Abstract
Majority of transplant recipients did not develop an appreciable humoral response following SARS‐CoV‐2 vaccine, in contrast to dialysis patients and healthy individuals. We analyzed the serologic response to BNT162b2 (Pfizer‐BioNTech) vaccine in a cohort of 19 kidney transplant recipients, vaccinated prior to transplantation, compare to 109 recipients vaccinated after transplantation, and to 39 healthcare workers, by determining the level of anti‐spike antibodies after transplantation. All controls and 17 of 19 (90%) of recipients vaccinated before transplant were seropositive, while only 49 of 109 (45%) recipients vaccinated post‐transplant had positive serology (P < .001). Median anti‐spike IgG in the group of kidney transplant recipients vaccinated after transplantation (10.7 AU/ml, [IQR 0–62.5]) was lower than the patients vaccinated before transplantation (66.2 AU/ml [21.6–138]), which was significantly lower than in the controls (156 AU/ml [99.7–215.5]). Negative humoral response was associated with vaccination post transplantation (odds ratio 22.4), older age (OR = 1.04), and longer time on dialysis (OR = 1.02), while higher lymphocyte count at time of vaccination was protective (OR = .52). Our findings of sustained superior humoral response to SARS‐CoV‐2 vaccine in kidney transplant recipients vaccinated prior to transplantation strongly support the recommendations of SARS‐CoV‐2 vaccination of transplant candidates, especially those younger than 60 years.
Keywords: COVID‐19, Kidney transplantation, SARS‐CoV‐2, vaccine
1. INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection and the resulting disease, coronavirus disease 2019 (COVID‐19), have spread to millions of people worldwide. COVID‐19 in solid organ transplant (SOT) recipients is associated with increased morbidity and mortality due to their multiple comorbidities and chronic immunosuppression state. 1 , 2
While SOT recipients were not enrolled in phase three studies of SARS‐CoV‐2 vaccines, 3 , 4 few studies demonstrated recently that the majority of SOT recipients did not have an appreciable immune response following a mRNA SARS‐CoV‐2 vaccine. 5 , 6 , 7 In contrast to that, most end‐stage renal disease (ESRD) patients on dialysis mount a serologic response to the mRNA vaccines, albeit they present lower post‐vaccination antibody levels than healthy controls. 8 , 9
The ideal timing of SARS‐CoV‐2 vaccination in the SOT setting is not known. The current recommendations of transplantation societies are to get vaccinated at least 2 weeks prior to transplantation. 10 , 11 , 12 However, data to support this approach in SARS‐CoV‐2 vaccines does not exist.
The present study was aimed to quantify the humoral response following full vaccination with the BNT162b2 (Pfizer‐BioNTech) SARS‐CoV‐2 vaccine in a cohort of kidney transplant recipients, vaccinated at least 1 month prior to transplantation, by determining the level of antibodies directed against the S (spike) antigen after transplantation, and to compare it to kidney transplant recipients vaccinated after transplantation, and to immunocompetent vaccinated healthcare workers. In order to eliminate cases with prior exposure to the virus and evaluate the effect of the vaccine itself, only participants with negative serology to SARS‐CoV‐2 nucleocapsid (N) protein were included.
2. METHODS
2.1. Study design
Three cohorts were included in the study: (1) Pre‐transplant vaccination group (Pre‐Tx Vac), composed of 19 consecutive adult kidney transplant recipients, who received full vaccination (two doses 21 days apart) at least 1 month prior to kidney transplantation, (2) Post‐transplant vaccination group (Post‐Tx Vac), composed of 116 kidney recipients who were vaccinated after transplantation, and (3) Control group, composed of 39 vaccinated healthcare workers.
Our group previously reported 6 an analysis on serology testing in a group of kidney transplant recipients (all vaccinated post‐transplant) and healthy controls, performed in February 2021. We offered all kidney transplant recipients, as well as healthcare workers, to repeat the serology test in April 2021, including individuals who did not perform it previously. In our current study, there is an overlap of participants in the Post‐Tx Vac group (84 of 116) and controls (27 of 39) from the previous study. All participants in the study had the second dose of vaccination between January 10, 2021 and February 4, 2021, and serology test between April 11, 2021 and May 30, 2021.
All transplant recipients are regularly followed in Tel Aviv Sourasky Medical Center (TASMC), SOT clinic, and had a routine visit during the study period. All study participants provided written informed consent.
The subjects were included if they had negative history of COVID‐19 and were never found to have positive polymerase chain reaction (PCR) to SARS‐CoV‐2. All participants had completed full vaccination with two doses of the BNT162b2 SARS‐CoV‐2 (Pfizer‐BioNTech) vaccine, with the recommended interval of 21 days between the two doses. The blood samples were collected at least1 month after the second vaccine dose injection and at least 1 month after transplantation in recipients vaccinated before transplantation (Pre‐Tx Vac group).
For the detection of anti‐SARS‐CoV‐2 antibodies we used the DiaSorin LIAISON SARSCoV‐2 S1/S2 IgG serology assay, 13 which was validated previously. 14
Freshly collected blood in clot activator and gel tube was centrifuged at 4500 rpm for 10 min. The sera were separated and stored at 4°C for analysis.
LIAISON SARS‐CoV‐2 S1/S2 IgG chemiluminescent assay (DiaSorin S.p.A., Saluggia, Italy) was used according to the manufacture instructions, to detect IgG antibodies directed against a recombinant S protein (S1/S2). 13 Samples displaying < 12.0 AU/ml were considered negative, those ranging between 12.0 and 15.0 AU/ml are equivocal, and those > 15 AU/ml were considered as positive. For the purpose of the analysis, participants with equivocal response (a total of two participants) were considered as negative.
In addition, in order to explore prior exposure to SARS‐CoV‐2, every participant was tested to IgG antibodies directed against the SARS‐CoV‐2 nucleocapsid protein, performed with an Architect i2000SR analyzer (Abbot Diagnostics, IL, USA) and Abbott chemistry according to the manufacture instructions. A cutoff of 1.4 index (S/C) was used. 14 Patients with detectable IgG antibodies for the SARS‐CoV‐2 nucleocapsid protein were excluded from the study.
All kidney transplant recipients received induction immunosuppression therapy with anti‐thymocyte globulin or basiliximab, according to patients’ risk of rejection, in addition to methylprednisolone intravenously, followed by triple maintenance immunosuppression including calcineurin inhibitors (CNIs; tacrolimus or cyclosporin), mycophenolate mofetil or mycophenolate sodium (MMF) and low dose prednisone (5 mg/day). This is consistent with the 2009 Kidney Disease: Improving Global Outcomes (KDIGO) 15 guideline for the Care of Kidney Transplant Recipients. According to the patient's risk stratification for rejection, side effects or other considerations, the maintenance regimen were intensified or reduced, including changing doses or suspending specific agent, adding or switching to mTOR inhibitors (everolimus or sirolimus) or azathioprine.
Clinical and epidemiological data were obtained from the medical charts. Data on the maintenance immunosuppression and laboratory tests was obtained from the last clinic visit prior the first vaccine dose. Serum creatinine was recorded on the day of blood sampling for the serology test for both recipients’ study groups.
Triple immunosuppression was defined as any combination of three different medications (including prednisone, CNIs, MMF, mTOR inhibitors, or azathioprine). Treatment with high dose corticosteroids was defined as a pulse of methylprednisolone (≥125 mg per day), or prednisone (≥40 mg per day). Low dose prednisone was defined as 10 mg or less per day.
Estimated glomerular filtration rate (eGFR) was calculated using MDRD formula 16 and adjusted to body surface area (Mosteller calculation). Body mass index (BMI) was defined as dry weight in kilograms divided by height in square meters.
The study was approved by the TASMC institutional ethical review board.
2.2. Statistical analysis
Continuous variables were first tested for normal distribution using the Kolmogorov‐Smirnov test and Q‐Q plots and were summarized and displayed as mean (standard deviation, SD) for normally distributed variables, and as median (IQR, interquartile range) for non‐normally distributed variables.
Categorical variables were displayed as number of patients and the percentage in each group. For all categorical variables, the Chi‐Square statistic was used to assess the statistical significance between groups. Continuous variables were compared by using a t‐test or ANOVA if normally distributed or by Kruskal Wallis/Mann‐Whitney test if non‐normally distributed.
Correlation between two continuous parameters was calculated by Spearman analysis. In order to identify which variables are affected by multicollinearity and the strength of the correlation, we calculated Variance Inflation Factors (VIF) and reported VIF above three. We fitted binary logistic regression models for the risk of negative serology test including the significant variables that were found in univariate analysis.
P < .05 was considered statistically significant for all analyses. IBM SPSS Statistics for Windows, version 22 (IBM Corp., Armonk, NY, USA) was used for all statistical analyses.
3. RESULTS
Seven transplant recipients (all of them vaccinated after transplantation) were excluded from further analysis due to finding of positive IgG antibodies to SARS‐CoV‐2 nucleocapsid protein as a serological marker of their remote exposure to the natural virus (all seven had positive levels of anti‐spike antibodies as well).
The group of Pre‐Tx Vac composed of 19 kidney transplant recipients transplanted from February 12, 2021 to May 4, 2021. The time range from completing the SARS‐CoV‐2 vaccination to kidney transplantation ranged from 30 to 84 days.
The group of Post‐Tx Vac composed of 109 participants, transplanted between January 1, 1996 and January 12, 2021. Seven of them had liver and kidney transplants, and nine–simultaneous kidney and pancreas transplantation (SPK). Twenty‐one patients in this group were transplanted during the 12 months preceding the vaccination, but none of them in the latest 3 months.
All participants in all cohorts were Caucasians except one liver and kidney recipient of East African (Ethiopian) origin.
As shown in Table 1, no significant differences were observed between subjects in the three study groups with similar mean age as well as similar timing of the serum sampling post vaccination (Table 1). However, the control group of healthcare workers had a higher percentage of females, and a lower prevalence of individuals with hypertension and diabetes, as compared to both transplant recipients’ groups.
TABLE 1.
Baseline characteristics of three study groups
| Parameter | Pre –Tx Vac group | Post –Tx Vac group | Control group | P |
|---|---|---|---|---|
| Number | 19 | 109 | 39 | |
| Age (years) | 54 (3.6) | 57 (12.9) | 53 (10.9) | .15 |
| Sex, female (%) | 8 (42) | 40 (37) | 23 (58.9)* | .02 |
| BMI (kg/m2) | 27.1 (6.4) | 27.2 (4.3) | 25.9 (3.9) | .65 |
| Time on dialysis before transplantation (months) | 33 (32.2) | 21 (27.4) | .16 | |
| Pre‐emptive transplantation, (%) | 6 (31) | 23 (21) | .37 | |
| Time since first transplantation (months) | 1 (.6) | 74 (97.7) | <.001 | |
| First transplant (%) | 19 (100) | 98 (90) | .36 | |
| Etiology for kidney failure | ||||
| Diabetes/nephrosclerosis | 8 (42) | 39 (36) | .59 | |
| Glomerulonephritis | 6 (31) | 29 (27) | ||
| Donor type, living (%) | 16 (84) | 68 (62) | .07 | |
| Hypertension, (%) | 16 (84) | 79 (72) | 6 (15)* | .001 |
| Diabetes mellitus, (%) | 7 (37) | 47 (43) | 0 (0)* | .001 |
| High dose steroids last 12 months, (%) | 19 (100) | 25 (23) | <.001 | |
| Anti‐thymocyte globulin, (%) | 14 (74) | 57 (57) | .12 | |
| Anti‐thymocyte globulin last 12 months, (%) | 14 (74) | 10 (9) | <.001 | |
| Rituximab last 12 months, (%) | 1 (5) | 6 (5) | 1.0 | |
| Low dose prednisone, (%) | 19 (100) | 101 (93) | .61 | |
| CNIs, (%) | 19 (100) | 97 (90) | .21 | |
| mTORs, (%) | 0 (0) | 7 (6) | .59 | |
| MMF, (%) | 19 (100) | 82 (75( | .01 | |
| Triple maintenance immunosuppression, (%) | 19 (100) | 92 (84) | .07 | |
| Hemoglobin (g/dl) | 11.2 (1.4) | 13.8 (1.8) | <.001 | |
| White blood cell count (10e3/μl) | 7.5 (1.9) | 8.3 (2.6) | .19 | |
| Neutrophils’ count (10e3/μl) | 4.9 (1.2) | 5.4 (1.9) | .36 | |
| Lymphocyte count (10e3/μl) | 1.9 (.9) | 1.7 (.8) | .13 | |
| Serum creatinine (mg/dl) | 1.2 (.4) | 1.3 (.7) | .66 | |
| eGFR (ml/min/m2) | 63 (10.1) | 66 (21.8) | .67 | |
| Days after second dose | 94 (15.2) | 95 (21.8) | 92 (11.9) | .91 |
Mean (SD), unless otherwise stated. (* indicates a significant statistical difference (P < .05) between one group to other two).
Abbreviations: BMI, body mass index; CNIs, calcineurin inhibitors (tacrolimus or cyclosporin); eGFR, estimated glomerular filtration rate; MMF, mycophenolate mofetil or mycophenolate sodium; mTORs, mammalian target of rapamycin inhibitors.
As expected, period of time following transplantation was significantly shorter in Pre‐Tx Vac group. There was a similar rate of pre‐emptive transplantations (without need for dialysis prior to kidney transplantation) in both groups.
Five patients in the Post‐Tx Vac group had active malignancies other than non‐melanomatous skin cancer at the time of vaccination (one renal cell carcinoma, one posttransplant lymphoproliferative disease (PTLD)‐both were on systemic chemotherapy), two prostatic carcinoma, one Kaposi sarcoma). Only one out of five was seropositive.
The type of induction immunosuppression was quite similar in Pre‐Tx Vac and Post‐Tx Vac groups: basilixmab vas used in 26% and 44% and anti‐thymocyte globulin in 74% and 57%, respectively, P = .12. Unsurprisingly, all recipients in Pre‐Tx Vac received high dose corticosteroids and had a higher rate of recent treatment with anti‐thymocytic globulin.
Maintenance immunosuppression was similar in both groups, apart of more frequent use of MMF in Pre‐Tx Vac group. All pre‐Tx Vac group were on a regimen includes tacrolimus, prednisone and MMF. Most recipients (84%) in post‐Tx Vac group were treated with a combination of three immunosuppressive medications as well, most of them with prednisone, tacrolimus and MMF (65, 71%); in two patients in this group the maintenance immunosuppression regimen was de‐intensified in the 30 days prior to the vaccine (MMF was temporarily suspended).
3.1. Clinical outcomes and adverse reactions after the vaccine administration
Three participants (all of them in Post‐Tx Vac group) developed COVID‐19 after vaccination. One patient, vaccinated 12 months after kidney transplantation, with undetectable antibody levels after full vaccination, expired due to PCR‐proven severe COVID‐19, 9 weeks after the second dose of vaccine. Another kidney transplant recipient vaccinated 19 months after transplantation had moderate COVID‐19 12 weeks following vaccination, and fully recovered. A third recipient with low positive level of antibodies against S protein (18.1 AU/ml) had an asymptomatic SARS‐CoV‐2 infection (she was tested due to exposure to her family member with COVID‐19). There were no proven infections with SARS‐CoV‐2 in the Pre‐Tx Vac group.
No biopsy‐proven acute rejections, new neurological diagnoses (Guillain‐Barre syndrome, Bell's palsy or other neuropathy), or severe allergic reactions were observed in all three cohorts. The most common side effect after the vaccine administration was local pain, in 92 of 167 (55%) participants. Systemic symptoms developed in 32 (19.1%) of participants. There was a similar rate of symptoms between seropositive and seronegative individuals, as well as between both recipients’ groups and immunocompetent controls. There was no correlation between presence of side effects and seropositivity.
Transplant recipients who were vaccinated before transplantation had a significantly lower mean hemoglobin level at time of vaccination. However, WBC, neutrophils and lymphocytes count were not different at time of vaccination.
3.2. Humoral response of kidney transplant recipients vaccinated before and after transplantation and controls
All participants in the control group had a positive antibody response to spike protein, 17 out of 19 (90%) of pre‐Tx Vac group had a positive response, while only 49 of 109 (45%) recipients from Post‐Tx Vac group had positive serology (P < .001) (Figure 1).
FIGURE 1.
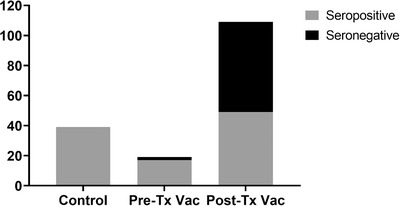
Negative and positive humoral response to vaccination in kidney recipients vaccinated before and after transplantation, and healthy controls. (P < .001 for Post‐Tx Vac vs. other groups)
The levels of anti‐spike IgG in the group of kidney transplant recipients vaccinated after transplantation (median = 10.7 AU/ml [IQR 0–62.5]) was significantly lower than in the patients vaccinated before transplantation (median = 66.2 AU/ml (IQR 21.6–138)), which was significantly lower than in the controls (median = 156 AU/ml (IQR 99.7–215.5)). A Kruskal Wallis test indicated that this difference was statistically significant (P < .001). When including only seropositive participants, mean antibody levels were similar in both groups of transplant recipients (median = 71.2 AU/ml [IQR 26.3–130] vs. 73.7 AU/ml [31–138], P = 1.0, in Pre‐Tx Vac vs Post‐Tx Vac group, respectively) and significantly lower compared to controls (156 AU/ml (IQR 99.7–156.5), P < .001) (Figure 2).
FIGURE 2.
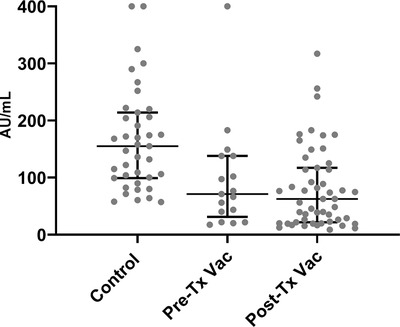
Scatter plot of IgG anti S in participants who were seropositive in three study groups. Control group differed significantly from both recipients’ groups, P < .001
3.3. Risk factors for negative humoral immune response
Table 2 demonstrates univariate and multivariate analysis of variables related to the risk of having negative humoral response to vaccination among kidney transplant recipients in both cohorts. Negative humoral response was significantly associated with post‐ kidney transplantation vaccination as compared to pre‐transplant, even after adjustment for other variables, with an odds ratio of 22.4. In addition, every year of age increased the risk of having a negative serology by 4%, and every month on dialysis before transplantation–by 2%, while higher lymphocyte count at time of vaccination had a significant association with positive serology. As significant multicollinearity was observed for type of donor and time on dialysis prior to vaccination (VIF = 6.9), the analysis was not further done on type of donor.
TABLE 2.
Univariate and multivariate analysis of variables related to risk of seronegative IgG anti S in kidney transplant recipients
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Variable | Exp (B) | 95% CI | P‐value | Exp (B) | 95% CI | P‐value |
| Vaccination before versus after transplantation | 10.4 | 2.9–15.6 | .002 | 22.4 | 3.6–35.8 | <.001 |
| Age (years) | 1.05 | 1.02–1.08 | <.001 | 1.04 | 1.01–1.1 | .02 |
| Sex | 1.06 | .54–2.2 | .78 | |||
| Donor (deceased vs. living) | 2.2 | 1.05–4.6 | .03 | |||
| BMI | .95 | .85–1.06 | .39 | |||
| Time on dialysis (months) | 1.013 | 1.01–1.27 | .02 | 1.02 | 1.01–1.04 | .05 |
| Diabetes mellitus | .93 | .5–1.9 | .82 | |||
| Lymphocyte count | .67 | .42–1.07 | .08 | .52 | .29–.96 | .04 |
| Anti‐thymocyte globulin last 12 months | .74 | .42–1.76 | .52 | |||
The significant inverse correlation between age and anti S levels was observed in all study groups (correlation coefficient −.15, P < .001; −.97, P = .002; and −.27, P = .05; for Pre‐Tx Vac, Post‐Tx Vac and controls, respectively). The differences in the magnitude of the humoral immune response to the vaccination as related to individuals’ age are shown in Figure 3. Pre‐Tx Vac patients aged below 60 years achieved significantly higher IgG levels when compared to the Post‐Tx Vac kidney recipients of the same age subgroup (P = .03). Actually, their anti S IgG levels were compatible to those in the control group (P = .31). However, in kidney transplant recipients above 60 years the differences between the two groups have disappeared and the anti S IgG levels in both cohorts were significantly lower when compared to the controls.
FIGURE 3.
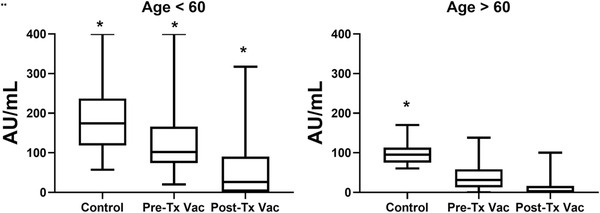
IgG anti S levels in three study cohorts subtracted to age groups of below and above 60 years
4. DISCUSSION
Data regarding response to SARS‐CoV‐2 vaccines are accumulating exponentially during the last months. Multiple studies found a robust serologic response to SARS‐CoV‐2 vaccine in healthy immunocompetent participants. 17 , 18 Individuals with ESRD on chronic dialysis also show a significant serologic response, 8 , 9 albeit lower than healthy controls. However, there is growing evidence that both humoral and cellular immune responses to SARS‐CoV‐2 vaccines are significantly reduced in kidney transplant recipients vaccinated after transplantation, compared to healthy controls and to ESRD patients. 19 , 20
Our study, for the first time to the best of our knowledge, provides a missing part of the puzzle by evaluating the post‐transplant level of humoral response to the SARS‐CoV‐2 vaccine in kidney transplant recipients vaccinated before transplantation. In the present study we demonstrated significantly higher rate (89.5%) of positive humoral response to BNT162b2 SARS‐CoV‐2 vaccine in kidney transplant recipients vaccinated before transplantation, as compared to individuals vaccinated after transplantation (44.9%), with appreciable anti‐spike antibody response sustained in pre‐transplant vaccinated patients after induction immunosuppression and high dose steroids treatment. Mean antibody levels were similar when including only seropositive recipients, suggesting a better chance of responding to the vaccine when administered pre transplantation. Unsurprisingly, the levels of protective antibodies in pre‐transplant vaccinated patients were compatible with those of dialysis patients, as was demonstrated in the previous studies 8 , 9 and lower than in healthy controls.
Our results support the current recommendations of transplantation societies for the SOT candidates to get vaccinated against SARS‐CoV‐2 at least 2 weeks prior to transplantation, 10 , 11 and reduce the concern that the diminished vaccine response in dialysis patients may become even more attenuated by post‐transplantation immunosuppression. 21
Previous studies demonstrated that the serological response to vaccination was related to the net burden of patients’ immunosuppression. 6 , 22 , 23 In our study kidney recipients vaccinated before transplantation had significant levels of immunosuppression at the time of the antibody levels evaluation, with the recent (less than 3 months) induction treatment and the maintenance regimen of triple immunosuppression. Even though, their humoral response to the mRNA SARS‐CoV‐2 vaccine remained significantly superior than in the cohort of kidney recipients vaccinated after transplantation while on significantly reduced net immunosuppression regimens. Unsurprisingly, in the multivariate analysis, vaccination before transplantation was most powerful predictor of positive serological response to the vaccine with an odds ratio of 22.4.
The predictors of negative serological response were advanced age, time on dialysis before transplantation, and lymphocyte count at the time of vaccination.
Advanced age was repeatedly defined as predictor for reduced antibody response in immunocompetent patients after COVID‐19, 24 as well as after mRNA SARS‐CoV‐2 vaccination. 25 , 26 Our finding indicates that even in immunosuppressed patients age remains an independent predictor of poor immunological response. In fact, in recipients older than 60 years, we could not find any advantage for the pre‐transplantation vaccination.
mRNA SARS‐CoV‐2 vaccines, similar to other vaccines, are noted to cause mild to moderate side effects in immunocompetent individuals. 27 In our current study, as well as previous studies, 6 , 28 the most common side effect was mild local pain, and none of the participants had a major adverse event, in a follow‐up period of about 90 days post second dose of vaccine. We could not find correlation of symptoms or severity of symptoms to humoral response, in all study groups.
Strengths of this study include its novelty. It is the first published data about maintenance of humoral response after transplantation in pre‐transplant SARS‐CoV‐2 vaccinated kidney recipients. The comparison between two groups was done at the similar time after vaccination, while the patients with IgG antibodies to nucleocapsid protein were excluded, therefore eliminating the possibility of humoral response to the former virus exposure and validating our results.
Limitations of the study include a relatively small sample size. A short follow‐up period and absence of assessing the cellular immune response to the vaccine preclude us to address full spectrum of its immunogenicity or clinical correlation to the viral protection, including estimation of asymptomatic infections. Furthermore, sequential titers over time as well as a longer‐term clinical follow‐up in this important setting is needed.
Despite that, the accumulating data of significantly reduced level of serologic response to SARS‐CoV‐2 vaccines in transplant recipients warrant prompt consideration and further studies about possible ways to improve immunogenicity in this vulnerable population.
Based on our findings of a superior humoral response to the SARS‐CoV‐2 vaccine in the kidney transplant recipients vaccinated prior to transplantation, we strongly suggest to adopt this approach whenever possible, especially for transplant candidates younger than 60 years.
CONFLICT OF INTEREST
The authors declare no conflict of interests.
AUTHORS’ CONTRIBUTIONS
A. Grupper participated in research design, data analysis, statistics and writing of the paper. E. Katchman participated in data analysis and writing of the paper. M. Ben‐Yehoyada Participated in writing of the paper, data collection and contributed to analytic tools. L. Rabinowich participated in research design and data analysis. D. Schwartz participated in drafting of the paper. I.F. Schwartz Participated in drafting and critical revision of the paper. M. Shashar participated in data analysis. T. Halperin contributed analytic tools. D. Turner participated in the performance of the research. Y. Goykhman Participated in the performance of the research and data collection. O. Shibolet Participated in research design and drafting of the paper. S. Levy participated in data collection. I. Houri participated in data collection. R. Baruch participated in data collection and drafting of the paper. H. Katchman participated in research design, data analysis, statistics, and critical revision of the paper.
ACKNOWLEDGMENT
The authors of this manuscript would to thank the Israeli minister of health for providing the LIAISON SARS‐CoV‐2 S1/S2 IgG chemiluminescent assay.
Grupper A, Katchman E, Ben‐Yehoyada M, et al. Kidney transplant recipients vaccinated before transplantation maintain superior humoral response to SARS‐CoV‐2 vaccine. Clin Transplant. 2021;35:e14478. 10.1111/ctr.14478
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Caillard S, Anglicheau D, Matignon M, et al., An initial report from the French SOT COVID Registry suggests high mortality due to COVID‐19 in recipients of kidney transplants. Kidney Int. 2020;98:1549‐1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Caillard S, Chavarot N, Francois H, et al. Is COVID‐19 infection more severe in kidney transplant recipients?. Am J Transplant. 2021;21:1295‐1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med. 2020;383:2603‐2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baden LR, EL Sahly HM, Essink B, et al. Efficacy and safety of the mRNA‐1273 SARS‐CoV‐2 vaccines. N Engl J Med. 2021;384:403‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boyarsky BJ, Werbel WA, Avery RK, et al. Immunogenicity of a single dose of SARS‐CoV‐2 messenger RNA vaccine in solid organ transplant recipients. JAMA. 2021;325:1784‐1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grupper A, Rabinowich L, Schwartz D, et al. Reduced humoral response to mRNA SARS‐CoV‐2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am J Transplant. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2‐dose SARS‐CoV‐2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325:2204‐2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grupper A, Sharon N, Finn T, et al. Humoral response to the pfizer BNT162b2 vaccine in patients undergoing maintenance hemodialysis. Clin J Am Soc Nephrol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jahn M, Korth J, Dorsch O, et al. Humoral response to SARS‐CoV‐2‐Vaccination with BNT162b2 (Pfizer‐BioNTech) in patients on hemodialysis. Vaccines (Basel). 2021;9:360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. SARS‐CoV‐2 Vaccination in Heart and Lung Transplantation: Recommendations from the ISHLT COVID‐19 Task Force. https://ishlt.org/ishlt/media/Documents/COVID19_Vaccine‐Recommendations_3‐15‐2021.pdf (Accessed: March 19, 2021). [Google Scholar]
- 11. American Society of Transplantation COVID‐19 Vaccination Guidance. http://www.myast.org/sites/default/files/AST‐COVID‐PtVaccine‐3.18.21‐final.pdf (Accessed: March 21, 2021). [Google Scholar]
- 12. Transplantation Society (TTS) , Transplant Infectious Disease (TID). Guidance on Coronavirus Disease 2019 (COVID‐19) for Transplant Clinicians. Updated 1 March 2021. https://tts.org/index.php?option=com_content&view=article&id=749&Itemid=140 [Google Scholar]
- 13. DiaSorin S p A . LIAISION®SARS‐CoV‐2 S1/S2 IgG package insert 2020‐04. 2020.
- 14. Perkmann T, Perkmann‐Nagele N, Breyer MK, et al. Side‐by‐side comparison of three fully automated SARS‐CoV‐2 antibody assays with a focus on specificity. Clin Chem. 2020;66:1405‐1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Disease Kidney. Improving global outcomes (KDIGO) transplant work group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9:S1‐155. [DOI] [PubMed] [Google Scholar]
- 16. Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461‐470. [DOI] [PubMed] [Google Scholar]
- 17. Walsh EE, Frenck RW, Falsey AR, et al. Safety and immunogenicity of two RNA‐based Covid‐19 vaccine candidates. N Engl J Med. 2020;383:2439‐2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS‐CoV‐2 ‐ preliminary report. N Engl J Med. 2020;383:1920‐1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sattler A, Schrezenmeier E, Weber UA, et al. Impaired humoral and cellular immunity after SARS‐CoV2 BNT162b2 (Tozinameran) prime‐boost vaccination in kidney transplant recipients. J Clin Invest. 2021:150175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bertrand D, Hamzaoui M, Lemée V, et al. Antibody and T cell response to SARS‐CoV‐2 messenger RNA BNT162b2 vaccine in kidney transplant recipients and hemodialysis patients. J Am Soc Nephrol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eckerle I, Rosenberger KD, Zwahlen M, Junghanss T. Serologic vaccination response after solid organ transplantation: a systematic review. PLoS One. 2013;8:e56974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kumar D, Blumberg EA, Danziger‐Isakov L, et al. Influenza vaccination in the organ transplant recipient: review and summary recommendations. Am J Transplant. 2011;11:2020‐2030. [DOI] [PubMed] [Google Scholar]
- 23. Fairhead T, Hendren E, Tinckam K, et al. Poor seroprotection but allosensitization after adjuvanted pandemic influenza H1N1 vaccine in kidney transplant recipients. Transpl Infect Dis. 2012;14:575‐583. [DOI] [PubMed] [Google Scholar]
- 24. Klein SL, Pekosz A, Park HS, et al. Sex, age and hospitalization drive antibody response in a COVID‐19 convalescent plasma donor population. J Clin Invest. 2020;130:6141‐6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Campillo NE, Jimenez M, Canelles M. COVID‐19 vaccine race: analysis of age‐dependent immune responses against SARS‐CoV‐2 indicates that more than just one strategy may be needed. Candersonurr Med Chem. 2020;27. [DOI] [PubMed] [Google Scholar]
- 26. Anderson EJ, Rouphael NG, Widge AT, et al. Safety and immunogenicity of SAR‐CoV‐2 mRNA vaccine in older adults. N Engl J Med. 2020;383:2427‐2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gee J, Marquez P, Su J, et al. First month of COVID‐19 vaccine safety monitoring‐United States, December 14, 2020‐January 13, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(8):283‐288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Boyarsky BJ, Ou MT, Greenberg RS, et al. Safety of the first dose of SARS‐CoV‐2 vaccination in solid organ transplant recipients. Transplantation. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


