Abstract
Since the emergence of the new coronavirus disease 19 (COVID‐19) pandemic, there has been a concern for the patients with chronic autoimmune diseases including dermatological conditions over the potential exacerbation of these underlying conditions after infection with severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV2). We performed a systematic review to evaluate presentations, postinfection change in the manifestation, diagnosis, and management of flare‐ups of underlying dermatologic disease in patients with COVID‐19. A total of 17 articles were recovered reporting on flare‐ups of dermatological disease including pemphigus vulgaris, psoriasis, subacute cutaneous lupus erythematosus, acrodermatitis continua of Hallopeau, systemic sclerosis sine scleroderma, and Sézary syndrome (SS). Out of these, psoriasis and alopecia areata were the most common conditions. However, most cases of psoriasis could have been attributed to either antimalarial agents that were initially used for the treatment of COVID‐19 or discontinuation of treatment following SARS‐CoV2 infection.
Keywords: COVID‐19, dermatology, exacerbation, flare‐up, pemphigus, psoriasis, SARS‐Cov2, worsening
1. INTRODUCTION
Aside from the fact that coronavirus diseases 2019 (COVID‐19) can present with cutaneous manifestations, there is a growing concern about the potential exacerbation of preexisting dermatological conditions following COVID‐19. Though several case reports have been published on this issue, it remains one of the least studied aspects of dermatology‐COVID‐19 intersection.
Viral infections are well‐known triggers for induction and exacerbation of autoimmune conditions. Several mechanisms have been proposed for this phenomenon including antigen mimicry, epitope spreading, cytokine imbalance, and the overwhelming of clearance mechanisms by amplified tissue destruction that increases the self‐antigens availability. Infection with the SARS‐CoV2 virus is associated with an exaggerated immune response that could derail the vulnerable immune balance in autoimmune diseases.
Though other factors related to the pandemic such as social isolation, loss‐of‐income, and non‐adherence to treatment could greatly influence disease severity, in this review, we only focus on the changes directly related to SARS‐CoV2 infection.
2. METHODOLOGY
We searched the PubMed, Scopus, and Google Scholar database with various combinations of three sets of keywords: COVID, SARS‐COV‐2, and coronavirus; flare‐up, exacerbation, magnification, worsening, and aggravation; and cutaneous, dermatologic, and the Mesh term for several specific dermatological diseases (Figure 1). The reference lists of selected studies were also explored. Databases were searched up to July 13, 2021. A final search was also done before paper submission.
FIGURE 1.
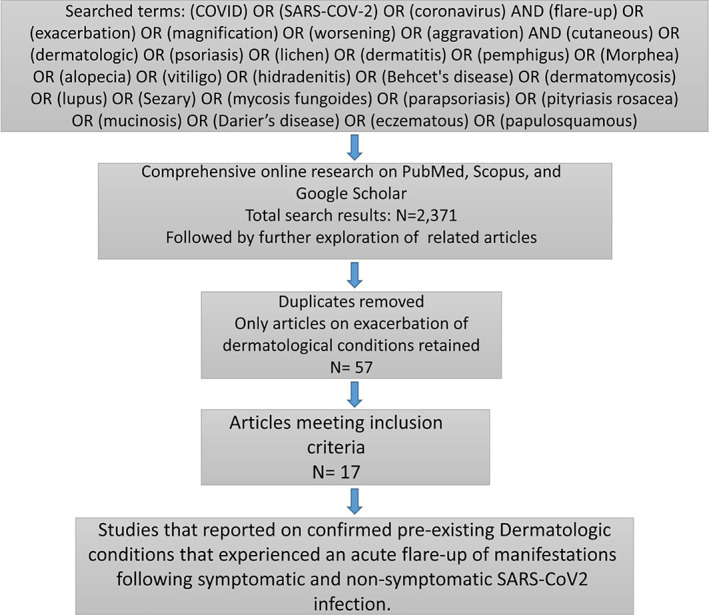
Flow chart of literature search
3. RESULTS
A total of 14 case reports were found. Nine patients had a history of psoriasis, and the others were diagnosed with pemphigus vulgaris, acrodermatitis continua of Hallopeau (ACH), systemic sclerosis sine scleroderma, SS, and cutaneous lupus erythematosus (CLE; Table 1). Of the nine patients with psoriasis, the flare‐ups in five cases could be attributed to either hydroxychloroquine or systemic corticosteroids that were initially used in the treatment of COVID‐19 and are well‐known causes of drug‐induced psoriasis. 1 , 2 , 3 , 6 , 9
TABLE 1.
A summary of cases reporting exacerbation of dermatological conditions following COVID‐19
| Age/gender | Pre‐existing skin condition | COVID‐19 test | Post‐infectious change in manifestations | Treatment | Reference |
|---|---|---|---|---|---|
| 47‐Year‐old woman | Pustular psoriasis | PCR | Pustular lesions on the trunk and extremities |
Hydroxychloroquine for COVID‐19 Treatment of exacerbation not mentioned |
Shakoei et al. 1 |
| 60‐Year‐old male | Psoriasis in childhood | PCR, Chest CT | Widespread erythematous patches with multiple pustules |
Hydroxychloroquine and prednisolone for COVID‐19 Oral acitretin 25 mg/day for the flare‐up |
Shahidi Dadras et al. 2 |
| 76‐Year‐old male patient | Psoriasis | Chest CT | Progression of erythematosquamous plaques on the scalp, trunk, limbs, and the T‐zone of the face |
Hydroxychloroquine for COVID‐19 Treatment of exacerbation not mentioned |
Sigha and Kouotou 3 |
| 38‐Year‐old man | Chronic plaque psoriasis with a single active psoriatic plaque on the right ankle | PCR | Multiple drops‐like well‐circumscribed salmon pink erythematous papules with fine‐scales consistent with guttate psoriasis |
No treatment for COVID‐19 Topical betamethasone 0.025% cream two times per day for the flare‐up |
Gananandan et al. 4 |
| 69‐Year‐old man | Psoriasis and psoriatic arthritis | PCR | Rapid onset of a mild pruritic erythematoedematous morbilliform rash spreading from arms to the trunk and lower limbs |
Discontinuation of Secukinumab after COVID‐19 Secukinumab restarted for the flare‐up |
Carugno et al. 5 |
| 48‐Year‐old female | Psoriasis | PCR, Chest CT | Active psoriatic lesions on the scalp, trunk, and extremities |
Hydroxychloroquine, azithromycin, oseltamivir, and inhaled ipratropium and budesonide for COVID‐19 Treatment of exacerbation not mentioned |
Ozaras et al. 6 |
| 44‐Year‐old male | Psoriasis | NA | Widespread psoriasis plaques | Treatment of COVID‐19 and flare‐up not mentioned | Al Abadie 7 |
| 45‐Year‐old male | Psoriasis | PCR | Severe erythroderma and ectropion, severe onycholysis |
Discontinuation of cyclosporine after COVID‐19 Acitretin 35 mg daily which was replaced by cyclosporine 100 mg twice daily and prednisolone 10 mg daily with the onset of acute knee arthritis |
Ghalamkarpour et al. 8 |
| 73‐Year‐old male | Psoriasis | PCR, Chest CT | Diffuse erythematous scaly plaques progressing to erythroderma |
Hydroxychloroquine for COVID‐19 was discontinued Cyclosporine 100 mg daily for psoriasis |
Nasiri et al. 9 |
| A 72‐year‐old woman | Acrodermatitis Continua of Hallopeau | PCR |
Generalized pustular eruption overlying erythematous plaques and patches that had appeared 2 weeks earlier Almost immediately after the first symptoms of COVID‐19 had appeared, pustular lesions which were confined to the digits and toes, started to eruptively spread to the trunk and extremities. Onycholysis of the fingers and toes was also present |
Acitretin 50 mg daily combined with intravenous hydrocortisone for 7 days followed by 5 mg/kg intravenous infliximab | Samotij et al. 10 |
| 50‐Year‐old female | Subacute cutaneous lupus erythematosus | PCR | Enlargement of pre‐existing plaques on the trunk and emergence of new lesions | NA | Abadías‐Granado et al. 11 |
| 40‐Year‐old female | Pemphigus vulgaris | PCR | Rapid aggravation of symptoms with involvement of nasal mucosa and appearance of multiple bullae on her skin | IVIG 25 g/day | Ghalamkarpour and Pourani 12 |
| 73‐Year‐old woman | Systemic sclerosis sine scleroderma | PCR, Chest CT | Raynaud's phenomenon | NA | Mariano et al. 13 |
| 56‐Year‐old woman | Sézary syndrome | PCR | Relapse of previously resolved pruritic erythroderma, and large bilateral inguinal lymph nodes | Failed Chemotherapy with gemcitabine | Gonzaga et al. 14 |
Abbreviation: NA, not available.
The exacerbation of psoriasis in two cases could have resulted from the discontinuation of treatments (secukinumab and cyclosporine). 5 , 8 In one case the COVID‐19 treatment was not mentioned. 7 Thus the exacerbation of psoriasis in only one case could be confidently linked to COVID‐19. 4
Various treatments were proposed to combat these exacerbations. For patients suffering from an exacerbation of psoriasis, these strategies included discontinuation of hydroxychloroquine and tapering of steroids accompanied by the initiation of topical steroids, oral acitretin, and cyclosporine and readministration of secukinumab (Table 1). The patient with pemphigus vulgaris was successfully treated with intravenous immunoglobulin (IVIG). 12 The patient with SS began a failed chemotherapy with gemcitabine. 14 The patient with ACH was initially started on oral acitretin and intravenous hydrocortisone which yielded unsatisfactory results. Intravenous infliximab was added to the treatment plan leading to improved lesions. 10
Aside from these case reports, several studies with larger sample sizes were able to assess the effect of COVID‐19 infection on the disease course. One questionnaire‐based study in the Netherlands assessed 1132 adult patients with atopic dermatitis and psoriasis and found 26% of the patients experienced worsening of their condition during symptomatic infection with SARS‐CoV2. However, they did not mention whether these patients received antimalarials and steroids or not. 15 Another study assessed 21 adults with atopic dermatitis who had also been infected with COVID‐19 and found out that 43% of these patients experienced disease exacerbation that did not require systemic intervention. The patients with severe atopic dermatitis who were receiving immunosuppressive therapy had milder exacerbations. 16
In a questionnaire‐based survey in Italy, the investigators contacted 475 patients previously diagnosed with alopecia areata (AA) to follow up on their conditions after COVID‐19. 42.5% of their participants who had been infected with SARS‐CoV2 experienced a relapse that occurred about 2 months after COVID‐19 (median of 2.14 months). Since only 12.5% of participants reported AA relapse in the absence of COVID‐19, the association between AA exacerbation and COVID‐19 seems to be significant.
4. DISCUSSION
Keratinocytes express the SARS‐CoV2 entry receptor, angiotensin‐converting enzyme 2 (ACE2) making them vulnerable to infection with the virus. 17 While possible through local infection of skin, the exacerbation of existing dermatologic pathologies is thought to be caused by systemic reactions of the immune system to infection elsewhere. After interiorization of viral particles, various agents of innate immunity stand to respond to the infection. 17 Dendritic cells respond to viral infection via type I interferon (IFN) secretion. Inadequacy in IFN production results in the activation of NF‐κB and NLRP3 inflammasome which lead to the uncontrolled expression of IL‐12, tumor necrosis factor‐alpha (TNFα), IFN‐γ, IL‐1β, IL‐6, IL‐8, IL‐17, IL‐18, IL‐33, and transforming growth factor‐beta (TGF‐β). 17 This creates a milieu of cytokines that could potentially trigger the exacerbation of many types of immune‐mediated diseases from both sides of the spectrum, humoral, and cellular.
Alopecia areata as one of the most commonly reported dermatological conditions to exacerbate after COVID‐19, results from a breach in the immune privilege of the hair follicles (HFs) in the anagen phase. 18
Viral infections besides SARS‐CoV2 have been shown to either induce or exacerbate AA. 19 A potential explanation for virus‐induced AA is antigen mimicry as demonstrated by a study investigating the association between AA and vaccination against hepatitis B. 19 The hepatitis B vaccine proteins share sequence homology to HF structural proteins such as SLC45A2 allowing the vaccine sensitized T‐cells to be able to recognize and attack this antigenic structure on HFs. Some vaccine peptides have similar structures to the NK cell inhibitory receptors, KIR3DL2 and KIR3DL1. The T‐cell response against these receptors could lead to the downregulation of KIR+ NK cells. As these receptors play a major role in preserving the immune privilege of HFs, their downregulation could facilitate an immune attack and induce AA. Perhaps a similar mechanism of antigen mimicry could happen in the setting of COVID‐19 infection.
The surge in IFN production following viral infection could also induce AA as IFN promotes the expression of MHC‐I molecules on HFs and thus facilitates the attack of T‐cells on HFs. 18
Though in most cases, the exacerbation of psoriasis could be attributed to the drugs used for the treatment of COVID‐19, viral infections have previously been documented as a possible trigger of a psoriasis flare‐up. 20 A French study was able to document 31 flare‐ups of psoriasis in a total of 25 patients following infection with influenza B, parainfluenza, rhinovirus, and subtypes of coronavirus. 20 They suggested that the activation of toll‐like receptors (especially TLR3) by viral particles could initiate an inflammatory cascade with overproduction of several cytokines and chemokines such as IL‐36 and CXCL8 that have been linked to the pathogenesis of psoriasis. The production of IL‐36 was reported to be enhanced after stimulation with polyinosinic–polycytidylic acid, a TLR3 agonist that mimics RNA of respiratory viruses. 20
5. CONCLUSION
There is a substantial paucity of literature on COVID‐19 and flare‐up of dermatological disease. We hope that the present review would inspire further larger epidemiological studies to assess the impacts of SARS‐CoV2 infection on the course of chronic dermatological diseases. This data could greatly benefit the risk assessment and management of patients with proper prophylactic and treatment strategies.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare. The article has never been presented anywhere else.
AUTHOR CONTRIBUTIONS
All authors contributed to the study's conception and design. Data collection was performed by Khashayar Aram and Fateme Rajabi. The first draft of the manuscript was written by Khashayar Aram and Fateme Rajabi and revised by Mohamad Goldust and Anant Patil. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
ETHICS STATEMENT
Not applicable.
INSTITUTIONAL BOARD APPROVAL
As this review does not directly involve human or animal subjects it did not require institutional board approval.
Aram K, Patil A, Goldust M, Rajabi F. COVID‐19 and exacerbation of dermatological diseases: A review of the available literature. Dermatologic Therapy. 2021;34(6):e15113. doi: 10.1111/dth.15113
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Shakoei S, Ghanadan A, Hamzelou S. Pustular psoriasis exacerbated by COVID‐19 in a patient with the history of psoriasis. Dermatol Ther. 2020;33(6):e14462. doi: 10.1111/dth.14462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shahidi Dadras M, Diab R, Ahadi M, Abdollahimajd F. Generalized pustular psoriasis following COVID‐19. Dermatol Ther. 2021;34(1): e14595. doi: 10.1111/dth.14595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sigha OB, Kouotou EA. COVID‐19 infection revealed by a flare‐up of psoriasis in an elderly Cameroonian: about a case; 2021.
- 4. Gananandan K, Sacks B, Ewing I. Guttate psoriasis secondary to COVID‐19. BMJ Case Rep. 2020;13(8):e237367. doi: 10.1136/bcr-2020-237367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carugno A, Gambini DM, Raponi F, et al. Coronavirus disease 2019 (COVID‐19) rash in a psoriatic patient treated with Secukinumab: is there a role for interleukin 17? Dermatol Ther. 2020;33(6):e14011. doi: 10.1111/dth.14011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ozaras R, Berk A, Ucar DH, Duman H, Kaya F, Mutlu H. Covid‐19 and exacerbation of psoriasis. Dermatol Ther. 2020;33(4):e13632. doi: 10.1111/dth.13632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Al Abadie MS, Al Abadie Mohammed Sami. COVID‐19 Infection Cause Moderate‐Severe Psoriasis Flare Up. European Journal of Medical and Health Sciences. 2020;2(3). doi: 10.24018/ejmed.2020.2.3.331 [DOI]
- 8. Ghalamkarpour F, Pourani MR, Abdollahimajd F, Zargari O. A case of severe psoriatic erythroderma with COVID‐19. J Dermatolog Treat. 2020;1‐3. [DOI] [PubMed] [Google Scholar]
- 9. Nasiri S, Araghi F, Tabary M, Gheisari M, Mahboubi‐Fooladi Z, Dadkhahfar S. A challenging case of psoriasis flare‐up after COVID‐19 infection. J Dermatolog Treat. 2020;31(5):448‐449. [DOI] [PubMed] [Google Scholar]
- 10. Samotij D, Gawron E, Szczęch J, Ostańska E, Reich A. Acrodermatitis continua of hallopeau evolving into generalized pustular psoriasis following covid‐19: a case report of a successful treatment with infliximab in combination with acitretin. Biol Targets Ther. 2021;15:107‐113. doi: 10.2147/BTT.S302164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Abadías‐Granado I, Navarro‐Bielsa A, Morales‐Callaghan AM, et al. COVID‐19‐associated cutaneous manifestations: does human herpesvirus 6 play an aetiological role? Br J Dermatol. 2021;184(6):1187‐1190. doi: 10.1111/bjd.19806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ghalamkarpour F, Pourani MR. Aggressive course of pemphigus vulgaris following COVID‐19 infection. Dermatol Ther. 2020;33(6): e14398. doi: 10.1111/dth.14398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mariano RZ, Del Rio APT, Reis F. COVID‐19 overlapping with systemic sclerosis. Rev Soc Bras Med Trop. 2020;53:1‐6. doi: 10.1590/0037-8682-0450-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gonzaga Y, Santos MBF, Silva MM, Nucci M. COVID‐19 infection in patients with Sézary syndrome: report of two cases. Dermatol Ther. 2020;33(6):e14042. doi: 10.1111/dth.14042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. de Wijs LEM, Joustra MM, Olydam JI, Nijsten T, Hijnen DJ. COVID‐19 in patients with cutaneous immune‐mediated diseases in The Netherlands: real‐world observational data. J Eur Acad Dermatology Venereol. 2021;35(3):e173‐e176. doi: 10.1111/jdv.17025 [DOI] [PubMed] [Google Scholar]
- 16. Miodońska M, Bogacz A, Mróz M, Mućka S, Bożek A. The effect of sars‐cov‐2 virus infection on the course of atopic dermatitis in patients. Med. 2021;57(6):521. doi: 10.3390/medicina57060521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Freeman TL, Swartz TH. Targeting the NLRP3 inflammasome in severe COVID‐19. Front Immunol. 2020;11:1518. doi: 10.3389/fimmu.2020.01518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rajabi F, Drake LA, Senna MM, Rezaei N. Alopecia areata: a review of disease pathogenesis. Br J Dermatol. 2018;179(5):1033‐1048. doi: 10.1111/bjd.16808 [DOI] [PubMed] [Google Scholar]
- 19. Richardson CT, Hayden MS, Gilmore ES, Poligone B. Evaluation of the relationship between alopecia areata and viral antigen exposure. Am J Clin Dermatol. 2018;19(1):119‐126. [DOI] [PubMed] [Google Scholar]
- 20. Sbidian E, Madrange M, Viguier M, et al. Respiratory virus infection triggers acute psoriasis flares across different clinical subtypes and genetic backgrounds. Br J Dermatol. 2019;181(6):1304‐1306. doi: 10.1111/bjd.18203 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


