Abstract
Solid organ transplant patients are at a higher risk for poor CoronaVirus Disease‐2019 (COVID‐19)‐related outcomes and have been included as a priority group in the vaccination strategy worldwide. We assessed the safety and efficacy of a two‐dose vaccination cycle with mRNA‐based COVID‐19 vaccine (BNT162b2) among 82 kidney transplant outpatients followed in our center in Rome, Italy. After a median of 43 post‐vaccine days, a SARS‐CoV‐2 anti‐Spike seroprevalence of 52.4% (n = 43/82) was observed. No impact of the vaccination on antibody‐mediated rejection or graft function was observed, and no significant safety concerns were reported. Moreover, no de novo HLA‐donor‐specific antibodies (DSA) were detected during the follow‐up period. Only one patient with pre‐vaccination HLA‐DSA did not experience an increased intensity of the existing HLA‐DSA. During the follow‐up, only one infection (mild COVID‐19) was observed in a patient after receiving the first vaccine dose. According to the multivariable logistic regression analysis, lack of seroconversion after two‐dose vaccination independently associated with patient age ≥60 years (OR = 4.50; P = .02) and use of anti‐metabolite as an immunosuppressant drug (OR = 5.26; P = .004). Among younger patients not taking anti‐metabolites, the seroconversion rate was high (92.9%). Further larger studies are needed to assess the best COVID‐19 vaccination strategy in transplanted patients.
Keywords: BNT162b2 vaccine, COVID‐19, graft rejection, HLA‐DSA, kidney transplantation, solid organ transplant patients
1. INTRODUCTION
Several studies on solid organ transplant patients affected by Severe Acute Respiratory Syndrome‐CoronaVirus‐2 (SARS‐CoV‐2) infection showed a higher risk for severe CoronaVirus Disease‐2019 (COVID‐19) with poor outcomes. 1 , 2 , 3 Thus, transplanted patients are among the priority population groups for SARS‐CoV‐2 vaccine‐based preventive activities worldwide. It should also be underlined that, in general, vaccine administration in transplanted patients may represent a nonspecific trigger factor for developing Donor‐Specific Antibodies against Human Leukocyte Antigens (HLA‐DSA) that are associated with acute organ rejection. 4 Moreover, response to various vaccines in transplanted patients is lower than in the general population. 5 Furthermore, transplanted patients were not included in the study population to register all approved COVID‐19 vaccines, including those based on mRNA technology (BNT162b2 ‐ Pfizer‐BioNTech; mRNA1273, Moderna). 6 , 7 According to real‐life data, transplanted patients receiving mRNA SARS‐CoV‐2 vaccines show significantly lower seroconversion and anti‐spike titers than the general population. 8 , 9 Moreover, to date, there are insufficient data on anti‐spike SARS‐CoV‐2 antibody levels in previously transplanted vaccinees beyond a period of one month after two‐dose vaccination.
The main objective of the present study is to assess the safety and efficacy of the mRNA SARS‐CoV‐2 vaccine (BNT162b2) after the second dose of the vaccine in a population of patients previously undergoing a specific organ transplant, namely the kidney transplantation (KT). The secondary objective is to evaluate the impact of the vaccination on rejection and graft function.
2. MATERIAL AND METHODS
2.1. Patients
A retrospective analysis was performed of the data of the KT patients transplanted and followed as outpatients at the Polyclinic Umberto I Hospital, Rome, and receiving COVID‐19 vaccination during January–May 2021. No patients presented any of the following exclusion criteria: (a) age < 18 years, (b) pregnancy or lactation status, (c) KT performed < one year before vaccination, (d) previous COVID‐19 positivity, and (e) symptomatic status for COVID‐19 during the period of the scheduled vaccination. The Local Ethics Board of Sapienza University of Rome approved the present study.
2.2. Vaccination policy in Italy for transplanted patients
According to the policy on COVID‐19 vaccination in Italy, vulnerable population groups, including solid organ transplant patients, were prioritized to receive mRNA SARS‐CoV‐2 vaccines. Each Italian region was in charge of the voluntary vaccination of residents in its territory. All KT patients transplanted at the Polyclinic Umberto I Hospital, Rome, and resident in the Lazio region, Italy, were contacted by phone to propose the vaccination. The vaccine used was the mRNA COVID‐19 vaccine (BNT162b2, Comirnaty, Pfizer‐ BioNTech) administered in two doses given three weeks apart. The vaccine was administered only in a hospital setting after written informed consent.
Among KT vaccinees, only those already followed as outpatients at Polyclinic Umberto I Hospital, Rome, were enrolled in the present study. Routine blood tests were performed as post‐transplant outpatient activity, including serum creatinine, serum electrolytes, serum level of calcineurin inhibitors, serum HLA‐DSA.
The search for HLA‐DSA antibodies was carried out using the multianalyte bead assay with the Luminex platform (Luminex, Austin, TX, USA), including Lifecode Screen and LSA I/II (Immucor, Inc., Norcross, Georgia). The results were expressed as mean fluorescence intensity (MFI); an MFI > 1000 was considered positive.
Following the second dose of the vaccine, serum anti‐spike SARS‐CoV‐2 antibodies were also assessed. LIAISON SARS‐CoV‐2 S1/S2 IgG chemiluminescent assay against a recombinant Spike (S) protein (S1/S2) (DiaSorin S.p.A., Saluggia, Italy) was used according to the manufacture instructions. Results below 12.0 AU/mL were considered negative. All the post‐vaccination adverse events were reported.
2.3. Statistical analysis
Continuous variables were reported as medians and interquartile ranges (IQR). Binary variables were reported as numbers and percentages. No missing data were reported in the investigated population. Mann–Whitney U test and Fisher's exact test were used for comparing continuous and categorical variables, respectively.
The last censoring of the investigated population was performed on June 30, 2021. A logistic regression analysis was adopted for identifying the variables independently associated with a poor anti‐spike response after vaccination. Potentially relevant variables were initially investigated using a univariable approach. Therefore, the relevant variables (P‐value < .20) were used to construct a multivariable logistic regression model. A backward wald approach was used for selecting the most relevant variables. Odds ratios (OR) and 95.0% confidence intervals (95.0%CI) were reported. Variables with a P‐value < .05 were considered statistically significant. SPSS statistical package version 25.0 (SPSS Inc., Chicago, IL, USA) was used.
3. RESULTS
All the 155 KT patients resident in the Lazio region, Italy, accepted and completed the voluntary vaccination against COVID‐19 during January‐May 2021 (vaccine acceptation rate: 100.0%). Among them, 82 patients (52.9%) were regularly followed as outpatients at the Polyclinic Umberto I, Rome (Table 1), while the remaining 73 patients (47.1%) were followed in peripheral nephrology centers. The median age of the enrolled patients was 58.5 years (IQR: 50.3‐65.0). Forty‐seven (57.3%) patients were male. The median time since the KT was 69 months (IQR: 35–143).
TABLE 1.
Demographic characteristics of the investigated population
| Median (IQR) or n (%) | ||||
|---|---|---|---|---|
| Antibody response | ||||
| Variables | Entire cohort (N = 82, 100.0%) | Detectable (n = 43, 52.4%) | Undetectable (n = 39, 47.6%) | P |
| Age, year | ||||
| 18–49 | 36 (43.9) | 13 (30.2) | 7 (17.9) | |
| 50–59 | 26 (31.7) | 17 (39.5) | 9 (23.1) | .03 |
| ≥60 | 20 (24.4) | 13 (30.2) | 23 (59.0) | |
| Male sex | 47 (57.3) | 24 (55.8) | 23 (59.0) | .83 |
| BMI | 25.8 (23.0‐28.4) | 26.8 (23.0‐28.8) | 25.2 (22.9‐27.8) | .41 |
| Time since KT, years | ||||
| 1–3 | 22 (26.8) | 8 (18.6) | 14 (35.9) | |
| 4–5 | 14 (17.1) | 5 (11.6) | 9 (23.1) | .07 |
| 6–10 | 22 (26.8) | 15 (34.9) | 7 (17.9) | |
| >10 | 24 (29.3) | 15 (34.9) | 9 (23.1) | |
| T2DM | 7 (8.5) | 5 (11.6) | 2 (5.1) | .44 |
| Hypertension | 70 (85.4) | 38 (88.4) | 32 (82.1) | .54 |
| Dyslipidemia | 43 (53.4) | 22 (51.2) | 21 (53.8) | .83 |
| Hyperuricemia | 32 (39.0) | 16 (37.2) | 16 (41.0) | .82 |
| Any side effect after vaccination | 36 (43.9) | 17 (39.5) | 19 (48.7) | .51 |
| Time 2nd dose‐Ab dosing, days | 43 (23‐63) | 42 (22‐62) | 43 (24‐63) | .60 |
| Steroid use | 75 (91.5) | 39 (90.7) | 36 (92.3) | 1.00 |
| Weekly steroid dose > 40 mg | 24 (29.3) | 12 (27.9%) | 12 (30.8) | .81 |
| Triple IS therapy | 59 (72.0) | 26 (60.5) | 33 (84.6) | .03 |
| Everolimus use | 10 (12.2) | 6 (14.0) | 4 (10.3) | .74 |
| Any CNI use | 80 (97.6) | 42 (97.7) | 38 (97.4) | 1.00 |
| Cyclosporine | 15 (18.3) | 10 (23.3) | 5 (12.8) | .26 |
| Tacrolimus bis‐die | 16 (19.5) | 9 (20.9) | 7 (17.9) | .79 |
| Tacrolimus mono‐die | 49 (59.8) | 24 (55.8) | 25 (64.1) | .50 |
| Anti‐metabolite | 57 (69.5) | 24 (55.8) | 33 (84.6) | .008 |
Abbreviations: Ab, antibody; BMI, body mass index; CNI, calcineurin inhibitor.;IQR, interquartile ranges; IS, immunosuppressive; KT, kidney transplantation; n, number; T2DM, type 2 diabetes mellitus.
All the patients received some immunosuppressive drugs at the time of vaccination. In detail, 75 patients (91.5%) received steroids, with 24 (29.3%) receiving a weekly dosage of steroids > 40 mg.
In 59 cases (72.0%), triple therapy was administrated. Calcineurin inhibitors represented the cornerstone of the therapy, being administrated in 80 cases (97.6%). Once‐daily tacrolimus was the most used drug (n = 49, 59.8%), followed by tacrolimus bis in die (n = 16, 19.5%), and cyclosporine (n = 15, 18.3%).
Everolimus use was reported in 10 patients (12.2%). An anti‐metabolite drug (azathioprine or derivatives of mycophenolic acid) was adopted in 57 patients (69.5%).
As for the analysis of the post‐vaccine side effects (Table 2), pain in the site of vaccine inoculation was reported in 34 (41.5%) and 27 (32.9%) patients after the first and the second dose, respectively. All the other symptoms were only occasionally reported, with only one case (1.2%) of fever reported after the first and the second dose, respectively. Similarly, flu‐like symptoms were reported in only four (4.9%) and one (1.2%) case after each dose administration.
TABLE 2.
Self‐reported reactions after the two doses of COVID‐19 vaccine (BNT162b2) in kidney transplant patients
| N (%) | |||
|---|---|---|---|
| Variables | First dose | Second dose | Cumulative |
| Pain in the site of inoculation | 34 (41.5) | 27 (32.9) | 36 (43.9) |
| Fever | 1 (1.2) | 1 (1.2) | 1 (1.2) |
| Flu‐like symptoms | 4 (4.9) | 1 (1.2) | 5 (6.1) |
Abbreviation: N, number.
In only one case (1.2%), a moderate COVID‐19 disease was reported after the first vaccine administration. The patient did not develop anti‐spike antibodies after the first vaccine administration. The COVID‐19 recovered rapidly after a short‐course hospitalization.
As for the graft function, only two cases (2.4%) of transient creatinine increase were reported immediately after the second vaccine dose. The creatinine levels spontaneously repaired in all the cases, excluding any suspect of clinically relevant acute rejection.
As for the immunological aspects, HLA‐DSA were not detectable before vaccination in all the patients but one. After the vaccine administration, no de novo HLA‐DSA were detected during the follow‐up period. Only one patient (1.2%) already presented HLA‐DSA before vaccination. In detail, an anti‐DQ2 was detected. No de novo HLA‐DSA were observed after vaccination, nor increased the intensity of the already existing HLA‐DSA. Before vaccination, the last MFI was 3588, while it was 3500 two months after the second dose of vaccine.
The median time passing from the second vaccine dose to the anti‐spike antibody assessment was 43 days (IQR: 23–63). After this period of follow‐up, 43 patients (52.4%) had detectable anti‐spike antibodies (Detectable Group), while 39 cases (47.6%) were seronegative (Undetectable Group) (Table 1).
Investigating the potential differences between the two groups of patients, we observed that the patients in the Undetectable Group were older with respect to the Detectable Group, with 59.0% vs 30.2% of the cases aged ≥60 years (P‐value = .03). No statistically relevant differences were observed concerning sex, body mass index, comorbidities, and side effects after the vaccination course. The time of KT only merged statistical relevance, with a more significant number of transplants performed more recently in the Undetectable Group (KT performed within 1–3 years: 35.9 vs 18.6%; P‐value = .07).
As for the immunosuppressive therapy, no relevant differences were observed in terms of steroid use, steroid dosing, and use of calcineurin inhibitors. In the Undetectable Group, triple therapy (84.6 vs 60.5%; P‐value .03) and antimetabolite drugs (84.6 vs 55.8%; P‐value = .008) were more common.
3.1. Variables independently associated with the undetectable titer after vaccination
The potential variables connected with an inadequate titer response after the two‐dose vaccination are reported in Supplementary Table 1 and Table 3. At univariable logistic regression analysis, patient age ≥60 years at the time of vaccination increased the odds of inadequate titer response, with an OR = 3.29 (95.0%CI = 1.05‐10.31; P‐value = .04).
TABLE 3.
Multivariable analysis for the risk factors of undetectable antibody response after COVID‐19 vaccination in kidney transplant patients: Backward conditional method
| 95.0%CI | |||||||
|---|---|---|---|---|---|---|---|
| Variables | Beta | SE | Wald | OR | Lower | Upper | P |
| Age, years | |||||||
| 18–49 | Ref. | 1.00 | |||||
| 50–59 | .26 | .66 | .15 | 1.29 | .36 | 4.69 | .70 |
| ≥60 | 1.50 | .63 | 5.71 | 4.50 | 1.31 | 15.46 | .02 |
| Anti‐metabolite | 1.66 | .58 | 8.18 | 5.26 | 1.69 | 16.42 | 004 |
| Constant | ‐.53 | .53 | 1.00 | .59 | – | – | .32 |
Hosmer‐Lameshow Test: .861.
Variables initially tested in the model: male sex, year since KT, age, anti‐metabolite, triple IS therapy.
Abbreviations: 95.0% CI, 95.0% confidence intervals.; OR, odds ratio;SE, standard error.
On the opposite, the longer was the time from KT, and the lower was the risk of inadequate response (time since KT 6–10 years: OR = .27, P‐value = .004; time since KT > 10 years: OR = .34, P‐value = .08). Triple immunosuppressive therapy (OR = 3.60, 95.0%CI = 1.24‐10.41; P‐value = .02) and the use of anti‐metabolites (OR = 4.35, 95.0%CI = 1.51‐12.54; P‐value = .006) both increased the odds of poor titer detection.
In Table 3, the results of the multivariable logistic regression analysis were reported. Only the patient age ≥60 years (OR = 4.50, 95.0%CI = 1.31‐15.46; P‐value = .02) and the use of anti‐metabolites (OR = 5.26, 95.0%CI = 1.69‐16.42; P‐value = .004) independently associated with undetectable titer after vaccination. Interestingly, when these two parameters were combined, we observed a vast difference in anti‐spike response in the patients with or without these variables (Figure 1).
FIGURE 1.
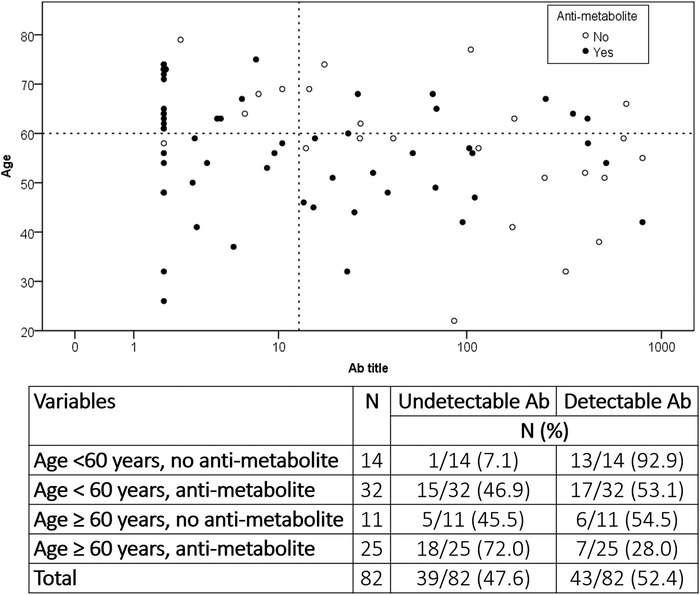
Scatter plot showing the distribution of the vaccinated patients according to their age, the anti‐spike title reached after the second vaccine dose, and the use of an anti‐metabolite as an immunosuppressive drug at the time of vaccination
In detail, patients aged < 60 years without use for anti‐metabolites at the time of vaccination showed a very high seroconversion rate (92.9% of cases), very similar to the general population receiving the mRNA SARS‐CoV‐2 vaccine. Patients having only one factor showed intermediate rates, with 53.1–54.5% of seropositive cases reported. Lastly, patients having both these factors showed very disappointing results, with only 28.0% of cases showing a post‐vaccination serological response.
4. DISCUSSION
In the present study performed on 82 KT, a seroconversion rate of 52.4% was assessed after a median period of 43 (IQR 23–63) days post‐second dose of mRNA‐based COVID‐19 vaccine (BNT162b2). This result is in line with previously published studies. 8 , 9 , 10 , 11 , 12 Age > 60 years and the use of anti‐metabolite drugs independently increased the odds of a lower seroconversion after COVID‐19 vaccination. Also in this case, the results of our study are in line with previous experiences. 8 , 9 Interestingly, when both advanced age and anti‐metabolites use were absent in our population, a high seroconversion rate (92.9%) was observed, suggesting the utility of a “tailored” use of the vaccine third dose.
Recent studies reported the efficacy for seroconversion of the administration of a third dose (around two months after the second dose) of the COVID‐19 vaccine among transplanted patients. 13 , 14 Overall, the seroconversion rate measured 2–4 weeks after the third dose of COVID‐19 vaccine improved in both studies, up to 66.7% 13 and 46.7%, 14 principally when no admixture of different vaccines was used. Furthermore, the third dose of the mRNA‐1273 vaccine induced a serologic response in 49% of 159 KT patients who did not respond after two doses. 15 In a randomized controlled trial performed on 120 transplanted patients receiving a third dose of mRNA‐1273 vaccine versus placebo, 55% versus 18% serologic response rates were reported (relative risk = 3.1; P < .001). 16 However, in light of our results, we can suggest not considering the entire population of solid organ transplant patients as systematically needing a third vaccine administration, mainly considering the small number of studies exploring this field.
As for the potential immunological risk of vaccination in transplanted patients, to the best of our knowledge, the risk of developing HLA‐DSA has been assessed among liver and heart transplanted patients receiving COVID‐19 vaccines 17 but never in KT ones. Up to now, only a post‐vaccine rejection case has been described in the literature, with a biopsy‐proven antibody‐mediated rejection episode reported in a heart‐transplant patient seven days after receiving the third dose of COVID‐19 vaccine. 14 In the present study, we assessed HLA‐DSA in all the participants before and after the vaccination, and no de‐novo development of HLA‐DSA was observed. Similar results were observed in liver and heart transplant patients. 17 Larger studies are necessary to clarify this specific aspect. Overall, no significant safety concerns were reported in this study (Table 2), similarly to what was observed in the registration trials of mRNA‐based COVID‐19 vaccines, 6 , 7 as well as in the studies assessing the efficacy of a third dose of the vaccines. 13 , 14
In literature, the rate of post‐vaccination infections among fully vaccinated transplanted patients has been estimated to be around .6% in two US studies. 18 , 19 This datum is substantially higher than the rate of .05% reported in the general population. 20 Although a limited follow‐up, we observed in our study that only one case of mild COVID‐19 occurred after the first dose of vaccine. The patient did not have seroconversion at the time of COVID‐19 diagnosis and fully recovered after the infection. No case was reported after the second vaccination administration, although only 52.4% of seroconversion rates were reported. This evidence is in line with the few reports of COVID‐19 in transplanted patients after two‐dose vaccination. 18 , 19 , 21
Interestingly, antibodies to SARS‐CoV‐2 seem not to be a surrogate indicator of the magnitude of memory T cells 22 in immunocompetent individuals, suggesting that antibody levels alone may not be a robust indicator of protection in subjects previously infected with SARS‐CoV‐2. 23 Thus, a low SARS‐CoV‐2 antibody titer after infection or vaccination does not necessarily mean a lack of protection. However, studies focused on this specific aspect are necessary to evaluate the impact on protecting the T‐cells response elicited by COVID‐19 vaccines, 23 particularly in the population of transplanted patients. A small study on seven KT non‐responders to two‐dose BNT162b2 vaccine and receiving triple immunosuppression regimen found SARS‐CoV‐2 Spike‐protein reactive T‐helper in all the patients, showing that vaccination might induce cellular immunity despite lack of serological response. 24
The main strengths of this work are: (1) the homogeneity of the study population (i.e., only KT patients); (2) the use of only one type of COVID‐19 vaccine; (3) the median availability of SARS‐CoV‐2 serology longer than one month after the second dose of the vaccine; (4) the assessment of the graft function; and (5) the evaluation of HLA‐DSA before and after the vaccination. The main limitations are: (1) the lack of an immunocompetent control group; (2) the relatively low number of participants; and (3) the lack of exploration of T‐ and B‐cells immune response after vaccination.
In conclusion, we identified age > 60 years and immunosuppressive anti‐rejection regimen containing an anti‐metabolite as factors independently increasing the odds of poor serological response to mRNA‐based COVID‐19 vaccine (BNT162b2) in KT patients. No impact of the vaccination was observed in terms of HLA‐DSA appearance and graft function worsening. The administration of the third dose of vaccine should be evaluated in selected transplanted patients. Further larger studies are needed for better clarifying the best COVID‐19 vaccination strategy to use in transplanted patients, including new generation vaccines with a broader repertoire of SARS‐CoV‐2 antigenic stimuli.
CONFLICTS OF INTEREST
The authors have no conflict of interest to declare, and any support has been received for the present study.
AUTHOR CONTRIBUTIONS
GR and RP contributed to conception and design of the study; QL, LP, MPP, AG, and MG contributed to acquisition of data; GR and QL analyzed and interpreted the data; GR and QL drafted the article; MR, CMM, and RP critically revised the manuscript; and all authors approved the final version.
Supporting information
Supporting material
ACKNOWLEDGMENT
Open Access Funding provided by Universita degli Studi di Roma La Sapienza within the CRUI‐CARE Agreement.
Russo G, Lai Q, Poli L, et al. SARS‐COV‐2 vaccination with BNT162B2 in renal transplant patients: Risk factors for impaired response and immunological implications. Clin Transplant. 2022;36:e14495. 10.1111/ctr.14495
Gianluca Russo and Quirino Lai contributed equally to the manuscript.
[Correction added on 16 May 2022, after first online publication: CRUI funding statement has been added.]
DATA AVAILABILITY STATEMENT
The data of this study are available on request from the corresponding author. The data are not publicly available because of privacy restrictions.
REFERENCES
- 1. Kates OS, Haydel BM, Florman SS, et al. for UW COVID‐19 SOT Study Team. COVID‐19 in solid organ transplant: a multi‐center cohort study. Clin Infect Dis. 2020:ciaa1097. [Google Scholar]
- 2. Raja MA, Mendoza MA, Villavicencio A, et al. COVID‐19 in solid organ transplant recipients: a systematic review and meta‐analysis of current literature. Transpl Rev (Orlando). 2021;35(1):100588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Trapani S, Masiero L, Puoti F, et al. For the Italian Network of Regional Transplant Coordinating Centers Collaborating group; Italian Surveillance System of Covid‐19, Italian Society for Organ Transplantation (SITO), The Italian Board of Experts in Liver Transplantation (I‐BELT) Study Group, Italian Association for the Study of the Liver (AISF), Italian Society of Nephrology (SIN), SIN‐SITO Study Group. Incidence and outcome of SARS‐CoV‐2 infection on solid organ transplantation recipients: a nationwide population‐based study. Am J Transplant. 2021;21(7):2509‐2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bohmig G, Regele H. Diagnosis and treatment of antibody‐mediated kidney allograft rejection. Transpl Int. 2003;16(11):773‐787. [DOI] [PubMed] [Google Scholar]
- 5. Eckerle I, Rosenberger KD, Zwahlen M, Junghanss T. Serologic vaccination response after solid organ transplantation: a systematic review. PLoS One. 2013;8(2):e56974. Epub 2013 Feb 22. PMID: 23451126. PMCID: PMC3579937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Polack FP, Thomas SJ, Kitchin N, et al. for the C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med. 2020;383(27):2603‐2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baden LR, El Sahly HM, Essink B, et al. for the COVE Study Group. Efficacy and safety of the mRNA‐1273 SARS‐CoV‐2 vaccine. N Engl J Med. 2021;384(5):403‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2‐dose SARS‐CoV‐2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325(21):2204‐2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rabinowich L, Grupper A, Baruch R, et al. Low immunogenicity to SARS‐CoV‐2 vaccination among liver transplant recipients. J Hepatol. 2021:S0168‐8278. (21)00255‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Korth J, Jahn M, Dorsch O, et al. Impaired humoral response in renal transplant recipients to SARS‐CoV‐2 vaccination with BNT162b2 (Pfizer‐BioNTech). Viruses. 2021;13(5):756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grupper A, Rabinowich L, Schwartz D, et al. Reduced humoral response to mRNA SARS‐CoV‐2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am J Transplant. 2021;21(8):2719‐27726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Benotmane I, Gautier‐Vargas G, Cognard N, et al. Low immunization rates among kidney transplant recipients who received 2 doses of the mRNA‐1273 SARS‐CoV‐2 vaccine. Kidney Int. 2021;99(6):1498‐1500. 10.1016/j.kint.2021.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A. Three doses of an mRNA Covid‐19 vaccine in solid‐organ transplant recipients. N Engl J Med. 2021;385(7):661‐662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Werbel WA, Boyarsky BJ, Ou MT, et al. Safety and immunogenicity of a third dose of SARS‐CoV‐2 vaccine in solid organ transplant recipients: a case series. Ann Intern Med. 2021;174(9):1330‐1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Benotmane I, Gautier G, Perrin P, et al. Antibody response after a third dose of the mRNA‐1273 SARS‐CoV‐2 vaccine in kidney transplant recipients with minimal serologic response to 2 doses. JAMA. 2021 Jul 23. doi: 10.1001/jama.2021.12339. Online ahead of print. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hall VG, Ferreira VH, Ku T, et al. Randomized Trial of a Third Dose of mRNA‐1273 Vaccine in Transplant Recipients. N Engl J Med. 2021:NEJMc2111462. 385(13):1244‐1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Herrera S, Colmenero J, Pascal M, et al. Cellular and humoral immune response after mRNA‐1273 SARS‐CoV‐2 vaccine in liver and heart transplant recipients. Am J Transplant. 2021 Jul 22. doi: 10.1111/ajt.16768. Online ahead of print. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wadei HM, Gonwa TA, Leoni JC, Shah SZ, Aslam N, Speicher LL. COVID‐19 infection in solid organ transplant recipients after SARS‐CoV‐2 vaccination. Am J Transplant. 2021 Apr 23;10.1111/ajt.16618. doi: 10.1111/ajt.16618. Online ahead of print. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Malinis M, Cohen E, Azar MM. Effectiveness of SARS‐CoV‐2 vaccination in fully‐vaccinated solid organ transplant recipients. Am J Transplant. 2021;21(8):2916‐2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Keehner J, Horton LE, Pfeffer MA, et al. SARS‐CoV‐2 infection after vaccination in health care workers in California. N Engl J Med. 2021;384(18):1774‐1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ali NM, Alnazari N, Mehta SA, et al. Development of COVID‐19 infection in transplant recipients after SARS‐CoV‐2 vaccination. Transplantation. 2021;105(9):e104‐e106. [DOI] [PubMed] [Google Scholar]
- 22. Dan JM, Mateus J, Kato Y, et al. Immunological memory to SARS‐CoV‐2 assessed for up to 8 months after infection. Science. 2021;371(6529):eabf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sette A, Crotty S. Adaptive immunity to SARS‐CoV‐2 and COVID‐19. Cell. 2021;184(4):861‐880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dolff S, Zhou B, Korth J, et al. Evidence of cell‐mediated immune response in kidney transplants with a negative mRNA vaccine antibody response. Kidney Int. 2021;100(2):479‐480. 10.1016/j.kint.2021.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting material
Data Availability Statement
The data of this study are available on request from the corresponding author. The data are not publicly available because of privacy restrictions.


