Abstract
Background
The survival time of amyotrophic lateral sclerosis (ALS) is greatly variable and protective or risk effects of the potential survival predictors are controversial. Thus, we aim to undertake a comprehensive meta-analysis of studies investigating non-genetic prognostic and survival factors in patients with ALS.
Methods
A search of relevant literature from PubMed, Embase, Cochrane library and other citations from 1st January 1966 to 1st December 020 was conducted. Random-effects models were conducted to pool the multivariable or adjusted hazard ratios (HR) by Stata MP 16.0. PROSPERO registration number: CRD42021256923.
Findings
A total of 5717 reports were identified, with 115 studies meeting pre-designed inclusion criteria involving 55,169 ALS patients. Five dimensions, including demographic, environmental or lifestyle, clinical manifestations, biochemical index, therapeutic factors or comorbidities were investigated. Twenty-five prediction factors, including twenty non-intervenable and five intervenable factors, were associated with ALS survival. Among them, NFL (HR:3.70, 6.80, in serum and CSF, respectively), FTD (HR:2.98), ALSFRS-R change (HR:2.37), respiratory subtype (HR:2.20), executive dysfunction (HR:2.10) and age of onset (HR:1.03) were superior predictors for poor prognosis, but pLMN or pUMN (HR:0.32), baseline ALSFRS-R score (HR:0.95), duration (HR:0.96), diagnostic delay (HR:0.97) were superior predictors for a good prognosis. Our results did not support the involvement of gender, education level, diabetes, hypertension, NIV, gastrostomy, and statins in ALS survival.
Interpretation
Our study provided a comprehensive and quantitative index for assessing the prognosis for ALS patients, and the identified non-intervenable or intervenable factors will facilitate the development of treatment strategies for ALS.
Funding
This study was supported by the National Natural Science Fund of China (Grant No. 81971188), the 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (Grant No. 2019HXFH046), and the Science and Technology Bureau Fund of Sichuan Province (No. 2019YFS0216).
Keywords: Amyotrophic lateral sclerosis, Survival, Outcome, Predictors, Hazard ratios
Research in Context.
Evidence before this study
Over the past decades, a great deal of attention has been devoted to study the prognosis and survival factors for amyotrophic lateral sclerosis (ALS) and some factors have been identified to have a potential influence on ALS survival. Previous meta-analyses assessed a limited number of prognosis factors for ALS, but many predictors of ALS survival have not yet been fully elucidated. A better and more comprehensive understanding of factors that influences ALS prognosis is extremely urgent. We searched PubMed, Embase, Cochrane library for English-language cohort and case-control studies, published until December 1st, 2020, that evaluated the hazard ratios of non-genetic factors on ALS survival and preformed the quality appraisal, bias analysis and heterogeneity assessment.
Added value of this study
From 115 studies involving 55,169 ALS patients, five dimensions, including demographic, environmental or lifestyle, clinical manifestations, biochemical index, therapeutic factors or comorbidities were investigated. Twenty-five prediction factors were associated with ALS survival. Among them, the superior predictors for poor prognosis were neurofilament light chain (NFL, both in serum and cerebrospinal fluid), frontotemporal dementia (FTD), changes in amyotrophic lateral sclerosis functional rating scale-revised (ALSFRS-R), respiratory subtype, executive dysfunction and age at onset. But pure lower motor neuron (pLMN) or pure upper motor neuron (pUMN), baseline ALSFRS-R score, duration, diagnostic delay were superior predictors for a good prognosis. Other factors may have insufficient evidence and our study did not support the involvement of gender, education level, diabetes, hypertension, noninvasive ventilation, gastrostomy, and statins in ALS survival
Implications of all the available evidence
This study provided a detailed summary of survival predictors of ALS which were then graded. Our work may help guide healthcare workers and ALS patients in scheduling disease management and in guiding clinical trials design. However, more high-quality observational studies and randomized clinical trials are needed to elucidate the survival factors of ALS and to try to delay the course of the disease or even cure it in the future.
Alt-text: Unlabelled box
1. Introduction
Amyotrophic lateral sclerosis (ALS) is characterized by degeneration of the upper and lower motor neurons and usually begins insidiously with focal weakness but spreads ruthlessly to involve most muscles [1,2]. Sporadic and familial are two primary classifications of ALS. Familial ALS (FALS) occurs in about 5% to 10% of patients with ALS, always due to dominant inheritance [1,3], whereas sporadic ALS (SALS) includes all other patients with ALS [1]. As one of the fatal neurodegenerative disorders, the survival time and associated factors are usually a hotspot of studying for neurologists and a focus of the attention for patients and their relatives.
In general, most patients with ALS survive 2 to 5 years [4]. Nonetheless, there were patients who live for 10 years and 20 years or even longer [5]. Although the prognostic factors of ALS have been wildly studied [6], these studies investigating the prediction of ALS survival involve limited samples or factors, and the results are usually not replicable with each other [7], [8], [9], [10], [11], [12]. Till now, the most comprehensive ALS survival prediction model consisted of only 10 non-intervenable or well-proven clinical predicting factors [11], but they were limited in that they did not encompass biochemical factors or combined medical conditions. Furthermore, although there were well-proven genetic factors associated with survival of ALS, such as C9orf72 C4G2 repeats and UNC13A variants, less than 10% of sporadic ALS may have genetic mutations [13] and specific genes tend to similar special clinical manifestations [14], [15], [16]. To avoid such confounding factors, studies exclusively investigating genetic factors for survival of ALS were not included in this meta-analysis.
The predictors of ALS have not yet been fully clarified. A better and more comprehensive understanding of factors that influence ALS outcomes is essential to guide healthcare workers and patients in scheduling therapeutic interventions and to guide clinical trials design [17]. Hence, the aim of this study was to identify non-genetic factors associated with ALS outcomes through a meta-analysis of observational studies.
2. Methods
2.1. Search strategy and selection criteria
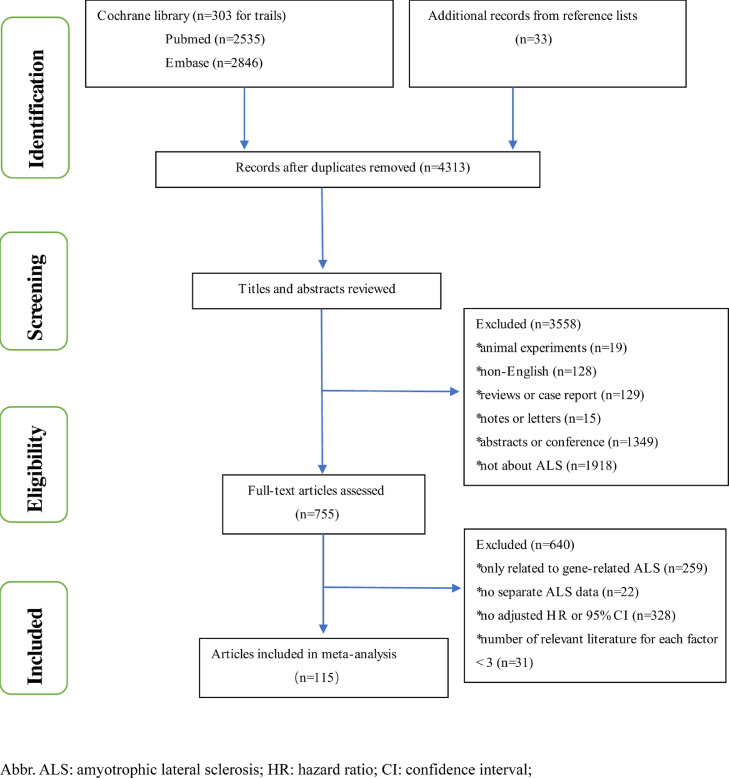
We followed the recommendations of the PRISMA (2009) guidelines for meta-analysis [18,19]. PubMed, Embase, and Cochrane library were searched from Jan 1,1966 to Dec 1, 2020 by terms “amyotrophic lateral sclerosis”, “motor neuron disease”, “Lou Gehrig's disease”, “Gehrig Disease” and “prognosis*”, “progress*”, “survival”, “outcome”, “mortality”, “death”, “hazards” for case-control or cohort studies by limiting “retrospective”, “prospective”, “cohort”, “case-control”, “consecutive” or “case control” in title or abstract. We also considered additional publications from reference lists of reports that were fully read. Previous survival researches in ALS usually use composite events as endpoints comprising both death and respiratory events [20,21]. In this research, survival was defined as time between the onset of symptoms and noninvasive ventilation (NIV) for more than 23 hours per day, or tracheostomy or death. The inclusion criteria are as follows: 1) Case-control or cohort studies published in English; 2) The literature reported factors on ALS survival outcome; 3) the articles’ context or supplementary materials should directly provide adjusted hazard ratio and 95% confidence intervals (CIs) through multivariate Cox hazard proportional model or other multivariate analysis; 4) As for the same reported factor, there must be at least three studies, considering only two studies may result in a more unreliable result; 5) To avoid repeated inclusion, only the largest sample size data were extracted for the same team's research on the same factor. We excluded these articles that only considered gene-related ALS or that didn't distinguish ALS from other motor neuron diseases (MNDs), such as progressive muscular atrophy (PMA), primary lateral sclerosis (PLS), and progressive bulbar palsy (PBP). The detailed flow chart was shown in Fig. 1 and bibliographies of relevant original studies were hand searched. Literature selection was performed by two investigators (WMS, YPC), and if there were any discrepancies, the conclusion would be determined by the third researcher (YFC). A protocol was registered with PROSPERO, registration number: CRD42021256923.
Figure 1.
flowchart of literature selection
Abbr. ALS: amyotrophic lateral sclerosis; HR: hazard ratio; CI: confidence interval;Figure 2 pool analysis of demographic factors and environmental and lifestyle factors
2.2. Data extraction
The nationality of ALS patients, the sample size of ALS cases, and the resulting adjusted hazard ratios and 95%CI were extracted. If a paper had multiple HRs for the same factor, we only included the results with the most adjustment variables. The data of following factors were extracted, including age at onset, gender, marital status, education level, duration, diagnostic delay, body mass index (BMI), smoking(current and former smoking), creatine kinase(CK in serum, creatinine in serum, neurofilament light chain (NFL, in serum and cerebrospinal fluid), albumin in serum, non-specific dementia, frontotemporal dementia (FTD), executive dysfunction, diabetes, hypertension, NIV, gastrostomy, statins, riluzole, multidisciplinary care, onset site, subtype, diagnostic level, amyotrophic lateral sclerosis functional rating scale-revised (ALSFRS-R), ALSFRS-R change, forced vital capacity (FVC), vital capacity (VC), manual muscle test score (MMT)). Except ALSFRS-R change which was time dependent, all the remaining variables were considered at diagnosis or at enrollment. In addition, since some identical factors (including age at onset, BMI, ALSFRS-R, duration, FVC, VC, MMT, ALSFRS-R change, diagnostic delay, CK, creatinine, NFL, and albumin) can be considered as both a continuous and categorical variable, we chose a type that is easy to merge for extraction, but three of them (FVC, ALSFRS-R change and diagnostic delay), which have enough literatures to conduct meta-analysis both for continuous and categorical variable according to the above mentioned criterion, were analyzed by two ways to verify whether the results were in the same direction. Finally, to avoid mistakes and bias, two authors extracted data separately (WMS, YPC), and a third (ZJ) verified the data.
2.3. Quality appraisal
The articles were appraised by two reviewers (WMS,YPC) according to the Newcastle–Ottawa Scale (NOS) [22]. And a score of 6 or above was considered to be of high quality. Similarly, in the event of disagreement, the two quality evaluators reached a consensus after consulting a third co-author (ZJ).
2.4. Statistical analysis
After considering interstudy variability, the adjusted hazard ratio (HR) and 95% confidence interval (CI) were log-transformed and effected using the random-effects model. We roundly explored the survival factors of ALS from the following five aspects: demographic, environmental or lifestyle, clinical manifestations, biochemical index, therapeutic factors or comorbidities. Heterogeneity was assessed by Q test and quantified by the I2 metric (“low”, “moderate”, “high” and “very high” corresponding to values of up to 25%, 50%, 75%, and 100%, respectively) [23]. Forest plots were used to visualize effect sizes and 95% CIs for each study and the pooled effects. As for publication bias, the assessment was conducted only when at least ten studies were available by Begg's test [24,25]. All statistical analyses were performed using Stata MP 16.0 and GraphPad Prism 9.0.0.
2.5. Role of funding source
The funders had no role in study design, data collection, analysis, interpretation, or writing of the report.
3. RESULTS
3.1. Literature results
A flowchart of the selection of eligible studies is presented in Fig. 1. The search of the PubMed database yielded 2535 articles and Embase provided 2846 entries using the terms mentioned above. And taking “amyotrophic lateral sclerosis” as keywords yields 303 trials in the Cochrane library. Besides, for studies retrieved in full-text, hand searching of reference lists and citation searches were performed. According to the predesigned inclusion criteria, finally, 5115 articles involving 55,169 ALS patients, were included. The NOS score ranged from 5 to 8 points in these included studies. The detailed quality evaluations of each study were shown in the supplementary material (Supp. Table 1), which also included characteristics of eligible studies. After reviewing the articles and extracted the data, a total of 32 candidate prognostic factors were conducted in this meta-analysis, including demographic factors (4), environmental and lifestyle factors (1), clinical manifestations (12), biochemical index (5), therapeutic factors and comorbidity (10).
3.2. Prognostic factors
3.2.1. Demographic factors
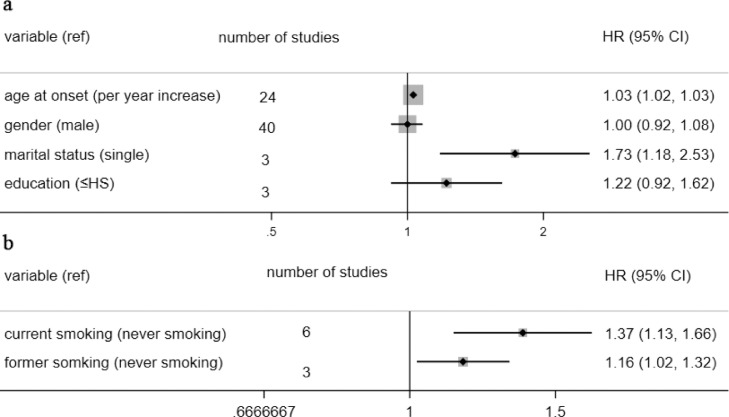
When analyzed as a continuous variable, the age at onset had a negative impact on the outcome of ALS patients, along with a 3% risk of early death increasing for every additional year (HR=1.03). Compared to ALS patients with a partner, single ones had 1.73 times the risk of dying early without significant overall interstudy heterogeneity (I2 = 47.5%, p = 0.149). Otherwise, neither gender nor education level made a difference in ALS survival. The overall results are presented in Fig. 2a, and the separate analysis of each ingredient is in the supplementary material (Supp. Fig. 1-4).
Figure 2.
pool analysis of demographic factors and environmental and lifestyle factors
Fig. 2a: pool analysis of demographic factors; Fig. 2b: pool analysis of environmental and lifestyle factors. Abbr. HS: high school; HR: hazard ratio; CI: confidence interval; note: we equated graduate equivalency diploma with high school.
3.2.2. Environmental and lifestyle factors
In the past, lots of energy has been devoted to researching the influence of environmental and lifestyle factors on the survival of ALS [26], but limited by the inclusion criteria, and only smoking was eligible. Compared to non-smokers, both current and former smokers had a higher risk of death (HR:1.37 and 1.16, respectively). The overall result is shown in Fig. 2b and forest plot is present in Supp. Fig. 5.
3.2.3. Clinical manifestations
Not surprisingly, many clinical manifestations and signs can predict poor prognoses, such as respiratory subtype, bulbar onset, more significant baseline impairment, and rapid disease progression [27], [28], [29], which were also identified in this study (Table 1). Most importantly, the respiratory subtype showed a powerful effect (HR:2.20) on ALS outcome (Supp. Fig. 6). Baseline respiratory function was an important factor affecting the survival of ALS. And we found that when FVC was lower than 85%, the risk of death in ALS patients increases by 0.86 times (HR: 1.86, Supp. Fig. 7). Compared with probable and possible ALS, definite ALS had a shorter life span righteously (Supp. Fig. 8). Patients with rapid functional deterioration tended to have shorter survival times, whether it was regarded as a categorical variable or continuous variable (HR: 1.48 or 2.37, respectively, Supp. Fig. 9). Besides, fifty-three articles examined the effect of bulbar onset on ALS survival. The pooled analysis revealed that bulbar onset is associated with a worse prognosis than spinal onset with HR =1.34 (95%CI: 1.22-1.46, Supp. Fig. 10).
Table 1.
meta-analysis of clinical manifestation factors.
| Survival factors | Numbers of studies | Pooled HR (95% CI) | I2 |
|---|---|---|---|
| Categorical variables | |||
| site of onset (ref: spinal) | |||
| bulbar | 54 | 1.35 (1.23,1.47) | 68.0% |
| subtype (ref: typical ALS) | |||
| flail arm or leg | 6 | 0.61 (0.50,0.73) | 14.6% |
| pUMN or pLMN ALS | 3 | 0.32 (0.22,0.45) | 0% |
| respiratory | 5 | 2.20 (1.15,4.22) | 79% |
| diagnostic level* (ref: definite) | |||
| probable | 7 | 0.73 (0.60,0.80) | 7.2% |
| possible | 10 | 0.60 (0.54,0.66) | 0% |
| ALSFRS-R change (ref:<1/month) | |||
| ≥1/month | 4 | 2.37 (1.68.3.35) | 81.7% |
| diagnostic delay (<12 months) | |||
| ≥12 months | 8 | 0.38 (0.29,0.52) | 89.7% |
| FVC(>85%) | |||
| ≤85% | 4 | 1.86 (1.6,2.17) | 0% |
| Continuous variable | |||
| MMT (points) | 3 | 0.98(0.97,0.98) | 65.3% |
| Time of duration(months) | 4 | 0.96(0.93,0.99) | 74.6% |
| FVC (%) | 10 | 0.98(0.98,0.99) | 34.6% |
| Diagnostic delay (months) | 12 | 0.97(0.95,0.98) | 95.7% |
| BMI (Kg/m2) | 17 | 0.97(0.95,0.99) | 51.0% |
| ALSFRS-R (points) ALSFRS-R change rate(1/month) |
19 4 |
0.96(0.93,0.98) 1.48(1.13,1.95) |
94.1% 81.7% |
Abbr. ref: reference; pUMN: pure upper motor neuron; pLMN: pure lower motor neuron; FVC: forced vital capacity; MMT: manual muscle test; BMI: body mass index; ALSFRS-R: amyotrophic lateral sclerosis functional rating scale-revised;
*:according to El Escorial diagnostic criteria.
As for the beneficial prognostic factors, patients with an atypical presentation of ALS, such as those with pure lower motor neuron (pLMN) or upper motor neuron type (pUMN) and flail arm or leg, have a better prognosis than those with ‘typical’ ALS (HR: 0.32 or 0.61, Supp. Fig. 11, respectively). Whether as a continuous variable or a binary variable (cut off: 12 months), the diagnosis delay time is related to the survival of ALS patients (HR:0.97 and 0.39, Supp. Fig. 12, respectively). And the disease duration was also an excellent prognostic factor (Supp. Fig. 13), partly because the longer the course of the disease tended to have a slower rate of progression. In addition, baseline clinical features (Supp. Fig. 14), including BMI (HR:0.97), MMT score (HR:0.98), ALSFRS-R score (HR:0.96), and FVC (HR:0.98) heralded a better prognosis.
3.2.4. Biochemical index
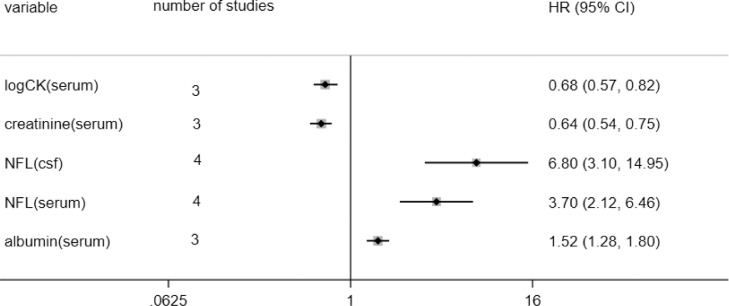
Although there have been many studies on biomarkers for ALS prognosis, it isn't easy to make a quantitative analysis due to the significant differences in the cut-off values of various studies. To initially explore the ability of biochemical indicators to predict the survival of ALS, we quantitatively analyzed potential biomarkers by extracting the HR and 95%CI for the subgroup with the highest concentration of biomarkers, although the cut-off values are not the same. CK, creatinine, albumin, and NFL were available limited by our predesigned criteria. The CK was analyzed in log form in three studies and all of them showed ALS patients with a higher serum log-CK levels had a better prognosis (HR: 0.68), and serum creatinine (HR:0.64) presented a similar effect. However, serum albumin (HR:1.52) indicated much poorer outcomes.
NFL as a potential biomarker of neurodegenerative diseases has been wildly studied in several studies [30]. It also showed extraordinary clinical significance on ALS survival. NFL was inversely associated with survival of ALS both in cerebrospinal fluid and serum. The former yielded a pooled HR of 6.80, and among four included studies, Lu et al. got the most significant result (HR=31.82) [31]. The higher serum NFL level may increase the risk of ALS death by 3.70. And the overall analysis of the biochemical index was shown in Fig. 3 and Supp. Fig. 15.
Figure 3.
pool analysis of biochemical index.
Abbr. CK: Creatine kinase; NFL: neurofilament light chain; csf: cerebrospinal fluid. HR: hazard ratio; CI: confidence interval; Note: for albumin and creatinine, there was an article provide male and female results without overall HR which was seen as different cohorts.
3.2.5. Comorbidity and Therapeutic factors
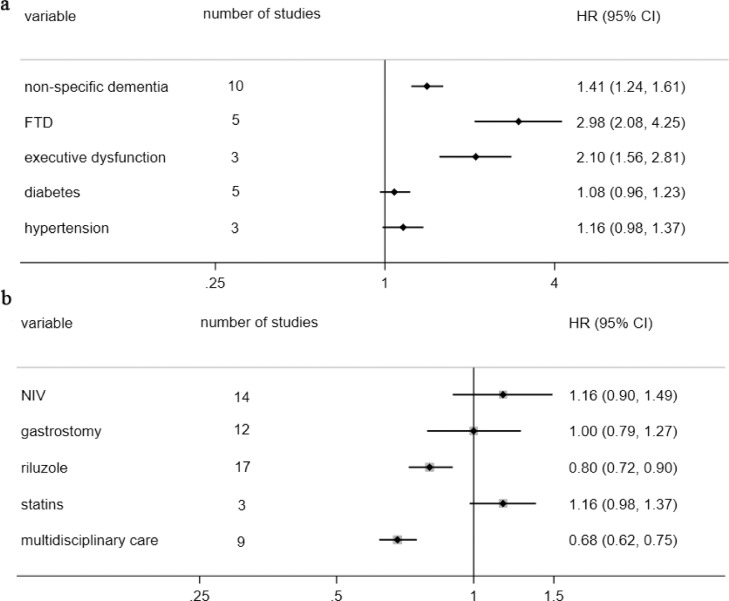
Cognitive and behavior changes are an intrinsic component of some forms [32], such as executive dysfunction, language impairment, and behavioral change [33]. It can be considered as one of the clinical features of ALS. Although cognitive impairment was analyzed as a comorbidity in this meta-analysis, it did not affect the results obtained. Frontotemporal dementia (FTD) was a strong predictor of poor prognosis in patients with ALS with nearly the two times risk of death increased (HR:2.98). There were ten articles that did not specify the type or degree of dementia, and we also got a meaningful result through meta-analysis without significant heterogeneity (HR: 1.41). The impaired executive function may play an important role which presented a more substantial effect than non-specific dementia (HR: 2.10). But risk factors for cardiovascular, such as diabetes and hypertension, made no difference on ALS survival (Fig. 4a and Supp. Fig. 16-17).
Figure 4.
pool analysis of comorbidity and therapeutic factors.
Fig. 4a: pool analysis of comorbidity; Fig. 4b: pool analysis of therapeutic factors. Abbr. FTD: Frontotemporal dementia. NIV: Noninvasive ventilation. HR: hazard ratio. CI: confidence interval. The “absence” was the reference level in all pool analysis.
The treatment of ALS has always been a challenging problem to break through. We only came out that riluzole (HR:0.80) and multidisciplinary (HR:0.68) could delay the patients’ death to some extent, but taking statins didn't affect the prognosis of ALS. Traditionally, ventilatory and nutritional support could improve the prognosis of ALS. However, disappointingly, not only percutaneous gastrostomy but also noninvasive ventilation did not confer any survival advantage than those who didn't use it in this study (Fig. 4b and Supp. Fig. 18). Patients taking NIV or PEG were more likely to be in the late stages of their natural course with more severe functional impairment and made it be an unreliable predictor of ALS survival.
3.3. Grading for prognostic factors
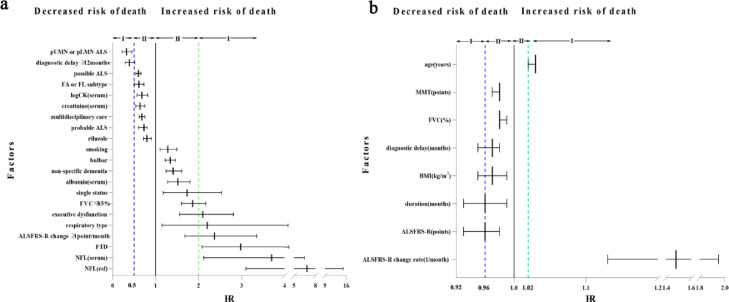
To assess which prognostic factors were more critical for ALS survival, we primarily graded all the prognostic factors. According to the following criterion, HR≥2 in categorical variables or HR ≥1.02 in continuous variables were defined as Class I, and the rest was Class II for poor prognosis factors. As a result, we identified six factors in Class I, including NFL (6.80 and 3.70 in CSF and serum, respectively), FTD (2.98), ALSFRS-R change (binary variable, 2.37; continuous variable, 1.48), respiratory subtype (2.20), executive dysfunction (2.10) and age of onset (1.03). As good ones, HR≤ 0.5 in categorical variables or HR ≤ 0.96 in continuous variables were defined as Class I, and the rest was Class II. Among them, four factors, including pLMN or pUMN (0.32), diagnosis delay (binary variable, 0.38; continuous variable, 0.97), duration (0.96) and baseline ALSFRS-R score (0.96) are classified in Class I. All of which were shown in Fig. 5 and Table 2.
Figure 5.
rating strength of predictors.
Fig. 5a rating strength of categorical variable. Fig. 5b rating strength of continuous variable. Abbr. NFL: neurofilament light chain; FTD: Frontotemporal dementia. csf: cerebrospinal fluid. FVC: forced vital capacity; pUMN: pure upper motor neuron; pLMN: pure lower motor neuron; ALSFRS-R: amyotrophic lateral sclerosis functional rating scale-revised; FA: flail arm; FL: flail leg; MMT: manual muscle test; BMI: body mass index; HR: hazard ratio. CI: confidence interval.
Table 2.
grade grouping of prognostic factors.
| Factors | Class I | Class II | |
|---|---|---|---|
| poor prognostic factors | non-intervenable | NFL, FTD, rate of progress, respiratory phenotype, executive dysfunction and age of onset | FVC≤85%, bulbar onset albumin and non-specific dementia |
| intervenable | none | Smoking and single statues | |
| good prognostic factors | non-intervenable | pLMN or pUMN phenotype, diagnostic delay, duration and higher ALSFRS-R | serum creatinine, creatine kinase, probable or possible ALS, FA or FL phenotype, higher FVC %, higher VC %, and higher MMT score |
| intervenable | BMI | riluzole and multidisciplinary care | |
Abbr. NFL: neurofilament light chain; FTD: Frontotemporal dementia. FVC: forced vital capacity; pUMN: pure upper motor neuron; pLMN: pure lower motor neuron; ALSFRS-R: amyotrophic lateral sclerosis functional rating scale-revised; FA: flail arm;FL: flail leg; MMT: manual muscle test; BMI: body mass index; HR: hazard ratio. CI: confidence interval.
3.4. Sensitivity analysis and publication bias
When the included literatures were greater than or equal to 10, further reporting bias was conducted by Begg's test (Supp. Fig. 19-25). And there was some heterogeneity between studies that reached the very high group (I2≥75%). Therefore, we further conducted a sensitivity analysis on them (Supp. Fig. 26-32 and Supp. Table 2-8). Heterogeneity analysis using the I2 statistic showed that whether as a continuous variable or a binary variable, the diagnostic delay both showed higher heterogeneity. Partly because the degree of diagnosis of ALS included in different literature was varied, and the variables analyzed by the multivariate Cox hazard proportional model were also quite different contributing to this result. But most included articles showed diagnostic delay was a good predictor for ALS outcome, and our sensitivity analyses yielded similar results. So, we still thought the outcome was reliable. For baseline ALSFRS-R was similar as well, its’ I2 was also up to 94.1%. In the same way, we did not get any contradictory results when we took sensitive risks to it. However, when we eliminated the result of Kaufmann et al.’s research on respiratory phenotype [34], I2 for respiratory phenotype reduced to 22.5% with HR=1.49. Besides, when Shepheard et al.’s study was omitted [35], the heterogeneity of ALSFRS-R change significantly reduced and its overall result was still positive that it might be due to the small sample size of the study.
4. Discussion
As far as we know, this is the most comprehensive meta-analysis investigating non-genetic survival factors of ALS patients, including five dimensions, demographic, environmental or lifestyle, clinical manifestations, biochemical index, therapeutic factors or comorbidities. Twenty-five prediction factors were identified to be associated with the survival in ALS. Among them, NFL, FTD, changes in ALSFRS-R, respiratory subtype, executive dysfunction, and age at onset are superior predictors of poor prognosis, but pLMN or pUMN, delay in diagnosis, duration and baseline ALSFRS-R score were predictors of good prognosis. However, gender, education level, diabetes, hypertension, NIV, gastrostomy, and statins did not affect the survival in ALS.
For poor predictors, in the current meta-analysis, we identified six factors in the top grade of predicting ALS survival. NFL, one of the markers of neurodegeneration, may have strong discriminatory power in diagnosing ALS [36]. And it was also found to be a reliable, independent survival predictor of ALS, when other prognostic factors were taken into account, and was found to have a better predictive performance for poor outcome of ALS in CSF than blood. This is partly due to the fact that NFL in cerebrospinal fluid was closer to the nerve tissue and may have a higher sensitivity to respond to neurodegeneration. Furthermore, the blood-brain barrier also prevents NFL from reaching the blood easily. Unfortunately, the number of studies that performed multivariate analysis was small. There were some differences in the cut-off values, which resulted in the specific threshold value of harmful NFL level was difficult to obtain. But it's worthy of knowing that a higher NFL level indicated a poor prognosis of ALS. The highest level in all studies has been proved to be negatively correlated with the outcome of ALS [31,[37], [38], [39], [40], [41], [42]], and patients with very long survival typically had low levels of NLF. In this meta-analysis, the HR of the highest level in CSF and serum NFL reached 6.80 and 3.7, respectively, and the heterogeneity of biochemical markers was acceptable. The current study was still insufficient, and more prospective studies with large samples were needed in the future. In addition, phosphorylated neurofilament-heavy (pNfH) could also serve as a prognostic factor of ALS [37,[43], [44], [45], [46]], it was not conducted a pooled analysis as fewer than three studies met the inclusion criteria. Comorbid with FTD or just presence of the executive dysfunction was also attributed to Class I of poor prognostic factors. A meta-analysis concluded that FTD-ALS had the shortest survival time (2.5 years) compared to other types of FTD [47]. In our study, the risk of early death was nearly three times higher in FTD-ALS patients than in ALS patients alone, and the presence of executive dysfunction in non-demented ALS patients might affect the longevity of survival. And our results might be explained by the fact that frontotemporal cortical involvement is likely to be associated with poor compliance with medical intervention [48]. Moreover, the decline in ALSFRS-R has been employed to determine the rate of disease progression in several population-based studies, which can reflect the rate of function loss concerning activities of daily living to some extent, and is thought to predict survival, but conflicting results have been reported [8,[49], [50], [51]]. However, in this meta-analysis which only included the results from multivariate analyses, it emerged as a strong predictor of survival as did FTD. Another strong poor prognosis predictor was the respiratory phenotype, which was the rarest phenotype (annual incidence rate: men 0.06/100 000; women 0.01/100 000) with median survival time of 1.4 years [52]. These patients had prevalent respiratory impairment at the onset, with orthopnoea or dyspnoea at rest or during exertion [53] and respiratory failure, pulmonary infection and nutritional deterioration would occur early. Many previous studies have confirmed that age was a risk factor for the onset of ALS [54]. Older age was also proven to be a poor prognosis predictor in the current research and previous researches suggested it was correlated with rapid progression of regional dysfunction [55], [56], [57], however, we need to realize the age at onset of the disease is intrinsically subjective which affects the accurate time of the measuring survival. In the Class II group of poor prognostic predictors, bulbar onset, FVC and changes in ALSFRS-R had no significant difference from previously published results [11]. However, it's worth noting that being married was associated with a reduced risk of death than unmarried status (divorced/separated, widowed, or single living without a stable partner). This may be partly resort to better care and less social pressure [58]. Environmental exposure factors have been continuously explored for the onset of ALS, and some of them suggested that smoking did not increase the risk of ALS [59], [60], [61]. While smoking has a negative effect on ALS survival, there may be a dose-response effect with the high pooled HR of current smokers. Smoking may contribute to an early death by worsening the respiratory function of ALS patients.
As for good predictors, four were identified to be in the Class I group. pLMN and pUMN patients had a longer survival than any other phenotype. They usually appeared among younger patients and had the most benign outcome [52]. The longer pLMN and pUMN are confined to the lower or upper motor neuron symptom, the longer is survival. Our previous study indicated a longer duration from an initial region to the involvement of a second region was associated with better prognosis [62]. This might also contribute to explain why the disease duration was a good prognostic factor in this study. Likewise, the time of diagnostic delay reflects the rate of progress. That is to say, if people with ALS had more than 12 months from the onset of the disease until they met the diagnostic criteria, their mortality risk was 61% lower (Supp. Fig. 12). Patients with less functional impairment at baseline had a lower risk of death. Both the total baseline ALSFRS-R score and the various parts of it had the purpose of predicting ALS patients’ outcome [34]. Beyond that, we also found some other good factors. The phenotype of flail arm or leg phenotype is relatively benign. They took a long time to appear significant functional involvement of bulbar muscles and had a long survival. BMI was estimated to be as high as 0.97 (95% CI: 0.95–0.99) for each additional BMI unit (Kg/m2) when it was considered a continuous variable, similar to previous result that only included time-to-event data [63]. In addition, we only found that riluzole was the sole medicine that can prolong ALS patients’ survival. In contrast, multidisciplinary care was more effective than general neurology care at improving the survival of patients with ALS. This may be related to solid and assertive communication between professionals, patients, caregivers, and families, which leads to enriched decision-making processes over the whole course of treatment rather than a consequence of the combined use of preconized interventions [64]. A previous report showed that lower baseline plasma creatinine was related to good prognosis [65]. In our study, baseline serum creatinine was also positively associated with survival of ALS when only HR from the multivariate analysis was included.
Our results didn't support that gender, education level, diabetes, hypertension, NIV, gastrostomy, and taking statins could predict ALS survival. Logically speaking, NIV and gastrostomy should help prolong survival in ALS. A previous systematic review from random controlled trials studies showed that the median survival in the NIV group was 48 days longer [66]. However, given that the current results only came from observational studies, it is inappropriate to assume that NIV and gastrostomy have no effect on ALS survival, especially when they are used in the relatively early stage of the disease. Some previous reports suggested that hyperlipidemia might be a prognostic factor for ALS [67, 68]. We found that taking statins did not increase the risk of ALS death, however, the number of articles conducting multivariate analyses of lipids and ALS survival are limited.
4.1. Strengths and limit
This is, hitherto, the most comprehensive and large-scale meta-analysis on predictors of ALS survival. We just included HR in multivariate analysis with less bias which made positive results more credible. Moreover, we only merged HR and did not conduct pooled analysis between risk ratio (RR) and HR. Only patients with definite, probable, or possible ALS were included, and those studies that included progressive muscular atrophy (PMA), primary lateral sclerosis (PLS), or progressive bulbar palsy (PBP) were excluded. This resulted in a more reliable source of data.
Despite all above, some weaknesses in this meta-analysis should be noted. First and foremost, we excluded several studies that did not report adjusted HR and didn't extract data from Kaplan-Meier curves. Those articles with different cut-off values of predictors were also excluded. Besides, some studies did not report insignificant results, and there were some differences in follow-up time, the included population, and the definition of outcomes, which may lead to publication bias. Moreover, the lack of commonality of prognostic factors investigated in different cox PH models is also a limitation. The absence of quantitative follow-up and missing data prevented us from adequately assessing the risk of attrition bias and sampling bias in some studies. In addition, the variables analyzed in different Cox models may affect the final results and the point-estimation of HR are difficult to assess, since they are influenced by the heterogeneity of the studies included in the analysis. The small number of prospective studies was also a limitation, and we did not include randomized controlled studies including interventions. And some factors, including marital status, education, former smoker, FVC, ALSFRS-R change, pUMN and pLMN, duration, hypertension and stains, reported in only three or four articles, the results should be explained with caution. Therefore, more high-quality prospective studies are warranted. Furthermore, cases in our study did not take the genetic factors into account, which might have an impact on the course and prognosis of ALS. It may not be most appropriate to assess the outcome of therapeutic interventions using this reporting method, but no treatments are particularly effective for ALS. In addition, increased health care awareness is certainly an important factor for the natural history of the disease. Moreover, although we excluded reports that do not distinguish PMA, PLS and PBP from ALS, but PLS may be included in the possible criteria for the revised El Escorial diagnostic criteria and PBP sometimes may be difficult to distinguish from bulbar onset ALS. Equally importantly, genetic studies were excluded from this study which may make us miss some information for ALS survival. Future comprehensive studies are needed to remedy this regret. Finally, rules of the review methodology of its restriction to articles published in English, and the low specificity of the search strategy, which increase the risk of missing relevant studies.
5. Conclusions
The present meta-analysis summarized and contrasted evidence for prognostic factors in patients with ALS. Demographic factors, environmental and lifestyle factors, clinical manifestations, biochemical index, therapeutic factors and comorbidities were identified independently to be associated with the prognosis for patients. Therefore, they could help predict the survival of ALS, and the identified non-intervenable or intervenable factors will benefit for guiding the therapy strategies for ALS.
Contributors
WMS and YPC conceived and designed the study. WMS, YPC, YFC and ZJ selected the articles and extracted and cross-checked the data. WMS, YPC, QQD and TMY contributed to the statistical analysis. WMS and YPC wrote the first draft of the manuscript. WMS, YPC and HFS revised and discussed the final edition. All authors read and approved the final version of the manuscript.
Data sharing statement
The study did not involve in the public datasets or code. Further data information in this study can be available from the corresponding author upon reasonable request.
Declaration of Competing Interest
The authors declare that there is no conflict of interest.
Acknowledgments
This study was supported the National Natural Science Fund of China (Grant No. 81971188), the 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (Grant No. 2019HXFH046), and the Science and Technology Bureau Fund of Sichuan Province (No. 2019YFS0216). The authors would like to thank the ALS patients in the included studies and all the researchers of them.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ebiom.2021.103732.
Contributor Information
Hui-Fang Shang, Email: hfshang2002@126.com.
Yong-Ping Chen, Email: yongping.chen@wchscu.cn.
Appendix. Supplementary materials
References
- 1.Brown R.H., Al-Chalabi A. Amyotrophic Lateral Sclerosis. N Engl J Med. 2017;377:162–172. doi: 10.1056/NEJMra1603471. [DOI] [PubMed] [Google Scholar]
- 2.Oskarsson B., Gendron T.F., Staff N.P. Amyotrophic Lateral Sclerosis: An Update for 2018. Mayo Clin Proc. 2018;93:1617–1628. doi: 10.1016/j.mayocp.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 3.Mulder D.W., Kurland L.T., Offord K.P., Beard C.M. Familial adult motor neuron disease: amyotrophic lateral sclerosis. Neurology. 1986;36:511–517. doi: 10.1212/wnl.36.4.511. [DOI] [PubMed] [Google Scholar]
- 4.Mehta P., Horton D.K., Kasarskis E.J., Tessaro E., Eisenberg M.S., Laird S., Iskander J. CDC Grand Rounds: National Amyotrophic Lateral Sclerosis (ALS) Registry Impact, Challenges, and Future Directions. MMWR Morb Mortal Wkly Rep. 2017;66:1379–1382. doi: 10.15585/mmwr.mm6650a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Testa D., Lovati R., Ferrarini M., Salmoiraghi F., Filippini G. Survival of 793 patients with amyotrophic lateral sclerosis diagnosed over a 28-year period. Amyotroph Lateral Scler Other Motor Neuron Disord. 2004;5:208–212. [PubMed] [Google Scholar]
- 6.Chiò A., Logroscino G., Hardiman O., Swingler R., Mitchell D., Beghi E., Traynor BG. Prognostic factors in ALS: A critical review. Amyotroph Lateral Scler. 2009;10:310–323. doi: 10.3109/17482960802566824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ackrivo J., Hansen-Flaschen J., Wileyto E.P., Schwab R.J., Elman L., Kawut S.M. Development of a prognostic model of respiratory insufficiency or death in amyotrophic lateral sclerosis. Eur Respir J. 2019;53 doi: 10.1183/13993003.02237-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elamin M., Bede P., Montuschi A., Pender N., Chio A., Hardiman O. Predicting prognosis in amyotrophic lateral sclerosis: a simple algorithm. J Neurol. 2015;262:1447–1454. doi: 10.1007/s00415-015-7731-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grollemund V., Chat G.L., Secchi-Buhour M.S., Delbot F., Pradat-Peyre J.F., Bede P., Pradat PF. Development and validation of a 1-year survival prognosis estimation model for Amyotrophic Lateral Sclerosis using manifold learning algorithm UMAP. Sci Rep. 2020;10:13378. doi: 10.1038/s41598-020-70125-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haverkamp L.J., Appel V., Appel SH. Natural history of amyotrophic lateral sclerosis in a database population. Validation of a scoring system and a model for survival prediction. Brain. 1995;118:707–719. doi: 10.1093/brain/118.3.707. (Pt 3) [DOI] [PubMed] [Google Scholar]
- 11.Westeneng H.J., Debray T.P.A., Visser A.E. Prognosis for patients with amyotrophic lateral sclerosis: development and validation of a personalised prediction model. Lancet Neurol. 2018;17:423–433. doi: 10.1016/S1474-4422(18)30089-9. [DOI] [PubMed] [Google Scholar]
- 12.Wei Q.Q., Chen Y., Chen X. Prognostic Nomogram Associated with Longer Survival in Amyotrophic Lateral Sclerosis Patients. Aging Dis. 2018;9:965–975. doi: 10.14336/AD.2017.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Es M.A., Hardiman O., Chio A., Al-Chalabi A., Pasterkamp R.J., Veldink J.H., van den Berg LH. Amyotrophic lateral sclerosis. The Lancet. 2017;390:2084–2098. doi: 10.1016/S0140-6736(17)31287-4. [DOI] [PubMed] [Google Scholar]
- 14.Hulisz D. Amyotrophic lateral sclerosis: disease state overview. Am J Manag Care. 2018;24:S320–S3S6. [PubMed] [Google Scholar]
- 15.Chiò A., Moglia C., Canosa A. ALS phenotype is influenced by age, sex, and genetics: A population-based study. Neurology. 2020;94:e802–ee10. doi: 10.1212/WNL.0000000000008869. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y.P., Yu S.H., Wei Q.Q. Role of genetics in amyotrophic lateral sclerosis: a large cohort study in Chinese mainland population. J Med Genet. 2021 doi: 10.1136/jmedgenet-2021-107965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDermott CJ. Clinical trials in amyotrophic lateral sclerosis. Curr Opin Neurol. 2019;32:758–763. doi: 10.1097/WCO.0000000000000731. [DOI] [PubMed] [Google Scholar]
- 18.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stroup D.F., Berlin J.A., Morton S.C. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 20.Miller R.G., Mitchell J.D., Moore DH. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND) Cochrane Database Syst Rev. 2012;2012 doi: 10.1002/14651858.CD001447.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cudkowicz M.E., van den Berg L.H., Shefner J.M. Dexpramipexole versus placebo for patients with amyotrophic lateral sclerosis (EMPOWER): a randomised, double-blind, phase 3 trial. Lancet Neurol. 2013;12:1059–1067. doi: 10.1016/S1474-4422(13)70221-7. [DOI] [PubMed] [Google Scholar]
- 22.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 23.Higgins J.P., Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 24.Begg C.B., Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 25.Egger M., Smith G.D., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj-Brit Med J. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keren N., Scott K.M., Tsuda M. Evidence of an environmental effect on survival in ALS. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15:528–533. doi: 10.3109/21678421.2014.911326. [DOI] [PubMed] [Google Scholar]
- 27.Kaufmann P., Levy G., Thompson J.L. The ALSFRSr predicts survival time in an ALS clinic population. Neurology. 2005;64:38–43. doi: 10.1212/01.WNL.0000148648.38313.64. [DOI] [PubMed] [Google Scholar]
- 28.Wolf J., Safer A., Wöhrle J.C., Palm F., Nix W.A., Maschke M., Grau AJ. Factors Predicting Survival in ALS Patients–Data from a Population-Based Registry in Rhineland-Palatinate, Germany. Neuroepidemiology. 2015;44:149–155. doi: 10.1159/000381625. [DOI] [PubMed] [Google Scholar]
- 29.Forbes R.B., Colvile S., Cran G.W., Swingler RJ. Unexpected decline in survival from amyotrophic lateral sclerosis/motor neurone disease. Journal of Neurology, Neurosurgery and Psychiatry. 2004;75:1753–1755. doi: 10.1136/jnnp.2003.024364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang S.Y., Chen W., Xu W. Neurofilament Light Chain in Cerebrospinal Fluid and Blood as a Biomarker for Neurodegenerative Diseases: A Systematic Review and Meta-Analysis. J Alzheimers Dis. 2019;72:1353–1361. doi: 10.3233/JAD-190615. [DOI] [PubMed] [Google Scholar]
- 31.Lu C.H., Macdonald-Wallis C., Gray E. Neurofilament light chain: A prognostic biomarker in amyotrophic lateral sclerosis. Neurology. 2015;84:2247–2257. doi: 10.1212/WNL.0000000000001642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swinnen B., Robberecht W. The phenotypic variability of amyotrophic lateral sclerosis. Nature Reviews Neurology. 2014;10:661–670. doi: 10.1038/nrneurol.2014.184. [DOI] [PubMed] [Google Scholar]
- 33.Pender N., Pinto-Grau M., Hardiman O. Cognitive and behavioural impairment in amyotrophic lateral sclerosis. Curr Opin Neurol. 2020;33:649–654. doi: 10.1097/WCO.0000000000000862. [DOI] [PubMed] [Google Scholar]
- 34.Kaufmann P., Levy G., Thompson J.L.P. The ALSFRSr predicts survival time in an ALS clinic population. Neurology. 2005;64:38–43. doi: 10.1212/01.WNL.0000148648.38313.64. [DOI] [PubMed] [Google Scholar]
- 35.Shepheard S.R., Wuu J., Cardoso M. Urinary p75ECD: A prognostic, disease progression, and pharmacodynamic biomarker in ALS. Neurology. 2017;88:1137–1143. doi: 10.1212/WNL.0000000000003741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Forgrave L.M., Ma M., Best J.R., DeMarco ML. The diagnostic performance of neurofilament light chain in CSF and blood for Alzheimer's disease, frontotemporal dementia, and amyotrophic lateral sclerosis: A systematic review and meta-analysis. Alzheimers Dement (Amst) 2019;11:730–743. doi: 10.1016/j.dadm.2019.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abu-Rumeileh S., Vacchiano V., Zenesini C. Diagnostic-prognostic value and electrophysiological correlates of CSF biomarkers of neurodegeneration and neuroinflammation in amyotrophic lateral sclerosis. Journal of Neurology. 2020;267:1699–1708. doi: 10.1007/s00415-020-09761-z. [DOI] [PubMed] [Google Scholar]
- 38.Huang F., Zhu Y., Hsiao-Nakamoto J. Longitudinal biomarkers in amyotrophic lateral sclerosis. Annals of Clinical and Translational Neurology. 2020;7:1103–1116. doi: 10.1002/acn3.51078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schreiber S., Spotorno N., Schreiber F. Significance of CSF NfL and tau in ALS. Journal of Neurology. 2018;265:2633–2645. doi: 10.1007/s00415-018-9043-0. [DOI] [PubMed] [Google Scholar]
- 40.Gille B., De Schaepdryver M., Goossens J. Serum neurofilament light chain levels as a marker of upper motor neuron degeneration in patients with Amyotrophic Lateral Sclerosis. Neuropathol Appl Neurobiol. 2019;45:291–304. doi: 10.1111/nan.12511. [DOI] [PubMed] [Google Scholar]
- 41.De Schaepdryver M., Lunetta C., Tarlarini C., Mosca L., Chio A., Van Damme P., Poesen K. Neurofilament light chain and C reactive protein explored as predictors of survival in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2020;91:436–437. doi: 10.1136/jnnp-2019-322309. [DOI] [PubMed] [Google Scholar]
- 42.Thouvenot E., Demattei C., Lehmann S. Serum neurofilament light chain at time of diagnosis is an independent prognostic factor of survival in amyotrophic lateral sclerosis. European Journal of Neurology. 2020;27:251–257. doi: 10.1111/ene.14063. [DOI] [PubMed] [Google Scholar]
- 43.Boylan K.B., Glass J.D., Crook J.E. Phosphorylated neurofilament heavy subunit (pNF-H) in peripheral blood and CSF as a potential prognostic biomarker in Amyotrophic Lateral Sclerosis. Journal of Neurology, Neurosurgery and Psychiatry. 2013;84:467–472. doi: 10.1136/jnnp-2012-303768. [DOI] [PubMed] [Google Scholar]
- 44.De Schaepdryver M., Goossens J., Jeromin A. Phosphorylated neurofilament heavy chains (pNfH) in blood as an early diagnostic and prognostic biomarker in ALS. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration. 2018;19:45–46. [Google Scholar]
- 45.Falzone Y.M., Domi T., Agosta F. Serum phosphorylated neurofilament heavy-chain levels reflect phenotypic heterogeneity and are an independent predictor of survival in motor neuron disease. Journal of Neurology. 2020;267:2272–2280. doi: 10.1007/s00415-020-09838-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gendron T.F., Daughrity L.M., Heckman M.G. Phosphorylated neurofilament heavy chain: A biomarker of survival for C9ORF72-associated amyotrophic lateral sclerosis. Ann Neurol. 2017;82:139–146. doi: 10.1002/ana.24980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kansal K., Mareddy M., Sloane K.L., Minc A.A., Rabins P.V., McGready J.B., Onyike CU. Survival in Frontotemporal Dementia Phenotypes: A Meta-Analysis. Dementia and Geriatric Cognitive Disorders. 2016;41:109–122. doi: 10.1159/000443205. [DOI] [PubMed] [Google Scholar]
- 48.Olney R.K., Murphy J., Forshew D. The effects of executive and behavioral dysfunction on the course of ALS. Neurology. 2005;65:1774–1777. doi: 10.1212/01.wnl.0000188759.87240.8b. [DOI] [PubMed] [Google Scholar]
- 49.Kimura F., Fujimura C., Ishida S. Progression rate of ALSFRS-R at time of diagnosis predicts survival time in ALS. Neurology. 2006;66:265–267. doi: 10.1212/01.wnl.0000194316.91908.8a. [DOI] [PubMed] [Google Scholar]
- 50.Labra J., Menon P., Byth K., Morrison S., Vucic S. Rate of disease progression: a prognostic biomarker in ALS. J Neurol Neurosurg Psychiatry. 2016;87:628–632. doi: 10.1136/jnnp-2015-310998. [DOI] [PubMed] [Google Scholar]
- 51.Gordon P.H., Cheung YK. Progression rate of ALSFRS-R at time of diagnosis predicts survival time in ALS. Neurology. 2006;67:1314–1315. doi: 10.1212/01.wnl.0000243812.25517.87. author reply -5. [DOI] [PubMed] [Google Scholar]
- 52.Chio A., Calvo A., Moglia C., Mazzini L., Mora G., group Ps. Phenotypic heterogeneity of amyotrophic lateral sclerosis: a population based study. J Neurol Neurosurg Psychiatry. 2011;82:740–746. doi: 10.1136/jnnp.2010.235952. [DOI] [PubMed] [Google Scholar]
- 53.Shoesmith C.L., Findlater K., Rowe A., Strong MJ. Prognosis of amyotrophic lateral sclerosis with respiratory onset. Journal of Neurology, Neurosurgery and Psychiatry. 2007;78:629–631. doi: 10.1136/jnnp.2006.103564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang M.D., Little J., Gomes J., Cashman N.R., Krewski D. Identification of risk factors associated with onset and progression of amyotrophic lateral sclerosis using systematic review and meta-analysis. Neurotoxicology. 2017;61:101–130. doi: 10.1016/j.neuro.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 55.Watanabe H., Atsuta N., Nakamura R. Factors affecting longitudinal functional decline and survival in amyotrophic lateral sclerosis patients. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration. 2014;15:129–130. doi: 10.3109/21678421.2014.990036. [DOI] [PubMed] [Google Scholar]
- 56.Yokoi D., Atsuta N., Watanabe H. Age of onset differentially influences the progression of regional dysfunction in sporadic amyotrophic lateral sclerosis. Journal of Neurology. 2016;263:1129–1136. doi: 10.1007/s00415-016-8109-0. [DOI] [PubMed] [Google Scholar]
- 57.Creemers H., Grupstra H., Nollet F., van den Berg L.H., Beelen A. Prognostic factors for the course of functional status of patients with ALS: a systematic review. J Neurol. 2015;262:1407–1423. doi: 10.1007/s00415-014-7564-8. [DOI] [PubMed] [Google Scholar]
- 58.Williams B.R., Sawyer P., Roseman J.M., Allman RM. Marital status and health: exploring pre-widowhood. J Palliat Med. 2008;11:848–856. doi: 10.1089/jpm.2007.0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.de Jong S.W., Huisman M.H., Sutedja N.A. Smoking, alcohol consumption, and the risk of amyotrophic lateral sclerosis: a population-based study. Am J Epidemiol. 2012;176:233–239. doi: 10.1093/aje/kws015. [DOI] [PubMed] [Google Scholar]
- 60.Fang F., Bellocco R., Hernán M.A., Ye W. Smoking, snuff dipping and the risk of amyotrophic lateral sclerosis - A prospective cohort study. Neuroepidemiology. 2006;27:217–221. doi: 10.1159/000096956. [DOI] [PubMed] [Google Scholar]
- 61.Alonso A., Logroscino G., Hernán MA. Smoking and the risk of amyotrophic lateral sclerosis: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2010;81:1249–1252. doi: 10.1136/jnnp.2009.180232. [DOI] [PubMed] [Google Scholar]
- 62.Chen X., Wei Q.Q., Chen Y. Clinical Staging of Amyotrophic Lateral Sclerosis in Chinese Patients. Front Neurol. 2018;9:442. doi: 10.3389/fneur.2018.00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dardiotis E., Siokas V., Sokratous M. Body mass index and survival from amyotrophic lateral sclerosis: A meta-analysis. Neurol Clin Pract. 2018;8:437–444. doi: 10.1212/CPJ.0000000000000521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.de Almeida F.E.O., Santana A.K.D., de Carvalho FO. Multidisciplinary care in Amyotrophic Lateral Sclerosis: a systematic review and meta-analysis. Neurological Sciences. 2021;42:911–923. doi: 10.1007/s10072-020-05011-2. [DOI] [PubMed] [Google Scholar]
- 65.Lanznaster D., Bejan-Angoulvant T., Patin F., Andres C.R., Vourc'h P., Corcia P., Blasco H. Plasma creatinine and amyotrophic lateral sclerosis prognosis: a systematic review and meta-analysis. Amyotroph Lateral Scler Frontotemporal Degener. 2019;20:199–206. doi: 10.1080/21678421.2019.1572192. [DOI] [PubMed] [Google Scholar]
- 66.Radunovic A., Annane D., Rafiq M.K., Brassington R., Mustfa N. Mechanical ventilation for amyotrophic lateral sclerosis/motor neuron disease. Cochrane Database Syst Rev. 2017;10 doi: 10.1002/14651858.CD004427.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dupuis L., Corcia P., Fergani A. Dyslipidemia is a protective factor in amyotrophic lateral sclerosis. Neurology. 2008;70:1004–1009. doi: 10.1212/01.wnl.0000285080.70324.27. [DOI] [PubMed] [Google Scholar]
- 68.Dorst J., Kühnlein P., Hendrich C., Kassubek J., Sperfeld A.D., Ludolph AC. Patients with elevated triglyceride and cholesterol serum levels have a prolonged survival in amyotrophic lateral sclerosis. Journal of Neurology. 2011;258:613–617. doi: 10.1007/s00415-010-5805-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.