Abstract
Purpose
Anosmia and dysgeusia (AD) are common amongst COVID‐19 patients. These symptoms are not frequently associated with rhinorrhea or nasal congestion and the underlying mechanism is unclear. Previous reports suggested that glucagon‐like peptide‐1 (GLP‐1) signalling plays a role in the modulation of olfaction and ageusia. We aimed to assess the correlation between GLP‐1 and COVID‐19‐associated AD.
Methods
Blood samples obtained from COVID‐19 patients with and without AD were tested for serum GLP‐1 levels using enzyme‐linked immunosorbent assay (ELISA). A second control group comprised of COVID‐19‐negative volunteers.
Results
Forty‐nine subjects were included in the study. Nineteen were positive for COVID‐19. Of the 19 patients, 10 had AD and 9 declined such complaints. Age and basic metabolic rate were similar amongst all study groups. Serum GLP‐1 levels were significantly lower amongst patients with AD compared with patients without AD and COVID‐19‐negative individuals (1820 pg/mL vs 3536 pg/mL vs 3014 pg/mL, respectively, P < .02).
Conclusion
COVID‐19 patients who reported AD had lower serum levels of GLP‐1 compared with those lacking AD symptoms and COVID‐19‐negative individuals. These results suggest that GLP‐1 may be involved in the pathogenesis of AD. However, further larger scale studies should corroborate our findings.
1. INTRODUCTION
Olfactory and taste disorders are known to be related to a wide range of viral infections including SARS‐CoV‐2. However, the pathophysiological mechanism has yet to be determined. Many viruses lead to anosmia and/or dysgeusia (AD) through an inflammatory reaction of the nasal mucosa leading to rhinorrhea and nasal congestion. However, amongst COVID‐19 patients, especially of mild severity, AD may present as an isolated complaint. 1 In a recent study, of 2013 COVID‐19 patients, 1754 patients (87%) reported the loss of smell, whereas 1136 (56%) reported taste dysfunction. Both complaints were more common amongst mild COVID‐19 patients. 2 Angiotensin‐converting enzyme‐2 (ACE2) is the main host cell receptor that mediates SARS‐CoV‐2 invasion to cells and is widely expressed in multiple tissues including the epithelial cells of the oral mucosa. In fact, this receptor was highly enriched in epithelial cells of the tongue. 3 An inflammatory response mediated by cytokines leading to oral and olfactory mucosal injury was suggested as a possible mechanism for AD. 4
Interestingly, the signalling pathway of taste modulation and olfaction involves several ligands, amongst them is glucagon‐like peptide‐1 (GLP‐1). 5 GLP‐1 is a pleiotropic gut hormone with numerous metabolic functions and has the main role in glucose homeostasis. GLP‐1 is released in the gut in response to nutrient ingestion and its activity is regulated by the enzyme dipeptidyl‐peptidase‐4. The taste cells, particularly L cells, produce GLP‐1 amongst other hormones. 6 Several reports suggest that GLP‐1 functions as a neurotransmitter mediating taste and olfaction. 5 , 7 , 8 Mice lacking the GLP‐1 receptor have significantly reduced taste sensitivity to sweeteners. 7 Pre‐pro‐glucagon (the precursor of GLP‐1) and GLP‐1 receptor mRNAs have been identified in cell layers in the olfactory bulb suggesting that GLP‐1 may mediate information flow from the olfactory epithelium to the brain. 8
In view of its role in the modulation of gustatory and olfaction functions, we aimed to examine the association between serum GLP‐1 levels amongst COVID‐19 patients reporting AD vs patients and COVID‐19‐negative individuals with intact olfaction and taste.
2. MATERIALS AND METHODS
This study took place in the COVID‐19 wards of Shaare Zedek Medical Center (SZMC), Jerusalem, Israel. We established a new bio‐bank of various blood‐derived products from hospitalised COVID‐19 patients. COVID‐19 was confirmed based on PCR‐positive nasopharyngeal swab specimen tested for SARS‐CoV‐2. Our study included COVID‐19 confirmed patients with AD symptoms (group A), without AD symptoms (group B) as well as COVID‐19‐negative asymptomatic subjects that served as a second control group (group C). Clinical and demographical data were retrieved from the computerised medical records or by interviewing the study participants. Those included age, sex, comorbidities, presence of anosmia/dysosmia and or/ dysgeusia and patients' medications. The severity of COVID‐19 was assessed using the NIH guidelines (https://www.covid19treatmentguidelines.nih.gov).
Blood samples were collected from COVID‐19‐confirmed patients (based on nasopharyngeal PCR testing) and from COVID‐19‐negative individuals in 5 mL CAT tubes (456018, Vacuette; Greiner Bio‐one). Samples were centrifuged at 1800 × g, R/T for 20 min according to protocol, sera were separated and aliquots were stored in a dedicated −80°C COVID‐19 freezer. Serum GLP‐1 levels were measured using a commercial enzyme‐linked immunosorbent assay (ELISA) according to the manufacturer instructions (Abcam, Cambridge, UK). Differences between groups were assessed using χ2 test for categorical variables and Welch analysis of variance (ANOVA) test for continuous variables with skewed distribution. P value of <.05 was considered statistically significant. Informed consent was obtained from all participants. The study was approved by the Institutional Review Board of SZMC.
3. RESULTS
Forty‐nine subjects were included in the study. Nineteen were COVID‐19‐confirmed hospitalised patients. Of them, 11 (57.9%) had mild disease, 3 (15.8%) had moderate disease and 5 (26.3%) had severe illness. One patient with severe COVID‐19 died. Overall, 9 COVID‐19 patients had complained of AD (group A) and 10 had no AD symptoms (group B). The third group of 30 asymptomatic COVID‐19‐negative subjects (group C) served as a second control group. The demographical and clinical characteristics of all subjects are shown in Table 1. None of the patients with AD had rhinitis or rhinorrhea.
TABLE 1.
Demographical and clinical characteristics of patients
| COVID‐19+/AD+ (Group A) n = 9 | COVID‐19+/AD− (Group B) n = 10 | COVID‐19‐/AD− (Group C) n = 30 | P value | |
|---|---|---|---|---|
| Age, median (range) | 52 (34‐62) | 64 (40‐80) | 60 (40‐69) | .06 |
| Male, n (%) | 5 (55.5) | 7 (70) | 16 (53.3) | .65 |
| Disease severity, n (%) | ||||
| Mild | 5 (55.5) | 6 (60) | NA | .75 |
| Moderate | 2 (22.2) | 1 (10) | NA | |
| Severe | 2 (22.2) | 3 (30) | NA | |
| BMI, kg/m 2 (mean ± SD) | 32.4 ± 12 | 32.3 ± 2 | 26.7 ± 0.8 | .2 |
| T2DM, n (%) a | 2 (22.2) | 4 (40) | 1 (3.3) | .01 |
| Other comorbidities b | 3 (33.3) | 5 (50) | 6 (20) | .18 |
Abbreviations: AD, anosmia/dysosmia and dysgeusia; SD, standard deviation; T2DM, type 2 diabetes mellitus.
A significant difference was detected between groups B and C with regard to the prevalence of diabetes (P = .01).
Other common comorbidities included hypertension, hypothyroidism and hyperlipidemia.
Age and BMI did not significantly differ between groups. The median age of patients in groups A, B and C were 52 (range 34‐62), 64 (range 40‐80) and 60 years (range 40‐69), respectively (P = .06); with male predominance (57.1%) in all groups. Height and weight data were missing in five subjects. COVID‐19 patients were more obese than COVID‐19 negative controls (Table 1).
Type 2 diabetes mellitus was reported in 22.2% (2/9) of patients in group A, 40% (4/10) in group B and 3.3% (1/30) in group C (Table 1). One diabetic patient with intact olfaction and ageusia was treated with GLP‐1 receptor analogue (liraglutide). Prevalence of co‐morbidities aside of diabetes (including primary hypertension, hypothyroidism and hyperlipidemia) was similar amongst groups A and B (Table 1).
The mean serum GLP‐1 levels amongst group A patients were significantly lower compared with GLP‐1 levels in groups B and C (1820 ± 226 pg/mL vs 3536 ± 449 pg/mL and 3014 ± 161 pg/mL, P < .002, respectively) (Figure 1). Serum GLP‐1 levels amongst groups B and C were similar (P = .65). GLP‐1 levels were also found significantly low amongst group A patients vs groups B and C after adjustment to age, sex, diabetes and BMI (P = .02). No correlation was found between the severity of disease and serum GLP‐1 levels.
FIGURE 1.
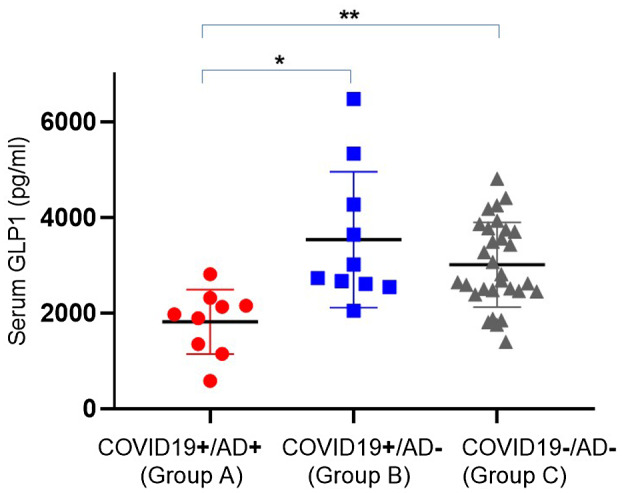
Serum GLP‐1 levels among the 3 groups. *P < .02; **P < .002
4. DISCUSSION
Amongst all symptoms that arise from COVID‐19 infection, the changes in taste and smell, although not life‐threatening, seem the most bothersome to many patients. However, the pathophysiology of anosmia and dysgeusia is not fully elucidated. In this study, we examined the association of serum GLP‐1 levels and AD amongst COVID‐19 patients. GLP‐1 levels were significantly lower amongst COVID‐19 patients with AD compared with patients without AD and COVID‐19‐negative subjects.
Prevalence of smell and/or taste impairment is high amongst COVID‐19 patients. 2 , 9 , 10 , 11 A proposed mechanism for this chemo‐sensitive dysfunction was local inflammation and cytokine release in the oral and olfactory mucosa induced by SARS‐CoV‐2 tissue invasion. 12 The high expression of ACE2 in the oral mucosa, especially the tongue and taste buds as well as the nasal cavity olfactory epithelium, supports this notion. 3 , 13 However, this theory is questionable in mild COVID‐19 cases, where the inflammatory response is less robust and rhinorrhea or nasal congestion are absent. 14 Indeed, these symptoms were not commonly reported in our cohort.
Others suggested that the interaction of SARS‐CoV‐2 with sialic acid receptors on taste buds impairs vital components of the salivary mucin. Reduced sialic acid in the saliva may accelerate the degradation of the gustatory particles. 12 , 15 However, this does not explain dysosmia.
Our findings may point to another intriguing mechanism of AD, which may be related to the gut hormone GLP‐1. 5 Aside from its major role as an incretin, enhancing insulin secretion and maintaining energy homeostasis, GLP‐1 has been reported to carry out protective and regulatory effects in numerous tissues, including heart, adipose, muscles, bones, kidneys, liver, lungs and brain as well as the olfactory epithelium and the taste buds on the tongue. 16 , 17 In fact, it functions as a neurotransmitter, coordinating communication between the peripheral organs and the brain. 5 , 18 , 19 GLP‐1 modulates taste sensitivity and affects taste preferences. 5 , 18 , 19 Expression of GLP‐1 was reported in two populations of taste cells (Type II and III cells), and its receptor has been identified in taste nerve fibres in mice and rodents. 18 GLP‐1‐producing cells and GLP‐1 receptors have also been found in the olfactory bulb. 20
In view of the above literature and in‐line with our findings, we propose that low serum levels of GLP‐1 may contribute to anosmia/hyposmia and dysgeusia amongst COVD‐19 patients.
No previous reports have examined the association between GLP‐1 and olfaction and ageusia during viral infections, and particularly amongst COVID‐19 patients.
The decrease in GLP‐1 may be explained either by increased degradation or decreased production of the hormone because of local inflammation in the oral and nasal cavities. Further research at the tissue and cellular level may shed light on this notion.
Our study has limitations. First, the retrospective design has inherent limitations including recall bias. Second, no histopathological analyses of samples from the oral and nasal cavity were performed. Third, because of the small sample size, our findings may not be generalizable, and subgroup analyses (based on BMI, diabetes, age) were not attainable. This may have affected our findings. However, it is unclear whether GLP‐1 secretion (either basal or postprandial) is decreased or enhanced under an obese or diabetic status. 21 Importantly, BMI was similar amongst the study groups and only one patient in the COVID‐19‐negative group was treated with GLP‐1 analogue.
It would be of interest to examine the degree of AD amongst diabetic COVID‐19 patients who are treated with GLP‐1 analogues or amongst patients postbariatric surgery. Both groups have relatively high GLP‐1 levels.
In summary, our preliminary findings point to a possible mechanism by which decreased GLP‐1 may be involved in the impairment of olfaction and ageusia amongst COVID‐19 patients. Further research is required to conclude a causal association.
CONFLICT OF INTEREST
None to declare.
CODE AVAILABILITY
Not applicable.
CONSENT TO PARTICIPATE
All study patients signed an informed consent permitting the collection of blood samples for research purposes.
CONSENT FOR PUBLICATION
All authors permit publication of the study by the publisher.
ETHICS APPROVAL
This study was approved by the SZMC institutional board (approval number SZMC‐02‐0278).
Ben‐Chetrit E, Ben‐Ya'acov A, Quitina A, et al. Anosmia and dysgeusia amongst COVID‐19 patients are associated with low levels of serum glucagon‐like peptide 1. Int J Clin Pract. 2021;75:e14996. doi: 10.1111/ijcp.14996
Funding information
This study was funded by Shaare Zedek Scientific Ltd. (Mada'it)
[Correction added on 10 November 2021, after first online publication: Ami Ben‐Ya'acov’s name was corrected in this current version.]
DATA AVAILABILITY STATEMENT
Raw data will be uploaded to Research Square.
REFERENCES
- 1. Lechien JR, Chiesa‐Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild‐to‐moderate forms of the coronavirus disease (COVID‐19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277:2251‐2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lechien JR, Chiesa‐Estomba CM, Hans S, Barillari MR, Jouffe L, Saussez S. Loss of smell and taste in 2013 European patients with mild to moderate COVID‐19. Ann Intern Med. 2020;173:672‐675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xu H, Zhong L, Deng J, et al. High expression of ACE2 receptor of 2019‐nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lozada‐Nur F, Chainani‐Wu N, Fortuna G, Sroussi H. Dysgeusia in COVID‐19: possible mechanisms and implications. Oral Surg Oral Med Oral Pathol Oral Radiol. 2020;130:344‐346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Martin B, Maudsley S, White CM, Egan JM. Hormones in the naso‐oropharynx: endocrine modulation of taste and smell. Trends Endocrinol Metab. 2009;20:163‐170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Müller TD, Finan B, Bloom SR, et al. Glucagon‐like peptide 1 (GLP‐1). Mol Metab. 2019;30:72‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Martin B, Dotson CD, Shin YK, et al. Modulation of taste sensitivity by GLP‐1 signaling in taste buds. Ann N Y Acad Sci. 2009;1170:98‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Merchenthaler I, Lane M, Shughrue P. Distribution of pre‐pro‐glucagon and glucagon‐like peptide‐1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol. 1999;403:261‐280. [DOI] [PubMed] [Google Scholar]
- 9. Aziz M, Perisetti A, Lee‐Smith WM, Gajendran M, Bansal P, Goyal H. Taste changes (dysgeusia) in COVID‐19: a systematic review and meta‐analysis. Gastroenterology. 2020;159:1132‐1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Spinato G, Fabbris C, Polesel J, et al. Alterations in smell or taste in mildly symptomatic outpatients with SARS‐CoV‐2 infection. JAMA. 2020;323:2089‐2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Speth MM, Singer‐Cornelius T, Oberle M, Gengler I, Brockmeier SJ, Sedaghat AR. Olfactory dysfunction and sinonasal symptomatology in COVID‐19: prevalence, severity, timing, and associated characteristics. Otolaryngol Head Neck Surg. 2020;163:114‐120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vaira LA, Salzano G, Fois AG, Piombino P, De Riu G. Potential pathogenesis of ageusia and anosmia in COVID‐19 patients. Int Forum Allergy Rhinol. 2020;10:1103‐1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Butowt R, Bilinska K. SARS‐CoV‐2: olfaction, brain infection, and the urgent need for clinical samples allowing earlier virus detection. ACS Chem Neurosci. 2020;11:1200‐1203. [DOI] [PubMed] [Google Scholar]
- 14. Dev N, Sankar J, Gupta N, et al. COVID‐19 with and without anosmia or dysgeusia: a case‐control study. J Med Virol. 2021;93:2499‐2504. [DOI] [PubMed] [Google Scholar]
- 15. Mirabelli M, Chiefari E, Puccio L, Foti DP, Brunetti A. Potential benefits and harms of novel antidiabetic drugs during COVID‐19 crisis. Int J Environ Res Public Health. 2020;17:3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Muscogiuri G, Cignarelli A, Giorgino F, et al. GLP‐1: benefits beyond pancreas. J Endocrinol Invest. 2014;37:1143‐1153. [DOI] [PubMed] [Google Scholar]
- 17. Thiebaud N, Llewellyn‐Smith IJ, Gribble F, Reimann F, Trapp S, Fadool DA. The incretin hormone glucagon‐like peptide 1 increases mitral cell excitability by decreasing conductance of a voltage‐dependent potassium channel. J Physiol. 2016;594:2607‐2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Takai S, Yasumatsu K, Inoue M, et al. Glucagon‐like peptide‐1 is specifically involved in sweet taste transmission. FASEB J. 2015;29:2268‐2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bagnoli E, FitzGerald U. Mitral cells and the glucagon‐like peptide 1 receptor: the sweet smell of success? Eur J Neurosci. 2019;49:422‐439. [DOI] [PubMed] [Google Scholar]
- 20. Fadool DA, Tucker K, Pedarzani P. Mitral cells of the olfactory bulb perform metabolic sensing and are disrupted by obesity at the level of the Kv1.3 ion channel. PLoS One. 2011;6:e24921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hira T, Pinyo J, Hara H. What is GLP‐1 really doing in obesity? Trends Endocrinol Metab. 2020;31:71‐80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw data will be uploaded to Research Square.


