Abstract
Background
Solid organ transplant recipients (SOTRs) are at increased risk for adverse outcomes with coronavirus disease 19 (COVID‐19). Early data show a lower severe acute respiratory syndrome virus 2 (SARS‐CoV‐2) spike antibody immune response among SOTRs leading to patient concerns about vaccine efficacy. Public health messaging has largely left out immunocompromized individuals leading to a higher risk of vaccine misinformation. The American Society of Transplantation recommends COVID‐19 vaccination for all SOTRs; however, patient concerns and beliefs about vaccination are largely unknown.
Methods
We conducted a transplant‐center‐based, pragmatic pilot trial to encourage COVID‐19 vaccination among 103 unvaccinated SOTRs. We assessed vaccine concerns, barriers to vaccination, answered questions about efficacy, side effects, and clinical recommendations.
Results
A total of 24% (n = 25) of SOTRs reported that they will schedule COVID‐19 vaccination after the study call, 46% reported that they will consider vaccination in the future, and 30% said they will not consider vaccination. Older age and White race were associated with lower willingness to schedule the vaccine, whereas Black race and longer time from transplant were associated with higher willingness. Common vaccine concerns included lack of long‐term data, inconsistent messaging from providers, scheduling inconvenience, and insufficient resources. Follow‐up approximately 1 month after the initial outreach found 52% (n = 13) of liver transplant recipients, and 10% (n = 3) of kidney transplant recipients subsequently received COVID‐19 vaccines for a vaccination rate of 29% among respondents.
Conclusion
Transplant center‐based vaccine outreach efforts can decrease misinformation and increase vaccination uptake; however, vaccine‐related mistrust remains high.
Keywords: health disparities, outreach, pragmatic study, solid organ transplant
Abbreviations
- CI
confidence interval
- COVID‐19
coronavirus disease 19
- IQR
interquartile range
- KT
kidney transplant
- LT
liver transplant
- SARS‐CoV‐2
severe acute respiratory syndrome virus 2
- SOTR
solid organ transplant recipient
1. INTRODUCTION
Solid organ transplant recipients (SOTRs) are at increased risk for adverse outcomes with coronavirus disease 19 (COVID‐19). 1 , 2 Two severe acute respiratory syndrome virus 2 (SARS‐CoV‐2) mRNA vaccines (BNT162b2 [Pfizer‐BioNTech], mRNA‐1273 [Moderna]) and one adenovirus vector‐based vaccine (Ad26.COV2.S [Johnson and Johnson]) have received emergency use authorization from the Food and Drug Administration. Given exclusion of SOTRs from clinical trials, the data about vaccine immunogenicity, clinical effectiveness, and safety are only beginning to emerge. To date, real world studies have shown lower humoral immune responses to the SARS‐CoV‐2 spike protein among SOTRs compared to the non‐transplant population. 3 , 4 Despite this diminished immunogenicity, a recent early report by Malinis et al. showed markedly decreased infection rates among SOTRs who received SARS‐CoV‐2 vaccination. 5
The American Society of Transplantation (AST) recommends COVID‐19 vaccination for all SOTRs whenever feasible, continuation of current immunosuppression regimens, and ongoing adherence to social distancing and protective measures regardless of vaccination status. 6 However, public health messaging has not specifically targeted immunocompromized patients and has been inconsistent among this group, potentially leading to confusion and vaccine hesitancy. Our transplant program, a large tertiary care center in the mid‐Atlantic, has reached out to SOTRs with several email blasts and messages urging vaccination. Additionally, vaccination is being uniformly encouraged by transplant clinicians. However, the effect of these initiatives is unknown. Our previous work showed that immunosuppressed patients had a high level of trust in their physicians with regard to COVID‐19 vaccination. 7 Therefore, we conducted a transplant‐center‐based, pragmatic outreach pilot to encourage SARS‐CoV‐2 vaccination among unvaccinated SOTRs using principles of motivational interviewing. Secondarily, we assessed vaccine beliefs, concerns, and barriers to vaccination among a cohort of liver and KTRs.
2. METHODS
2.1. Data source and patients
On May 13th, 2021, we queried the electronic health record (EHR) of the University of Pennsylvania Health System (UPHS) for all living patients aged 18 or older who had received a liver or kidney transplant at the Penn Transplant Institute. We generated a health‐system‐wide report of patients documented to have received COVID‐19 vaccinations at either UPHS or a non‐UPHS facility. Liver or kidney recipients not documented in the report to have received COVID‐19 vaccine were considered to be potentially unvaccinated, our population of interest. Patients who responded “yes” to a screening question of whether they already received or scheduled a COVID‐19 vaccine were excluded from the analysis, although they were invited to ask questions about the vaccine. A priori, we planned to enroll 50‐liver and 50‐kidney transplant recipients (KTRs) who were potentially unvaccinated. Given disproportionately low rates of COVID‐19 vaccination in the general population of Black and Hispanic individuals nationally compared to White, 8 we planned to enroll 50% of transplant recipients of Black or Hispanic, race/ethnicity.
2.2. Study procedures
This was a pragmatic study designed to be integrated into clinical care with brief and targeted assessments. Potentially unvaccinated patients were called at random by transplant center staff (nurse coordinators or research coordinators) and were asked a screening question, and patients who responded “YES” were excluded from the analysis. If they answered “NO,” the study team used principles of motivational interviewing to assess vaccine concerns, reasons for not yet getting the vaccine, provide further clinical information on antibody response, known side effects, clinical effectiveness, and up‐to‐date AST recommendations. 6 Motivational interviewing in this study followed the three core tasks in the three‐component model: Exploring, Guiding, and Choosing. 9 The research staff first attempted to understand the participants’ thought processes and causes for hesitation, then objective vaccine data were provided when the root causes for hesitation stemmed from limited knowledge or doubts about efficacy of COVID‐19 vaccines in transplant recipients. If willing to schedule their own vaccine, patients were provided scheduling information within the health system or locally using vaccines.gov. Vaccine scheduling assistance during the phone call was also provided. Initial phone calls were made from May 19th to June 13th, 2021. Follow‐up calls to patients who reported willingness to schedule the COVID‐19 vaccines on their own or considering the vaccines were made between July 23rd and July 29th, 2021 to assess whether they received the vaccines. The study was reviewed by the Institutional Review Board at the University of Pennsylvania and deemed to be under the umbrella of quality improvement; therefore, no consent was required.
2.3. Study outcomes
We evaluated the following COVID‐19 vaccination outcomes during the phone call: (1) vaccine scheduled during call, (2) patient to schedule vaccine on their own, (3) will consider vaccination in the future, and (4) not considering vaccination at this time. We created a composite variable for willingness to be vaccinated by combining the outcomes “vaccine was scheduled during call” and “patient to schedule vaccine on their own.” We also collected semi‐structured patient feedback regarding vaccination concerns or barriers.
2.4. Other variables
Using our previous methodology, 7 we obtained information on age, sex, self‐reported race/ethnicity, median zip‐code estimated household income, organ transplanted (liver or kidney; simultaneous liver/kidney was categorized as liver transplant), time from most recent transplant, and insurance.
2.5. Statistical analysis
Descriptive statistics including proportions as well as median, interquartile range were calculated for categorical and continuous variables as appropriate. Bivariate comparisons by age, race/ethnicity, sex, organ (liver vs. kidney), income, insurance type, and time from transplant were conducted with chi‐squared or Fisher's exact tests where appropriate. Analyses were conducted using IBM SPSS 26 (IBM SPSS Statistics for Windows, Version 24.0.; IBM Corp., Armonk, NY, USA).
3. RESULTS
3.1. Study population
Figure 1 shows the study flow diagram of the entire population of SOTRs within the health system. A total of 4780 SOTRs; 1708 liver transplant recipients (LTRs), and 3071 KTRs were reported alive within the health system. The median age was 61 years, interquartile range (IQR) 49–69, 39% were female, 60% were White, 23% were Black, 5% were Hispanic, 5% were Asian, and 7% were of unknown race/ethnicity. Based on patient zip code, the median estimated household income was $20,000–44,999 in 17%, $45,000–139,999 in 70%, and $140,000 or greater in 9% of SOTRs. A total of 48% had medicare, 6% had medicaid, 41% had commercial insurance, and 5% had unknown insurance type.
FIGURE 1.
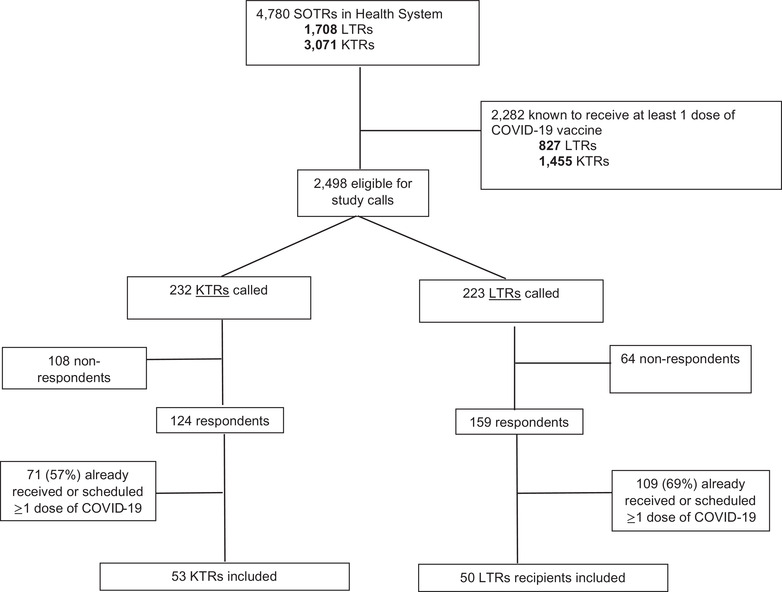
Study flow diagram
Based on the EHR query 2282 (47.7%) were known to have received at least once vaccine dose (97% mRNA, 3% vector‐based adenovirus) leaving a sample of 2498 eligible for study calls. A total of 223 phone calls were conducted with LTRs and 232 with KTRs. At the time of the call, 71 KTRs (57%) and 109 LTRs (67%) among the respondents had already received one dose of vaccine or had been scheduled; these were not enrolled. The final study sample was 103 (53 KTRs and 50 LTRs not yet vaccinated or scheduled); this was slightly larger than 100 as multiple team members were making calls simultaneously.
3.2. Pilot outreach sample
Table 1 shows the characteristics of unvaccinated SOTRs stratified by organ type. The study sample was 53 (52%) White, 31 (30%) Black, 10 (10%) Hispanic, two (2%) Asian, and seven (7%) unknown race/ethnicity. A total of 43 (42%) were medicare‐insured, 44 (45%) had commercial insurance, 13 (13%) had medicaid; 14 (14%) had a median estimated annual household income based on zip code of less than $45,000. LTRs were older (median 59 years; IQR; [36–56]) compared to KTRs (median 42 years [IQR: 40–67]) and with longer time since transplant (LTR: 5.8 years [IQR 1.8–12.1]; KTR: 1.6 years [IQR 0.7–3.4]). There were no other significant differences in patient demographics or insurance type by organ.
TABLE 1.
Characteristics of unvaccinated solid organ transplant recipients stratified by organ
| Variable | Total (n = 103) | Liver (n = 50) | Kidney (n = 53) | p value |
|---|---|---|---|---|
| Age (years), median (IQR) | 51 (37–63) | 59 (40–67) | 42 (36–56) | 0.001 |
| Female | 58 (56.3%) | 26 (52%) | 32 (60%) | 0.556 |
| Race/ethnicity | 0.056 | |||
| White | 53 (52%) | 28 (56%) | 25 (47%) | |
| Black | 31 (30%) | 9 (18%) | 22 (42%) | |
| Hispanic | 10 (10%) | 7 (14%) | 3 (6%) | |
| Asian | 2 (2%) | 1 (2%) | 1 (2%) | |
| Other/Unknown | 7 (7%) | 5 (10%) | 2 (4%) | |
| Time from transplant (years), median (IQR) | 2.3 (0.93–6.7) | 5.8 (1.8–12.1) | 1.6 (0.7–3.4) | <0.001 |
| Insurance | 0.980 | |||
| Medicare | 43 (42%) | 21 (42%) | 22 (42%) | |
| Commercial Insurance | 45 (44%) | 21 (42%) | 24 (45%) | |
| Medicaid | 13 (13%) | 7 (14%) | 6 (11%) | |
| Median estimated household income based on zip code | 0.776 | |||
| $20,000–$44,999 | 14 (14%) | 5 (10%) | 9 (17%) | |
| $45,000–$139,999 | 74 (72%) | 37 (74%) | 37 (70%) | |
| $140,000 or greater | 2 (2%) | 1 (2%) | 1 (2%) | |
| Vaccine outcomes | ||||
| Scheduled with help | 3 (3%) | 3 (6%) | 0 (0%) | 0.111 |
| Willing to schedule | 22 (21%) | 11 (22%) | 11 (21%) | 0.878 |
| Will consider | 47 (46%) | 16 (32%) | 31 (59%) | 0.010 |
| Will not consider | 31 (30%) | 20 (40%) | 11 (21%) | 0.052 |
Abbreviation: IQR, interquartile range.
3.3. COVID‐19 vaccination outcomes
Vaccine outcomes stratified by organ type are shown in Table 1 and in Figure 2A. A total of three (3%) of SOTRs were willing to be scheduled for COVID‐19 vaccination during the calls, 22 (21%) were willing to schedule on their own, 47 (46%) reported that they will consider vaccination in the future, and 31 (30%) reported that they will not consider vaccination at this time. A higher proportion of KTRs were reported they would consider vaccination in the future (59%) compared to LTRs (34%); p = 0.018. There were no statistically significant differences in other vaccine outcomes by organ type. Univariable factors (Figure 3) associated with lower willingness to schedule the vaccine were older age (age ≥ 55 vs. < 55, odds ratio (OR) 0.34, 95% CI 0.13–0.90, p = 0.030) and White race (vs. all others; OR 0.32, 95% CI 0.12–0.82, p = 0.017). Black race (vs. all others; OR 1.6, 95% CI 1.02–2.6, p = 0.042) and years from transplant (per year increase, OR 1.09, 95% CI 1.02–1.17, p = 0.017) were associated with higher willingness to be scheduled during the call or schedule on their own. No significant differences in willingness to schedule the vaccine were noted by Hispanic or Asian race, insurance type, income, or organ type. Detailed data on patient characteristics stratified by vaccine outcomes are in Table S1. The proportion of SOTRs who were Black and Hispanic included in the study did not differ from non‐respondents (Table S2). In exploratory analyses, we assessed whether COVID‐19 vaccination status was associated with previous influenza vaccination in 2019 and 2020 and found no significant associations (Table S3).
FIGURE 2.
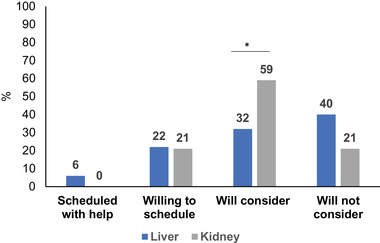
Percentage COVID‐19 vaccination outcomes by organ type (n = 50 LTR, n = 53 KTR)
*p < 0.05 in bivariate comparisons liver versus kidney transplant recipients. Abbreviations: KTR, kidney transplant; LTR, liver transplant
FIGURE 3.
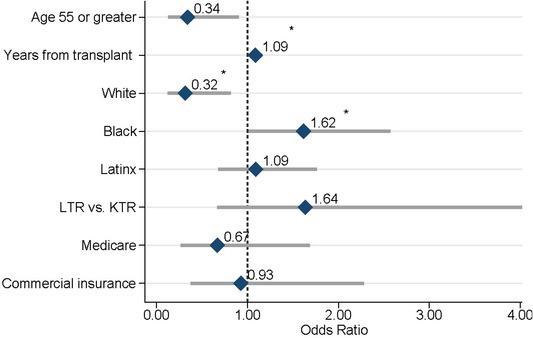
Univariable factors associated with willingness to schedule the COVID‐19 vaccine (n = 50 LTR, n = 53 KTR). *p < 0.05. Abbreviations: KTR‐kidney transplant; LTR‐liver transplant
3.4. Barriers to and concerns about COVID‐19 vaccination
Concern about lack of efficacy and safety data of the COVID‐19 vaccine in patients with transplants was cited with the most frequency in all SOTRs (n = 7 in LTRs; n = 13 in KTRs). Related to this concern were uncertainties about the duration of the immunity in transplant recipients, specifically, and the interactions between the vaccinations and immunosuppressive regimens. LTRs attributed their hesitation to lack of long‐term vaccination data (n = 6, 6%), distrust in the vaccine development process and governmental agencies (n = 4, 4%), recommendations from their healthcare professionals to not be vaccinated (n = 3, 3%), potential side effects (n = 2, 2%), and inconsistent information about the vaccines (n = 2, 2%). KTRs have cited further instructions from their healthcare providers (n = 6, 6%), uncertainties about vaccination from a medical standpoint (n = 4, 4%), and distrust toward the COVID‐19 vaccines (n = 3, 3%) as reasons for not being vaccinated. Among those who expressed willingness to be vaccinated, 30% of the respondents expressed they were “too busy” or had insufficient resources to schedule for vaccination prior to the calls.
3.5. Follow‐up phone calls
About 1 month after the pilot study, subsequent outreach to SOTRs was made to patients who stated they would schedule the vaccine on their own or stated they would consider the COVID‐19 vaccine in the future (Figure 4). Calls were made to 27 LTRs and 42 KTRs; 25 (93%) LTRs and 31 (74%) KTRs responded to the follow‐up attempts. Among the 25 LTRs and 31 KTRs, a total of 13 (52%) LTRs and three (10%) KTRs subsequently received the vaccine for a total documented vaccination rate of 29% among those reached in follow‐up. Specifically, of 13 LTRs and eight KTRs initially indicating willingness to schedule the vaccine on their own, 10 (77%) and one (13%) received the vaccine, respectively. In addition, five of 15 LTRs (33%) and two of 21 (10%) KTRs who expressed willingness to consider the vaccine in the future did receive the COVID‐19 vaccines at follow‐up. KTRs were less likely to have follow‐up vaccinations than LTRs (p < 0.05 in bivariate comparisons). None of the patients indicating “not vaccinated” at follow‐up have upcoming vaccination appointments.
FIGURE 4.
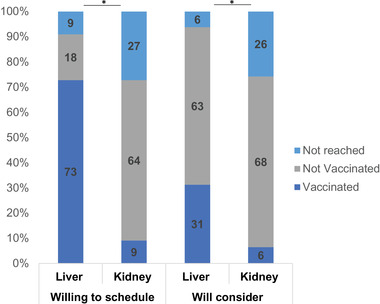
Percentage outcomes by organ type during follow‐up phone calls (n = 27 LTR, n = 42 KTR). *p < 0.05 in bivariate comparisons liver versus kidney transplant recipients. Abbreviations: KTR, kidney transplant; LTR, liver transplant
4. DISCUSSION
We characterized and intervened upon a sample of patients with abdominal organ transplantation who had not yet received the COVID‐19 vaccination through a pragmatic outreach initiative at a large transplant center. First, we were encouraged to find that about one third of SOTRs were willing to schedule the vaccine and appreciated the information provided to help with scheduling. However, nearly half of sample patients reported ongoing hesitancy and one third reported not considering vaccination at this time. Second, we identified older age and White race as factors associated with lower willingness to receive the vaccination, while Black race and a longer time from transplant factors associated with higher willingness to receive the COVID‐19 vaccination. Third, we found that among those not willing to be vaccinated, the most prominent vaccine‐related concerns were lack of data in transplant recipients (about 20%) and distrust toward the vaccine and process associated with its development (7%). Concerns about side effects and inconsistent clinician recommendations were also reported. Being busy, having insufficient resources, or lack of knowledge of how to schedule vaccination were barriers among those willing to be vaccinated, which could be readily overcome with outreach programs. Our follow‐up phone calls showed that about one third of SOTRs that could be reached were subsequently vaccinated; importantly we noted this among those who expressed willingness to schedule on their own as well as those who were still considering vaccination at the time of the initial call. Subsequent vaccination was higher among LTRs; specific reasons for this are unclear and need to be further explored.
Our findings demonstrate patient's desire for additional short‐term and long‐term efficacy and safety data of COVID‐19 vaccines in SOTRs. Patients are keenly interested in better understanding the duration of immunity, the need for regular evaluation of antibody titers, and any changes required in immunosuppression. However, as the evidence base for the effectiveness of vaccination is still building, clear and consistent messaging to transplant recipients and removing vaccination barriers may be effective strategies to increase vaccination among more difficult to reach groups. Guidance for transplant patients under various circumstances, including those with recent transplantation, recent treatment for infections, should be clearly defined and communicated by transplant providers and other clinicians. Additional efforts could include leveraging different forms of communication such as text messaging, behavioral nudges with opt‐out framing, peer‐support, and bundling vaccinations with routine office visits.
Despite more adults ≥18 years old receiving the influenza vaccination in 2019–2020 than those in the year prior, vaccine hesitancy persists 10 and likely stems from the perceived increased incidence of adverse effects. The World Health Organization (WHO) cited negative attitudes toward influenza vaccinations and fewer previous vaccinations as primary reasons for hesitancy. 11 Vaccine hesitancy and lower vaccine uptake among Black, Indigenous, and People of Color community stem from lack of trust in the healthcare system due to personal experiences and history of systemic racism as well as oppressive and unethical research studies. It is also important to note significant barriers such as socioeconomic status (SES) in access to vaccines. Several studies have demonstrated the association of SES, reflected by markers such as insurance status and education levels, with influenza vaccination. 12 , 13
Nationally, the proportion of Black and Hispanic people who have received COVID‐19 vaccination is lower compared to their proportion of the total population in most states; although these gaps have been narrowing as of July 2021. 8 We specifically designed the program to reach out to at least 50% of Black and Hispanic due to these gaps. In our cohort, however, we found Black race was associated with a higher willingness to schedule the vaccine once information was provided. This finding of higher willingness among Black SOTRs may suggest that the lack of vaccination in these groups may be more likely due to systemic barriers such as knowledge and access rather than entrenched vaccine beliefs. Therefore, transplant‐based vaccination outreach efforts may particularly have high yield for certain populations, in geographic areas where vaccination rates may be low, and access and knowledge barriers persist.
We must note certain study limitations. This was an outreach pilot and limited in scope. However, the outreach effort was efficient and could be easily implemented within transplant center workflows. Our study was conducted in a large transplant center in the mid‐Atlantic with a relatively high uptake of COVID‐19 vaccination; findings may not be generalizable to other areas. On the other hand, transplant centers located in geographic regions with low vaccine uptake may experience higher conversions to vaccination with outreach, particularly if public health messaging in those areas remains confusing or inconsistent. Given the cross‐sectional nature of the study, we have not yet been able to verify whether willingness to schedule vaccination led to vaccination. We explored correlations between COVID‐19 vaccine hesitancy and the annual influenza vaccine and noted there was no significant association (Table S3) and showed there was no significant association suggesting the fact that misinformation or unclear messaging may be playing a role in COVID‐19 vaccination hesitancy among those who are otherwise willing to be vaccinated for influenza. Research staff involved in outreach lacked formal training in motivational interviewing. However, staff were provided background reading on principles of motivational interviewing, given the time‐sensitive nature of the initiative to educate SOTRs regarding the COVID vaccines. There are current challenges with accurately capturing data on vaccinations conducted outside the health system resulting in phone calls made to already vaccinated individuals.
In conclusion, our transplant center‐based vaccine outreach effort was pragmatic, can decrease misinformation and increase vaccination uptake; however, vaccine‐related mistrust remains high. Identifying unvaccinated SOTRs and providing clear and consistent guidance and also navigation toward vaccination may increase vaccination among SOTRs.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
AUTHOR CONTRIBUTIONS
Study design: Marina Serper, Emily A. Blumberg, K. Rajender Reddy, and Shivan J. Mehta. Statistical analysis: Marina Serper and Chung‐Heng Liu. Drafting of the manuscript: Marina Serper, Chung‐Heng Liu. Data collection: Chung‐Heng Liu, Alexander E. Burdzy, Stephanie Veasey, Samantha Halpern, Elaine Lander, and Matthew Sigafus. Study concept and idea: Shivan J. Mehta. Revision of manuscript for important intellectual content: Emily A. Blumberg, Alexander E. Burdzy, Stephanie Veasey, Samantha Halpern, Elaine Lander, Matthew Sigafus, Roy D. Bloom, Ty Dunn, Peter Abt, K. Rajender Reddy, and Shivan J. Mehta.
Supporting information
Supporting information
ACKNOWLEDGMENTS
We would like to thank Christopher Snider and Mary Williams for assistance with data acquisition and Renee Westmoreland for assistance with study coordination. Marina Serper and Shivan Mehta receive funding from the National Cancer Institute (grant number: 1K08CA234326) and National Institute of Diabetes and Digestive and Kidney Diseases (grant number: 5K23DK115897), respectively.
Serper M, Liu C‐H, Blumberg EA, et al. A pragmatic outreach pilot to understand and overcome barriers to COVID‐19 vaccination in abdominal organ transplant. Transpl Infect Dis. 2021;23:e13722. 10.1111/tid.13722
REFERENCES
- 1. Webb GJ, Marjot T, Cook JA, et al. Outcomes following SARS‐CoV‐2 infection in liver transplant recipients: an international registry study. Lancet Gastroenterol Hepatol. 2020;5:1008‐1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Caillard S, Chavarot N, Francois H, et al. Is COVID‐19 infection more severe in kidney transplant recipients?. Am J Transplant. 2021;21:1295‐1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2‐dose SARS‐CoV‐2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325:2204‐2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grupper A, Rabinowich L, Schwartz D, et al. Reduced humoral response to mRNA SARS‐CoV‐2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am J Transplant. 2021;21:2719‐2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Malinis M, Cohen E, Azar MM. Effectiveness of SARS‐CoV‐2 vaccination in fully‐vaccinated solid organ transplant recipients. Am J Transplant. 2021;21:2916‐2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Habka D, Mann D, Landes R, Soto‐Gutierrez A. Future economics of liver transplantation: a 20‐Year cost modeling forecast and the prospect of bioengineering autologous liver grafts. PLoS One. 2015;10:e0131764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Serper M, Reddy KR, Bewtra M, Ahmad N, Mehta SJ. COVID‐19 vaccine perceptions among patients with chronic disease in a large gastroenterology and hepatology practice. Am J Gastroenterol. 2021;116:1345‐1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Latest data on COVID‐19 vaccinations by race/ethnicity. Kaiser Family Foundation. https://www.kff.org/coronavirus‐covid‐19/issue‐brief/latest‐data‐on‐covid‐19‐vaccinations‐race‐ethnicity/. Accessed August 4, 2021. [Google Scholar]
- 9. Resnicow K, McMaster F. Motivational interviewing: moving from why to how with autonomy support. Int J Behav Nutr Phys Act. 2012;9:19‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Flu Vaccination Coverage, United States, 2019–20 Influenza Season . Centers for Disease Control and Prevention (CDC). 2020. cdc.gov/flu/fluvaxview/coverage‐1920estimates.htm. [Google Scholar]
- 11. World Health Organization (2016). Barriers of influenza vaccination intention and behavior: a systematic review of influenza vaccine hesitancy 2005–2016. World Health Organization. https://apps.who.int/iris/handle/10665/251671, Accessed September 10th, 2021. [Google Scholar]
- 12. Lucyk K, Simmonds KA, Lorenzetti DL, et al. The association between influenza vaccination and socioeconomic status in high income countries varies by the measure used: a systematic review. BMC Med Res Methodol. 2019;19:153‐153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Suryadevara M, Bonville CA, Rosenbaum PF, Domachowske JB. Influenza vaccine hesitancy in a low‐income community in central New York state. Hum Vaccin Immunother. 2014;10:2098‐2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information


