To The Editor:
Emerging SARS‐CoV‐2 variants have raised concerns about levels of immunity to variant Spike proteins after prior SARS‐CoV‐2 infection or vaccination. Here, we report distinct cross‐reactivity of Wuhan‐HA‐1 (WA1)‐induced antibodies upon BNT162b2 mRNA vaccination and SARS‐CoV‐2 infection. We show that neutralizing antibodies against WA1 strongly correlate with Delta neutralization, and that SARS‐CoV‐2 infection‐induced antibodies have better neutralization capability for the Delta variant compared to vaccination.
We measured the magnitude and breadth of Spike antibody responses against the original WA1 and three variants (Alpha [B.1.1.7], Beta [B.1.351], and Delta [B.1.617.2]) of SARS‐CoV‐2 in three cohorts: (i) SARS‐CoV‐2 naïve recipients of two doses of BNT162b2 mRNA (Pfizer/BioNTech) vaccine (day 90 post first dose; n = 55); (ii) COVID‐19 convalescent patients who received the first dose of the BNT162b2 mRNA vaccine at 6–10 months postsymptom onset (day 90 post first dose; n = 5); (iii) COVID‐19 convalescent patients (n = 23) at peak postinfection at a median of 2 months postsymptom onset. We examined the levels and breadth of anti‐Spike antibodies and their neutralizing ability against WA1 and the variants Alpha, Beta, and Delta, which differ by one to three amino acids (AA) within the receptor‐binding domain (RBD) (Figure 1A).
FIGURE 1.
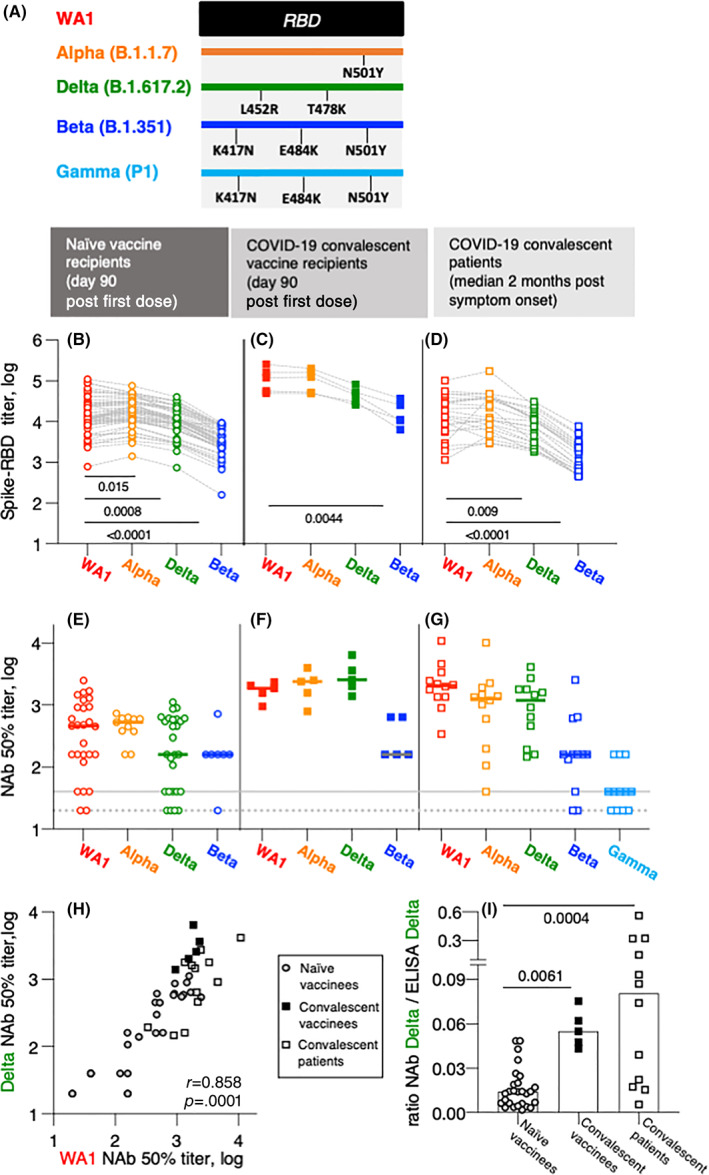
Anti‐Spike antibody characterization in BNT162b2 mRNA vaccinated and CoV‐2 infected persons. (A) The cartoon depicts AA changes in RBD (AA 332–532). Beta and Gamma share the identical RBD but differ in several AA within Spike used in neutralization assay. (B–D) Wuhan‐Spike induced antibodies were measured by in‐house ELISA using a panel of purified Spike‐RBD proteins. (B and C) CoV‐2 naïve volunteers 1 and COVID‐19 convalescent patients 1 received the BNT162b2 mRNA vaccine and were analyzed at day 90. The convalescent vaccine recipients 1 had detectable Spike‐RBD antibodies (median titer 2.8 log, range 2.6–3.9) at the day of the first dose. (D) SARS‐CoV‐2‐infected patients 2 , 3 were analyzed at a median of 2 months postsymptom onset. Comparison between the groups were made using ANOVA Friedmann's multiple comparison test. (E–G) Neutralization was performed with the samples shown in panels (B–D) using a pseudotyped HIVNLΔEnv‐Nanoluc assay carrying a panel of Spike (AA 1–1254) proteins. Threshold of detection in gray solid line; threshold of quantification in gray dotted line. (H) Correlation of NAb to WA1 and Delta in the three cohorts described in panels (E–G). Spearman r and p value are given. (I) Ratios of Delta NAb (from panels E–G) and Delta antibody titers (panel B–D) for the different cohorts were calculated using linear values. The p values are from ANOVA Kruskal–Wallis test. ANOVA, analysis of variance
The study participants including patients and volunteers are described in NCT04408209 and NCT04743388. CoV‐2 naïve volunteers and COVID‐19 convalescent patients received two doses of BNT162b2 mRNA vaccine at day 1 and 21, respectively. 1 The convalescent vaccine recipients received first dose at 6–10 months postsymptom onset. 1 SARS‐CoV‐2‐infected patients analyzed at a median of 2 months postsymptom onset have been described. 2 , 3 In‐house ELISA using a panel of purified Spike‐RBD proteins (AA 319–525) were detailed elsewhere. 2 , 3 , 4 Neutralization was performed using a pseudotyped HIVNLΔEnv‐Nanoluc assay 5 , 6 carrying a panel of Spike (AA 1–1254) proteins as described. 2 , 3 , 4 Statistical analyses were performed using analysis of variance and Spearman correlation (GraphPad Prism Version 9.0.2 X; GraphPad Software, Inc.).
All three cohorts showed robust humoral response to WA1 Spike‐RBD. Similar antibody levels were detected in the naïve BNT162b2 mRNA vaccine recipients at day 90 and in the COVID‐19 convalescent patients (2 months postinfection). A 12‐fold higher Ab level was detected in the convalescent vaccinees, 1 as a result of a strong anamnestic response in this cohort 1 , 7 , 8 , 9 (Figure 1B–D). Compared to the responses against WA1, the vaccine‐induced antibodies showed significantly lower recognition of Alpha and Delta Spike‐RBD and greatly reduced binding of Beta Spike‐RBD in the naïve vaccines (Figure 1B). In contrast, the Spike‐RBD antibodies in the SARS‐CoV‐2 convalescent vaccine recipients (Figure 1C) showed similar strong binding to Beta and Delta indicating some improved breadth. Despite the increased humoral responses, recognition of Beta Spike‐RBD was significantly reduced (Figure 1C). We further compared the anti‐Spike‐antibody breadth in a cohort of COVID‐19 convalescent patients (Figure 1D). We noted a similar ranking of responses as in the cohort of naïve vaccine recipients with reduced recognition of Delta and Beta Spike‐RBD. Together, our data suggest a strong benefit of vaccination for COVID‐19 convalescent patients. Our data further point to two AA changes (K417N and E484K; Figure 1A), which play a key role for RBD recognition. These data mirror our recent findings from nonhuman primates which received WA1 Spike‐based DNA vaccines. 4 Importantly, increased Spike‐RBD antibody magnitude cannot compensate for this. Thus, a booster vaccination including different variants may be beneficial and should be considered.
Next, we tested the neutralizing capability of the Spike antibodies in the different groups (Figure 1E–G). Sera were tested for their ability to neutralize infection by reporter viruses pseudotyped with different Spike variants. Analysis of both the naïve vaccine recipients (Figure 1E; n = 27) and the convalescent vaccine recipients (Figure 1F; n = 5) showed that WA1, Alpha and Delta Spike pseudotyped viruses were most susceptible. Similar data were found in the convalescent cohort (Figure 1G; n = 12). A drastic reduction in the ability to neutralize Beta was found in all the groups. These data were further supported by the testing of neutralization of Gamma which shares with Beta the same AA changes in RBD but differs in several AA in Spike (Figure 1A). The convalescent cohort (Figure 1G) also showed a strong reduction in the neutralization ability of Gamma. These data support the key role of the AA K417N and E484K (Figure 1A) for both binding and neutralization by WA1‐induced antibodies. Our data on the cross‐reactive recognition of Spike variants are in overall agreement with other recent reports analyzing the Moderna mRNA‐1273, Pfizer BNT162b2, or AstraZeneca vaccines in healthy vaccine recipients or in COVID‐19 convalescent vaccine recipients 10 , 11 , 12 , 13 , 14 , 15 and in our DNA vaccine study in nonhuman primates. 4
Because of the practical importance of the ability of WA1‐induced antibodies to control infection by the Delta variant, we compared neutralization abilities of antibodies against WA1 and Delta in the three cohorts (Figure 1H). We found a strong direct correlation (Spearman r = 0.8586, p < .0001), supporting the notion that individuals with robust NAb against WA1 also strongly neutralize Delta. Recent findings of breakthrough infections in individuals with vaccine‐ or infection‐induced SARS‐CoV‐2 immunity 16 , 17 by the circulating Delta strain may occur in individuals with low anti‐WA1 antibodies. Our data indicate that a WA1‐based booster vaccination will greatly improve anti‐Delta immune response.
To further characterize the WA1 vaccine‐induced and the infection‐induced Delta‐specific antibodies, we compared the ratio of the respective NAb and binding antibodies in the three cohorts (Figure 1I). Interestingly, compared to the naïve vaccine recipients, we found significant higher ratios in the convalescent cohort and in the convalescent vaccine recipients. These data support the conclusion that antibodies induced by SARS‐CoV‐2 infection have better Delta‐specific neutralization function. The subsequent vaccination of the convalescent patients was able to increase the level and maintain the breadth of anti‐Delta neutralizing Ab.
The report presented here shows a side‐by‐side comparison of characteristics of Spike antibodies induced upon BNT162b2 mRNA vaccination, upon CoV‐2 infection or a combination thereof using the same methodologies. We found that anti‐Spike antibodies from the vaccine recipients (naïve and convalescent) and SARS‐CoV‐2 convalescent patients show a strong ability to recognize and neutralize the autologous WA1, as well as the Alpha and Delta Spike variants but that they show greatly impaired recognition of Beta, in agreement with others, 10 , 11 , 12 , 13 , 14 , 15 pointing to the importance of AA changes within RBD. Recently emerging variants of concern show a multitude of such changes which will likely affect NAb function of the WA1‐induced antibodies. Interestingly, our data further showed that SARS‐CoV‐2 infection‐induced antibodies show better neutralization capability of the Delta variant. 10 , 11 , 12 , 13 , 14 , 15 This finding points to subtle but critical differences in antibody development perhaps guided through continuous antigen exposure upon infection, whereas vaccination provides a limited number of short‐lived exposures.
SARS‐CoV‐2 booster vaccination using WA1 is being implemented in many countries to increase the anti‐Spike antibody titers, and our data support its merit to improve anti‐WA1 antibody magnitude and thereby increased ability to recognize Delta. However, our data also show that the magnitude alone is not sufficient to neutralize effectively current SARS‐CoV‐2 variants of concern. Thus, future booster vaccinations with variant Spike vaccines, including Delta and Beta, should be considered to increase the breadth of the immune responses. The nucleic acid vaccine platform offers the necessary versatility to rapidly adapt to emerging variants of concern, which need to be considered in the future to improve vaccine efficacy.
CONFLICT OF INTEREST
The authors have declared that no conflict of interest exists.
ACKNOWLEDGMENTS
The authors thank D. Esposito (Protein Expression lab; NCI) for SARS‐CoV‐2 Spike‐RBD proteins; members of the Felber and Pavlakis labs for discussion, and T. Jones for assistance. This work was supported by funding from the Intramural Research Program, National Institutes of Health, National Cancer Institute, Center for Cancer Research to George N. Pavlakis and Barbara K. Felber. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Margherita Rosati and Evangelos Terpos contributed equally as first authors.
Funding information Department of Health and Human Services; National Cancer Institute; National Institutes of Health
Contributor Information
Evangelos Terpos, Email: eterpos@med.uoa.gr.
Barbara K. Felber, Email: barbara.felber@nih.gov.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Bergamaschi C, Terpos E, Rosati M, et al. Systemic IL‐15, IFN‐gamma, and IP‐10/CXCL10 signature associated with effective immune response to SARS‐CoV‐2 in BNT162b2 mRNA vaccine recipients. Cell Rep. 2021;36:109504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Terpos E, Politou M, Sergentanis TN, et al. Anti‐SARS‐CoV‐2 antibody responses in convalescent plasma donors are increased in hospitalized Patients; Subanalyses of a phase 2 clinical study. Microorganisms. 2020;8:1885. [DOI] [PMC free article] [PubMed]
- 3.Terpos E, Stellas D, Rosati M, et al. SARS‐CoV‐2 antibody kinetics eight months from COVID‐19 οnset: persistence of spike antibodies but loss of neutralizing antibodies in 24% of convalescent plasma donors. Eur J Intern Med. 2021;89:87‐96. [DOI] [PMC free article] [PubMed]
- 4. Rosati M, Agarwal A, Hu X, et al. Control of SARS‐CoV‐2 infection after spike DNA or spike DNA+protein co‐immunization in rhesus macaques. PLOS Path. 2021;17(9):e1009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robbiani DF, Gaebler C, Muecksch F, et al. Convergent antibody responses to SARS‐CoV‐2 in convalescent individuals. Nature. 2020;584:437‐442. [DOI] [PMC free article] [PubMed]
- 6.Schmidt F, Weisblum Y, Muecksch F, et al. Measuring SARS‐CoV‐2 neutralizing antibody activity using pseudotyped and chimeric viruses. J Exp Med. 2020;217:e20201181. [DOI] [PMC free article] [PubMed]
- 7.Lozano‐Ojalvo D, Camara C, Lopez‐Granados E, et al. Differential effects of the second SARS‐CoV‐2 mRNA vaccine dose on T cell immunity in naive and COVID‐19 recovered individuals. Cell Rep. 2021;36:109570. [DOI] [PMC free article] [PubMed]
- 8. Kremsner PG, Mann P, Kroidl A, et al. Safety and immunogenicity of an mRNA‐lipid nanoparticle vaccine candidate against SARS‐CoV‐2: a phase 1 randomized clinical trial. Wien Klin Wochenschr. 2021;133(17‐18):931‐941. doi: 10.1007/s00508-021-01922-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Urbanowicz RA, Tsoleridis T, Jackson HJ, et al. Two doses of the SARS‐CoV‐2 BNT162b2 vaccine enhances antibody responses to variants in individuals with prior SARS‐CoV‐2 infection. Sci Transl Med. 2021;13(609):eabj0847. doi: 10.1126/scitranslmed.abj0847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pegu A, O'Connell S, Schmidt SD, et al. Durability of mRNA‐1273 vaccine‐induced antibodies against SARS‐CoV‐2 variants. Science. 2021;373(6561):1372‐1377. doi: 10.1126/science.abj4176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Planas D, Veyer D, Baidaliuk A, et al. Reduced sensitivity of SARS‐CoV‐2 variant delta to antibody neutralization. Nature. 2021;596:276‐280. [DOI] [PubMed] [Google Scholar]
- 12. Betton M, Livrozet M, Planas D, et al. Sera neutralizing activities against SARS‐CoV‐2 and multiple variants six month after hospitalization for COVID‐19. Clin Infect Dis. 2021;73(6):e1337‐e1344. doi: 10.1093/cid/ciab308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang Z, Schmidt F, Weisblum Y, et al. mRNA vaccine‐elicited antibodies to SARS‐CoV‐2 and circulating variants. Nature. 2021;592:616‐622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Collier DA, De Marco A, Ferreira I, et al. Sensitivity of SARS‐CoV‐2 B.1.1.7 to mRNA vaccine‐elicited antibodies. Nature. 2021;593:136‐141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bergwerk M, Gonen T, Lustig Y, et al. Covid‐19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021;385:1474‐1484. doi: 10.1056/NEJMoa2109072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brown CM, Vostok J, Johnson H, et al. Outbreak of SARS‐CoV‐2 infections, including COVID‐19 vaccine breakthrough infections, associated with large public gatherings ‐ Barnstable County, Massachusetts, July 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1059‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Farinholt T, Doddapaneni H, Qin X, et al. Transmission event of SARS‐CoV‐2 Delta variant reveals multiple vaccine breakthrough infections. BMC Med. 2021;19:255. doi: 10.1101/2021.06.28.21258780 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


