Abstract
Background
Gastrointestinal complications of COVID‐19 have been reported over the last year. One such manifestation is bowel ischaemia. This study thus aims to provide a more holistic review of our current understanding of COVID‐19‐induced bowel ischaemia.
Method and Results
A meticulous search was performed using different keywords in PubMed and Google Scholar. Fifty‐two articles were included in our study after applying inclusion and exclusion criteria and performing the qualitative assessment of the studies. A total of 25 702 patients were included in our study after the completion of the qualitative assessment.
Discussion
The common symptoms of GIT in COVID‐19 patients are as diarrhoea, vomiting, nausea and abdominal pain. The mechanism of bowel ischaemia is associated with the formation of emboli which is related to COVID‐19’s high affinity for angiotensin‐converting enzyme‐2 on enterocytes, affecting the superior mesenteric vessels. Clinically, patients present with abdominal pain and vomiting. CT angiography of the abdomen and pelvis showed acute intestinal ischaemia (mesenteric). Management is usually initiated with gastric decompression, fluid resuscitation, and haemodynamic support. Surgical intervention is also sought.
Conclusion
Intestinal ischaemia presenting in patients with COVID‐19 has to be considered when symptoms of severe abdominal pain are present. More research and guidelines are required to triage patients with COVID‐19 to suspect intestinal ischaemia and to help in diagnosis and management.
Review Criteria
We gathered information with the help of an electronic search using PubMed and Google Scholar by using keywords either in combination or alone.
Keywords used were “COVID‐19,” “Bowel ischemia,” “Mesenteric ischemia,” and “pathophysiology,” “Management,” “Sequelae.”
Articles went through our inclusion and exclusion criteria mentioned in the methods section to decide whether to include them in our review.
Clinical Appraisal tools were later applied to each of the articles by the authors for quality analysis.
Message for the clinic
We intend to provide an insight to the clinician so that an early diagnosis of bowel ischaemia is made and none of the cases are missed.
We have included even very rare presentations of bowel ischaemia and methods of their diagnosis to guide the clinician in their management.
Bowel ischaemia should not be taken lightly as it has a very high mortality rate. It requires the immediate attention of the clinician while managing the patient of COVID‐19.
1. INTRODUCTION AND BACKGROUND
The world has not been the same after the universal spread of the COVID‐19 pandemic which dawned in Wuhan, China at the end of 2019. COVID‐19 has been known to be transmitted through respiratory droplets. The manifestations of SARS‐CoV‐2 are so variable ranging from being asymptomatic to severe respiratory distress syndrome (ARDS). 1 It has affected millions of people all over the world because of its rapid aerosol transmission. This compelled the World Health Organization (WHO) to declare COVID‐19 as a health emergency on 30 January 2020. Later, declaring it as a full‐blown pandemic on 11 March 2020. COVID‐19 has various systemic manifestations that are vital to understanding and preventing significant morbidity and mortality. SARS‐CoV‐2 creates a habitat for a prothrombotic state in the human body and causes arterial, venous, and catheter‐related thrombosis. 2 This hypercoagulable state leads to a variety of manifestations on the cardiovascular system, nervous system, renal and gastrointestinal systems. Arterial thrombosis caused by COVID‐19 includes limb and mesenteric ischaemia, stroke, and acute coronary syndrome. 3 , 4 , 5
Plenty of other gastrointestinal complications were reported in COVID‐19 patients like ileus, hepatic necrosis, acalculous cholecystitis as well. There is very high mortality in bowel ischaemia and limited data available make it very essential to predict and prevent COVID‐19‐related bowel ischaemia. Our study specifically focuses on bowel ischaemia as a gastrointestinal complication of Coronavirus. Apart from hypercoagulation, several other mechanisms like inflammation, vasculopathy and immobilisation have been proposed to explain the occurrence of GI ischaemia, but the exact cause is still very unclear. 2
The clinician should have a high index of suspicion to prevent such a deadly complication. Our systematic review aims at exploring the occurrence, course, symptomatology and outcome of bowel ischaemia in COVID‐19. This will help clinicians to predict this specific complication and treat it in the earliest possible way to reduce significant morbidity and mortality.
2. METHODS
2.1. Electronic search
Preferred Reporting Items for Systematic Review and Meta‐Analysis (PRISMA) guideline was strictly followed for the conduct of our systematic review. The authors SP, RS, DV systematically searched PubMed and Google Scholar using an advanced search strategy to find relevant articles for our study. The keywords used for the search strategy were “COVID‐19,” “Bowel ischemia,” “Mesenteric ischemia,” “pathophysiology,” “Management,” and “Sequelae.” The keywords were used either alone or in combination. In addition, many publications were identified using the “Snowball Method,” that is, articles were identified from reference lists of relevant articles.
2.2. Eligibility criteria
The eligibility criteria that we applied for the studies to be included in our review article were: Laboratory‐confirmed SARS‐CoV‐2/COVID‐positive cases, cases reporting a diagnosis of AMI/bowel ischaemia, articles published in English language. Animal trial studies, in vitro studies, articles with no abstracts were excluded.
2.3. Study selection and quality assessment
The authors MW, CP and UA evaluated the title and abstracts of the articles extracted from databases and ascertained the studies based on predominant eligibility criteria. Quality assessment of the selected studies was done using standardised quality appraisal tools based on the type of study. The authors RA, NB, DS, RS, SP, CP and MW did the quality assessment of the studies. The standard tools used were:
Observational studies: New‐Castle Ottawa Scale
Systematic Reviews/Meta‐Analysis: AMSTAR Checklist
Case Reports and Case Series: Joanna Briggs Institute Checklist
Narrative Reviews: SANRA
Studies were ranked as good, fair and poor quality based on the assessment scores of the studies.
2.4. Data extraction
A standardised data abstraction form was adopted to extract the data from databases. The authors DV, SP and RA extracted the data manually. Any difference in opinion was resolved through mutual discussion among the authors. The following information was extracted: authors, year of publication, no of cases or participants in the study, study design, laboratory findings and treatment procedure adopted for the management, complication and outcome in the patients following the treatment.
3. RESULTS
An initial search of the databases yielded 180 826 articles using advanced search strategies which included Medical Subjects Heading [MeSH] as well. The articles were then screened by applying eligibility criteria as discussed above which left us with 92 articles and 180 734 articles were excluded. Ninety‐two articles were screened and 32 articles were removed because of irrelevant or insufficient information or not having a full‐text article or abstract. Sixty articles were then assessed for eligibility.
The eligibility was determined by the use of various critical appraisal tools as mentioned above. Fifty‐two articles qualified for the critical appraisal assessment and were included in our study. Eight articles were excluded for not qualifying the critical appraisal assessment.
Figure 1 below shows the PRISMA flow diagram mentioning the result of our study. 6
FIGURE 1.
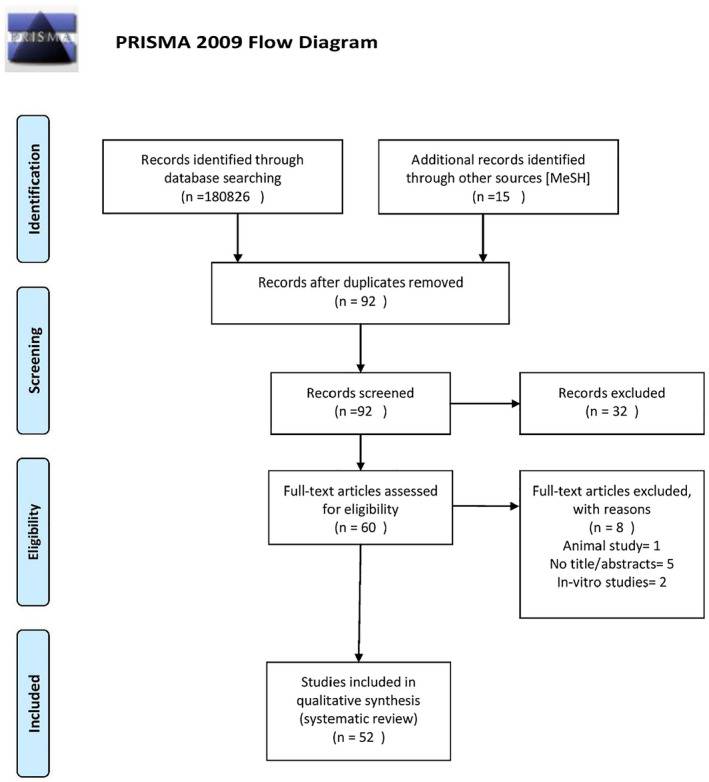
PRISMA 2009 flow diagram
A total of 25 702 patients were included in our study from 10 observational studies, three systematic reviews, meta‐analysis, and 24 case reports. Fever was the most predominant presenting complaint reported. Most of the studies reported the presence of acute abdomen, nausea, loss of appetite, diarrhoea along with sore throat, cough, confusion and sepsis in severely ill patients.
3.1. Diagnostic findings
All the cases included in our study were laboratory‐confirmed SARS‐CoV‐2 positive. We tried to explore the diagnostic findings suggesting bowel ischaemia. Most of the cases did not report any laboratory findings supporting the diagnosis of bowel ischaemia. Some studies reported elevation of D‐dimer and lactate dehydrogenase. Table 1 below summarises the presenting complaints and laboratory findings in various studies.
TABLE 1.
Studies depicting presentation and lab values, specifically D‐dimer and LDH values, of COVID‐19 patients
| Reference | Author | Year of publication | Type of study | n | SARSCoV2 status | Age (years) | Presenting symptoms | d‐dimer | LDH |
|---|---|---|---|---|---|---|---|---|---|
| 4 | Kaur P et al | 2020 | Case report | 1 | Positive | 43 | Shortness of breath and acute right leg pain | >20 (reference: <0.5) | 718 U/L (reference: 140‐271 U/L) |
| 5 | Singh B et al | 2020 | Case report | 1 | Positive | 77 | Shortness of breath and pain, discoloration, and swelling of the left leg | 2.77 (reference: <0.5) | 392 U/L (reference: 140‐271 U/L) |
| 7 | Wang D et al | 2020 | Retrospective single‐centre case series | 138 | Positive | 56 (mean age) | Fever, fatigue, and dry cough | 203 U/L (125‐243) [IQR] | 261 U/L [125‐243] [IQR] |
| 8 | Guan W et al | 2020 | Retrospective cohort study | 1099 | Positive | 47 (mean age) | Fever and cough, diarrhoea | No of patients with d‐dimer ≥0.5 mg/L ‐ 46.4% a | No of patients with LDH ≥250 U/L ‐ 41.0% |
| 9 | Pan L et al | 2020 | Descriptive, cross‐sectional, multicentre study | 204 | Positive | 52.9 (mean age) | Digestive manifestations, including lack of appetite, diarrhoea, vomiting, and abdominal pain | N/A | N/A |
| 10 | Cheung KS et al | 2020 | Systematic review and meta‐analysis | 59 | Positive | 58.5 (mean age) | Fever, cough, dyspnoea, diarrhoea, vomiting, abdominal pain/discomfort | N/A | N/A |
| 12 | Han C et al | 2020 | Retrospective cohort study | 206 | Positive | 62.5 (mean age) | Diarrhoea | N/A | N/A |
| 13 | Mao R et al | 2020 | Systematic review and meta‐analysis | 6064 | Positive | N/A | Nausea, vomiting, diarrhoea, loss of appetite, abdominal pain, fever | N/A | N/A |
| 14 | Noda S et al | 2021 | Case report | 1 | Positive | 17 | Abdominal pain, vomiting and fever | 2.2 mcg/mL, normal ≤0.5 mcg/mL | N/A |
| 15 | Laski D et al | 2021 | Case report | 1 | Positive | 39 | Fever, pain in upper abdomen, nausea, vomiting | N/A | N/A |
| 16 | Zeng W et al | 2021 | Systematic review | 5285 | Positive | N/A | Diarrhoea, abdominal pain | N/A | N/A |
| 17 | Chen N et al | 2020 | Retrospective, single‐centre study | 99 | Positive | 55.5 (mean age) | Fever, cough, shortness of breath. Muscle ache, diarrhoea | 0.9 (normal range 0.0‐1.5) | 336.0 (normal range 120.0‐250.0) |
| 18 | Kim JY et al | 2020 | Case report | 1 | Positive | 35 | Fever, chills, myalgia | N/A | 561 U/L (highest value recorded) |
| 19 | Tang A et al | 2020 | Case report | 1 | Positive | 10 | Asymptomatic | N/A | N/A |
| 22 | Singh B et al | 2021 | Systematic review | 13 | Positive | 56 (mean age) | Nausea, vomiting, fever, abdominal pain | N/A | N/A |
| 23 | Rodriguez‐Nakamura RM et al | 2020 | Case report and literature review | 2 | Positive | 43.5 (mean age) | Case 1 ‐ Severe gastric pain | Case 1 ‐ 1450 mcg/L | N/A |
| Case 2 ‐ spontaneous abdominal pain | Case 2 – 14 407 mcg/L | ||||||||
| 25 | Ignat M et al | 2020 | Case series | 3 | Positive | 50.3 (mean age) | Case 1 ‐ Abdominal pain | N/A | N/A |
| Case 2,3 ‐ ARDS | |||||||||
| 26 | Paul T et al | 2021 | Case report | 1 | Positive | 66 | Fever, cough, loss of smell and taste | N/A | N/A |
| 27 | Swami GA et al | 2021 | Case report | 1 | Positive | 54 | Fever, cough | 1245 ng/mL (normal ‐ <250 ng/mL) | 745 IU/L (n = 50‐250) |
| 28 | Calcagno E et al | 2021 | Case report | 1 | Positive | 36 | Abdominal pain | N/A | N/A |
| 32 | Holleb P et al | 2021 | Case report | 1 | Positive | 54 | Nausea, vomiting, abdominal pain, diarrhoea | N/A | N/A |
| 34 | Gartland M et al | 2020 | Case report | 1 | Positive | 47 | Fever, shortness of breath | N/A | N/A |
| 35 | JHQ Pang et al | 2020 | Case report | 1 | Positive | 30 | Abdominal pain associated with vomiting | d‐dimer >20.0 μg/mL | N/A |
| 36 | Al Argan RJ et al | 2021 | Case series | 3 | Positive | 59.33(mean age) | Case 1 ‐ Dry cough and fever | Case 1 ‐ 0.6 | Case 1 ‐ 434 |
| Case 2 ‐ Shortness of breath | Case 2 ‐ 0.41 | Case 2 ‐ 442 | |||||||
| Case 3 ‐ Abdominal pain | Case 3 ‐ 1.66 (Normal ‐ ≤0.5 μg/mL) | Case 3 ‐ 617 (Normal ‐ (81‐234) U/L) | |||||||
| 37 | Varshney R et al | 2021 | Case report | 1 | Positive | 50 | Abdominal pain and constipation | N/A | N/A |
| 38 | Drakos, P. et al | 2021 | retrospective cohort study | 218 | Positive | 59.8 (mean age) | Listed as acute gastrointestinal injury | Increased based on grading of acute gastrointestinal injury | N/A |
| 33 | Cheung S et al | 2020 | Case report | 1 | Positive | 55 | Nausea, vomiting, generalised abdominal pain | 3.4 nmol/L | N/A |
| 39 | Krothapalli N et al | 2021 | Case report | 1 | Positive | 76 | Shortness of breath | 2159 ng/L (normal ‐ <250 ng/mL) | N/A |
| 40 | Bannazadeh M et al | 2021 | Case report | 1 | Positive | 55 | Severe abdominal pain | 2400 ng/L (normal ‐ <250 ng/mL) | N/A |
| 41 | Alharthy A et al | 2020 | Case report | 1 | Positive | 54 | Fever, cough, dyspnea, diarrhoea | 4.1 μg/mL; reference range, 0 to 0.5 μg/mL | 997 U/L; reference range, 100‐190 U/L) |
| 43 | Tang N et al | 2020 | Retrospective cohort study | 449 | Positive | 65.1 (mean age) | Diarrhoea, acute abdomen | 1.94 Reference ‐ 0 to 0.5 μg/mL) | N/A |
| 44 | Dinoto E et al | 2021 | Case report | 1 | positive | 84 | Acute abdomen | 6937 ng/mL (normal ‐ <250 ng/mL) | 431 U/L (n = 50‐250) |
| 45 | Balani P et al | 2021 | Case report | 1 | Positive | 37 | Abdominal pain, vomiting | 3.1 Reference ‐ 0 to 0.5 μg/mL | N/A |
| 47 | Shaikh DH et al | 2021 | Case report | 1 | Positive | 73 | Acute abdomen, distension of abdomen | 2757 (normal ‐ <250 ng/mL) | 236 (normal = 50‐250) |
Abbreviations: IQR, interquartile range; n, no. of patients; N/A, not available.
No./total no. (%).
4. DISCUSSION
4.1. General GI manifestations of COVID‐19
Patients infected with COVID‐19 have shown GI symptoms (2%‐10%); however, those numbers, in reality, appear to be much higher. 7 , 8 About 50.5% of patients reported various gastrointestinal signs and symptoms during a study of COVID‐19 patients in China. 9 A meta‐analysis consisting of 60 studies and 4243 patients demonstrated a pooled prevalence of GI symptoms of 16.1% and 33.4% in studies from China and other countries, respectively. 10 In another meta‐analysis including 47 studies and 10 890 unique patients, GI symptoms were present in less than 10% of patients, but rates were higher in studies outside of China. 11 GI symptoms as a result of COVID‐19 were suggested to be more common in females according to Han et al (65.7% in females and 51.1% in males), but no alternate studies showed similar figures. 12 COVID‐19 presents as anorexia (21%), diarrhoea (9%), nausea/vomiting (7%), and abdominal pain/discomfort (3%) when presenting in the GI. 13 A minority of patients present with an acute abdomen with aetiologies such as acute pancreatitis, acute appendicitis, intestinal obstruction, bowel ischaemia, haemoperitoneum, abdominal compartment syndrome, bowel perforation, hepatic necrosis, acalculous cholecystitis, and colitis. Very rare GI presentations of the COVID‐19 have also been noted like mesenteric adenopathy 14 and pneumatosis intestinalis. 15
There is a strong relationship between the severity of COVID‐19 and gastrointestinal symptoms which can be illustrated by: (1) 40% of severe COVID‐19 positive patients have gastrointestinal symptoms; (2) Abdominal pain is associated with 2.8‐fold increase in the risk of severe COVID‐19. 16
Like other respiratory virus infections, the majority of patients with COVID‐19 present with acute respiratory symptoms. 17 , 18 Many times COVID‐19 patients are asymptomatic so there are very high chances of these cases being missed out which need more investigation like stool PCR. 19 Diarrhoea, nausea and vomiting while being common GI symptoms in general did not seem to be present in COVID‐19 in initial studies. 20 However, with the growing number of studies on COVID‐19, up to 48.5% (204 patients) in China reported presenting with GI symptoms. They presented with symptoms such as anorexia, diarrhoea, vomiting and abdominal pain. 9
Patients with underlying digestive diseases and COVID‐19 infection may present with a severe infection in chronic liver diseases such as viral hepatitis B or C. 21 Sometimes damage to the alimentary canal leads to inflammation that causes malabsorption and damage to the mucosal integrity of the canal, activation of the enteric nervous system, and an imbalance in the secretion of the alimentary canal. Usually, the symptoms are self‐limited but, at times surgery may be required depending on the extent of the damage.
4.2. Pathophysiology
Studies indicate the involvement of multiple mechanisms in the causation of acute mesenteric ischaemia (AMI) in COVID‐19 patients.
Firstly, the SARS‐CoV‐2 virus has a high affinity and tropism for angiotensin‐converting enzyme‐2 (ACE2) receptors on enterocytes. This can lead to the tropism of the virus into the enterocytes causing direct damage to the bowel tissue. 22 This binding of the virus to the ACE2 receptors decreases the breakdown of ACE2 which increases IL‐6 levels, leading to the development of a cytokine storm. Endothelial cells increase the expression of tissue factor and plasminogen activator inhibitor‐1 because of the hypercoagulability caused by angiotensin 2. 23 Second, a viral infection of the endothelial cells can lead to their inflammation and dysfunction. This endothelial damage increases the formation of procoagulant factors like factor VIII, von Willebrand factor secreted by the Weibel Palade bodies, and fibrinogen. The hypercoagulable state in COVID‐19 can cause thrombosis of small mesenteric vessels and bowel ischaemia. 24 This mucosal ischaemia can lead to a considerable spread of the virus from the bowel to other organ systems causing a rapid clinical deterioration in the patient's condition. 25 Venous thromboembolism is more commonly observed than arterial thrombosis in patients with COVID‐19. 26 Hypercoagulability can also be a result of the circulating microvesicles that can originate from platelets, monocytes or neutrophil extracellular traps released from activated neutrophils. 22 Lastly, patients with severe COVID‐19 pneumonia might require vasopressors like norepinephrine and high‐dose epinephrine to treat shock and haemodynamic instability. The resulting vasoconstriction and decreased mesenteric blood circulation can lead to non‐occlusive mesenteric ischaemia. 26 Figure 2 below summarises the mechanism involved in AMI. Table 2 below summarises the pathophysiological mechanism involved.
FIGURE 2.
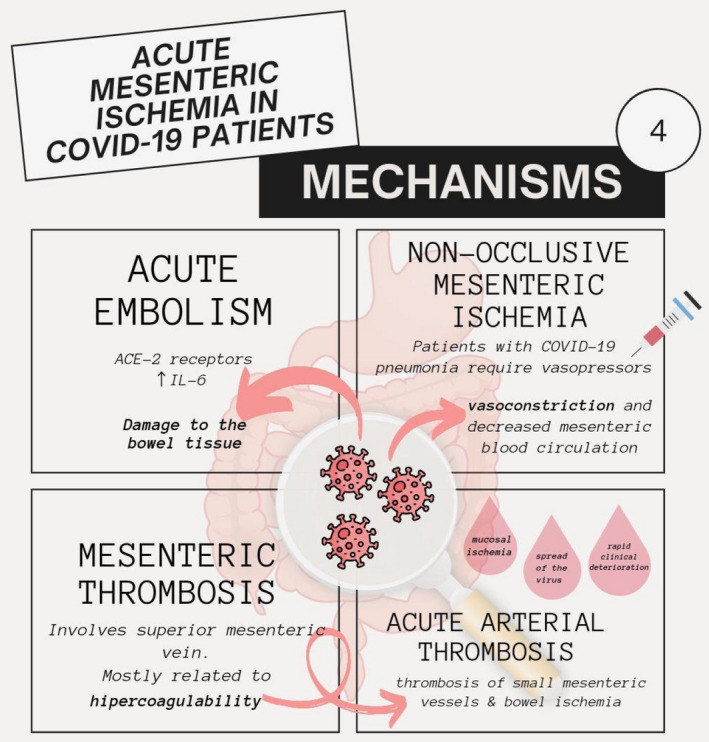
Flow chart of mechanisms of acute mesenteric ischaemia in COVID‐19. ACE‐2, angiotensin‐converting enzyme 2; IL‐6, interleukin‐6
TABLE 2.
Pathophysiology involved in bowel ischaemia
| Author | Year | Type | Purpose of study | Place of study | Conclusion |
|---|---|---|---|---|---|
| Singh B et al 22 | 2021 | Letter to the editor | To identify the possible mechanisms and diagnostic pathways for AMI in Severe Coronavirus‐19 infection. | USA | Detailed understanding of the occurrence of AMI in COVID‐19 patients will aid in carrying out appropriate diagnostic tests at an early stage and making swift decisions regarding the intensity of thromboprophylaxis to decrease the risk of morbidity and mortality |
| Rodriguez N et al 23 | 2020 | Case report and literature review | 2 cases of COVID‐19‐induced ischaemia leading to acute mesenteric thrombosis. The SCARE criteria have been utilised to report the work in the study | Mexico | Suspicion for rare pathologies like mesenteric thrombosis in COVID‐19 should be raised in patients who present with an unclear clinical picture |
| Parry A et al 24 | 2020 | Letter to the editor | To identify the possible mechanisms and diagnostic pathways for AMI in Severe Coronavirus‐19 infection | India | Suitable diagnostic tests at an early stage of COVID‐19 can be helpful in making swift decisions regarding the intensity of thromboprophylaxis to decrease the risk of morbidity and mortality linked with the disorder |
| Ignat M et al 25 | 2020 | Case series | To describe the clinical and the CT features of 3 patients presenting with an acute abdomen induced by SARS‐CoV‐2 infection | France | If a patient with COVID‐19 worsens and the cause is undetermined, abdominal CT can be considered. Exploratory laparotomy and bowel resection may be deemed necessary in the event of small bowel involvement |
| Paul T et al 26 | 2020 | Case report | Qatar | Severe COVID‐19 pneumonia should raise concern for a hypercoagulable state. Diagnosing and treating such patients early in the disease course has shown better outcomes |
4.3. Diagnosis, sequela and prognosis
The SARS‐CoV‐2 virus has been found to cause pneumonia as well as a variety of other symptoms in the gastrointestinal, cardiovascular, and nervous systems as part of a multi‐inflammatory syndrome, which is becoming more widely recognized as a part of the disease spectrum. Coagulopathy associated with COVID‐19 has become a major contributor to high mortality and morbidity, with ischemic bowel disease being one of the outcomes. 27 Because clinical factors are ineffective at predicting or suspecting coagulopathy and associated consequences, it is critical to be aware of imaging symptoms of COVID‐19 coagulopathy sequelae. 28 The following imaging findings are often documented on CT abdomen of COVID‐19 positive patients with GI symptoms:thrombosis of vessels, pneumoperitoneum, gut wall thickening, fluid‐filled dilated colon, pneumatosis, mesenteric ischemia, and intussusception. 29
Mesenteric ischemia can be either acute (95% of the cases) or chronic (remaining 5% of the cases). Mesenteric ischemia is not detected by laboratory testing since they are neither sensitive nor specific. 30 Increased WBC count, metabolic acidosis, hemoconcentration, and elevated levels of lactate, d‐dimer, liver enzymes, and amylase are some of the abnormal laboratory findings associated with ischemia. The first‐line imaging test for suspected Acute and chronic mesenteric ischemia is multiplanar biphasic CT angiography of the abdomen and pelvis. Thin sections, multiplanar reformation, and three‐dimensional rendering should all be used while performing CT angiography. 31 The initial sign of acute mesenteric ischemia (AMI) is a dynamic ileus, in which the intestine is dilated and frequently filled with fluid. This might be due to ischemia‐induced aperistalsis or infarction‐induced lack of contractility. The most prevalent sign in AMI is circumferential edematous intestinal wall thickening. When compared to other forms of mesenteric ischemia, in venous ischemia wall thickening is most noticeable,with a striated pattern that corresponds to the “target” & “halo sign”. Edema or hemorrhage can cause the thickened intestinal wall to hypoattenuate or hyperattenuated respectively. In individuals with arterial AMI, the gut wall may also be thin. In individuals with AMI, bowel wall enhancement may be enhanced, reduced, or nonexistent. Mural hyperenhancement generally indicates that gut is viable and is associated with venous drainage problems, shock bowel, or acute arterial occlusion with reperfusion. 30
One of the suggested mechanisms for paralytic ileus/small bowel obstruction is through the inflammatory process from the virus entry into the small intestine and leading to micro thrombosis of the microcirculation. 32 Studies have shown that in patients infected with coronavirus gastrointestinal involvement is seen in at least two‐thirds of patients. Coagulation abnormalities and elevated D‐dimer levels were noted in patients with COVID‐19 and are related to a poor clinical outcome and a very high inpatient mortality rate. 33 A case report in a COVID‐19‐positive patient who had patent mesenteric vessels associated with catastrophic bowel necrosis suggested that it can be caused by microvascular thrombosis and inflammation. It is considered as one of the proposed mechanisms in such patients known to have hypercoagulability. 34 Another case report in a patient with COVID‐19 has shown how superior mesenteric thrombosis can present as intestinal obstruction from congenital adhesion, which is a rare surgical condition. This patient had an acute presentation from small bowel stricture which can be one of the sequelae secondary to bowel ischaemia. 35 Cases have been reported in which COVID‐19 led to gastric wall perforation, caecal perforation and sigmoid diverticulitis with perforation which were treated conservatively and surgically. 36 Varshney et al reported a case in which COVID‐19 resulted in sigmoid and descending colonic gangrene further requiring colectomy. 37 Drakos et al performed Kaplan‐Meier survival analysis, various other statistical tests and determined that the development of acute gastrointestinal injury (AGI) and intolerance to feeds during the initial days can serve as prognostic tools for predicting outcomes in critically ill COVID‐19 ICU patients. Higher grades of AGI also correlated with the increased levels of d‐dimer and C‐reactive protein as shown in the Figure 3. 38
FIGURE 3.
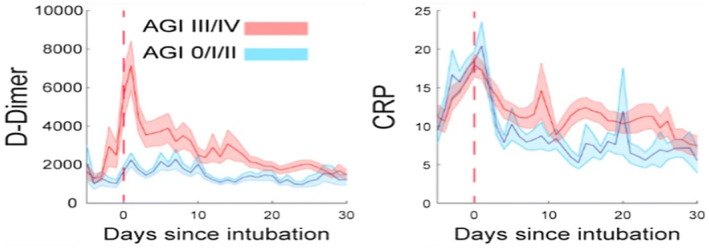
Co‐relation of AGI with d‐dimer and CRP
4.4. Management of bowel ischaemia in COVID‐19
It has been documented that COVID‐19 leads AMI. 33 , 39 This has often been attributed to the hypercoagulable state that the novel coronavirus puts the patient's body in, which eventually leads to bowel ischaemia. 40 The importance of early diagnosis and prevention cannot be stated enough. The cornerstone of management of such an acute emergency is the immediate restoration of blood flow, and removal of any necrotic bowel. 41 , 42
This is an evolving topic and there are no clear guidelines specific to COVID‐19‐related bowel ischaemia when it comes to management. It is in the interest of healthcare providers to aid in the prevention of such severe complications as bowel ischaemia. Based on the understanding of the increased incidence of thrombotic events in patients with COVID‐19, the use of prophylaxis as well as out and out anticoagulation strategies have been tried and tested with favourable outcomes. 43 Screening with CT imaging and prothrombotic workup also plays a role in the early diagnosis of bowel ischaemia. Supportive and conservative therapy includes gastric decompression, fluid resuscitation, and hemodynamic support. 44 , 45 Also, early diagnosis and systemic thrombolysis have been shown to produce better outcomes in terms of bowel salvage. 44 , 45
In terms of surgical interventions, exploratory laparotomy followed by bowel resection was often sought because of the acute presentation of the case. Another treatment modality involves an endovascular approach such as superior mesenteric artery (SMA) thrombectomy with stent repair, or catheter‐directed thrombolysis with thrombus aspiration. 45 Tocilizumab, a monoclonal antibody acting against the IL‐6 receptor, has been used to treat hospitalised patients with severe COVID‐19. 46 Shaikh DH et al mentions its use as an infusion, in COVID‐19‐induced small bowel ischaemia, after a patient underwent surgical intervention. Good recovery was seen. In this case, it was also noted that convalescent plasma therapy along with tocilizumab infusion, was used post‐op. 47 But, symptomatic and supportive care is the mainstay of therapy. In a general sense as well, more studies will help throw visibility on this unique but drastic complication of COVID‐19 so that better treatment modalities may be identified. Table 3 presented below outlines the various management strategies involved in the treatment of bowel ischaemia secondary to COVID‐19.
TABLE 3.
Management strategies for bowel ischaemia secondary to COVID‐19
| Author | Year | Type | Purpose of study | Place of study | Conclusion |
|---|---|---|---|---|---|
| Cheung S et al 33 | 2020 | Case reports | COVID‐19‐induced thrombosis of the superior mesenteric artery. | USA | This case was managed with exploratory laparotomy, thrombectomy and resection of the ischaemic bowel |
| Krothapalli N et al 39 | 2021 | Case report and literature review | Discusses aetiology, clinical picture and laboratory findings of mesenteric ischaemia in COVID‐19‐positive patients and explains a case of it | USA | Surgical resection of the bowel is aided with gastric decompression and fluid resuscitation. This complication is best prevented by prophylactic thrombolytics |
| Bannazadeh M et al 40 | 2021 | Case report | Case report discussing the presentation of mesenteric artery thrombosis in a patient with COVID‐19, 1 week after discharge | USA | Therapeutic enoxaparin for 3 months following bowel resection and end‐to‐end anastomosis will help |
| Alharthy A et al 41 | 2020 | Case report | To examine a case of COVID‐19 presenting with acute abdomen and sepsis. | Saudi Arabia | Management mainly involves exploratory laparotomy, resection of the ischaemic area and anticoagulation in the post‐op period. Based on renal function, enoxaparin is recommended for therapeutic anticoagulation |
| Kariyawasam JC et al 42 | 2021 | Review article | Presents the gastrointestinal manifestations of COVID‐19 | Sri Lanka | Cases of acute abdomen and GI bleeding may warrant surgical or endoscopic modalities for management |
| Tang N et al 43 | 2020 | Retrospective cohort study | To study the efficacy of low molecular weight heparin in decreasing the risk of disseminated intravascular coagulation and venous thromboembolism | China | Low molecular weight heparin appears to be the mainstay for therapeutic anticoagulation in severe COVID‐19 patients with elevated d‐dimer |
| Dinoto E et al 44 | 2021 | Case report | Introduces a case of mesenteric ischaemia which was followed by acute limb ischaemia in a patient with COVID‐19 | Italy | Endovascular management can be used as it has reduced mortality and morbidity rates. Different options exist such as mechanical thrombectomy, local thrombolysis and PTA stenting |
| Balani P et al 45 | 2021 | Case Report and literature review | Discusses early detection and management of bowel ischaemia in a patient with COVID‐19 | India | Catheter‐directed thrombolysis with thrombus aspiration is a possible treatment modality for superior mesenteric thrombus if caught early |
| Mariette X et al 46 | 2021 | Research Letter | Follow‐up article to a trial of tocilizumab in hospitalised patients of COVID‐19 infection | France | Treatment of moderate‐to‐severe COVID‐19 with elevated CRP levels can include the use of tocilizumab |
| Shaikh DH et al 47 | 2021 | Case report and literature review | To explain a case of COVID‐19‐related colitis along with distention and ischaemia | USA | Tocilizumab infusion can also be added after bowel resection to the management to treat COVID‐19 |
4.5. Limitations
This study is not without its limitations. COVID‐19 is still very much under investigation. Currently, there is not enough material for us to confidently establish causation and management of COVID‐19‐induced bowel ischaemia. Another point to note is that this is a retrospective study. While we are able to identify cases where AMI has occurred, we are unable to identify the baseline exposure status of such patients. Without this, we cannot point out the factors that lead to patients developing mesenteric ischaemia. Prospective studies in this direction will go a long way in understanding this complication. In addition, the pathophysiology of COVID‐19‐induced bowel ischaemia is not well‐established. This is an important area of research that requires further exploration to help define this mechanism.
5. CONCLUSION
Acute mesenteric ischemia does seem to be a rare complication of COVID‐19. This review article underscores the importance of placing AMI in the workup of such a patient with COVID‐19 presenting with manifestations of the gut, most commonly acute abdomen. Elevated lactate, LDH, and d‐dimer levels can be considered while assessing a patient of COVID‐19 with abdominal pain for mesenteric ischaemia. Ultimately, Computed Tomography of the abdomen helps clinch the diagnosis. However, more prospective studies are required to assess the laboratory diagnostics to help suspect as well as confirm bowel ischaemia in a patient with COVID‐19. Immediate intervention may help provide better outcomes. It is important to acknowledge that adequate thrombolysis and hemodynamic support should be implemented as they may help maintain homeostasis thereby aiding in early diagnosis or even preventing this complication.
DISCLOSURES
No author has any conflicts of interest.
Patel S, Parikh C, Verma D, et al. Bowel ischaemia in COVID‐19: A systematic review. Int J Clin Pract. 2021;75:e14930. doi: 10.1111/ijcp.14930
DATA AVAILABILITY STATEMENT
Data used in this study were a re‐analysis of existing data, which are openly available at locations cited in the reference section. This data are publicly available to everyone at Pubmed Central [https://pubmed.ncbi.nlm.nih.gov/] and Google scholar [https://scholar.google.com/]. No datasets were generated or analysed during this study.
REFERENCES
- 1. Waleed MS, Sadiq W, Azmat M. Understanding the mosaic of COVID‐19: a review of the ongoing crisis. Cureus. 2020;12(3):e7366. 10.7759/cureus.7366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abou‐Ismail MY, Diamond A, Kapoor S, Arafah Y, Nayak L. The hypercoagulable state in COVID‐19: incidence, pathophysiology, and management. Thromb Res. 2020;19:101‐115. 10.1016/j.thromres.2020.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kaur S, Bansal R, Kollimuttathuillam S, et al. The looming storm: blood and cytokines in COVID‐19. Blood Rev. 2021;46:100743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kaur P, Posimreddy S, Singh B, Qaqa F, Habib HA, Maroules M. COVID‐19 presenting as acute limb ischaemia. Eur J Case Rep Intern Med. 2020;7(6):e001724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Singh B, Kaur P, Ajdir N, Gupta S, Maroules M. COVID‐19 presenting as acute limb ischemia. Cureus. 2020;12(7):e9344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine. 2009;6(7):e1000097. 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang D, Hu BO, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus—infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061‐1069. 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708‐1720. 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pan L, Mu MI, Yang P, et al. Clinical characteristics of COVID‐19 patients with digestive symptoms in Hubei, China: a descriptive, cross‐sectional, multicenter study. Am J Gastroenterol. 2020;115(5):766‐773. 10.14309/ajg.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cheung KS, Hung IF, Chan PP, et al. Gastrointestinal manifestations of SARS‐CoV‐2 infection and virus load in fecal samples from the Hong Kong cohort and systematic review and meta‐analysis. Gastroenterology 2020;159(1):81‐95. 10.1053/j.gastro.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sultan S, Altayar O, Siddique SM, et al. AGA institute rapid review of the GI and liver manifestations of COVID‐19, meta‐analysis of international data, and recommendations for the consultative management of patients with COVID‐19. Gastroenterology. 2020;159(1):320‐334.e27. 10.1053/j.gastro.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Han C, Duan C, Zhang S, et al. Digestive symptoms in COVID‐19 patients with mild disease severity: clinical presentation, stool viral RNA testing, and outcomes. Am J Gastroenterol. 2020;115(6):916‐923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mao R, Qiu Y, He J‐S, et al. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID‐19: a systematic review and meta‐analysis. Lancet Gastroenterol Hepatol. 2020;5(7):667‐678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Noda S, Ma J, Romberg EK, et al. Severe COVID‐19 initially presenting as mesenteric adenopathy. Pediatr Radiol. 2021;51:140‐143. 10.1007/s00247-020-04789-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Łaski D, Biernat K, Kaska Ł. Pneumatosis intestinalis due to COVID‐19 infection in kidney transplant recipient: a case report. Transplant Proc. 2021;53(4):1215‐1218. 10.1016/j.transproceed.2021.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zeng W, Qi K, Ye M, et al. Gastrointestinal symptoms are associated with severity of coronavirus disease 2019. Eur J Gastroenterol Hepatol. 2021. Feb 25. 10.1097/MEG.0000000000002072. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 17. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim JY, Choe PG, Oh Y, et al. The first case of 2019 novel coronavirus pneumonia imported into Korea from Wuhan, China: implication for infection prevention and control measures. J Korean Med Sci. 2020;35:e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tang A, Tong ZD, Wang HL, et al. Detection of novel coronavirus by RT‐PCR in stool specimen from asymptomatic child, China. Emerg Infect Dis. 2020;26:1337‐1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323:1061‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fix OK, Hameed B, Fontana RJ, et al. Clinical best practice advice for hepatology and liver transplant providers during the COVID‐19 pandemic: AASLD Expert Panel Consensus Statement. Hepatology. 2020;72:287‐304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Singh B, Kaur P. COVID‐19 and acute mesenteric ischemia: a review of literature. Hematol Transfus Cell Ther. 2021;43:112‐116. 10.1016/j.htct.2020.10.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rodriguez‐Nakamura RM, Gonzalez‐Calatayud M, Martinez Martinez AR. Acute mesenteric thrombosis in two patients with COVID‐19. Two cases report and literature review. Int J Surg Case Rep. 2020;76:409‐414. 10.1016/j.ijscr.2020.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Parry AH, Wani AH, Yaseen M. Acute mesenteric ischemia in severe coronavirus‐19 (COVID‐19): possible mechanisms and diagnostic pathway. Acad Radiol. 2020;27(8):1190. 10.1016/j.acra.2020.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ignat M, Philouze G, Aussenac‐Belle L, et al. Small bowel ischemia and SARS‐CoV‐2 infection: an underdiagnosed distinct clinical entity. Surgery. 2020;168(1):14‐16. 10.1016/j.surg.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Paul T, Joy AR, Alsoub HARS, Parambil JV. Case report: ischemic colitis in severe COVID‐19 pneumonia: an unforeseen gastrointestinal complication. Am J Trop Med Hyg. 2021;104(1):63‐65. 10.4269/ajtmh.20-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Swami GA, Shinde SK, Chandrashekha SH, Asawa G. Gangrenous bowel ischemia‐a complication of COVID‐19: a case report. Int Surg J. 2021;8(5):2349‐2902. 10.18203/2349-2902.isj20211854. [DOI] [Google Scholar]
- 28. Calcagno E, Sogunro O, Nepal P, Assaker R, Sapire J. COVID‐19 induced mesenteric venous infarction. Radiol Case Rep. 2021;16(8):1999‐2002. 10.1016/j.radcr.2021.04.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lui K, Wilson MP, Low G. Abdominal imaging findings in patients with SARS‐CoV‐2 infection: a scoping review. Abdom Radiol. 2021;46(3):1249‐1255. 10.1007/s00261-020-02739-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Costa AF, Chidambaram V, Lee JJ, Asquith J, Skaff ER, Thipphavong S. Multidetector computed tomography of mesenteric ischaemia. Insights Imaging. 2014;5(6):657‐666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Expert Panels on Vascular Imaging and Gastrointestinal Imaging , Ginsburg M, Obara P, et al. ACR appropriateness criteria® imaging of mesenteric ischemia. J Am Coll Radiol. 2018;15(11S):S332‐S340. 10.1016/j.jacr.2018.09.018. [DOI] [PubMed] [Google Scholar]
- 32. Holleb P, Patel P, Saxena P, Beniwal J, Zuberi J. Acute abdomen in a 54‐year‐old COVID‐19 patient: a case report. J Surg Case Rep. 2021;2021:rjab198. 10.1093/jscr/rjab198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cheung S, Quiwa JC, Pillai A, Onwu C, Tharayil ZJ, Gupta R. Superior mesenteric artery thrombosis and acute intestinal ischemia as a consequence of COVID‐19 infection. Am J Case Rep. 2020;21:e925753. 10.12659/AJCR.925753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gartland RM, Velmahos GC. Bowel necrosis in the setting of COVID‐19. J Gastrointest Surg. 2020;24:2888‐2889. 10.1007/s11605-020-04632-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pang JHQ, Tang JH, Eugene‐Fan B, Lee CL, Low JK. A peculiar case of small bowel stricture in a corona virus disease 2019 patient with congenital adhesion of small bowel stricture in a corona virus disease 2019 patient with congenital adhesion band and superior mesenteric vein hrombosis. Ann Vasc Surg. 2021;70:286‐289. 10.1016/j.avsg.2020.08.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Al Argan RJ, Alqatari SG, Al Said AH, et al. Gastrointestinal perforation secondary to COVID‐19. Medicine. 2021;100(19):e25771. 10.1097/MD.0000000000025771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Varshney R, Bansal N, Khanduri A, Gupta J, Gupta R. Colonic gangrene: a sequela of coronavirus disease 2019. Cureus. 2021;13(4):e14687. 10.7759/cureus.14687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Drakos P, Volteas P, Cleri NA, et al. Acute gastrointestinal injury and feeding intolerance as prognostic factors in critically ill COVID‐19 patients. J Gastrointest Surg. 2021. Apr 27. 10.1007/s11605-021-05015-z. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Krothapalli N, Jacob J. A rare case of acute mesenteric ischemia in the setting of COVID‐19 infection. Cureus. 2021;13(3):e14174. 10.7759/cureus.14174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bannazadeh M, Tassiopoulos A, Koullias G. Acute superior mesenteric artery thrombosis seven days after discharge for novel coronavirus pneumonia (NCP). J Vasc Surg Cases Innov Tech. 2021;7:586–588. 10.1016/j.jvscit.2020.12.002. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Alharthy A, Balhamar A, Faqihi F, et al. Rare case of COVID‐19 presenting as acute abdomen and sepsis. New Microbes New Infect. 2020;38:100818. 10.1016/j.nmni.2020.100818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kariyawasam JC, Jayarajah U, Riza R, Abeysuriya V, Seneviratne SL. Gastrointestinal manifestations in COVID‐19. Trans R Soc Trop Med Hyg. 2021;115:1362–1388. trab042. 10.1093/trstmh/trab042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tang N. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094‐1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dinoto E, Ferlito F, La Marca MA, Mirabella D, Bajardi G, Pecoraro F. Staged acute mesenteric and peripheral ischemia treatment in COVID‐19 patient: case report. Int J Surg Case Rep. 2021;84:106105. 10.1016/j.ijscr.2021.106105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Balani P, Bhuiyan AS, Dalal VN, Maheshwari GS. Early detection and successful management of acute mesenteric ischaemia in symptomatic COVID‐19 patient. Indian J Surg. 2021;2:1‐3. 10.1007/s12262-021-02839-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mariette X, Hermine O, Tharaux P, et al. Effectiveness of tocilizumab in patients hospitalized with COVID‐19: a follow‐up of the CORIMUNO‐TOCI‐1 randomized clinical trial. JAMA Intern Med. 2021;181(9):1241. 10.1001/jamainternmed.2021.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shaikh DH, Patel H, Makker J, Badipatla K, Chilimuri S. Colonic ileus, distension, and ischemia due to COVID‐19‐related colitis: a case report and literature review. Cureus. 2021;13(2):e13236. 10.7759/cureus.13236. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data used in this study were a re‐analysis of existing data, which are openly available at locations cited in the reference section. This data are publicly available to everyone at Pubmed Central [https://pubmed.ncbi.nlm.nih.gov/] and Google scholar [https://scholar.google.com/]. No datasets were generated or analysed during this study.


