Dear Editor,
Delayed immunologic reactions have been described in association with the novel mRNA COVID‐19 vaccines. We report the case of a 40‐year‐old male who presented with widespread pruritic urticarial indurated papules and plaques on the arms, legs, palms, and face 9 days following his first Moderna vaccination (Figure 1A,B). He denied constitutional symptoms, new medications, recent illnesses, or new personal care products. Punch biopsy demonstrated spongiosis with papillary dermal edema and a perivascular lymphocytic infiltrate with occasional eosinophils, compatible with a dermal hypersensitivity reaction (Figure 1C,D). The patient was treated with a 14‐day oral prednisone taper, with resolution of the reaction within 2 days of initiation. The patient did not have recurrence of lesions after the second inoculation.
FIGURE 1.
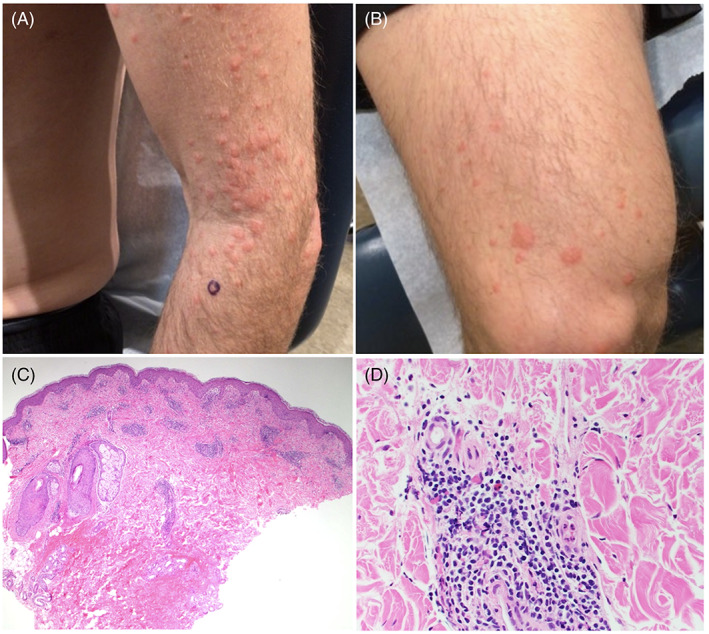
(A) Urticarial papules and plaques on the left upper extremity and (B) right lower extremity 9 days following Moderna COVID‐19 vaccination. Histopathology shows papillary dermal edema with a brisk superficial and deep perivascular lymphoid infiltrate with occasional eosinophils, hematoxylin and eosin, (C) ×40 and (D) ×400
There are multiple reports of local immunologic reactions to the Moderna and Pfizer‐BioNTech COVID‐19 vaccines. 1 , 2 , 3 , 4 , 5 , 6 These delayed injection‐site reactions, nicknamed “COVID‐arm,” are thought to represent a dermal hypersensitivity response. In a retrospective study of 4775 Pfizer‐BioNTech COVID‐19 vaccine recipients, delayed injection‐site reactions were observed in 103 subjects (2.1%). 2 Microscopic analysis of these sites revealed an inflammatory infiltrate consistent with a hypersensitivity reaction. 2 Blumenthal et al. also sampled areas of delayed injection‐site reactions, confirming suspicion of a T‐cell‐mediated hypersensitivity. 3 Delayed local reactions are typically self‐limiting and are the most common cutaneous adverse event in association with the novel COVID‐19 vaccines. 5
Though we now have an understanding of “COVID‐arm,” delayed widespread reactions remain poorly characterized. In an international registry study, McMahon et al. report 34 instances of urticarial eruption that occurred more than 24 h after Moderna or Pfizer‐BioNTech COVID‐19 vaccination. Although histopathologic correlation was not assessed, the authors suggest the development of a delayed host immune response. 5 Additional cases of generalized delayed urticarial eruptions have been reported; 2 however, microscopic analysis was not performed and mechanism remains speculated. In our case, histopathologic analysis supports a delayed dermal hypersensitivity reaction. Interestingly, these delayed urticarial reactions resemble the urticarial eruptions that have been observed in individuals infected with COVID‐19, 7 raising the possibility that an immunologic response is mounted to the viral mRNA protein product or cytokines from stimulated immune cells after vaccination.
Delayed hypersensitivity reactions typically peak within 72–96 h of vaccination. 8 This report highlights a very delayed hypersensitivity response, occurring 9 days after inoculation. Interestingly, this late timing is consistent with other COVID‐19 vaccine‐related hypersensitivities. A case series outlining delayed large local reactions with Pfizer‐BioNTech COVID‐19 vaccination reported a median onset of 8 days following initial dose. 3 Subsequent studies report a median onset of 7 days following initial dose. 4 , 5 , 6 These findings suggest that the late appearance of skin lesions may actually be characteristic in this clinical context.
Widespread reactions may cause concern for both patients and healthcare professionals. The difference between type I IgE‐mediated hypersensitivity reactions, which are associated with anaphylaxis and occur within hours of inoculation, and type IV delayed hypersensitivity reactions, which are driven by a T‐cell mediated inflammatory response, should be emphasized. In our case, the presumed type IV delayed hypersensitivity eruption was not associated with additional symptoms and did not recur after the second vaccination. These findings are consistent with those of McMahon et al., who emphasize that in cases of delayed urticarial reactions, there were no reports of anaphylaxis and only four individuals experienced urticarial eruption after both doses. 5 Given these points, we suggest that delayed generalized hypersensitivity following initial inoculation should not be a contraindication to subsequent COVID‐19 vaccination.
In summary, delayed generalized hypersensitivity reactions may be observed in association with the novel mRNA COVID‐19 vaccines. However, reports remain limited and further characterization of incidence, clinical course, and effective treatment will be crucial in understanding this entity.
CONFLICT OF INTEREST
All authors report no conflict of interest.
AUTHOR CONTRIBUTIONS
Conceptualization: Sandra Leyo DuPont and Caitlyn N. Myrdal. Resources: Keliegh S. Culpepper, and Sandra Leyo DuPont. Supervision: Sandra Leyo DuPont. Writing—original draft: Caitlyn N. Myrdal. Writing—review & editing: Keliegh S. Culpepper, Sandra Leyo DuPont, and Caitlyn N. Myrdal.
ACKNOWLEDGMENTS
We would like to thank the patient for their permission in publication of this work. No funding sources supported this work.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Wei N, Fishman M, Wattenberg D, Gordon M, Lebwohl M. “COVID arm”: a reaction to the Moderna vaccine. JAAD Case Rep. 2021;10:92‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fernandez‐Nieto D, Hammerle J, Fernandez‐Escribano M, et al. Skin manifestations of the BNT162b2 mRNA COVID‐19 vaccine in healthcare workers. “COVID‐arm”: a clinical and histological characterization. J Eur Acad Dermatol Venereol. 2021;35(7):e425‐e427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blumenthal KG, Freeman EE, Saff RR, et al. Delayed large local reactions to mRNA‐1273 vaccine against SARS‐CoV‐2. N Engl J Med. 2021;384(13):1273‐1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ramos CL, Kelso JM. “COVID arm”: very delayed large injection site reactions to mRNA COVID‐19 vaccines. J Allergy Clin Immunol Pract. 2021;9(6):2480‐2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McMahon DE, Amerson E, Rosenbach M, et al. Cutaneous reactions reported after Moderna and Pfizer COVID‐19 vaccination: a registry‐based study of 414 cases. J Am Acad Dermatol. 2021;85(1):46‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Johnston MS, Galan A, Watsky KL, Little AJ. Delayed localized hypersensitivity reactions to the Moderna COVID‐19 vaccine: a case series. JAMA Dermatol. 2021;157(6):716‐720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Abuelgasim E, Dona ACM, Sondh RS, Harky A. Management of urticaria in covid‐19 patients: a systematic review. Dermatol Ther. 2021;34(1):e14328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Caballero M, Quirce S. Delayed hypersensitivity reactions caused by drug excipients: a literature review. J Investig Allergol Clin Immunol. 2020;30(6):400‐408. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


