plasma glucose and hemoglobin A1c values. Arch. Intern. Med. 2004;164:1627–1632. doi: 10.1001/archinte.164.15.1627. [DOI] [PubMed] [Google Scholar]
- Yoshikawa K., Takahashi K., Matsuchika K., Chang H.C., Wang J.D. Structures of alternosides XI-XIX from Gymnema alternifolium. Chem. Pharm. Bull. 1999;47:1598–1603. doi: 10.1248/cpb.47.798. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.