The recent successful development of COVID-19 vaccines using mRNA technology clearly shows the great potential for this novel modality. In addition to prophylactic mRNA vaccines for infectious diseases and therapeutic mRNA vaccines for cancer, there is substantial interest in mRNA therapeutics. The first potential ‘secreted protein’ mRNA drug — designed to deliver vascular endothelial growth factor A (VEGF-A) to induce regenerative angiogenesis in patients with cardiovascular disease — was advanced into phase I trials in 2016 (NCT02935712) (Mullard, A. mRNA-based drug approaches phase I milestone. Nat. Rev. Drug Discov. 15, 595 (2016))1. Here, we highlight the history and clinical development of this agent (known as AZD8601), with the aim of providing insights that may be useful for the further development of mRNA therapeutics.
Therapeutic rationale
VEGF-A has long been recognized as a potential vascular and proliferative regenerative therapeutic in patients with ischaemic heart disease2,3. However, unlocking its therapeutic potential has been confounded by the challenges of the relatively short half-life of the systemically administrated protein, side effects from chronic long-term exposure and the delivery challenges to the intact heart in vivo to achieve the desired therapeutic threshold.
The modified mRNA technology used in COVID-19 vaccines also holds great potential for therapeutic applications requiring controlled spatial and temporal delivery of paracrine factors such as VEGF-A4. This potential supported the investigation of VEGFA mRNA to induce therapeutic angiogenesis and proliferation in the context of heart disease5.
Drug delivery and pharmacokinetics
Given the concern that VEGFA mRNA formulated in lipid nanoparticles (such as those used in COVID-19 vaccines) could potentially lead to VEGFA mRNA leaking from the injection site into circulation, we explored alternative formulations. We discovered that therapeutic VEGFA mRNA that had 100% replacement of uridine with pseudouridine (similar to the COVID-19 mRNA vaccines) does not require lipid-based carriers for efficient spontaneous transfection of cardiomyocytes when injected directly into the myocardium of the heart, and caused no activation of the innate immune system in multiple species6. Furthermore, VEGF-A protein expression by cardiomyocytes has a transient and pulse-like profile following intracardial administration of mRNA formulated in a simple citrate saline buffer solution6. Following intracardiac injection of 100 μg of VEGFA mRNA in rats, the Cmax of VEGF-A (6,340 pg per tissue sample) was 6 hours and the terminal half-life was 28 hours6. This more transient pharmacokinetic profile differs fundamentally from that of gene therapy modalities and may finally unlock the therapeutic potential of VEGF-A.
Effects in animal models
VEGFA mRNA induces cardiovascular regeneration by increasing the capillary density in the injured muscle6. We investigated the therapeutic potential of VEGFA mRNA in mouse, rat and pig models of myocardial infarction5,6. In rats, direct intramyocardial injection of VEGFA mRNA at the same time as induction of myocardial infarction led to a significant increase in left ventricular ejection fraction (LVEF) 1 week after administration, as assessed by cardiac MRI6. In a pig model, VEGFA mRNA was injected into ~20 sites across the peri-infarct region 7 days after induction of myocardial infarction. Two months after treatment, LVEF showed a statistically significant improvement in the VEGFA mRNA group compared with the citrate saline control group (52% versus 47%). We also noted improvement in haemodynamic parameters and myocardial compliance in pigs treated with VEGFA mRNA. Histological examination revealed increased capillary and arteriole density and reduced fibrosis in the peri-infarct region of pigs treated with VEGFA mRNA compared with control animals6. These promising findings supported progression of VEGFA mRNA into clinical trials.
Studies in humans
The first VEGFA mRNA clinical trial was a randomized phase I study in otherwise healthy volunteers with type 2 diabetes mellitus (NCT02935712), aiming to assess safety and tolerability and provide proof of the therapeutic principle7. Injection of VEGFA mRNA formulated in citrate saline buffer induced VEGF-A production in humans without causing serious side effects. Skin microdialysis revealed that local VEGF-A levels at mRNA-treated injection sites were increased compared with placebo. Injection of 360 μg of VEGFA mRNA (the highest dose evaluated) resulted in VEGF-A levels of 300 pg ml–1, peaking at 3.5–5 hours and sustained for 24 hours, compared with 170 pg ml–1 for placebo. By contrast, systemic VEGF-A levels were not elevated and were consistent with previously published normal levels. Treatment was well tolerated, and the only adverse events causally related to VEGFA mRNA were mild injection site reactions.
Conducting the phase I study in patients with type 2 diabetes mellitus, who tend to have impaired blood flow in multiple organs, including the skin, enabled detection of increases in skin blood flow to provide proof of the principle that VEGFA mRNA may have potentially beneficial angiogenic effects. Laser Doppler fluximetry and imaging revealed a twofold enhancement in basal skin blood flow 4 hours after administration, which was sustained 7 days after administration, at VEGFA mRNA-treated injection sites compared with placebo-treated sites7. The magnitude of this improvement was similar to the magnitude of the difference in basal blood flow between patients with type 2 diabetes mellitus and healthy volunteers in the previous methodological study7, suggesting transient normalization of basal skin blood flow at VEGFA mRNA-treated injection sites. These findings confirmed the therapeutic potential of VEGFA mRNA and supported further clinical development.
The second clinical trial of VEGFA mRNA was a phase IIa safety and exploratory efficacy study in patients with reduced LVEF who were undergoing coronary artery bypass grafting (NCT03370887; known as EPICCURE)8. In this randomized, double-blind trial, patients received 30 epicardial injections of VEGFA mRNA or placebo in a short extension of the surgical procedure. These injections were targeted to specific myocardial regions via a personalized injection map created for each patient using [15O]-water PET imaging of myocardial blood flow. For each injection, the volume was 0.2 ml and the dose was 0.1 mg, resulting in a total dose of 3 mg of VEGFA mRNA.
The planned enrolment for the phase IIa EPICCURE study was 24 patients, but the study was terminated after 11 patients had been randomized because of slow recruitment rates. The slow recruitment was due to several factors. Only a few countries have approved isotope labelling facilities for 15[O]-water PET, and the study was conducted in specific sites in Finland and Germany where the number of patients undergoing elective coronary artery bypass grafting is limited. In addition, after the COVID-19 pandemic started, the frequency of elective surgery was substantially reduced. At the time of writing, all these patients have completed the study and no deaths or serious treatment-related adverse events have occurred. Preliminary data on adverse events, echocardiography and clinical laboratory findings indicate no safety signals of concern. High-level results presented at the American Heart Association conference in November 2021 showed that the primary endpoint of safety and tolerability was met, and exploratory efficacy analyses support further clinical development of VEGFA mRNA. Full data analyses are being performed and a manuscript on the EPICCURE data is in preparation.
Conclusion
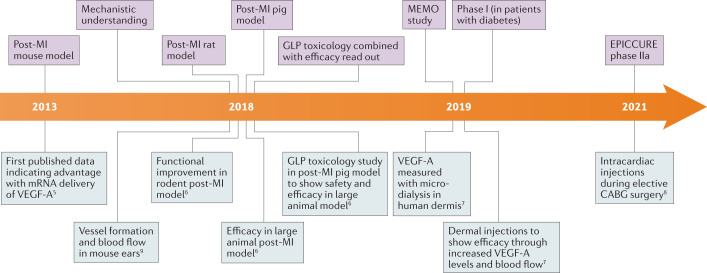
We have presented the development journey of one of the first mRNA therapeutic molecules to enter clinical trials (Fig. 1). Its early clinical development has built on a strong platform of preclinical experiments in several animal species, and it has the potential to expand into further clinical development to benefit patients with heart failure.
Fig. 1. Development timeline for VEGFA mRNA.
The project progression from preclinical studies to clinical trials illustrates the importance of building a comprehensive data package in a step-wise manner for a novel therapeutic modality, such as mRNA5–9. CABG, coronary artery bypass graft; GLP, good laboratory practice; MI, myocardial infarction; VEGF-A, vascular endothelial growth factor A.
Competing interests
A.C., N.B., L.-M.G. and R.F.-D. are employees of AstraZeneca and may own stock or stock options. S.H. is an employee of Moderna, Inc. and holds stock or stock options. K.R.C. is an advisor and chair of the External Science Panel for AstraZeneca and a member of the Karolinska Institutet/AstraZeneca Integrated Cardio Metabolic Center in Huddinge, and receives support for these services, as well as research support through the Karolinska Institutet Center. He is also a co-founder and equity holder of Moderna, Inc.
Footnotes
Peer review information
Nature Reviews Drug Discovery thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mullard A. mRNA-based drug approaches phase I milestone. Nat. Rev. Drug Discov. 2016;15:595. doi: 10.1038/nrd.2016.182. [DOI] [PubMed] [Google Scholar]
- 2.Chien KR, Zangi L, Lui KO. Synthetic chemically modified mRNA (modRNA): toward a new technology platform for cardiovascular biology and medicine. Cold Spring Harb. Perspect. Med. 2014;5:a014035. doi: 10.1101/cshperspect.a014035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yla-Herttuala S, Bridges C, Katz MG, Korpisalo P. Angiogenic gene therapy in cardiovascular diseases: dream or vision? Eur. Heart J. 2017;38:1365–1371. doi: 10.1093/eurheartj/ehw547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie W, Chen B, Wong J. Evolution of the market for mRNA technology. Nat. Rev. Drug Discov. 2021;20:735–736. doi: 10.1038/d41573-021-00147-y. [DOI] [PubMed] [Google Scholar]
- 5.Zangi L, et al. Modified mRNA directs the fate of heart progenitor cells and induces vascular regeneration after myocardial infarction. Nat. Biotechnol. 2013;31:898–907. doi: 10.1038/nbt.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carlsson L, et al. Biocompatible, purified VEGF-A mRNA improves cardiac function after intracardiac injection 1 week post-myocardial infarction in swine. Mol. Ther. Methods Clin. Dev. 2018;9:330–346. doi: 10.1016/j.omtm.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gan LM, et al. Intradermal delivery of modified mRNA encoding VEGF-A in patients with type 2 diabetes. Nat. Commun. 2019;10:871. doi: 10.1038/s41467-019-08852-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anttila V, et al. Synthetic mRNA encoding VEGF-A in patients undergoing coronary artery bypass grafting: design of a phase 2a clinical trial. Mol. Ther. Methods Clin. Dev. 2020;18:464–472. doi: 10.1016/j.omtm.2020.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun N, et al. Modified VEGF-A mRNA induces sustained multifaceted microvascular response and accelerates diabetic wound healing. Sci. Rep. 2018;8:17509–17520. doi: 10.1038/s41598-018-35570-6. [DOI] [PMC free article] [PubMed] [Google Scholar]