Abstract
Aim
In this study, we aimed to define the predictive role of liver function tests at admission to the hospital in outcomes of hospitalised patients with COVID‐19.
Material and Method
In this multicentric retrospective study, a total of 269 adult patients (≥18 years of age) with confirmed COVID‐19 who were hospitalised for the treatment were enrolled. Demographic features, complete medical history and laboratory findings of the study participants at admission were obtained from the medical records. Patients were grouped regarding their intensive care unit (ICU) requirements during their hospitalisation periods.
Results
Among all 269 participants, 106 were hospitalised in the ICU and 66 died. The patients hospitalised in ICU were older than patients hospitalised in wards (P = .001) and expired patients were older than alive patients (P = .001). Age, elevated serum D‐dimer, creatinine and gamma‐glutamyl transferase (GGT) levels at admission were independent factors predicting ICU hospitalisation and mortality in COVID‐19 patients.
Conclusion
In conclusion, in hospitalised patients with COVID‐19, laboratory data on admission, including serum, creatinine, GGT and d‐dimer levels have an important predictive role for the ICU requirement and mortality. Since these tests are readily available in all hospitals and inexpensive, some predictive formulas may be calculated with these parameters at admission, to define the patients requiring intensive care.
What’s known
Coronavirus disease 2019 (COVID‐19) is an acute respiratory infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2).
What’s new
GGT, creatine, and d‐dimer levels have an important predictive role for the ICU requirement and mortality.
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19) is an acute respiratory infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). 1 , 2 Since the patients can suddenly get worsen requiring intensive care unit (ICU), early identification and timely intervention of patients with a tendency to become severe is essential to improve outcomes. 1 Up to now, August 2021, approximately 225 million COVID‐19 confirmed cases and 4.5 million associated deaths were reported worldwide; while 6.5 million confirmed cases and 59 thousand deaths associated with this disease were reported in our country. 3
The clinical findings of the patients may vary according to the age and sex. However, the most commonly reported symptoms include headache, loss of smell, nasal obstruction, cough, asthenia, myalgia, rhinorrhea and sore throat. In approximately half of the patients, fever was present at admission. 4 The severity of the disease was associated with some comorbidities such as hypertension, bilateral lung involvement, older age, high C‐reactive protein levels and decreased lymphocyte count. 4 , 5
Liver function tests (LFTs) including aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma‐glutamyl transferase (GGT), alkaline phosphatase (ALP) and bilirubin levels were studied in COVID‐19 in some previous studies. 6 , 7 As with many viral infections, Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is also shown to be associated with abnormal liver function tests. 8
In hospitalised patients, older age, male sex, comorbidity, and signs of dyspnea were reported as higher risk factors for severe disease. 9 , 10 , 11 Angiotensin‐converting enzyme 2 (ACE2) is known to be the host cell receptor for SARS‐CoV‐2. It has been shown that there is a significant enrichment of ACE2 expression in a major portion of the cholangiocyte clusters. GGT is a diagnostic biomarker for cholangiocyte injury and thus may be suggested as a marker of cholangiocyte involvement in COVID‐19. 12 , 13 Patients with elevated liver function tests were related to more severe disease in COVID‐19. 14 , 15
In this study, we aimed to define the predictive role of liver function tests at admission to the hospital in outcomes of hospitalised patients with Covid‐19. In this way, we aimed to define an effective tool for the early clinical detection of severe cases in the COVID‐19 outbreak.
2. MATERIAL AND METHODS
In this multicentric retrospective study, adult patients (≥18 years of age) with confirmed COVID‐19 who were hospitalised for the treatment were enrolled.
2.1. Case definition for COVID‐19
Diagnosis of COVID‐19 was assessed by using nasopharyngeal swap reverse transcriptase‐polymerase chain reaction (Real‐Time PCR) according to WHO guideline. 16 Patients were treated in line with the recommendations of Turkey's Health Ministry COVID‐19 adult patient treatment guidelines. 17
2.2. Data collection and participants
Demographic features, complete medical history, and laboratory findings of the study participants at admission are obtained from the hospital records. The laboratory data including haemoglobin level, white blood cell count, neutrophil and lymphocyte counts, ALT, AST, GGT, and ALP levels, serum creatinine level, concentrations of D‐dimer and C‐reactive protein (CRP) levels were recorded. All data were obtained at the admission of the patients before treatment.
2.3. Inclusion and exclusion criteria
Patients with missing data, having any known chronic hepatobiliary diseases, non‐alcoholic fatty liver disease, patients with alcohol abuse, hepatobiliary malignancies and receiving drugs known to have cholestatic effects other than those used in COVID‐19 management, end‐stage renal disease and patients on haemodialysis were excluded from the study. 483 patients were evaluated and it was decided to include 269 patients because of strict exclusion criteria.
Patients were grouped regarding their ICU requirements during their hospitalisation periods. Moreover, the outcomes of the patients at the end of the treatment were also recorded. Patients whose treatment or hospitalisation periods were continuing during the data collection were not included in the study.
2.4. Statistical analysis
Statistical analyses were performed using SPSS 20.0 software. Categorical variables were described as frequency rates and percentages, and continuous variables were described using mean values. Mean values for continuous variables were compared using independent group t‐tests when the data were normally distributed. Multivariate logistic regression analysis was performed to calculate adjusted odds ratios (OR) with its 95% confidence interval (CI) for ICU hospitalisation and mortality in COVID‐19 patients. ROC curves were obtained for the parameters that were significantly affecting mortality. P < .05 was considered statistically significant.
2.5. Ethics statement
The study was approved by the local ethics committee of Lokman Hekim University with the number of 2021/065. Informed consent was obtained from all of the patients.
3. RESULTS
Totally 269 patients (95 female and 174 male) hospitalised for COVID‐19 were included in the study. Among participants, 106 were hospitalised in the intensive care unit and 66 of them died. Thorax tomography images of two patients hospitalised in ICU are shown in Figures 1 and 2. Demographic features of study participants are summarised in Table 1. There was no significant difference between hospitalised patients in ICU or wards and alive or death patients regarding gender. However, patients hospitalised in ICU were older than patients hospitalised in wards (P: .001); and expired patients were older than alive patients (P: .001).
FIGURE 1.
Thorax tomography image of a 79‐year‐old female patient with Covid pneumonia
FIGURE 2.
Thorax tomography image of a 73‐year‐old male patient with Covid pneumonia
TABLE 1.
Demographic features of study participants
| Hospitalisation in ICU | Outcomes | |||||
|---|---|---|---|---|---|---|
| Hospitalised in Wards (n: 163) | Hospitalised in ICU (n: 106) | P a | Alive (n: 203) | Death (n: 66) | P | |
| Gender (F/M) | 56 /107 | 39/67 | .69 | 68/135 | 27/39 | .30 |
| Age (y) | 49.18 ± 20.72 | 68.30 ± 14.98 | .001 | 52.08 ± 20.72 | 70.95 ± 13.66 | .001 |
Abbreviations: F, female; ICU, intensive care unit; M, male.
Chi Square and Independent Sample T test.
Statistically significant P values are indicated in bold.
Laboratory data of study participants are summarised in Table 2. Haemoglobin, white blood cell count, liver and renal function tests, D‐dimer and CRP levels of study participants are summarised in Table 2. While AST, GGT, white blood cell count, creatinine, D‐Dimer and CRP levels were significantly higher in patients hospitalised in intensive care units than in patients hospitalised in wards, Hb levels were significantly lower. These laboratory parameters showed similar changes in expired patients compared with survivors.
TABLE 2.
Laboratory data of study participants
| Hospitalisation in ICU | Outcomes | |||||
|---|---|---|---|---|---|---|
| Hospitalised in Wards (n: 163) | Hospitalised in ICU (n: 106) | P a | Alive (n: 203) | Death (n: 66) | P a | |
| Haemoglobin | 13.38 ± 1.99 | 11.96 ± 2.66 | .001 | 13.10 ± 2.27 | 11.95 ± 2.53 | .001 |
| WBC count (×109/L) | 6.71 ± 2.04 | 1.01 ± 5.57 | .001 | 7.17 ± 3.48 | 1.09 ± 6.04 | .001 |
| Neutrophil (×109/L) | 4.66 ± 2.81 | 8.11 ± 5.38 | .001 | 5.19 ± 2.56 | 8.57 ± 5.48 | .001 |
| Lymphocyte (×109/L) | 1.48 ± 0.78 | 1.42 ± 1.00 | .65 | 1.47 ± 0.90 | 1.40 ± 0.93 | .62 |
| ALT (U/L) | 30.68 ± 31.04 | 46.22 ± 38.28 | .061 | 31.47 ± 22.62 | 53.20 ± 40.72 | .021 |
| AST(U/L) | 26.63 ± 17.81 | 81.52 ± 40.11 | .023 | 30.10 ± 27.766 | 104.12 ± 80.51 | .001 |
| GGT(U/L) | 23.62 ± 14.98 | 43.27 ± 37.075 | .001 | 26.02 ± 16.431 | 47.85 ± 34.24 | .001 |
| ALP(U/L) | 70.63 ± 23.97 | 74.68 ± 27.42 | .15 | 72.16 ± 25.6 | 79.45 ± 29.44 | .12 |
| Creatinine (mg/dl) | 0.95 ± 0.22 | 1.30 ± 0.98 | .001 | 0.97 ± 0.32 | 1.45 ± 1.14 | .001 |
| D‐dimer (ng/mL) | 1823.33 ± 1020.73 | 3722.52 ± 1236.17 | .001 | 1762.41 ± 975.53 | 4943.288 ± 1191.26 | .001 |
| CRP(mg/L) | 44.22 ± 31.77 | 110.589 ± 88.50 | .001 | 51.95 ± 43.43 | 127.03 ± 98.45 | .001 |
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CRP, C‐reactive protein; GGT, gamma‐glutamyl‐transferase; ICU, intensive care unitWBC, white blood cell.
Independent sample T test.
Statistically significant P values are indicated in bold.
Risk factors associated with the ICU hospitalisation and mortality in COVID‐19 patients were analysed with the logistic regression analysis (Table 3). Regarding these findings, age (>65) (P: .001, OR: 4.05), elevated serum d‐dimer (>500) (P: .001, OR: 4.046), creatinine (>1,1) (P: .023, OR: 2.165) and GGT levels (>40) (P: .015, OR: 2.374) at admission were independent factors predicting mortality in COVID‐19 patients.
TABLE 3.
Risk factors associated with the ICU hospitalisation and mortality in COVID‐19
| ICU hospitalisation | Mortality | |||
|---|---|---|---|---|
| OR (95% CI) | P a | OR (95% CI) | P a | |
| Age (>65) | 3.27 (1.64‐6.61) | .001 | 4.050 (1.918‐8.551) | .001 |
| Haemoglobin (<12) | 0.867 (0.434‐1.729) | .685 | 1.089 (0.539‐2.199) | .813 |
| WBC count (>9 × 109/L) | 0. 82 (0.68‐1.29) | .207 | 2.280 (1.156‐4.496) | .017 |
| AST(>35 U/L) | 0.641 (0.297‐1.383) | .257 | 1.036 (0.478‐2.248) | .928 |
| GGT(>40 U/L) | 2.731 (1.26‐5.90) | .011 | 2.374 (1.23‐4.48) | .015 |
| Creatinine (mg/dL) (>1.1) | 2.19 (1.11‐4.29) | .022 | 2.165 (1.115‐4.206) | .023 |
| D‐dimer (ng/mL) (>500) | 3.84 (1.94‐7.57) | .001 | 4.046 (1.821‐8.989) | .001 |
| CRP (mg/L) (>10) | 3.95 (1.52‐10.17) | .004 | 1.273 (0.690‐2.645) | .205 |
Abbreviations: AST, aspartate aminotransferase; CRP, C‐reactive protein; GGT, gamma‐glutamyl‐transferase; ICU, Intensive Care Unit; OR, odds‐ratio; WBC, white blood cell.
Multivariate logistic regression analysis.
Statistically significant P values are indicated in bold.
In ROC curve analysis, the areas under the curve were calculated for these variables and summarised in Table 4, Figure 3.
TABLE 4.
The results of ROC curve analyses
| AUC | Asymptotic 95% confidence interval | ||
|---|---|---|---|
| Lower bound | Upper bound | ||
| Age | 0.728 | 0.703 | 0.807 |
| GGT | 0.759 | 0.69 | 0.827 |
| Creatinine | 0.698 | 0.615 | 0.782 |
| CRP | 0.758 | 0.692 | 0.824 |
Abbreviations: CRP, C‐reactive protein; GGT, gamma‐glutamyl‐transferase.
FIGURE 3.
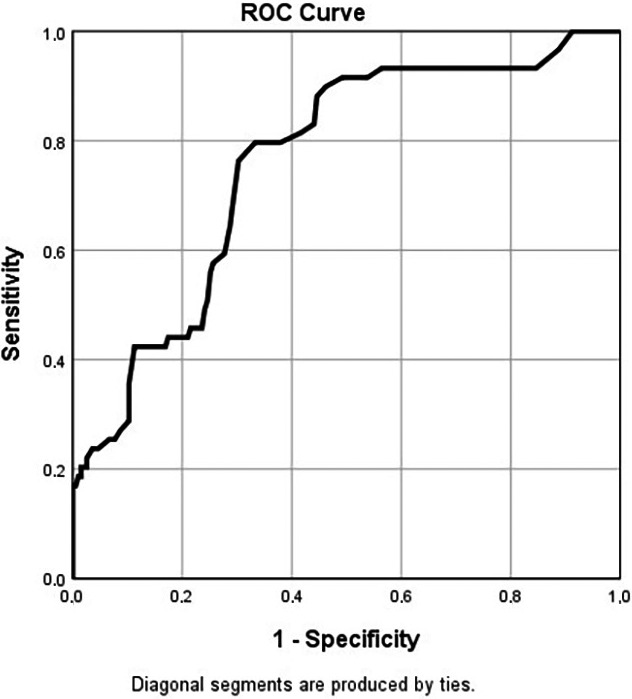
ROC curve for GGT in prediction of mortality
Since the AUC was the highest for GGT, we calculated some GGT values to predict the mortality in ROC curve with different sensitivity and specificity values. Regarding these results, 30.5 U/L has the best sensitivity (76.3%) and specificity (69.7%) combination.
4. DISCUSSION
In this study, we analysed the factors affecting ICU hospitalisation and mortality in patients with Covid‐19 and we determined that age older than 65 years, higher serum D‐dimer, creatinine, and GGT levels than the upper limits of the laboratory at admission were independent factors predicting ICU hospitalisation and mortality in COVID‐19 patients. Moreover, in ROC analysis, we determined that GGT values higher than 30 U/L at admission should be regarded as a risk factor for mortality and these patients should be evaluated more intensely.
Since an early identification and timely intervention of patients with a tendency to become severe is essential to improve outcomes in COVID‐19 patients, there are many studies in previous literature evaluating the predictive factors of outcomes. Jinrui et al reported that the higher level of CRP and ALT levels and chronic comorbidities were the risk factors for the progression into severe pneumonia in COVID‐19 patients. 18 Ullah et al defined elevated CRP and D‐dimer levels as independent predictors of in‐hospital mortality. 19 Supporting our results, in a meta‐analysis, age, and CRP levels were also defined as the main risk factors in predicting severe COVID‐19 outcomes. 20 Unlike this study, in our study, it was determined that GGT is important in predicting the outcome. Our strict exclusion criteria and the fact that the study was conducted in a larger population may explain this difference between these two studies.
Based on the admission data, in elderly patients, serum albumin and D‐dimer levels and onset to hospitalisation time were reported as significant predictors for the severity of COVID‐19. 21 In another meta‐analysis performed to identify the predictors associated with poor clinical outcomes in patients with COVID‐19, severe COVID‐19 was associated with lower levels of lymphocytes and haemoglobin; elevated levels of ALT, AST, creatinine, high‐sensitivity CRP, D‐dimer, ferritin and LDH levels. 22 Our results were supporting these previous findings and we also determined that older age and elevated serum d‐dimer, creatinine, and GGT levels at admission were independent factors predicting ICU hospitalisation and mortality in COVID‐19 patients. Admission serum CRP levels were associated with the ICU hospitalisation but not with the mortality in our study. This may be associated with the late elevation of CRP levels requiring time for the synthesis during the hospitalisation of patients.
In previous literature, patients with elevated liver function tests were related to more severe disease in COVID‐19. 14 , 15 However, Ramachandran et al reported that elevated AST or ALT levels among hospitalised COVID‐19 patients were associated with higher rates of mechanical ventilation but were not a significant independent predictor of more severe disease. 23 In a recent study from Turkey, elevated serum ALT, AST levels and AST/ALT ratio >1 were associated with the more severe course and increased mortality in COVID‐19. 24 However, Ponziani et al reported that baseline liver test abnormalities were associated with the increased risk of ICU admission but not the outcomes. 25 Similarly, Monterde et al reported that abnormalities in liver function tests on admission were not associated with survival but with respiratory complications at admission. However, an increase during hospitalisation in GGT, and ALP levels was associated with reduced survival. 26 Zhang et al did not determine any role of ALT, AST or GGT levels in predicting severe COVID‐19 infection. 27
In a clinical epidemiological study, Shao et al reported that elevated GGT and CRP levels were associated with a longer length of hospital stay. 28 Very recently, abnormal liver biochemical tests at admission were reported to be closely related to the severity and prognosis of COVID‐19 patients, supporting our findings. 29 In this study, we determined that elevated serum GGT levels, but not aminotransferases, at admission were associated with the increased risk for ICU hospitalisation and mortality.
There are some limitations to this study that should be mentioned. First, this is a retrospective study carrying some bias of this type of investigation. Second, we did not analyse the effects of comorbidities in this group of patients, which may also affect the outcomes. Although we could not exclude all factors that may cause GGT elevation in participants, multicentered design of study and using same treatment algorithms were the strong ways of this current research.
In conclusion, in hospitalised patients with COVID‐19, laboratory data on admission, including serum GGT, creatine and d‐dimer levels have an important predictive role for the ICU requirement and mortality. Since these tests are readily available in all hospitals and inexpensive, some predictive formulas may be calculated with these parameters at admission, to define the patients requiring more intense treatments.
DISCLOSURES
Authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTIONS
B.K and MK contributed to study design. A.Y, A.T, G.C and YSS contributed to data collection. B.K, A.Y, A.T, G.C and YSS contributed to data analysis. B.K, A.Y and MK contributed to manuscript preparation.
GUARANTOR OF THE ARTICLE
Corresponding author is the guarantor.
Kasapoglu B, Yozgat A, Tanoglu A, Can G, Sakin YS, Kekilli M. Gamma‐glutamyl‐transferase may predict COVID‐19 outcomes in hospitalised patients. Int J Clin Pract. 2021;75:e14933. doi: 10.1111/ijcp.14933
Funding information
None.
REFERENCES
- 1. Mannucci E, Silverii A, Monami M. Association between different screening strategies for SARS‐CoV‐2 and deaths and severe disease in Italy. Int J Clin Pract. 2021;75:e13867. doi: 10.1111/ijcp.13867 [DOI] [PubMed] [Google Scholar]
- 2. Ozcelik F, Tanoglu A, Guven BB, Keskin U, Kaplan M. Assessment of severity and mortality of COVID‐19 with anti‐A1 and B IgM isohemagglutinins, a reflection of the innate immune status. Int J Clin Pract. 2021:e14624. doi: 10.1111/ijcp.14624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Control ECfDPa. European Union . https://www.ecdc.europa.eu/en/geographical‐distribution‐2019‐ncov‐cases. Accessed September 19, 2021.
- 4. Lechien JR, Chiesa‐Estomba CM, Place S, et al. Clinical and epidemiological characteristics of 1420 European patients with mild‐to‐moderate coronavirus disease 2019. J Intern Med. 2020;288:335‐344. doi: 10.1111/joim.13089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zheng F, Tang W, Li H, Huang YX, Xie YL, Zhou ZG. Clinical characteristics of 161 cases of corona virus disease 2019 (COVID‐19) in Changsha. Eur Rev Med Pharmacol Sci. 2020;24:3404‐3410. doi: 10.26355/eurrev_202003_20711 [DOI] [PubMed] [Google Scholar]
- 6. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City area. JAMA. 2020;323:2052‐2059. doi: 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. The N Engl J Med. 2020;382:1708‐1720. doi: 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bertolini A, van de Peppel IP, Bodewes F, et al. Abnormal liver function tests in patients with COVID‐19: relevance and potential pathogenesis. Hepatology. 2020;72:1864‐1872. doi: 10.1002/hep.31480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Popov GT, Baymakova M, Vaseva V, Kundurzhiev T, Mutafchiyski V. Clinical characteristics of hospitalized patients with COVID‐19 in Sofia, Bulgaria. Vector Borne Zoonotic Dis. 2020;20:910‐915. doi: 10.1089/vbz.2020.2679 [DOI] [PubMed] [Google Scholar]
- 10. Li R, Tian J, Yang F, et al. Clinical characteristics of 225 patients with COVID‐19 in a tertiary Hospital near Wuhan, China. J Clin Virol. 2020;127:104363. doi: 10.1016/j.jcv.2020.104363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323:1061‐1069. doi: 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li W, Moore MJ, Vasilieva N, et al. Angiotensin‐converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450‐454. doi: 10.1038/nature02145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271‐280.e8. doi: 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ali N, Hossain K. Liver injury in severe COVID‐19 infection: current insights and challenges. Expert Rev Gastroenterol Hepatol. 2020;14:879‐884. doi: 10.1080/17474124.2020.1794812 [DOI] [PubMed] [Google Scholar]
- 15. Yang R, Gui X, Ke H, Gao S, Luo M, Xiong Y. The indicative role of markers for liver injury on the severity and prognosis of coronavirus disease 2019 patients. Eur J Gastroenterol Hepatol. 2020. doi: 10.1097/MEG.0000000000001968 [DOI] [PubMed] [Google Scholar]
- 16. Organization WH . Assessment tool for laboratories implementing COVID‐19 virus testing: interim guidance, 8 April 2020. https://apps.who.int/iris/handle/10665/331714. Accessed December 1, 2020.
- 17. Bakanligi TCS . COVID‐19 (SARS‐CoV‐2 ENFEKSİYONU) ERİŞKİN HASTA TEDAVİSİ. October 2020. https://covid19.saglik.gov.tr/TR‐66926/eriskin‐hasta‐tedavisi.html. Accessed December 1, 2020.
- 18. Gao J, Huang X, Gu H, Lou L, Xu Z. Predictive criteria of severe cases in COVID‐19 patients of early stage: a retrospective observational study. J Clin Lab Anal. 2020;34:e23562. doi: 10.1002/jcla.23562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ullah W, Thalambedu N, Haq S, et al. Predictability of CRP and D‐Dimer levels for in‐hospital outcomes and mortality of COVID‐19. J Community Hosp Internal Med Perspect. 2020;10:402‐408. doi: 10.1080/20009666.2020.1798141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Katzenschlager S, Zimmer AJ, Gottschalk C, et al. Can we predict the severe course of COVID‐19 ‐ a systematic review and meta‐analysis of indicators of clinical outcome? medRxiv: the preprint server for health sciences. 2020. doi: 10.1101/2020.11.09.20228858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zeng F, Deng G, Cui Y, et al. A predictive model for the severity of COVID‐19 in elderly patients. Aging. 2020;12:20982‐20996. doi: 10.18632/aging.103980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mudatsir M, Fajar JK, Wulandari L, et al. Predictors of COVID‐19 severity: a systematic review and meta‐analysis. F1000Research. 2020;9:1107. doi: 10.12688/f1000research.26186.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ramachandran P, Perisetti A, Gajendran M, Chakraborti A, Narh JT, Goyal H. Increased serum aminotransferase activity and clinical outcomes in coronavirus disease 2019. J Clin Exp Hepatol. 2020;10:533‐539. doi: 10.1016/j.jceh.2020.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Medetalibeyoglu A, Catma Y, Senkal N, et al. The effect of liver test abnormalities on the prognosis of COVID‐19. Ann Hepatol. 2020;19:614‐621. doi: 10.1016/j.aohep.2020.08.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ponziani FR, Del Zompo F, Nesci A, et al. Liver involvement is not associated with mortality: results from a large cohort of SARS‐CoV‐2‐positive patients. Aliment Pharmacol Ther. 2020;52:1060‐1068. doi: 10.1111/apt.15996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bernal‐Monterde V, Casas‐Deza D, Letona‐Gimenez L, et al. SARS‐CoV‐2 infection induces a dual response in liver function tests: association with mortality during hospitalization. Biomedicines. 2020;8:328. doi: 10.3390/biomedicines8090328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang Y, Zheng L, Liu L, Zhao M, Xiao J, Zhao Q. Liver impairment in COVID‐19 patients: a retrospective analysis of 115 cases from a single centre in Wuhan city, China. Liver Int. 2020;40:2095‐2103. doi: 10.1111/liv.14455 [DOI] [PubMed] [Google Scholar]
- 28. Shao T, Tong Y, Lu S, et al. gamma‐Glutamyltransferase elevations are frequent in patients with COVID‐19: a clinical epidemiologic study. Hepatol Commun. 2020;4:1744‐1750. doi: 10.1002/hep4.1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wu Y, Li H, Guo X, et al. Incidence, risk factors, and prognosis of abnormal liver biochemical tests in COVID‐19 patients: a systematic review and meta‐analysis. Hep Int. 2020;14:621‐637. doi: 10.1007/s12072-020-10074-6 [DOI] [PMC free article] [PubMed] [Google Scholar]


