Abstract
Aim
To establish the responses to the Sinopharm HB02 COVID‐19 vaccination in the dialysis population, which are not well established. We examined the humoral responses to the Sinopharm COVID vaccine in haemodialysis patients.
Methods
Standard vaccinations (two doses at interval of ~21 days) were given to all consenting haemodialysis patients on dialysis (n = 1296). We measured the antibody responses at 14–21 days after the second vaccine to define the development of anti‐spike antibodies >15 AU/ml after vaccination and observed the clinical effects of vaccination.
Results
Vaccination was very well tolerated with few side‐effects. In those who consented to antibody measurements, (n = 446) baseline sampling showed 77 had positive antibodies, yet received full vaccination without any apparent adverse events. Positive anti‐spike antibodies developed in 50% of the 270 baseline negative patients who had full sampling, compared with 78.1% in the general population. COVID infection continues to occur in both vaccinated and unvaccinated individuals, but in the whole group vaccination appears to have been associated with a reduction in the case fatality rate.
Conclusion
The humoral immune responses to standard HB02 vaccination schedules are attenuated in a haemodialysis cohort, but likely the vaccine saves lives. We suggest that an enhanced HB02 vaccination course or antibody checking may be prudent to protect this vulnerable group of patients.
We suggest a booster dose of this vaccine at 3 months should be given to all dialysis patients, on the grounds that it is well tolerated even in those with good antibody levels and there may be a survival advantage.
Keywords: COVID‐19, haemodialysis, Sinopharm, vaccination
SUMMARY AT A GLANCE
In this analysis of humoral response to the Sinopharm COVID‐19 vaccine in over 400 haemodialysis patients in United Arab Emirates, positive anti‐spike antibodies (>15 AU/ml) developed in 50% of 270 COVID‐19 sero‐naive patients who had full sampling, compared with 78.1% in the general population. Side effects were remarkably few.
1. INTRODUCTION
The coronavirus (SARS‐CoV‐2), causing COVID‐19 infection started to spread rapidly across the world in December 2019 and has caused an international clinical and public health emergency.
The worldwide response to this pandemic has included the rapid development of vaccines. There are now several vaccines that are now approved by the WHO for emergency use across the globe including using standard technologies (e.g., inactivated virus or purified protein) and novel vaccine approaches (mRNA or adenovirus vectors). Although registered for human use in the United Arab Emirates (UAE) since September 2020, the Sinopharm vaccine trial results, have recently been reported and noted it efficacy to prevent symptomatic and hospitalized disease to be ~78.1% for all age groups 1 without comorbidities.
The prognosis for the elderly and those with chronic diseases is worse than for younger and healthier individuals, but of particular concern are those with chronic kidney disease (CKD), in whom the outcomes are much worse than for others affected with other chronic diseases. 2 In particular, the case fatality rate is much higher in dialysis patients, with mortality estimates for dialysis patients developing SARS‐CoV‐2 (COVID19) virus infection as high as 31%. 3 We reported previously a lower mortality 4 during the first wave of the virus in the Abu Dhabi. 4 Nevertheless, we were keen to vaccinate dialysis patients early and the Abu Dhabi Department of Health made trials available in July 2020 and made the Sinopharm HB02 vaccine freely available to any resident of the UAE in late 2020, and this early and aggressive vaccination campaign allowed the UAE to achieve internationally very high vaccination rates. No other vaccines were freely or widely available within the Emirate of Abu Dhabi during the antibody study period. Other groups with renal impairment, for example, after renal transplantation 5 and with advanced CKD 6 are also at high risk. 7 Many studies report infection in a high percentage of dialysis patients with COVID‐19 (~19%, range of 10%–24%) among its dialysis centres. 8 The overall mortality of COVID‐19 infection in a dialysis population reported at 14%–31%. 9 , 10 , 11 However, median ages in these studies was generally older than the UAE dialysis population. One study reported 66% of those who died were above the age of 75 years. 11
Despite screening, contact avoidance, distancing, isolation and droplet control precautions (through extensive use of PPE), patients with COVID‐19 can infect others at any time, especially during transport to, and at haemodialysis centres during frequent close contact in dialysis units. This makes this highly comorbid population especially vulnerable. Thus, this infectious disease requires specific control measures to avoid community and family transmission in areas in and around renal units, that are now well described. 12
Vaccination in dialysis patients is generally less successful, and circulating antibody response less durable, in renal patients as exemplified by the increased dosing requirements and boosters for hepatitis B vacation. 13
Many studies have reported showing that standard COVID vaccination antibody response with the Pfizer/BioNTech, Astra‐Zenica vaccine in haemodialysis (HD) patients was suboptimal but provides good antibody responses in a number of patients. 14 Yet there is little data on the efficacy of the Sinopharm vaccine, nor on its protective effects. This study set out to examine the antibody response to this vaccine in a HD cohort in Abu Dhabi, UAE.
2. METHODS
We have monitored all COVID19 infection since our first case in March 2020 and started vaccinating haemodialysis patients as soon as a vaccine became available in January 2021, and all patients were offered vaccination at our centre. We report a prospective, concurrent cohort study of antibody responses in patients undergoing consultation and haemodialysis at the SEHA Kidney Care which encompasses many facilities throughout Abu Dhabi, as well as describing the clinical events around the pandemic in this patient cohort. The epidemiological data on COVID infection is described from March 2020 to June 2021 but the antibody study ran from January 2021 to the current data and data was censored at the end of June 2021. The inclusion criteria for vaccination (issued by the Department of Health, Abu Dhabi) were 18 years old and above; hemodynamically stable at the time of vaccination; and controlled chronic disease/s including autoimmune disease. The exclusion criteria were anyone with history of moderate to severe COVID 19 infection needing oxygen during admission; known to be pregnant (based on clinical history and/or pregnancy test); previous severe allergic reaction to vaccination; recent history of convulsion, epilepsy, encephalopathy, or severe coagulation dysfunction; receiving any live attenuated vaccine within 1 month before this vaccination or other vaccines types within 14 days before this vaccination; recent history of Guillain Barre Syndrome (GBS) or transverse myelitis; uncontrolled symptomatic autoimmune disease, immunocompromised conditions, active cancer or in remission for less than 6 months, or inability or refusal to give informed consent.
The primary outcome of the antibody study was development of an anti‐spike IgG response after a standard vaccination schedule with the HB02 vaccine, a fourfold increase geometric mean of anti‐SARS‐CoV‐2 spike antibody 14–21 days after two doses of immunization. Secondary outcomes were acquiring COVID 19 virus infection; severe side effects precluding second dosing; the incidence of adverse events within 30 min after each dose of vaccine; incidence of events within D0 ~ 28 after each dose of vaccination; and incidence of serious adverse events from the beginning of the first dose to 3 months after the whole course of immunization.
Institutional review board including an ethics and scientific committee approval was obtained for this study to obtain serum for antibody analysis (Abu Dhabi Department of Health's COVID‐19 Research Ethics Committee (CVDC‐ADHRTC‐25 15 ). Patients were consented separately to receive the vaccine and to allow us to study the antibody response.
2.1. Vaccine
The HB02 vaccine is an inactivated SARS‐CoV‐2 and is prepared by inoculating Verda Reno cells (Vero cells) with SARS‐CoV‐2 HB02 strain, culturing, harvesting, inactivating, clarifying, concentrating, purifying, and adding aluminium hydroxide as an adjuvant. This vaccine was approved for clinical use by The Government of Abu Dhabi in September 2020 and was used to help protect the population of the UAE since early 2021 when the general vaccination campaign started. Following trials 1 this vaccine was approved by the WHO as safe and effective for patients without comorbidities 29th April 2021, but there was insufficient data to endorse use in higher risk groups.
Two doses of HB02 vaccine were inoculated to the deltoid muscle of the upper arm according to the immunization schedule at day 0 (T0) and day 21 (T1) (±7 days). These were given on routine HD sessions, and without dialysis anticoagulation withdrawal. Subjects gave a blood sample at baseline before immunization (Baseline), before the second dose T2) and between 14 and 28 days after the second dose (T3).
3. LABORATORY ANALYSIS
Samples were batched and assayed retrospectively or antibodies to the S1/S2 spike protein as well as a ‘neutralizing’ antibody response. COVID‐19 reverse transcription polymerase chain reaction (RT‐PCR) were performed using Cobas SARS‐CoV‐2 test by Roche Diagnostics (Roche Molecular Systems, Branchburg, NJ, United States). COVID‐19 IgG antibody testing was performed using the LIAISON® SARS CoV‐2 S1/S2 IgG assay (LIAISON® XL Analyser, DiaSorin Inc.1951, USA) which uses a chemiluminescence immunoassay (CLIA) technology for the quantitative determination of anti‐S1 and anti‐S2 specific IgG antibodies to SARS‐CoV‐2 in human serum or plasma samples. Antibody titres <12 AU/ml were considered negative, 12–14.9 AU/ml equivocal, and ≥ 15 AU/ml positive as per the manufacturer's instructions. The lower limit of quantitation (LLoQ) was 3.8 AU/ml and upper limit of quantitation (ULoQ) 400 AU/ml. Samples above the ULoQ were dealt with by assigning them the value of the ULoQ. Neutralizing antibody testing was performed using cPass™ SARS‐CoV‐2 Neutralization antibody detection kit by GenScript USA, Inc, which is a blocking enzyme‐linked immunosorbent assay intended for qualitative direct detection of total neutralizing antibodies to SARS‐CoV‐2 in human serum and K2‐EDTA plasma and were reported a percentage, with a LLoQ of 1%. Neutralizing antibody levels >30% were considered positive, as per FDA recommendation. Both antibody detection kits are FDA approved and performance indicators have been verified and approved in Bioegnix laboratory for use on clinical samples.
3.1. Analytical plan
Spike Antibody results were expressed as AU/ml and neutralizing as %. Levels below the LLoQ were dealt with by assigning them the value of LLoQ/2 and those above the ULoQ were assigned the value of the ULoQ. 16 The primary end‐point was the presence of anti‐SARS‐CoV‐2 antibody at a fourfold growth rate or more in antibody titres after vaccination versus baseline. Patients with positive spike antibodies at baseline were excluded from further analysis. Continuous variables are expressed as mean (SE), if parametric or medians (with interquartile range) if non‐parametric. Normality was assessed by D'Agostino & Pearson test. For ordinal data the non‐parametric Frieman test was used to examine the paired values at baseline, and after the first and second samplings. Fisher's exact test was used for contingency tables. Sample size: Based upon the fact that some dialysis patients fail to seroconvert with an accelerated hepatitis B vaccine, we hypothesized a lower rate of seroconversion. Assuming a relatively optimistic seroconversion rate of 70% in HD patients, and a general population seroconversion rate of 80%, we calculated that we would require a sample size of 188 to detect a difference in seroconversion rates with an anti‐spike antibody response, with power of 90% and α of .05.
Data collection was performed in Microsoft Excel with statistical testing and graphing performed using GraphPad Prism 9.1.2 (225) for Mac, GraphPad Software, San Diego, CA, United States, www.graphpad.com. Statistical significance was determined at α = .05 level.
4. RESULTS
The antibody study flow, and data, are summarized graphically in Figure 1. Baseline data for the whole cohort and those with all antibody tests suggest that the sample with antibody tests was relatively well representative of the entire dialysis cohort (Table 1 and Figure 2). Of 1296 dialysis patients offered a vaccine, 1056 agreed to be vaccinated (81%), with 240 declining vaccination or having a contraindication to vaccination, reflecting a relatively high level of vaccine hesitancy (nearly 20%). Seven hundred and thirty‐three patients consented to allow us to use their antibody result, but only 446 patients underwent baseline sampling. Seventy‐seven of these were found to have COVID‐19 antibodies pre‐existing, of whom 52 (68%) had had known COVID‐19 infection previously. Interestingly 25 (32%) of these had anti‐spike antibodies but without a history of COVID‐19 infection despite undergoing regular COVID‐19 PCR screening at our facility. These patients, who had good antibody levels at baseline, still underwent full vaccination (because antibody levels were analysed retrospectively) and suffered no apparent ill‐effects. The geometric mean (GM) of these baseline‐positive patients for anti‐spike antibodies was 83 (95%CI 68–101) AU/ml and for neutralizing 67% (95%CI 61–73). These patients were removed from the cohort analysis of the effects of vaccination.
FIGURE 1.
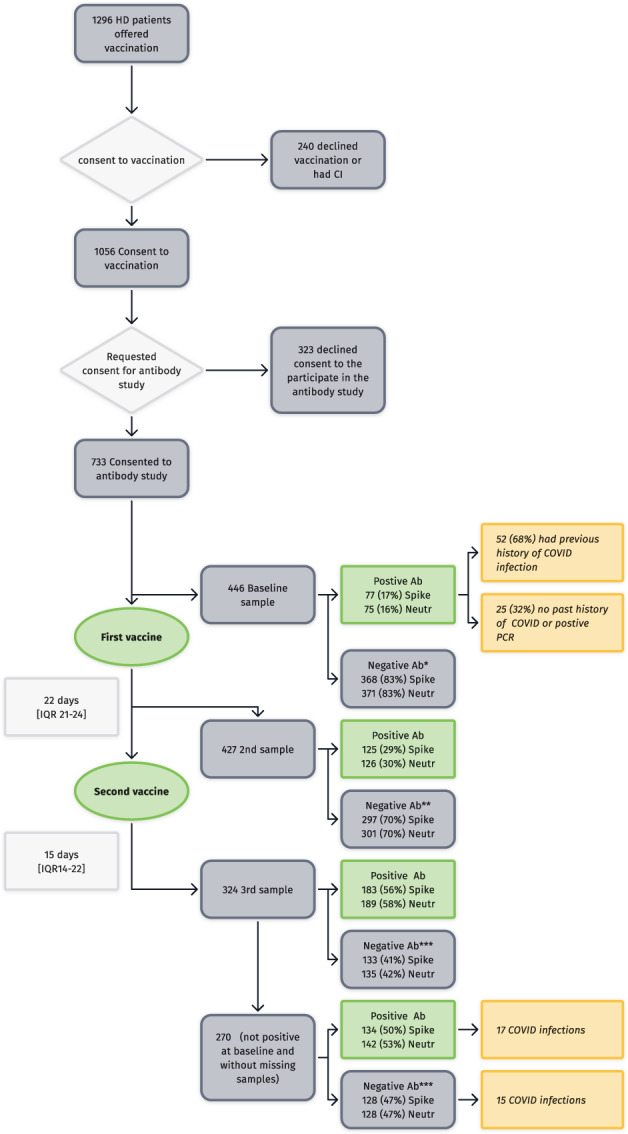
Flow diagram of the study with main results. (*1 equivocal result, **5 equivocal results, ***8 equivocal results)
TABLE 1.
Baseline demographics of the samples
| Baseline data | Full sample | Full Antibody data | p |
|---|---|---|---|
| n | 1296 | 270 | |
| Male | 832 (64%) | 167 (62%) | ns |
| Female | 464 (36%) | 103 (38%) | ns |
| Age distribution (median [IQR]) | 57 [45–66] | 57 [45–66] | ns |
| Nationality | % | % | |
| Emirati | 24 | 21 | |
| Philippines | 11 | 19 | |
| Pakistani | 9 | 8 | |
| Bangladeshi | 8 | 7 | |
| Indian | 7 | 5 | |
| Yemen | 6 | 7 | |
| Sudan | 5 | 4 | |
| Others (all <5% each) | 30 | 29 |
FIGURE 2.
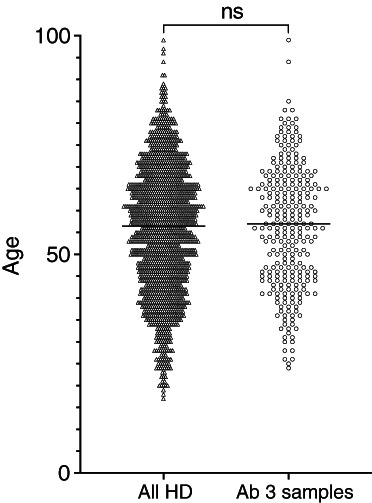
Age ranges of the full dialysis population (n = 1296) and the sample with full antibody results (n = 270). p = ns
The GM of the baseline negative antibody samples was 2.2 (95%CI 2.0–2.3) AU/ml and for neutralizing 2.6 (95%CI 2.2–3.1)%, 1 patient had equivocal results. A total of 427 subjects underwent antibody sampling before the second vaccination a median of 22 days (IQR 21–24) days after the first vaccination. Of those, 125 (29%) had positive anti‐spike antibodies (with 4 equivocal results) and GM titre of 3.4 (95%CI 2.9–2.9) AU/ml, with 126 (30%) had positive neutralizing antibodies with GM of 5.0 (95%CI 4.1–6.1)% from a single vaccination.
Three hundred and twenty‐four patients underwent third sampling 15 (IQR14‐21) days after the second vaccine. Of those, 183 (~56.5%) had positive anti‐spike antibodies, with 8 (~2.5%) equivocal results, but others (133%–41%) had suboptimal antibody levels. Overall GM antibody levels at the third time point were 13.3 (95%CI11.1–15.8) AU/ml and neutralizing 13.0 (95%CI 10.9–15.6)%. However, in the (183) patients who developed a positive response, the anti‐spike antibody titres appeared good GM 70 (95%CI 60–81) AU/ml, neutralizing 58 (95%CI 58–66)%.
Excluding patients who were positive at first sampling and those with missing interim samples, 269 patients had a complete set of 3 antibody results for pairwise comparisons, and this data is shown in Figure 3A,B. Antibody levels were not normally distributed and are shown on log scales. These show a similar pattern of significant increasing antibody acquisition after vaccination) p < .0001. There was generally good agreement between anti‐spike and neutralizing antibody responses in all assays (94% agreement if we consider borderline anti‐Spike samples as negative). In the final 270 patients will full data, anti‐Spike antibodies were recorded as positive in 134 (50%) of the patients with GM 12.2 [95%CI 11.1–16.9] and positive neutralizing antibodies in 142 (53%) with GM 21.8% [95%CI 18.5–25.8]. Patients who did not have three antibody results were excluded from the main analysis, but data from those with incomplete data was used to check that this group were congruent with the full results group (Figure S1).
FIGURE 3.
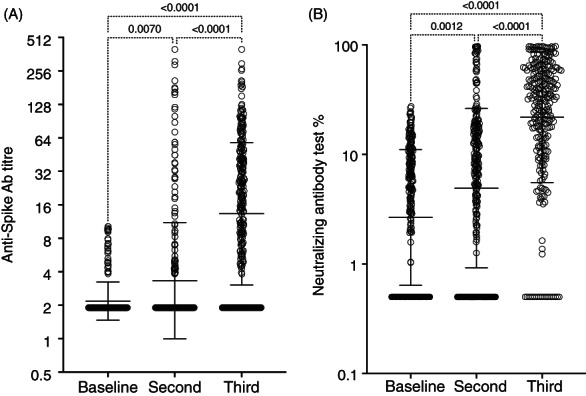
(A) Antibody results from the patients completing all three follow up. The data is not normally distributed, and titres are shown on a log2 axis. Significance was assessed by Friedman test and shown in dotted brackets. Geometric mean and confidence intervals are shown as large and small bars respectively (n = 269). (B) Anti‐neutralizing antibody assay percentage. Axis Log10. Geometric mean and CI plotted as wide and narrow bars at baseline, After 1 and 2 doses of the vaccine. Significance was assessed by Friedman test and shown in dotted brackets (n = 269)
4.1. Factors affecting antibody seroconversion
Comparing responders with non‐responders (ignoring borderline patients) there was significant difference in median age 53[IQR 43–62] versus 61[IQR 49–69], p < .0001, and female sex being associated with non‐response (non‐responders M:F 74:59 vs. responders 129:42, p < .0001).
4.2. Side effects
Despite being given an intramuscular injection during the dialysis procedure and with standard dialysis anticoagulation, there were remarkably few side effects. Few patients experienced any side effects from the vaccination itself and no bleeding issues were noted. There was one recorded immediate moderate side‐effect of pain in 1986 recorded administrations (<0.05%), and three more reported mild pain, as all patients given vaccination had immediate side effects recorded. One patient developed a moderately severe maculopapular rash, 6 days after vaccination.
4.3. Antibody response and COVID disease
Within the cohort between March 14 2020 and August 22 2021 we had 512 patients with a positive COVID PCR, 323 (63%) were unvaccinated and 189 (37%) were vaccinated. Antibody results were available in some of these patients with the others either not having had antibody levels taken or refused consent. In those in whom we had three antibody levels (n = 32), there was no overall difference in antibody levels between those with and without COVID. Fifteen patients with ‘negative’ anti‐spike antibody levels and 17 with ‘positive’ anti‐spike antibodies became COVID positive.
There did appear to be a significant difference in length of COVID positive days by PCR testing between those vaccinated (median 14 days (IQR 8–19) and those unvaccinated (17 [IQR 9–29], p = .0001) with vaccination seeming to shorten the time to the second negative PCR (Figure S3).
4.4. Mortality
Overall mortality rates regardless of vaccination status was 36/511 cases (7%). From the start of the pandemic in our units (March 14 2020) until we started the vaccination programme (January 25 2021) the case fatality ratio was ~8.9% (17/190). During the initial vaccination period (January 25 2021–March 30 2021) we had 6 deaths in 71 cases (8.5%). After vaccination (March 30 2021–August 22 2021) we have had 13 deaths in 250 cases (5.2%).
Overall case fatality rate in unvaccinated individuals March 14 2020 to August 22 2021 was 28/324 = 8.7% and in vaccinated 8/187 = 4.3% (p = .1, ns).
Figure 4 shows the survival curves to 60 days for vaccinated and unvaccinated patients who contracted COVID‐19 (with Table S1 showing the numbers at risk). The censoring data for this data was June 22 2021 (so we have full 60 day mortality data available). Figure 5 shows the ages of patients who died (median 71 [IQR 57–78] years) and those who survived (54 [IQR43–64] years), p < .0001, in addition to ages of those vaccinated and unvaccinated who died (78 [IQR 71–80] years vs. 66 [IQR 55–75] years), p = .058.
FIGURE 4.
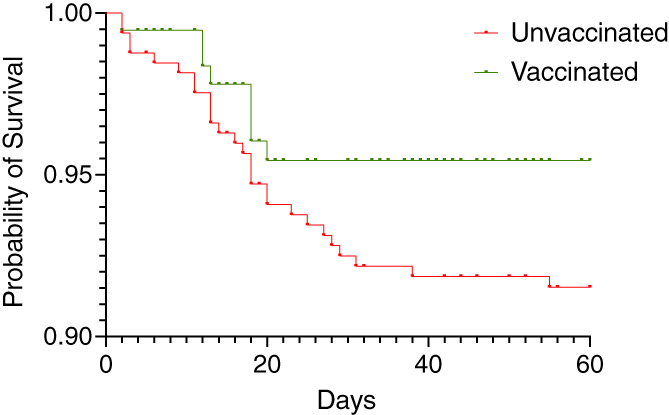
Probability of survival after COVID diagnosis by polymerase chain reaction (PCR) in vaccinated and unvaccinated haemodialysis patients between March 14 2021 and August 22 2021. Whilst there was no significant differences in the survival curves at 60 days from COVID related deaths (Log‐rank [Mantel‐Cox] test p = ns), the unvaccinated death rate is low and curves diverge after ~20 days (number at risk shown in Table S1) and hazard ratio is 1.71 (95%CI 0.91–3.64)
FIGURE 5.
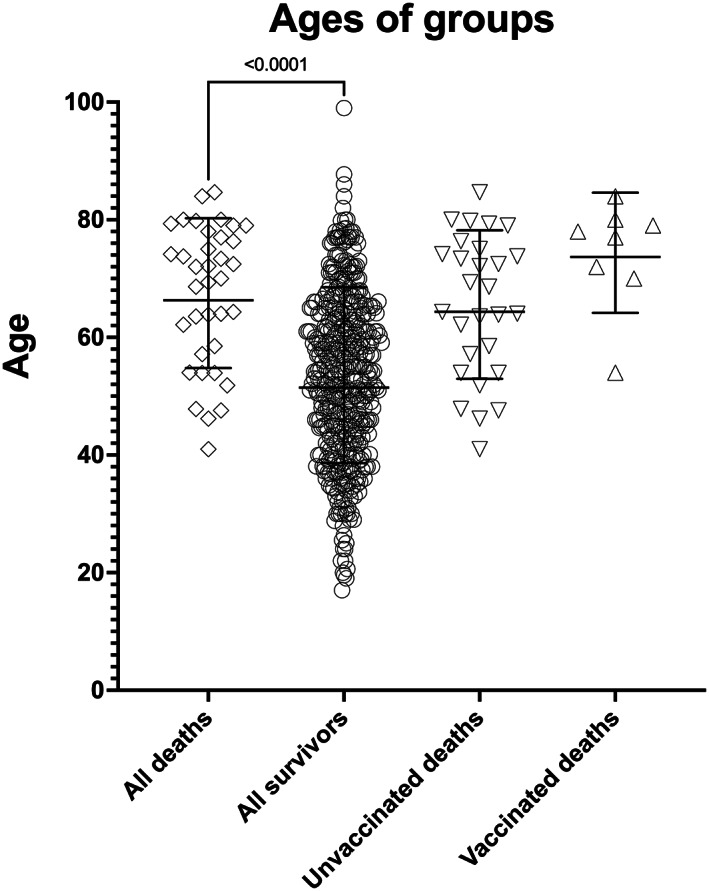
Ages of patients who died (open diamonds) and those who survived COVID where outcome is known (n = 479) (open circles), with the rest still unrecovered at 22 August 2021). Overall, those who died were older (p < .0001). When we compared the ages of those who died without vaccination (n = 27, open downward triangles) and with vaccination (open upwards triangles, n = 8) showing a trend towards those who died being older if they were vaccinated (but p = ns)
5. DISCUSSION
The first Coronavirus infection (SARS‐CoV‐1) was noted in 2003 but affected only a relatively small number of patients. The second coronavirus pandemic (SARS‐CoV‐2) began in 2019 17 and has rapidly become a global health emergency, causing countless infections and deaths worldwide. Patients with chronic diseases, but especially those with kidney disease, are uniquely vulnerable to COVID‐19 infection. Perhaps infection rates in this group are related to the need of this patient group to be in frequent contact with family members that they rely upon, dialysis units, and other parts of the health‐care system. The requirement life‐supporting and other treatments including, but not limited to, dialysis or transplantation brings them in frequent contact with health‐care services. Mortality for this patient group is high and thus vaccination may be a game changer in the management of these patients. There is an urgent need to know how best to administer and monitor responses to vaccination.
Most countries have responded by attempting mass vaccination programmes in their populations with different degrees of success. The vaccines that are now recognized by the WHO for emergency use across the globe include (approval date) the Pfizer/BioNTech vaccine (December 31 2020); AstraZeneca/Oxford (February 15 2021); Janssen Ad26.COV2.S (Johnson & Johnson) (March 12 2021); Moderna (April 30 2021), Sinopharm (May 7 2021). The UAE instituted population vaccination early with the Sinopharm vaccine and has achieved a high level of vaccination in its population, currently sitting in the world top 10 in terms of percentage of the population vaccinated. 18 Whilst overall the SinoPharm vaccine appears efficacious in terms of antibody seroconversion (~78.1%), for all age groups, efficacy trials included few older adults (over 60 years), or those with comorbid disease. Nevertheless, WHO is not recommending an upper age limit for the vaccine because preliminary data and supportive immunogenicity data suggest the vaccine is likely to have a protective effect in older and comorbid people. There is no theoretical reason to believe that the vaccine has a different safety profile in older or younger populations.
Vaccination in dialysis groups is usually less efficacious than in the general population but a number of relatively small early studies from the mRNA and adenoviral vector vaccines (recently summarized by Carr et al. 19 ) have shown good seroconversion rates in dialysis cohorts from full vaccination of 71%–97%, 19 but little data on SinoPharm vaccine exists in this group.
We have noted that the Sinopharm vaccination in dialysis patients was really very well tolerated when given on dialysis and there was no need to change the anticoagulation to give the dose on dialysis. We had only a single case of moderate side‐effect attributed to the vaccine with a rash, and it was remarkable that most patients reported no immediate problems, to the point that anecdotally some patients even doubted that they had actually been given real vaccine. However, the seroconversion was lower than the general population (~78%) at ~50% in the HD patients.
The mortality rate in the entire HD group appears lower after vaccination, but the low numbers in both groups mean that statistical significance overall was difficult to demonstrate, but the death rate in the dialysis cohort generally low compared with reported cases elsewhere in the literature which has varied between ~14% and 30% in unvaccinated individuals in early reports. 9 , 10 , 11 We previously reported the development of generally good antibody responses to COVID19 infection, before vaccination was available, in our dialysis cohort in the first wave. 4 However, we also noted a lack of antibodies in some patients, further increasing our anxiety around the effectiveness of the vaccination in this group of patients. Even though we reported an internationally low death rate before the vaccine was available it was still much higher than the general population, so we were anxious to protect this very vulnerable group from second and subsequent waves of COVID infection. The relatively low death rate is reported here, this may reflect a relatively lower age than many countries dialysis populations.
We have shown that the COVID vaccine given at standard doses and dosing intervals is moderately successful in provoking a protective immune response with Anti‐S antibodies in around half our patients (50%), but the antibody responses in many patients were suboptimal, and not demonstrated at all in some. The fact that HD patients have a less robust response to vaccination is not an unexpected result and it has recently been reported that patients who received the Pfizer vaccine in a much smaller cohort of HD patients, had a poor antibody response. 14 In the latter study 73% seroconverted using the Pfizer study in a much smaller cohort, but a summary of published seroconversion frequencies showed better seroconversion rates. 19 No data yet exists on mixing vaccine types or increasing the dose or dosing frequency for particular populations. It is also interesting to note that this vaccine was well tolerated even by those who already possessed good antibody levels, suggesting that augmented dosing of this vaccine may not be problematic. Our results may also suggest, since we have a relatively young dialysis population, that we might expect an even lower seroconversion rate in older populations to a standard vaccination regimen.
Other vaccination associated antibody titres wane more rapidly in dialysis patients suggesting that vaccine induced immunity wanes quickly. However, based on these and other data we are now providing early booster doses to all patients with this vaccine.
The fact that we have recently seen a rise in the number of vaccinated patients developing a positive COVID test may relate to new variants which may partially evade the antibody protection provided by the vaccine. 20
The strengths of this study were that it included a relatively large cohort of dialysis patients and had full antibody results at baseline, prior the first and second vaccine. We have also shown that vaccination appears to reduce the duration COVID PCR positivity to the second negative PCR (Figure S3).
The limitations of the study were the relatively large study non‐consent rate. There were significant numbers of patients who had a baseline sample but did not complete 3 blood test results. These samples showed a similar trend to the main cohort with slightly, but significantly higher second sample titres (6.0[95%CI 4.3–8.3]) versus 3.3 (95%CI 2.9–3.8) AU/ml in the cohort with full data. However, 31% of these had values considered positive at the first sampling period, whereas in the full cohort this was only 16%. Suggesting that the sampling errors may have contributed to a slight under reporting of positivity rate. We do not know if the positivity rate at the third sampling would have been similar, yet even if we assign a 70% positivity rate at the third sampling, this would make the overall seroconversion rate ~56%, still well below the figure quoted for the general population. (see Figure S1A,B).
We also urge caution in the interpretation of the ‘neutralization’ assay which has been reported to have some sensitivity issues so caution should be applied in its interpretation and results should not be conflated with true microneutralisation assays. 21
Nevertheless, we are encouraged by case fatality reduction and the diverging mortality curves as well as the data suggesting that vaccination seemed to be effective at protecting younger patients and limiting the length of disease in survivors. However, we cannot exclude advances in our care being responsible for the improvement in mortality in later patients. Additionally, we cannot exclude the fact that the first and second waves of COVID infection may have left a more physiologically robust dialysis cohort. Most worryingly, we do not have information on the variants of SARS‐Co‐2 which may include a number that may be relatively resistant to vaccine directed antibodies and may explain the rise in COVID cases more recently among vaccinated patients (Figure S2). Whilst COVID positive patients were generally PCR tested every other day whilst hospitalized, the frequency of testing at discharge was with dialysis sessions following discharge, and was generally performed on every session until two negatives were obtained (in order to help with isolation decisions). Although we showed that vaccination appeared to reduce the median time to COVID PCR negativity, we cannot exclude the possibility that this was related to differing testing intervals. This was especially true for those who remained PCR positive for some time and who were symptomatically well, where testing frequency may well have been reduced. Of course, PCR tests do not measure replicant competent virus, and we do expect infectivity to have reduced before PCR negativity, 22 so the clinical significance of this finding is uncertain but consistent with possible faster viral clearance. Testing for viral genotype is being considered to further clarify some of the more recent positive patients despite apparently good antibody levels antibody.
6. CONCLUSIONS
The Sinopharm COVID HB02 vaccine given in standard doses on dialysis does induce antibody response in the haemodialysis population in around half of all subjects. Seroconversion, is not as good as in the general population (50% vs. 78%) but this was an expected result and this response does not appear to be as good as mRNA or adenovirus induced seroconversion, but this vaccine may be better tolerated. This has prompted us to intensifying the dosing schedule in these patients. Importantly vaccination does appear to offer some protection from death, as the case fatality rate has halved from community acquired infection since the vaccination roll out.
Dialysis patients may have a requirement for an augmented standard vaccination schedule with this vaccine to provide more protection to this vulnerable group.
CONFLICT OF INTEREST
The authors declared no conflict of interest.
AUTHOR CONTRIBUTIONS
Substantial contributions to the conception and design of the work, acquisition, analysis and interpretation of data for the work; drafting the work and revising it critically for important intellectual content; final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: Stephen Geoffrey Holt. Substantial contributions to the conception and design of the work; critically revising for important intellectual content; Final approval of the version; agreement to be accountable for all aspects of the work: Islam Eltantawy. Sample analysis and laboratory procedures, critically revising the manuscript for important intellectual content. Final approval of the version; agreement to be accountable for all aspects of the work: Sally Mahmoud. Substantial contributions to the conception and design of the work, acquisition, critically revising; final approval of the version to be published; agreement to be accountable for all aspects of the work: Ayman Kamal Al Madani. Substantial contributions to the conception and design of the work, acquisition, critically revising; final approval of the version to be published; agreement to be accountable for all aspects of the work: Walid Abbas Zaher. Substantial contributions to the conception and design of the work, acquisition, critically revising; final approval of the version to be published; agreement to be accountable for all aspects of the work: Wasim Ahmed. Substantial contributions to the conception and design of the work, acquisition, critically revising; final approval of the version to be published; agreement to be accountable for all aspects of the work: Ali Abdulkareem Al Obaidli. Critically revising; final approval of the version to be published; agreement to be accountable for all aspects of the work: Gareth John Goodier. Critically revising; final approval of the version to be published; agreement to be accountable for all aspects of the work: Nawal Ahmed Al Kaabi. Analysis and interpretation of data for the work; critical revisions and important intellectual content; statistical review. final appraisal of the version to be published; agreement to be accountable for all aspects of the work: Juan Manuel Acuna.
Supporting information
TABLE S1: Days at risk and survival to 60 days
FIGURE S1A: Anti Spike antibodies in patients excluded due to not having full samples. (n = 99‐ baseline samples, n = 85 second samples). The first three columns are recapitulated from Figure 3 (open circles). Excluded samples are shown as squares. Excluded baseline samples (n = 99) were not significantly different to the group with full sampling(p = ns). Those with a baseline and second sample(n = 85) but no third sample showed a slightly higher median anti‐spike titre than those with 3 samples (6.0 (95% CI 4.3–8.3) vs 3.3 (95%CI 2.9–3.8) AU/ml p = 0.0006
FIGURE S1B: neutralizing antibodies in patients excluded (n = 99‐ baseline samples, n = 85 second samples) from the cohort analysis by virtue of incomplete data and not positive at baseline (square markers) along with cohort data (round markers). These show similar trends to the main cohort but without significantly higher second sample titres. The geometric mean titre for the excluded second samples was 7.0 (95%CI 4.3–11) vs 4.9 (4–6)% in the cohort with full data.
FIGURE S2: Active cases of vaccinated and unvaccinated patients showing three ‘waves’ of infection in our patients in the UAE. The third wave may have represented new variant infection (eg the delta variant) and although vaccination did not appear to prevent infection completely may have reduced infection time and mortality.
FIGURE S3: Days from first PCR to second negative PCR in dialysis patients vaccinated and unvaccinated (n = 449, linear axis) where an outcome was known (i.e. two negatives PCR value and excluding deaths) showing a significant difference (p < .0001). The patients with <3 days negative may have been false positives
ACKNOWLEDGEMENTS
The Authors would like to thank Sheena Rajan, Dr Marie Kim James, Dexie Marquez, Annie Valois, Allum Rezqallah, Azmi Horani and all the SKC nursing team, in addition to Dr Samuel Fong and Nastaran Shekary, for their invaluable help in the preparation, execution, and contribution to this study. The UAE government is acknowledged and thanked for providing rapid and continuous support for vaccination and treatment of COVID‐19 infection to all residents of the UAE. Both SEHA Kidney Care and G42 provided in kind funding for this study – SKC provided clinical time, materials, nursing and medical expertise. G42 provided antibody testing and laboratory expertise.
Holt SG, Mahmoud S, Ahmed W, et al. An analysis of antibody responses and clinical sequalae of the Sinopharm HB02 COVID19 vaccine in dialysis patients in the United Arab Emirates . Nephrology. 2022;27(3):260‐268. doi: 10.1111/nep.13980
REFERENCES
- 1. Al Kaabi N, Zhang Y, Xia S, et al. Effect of 2 inactivated SARS‐CoV‐2 vaccines on symptomatic COVID‐19 infection in adults. JAMA. 2021;326(1):35‐45. doi: 10.1001/jama.2021.8565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gasparini M, Khan S, Patel JM, et al. Renal impairment and its impact on clinical outcomes in patients who are critically ill with COVID‐19: a multicentre observational study. Anaesthesia. 2021;76(3):320‐326. [DOI] [PubMed] [Google Scholar]
- 3. Wu J, Li J, Zhu G, et al. Clinical features of maintenance hemodialysis patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. Clin J Am Soc Nephrol. 2020;15(8):1139‐1145. doi: 10.2215/CJN.04160320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ahmed W, al Obaidli AAK, Joseph P, et al. Outcomes of patients with end stage kidney disease on dialysis with COVID‐19 in Abu Dhabi, United Arab Emirates; from PCR to antibody. BMC Nephrol. 2021;22(1):198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Akalin E, Azzi Y, Bartash R, et al. Covid‐19 and kidney transplantation. N Engl J Med. 2020;382(25):2475‐2477. doi: 10.1056/NEJMc2011117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Flythe JE, Assimon MM, Tugman MJ, et al. Characteristics and outcomes of individuals with pre‐existing kidney disease and COVID‐19 admitted to intensive care units in the United States. Am J Kidney Dis. 2021;77(2):190‐203.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Valeri AM, Robbins‐Juarez SY, Stevens JS, et al. Presentation and outcomes of patients with ESKD and COVID‐19. J Am Soc Nephrol. 2020;31(7):1409‐1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Savino M, Casula A, Santhakumaran S, et al. Sociodemographic features and mortality of individuals on haemodialysis treatment who test positive for SARS‐CoV‐2: a UK renal registry data analysis. PLoS One. 2020;15:4‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.UK Kidney Association Association. https://ukkidney.org/health-professionals/covid-19. Accessed October 1, 2021.
- 10. Goicoechea M, Sánchez Cámara LA, Macías N, et al. COVID‐19: clinical course and outcomes of 36 hemodialysis patients in Spain. Kidney Int. 2020;98(1):27‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Keller N, Chantrel F, Krummel T, et al. Impact of first‐wave COronaVIrus disease 2019 infection in patients on haemoDIALysis in Alsace: the observational COVIDIAL study. Nephrol Dial Transplant. 2020;35(8):1338‐1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Connealy MB, Lew SQ, Alsamman M, Lange JJ, Pourmand A. The emergency department care for hemodialysis patient during the COVID‐19 pandemic. Am J Emerg Med. 2021;40:47‐54. doi: 10.1016/10.1016/j.ajem.2020.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Holt SG, Locarnini S, Sasadeusz J. Hepatitis B related dilemmas in the renal unit. Nephrology. 2020;26(4):287‐293. doi: 10.1111/nep.13815 [DOI] [PubMed] [Google Scholar]
- 14. Simon B, Rubey H, Treipl A, et al. Haemodialysis patients show a highly diminished antibody response after COVID‐19 mRNA vaccination compared to healthy controls. Nephrol Dial Transplant. 2021;4(1):1‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Abu Dhabi Department of Health . https://www.doh.gov.ae/en/covid‐19/Research‐Registry/ResearchAndRegistryData/251‐ResearchData‐254. Accessed October 1, 2021.
- 16. Bergstrand M, Karlsson MO. Handling data below the limit of quantification in mixed effect models. AAPS J. 2009;11(2):371‐380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chan JFW, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person‐to‐person transmission: a study of a family cluster. Lancet. 2020;395(10223):514‐523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Global Change Data Lab . Our World in Data. https://ourworldindata.org/covid-vaccinations. Accessed October 1, 2021.
- 19. Carr EJ, Kronbichler A, Graham‐Brown M, et al. Review of early immune response to SARS‐CoV‐2 vaccination among patients with CKD. Kidney Int Rep. 2021;6:1‐13. doi: 10.1016/10.1016/j.ekir.2021.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hacisuleyman E, Hale C, Saito Y, et al. Vaccine breakthrough infections with SARS‐CoV‐2 variants. N Engl J Med. 2021;384(23):2212‐2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Papenburg J, Cheng MP, Corsini R, et al. Evaluation of a commercial culture‐free neutralization antibody detection kit for severe acute respiratory syndrome‐related coronavirus‐2 and comparison with an antireceptor‐binding domain enzyme‐linked immunosorbent assay. Open Forum Infect Dis. 2021;8(6):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van Kampen JJA, van de Vijver DAMC, Fraaij PLA, et al. Duration and key determinants of infectious virus shedding in hospitalized patients with coronavirus disease‐2019 (COVID‐19). Nat Commun. 2021;12(1):8‐13. doi: 10.1038/s41467-020-20568-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1: Days at risk and survival to 60 days
FIGURE S1A: Anti Spike antibodies in patients excluded due to not having full samples. (n = 99‐ baseline samples, n = 85 second samples). The first three columns are recapitulated from Figure 3 (open circles). Excluded samples are shown as squares. Excluded baseline samples (n = 99) were not significantly different to the group with full sampling(p = ns). Those with a baseline and second sample(n = 85) but no third sample showed a slightly higher median anti‐spike titre than those with 3 samples (6.0 (95% CI 4.3–8.3) vs 3.3 (95%CI 2.9–3.8) AU/ml p = 0.0006
FIGURE S1B: neutralizing antibodies in patients excluded (n = 99‐ baseline samples, n = 85 second samples) from the cohort analysis by virtue of incomplete data and not positive at baseline (square markers) along with cohort data (round markers). These show similar trends to the main cohort but without significantly higher second sample titres. The geometric mean titre for the excluded second samples was 7.0 (95%CI 4.3–11) vs 4.9 (4–6)% in the cohort with full data.
FIGURE S2: Active cases of vaccinated and unvaccinated patients showing three ‘waves’ of infection in our patients in the UAE. The third wave may have represented new variant infection (eg the delta variant) and although vaccination did not appear to prevent infection completely may have reduced infection time and mortality.
FIGURE S3: Days from first PCR to second negative PCR in dialysis patients vaccinated and unvaccinated (n = 449, linear axis) where an outcome was known (i.e. two negatives PCR value and excluding deaths) showing a significant difference (p < .0001). The patients with <3 days negative may have been false positives


