Abstract
Tachyzoite forms of Toxoplasma gondii were subjected to a sequential organic solvent extraction, which allows fractionation of membrane components according to their degrees of hydrophobicity, yielding three fractions named F1 (most hydrophobic) to F3 (least hydrophobic). Fractions F2 (80.85% specificity and 86.95% sensitivity) and F3 (89.36% specificity and 93.61% sensitivity) gave the best results, being preferentially recognized by immunoglobulin M (IgM) and IgG in sera from patients with acute and chronic toxoplasmosis, respectively. Improved scores of specificity (100%) and sensitivity (100%) were achieved when a secondary antibody against human IgG1 instead of total IgG was employed to measure the reactivity of IgG antibodies with the F3 fraction. To purify tachyzoite antigens recognized by human IgM or IgG antibodies, the F2 or F3 fraction was loaded onto an octyl-Sepharose column and eluted with a propan-1-ol gradient. The main antigen(s) recognized by IgM or IgG eluted in a single peak from the octyl-Sepharose resin loaded with either F2 (30 to 50% propan-1-ol) or F3 (15 to 35% propan-1-ol), respectively. These semipurified fractions gave improved scores when used to detect T. gondii-specific IgM (95.7% specificity and 81.8% sensitivity) or IgG (100% specificity and 93.75% sensitivity) in an enzyme-linked immunosorbent assay. Further biochemical and immunological analyses of antigens partially purified from F2 and F3 indicate that glycoinositolphospholipids are preferentially recognized by IgM, whereas proteins of approximately 30 to 40 kDa are recognized by IgG, elicited during T. gondii infection in humans.
Toxoplasma gondii is widespread throughout the world, with no geographic or zoological boundaries, so that human populations are constantly exposed to and infected with this parasite (7). It is estimated that toxoplasmosis exists in a chronic, asymptomatic form in 500 million to 1 billion of the world's human population (17). Whereas infection with T. gondii is usually innocuous or asymptomatic in most individuals, it causes serious morbidity and mortality in fetuses of primarily infected pregnant women (19) and in immunocompromised individuals (4). The simultaneous infection with T. gondii and human immunodeficiency virus type 1 is of increasing concern, since it is reported that this parasite is the major infectious cause of encephalitis in AIDS patients, being among the top 10 opportunistic infections which are more often encountered as AIDS-defining illness (22).
Therefore, there are at least two major situations in which the diagnosis of T. gondii infection, leading to therapeutic intervention, is of medical importance. The first one is the detection of T. gondii-specific immunoglobulin M (IgM) in sera from pregnant women, who, if not treated with specific chemotherapy, may have serious fetal problems, including malformation or abortion (19). Second, different studies indicate that up to 15% of AIDS patients who have positive serological tests for T. gondii may develop toxoplasmic encephalitis. Toxoplasmic encephalitis is often difficult to diagnose and has to be treated immediately after the initial symptoms to avoid fatality (2, 16).
Different studies have defined the major targets for T. gondii-specific IgM or IgG antibodies found in sera from acutely or chronically infected individuals (6, 19). However, most serological tests used in the laboratory employ parasite extracts rather than purified or recombinant antigens. This is especially true in the case of tests to detect T. gondii-specific IgM that target complex glycolipids that are difficult to synthesize in the laboratory. In addition, false-positive and false-negative results, using commercial kits for parasite-specific IgM detection, are often reported (15). Even in tests for detection of tachyzoite-specific IgG, the vast majority of which recognize parasite proteins, the use of recombinant protein or synthetic peptides has been problematic (23), also yielding dubious results.
In the present study, we used a methodology that employs a sequential organic solvent extraction, which allows the fractionation of membrane components according to their degrees of hydrophobicity (1, 10). This methodology yielded two distinct fractions, named F2 and F3, which were preferentially recognized by IgM and IgG present in sera from patients with acute and chronic toxoplasmosis, respectively. Because the major targets for either IgM or IgG have been defined as a specific subset of glycoinositolphospholipids (GIPLs) (21, 24) or glycosylphosphatidylinositol (GPI)-anchored proteins (14, 25), respectively, we used hydrophobic interaction chromatography to further purify the parasite molecules which are major targets for human antibodies. The antigens recognized by IgM or IgG were eluted as a single peak from octyl-Sepharose resin loaded with either F2 (30 to 50% propan-1-ol) or F3 (15 to 35% propan-1-ol) and highly enriched. The fractions obtained from octyl-Sepharose loaded with F2 and F3, when used in an enzyme-linked immunosorbent assay (ELISA), resulted in an assay of much higher specificity and approximately the same sensitivity to detect T. gondii-specific IgM and IgG, respectively.
MATERIALS AND METHODS
Population studied.
Coded serum samples were obtained from 23 patients with acute T. gondii infection (IgG positive and IgM positive) and 47 patients with chronic T. gondii infection (IgG positive and IgM negative). Sera from 47 uninfected individuals (IgG and IgM negative) were used as controls. The patients with acute infection were further classified as high (n = 5; average serum titer, 1:960) and low (n = 18; average serum titer, 1:126) IgM producers, as indicative of early and late acute toxoplasmosis, respectively. Toxoplasma-specific IgM serological testing was performed with a commercially available immunofluorescence assay (IFA) kit with fixed tachyzoites (Imunotoxo; bioMérieux, Marcy l'Etoile, France), using the anti-human IgM (whole-molecule) fluorescein isothiocyanate conjugate (Fluoline H; bioMérieux) (3). Toxoplasma-specific IgG serological testing was performed using a ELISA kit employing a tachyzoite extract (Toxonostika IgG; Organon, Boxtel, The Netherlands) (9). All of the patients with chronic toxoplasmosis were asymptomatic. In contrast, patients with acute infection presented variable clinical symptoms, ranging from no symptoms to fever, headache, lymphoadenopathy, and/or pneumonia.
Parasites.
Tachyzoites of RH strains of T. gondii were maintained by in vitro passage in human foreskin fibroblasts at 37°C (12). Tachyzoites were harvested at 4 to 5 days postinfection, centrifuged at 70 × g for 10 min in order to remove cell debris, and then pelleted at 590 × g for 10 min. The parasite pellet was washed twice by resuspension in cold phosphate-buffered saline (PBS) and centrifugation at 590 × g for 10 min. The final pellet was stored at −70°C until used for sequential organic solvent extraction.
Sequential organic solvent extraction of tachyzoite membrane components.
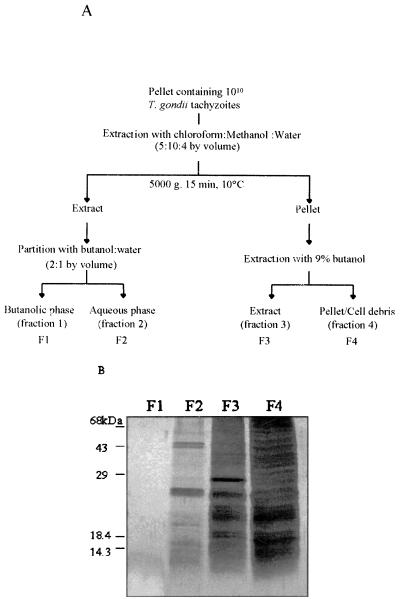
The tachyzoite pellet frozen at −70°C was lyophilized and subjected to extraction with chloroform-methanol-water (5/10/4, vol/vol) (Fig. 1A) (1, 10). Ten volumes of chloroform-methanol-water was added to the parasite pellet and sonicated for 15 min, followed by centrifugation at 5,000 × g for 15 min at 10°C. The resulting pellet was subjected to same protocol twice more, and the supernatants were pooled, dried in a speed vacuum (Savant Instruments Inc., Farmingdale, N.Y.), and subjected to a butan-1-ol–water (1/1, vol/vol) partition. The butanolic and aqueous phases generated by the butan-1-ol–water partition were named F1 and F2, respectively. The pellet obtained after the chloroform-methanol-water extractions was dried in a speed vacuum and extracted three times with 10 volumes of 9% butan-1-ol for 3 h with shaking at room temperature, followed by centrifugation at 5,000 × g for 15 min at 10°C. The 9% butan-1-ol supernatants were pooled and named F3. The resulting pellet (cell debris) and fractions F1 to F3 were all dried and resuspended in water, and their protein concentrations were determined by the Bradford method (Bio-Rad Laboratories, Richmond, Calif.) using bovine serum albumin as a standard. Cell debris and F1 to F3 samples were then stored at −70°C until used in the ELISA and Western blotting assay.
FIG. 1.
(A) Strategy used for fractionating components from tachyzoite parasites based on their hydrophobicity-hydrophilicity properties. (B) Ten micrograms of F1, F2, F3, or cell debris (F4) was run on an SDS–15% polyacrylamide gel and silver stained. The numbers on the left indicate the molecular masses of proteins used as standard markers.
Octyl-Sepharose chromatography.
Frozen F2 and F3 fractions were resuspended in 100 mM ammonium acetate containing 5% propan-1-ol and subjected to hydrophobic interaction chromatography using octyl-Sepharose resin (Pharmacia Biotech, Uppsala, Sweden) elution with a propan-1-ol (5 to 60%) gradient. Two-milliliter fractions were collected and assayed for myo-inositol content, protein concentration, and the ability to bind to IgM and IgG present in sera from patients with acute and chronic toxoplasmosis, respectively.
myo-Inositol measurements.
Briefly, samples were preincubated with 40 pmol of deuterated myo-inositol, dried in a SpeedVac centrifuge (Savant Instruments), resuspended in 50 μl of deionized water, and transferred to glass capillary tubes. Samples were dried again, and 50 μl of 6 N HCl was added. The capillary tubes were then sealed under vacuum and subjected to hydrolysis at 110°C for 16 to 18 h. Samples were dried under vacuum, and the residual HCl was removed by evaporation after addition of 50 μl of water. For dehydration, 50 μl of methanol was added to each sample and dried under vacuum. The samples were then incubated with fresh trimethylsilyl (TMS) reagent for 15 to 30 min at room temperature. TMS derivatives were analyzed (1 μl per sample) in an SE-54 (0.25 mm by 30 m; Alltech) capillary column using a temperature gradient of 140°C for 1 min, 140 to 250°C for 7.3 min (15°C/min), and 250°C for 5 min. Selective ion monitoring was carried out for TMS derivatives of d6-myo-inositol at 307 and 321 m/z and of myo-inositol at 305 and 318 m/z (5).
ELISA.
Immulon-2 plates (Dynatech Laboratories, McLean, Va.) were coated with 100 μl of either F1, F2, or F3 at a protein concentration of 10 μg/ml in 0.05 M carbonate-bicarbonate buffer, pH 9.6. Alternatively, 0.5 pmol of F2-derived eluate F, or 1.0 pmol of F3-derived eluates E and F, per well in 50 μl of 0.05 M carbonate-bicarbonate buffer (pH 9.6) was used to coat the Immulon-2 plates. Plates were incubated overnight at 4°C, blocked with 2% casein (Calbiochem, La Jolla, Calif.) for 2 h at 37°C, and then washed four times with 0.15 M PBS (pH 7.2)–0.05% Tween 20 (Sigma, St. Louis, Mo.) (PBS-T). One-hundred-microliter portions of sera at dilutions of 1:50 to 1:200 in PBS-T–1% bovine serum albumin (Biobras, Montes Claros, Brazil) were added and incubated for 1 h at 37°C. Plates were then incubated with biotinylated conjugates of anti-human IgG, IgG1, IgG2, IgG3, IgG4, or IgM (Sigma) at 1:20,000 in PBS-T for additional 1 h at 37°C and washed with PBS-T. Streptavidin-peroxidase conjugate (Sigma) at a 1:1,000 dilution was added and incubated for 30 min at 37°C. The plates were then washed with PBS-T and developed using ABTS [2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid)] as a substrate. The reaction was terminated by the addition of 50 μl of 1% sodium dodecyl sulfate (SDS) solution, and results were read at 405 nm.
SDS-PAGE.
Different tachyzoite antigen preparations were resolved by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) on 10 or 15% gels under reducing conditions as previously described (8). Gels were silver stained after fixation for 30 min in 50% methanol–10% acetic acid and for 30 min in 5% methanol–7% acetic acid, followed by 30 min of incubation with 20 mM dithiothreitol and 0.1% AgNO3. The gels were developed in 3% Na2CO3–0.01% formaldehyde.
Immunoblotting.
Proteins separated by SDS-PAGE were transferred to nitrocellulose paper (27), using the Mini-Protean system (Bio-Rad). Alternatively, 5 μl of parasite antigen suspension in PBS was added to a nitrocellulose paper, which was left drying for 10 min. Blots or dot blots were first soaked in 2% casein–PBS-T for 1 h at room temperature to block free binding sites. The blots were then incubated overnight at 4°C with a pool of sera, at a 1:200 dilution, from patients with either acute or chronic toxoplasmosis or from individuals who did not have any evidence of T. gondii infection. The nitrocellulose sheets were then incubated with biotin-conjugated goat anti-human IgM or IgG antibody (Sigma) for 1 h at room temperature and then reincubated for 30 min at room temperature with streptavidin-peroxidase conjugate (Sigma) at a 1:1,000 dilution. After each incubation, the membranes were washed three times with PBS-T. Finally, after being rinsed with 0.05 M carbonate-bicarbonate buffer (pH 9.6), blots were incubated with ECL reagent (Amersham, Little Chalfont, England) and exposed to X-ray films.
ES-MS analysis.
Electrospray-mass spectrometry (ES-MS) analysis was carried out on a Quattro apparatus (Micromass, Manchester, United Kingdom) in negative mode. Samples diluted in 50% propan-1-ol–0.2% formic acid were introduced into the ES source at 5 μl/min using a Harvard syringe pump. The capillary voltage was kept at 2.3 kV, the cone voltage was kept at 40 V, and the cone/skimmer offset was kept at 5 V.
Statistical analysis.
The antigen concentration and serum dilution were defined by analysis of variance with 10 samples from individuals with either acute or chronic toxoplasmosis. The positive-negative borderline was calculated by Z distribution. For IgM assays, 23 acute, 24 chronic, and 23 unreactive samples were used; for IgG assays, 47 chronic and 47 negative samples were used. The sensitivity and specificity of our assays with each antigen were calculated by the chi-square test. Analyses were performed using the Statistic software (version 4.5).
RESULTS
Preparation of tachyzoite extracts according to their hydrophobic-hydrophilic properties.
As shown in Fig. 1, we used a strategy that yields three different fractions (F1, F2, and F3) based on their degrees of hydrophobicity (Fig. 1A), where F1 and F3 were the most and least hydrophobic fractions, respectively. F4 was considered cell debris; it was not solubilized by the solvent system used. The results presented in Fig. 1B show that, except for F1, the fractions generated by this protocol still presented a complex protein profile when analyzed by SDS-PAGE. When analyzed for their ability to be recognized by human sera, F2 or F3 was preferentially recognized by IgM or IgG antibodies present in sera from acutely or chronically infected individuals, respectively (see below).
Identification of tachyzoite antigens that are preferentially recognized by IgM antibodies from sera of patients acutely infected with T. gondii.
The results presented in Table 1 show the ability of the F2 fraction (80.85 specificity and 86.95% sensitivity) to detect specific IgM antibodies present in sera of patients with acute toxoplasmosis. However, a high number of false-positive results were observed in the experiments using the F2 fraction, as indicated by the relatively low (80.85%) specificity of the assay.
TABLE 1.
Specificity and sensitivity of the ELISA using F2 tachyzoite extract to identify patients with acute toxoplasmosis and high levels of anti-T. gondii IgMa
| Patient group (n) | OD405
|
|
|---|---|---|
| Median | SD | |
| Uninfected (24) | 0.129 | 0.06 |
| Acutely infected (23) | 0.503 | 0.17 |
| Chronically infected (23) | 0.214 | 0.09 |
The ELISA was developed as described in Materials and Methods. The optical densities at 405 nm (OD405) were obtained from individual sera from patients from the same group. The sensitivity (86.95%) and specificity (80.85%) were calculated using the chi-square test to compare the ELISA using the F2 fraction and anti-IgM conjugate with IFA (P < 0.001).
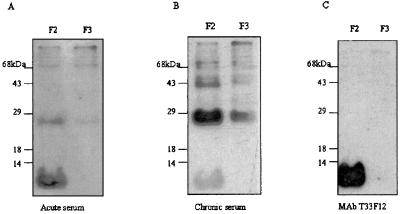
The pattern of antigen complexity of this fraction was further analyzed by immunoblotting analysis, using sera from patients with acute toxoplasmosis. Figure 2A shows that the main antigen recognized in the F2 fraction by the IgM antibodies present in sera from acutely infected patients was an antigen with a diffuse pattern of migration and an apparent molecular mass of below 14 kDa. This antigen was also recognized by a monoclonal antibody (MAb), T33F12, against GIPLs from T. gondii tachyzoites (Fig. 2C) (23). A less diffuse band of approximately 30 kDa and a more defined band at 70 kDa present in the F2 fraction were also recognized by IgM antibodies present in sera from acutely infected patients.
FIG. 2.
Western blotting analysis of F2 and F3 fractions developed with a pool of sera (dilution, 1:200) from patients with acute toxoplasmosis (A), a pool of sera (dilution, 1:100) from patients with chronic toxoplasmosis and (B), MAb T33F12, specific for tachyzoite-derived GIPLs (C). Ten micrograms of F2 or F3 was run on an SDS–15% polyacrylamide gel, transferred to a nitrocellulose sheet, incubated with specific antibodies, and then developed using an ECL kit. The numbers on the left indicate the molecular masses of proteins used as standard markers.
Identification of tachyzoite antigens that are preferentially recognized by IgG antibodies from sera of patients chronically infected with T. gondii.
Our experiments also indicate that F3 gave the best results for IgG detection, with higher and lower averages for infected and uninfected individuals as well as the best sensitivity (93.61%) and specificity (89.36%) scores (Table 2).
TABLE 2.
Specificity and sensitivity of the ELISA using the F3 fraction to identify patients with chronic toxoplasmosis and high levels of anti-T. gondii IgGa
| IgG isotype | OD405 for:
|
Sensitivity (%) | Specificity (%) | P | |||
|---|---|---|---|---|---|---|---|
| Uninfected patients
|
Chronically infected patients
|
||||||
| Median | SD | Median | SD | ||||
| Total IgG | 0.209b | 0.12 | 1.036b | 0.25 | 93.61 | 89.36 | <0.001 |
| IgG1 | 0.165 | 0.04 | 1.418 | 0.82 | 100 | 100 | <0.001 |
| IgG2 | 0.171 | 0.06 | 0.263 | 0.25 | 75.8 | 80 | 0.06 |
| IgG3 | 0.218 | 0.04 | 0.354 | 0.14 | 75.8 | 83.3 | <0.001 |
| IgG4 | 0.158 | 0.03 | 0.349 | 0.45 | 82.7 | 73.3 | 0.012 |
The ELISA was developed as described in Materials and Methods. The optical densities at 405 nm (OD405) were obtained from individual sera from the same group. The sensitivity and specificity were calculated using the chi-square test to compare ELISA using the F3 fraction and either anti-total IgG, anti-IgG1, anti-IgG2, anti-IgG3, or anti-IgG4 conjugates with a commercially available test to detect T. gondii-specific IgG antibodies. Unless otherwise indicated, n = 30 for uninfected patients and n = 29 for chronically infected patients.
n = 47.
Figure 2B shows that the pattern of recognition of different fractions by IgG was more complex than the pattern generated by IgM antibodies. A band of approximately 30 kDa was the major antigen recognized by T. gondii-specific IgG from sera of patients with chronic toxoplasmosis. This antigen was present in both F2 and F3. In addition to the 30-kDa antigen, many other antigens with molecular masses of above 30 kDa were also recognized by IgG antibodies in sera from chronically infected individuals. The same 30-kDa antigen appears to be recognized by IgM antibodies, but with much lower intensity (Fig. 2A and B) than IgG antibodies, from chronically infected patients. Accordingly, in the ELISA the total tachyzoite sonicate and F3 fraction were poorly recognized by T. gondii-specific IgM antibodies compared to the F2 fraction.
We also determined the main IgG isotype present in sera of patients with chronic toxoplasmosis that recognized the tachyzoite antigens present in F3. The results presented in Table 2 show a clear dominance of the IgG1 isotype among IgG antibodies specific for T. gondii antigens. Importantly, the use of anti-IgG1 instead of anti-total IgG secondary antibody also resulted in an increased specificity (100%) and sensitivity (100%) to discriminate infected from uninfected individuals.
Purification and partial characterization of tachyzoite molecules recognized by human IgM and IgG from sera of patients with acute or chronic toxoplasmosis.
In order to improve the scores of our ELISA test, we decided to further purify components of the tachyzoite membrane by hydrophobic interaction chromatography using octyl-Sepharose. In fact, different studies suggest that GIPLs and GPI-linked proteins are the main targets of IgM (19, 20, 22) and IgG (13, 23) antibodies present in sera from humans infected with T. gondii.
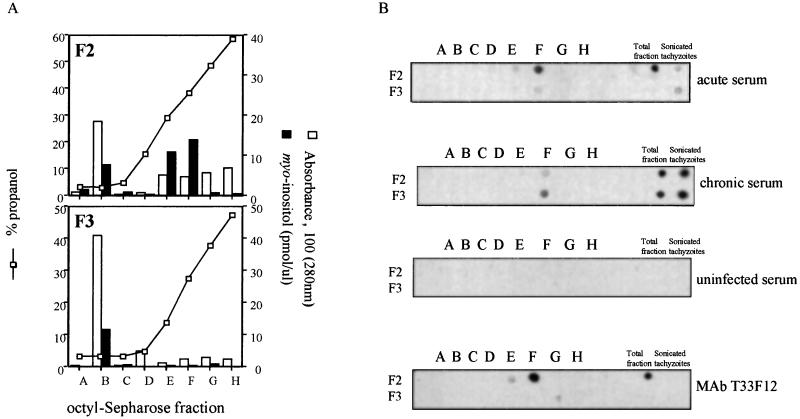
Figure 3A shows protein concentrations (absorbance at 280 nm) and myo-inositol concentrations of fractions A to H released during the propan-1-ol gradient treatment used to release the tachyzoite molecules from octyl-Sepharose columns loaded with F2 and F3. Major protein and myo-inositol peaks were observed in eluate B (5% propan-1-ol) for the column loaded with either F2 or F3, and these correspond to unbound material. Two additional major myo-inositol peaks were detected in eluates E (30% propan-1-ol) and F (40% propan-1-ol) from the octyl-Sepharose column loaded with F2. Minor myo-inositol peaks were also observed in eluates E (15% propan-1-ol), F (25% propan-1-ol), and G (35% propan-1-ol) from the octyl-Sepharose column loaded with F3.
FIG. 3.
Purification of tachyzoite molecules recognized by human IgM and IgG present in sera of patients with acute and chronic toxoplasmosis. (A) Fraction F2 or F3 was loaded into an octyl-Sepharose column and eluted in a gradient of propan-1-ol. The octyl-Sepharose eluates were pooled in eight distinct fractions (A to H), and the propan-1-ol, myo-inositol, and protein (absorbance at 280 nm) concentrations in each were measured. (B) Two microliters of each eluate (A to H), unfractionated F2 or F3, or total parasite extract was dotted on a nitrocellulose sheet that was then incubated with specific antibodies, i.e., a pool of acute sera (dilution, 1:200), a pool of chronic sera (dilution, 1:100), a pool of sera from noninfected individuals (dilution, 1:100), or MAb T33F12 (dilution, 1:100). Dot immunoblotting was performed with an ECL kit.
Each of the eluates obtained from octyl-Sepharose columns loaded with F2 and F3 were also characterized for their ability to be recognized by IgM and IgG from sera of patients with acute or chronic toxoplasmosis (Fig. 3B). These studies were performed using the dot immunoblotting analysis. Our results show that the eluate F and, to a lesser extent, eluate E eluted from octyl-Sepharose loaded with F2 were preferentially recognized by IgM antibodies present in sera from patients acutely infected with T. gondii. This same eluate F was recognized specifically by MAb T33F12. In contrast, eluate F obtained from the octyl-Sepharose column loaded with F3 reacted preferentially with IgG antibodies from sera of patients chronically infected with T. gondii. No reactivity with any of the eluates was observed when we used sera from uninfected individuals.
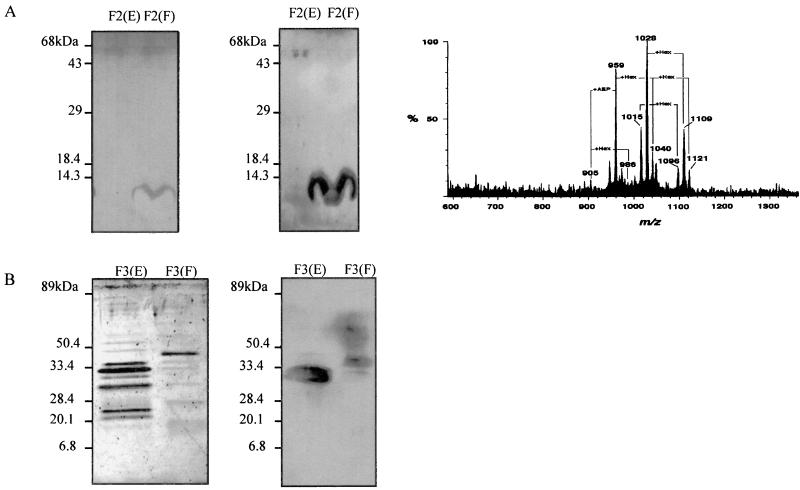
Each of the fractions that showed reactivity with either human IgM or IgG was further analyzed by SDS-PAGE and immunoblotting analysis. Our results demonstrate that eluates E and F did not present a single protein band when silver stained. Only a major diffuse band with molecular mass of below 14 kDa was prominent in eluate F obtained from the column loaded with F2 (Fig. 4A, left panel). This low-molecular-mass diffuse band was recognized by IgM antibodies from sera of patients with acute toxoplasmosis (Fig. 4A, middle panel). After a further butan-1-ol–water partition, the butanolic phase of F2-derived eluate F showed by ES-MS (negative mode) a group of doubly charged [(M − 2H)2−] pseudomolecular ions at m/z 900 to 1150. At least four major species at m/z 905, 959, 1015, and 1028 were observed. Interestingly, two of the less abundant species, at m/z 905 and 986, have estimated molecular masses (1,812 and 1,974 Da, respectively) consistent with two major GIPL structures previously reported (Fig. 4A, right panel) (23). These structures correspond to (i) (ethanolamine-PO4)-Manα1-2Manα1-6(GalNAcβ1-4) Manα1-4GlcNα-inositol-PO4-diacyl(C16:0/C18:0)-glycerol (molecular mass, 1,812 Da) and (ii) (ethanolamine-PO4)-Manα1- 2Manα1-6(Glcα1-4GalNAcβ1-4)Manα1-4GlcNα-inositol-PO4-diacyl(C16:0/C18:0)-glycerol (molecular mass, 1,974 Da). In fact, most of the major doubly charged species observed (m/z 959, 1015, 1028, 1040, 1096, 1109, and 1121) could be derived from the species at m/z 905 and 986, as indicated in Fig. 4A (right panel) and Table 3.
FIG. 4.
Gel electrophoresis, immunoblot, and mass spectrometry analyses of F2-derived eluates E and F and F3-derived eluates E and F. (A) Ten micrograms of F2-derived eluate E or F was run on an SDS–15% polyacrylamide gel and silver stained (left panel) or transferred to a nitrocellulose sheet for immunoblotting analysis using a pool of sera from patients with acute toxoplasmosis (dilution, 1:200) and an anti-human IgM secondary antibody (middle panel). The right panel shows the ES-MS profile of a GIPL preparation from tachyzoite membranes, which is highly reactive with human IgM MAbs and MAb T33F12. (B) Ten micrograms of F3-derived eluate E or F was run on an SDS–15% polyacrylamide gel and silver stained (left panel) or transferred to a nitrocellulose sheet for immunoblotting analysis using a pool of sera from patients with chronic toxoplasmosis (dilution, 1:100) and a secondary anti-human IgG antibody (right panel). The numbers on the left indicate the molecular masses of proteins used as standard markers.
TABLE 3.
Proposed assignments for Toxoplasma GIPL species observed by ES-MS
| GIPL series | [(M − 2H)2−] (m/z) | Mass (Da) | Proposed assignmenta |
|---|---|---|---|
| A | 905 | 1,812 | (Hex3HexNAc)(EtNP)-HexN-InsP-(C16:0/C18:0)DAGb |
| 986 | 1,974 | (Hex4HexNAc)(EtNP)-HexN-InsP-(C16:0/C18:0)DAGb | |
| B | 959 | 1,920 | (Hex3HexNAc)(EtNP)(AEP)-HexN-InsP-(C16:0/C18:0)DAG |
| 1,040 | 2,082 | (Hex4HexNAc)(EtNP)(AEP)-HexN-InsP-(C16:0/C18:0)DAG | |
| 1,121 | 2,244 | (Hex5HexNAc)(EtNP)(AEP)-HexN-InsP-(C16:0/C18:0)DAG | |
| C | 1,015 | 2,032 | NDc |
| 1,096 | 2,194 | ND | |
| D | 1,028 | 2,058 | (Hex4HexNAc)(EtNP)-HexN-InsP-(C16:0/22:0)DAG |
| 1,109 | 2,220 | (Hex5HexNAc)(EtNP)-HexN-InsP-(C16:0/22:0)DAG |
Hex, hexose; HexNAc, N-acetylhexosamine; EtNP, ethanolaminephosphate; HexN, hexosamine; InsP, myo-inositol-phosphate; DAG, diacylglycerol; AEP, 2-aminoethylphosphonate.
Based on structures described by Striepen et al. (21).
ND, not determined.
Eluates E and F obtained from the column loaded with F3 showed an intense reactivity with sera from chronically infected individuals (Fig. 4B). Analysis of silver-stained polyacrylamide gels showed that whereas eluate E had multiple bands with apparent molecular masses of 20 to 33 kDa, eluate F had a major protein band of approximately 40 kDa. As shown by immunoblotting analysis, a band with average molecular mass of approximately 30 kDa was the main IgG target present in eluate E (Fig. 4B, right panel). In eluate F, we observed two bands, at 33 and 40 kDa, with strong reactivity with sera of chronically infected individuals.
Improvement of ELISA scores using eluates from octyl-Sepharose columns loaded with either fraction F2 or F3.
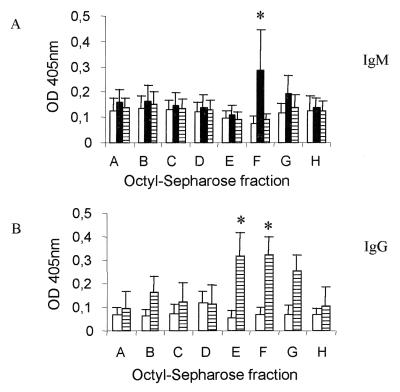
As shown in Fig. 5A, each of the eluates from the octyl-Sepharose column loaded with F2 was tested for the ability to react with sera from infected as well as uninfected individuals. Our results show that for discriminating acutely infected from chronically infected and uninfected individuals, eluate F had the best performance (Fig. 5A). Importantly, even the sera from individuals producing low levels of tachyzoite-specific IgM were readily detected in our ELISA using the F2 eluate F (data not shown). Comparing the total F2 fraction with eluate F, the sensitivity of the assay persisted in the range of 81.8%; however, the specificity of the assay improved from 80.85 to 95.7% (Fig. 5A). Periodate treatment destroyed most of the reactivity of F2 eluate F with IgM from sera of patients with acute toxoplasmosis (data not shown), indicating the carbohydrate nature of these epitopes.
FIG. 5.
Human IgG and IgM recognition of eluates derived from octyl-Sepharose columns loaded with F2 and F3 fractions. (A) Fraction F2 was loaded onto an octyl-Sepharose column and eluted in a gradient of propan-1-ol, as described for Fig. 3A. The octyl-Sepharose eluates were tested for their ability to be bound by human IgM present in sera (dilution, 1:200) from patients with acute toxoplasmosis. The results represent the means and standard deviations for sera from 23 uninfected controls (□), 23 patients with acute toxoplasmosis (▪), and 24 patients with chronic toxoplasmosis (▥). (B) Fraction F3 was loaded onto an octyl-Sepharose column and eluted in a gradient of propan-1-ol as described for Fig. 3A. The octyl-Sepharose eluates were tested for their ability to be bound by human IgG present in sera (dilution, 1:100) from patients with chronic toxoplasmosis. The results represent the means and standard deviations for sera from 29 uninfected controls and 32 patients with chronic toxoplasmosis. The ELISA was developed as described in Materials and Methods. The means and standard deviations were obtained from optical densities (OD) at 405 nm obtained from individual sera from patients of the same group. Asterisks indicate eluates with higher performance in discriminating sera from patients at different stages of infection with T. gondii, as determined by the chi-square test.
An improvement was also observed when we compared the eluates obtained from the octyl-Sepharose column loaded with F3 in regard to their abilities to be recognized by sera from chronically infected but not from uninfected individuals. The results presented in Fig. 5B demonstrate that eluates E and F were highly effective in discriminating sera from chronically infected and uninfected individuals, as seen by their high specificity (100%) and sensitivity (93.75%) scores. Treatment with proteinase K destroyed most reactivity of F3 eluates E and F with IgG from sera of patients with chronic toxoplasmosis (data not shown), indicating the proteinaceous nature of these epitopes.
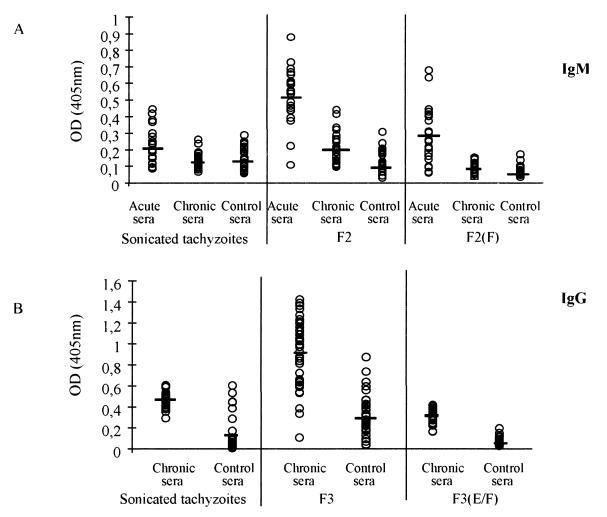
The results presented in Fig. 6 show the individual values of parasite-specific IgM (Fig. 6A) or IgG (Fig. 6B) in ELISA using different antigen preparations as well as sera from patients with acute toxoplasmosis, patients with chronic toxoplasmosis, and uninfected controls. These results show an already substantial improvement after the sequential organic extraction when comparing the serology results using F2 (IgM) and F3 (IgG) with total tachyzoite extracts. The data were further improved when eluate F from F2 and eluates E and F from F3 were used to measure IgM and IgG specific for tachyzoite antigens, respectively. The latter improvement was mainly due to an increase in the specificity of the assay, i.e., a decrease in the number of false-positive results with sera from chronically infected patients in the ELISA to measure T. gondii-specific IgM as well as a reduction in the number of false-positive results with sera from uninfected controls in the assay used to measure parasite-specific IgG.
FIG. 6.
Individual serological tests for parasite-specific IgM and IgG using tachyzoite-derived preparations after different steps of purification. (A) Sera (dilution, 1:200) from patients with acute (n = 23) and chronic (n = 24) toxoplasmosis and from uninfected individuals (n = 23) were tested for sonicated tachyzoites, F2, and the F2 eluate F. (B) Sera (dilution, 1:100) from patients with chronic toxoplasmosis (n = 32) and uninfected individuals (n = 29) were tested for sonicated tachyzoites, F3, and F3 eluate E or F. Results for F3 eluate F are not shown but were identical to those obtained with F3 eluate E [indicated as F3(E/F)]. The ELISAs were developed as described in Materials and Methods. The means and standard deviations were obtained from optical densities (OD) at 405 nm obtained from individual sera from patients of the same group.
DISCUSSION
Despite major advances in the field of DNA technology, most serological tests used for diagnosis of T. gondii infection still employ paraformaldehyde-fixed parasites or crude extracts from tachyzoites instead of parasite recombinant antigens. Thus, in addition to the Sabin-Feldmen test (17), which is considered the standard serological test for toxoplasmosis, immunofluorescence of fixed parasites is used for detection of IgM present in sera of acutely infected patients. For detection of IgG present in sera of chronically infected patients, an ELISA using total tachyzoite extracts is the most usual method employed. The failure of recombinant antigens to provide a test with high specificity and sensitivity scores may be in part attributed to the facts that (i) carbohydrates instead of peptides are the major targets for IgM antibodies elicited during the acute infection with T. gondii and (ii) improper folding of recombinant antigens may result in a dramatic reduction in the binding of a considerable amount of anti-Toxoplasma IgG antibodies, which may recognize tertiary rather than primary peptide structures.
As previously established, patients in the early stages of acute toxoplasmosis produce high levels of parasite-specific IgM (3). Therefore, our acutely infected patients were divided into those producing high and low levels of T. gondii-specific IgM, independent of the levels of parasite-specific IgG. The sera from uninfected controls were all negative for T. gondii-specific IgM and IgG, whereas sera from patients with chronic toxoplasmosis were all IgM negative and IgG positive as determined by parasite-specific IFA and ELISA, respectively. In the present study we compared different extracts prepared from tachyzoite antigens in regard to their ability to discriminate sera from patients acutely or chronically infected with T. gondii from those from uninfected individuals.
Several studies suggest that the main targets for antibody production during the acute and chronic phases of infection are the surface antigens present in the tachyzoite membrane. More precisely, in humans most of the IgM responses against T. gondii are directed against the carbohydrates (11), which were recently shown to be a branch derived from the glycan core of a unique GIPL structure (21). In addition, the surface antigens of approximately 20 (SAG-2), 30 (SAG-1), and 40 (SAG-3) kDa have also been shown to be major targets for IgG responses during chronic infection with T. gondii in humans; several studies suggest a dominant response to SAG-1 (6). It is noteworthy that most of the surface molecules are linked to the tachyzoite surface through GPI anchors (13, 18, 25, 26). The strategy used to prepare tachyzoite extracts was the adaptation of a protocol first used for fractionation of Leishmania donovani (10) and Trypanosoma cruzi (1) membrane components based on their hydrophobicities. As described in Materials and Methods, this protocol generates three fractions, F1 to F3, which consist of highly hydrophobic molecules (F1) (e.g., phospholipids), amphipathic components (F2) (e.g., GIPLs), and hydrophilic molecules (F3) (e.g., GPI-linked glycoproteins).
This study shows that by using sequential organic solvent extraction, we were able to produce a tachyzoite extract, named F2, which was highly enriched for GIPLs and displayed a pronounced ability to identify sera from patients with high Toxoplasma-specific IgM titers. However, this fraction still gave a high number of false-positive results and therefore low score for specificity (80.85%). In contrast, the F3 extract gave excellent results in discriminating sera from T. gondii-infected individuals from those from uninfected individuals, with specificity and sensitivity scores in the ranges of 89.36 and 93.61%, respectively; both are within the values required for standard serological methods for toxoplasmosis. Further improvement in discriminating sera from chronically infected individuals from those from uninfected controls in the ELISA was obtained from the use of the F3 fraction and a secondary antibody against IgG1, instead of total IgG, which resulted in a test with 100% sensitivity as well as 100% specificity.
Further improvements of our ELISA scores were obtained after fractionation of the F2 and F3 extracts using an octyl-Sepharose column. Thus, the specificity and sensitivity of our ELISA for IgM, employing eluate F, were 95.7 and 81.8%, respectively. The biochemical and immunochemical data are consistent with the fact that eluate F, obtained from the octyl-Sepharose column loaded with F2, consisted mainly of GIPLs derived from tachyzoite membranes. Furthermore, this is in agreement with previous studies showing that the IgM antibodies from acutely infected patients recognize mainly carbohydrate epitopes (11).
We also observed a small increase in the specificity score when F3-derived eluates E (100%) and F (100%) were used instead of the F3 extract to discriminate sera of chronically infected individuals from those of uninfected individuals. These eluates E and F consisted mainly of protein of approximately 30 and 40 kDa, respectively. In contrast to the antibodies of the IgM isotype, the IgG antibodies were directed mainly against proteinaceous epitopes, as previously suggested by Hadman et al. (6) and Noat et al. (14).
Thus, our study shows that by using a simple biochemical procedure we can fractionate the major membrane components of the tachyzoite membrane. The use of these proteins of 30 and 40 kDa (eluates E and F from F3) leads to an improvement of the specificity and sensitivity scores of the ELISA for detecting sera from patients with chronic toxoplasmosis. In addition, false-negative results are common finding in IFA used to detect tachyzoite-specific IgMs. The main reason for the false-negative results is the saturation of IgM binding sites by IgG antibodies. In order to avoid this problem, the use of IgM capture assays to measure T. gondii-specific antibodies has been recommended. Our data indicate that the direct recognition of fraction F2 eluate F by IgM is minimally affected by tachyzoite-specific IgG. Therefore, the chemical isolation of a fraction highly enriched for tachyzoite-derived GIPLs that are preferentially recognized by IgM, but not IgG, antibodies may help in the development of a simpler direct ELISA for detecting T. gondii-specific IgM antibodies, with high specificity and fewer problems with false-negative results.
ACKNOWLEDGMENTS
We thank Jean Francois Drubemetz and Striepen Boris for providing MAb T33F12 and Leonides Resende, Jr., for providing human sera tested for T. gondii-specific IgG and/or IgM.
This work was supported in part by CNPq/PADCT SBIO (62.0106/95-6). R.T.G. is a research fellow of the CNPq. M.G. and H.C. are graduate students with scholarships from COLCIENCIAS and CAPES, respectively. I.C.A. was a postdoctoral fellow with a fellowship (no. 96/04260-0) from FAPESP.
REFERENCES
- 1.Almeida I C, Ferguson M A J, Schenkman S, Travassos L R. Lytic anti-α-galactosyl antibodies from patients with chronic Chagas' disease recognize novel O-linked oligosaccharides on mucin-like glycosyl-phosphatidylinositol-anchored glycoproteins of Trypanosoma cruzi. Biochem J. 1994;304:793–802. doi: 10.1042/bj3040793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ammassari A, Murri R, Cingolani A, DeLuca A, Antinori A. AIDS-associated cerebral toxoplasmosis: an update on diagnosis and treatment. Curr Topics Microbiol Immunol. 1996;219:209–222. doi: 10.1007/978-3-642-51014-4_19. [DOI] [PubMed] [Google Scholar]
- 3.Camargo M E, Leser P G. Diagnostic information from serological tests in human toxoplasmosis. Rev Inst Med Trop São Paulo. 1976;18:227–238. [PubMed] [Google Scholar]
- 4.Dubey J P. Advances in the life cycle of Toxoplasma gondii. Int J Parasitol. 1998;28:1019–1024. doi: 10.1016/s0020-7519(98)00023-x. [DOI] [PubMed] [Google Scholar]
- 5.Ferguson M A J. Chemical and enzymatic analysis of glicosyl-phosphatidylinositol anchors. In: Hooper N M, Turner A J, editors. The chemical and enzymatic analysis of GPI fine structure. Lipid modification of proteins: a practical approach. Oxford, United Kingdom: IRL Press; 1992. pp. 196–230. [Google Scholar]
- 6.Hadman E, Goding J W, Remington J S. Detection and characterization of membrane antigens of Toxoplasma gondii. J Immunol. 1980;124:2578–2583. [PubMed] [Google Scholar]
- 7.Joiner K A, Dubremetz J F. Toxoplasma gondii: a parasite for the nineties. Infect Immun. 1993;61:1169–1172. doi: 10.1128/iai.61.4.1169-1172.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 9.McCabe R E, Remington J S. The diagnosis and treatment of toxoplasmosis. Eur J Clin Microbiol. 1983;2:95–104. doi: 10.1007/BF02001573. [DOI] [PubMed] [Google Scholar]
- 10.McConville M J, Blackwell J M. Developmental changes in the glycosylated phosphatidylinositols of Leishamania donovani. Characterization of the promastigote and amastigote glycolipids. J Biol Chem. 1991;266:15170–15179. [PubMed] [Google Scholar]
- 11.Mineo J R, Camargo M E, Ferreira A W. Enzyme-linked immunosorbent assay for antibodies to Toxoplasma gondii polysaccharides in human toxoplasmosis. Infect Immun. 1980;27:283–287. doi: 10.1128/iai.27.2.283-287.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagel S D, Boothroyd J C. The alpha and beta tubulins of Toxoplasma gondii are encoded by single copy genes containing multiple introns. Mol Biochem Parasitol. 1988;29:261–273. doi: 10.1016/0166-6851(88)90081-3. [DOI] [PubMed] [Google Scholar]
- 13.Nagel S D, Boothroyd J C. The major surface antigen, P30, of Toxoplasma gondii is anchored by a glycolipid. J Biol Chem. 1989;264:5569–5574. [PubMed] [Google Scholar]
- 14.Noat Y, Guptill D R, Mullenax J, Remington J S. Characterization of Toxoplasma gondii antigens that react with human immunoglobulin M and immunoglobulin G antibodies. Infect Immun. 1983;41:331–338. doi: 10.1128/iai.41.1.331-338.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noat Y, Remington J S. An enzyme-linked immunosorbent assay for detection of IgM antibodies of Toxoplasma gondii: use for diagnosis of acute acquired toxoplasmosis. J Infect Dis. 1980;142:757–766. doi: 10.1093/infdis/142.5.757. [DOI] [PubMed] [Google Scholar]
- 16.Porter S B, Sande M A. Toxoplasmosis of the central nervous system in the acquired immunodeficiency syndrome. N Engl J Med. 1992;327:1643–1648. doi: 10.1056/NEJM199212033272306. [DOI] [PubMed] [Google Scholar]
- 17.Savva D. Toxoplasma. In: Myint S, Cann A, editors. Molecular and cell biology of opportunistic infections in AIDS. London, United Kingdom: Chapman & Hall; 1992. pp. 163–185. [Google Scholar]
- 18.Schwarz R T, Tomavo S. The current status of the glycobiology of Toxoplasma gondii: glycosylphosphatidylinositols, N- and O-linked glycans. Res Immunol. 1993;144:24–31. doi: 10.1016/s0923-2494(05)80092-6. [DOI] [PubMed] [Google Scholar]
- 19.Sharma S D. Immunology of toxoplasmosis. In: Wyler D J, editor. Modern parasite biology cellular, immunological and molecular aspects. W. H. New York, N.Y: Freeman and Co.; 1990. pp. 184–199. [Google Scholar]
- 20.Sharma S D, Mullenax J, Araujo F G, Erlich H A, Remington J S. Western blot analysis of the antigens of Toxoplasma gondii recognized by human IgM and IgG antibodies. J Immunol. 1983;131:977–983. [PubMed] [Google Scholar]
- 21.Striepen B, Zinecker C F, Damm J B L, Melgers P A T, Gerwig G J, Koolen M, Vliegenthart J F G, Dubremetz J F, Schwarz R T. Molecular structure of the “low molecular weight antigen” of Toxoplasma gondii: a glucose α1-4 N-acetylgalactosamine makes free glycosyl-phosphatidylinositols highly immunogenic. J Mol Biol. 1997;266:797–813. doi: 10.1006/jmbi.1996.0806. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki Y, Remington J S. Toxoplasmic encephalitis in AIDS patients and experimental models for study of the disease and its treatment. Res Immunol. 1993;144:66–67. doi: 10.1016/s0923-2494(05)80102-6. [DOI] [PubMed] [Google Scholar]
- 23.Tenter A M, Johnson A M. Recognition of recombinant Toxoplasma gondii antigens by human sera in an ELISA. Parasitol Res. 1991;77:197–203. doi: 10.1007/BF00930858. [DOI] [PubMed] [Google Scholar]
- 24.Tomavo S, Couvreur G, Leriche M A, Sadak A, Achbarou A, Fortier B, Dubremetz J F. Immunolocalization and characterization of the low molecular weight antigen (4-5 kDa) of Toxoplasma gondii that elicits an early IgM response upon primary infection. Parasitology. 1994;108:139–145. doi: 10.1017/s0031182000068220. [DOI] [PubMed] [Google Scholar]
- 25.Tomavo S, Dubremetz J F, Schwarz R T. A family of glycolipids from Toxoplasma gondii. Identification of candidate glycolipid precursor(s) for Toxoplasma gondii glycosylphosphatidylinositol membrane anchors. J Biol Chem. 1992;267:11721–11728. [PubMed] [Google Scholar]
- 26.Tomavo S, Schwarz R T, Dubremetz J F. Evidence for glycosyl-phosphatidylinositol anchoring of Toxoplasma gondii major surface antigens. Mol Cell Biol. 1989;9:4576–4580. doi: 10.1128/mcb.9.10.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]