Abstract
Objective
To identify barriers and enablers to COVID‐19 vaccination in renal transplant recipients who are undecided about vaccination.
Methods
An online survey was distributed to 876 adult kidney transplant recipients at a tertiary referral service, who had not been vaccinated against COVID‐19. The survey assessed willingness to be vaccinated, attitudes toward COVID‐19 vaccines, and barriers and enablers to proceeding with vaccination.
Results
The survey response rate was 54% (473/876). Three hundred and forty‐six (73.1%) participants planned to receive vaccination (yes group), 105 (22.2%) were undecided, and 22 (4.7%) refused vaccination. The undecided group were younger but were not different in other demographic characteristics to the yes group. The undecided group were less positive toward (34.29% vs. 91.3%, p < .001) and more concerned about (93.3% vs. 25.1%, p < .001) vaccination than the yes group. Their concerns related to vaccine safety (including harm to their transplant), poor efficacy, and a lack of rigorous testing in transplant recipients. Undecided recipients had received less vaccine‐specific information from medical specialists than the yes group. Most undecided participants (95.1%) were willing to proceed with vaccination with appropriate supports. The most desired supports were information and a recommendation to proceed with vaccination from their treating transplant specialist and team.
Conclusion(s)
Concerns about vaccine safety (including harm to transplant), poor vaccine efficacy, and lack of rigorous testing were barriers to vaccine uptake. Most undecided recipients would proceed with vaccination with specific recommendations and vaccine information provided by their transplant specialist/team. These simple interventions can be readily implemented to optimize vaccine uptake.
Keywords: renal transplantation, SARS‐CoV2 vaccine, vaccination promotion, vaccine hesitancy, vaccine intention, vaccine refusal
22.2% of transplant recipients were undecided about having the COVID‐19 vaccine. This group were less positive and more concerned about the vaccine. Concerns about vaccine safety, insufficient vaccine information and concerns about vaccine efficacy were barriers to vaccine uptake whereas information and recommendation to have the vaccine by their transplant specialist/team and transplant clinic consultation were enablers of vaccine uptake. The majority (95.1%) would proceed with vaccination with the right supports.

1. INTRODUCTION
Vaccine hesitancy is defined by the World Health Organization (WHO) as a ‘delay in acceptance or refusal of vaccines despite availability of vaccination services,’ and was declared by the organization to be one of the top 10 global threats to health in 2019. 1 , 2 The current COVID‐19 pandemic has amplified the significance of vaccine hesitancy as improving national and global health and economic status relies heavily on high vaccine uptake. The emergence of COVID‐19 variants with enhanced transmissibility (such as delta variant) has highlighted the need to vaccinate populations comprehensively and quickly.
At the time of this study, the vaccination program in Australia had just commenced, however, by September 29, 2021, 52.6% of the Australian population aged 16 had been double vaccinated. 3
Solid organ transplant recipients (SOTR) are at risk of severe disease and high mortality from COVID‐19 but the data are unfolding and complicated. 4 , 5 Earlier observational studies reported a much higher mortality rate in transplant recipients with COVID‐19 infection compared to the general population. 6 , 7 , 8 However, several studies have suggested that short‐term mortality in SOTR from COVID‐19 is similar to immunocompetent patients, with the postulated reason being protection by blunting of the immune responses due to chronic immunosuppression. 4 There are emerging reports that certain subgroups of SOTR may face worse outcomes in terms of greater morbidity. 4
Responses to COVID‐19 vaccination are diminished in SOTR compared with immunocompetent adults. 9 It is possible that vaccination may still offer some protection against severe COVID‐19 despite incomplete humoral responses and vaccination is widely recommended for transplant recipients. 4
Vaccine hesitancy needs to be addressed for SOTR for both their own protection from severe COVID‐19 but additionally, increased and prolonged viral shedding seen in transplant recipients and other immunosuppressed cohorts increases the risk of viral transmission and development of viral variants. 10 , 11 , 12
Hesitancy to COVID‐19 vaccination in Australia was recently estimated to be 36% of the general population. 13 Complacency about the perceived threat of the vaccine‐preventable disease, inconvenience associated with vaccine access, vaccine misinformation, and lack of confidence in the vaccine and public health officials have impacted vaccine uptake in general. 2 , 14 As transplant recipients were excluded from the major vaccine trials, transplant recipients may be uncertain about vaccine safety, potentially increasing hesitancy. 15 , 16 , 17 Vaccine hesitant populations are comprised of a larger undecided group and a smaller outright refusal group. Those who refuse have firmly held views which are largely unamenable to change while the undecided group are potentially open to vaccination. 18 A recent population‐based study of COVID‐19 vaccine hesitancy found that education that addressed the individual risk–benefit balance of COVID‐19 vaccination was more effective at reducing hesitancy than addressing the collective benefits of vaccination or seriousness of the pandemic. 19
Understanding the complex and multifactorial reasons behind vaccine hesitancy in SOTR and channeling resources to address these is crucial to optimize uptake. We sought to assess understanding, attitudes, and hesitancy to COVID‐19 vaccination in kidney transplant recipients. We focused on those who were undecided about vaccination in order to identify barriers and enablers of vaccine acceptance that can be addressed to optimize vaccine coverage in this susceptible population.
2. PATIENTS AND METHODS
2.1. Study design and population
We conducted a cross‐sectional survey of kidney and kidney/pancreas transplant recipients at Monash Health, a tertiary referral center in Victoria, Australia. Monash Health is a 1500‐bed academic health service that performs approximately 100 transplants annually, comprised of approximately 85 kidney and 15 simultaneous pancreas and kidney (SPK) transplants. At the time of the survey, we were providing ongoing care for 978 prevalent transplant recipients of whom 868 were kidney transplant recipients and 110 were SPK transplant recipients. All prevalent adult (age ≥18 years) transplant recipients for whom Monash Health was providing ongoing care were eligible to participate if they had not yet received COVID‐19 vaccination. In Australia, most transplant recipients were deemed eligible for COVID‐19 vaccination as of March 22, 2021 (classed as Category 1b). Of our transplant cohort, only 12 had received COVID‐19 vaccination when the study commenced, due to their early eligibility as quarantine/frontline/healthcare/aged care workers (Category 1a). The survey was delivered electronically to all transplant recipients for whom we were able to obtain valid email addresses prior to study commencement (876 of 978 (90%)). Reminder emails were sent twice to survey nonresponders to increase the response rate. The survey was not delivered by any other means. At the time of the survey, there had not been any systematic education regarding the vaccine provided to transplant recipients by Monash Health. The study took place over 2 weeks (March 17 to April 1, 2021) and was approved by the human research ethics committee of Monash Health (Project number: RES‐21‐0000‐148Q‐74425).
2.2. Survey and data collection
The survey was adapted from a survey designed to examine vaccine hesitancy in rheumatic disease. 20 The investigators adapted the survey questions to be relevant to transplant recipients while ensuring they incorporated the three main domains of vaccine hesitancy determinants (confidence, complacency, and convenience), and the identified barriers and enablers to vaccine acceptance as specified by the WHO's SAGE vaccine hesitancy working group. 2
Questions were presented in yes/no, multiple choice, and 5‐point Likert scale formats and assessed participant demographic and clinical characteristics, intention to receive vaccination, vaccine attitudes and understanding, information sources, and barriers and enablers of vaccination. Questions pertaining to barriers and enablers addressed the undecided cohort only. Free text fields were available for participants to express their thoughts in more detail. All responses were anonymous.
2.3. Definitions
Participants were categorized into three groups by intention to receive vaccination: yes (planning to have vaccination), undecided (unsure if will have vaccination), and no (planning not to have vaccination). Because our study was interested in contrasts to vaccine uptake in undecided individuals to those in the yes group, those refusing vaccination (no group) were not further considered.
2.4. Statistical analysis
Categorical variables were summarized using frequency and percentage. Continuous variables were summarized using mean and standard deviation (SD) or median and interquartile range (IQR) as appropriate. Comparisons of proportions between the yes and undecided groups were made using Pearson's Chi‐squared test. Comparison of two independent samples used the Student's t‐test. For ordered responses the Cochrane–Armitage test for trend was used. Finally, for two‐way comparisons of multiple responses, p‐values were adjusted for multiple testing (Bonferroni method). Analyses were conducted using Stata version 16 (StataCorp., College Station, TX, USA).
3. RESULTS
3.1. Study participants
Of the 876 surveys sent, 473 were returned giving a response rate of 54%. Of the 37 survey questions, 33 had five or less missing responses. Three questions had eight missing responses and one question had 17 missing responses. All results shown are the proportion of the number of responses received for each question.
Comparison of the characteristics of participants categorized by the yes (would have vaccination) group (n = 346) and undecided group (n = 105) is presented in Table 1. The two groups were largely similar except for age where the undecided group were younger (mean age 54.7 years, SD 12.5) than the yes group (58.5 years, SD 12.1, p = .01), and the yes group were more likely to have been vaccinated to influenza in the preceding year (n = 303, 87.6% vs. n = 82, 78.1%, respectively, p = .02). No participant had previously contracted COVID‐19 infection while three had prior contact with a confirmed COVID‐19 positive case (one in the yes group and two in the undecided group).
TABLE 1.
Participant characteristics according to yes and undecided groups
| Demographic categories | Yes (n = 346) | Undecided (n = 105) | p‐Value |
|---|---|---|---|
| Female | 208 (60.6) | 57 (54.3) | .25 |
| Age | 58.53 (12.09) | 54.70 (12.49) | .01 |
| Transplant vintage | .51 | ||
| 2011–2021 | 232 (68.4) | 71 (68.3) | |
| 2001–2010 | 74 (21.8) | 21 (20.2) | |
| 1991–2000 | 28 (8.3) | 8 (7.7) | |
| 1976–1990 | 5 (1.5) | 4 (3.9) | |
| Immunosuppression | |||
| Mycophenolate | 289 (83.5) | 82 (78.1) | .20 |
| Tacrolimus | 297 (85.8) | 91 (86.7) | .83 |
| Prednisolone | 286 (82.7) | 80 (76.2) | .14 |
| Azathioprine | 33 (9.5) | 13 (12.4) | .40 |
| Cyclosporine | 36 (10.4) | 9 (8.6) | .58 |
| mtor inhibitor a | 13 (3.8) | 1 (1) | .15 |
| Medical comorbidities | |||
| Cardiovascular disease | 58 (16.8) | 16 (15.2) | .71 |
| Hypertension | 205 (59.3) | 63 (60) | .89 |
| Chronic lung disease | 19 (5.5) | 6 (5.7) | .93 |
| Diabetes mellitus | 99 (28.6) | 28 (26.7) | .70 |
| Past influenza vaccination | 303 (87.6) | 82 (78.1) | .02 |
| Highest level of education | .63 | ||
| Primary school | 8 (2.3) | 2 (1.9) | |
| High school | 118 (34.3) | 45 (43.3) | |
| Certificate | 58 (16.9) | 15 (14.4) | |
| Diploma | 64 (18.6) | 14 (13.5) | |
| Bachelor's degree | 58 (16.9) | 18 (17.3) | |
| Postgraduate degree | 38 (11.1) | 10 (9.6) | |
| Occupation | .06 | ||
| Student | 3 (0.9) | 1 (1) | |
| Employed | 141 (41.2) | 42 (40) | |
| Unemployed | 43 (12.6) | 24 (22.9) | |
| Retired | 155 (45.3) | 38 (36.2) | |
Note: Data are presented as mean (standard deviation) for continuous measures, and n (%) for categorical measures.
mtor inhibitor: mammalian target of rapamycin Inhibitor.
Twenty‐two participants (4.7% of the returned surveys) responded that they would not have the vaccine (refusal) and are not considered further. For completeness however, relative to the other two groups they were on average younger (47.5 years, SD 15.0), more likely to be a student or unemployed and less likely to have received other vaccines previously, but were not different in terms of gender ratio, education level, and medical comorbidities.
3.2. Attitudes to COVID‐19 vaccination
While the two groups expressed similar levels of concern about their risk of contracting COVID‐19 (Figure S1) there were differences in their attitudes to vaccination. The majority of the yes group had positive feelings and felt relieved (n = 314, 91%) that the vaccine was available compared to 34.3% (n = 36) and 51.5% (n = 53), respectively, in the undecided group (p < .001 for both). Likewise, 98 (93.3%) of the undecided group had concerns about vaccination compared with 86 (25.1%) in the yes group (p < .001) (Figure 1). Overall, the yes group were overwhelmingly in favor of receiving both the influenza and COVID‐19 vaccinations (n = 338, 98.8%). Just over half (n = 51, 55.4%) of the undecided participants were willing to have influenza vaccination but not COVID‐19 vaccination while 35.9% (n = 33) were prepared to have both and 8.7% (n = 8) would not commit to either.
FIGURE 1.
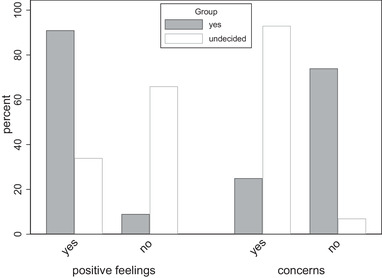
Attitudes toward the COVID‐19 vaccine by vaccine intention group
3.3. Vaccine knowledge by vaccination intention
Agreement with various aspects of vaccine knowledge is presented in Figure 2. Participants in the yes group were more likely to agree that the vaccines were effective and safe as well as being less concerned about vaccine side effects than the undecided group (p < .001 for all). Despite opting to have vaccination, 127 (36.8%) participants in the yes group agreed that they had concerns that vaccination could cause harm to their transplant. This concern was even more prevalent in the undecided group (n = 78, 75%, p < .001). One‐third of the yes group agreed that there had been insufficient vaccine testing (n = 109, 31.5%) compared to 76% (n = 79) of the undecided group (p < .001).
FIGURE 2.
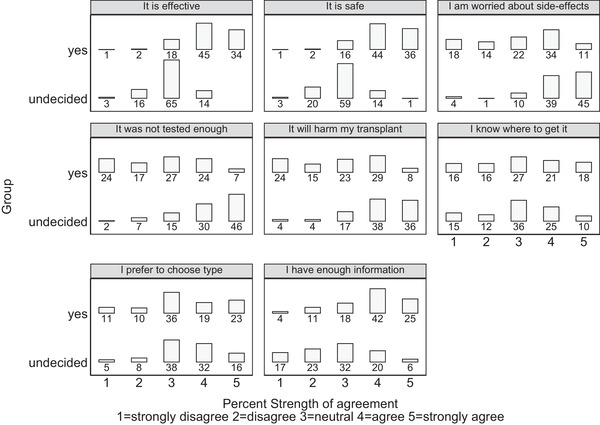
COVID‐19 vaccine knowledge by intention to receive vaccine
Agreement between the two groups was similar regarding preference in the choice of vaccine as well as knowledge regarding when and where to get vaccinated.
When asked specifically about where they anticipated having the vaccine, most recipients from the yes and undecided groups preferred to have the vaccine at the hospital transplant or vaccination clinic (n = 221, 63.9% and n = 52, 51%, respectively, p = .10) while most of the remainder were unsure (n = 105, 30.4% and n = 44, 43.1%, respectively).
3.4. Vaccine information by vaccination intention
The majority of participants in both groups had received some form of vaccine‐specific information (n = 240, 71% vs. n = 69, 65.7% yes versus undecided groups, respectively, p = .30). More than 67% (n = 232) of the yes group agreed that they had received enough vaccine information compared with just 26.2% (n = 27) in the undecided group (p < .001).
Kidney/other medical specialist (n = 237, 60.6%), news services (n = 164, 41.9%), and government sources (n = 144, 36.8%) were the most common sources of vaccine information cited by participants (Figure S2). Compared to the undecided group, the yes group were more likely to have received vaccine‐specific information from their nephrologist/other medical specialist (n = 189, 54.6% vs. n = 38, 36.2%, p = .007). There were no significant differences in information obtained from other sources.
3.5. Vaccination and other infection prevention strategies
Prior to vaccine availability, most participants from the yes and undecided groups practiced social distancing (n = 318 (91.9%) vs. n = 93 (88.6%), p = .3), mask wearing (n = 329 (95.1%) vs. n = 98 (93.3%), p = .48), and hand hygiene (n = 336 (97.1%) vs. n = 101 (96.2%), p = .63).
Participants were asked to rate the efficacy of vaccination and other infection prevention strategies to reduce the risk of contracting and transmitting COVID‐19 from 1 (least effective) to 5 (most effective). Results are presented in Figure 3. Of the total cohort, 318 (68.4%) participants rated vaccination as effective (score of 4 or 5), while hand washing, social distancing, mask wearing, and the combination of these three measures were consistently rated as more effective at 85.4%, 82.8%, 77.9%, and 92.8%, respectively.
FIGURE 3.
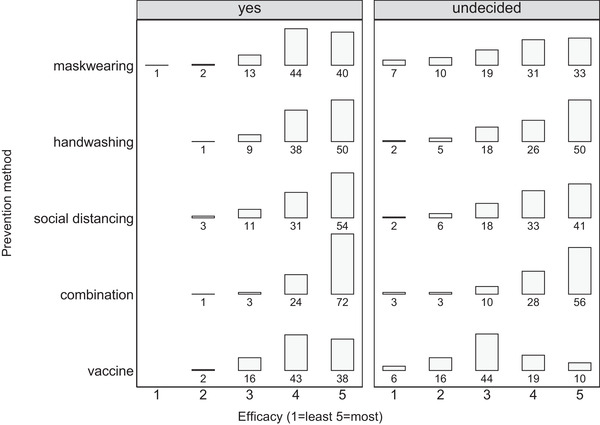
Percent perceived efficacy of COVID‐19 infection prevention methods by vaccination intention. Combination = mask wearing + hand washing + social distancing
In general, a significantly lower proportion in the undecided group rated the nonvaccination approaches as effective compared with the yes group (Figure 3), all p‐values < .05. However, the most marked difference between groups related to vaccination, with only 31% (n = 31) of the undecided group rating it 4 or 5 compared to 81.9% (n = 281) of yes group, p < .001.
3.6. Enablers and barriers to vaccination
Undecided participants were asked about barriers and enablers to vaccination (Figure 4). The main perceived barrier to vaccine uptake was vaccine safety (n = 84, 80%) with concerns largely relating to the COVID‐19 vaccine and/or their transplant. The second most frequent barrier was a lack of knowledge about the vaccines (n = 66, 62.9%), while concern about poor vaccine efficacy in the transplant population (n = 43, 41%) was also common. Other potential barriers to vaccination such as a perceived low risk of COVID‐19 infection (3.2%), presence of medical comorbidities (4.6%), a dislike of injections (2.7%), not knowing where to get vaccinated (5.7%), inconvenience of travel (0%), and fear of allergic reactions (1.4%) were not a major concern for most participants.
FIGURE 4.
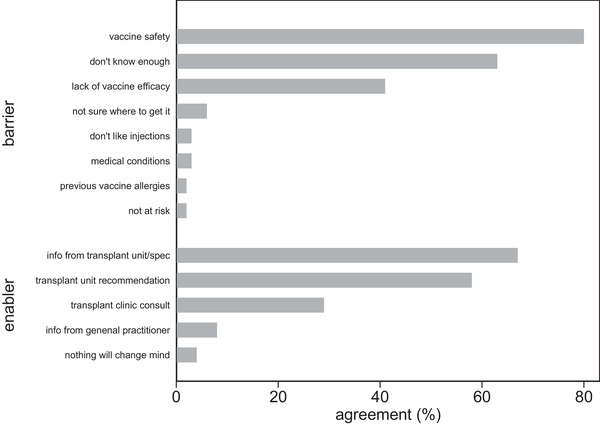
Barriers and enablers of COVID‐19 vaccine acceptance in undecided recipients (% per category)
Vaccine‐specific information provided by their kidney specialist/transplant team (n = 70, 66.7%), recommendations to have vaccination by their kidney specialist/transplant team (n = 61, 58.1%), and a transplant clinic consultation (n = 30, 28.6%) were identified as important factors to increase the likelihood of vaccine acceptance. A smaller proportion (n = 8, 7.6%) felt that information from their general practitioner would be beneficial. Only four (3.8%) undecided participants felt that no additional supports would change their minds regarding vaccination.
4. DISCUSSION
Vaccine hesitancy is a growing concern that undermines national and global efforts to control the COVID‐19 pandemic. We present emerging data regarding COVID‐19 vaccine attitudes and intentions in a large kidney transplant cohort. The major finding from this study is substantial vaccine hesitancy, primarily comprised of vaccination undecided individuals. Although undecided transplant recipients felt less positive and more concerned about COVID‐19 vaccines, more than 95% were open to vaccination given appropriate supports.
In international studies in the general population, vaccine hesitancy has ranged from 3% to 41.1%. 21 , 22 , 23 A recent Australian survey found 36% of the population were vaccine hesitant. 13 There is limited data on international COVID‐19 vaccine hesitancy in transplant populations. Two studies of US cohorts report vaccine hesitancy rates in SOTR to be 34% and 49%. 24 , 25 By comparison, we found 26.9% to be hesitant with 83% of those, undecided rather than outright refusers. While this is lower than the general Australian population and the reported solid organ transplant cohorts in the United States, it remains very concerning considering the substantially increased risk of infection and death in immunosuppressed individuals.
Since declaration of the pandemic, the comparatively lower total COVID‐19 cases and deaths in Australia has likely contributed significantly to the higher vaccine hesitancy and lower vaccination rates. 26 By contrast countries with higher infection rates such as the United States, United Kingdom, Canada, Israel, Spain, and Italy along with many other countries (predominantly in Europe) have higher total population vaccination rates. 26 Irrespective of local case numbers and outbreak status, in order to achieve control over the far‐reaching health, psychosocial and economic impacts of COVID‐19 it is critical that we continue to assess and address vaccine hesitancy, particularly in the low uptake countries and vulnerable subpopulations.
Similar to previous studies, younger age was associated with vaccine hesitancy, however we did not find an association with female gender, employment status, or education levels. 21 , 27 , 28 Additionally, poor uptake of other vaccines has been reported to correlate with current vaccine hesitancy. 29 , 30 Among COVID‐19 vaccine‐undecided transplant recipients in the current study, most had received influenza vaccination in previous years and planned to do so again, revealing a lack of anti‐vaccine sentiments but rather concerns specific to COVID‐19 vaccination.
Three categories of vaccine hesitancy determinants: complacency, convenience, and confidence are described by the WHO. 2 , 14 Complacency and convenience were not significant factors affecting vaccine uptake in this cohort. Participants were highly concerned about the risk of COVID‐19 infection and did not find logistical issues a barrier. Confidence, however, was the significant factor, particularly in the undecided group. Like the general population, this chiefly related to concerns about safety, including a perceived lack of rigorous vaccine testing and a perception of poor efficacy. Specific to transplant recipients was concern regarding harm to their transplant, including transplant rejection. A large proportion of the nonhesitant group also had concerns about side effects and harm to their transplant, underlining the importance of addressing this specific issue in transplant recipients.
Poor vaccine efficacy was a significant barrier to vaccine uptake, which coupled with the perceived risk of harm threatens to sway the perceived risk–benefit balance against vaccine acceptance in the undecided cohort.
Some of the concerns expressed, pertaining to harm and poor vaccine efficacy were valid at the time of the survey given the lack of trial data involving transplant recipients and many preliminary studies showing reduced humoral and cellular vaccine responses in transplant recipients. 9 , 31 , 32 Promising evidence of improved sero‐responses through booster doses may help alleviate efficacy concerns. 33 , 34 With respect to harm, reassuringly, increased adverse immunological sequalae, including transplant rejection, have not been convincingly demonstrated following vaccination against other pathogens or thus far against COVID‐19. 35 , 36
Transplant recipients rated mask wearing, social distancing, and hand hygiene as more effective than vaccination, reinforcing the finding of their lack of confidence in vaccine efficacy. Again, this view may be accurate if vaccine responses are inadequate in immunosuppressed populations and data specific to transplant recipients will aid in improving confidence in vaccination.
A strong and persistent physician recommendation to undergo vaccination is reported to be a highly effective method of increasing vaccine acceptance. 14 , 37 Undecided participants in this study felt that they lacked sufficient information regarding COVID‐19 vaccination and felt that information and a recommendation to proceed with vaccination from their specialist or the transplant team, particularly in a transplant clinic consultation, would increase their likelihood of proceeding with vaccination. This implies a desire for tailored information relevant to their specific medical circumstance delivered by their usual treating team. We are now addressing this issue to optimize the proportion of our patients who are vaccinated while encouraging ongoing use of other infection prevention measures. Finally, in this study, we did not examine responses from participants who were absolutely against vaccination. They represent a small group who are highly resistant to vaccination and present a substantial challenge to protect from COVID‐19 infection. Further studies are needed to specifically address this group to determine what interventions or supports might be of use in getting them to change their views on vaccination.
5. LIMITATIONS
Vaccine attitudes and uptake intentions are likely to vary over time and place, depending on the perceived risk–benefit ratio following the reporting of side effects and local case rates and outbreaks. The survey was undertaken prior to age restrictions imposed on access to the AstraZeneca vaccine in Australia. While the response rate was very good it is possible that not all subgroups of the target population were represented given it was only delivered in English and a higher response rate might have yielded further themes relating to vaccine hesitancy. As the survey was anonymous, we were unable to define the characteristics of the nonresponder group to examine this. Additionally, while we did assess employment status and level of education, we did not collect additional socioeconomic factors, residential remoteness, or specific health literacy, which may influence vaccine uptake.
6. CONCLUSION
Vaccine hesitancy is a major public health concern, greatly impacting efforts to achieve herd immunity against COVID‐19 and places vulnerable populations at greater risk. Undecided transplant recipients had concerns pertaining to vaccine safety, effectiveness, lack of vaccine information, and inadequate vaccine research. Tailored vaccine‐specific information and a recommendation from their specialist or transplant team were identified as key mechanisms to increase vaccine acceptance. The overwhelming majority of the undecided transplant recipients were willing to proceed to vaccination, suggesting that investing in targeted interventions in this vulnerable population would likely be highly effective.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
Supplementary Figure 1. Bar graph comparing the strength of agreement to question ‘I am worried about getting COVID’ in the yes and undecided groups.
Supplementary Figure 2. Comparing the sources of vaccine information (proportions) among the yes and undecided groups.
Tharmaraj D, Dendle C, Polkinghorne KR, Mulley WR. Kidney transplant recipients’ attitudes toward COVID‐19 vaccination and barriers and enablers to vaccine acceptance. Transpl Infect Dis. 2022;24:e13749. 10.1111/tid.13749
REFERENCES
- 1. Friedrich M. WHO's top health threats for 2019. JAMA. 2019;321(11):1041. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization: SAGE Working Group on Vaccine Hesitancy . Report of the sage working group on vaccine hesitancy. 2014.. Accessed April 21, 2021. https://www.who.int/immunization/sage/meetings/2014/october/1_Report_WORKING_GROUP_vaccine_hesitancy_final.pdf
- 3.Australian Government; Department of Health. COVID‐19 vaccines. 2021.. Accessed September 29, 2021. https://www.health.gov.au/initiatives-and-programs/covid-19-vaccines?gclid=Cj0KCQjwwNWKBhDAARIsAJ8HkhdlMIqLgOEE5Xx19iIPbzfcYKVOZ1FOdmIEp8wKkQCK7la1cWRnRisaAvu5EALw_wcB
- 4. Heldman MR, Kates OS. COVID‐19 in solid organ transplant recipients: a review of the current literature. Curr Treat Options Infect Dis. 2021;13(3):67‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pereira MR, Arcasoy S, Farr MA, et al. Outcomes of COVID‐19 in solid organ transplant recipients: a matched cohort study. Transpl Infect Dis. 2021;23(4):e13637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Akalin E, Azzi Y, Bartash R, et al. Covid‐19 and kidney transplantation. N Engl J Med. 2020;382(25):2475‐2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Elias M, Pievani D, Randoux C, et al. COVID‐19 infection in kidney transplant recipients: disease incidence and clinical outcomes. J Am Soc Nephrol. 2020;31(10):2413‐2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fernández‐Ruiz M, Andrés A, Loinaz C, et al. COVID‐19 in solid organ transplant recipients: a single‐center case series from Spain. Am J Transplant. 2020;20(7):1849‐1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2‐dose SARS‐CoV‐2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325(21):2204‐2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aydillo T, Gonzalez‐Reiche AS, Aslam S, et al. Shedding of viable SARS‐CoV‐2 after immunosuppressive therapy for cancer. N Engl J Med. 2020;383(26):2586‐2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Italiano J, Bush R, Acharya R, Upadhyay K. Persistent viral shedding despite seroconversion in a kidney transplant recipient with severe extrapulmonary COVID‐19. BMJ Case Rep. 2020;13(11):e239612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Man Z, Jing Z, Huibo S, Bin L, Fanjun Z. Viral shedding prolongation in a kidney transplant patient with COVID‐19 pneumonia. Am J Transplant. 2020;20(9):2626‐2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Edwards B, Biddle N, Gray M, Sollis K. COVID‐19 vaccine hesitancy and resistance: correlates in a nationally representative longitudinal survey of the Australian population. PLoS One. 2021;16(3):e0248892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Macdonald NE, SAGE Working Group on Vaccine Hesitancy . Vaccine hesitancy: definition, scope and determinants. Vaccine. 2015;33(34):4161‐4164. [DOI] [PubMed] [Google Scholar]
- 15. Major R, Selvaskandan H, Makkeyah YM, Hull K, Kuverji A, Graham‐Brown M. The exclusion of patients with CKD in prospectively registered interventional trials for COVID‐19 – a rapid review of international registry data. J Am Soc Nephrol. 2020;31(10):2250‐2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mulligan MJ, Lyke KE, Kitchin N, et al. Phase I/II study of COVID‐19 RNA vaccine BNT162b1 in adults [published correction appears in Nature. 2021;590(7844):e26]. Nature. 2020;586(7830):589‐593. [DOI] [PubMed] [Google Scholar]
- 17. Walsh EE, Frenck RW, Falsey AR, et al. Safety and immunogenicity of two RNA‐based Covid‐19 vaccine candidates. N Engl J Med. 2020;383(25):2439‐2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dubé È, Ward JK, Verger P, Macdonald NE. Vaccine hesitancy, acceptance, and anti‐vaccination: trends and future prospects for public health. Annu Rev Public Health. 2021;42:175‐191. [DOI] [PubMed] [Google Scholar]
- 19. Schwarzinger M, Luchini S. Addressing COVID‐19 vaccine hesitancy: is official communication the key? Lancet Public Health. 2021;6(6):e353‐e354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ko T, Dendle C, Woolley I, Morand E, Antony A. SARS‐COV‐2 vaccine acceptance in patients with rheumatic diseases: a cross‐sectional study. Hum Vaccin Immunother. Published online August 6, 2021. 10.1080/21645515.2021.1958611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Robertson E, Reeve KS, Niedzwiedz CL, et al. Predictors of COVID‐19 vaccine hesitancy in the UK household longitudinal study. Brain Behav Immun. 2021;94:41‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sallam M. COVID‐19 vaccine hesitancy worldwide: a concise systematic review of vaccine acceptance rates. Vaccines (Basel). 2021;9(2):160.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. United States Census Bureau . How do COVID‐19 vaccination and vaccine hesitancy rates vary over time? 2021.. Accessed June 20, 2021. https://www.census.gov/library/stories/2021/04/how‐do‐covid‐19‐vaccination‐and‐vaccine‐hesitancy‐rates‐vary‐over‐time.html
- 24. Ou MT, Boyarsky BJ, Zeiser LB, et al. Kidney transplant recipient attitudes toward a SARS‐CoV‐2 vaccine. Transplant Direct. 2021;7(7):e713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tsapepas D, Husain SA, King KL, Burgos Y, Cohen DJ, Mohan S. Perspectives on COVID‐19 vaccination among kidney and pancreas transplant recipients living in New York City. Am J Health Syst Pharm. Published online June 29, 2021.. doi:10.1093/ajhp/zxab272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Our World in Data . Statistics and research: coronavirus (COVID‐19) vaccinations. 2021. Accessed September 29, 2021. https://ourworldindata.org/covid‐vaccinations?country=OWID_WRL
- 27. KFF: COVID‐19 vaccine monitor . Does the public want to get a COVID‐19 vaccine? When? 2021.. Accessed June 1, 2021. https://www.kff.org/coronavirus-covid-19/dashboard/kff-covid-19-vaccine‐monitor‐dashboard/
- 28. Razai MS, Chaudhry UAR, Doerholt K, Bauld L, Majeed A. Covid‐19 vaccination hesitancy. BMJ. 2021;373:n1138. [DOI] [PubMed] [Google Scholar]
- 29. Lin C, Tu P, Beitsch LM. Confidence and receptivity for COVID‐19 vaccines: a rapid systematic review. Vaccines (Basel). 2020;9(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schwarzinger M, Watson V, Arwidson P, Alla F, Luchini S. COVID‐19 vaccine hesitancy in a representative working‐age population in France: a survey experiment based on vaccine characteristics. Lancet Public Health. 2021;6(4):e210‐e221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bertrand D, Hamzaoui M, Lemée V, et al. Antibody and T cell response to SARS‐CoV‐2 messenger RNA BNT162b2 vaccine in kidney transplant recipients and hemodialysis patients. J Am Soc Nephrol. 2021;32(9):2147‐2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stumpf J, Siepmann T, Lindner T, et al. Humoral and cellular immunity to SARS‐CoV‐2 vaccination in renal transplant versus dialysis patients: a prospective, multicenter observational study using mRNA‐1273 or BNT162b2 mRNA vaccine. Lancet Reg Health Eur. 2021;9:100178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Benotmane I, Gautier G, Perrin P, et al. Antibody response after a third dose of the mRNA‐1273 SARS‐CoV‐2 vaccine in kidney transplant recipients with minimal serologic response to 2 doses. JAMA. 2021;326(11):1063‐1065. 10.1001/jama.2021.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A. Safety of the first dose of SARS‐CoV‐2 vaccination in solid organ transplant recipients. Transplantation. 2021;105(5):e56‐e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Boyarsky BJ, Ou MT, Greenberg RS, et al. Safety of the first dose of SARS‐CoV‐2 vaccination in solid organ transplant recipients. Transplantation. 2021;105(5):e56‐e57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mulley WR, Dendle C, Ling JEH, Knight SR. Does vaccination in solid‐organ transplant recipients result in adverse immunologic sequelae? A systematic review and meta‐analysis. J Heart Lung Transplant. 2018;37(7):844‐852. [DOI] [PubMed] [Google Scholar]
- 37. Gargano LM, Herbert NL, Painter JE, et al. Impact of a physician recommendation and parental immunization attitudes on receipt or intention to receive adolescent vaccines [published correction appears in Hum Vaccin Immunother. 2014;10(9):2631]. Hum Vaccin Immunother. 2013;9(12):2627‐2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Bar graph comparing the strength of agreement to question ‘I am worried about getting COVID’ in the yes and undecided groups.
Supplementary Figure 2. Comparing the sources of vaccine information (proportions) among the yes and undecided groups.


