Abstract
Purpose
Coronavirus disease 2019 (COVID‐19) is associated with hypercoagulability and increased thrombotic risk. The impact of prehospital antiplatelet therapy on in‐hospital mortality is uncertain.
Methods
This was an observational cohort study of 34 675 patients ≥50 years old from 90 health systems in the United States. Patients were hospitalized with laboratory‐confirmed COVID‐19 between February 2020 and September 2020. For all patients, the propensity to receive prehospital antiplatelet therapy was calculated using demographics and comorbidities. Patients were matched based on propensity scores, and in‐hospital mortality was compared between the antiplatelet and non‐antiplatelet groups.
Results
The propensity score‐matched cohort of 17 347 patients comprised of 6781 and 10 566 patients in the antiplatelet and non‐antiplatelet therapy groups, respectively. In‐hospital mortality was significantly lower in patients receiving prehospital antiplatelet therapy (18.9% vs. 21.5%, p < .001), resulting in a 2.6% absolute reduction in mortality (HR: 0.81, 95% CI: 0.76–0.87, p < .005). On average, 39 patients needed to be treated to prevent one in‐hospital death. In the antiplatelet therapy group, there was a significantly lower rate of pulmonary embolism (2.2% vs. 3.0%, p = .002) and higher rate of epistaxis (0.9% vs. 0.4%, p < .001). There was no difference in the rate of other hemorrhagic or thrombotic complications.
Conclusions
In the largest observational study to date of prehospital antiplatelet therapy in patients with COVID‐19, there was an association with significantly lower in‐hospital mortality. Randomized controlled trials in diverse patient populations with high rates of baseline comorbidities are needed to determine the ultimate utility of antiplatelet therapy in COVID‐19.
Keywords: antiplatelet therapy, aspirin, clopidogrel, COVID‐19, dipyridamole, prasugrel, SARS‐CoV‐2, ticagrelor
Essentials
-
•
Pre‐hospital antiplatelet therapy (APT) may be associated with benefits in COVID‐19.
-
•
In 34 675 patients ≥50 years old with COVID‐19, 17 292 patients were propensity matched.
-
•
APT prior to admission was associated with a lower rate of in‐hospital mortality.
-
•
APT may have protective effects and reduce the risk of in‐hospital mortality in COVID‐19.
Alt-text: Unlabelled Box
1. INTRODUCTION
Severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2), the virus that causes coronavirus disease 2019 (COVID‐19), has infected more than 222 million people worldwide and has led to more than 4.6 million deaths globally.1 Despite an unprecedented vaccination effort with more than 5.5 billion doses administered to date, novel SARS‐CoV‐2 variants with increased infectivity have accelerated the spread of the disease. New cases have been recorded at a rate of more than 412 000 infections per day in countries such as India, and new deaths have exceeded 12 400 patients per day worldwide.2., 3.
Numerous studies have found that COVID‐19 is not only a respiratory disease that can cause acute respiratory distress syndrome, but also a disease characterized by endothelial cell dysfunction.4 Patients with severe COVID‐19 are now well known to have hypercoagulability and an increased risk for venous thromboembolism (VTE) and arterial thrombosis.5., 6., 7., 8. VTEs in patients hospitalized with COVID‐19 have been reported at a rate ranging from 20% to 69%, with one study finding that VTEs were associated with a 2.4‐fold increase in the risk of death.9., 10., 11. Therapeutic anticoagulation and intermediate‐ to high‐dose deep vein thrombosis (DVT) prophylaxis have been used at many institutions, and small retrospective studies early in the pandemic found that these interventions were associated with improved outcomes.12 Interim results from larger studies such as the Accelerating Covid‐19 Therapeutic Interventions and Vaccines‐4 Antithrombotics Inpatient trial found that although therapeutic anticoagulation decreased the need for mechanical ventilation in moderately ill patients, this benefit was not observed in critically ill patients.13., 14.
Because increased megakaryocytes have been observed in multiple organs on autopsy in COVID‐19 patients, and because of the high rate of thromboembolic events, there may be a role for antiplatelet therapy in the treatment of this disease.15., 16., 17. The first study of aspirin use in COVID‐19 found that in 412 patients, aspirin was associated with a significantly decreased risk of mechanical ventilation, intensive care unit admission, and in‐hospital mortality.15 Another study of 638 propensity‐matched patients found that aspirin was associated with a lower incidence of in‐hospital mortality.16 In a large propensity‐matched study examining 12 600 predominately male patients in the Veterans Affairs (VA) health system, preadmission aspirin use was also associated with decreased 30‐day mortality.17
Given these data related to aspirin use in patients with COVID‐19, our study objective was to explore the effect of prehospital antiplatelet therapy on mortality in hospitalized patients with COVID‐19. We hypothesized that antiplatelet therapy would be associated with a reduced risk of in‐hospital mortality.
2. METHODS
2.1. Patients
The COVID‐19 Analysis to Assess the Mortality Impact of Antiplatelet Regimens at North American centers (CATAMARAN) study analyzed 34 675 COVID‐19‐positive patients from 90 health systems and 26 states in the United States. Patients were abstracted from the Cerner Network Real‐World Dataset, which contains deidentified data of more than 89 million patients.18 The dataset contains more than 17 million inpatient encounters, 161 million procedures, 6 million microbiology results, 903 million medications, and 11 billion laboratory results. These data concepts were cleansed, standardized, and deidentified with Health Insurance Portability and Accountability Act‐compliant operating policies before publication in the Cerner Network Real‐World data mart.
The study was deemed exempt by the institutional review board at the George Washington University under 45 CFR 46.101(b). The study was conducted in accordance with the ethical principles described in the Declaration of Helsinki. Patients were included in this study if they were ≥50 years old and hospitalized between February 2020 and September 2020 with a confirmed diagnosis of COVID‐19. Patients with COVID‐19 were identified using the ICD‐10 code U07.1. This code is given only to patients with a confirmed diagnosis of COVID‐19, which is defined as a positive COVID‐19 test result or documentation by the patient's clinician.19 Patients with suspected, possible, probable, or inconclusive COVID‐19 are not coded with U07.1. This diagnosis code has been shown to have 98.0% sensitivity and 99.0% specificity for a positive SARS‐CoV‐2 polymerase chain reaction test.20 Patients were excluded if they were <50 years old, not hospitalized, or if COVID‐19 positivity was not confirmed with a positive test result.
2.2. Study data
Patient demographics, comorbidities, preadmission antiplatelet medication use, in‐hospital therapeutics, and outcome data were collected for each patient. Antiplatelet use was defined as the use of aspirin, clopidogrel, dipyridamole, ticagrelor, or prasugrel before hospital admission. The study's primary outcome was in‐hospital mortality. Based on previous studies, we hypothesized that antiplatelet therapy would be associated with decreased in‐hospital mortality. For measures of risks and safety, we examined differences in the rate of hemorrhagic complications between the groups. Hemorrhagic complications were identified with procedure codes for blood transfusion as well as with ICD‐10 codes for cerebral hemorrhage, gastrointestinal bleeding, epistaxis, and pulmonary hemorrhage. In addition, we also analyzed differences in rates of thrombotic complications, such as ST‐segment elevation myocardial infarction, pulmonary embolism (PE), acute DVT, arterial thrombosis, and thrombotic or embolic stroke.
2.3. Statistical analysis
To reduce bias, propensity score analysis was used with the Scikit‐learn Python Library.21 Specifically, for each patient, we created a propensity score for receiving prehospital antiplatelet therapy. The independent variables in the propensity score model included age, gender, body mass index, race, chronic kidney disease, asthma, chronic obstructive pulmonary disease, heart disease, hypertension, diabetes, prior stroke, and prior PE. Patients were then matched by propensity score in a one‐to‐two ratio using the nearest‐neighbor approach with no replacement and a caliper size of 0.1. A matched dataset containing 17 347 patients was created. The standardized mean difference (SMD) for each covariate was calculated to evaluate the balance in baseline characteristics before and after matching. In addition, to further assess balance, the variance ratio of the continuous covariates in the antiplatelet and non‐antiplatelet groups were calculated.22 Variables with a SMD <0.1 and variance ratio <1 were considered to be well‐balanced between the antiplatelet and non‐antiplatelet groups.22
The Mann‐Whitney U test was used for nonparametric continuous variables such as admission vital signs, quick sequential organ failure assessment score, and laboratory values. The chi‐square test was applied for categorical variables such as demographics and medical comorbidities. p values <.05 were considered statistically significant for all comparisons.
Differences in study outcomes between groups were tested for using the chi‐square test. For the primary outcome, Kaplan‐Meier estimates of cumulative survival were calculated along with the hazard ratio and 95% confidence interval. For in‐hospital mortality, the number needed to treat (NNT) was calculated from the absolute risk reduction with antiplatelet therapy. Additional subgroup analysis exploring differences in outcomes in the aspirin, clopidogrel, and dual antiplatelet therapy groups were performed. Finally, a sensitivity analysis with calculation of an E‐Value was performed to estimate the strength of an unadjusted confounding variable associated with both antiplatelet therapy and in‐hospital mortality that would be needed to nullify the association between the two variables.23
Our study was retrospective, and an a priori sample size calculation was not performed. However, post hoc calculations found that with the 17 347 patients in the matched cohort and with the observed in‐hospital mortality rate of 21.5% in the non‐antiplatelet therapy group, our sample size allowed for the detection of a 1.3% absolute mortality reduction and 5.8% relative mortality reduction, given an alpha of 5% and 80% power. Statistical analysis was performed using Python 3 (Python Software Foundation).
3. RESULTS
The study included 34 675 patients from 90 health systems who were admitted with COVID‐19. Median age was 69 years (interquartile range 60, 78) and 52.3% of patients were male (Table 1 ). Prehospital antiplatelet therapy was present in 6781 (19.6%) patients, whereas 27 894 (80.4%) patients were not on antiplatelet therapy at the time of admission. Aspirin was the most common antiplatelet agent administered (83.9%), followed by clopidogrel (8.2%), ticagrelor (0.3%), and prasugrel (0.1%). Dual antiplatelet therapy was used in 7.4% of patients. In the group who did not receive prehospital antiplatelet therapy, 29.4% of patients received in‐hospital antiplatelet therapy during their hospitalization.
TABLE 1.
Baseline demographics and characteristics in the overall and matched cohort
| Variable | Overall Cohort (N = 34 675) | Matched Cohort (N = 17 347) |
|||
|---|---|---|---|---|---|
| Antiplatelet Treatment (N = 6781) | No Antiplatelet Treatment (N = 10 566) | SMD | p* | ||
| Demographics | |||||
| Age, years | 69 (60–78) | 72 (64–80) | 72 (64–80) | 0.02 | |
| Male | 18143 (52.3) | 3696 (54.5) | 5635 (53.3) | 0.02 | |
| BMI (kg/m2) | 29.0 (25.0–34.1) | 28.7 (24.7–33.8) | 28.9 (24.9–34.0) | 0.002 | |
| Race | |||||
| American Indian or Alaskan Native | 604 (1.7) | 175 (2.6) | 162 (1.5) | 0.07 | |
| Asian | 610 (1.8) | 106 (1.6) | 207 (2.0) | 0.03 | |
| African American | 6585 (19) | 1327 (19.6) | 2382 (22.5) | 0.07 | |
| Native Hawaiian or Other Pacific Islander | 88 (0.3) | 16 (0.2) | 25 (0.2) | 0.004 | |
| Other | 3747 (10.8) | 566 (8.3) | 976 (9.2) | 0.03 | |
| Unknown | 830 (2.4) | 171 (2.5) | 230 (2.2) | 0.02 | |
| White | 22 211 (64.1) | 4420 (65.2) | 6584 (62.3) | 0.06 | |
| Comorbidities | |||||
| Chronic kidney disease | 6326 (18.2) | 1780 (26.2) | 2704 (25.6) | 0.02 | |
| Asthma or COPD | 6040 (17.4) | 1502 (22.2) | 2298 (21.7) | 0.01 | |
| Heart disease | 22 020 (63.5) | 5226 (77.1) | 8039 (76.1) | 0.02 | |
| Hypertension | 19 327 (55.7) | 4477 (66.0) | 6948 (65.8) | 0.01 | |
| Diabetes mellitus | 13 875 (40.0) | 3391 (50.0) | 5171 (48.9) | 0.02 | |
| Prior stroke | 1706 (4.9) | 570 (8.4) | 743 (7.0) | 0.05 | |
| Prior pulmonary embolus | 2103 (6.1) | 510 (7.5) | 715 (6.8) | 0.03 | |
| Prehospital antiplatelet therapies | |||||
| Aspirin | 5659 (16.3) | 5690 (83.9) | |||
| Clopidogrel | 555 (1.6) | 555 (8.2) | |||
| Dipyridamole | 0 (0.0) | 0 (0.0) | |||
| Ticagrelor | 23 (0.1) | 23 (0.3) | |||
| Prasugrel | 8 (0.0) | 8 (0.1) | |||
| Dual antiplatelet therapy | 505 (1.5) | 505 (7.4) | |||
| Prehospital antiplatelet doses | |||||
| Aspirin | 81 (81–81) | 81 (81–81) | |||
| Clopidogrel | 75 (75–75) | 75 (75–75) | |||
| Dipyridamole | 200 (200–200) | 200 (200–200) | |||
| Ticagrelor | 90 (90–90) | 90 (90–90) | |||
| Prasugrel | 10 (10–10) | 10 (10–10) | |||
| Admission vital signs and prediction scores | |||||
| Admission qSOFA | 1 (0–1) | 1 (0–1) | 1 (0–1) | .29 | |
| Systolic BP, mmHg | 132 (116–148) | 132 (116–149) | 132 (116–149) | .38 | |
| Diastolic BP, mmHg | 74 (65–83) | 73 (64 −83) | 73 (64–83) | .27 | |
| HR, beats/min | 89 (78 −102) | 88 (76–102) | 88 (77 −101) | .25 | |
| RR | 20 (18 −24) | 20 (18–24) | 20 (18 −24) | .004 | |
| SpO2, % | 94 (91–97) | 95 (91 −97) | 94 (91–97) | <.001 | |
| Temperature, °C | 37.0 (36.7–37.6) | 37.0 (36.7–37.6) | 37.0 (36.7–37.6) | .45 | |
| Initial laboratory values | |||||
| WBC, K/μl | 7.4 (5.4–10.4) | 7.4 (5.4–10.5) | 7.4 (5.4–10.5) | .28 | |
| Lymphocytes, K/μl | 0.9 (0.6–1.3) | 0.9 (0.6–1.3) | 0.9 (0.6–1.3) | .27 | |
| Hemoglobin, g/dl | 12.9 (11.3–14.2) | 12.5 (11.0–14.0) | 12.7 (11.1–14.1) | <.001 | |
| Platelets, K/μl | 210 (160–275) | 209 (157–275) | 208 (158–272) | .24 | |
| INR | 1.12 (1.03–1.24) | 1.09 (1.00–1.19) | 1.12 (1.02–1.26) | <.001 | |
| PT, s | 13.5 (12.3–15.0) | 13.6 (12.4–15.1) | 13.6 (12.4–15.3) | .10 | |
| PTT, s | 31.5 (28.1–36.4) | 31.8 (28.3–36.6) | 31.9 (28.4–37.0) | .33 | |
| Fibrinogen, ng/ml | 544 (427–673) | 542 (422–677) | 533 (417–662) | .08 | |
| Lactate, mmol/L | 1.5 (1.1–2.2) | 1.5 (1.1–2.2) | 1.6 (1.2–2.2) | .03 | |
| Receipt of other therapeutics | |||||
| Dexamethasone | 18 942 (54.6) | 3559 (52.5) | 5645 (53.4) | .41 | |
| Remdesivir | 8546 (24.6) | 1652 (24.4) | 2455 (23.2) | .14 | |
Abbreviations: BMI, body mass index; BP, blood pressure; COPD, chronic obstructive pulmonary disease; HR, heart rate; INR, international normalized ratio, PT, prothrombin time; PTT, partial thromboplastin time; qSOFA, quick sequential organ failure assessment; SMD, standardized mean difference; SpO2, peripheral capillary oxygen saturation, WBC, white blood cell.
*Categorical variables are reported as number (percent). Continuous variables are represented as median (interquartile range). Chi‐squared test for categorical variables, Mann‐Whitney U test for continuous variables, pairwise comparison between antiplatelet therapy and non‐antiplatelet therapy groups.
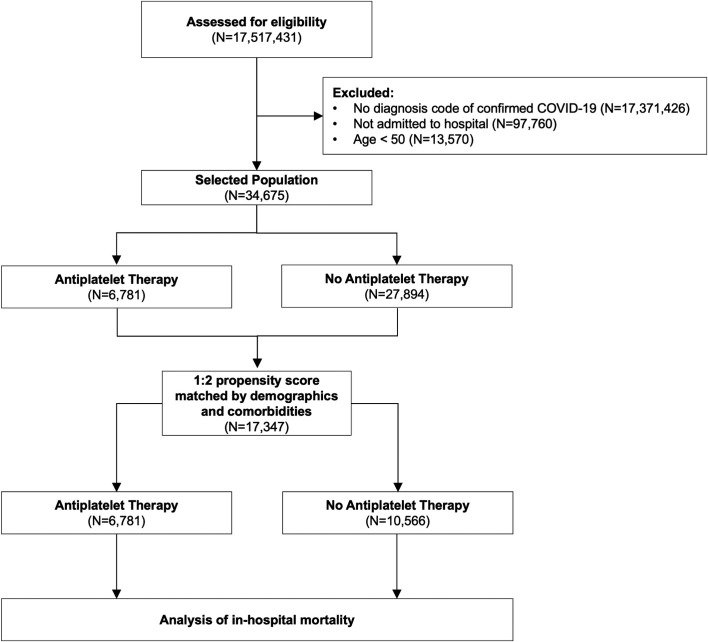
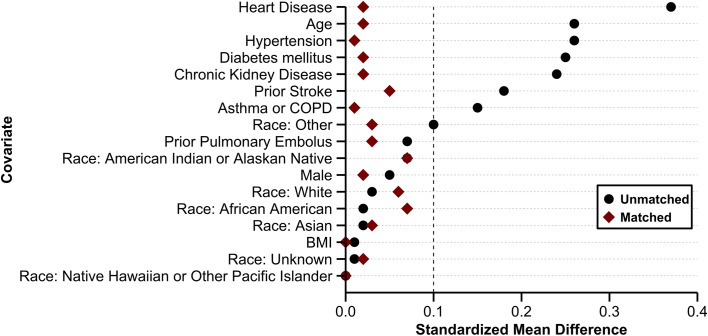
Propensity score matching using the previously described methodology was performed and 17 347 matched patients were included in the final analysis cohort (Figure 1 ). The SMDs in the unmatched sample were ≥0.1 in eight of the 17 covariates (47.1%). In contrast, after propensity matching, all SMDs were <0.1 and all variance ratios were <1 (Figure 2 ). This indicated that the matched cohort was well balanced with respect with demographics and comorbidities.22
FIGURE 1.
Flow diagram depicting the phases of enrollment, exclusion, and data analysis. Abbreviations: COVID‐19, coronavirus disease 2019
FIGURE 2.
Standardized mean differences of covariates before and after propensity matching. The dashed vertical line denotes a standardized mean difference of 0.1. The standardized mean differences in the unmatched sample were ≥0.1 in eight of the 17 covariates (47.1%). After propensity matching, all standardized mean differences were <0.1, indicating that the baseline demographics and comorbidities were well balanced between the antiplatelet and non‐antiplatelet groups. Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease
At the time of hospital admission, there was no difference in the admission quick sequential organ failure assessment score. Blood pressure, heart rate, and temperature were not different between the groups, although patients on antiplatelet therapy had higher SpO2 on admission (95% vs 94%). Patients receiving antiplatelet therapy presented with lower hemoglobin (12.5 g/dl vs 12.7), lower international normalized ratio (1.09 vs 1.12), and lower lactate (1.5 mmol/L vs 1.6) than patients not receiving antiplatelet therapy. Receipt of other therapeutics that have been shown in large inpatient randomized controlled trials to have a mortality benefit, such as dexamethasone and remdesivir, was not different between the two groups.24., 25., 26., 27.
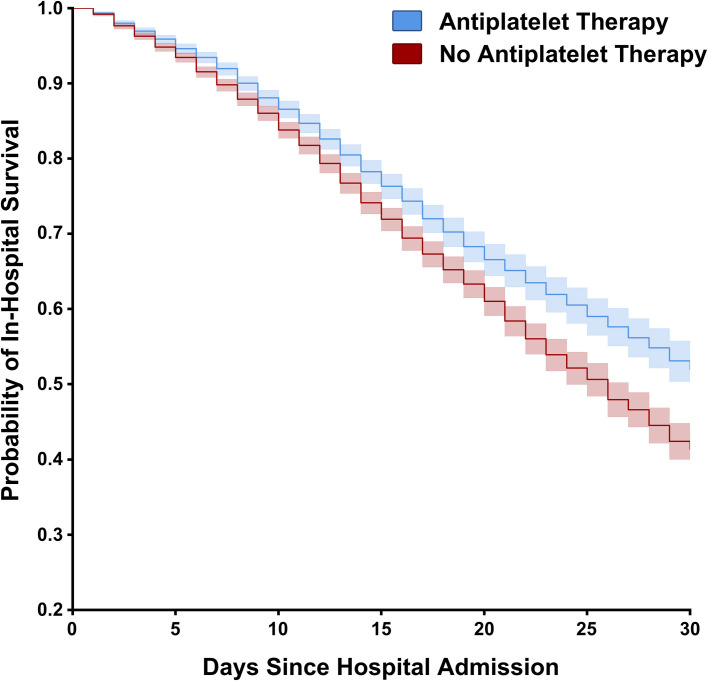
In the matched cohort, in‐hospital mortality was significantly lower for patients who were on prehospital antiplatelet therapy (18.9% mortality antiplatelet therapy vs 21.5% non‐antiplatelet therapy, p < .001; Table 2 ). This difference equated to a 2.6% absolute mortality reduction (95% CI, 1.4‐3.8) and a 12.1% relative mortality reduction (95% CI, 6.6‐17.4). On average, 39 patients needed to be treated with prehospital antiplatelet therapy to prevent one in‐hospital death. A Kaplan‐Meier survival curve was created to depict the differences in survival between the groups (adjusted HR = 0.81; 95% CI, 0.76‐0.87; p < .005; Figure 3 ). The E‐value indicated that an unmeasured confounder would need to be associated with both prehospital antiplatelet therapy and in‐hospital mortality at a minimum risk ratio of 1.77 (upper confidence limit 1.56).
TABLE 2.
Complications and outcomes
| Variable | Overall Cohort (N = 34 675) | Matched Cohort (N = 17 347) |
||
|---|---|---|---|---|
| Antiplatelet Treatment (N = 6781) | No Antiplatelet Treatment (N = 10 566) | p* | ||
| Thrombotic complications | ||||
| Thrombotic/embolic stroke | 176 (0.5) | 57 (0.8) | 72 (0.7) | .36 |
| PE | 876 (2.5) | 151 (2.2) | 321 (3.0) | .002 |
| STEMI | 114 (0.3) | 33 (0.5) | 38 (0.4) | .20 |
| Acute deep vein thrombosis | 874 (2.5) | 176 (2.6) | 289 (2.7) | .58 |
| Arterial thrombosis | 54 (0.2) | 15 (0.2) | 16 (0.2) | .29 |
| Hemorrhagic complications | ||||
| Cerebral hemorrhage | 122 (0.4) | 35 (0.5) | 43 (0.4) | .30 |
| Gastrointestinal hemorrhage | 822 (2.4) | 211 (3.1) | 283 (2.7) | .10 |
| Pulmonary hemorrhage | 176 (0.5) | 43 (0.6) | 56 (0.5) | .38 |
| Epistaxis | 169 (0.5) | 61 (0.9) | 46 (0.4) | <.001 |
| Blood transfusion | 1395 (4.0) | 187 (2.8) | 418 (4.0) | <.001 |
| Outcomes | ||||
| Hospital LOS, days | 8 (5–14) | 8 (5–15) | 8 (5–14) | <.001 |
| Mechanical ventilation | 10 633 (30.7) | 2122 (31.3) | 3403 (32.2) | .30 |
| In‐hospital mortality | 6510 (18.8) | 1280 (18.9) | 2271 (21.5) | <.001 |
Abbreviations: LOS, length of stay; PE, pulmonary embolism; STEMI, ST‐segment elevation myocardial infarction.
*Categorical variables are reported as number (percent). Continuous variables are represented as median (interquartile range). Chi‐squared test for categorical variables, Mann‐Whitney U test for continuous variables, pairwise comparison between antiplatelet therapy and non‐antiplatelet therapy groups.
FIGURE 3.
Kaplan‐Meier estimates of cumulative survival. Patients are stratified by antiplatelet therapy. Patients discharged within the study period are right‐censored. The 95% CIs of estimated cumulative survival for each group shown in the shaded areas. Antiplatelet use was associated with a decreased hazard for in‐hospital mortality (adjusted HR = 0.81; 95% CI, 0.76–0.87, p < .005)
When analyzing thrombotic complications, patients on antiplatelet therapy had a significantly lower rate of PE (2.2% antiplatelet therapy vs 3.0% non‐antiplatelet therapy, p = .002), although there was no difference in the rate of stroke, ST‐segment elevation myocardial infarction, acute DVT, or arterial thrombosis. When examining the rate of hemorrhagic complications, the rate of epistaxis was significantly higher in the antiplatelet therapy group (0.9% antiplatelet therapy vs 0.4% non‐antiplatelet therapy, p < .001), although the number of patients requiring blood transfusions were significantly lower in the antiplatelet group (2.8% antiplatelet vs 4.0% non‐antiplatelet, p < .001). The rate of cerebral, gastrointestinal, and pulmonary hemorrhage was not different between the groups.
Because P2Y12 inhibitors are either cost‐prohibitive or unavailable in some countries, a subgroup analysis was performed examining the outcomes in those receiving aspirin (n = 5690), clopidogrel (n = 555), or dual antiplatelet therapy (n = 505). Bonferroni correction of the five exploratory outcomes was performed (p threshold ≤ .01), and when comparing those receiving only aspirin against those receiving no antiplatelet therapy, in‐hospital mortality was significantly lower in the aspirin group (18.5% aspirin vs 21.5% no antiplatelet therapy, p < .001). This resulted in an absolute risk reduction of 3.0% and NNT of 33. In contrast, for those receiving only clopidogrel and for those receiving only dual antiplatelet therapy, the difference in in‐hospital mortality was not significant when compared with those receiving no antiplatelet therapy (22.0% clopidogrel vs 21.5% no antiplatelet therapy, p = .81; 19.2% dual antiplatelet therapy vs 21.5% no antiplatelet therapy, p = .28). Similarly, when performing head‐to‐head comparisons of outcomes in the aspirin, clopidogrel, and dual antiplatelet therapy groups, in‐hospital mortality was not significantly different for aspirin versus clopidogrel (18.5% aspirin vs. 22.0% clopidogrel, p = .05), and for aspirin versus dual antiplatelet therapy (18.5% aspirin vs. 19.2% dual antiplatelet therapy, p = .73). However, given the reduced sample size in the clopidogrel and dual antiplatelet therapy groups, and assuming an alpha of 0.05 and power of 0.8, any analysis involving the clopidogrel or dual antiplatelet therapy groups was only powered to detect a 3.5% to 3.9% absolute risk reduction in in‐hospital mortality in these groups.
4. DISCUSSION
In a multicenter, retrospective, observational cohort study of 17 292 propensity‐matched patients with COVID‐19, prehospital antiplatelet therapy was associated with a significant decrease in in‐hospital mortality and PE. In a disease that has devastated the global population, the observed 2.6% absolute risk reduction in mortality and number needed to treat of 39 could translate into a large number of patients who could benefit from antiplatelet therapy. Importantly, most patients in the antiplatelet therapy arm received aspirin, which is a widely available medication that is inexpensive and has a well described risk profile.
Antiplatelet drugs act by antagonizing the P2Y12 receptor (clopidogrel, ticagrelor, and prasugrel), inhibiting phosphodiesterase (dipyridamole), or inhibiting cyclooxygenase‐1 (COX‐1) (aspirin), all of which are critical pathways for platelet activation and aggregation.28 Activation of COX‐1 leads to the production of thromboxane A2 and increased platelet reactivity.28 Aspirin irreversibly inhibits platelet function by inhibiting COX‐1. Similarly, the P2Y12 receptor leads to activation of the glycoprotein IIb/IIIa receptor, which results in platelet degranulation, thromboxane A2 production, and platelet aggregation.29 Clopidogrel, ticagrelor, and prasugrel act on this pathway to antagonize the P2Y12 receptor and decrease platelet aggregation.29
COVID‐19 leads to microthrombosis and is associated with megakaryocyte proliferation in the heart, lungs, and kidneys.8., 30. In one study, one‐third of postmortem patients had PE as the cause of death.31 VTEs were also present in more than 50% of these patients, even though they were not suspected before death.31 In another study of 219 patients, 4.6% of patients had suffered from acute ischemic stroke, which was associated with a 50% mortality rate.32 Finally, in a systemic review of 27 studies, the rate of arterial thrombosis was 4.4% and was not limited to any one organ system.33 Our study demonstrated a significant reduction in the rate of PE in the antiplatelet therapy group. However, we did not detect a significant reduction in other thrombotic complications such as DVT or arterial thrombosis. Because this was a retrospective study examining standardized, deidentified data, these complications could only be identified with ICD‐10 codes, and is a limitation to our analysis. It may be easier to identify and code a complication in the medical record with high clinical significance, such as a PE. Complications with lower clinical significance may be more difficult to identify and code accurately, which may explain why we observed a significant reduction in the rate of PE, but not in the rate of DVT. In addition, in patients presenting with dyspnea, computed tomography angiography is commonly used in the emergency department to diagnose PE. However, lower extremity Doppler studies are not routinely performed to diagnose DVT, especially in the setting of limiting health care worker exposure to COVID‐19. Additionally, in a patient with a PE, may clinicians may not perform Doppler studies because it would not alter the treatment course, which is another reason that may explain the insignificant difference in the rate of DVTs between the two groups.
4.1. Previous studies
Our in‐hospital mortality findings on antiplatelet therapy in COVID‐19 are consistent with three recently published observational studies and one randomized controlled trial. The first study was reported from the multicenter Collaborative Registry to Understand the Sequelae of Harm (CRUSH) COVID Registry, which included 412 COVID‐19 patients.15 Mechanical ventilation (adjusted HR: 0.56; 95% CI, 0.37‐0.85, p = .007), intensive care unit admission (adjusted HR: 0.57; 95% CI, 0.38‐0.85, p = .005), and in‐hospital mortality rates (adjusted HR: 0.53; 95% CI, 0.31‐0.90, p = .02) were more favorable in patients taking aspirin in the 7 days before admission or in the first 24 hours of admission. Those in the aspirin group did not have a significant increase in major bleeding complications (p = .69). However, this study was limited by its relatively small size, and the results may be difficult to discern because of the inclusion of both patients who were chronically taking aspirin and patients who acutely received aspirin in the first 24 hours of admission. Although the CRUSH COVID study demonstrated a larger effect size for aspirin than the present study, it also included more critically ill patients.
Another study of 638 propensity‐matched patients found that in‐hospital aspirin use was associated with a lower incidence of in‐hospital mortality (HR: 0.522; 95% CI, 0.336‐0.812).16 Even after accounting for the confounding effect of time inherent in the before‐after study design, the sensitivity analysis demonstrated significantly lower in‐hospital mortality in the aspirin group (HR: 0.036; 95% CI, 0.002‐0.576).16
Before our study, the largest investigation of aspirin in COVID‐19 involved 12 600 propensity‐matched patients from the VA health system.17 The odds of 30‐day mortality were significantly lower in patients with an active outpatient aspirin prescription (OR: 0.38; 95% CI, 0.33‐0.45).17 Mortality was reduced from 10.5% in the nonaspirin group to 4.3% in the aspirin group, which represented a 59.0% relative risk reduction in mortality. With its large sample size, this study further provides a strong rationale for the use of aspirin in COVID‐19. However, as expected in many VA health system studies, the matched cohort comprised of 95.8% male veterans, and the results may not be generalizable to a more diverse patient population.
The Randomized Evaluation of COVID‐19 therapy (RECOVERY) trial randomized 14 892 patients to either aspirin or usual care.34 Although all‐cause 28‐day mortality was no different between the groups (rate ratio 0.96; 95% CI, 0.89‐1.04, p = .35), aspirin was associated with a reduction in hospital length of stay (median 8 days vs 9 days) and a modest improvement in 28‐day in‐hospital survival (74.8% aspirin vs 73.6% nonaspirin, rate ratio 1.06; 95% CI, 1.02‐1.10, p = .006).34 This represents a 1.2% absolute reduction in in‐hospital mortality, which is similar to the 2.6% absolute reduction found in our study. However, there are several important distinctions between the populations of RECOVERY and our study, CATAMARAN. CATAMARAN examined patients ≥50 years of age, whereas RECOVERY enrolled adults ≥18 years of age, which resulted in older patients in our study (median age 72 vs 59 years). In addition, patients in CATAMARAN were more ethnically diverse (non‐White 34.3% vs 16.0%) and had higher rates of comorbidities such as diabetes (49.4% vs. 21.8%), cardiovascular disease (76.5% vs. 10.5%), lung disease (21.9% vs. 19.0%), and renal disease (25.8% vs. 3.1%).34 Likewise, patients in CATAMARAN had more severe illness, as evidenced by a higher mechanical ventilation rate (31.8% vs 4.9%). These reasons may explain why the effect size of antiplatelet therapy is larger in CATAMARAN than in RECOVERY. Finally, an overwhelming majority of patients in RECOVERY received corticosteroids (94.1% vs 53.1%), which may decrease the observed effect size of aspirin. Nonetheless, the prior studies on aspirin therapy, in combination with the results from RECOVERY and now CATAMARAN, provide evidence that antiplatelet agents may hold therapeutic value in the treatment of high‐risk COVID‐19 patients.
4.2. Adverse events
The administration of antiplatelet drugs is not without complications and studies in non‐COVID‐19 populations have found an increased risk of bleeding in patients receiving aspirin for primary prevention of cardiovascular disease.35., 36. Patients in the Aspirin in Reducing Events in the Elderly randomized controlled trial were 1.38 times more likely to have a major hemorrhagic complication, and a systematic review found that patients were 1.43 times more likely to have a serious bleeding complication while on aspirin.35., 36. We did not find that antiplatelet therapy resulted in an increase in blood transfusions during hospitalization. With the exception of epistaxis, we also did not find an increased risk of serious hemorrhagic complications such as cerebral, gastrointestinal, and pulmonary hemorrhage. Although this is contrary to previous cardiovascular disease studies, it consistent with previous COVID‐19 studies, possibly because COVID‐19 is such a prothrombotic disease. These data suggest that although aspirin use may be associated with significant benefits in COVID‐19, the bleeding risks should be carefully considered in each patient before therapy is initiated.
4.3. Limitations
Although our study is strengthened by a diverse population of patients from 90 health care systems in the United States, it is not without limitations. Our dataset relied on data extraction from the electronic medical record, which then underwent data cleaning and standardization. The reliability of the data is therefore subject to the accurate reporting of antiplatelet medications by patients and the clinicians who treated those patients. It is possible that clinicians may have failed to document the presence of an antiplatelet medication at admission. In this case, a patient would be misallocated to the non‐antiplatelet therapy cohort, thus reducing the true effect size of antiplatelet therapy.
Our study is also limited by the bias inherent in retrospective, observational studies. The matching process intrinsically results in the loss of some patients, which could affect the generalizability of our study's results.37 The target matching ratio was 1:2, but not all patients in the treatment group could be matched with exactly two control patients, resulting in an actual matching ratio of 1:1.6. Perfect 1:2 matching of the 6781 patients in the antiplatelet therapy group requires 13 562 patients in the non‐antiplatelet therapy group to be matched. With an available pool of 27 894 non‐antiplatelet therapy patients, almost 50% of these patients need to be matched to attain this 1:2 matching ratio. Patients were matched on propensity to receive prehospital antiplatelet therapy, and with antiplatelet therapy so widely used in the United States, especially in patients with multiple comorbidities who are older than 50 years of age, it may have been difficult to match patients with similar demographics and comorbidities who were not already on an antiplatelet agent. Although propensity score matching can account for observed biases, it cannot account for unmeasured confounders such as the quality of care provided to individual patients.37 It is possible that unmeasured confounders could have introduced treatment bias to patients in our cohort. However, in our sensitivity analysis, the E‐value indicated that any confounding variable would have to have a minimum risk ratio of 1.77 to nullify the relationship between antiplatelet therapy and mortality that was observed in our study. Finally, some patients in the control group received in‐hospital antiplatelet therapy, and this may have led to an underestimation of the true effect size of prehospital antiplatelet therapy.
5. FUTURE INVESTIGATION
Finally, because each class of antiplatelet agent has a unique mechanism of action, it is plausible that they may influence mortality outcomes differently. Our subgroup analysis found significant mortality improvements in the aspirin versus the non‐antiplatelet therapy groups. However, the differences in mortality between the clopidogrel versus non‐antiplatelet groups and in the dual antiplatelet therapy versus non‐antiplatelet therapy groups were not significant. Similarly, head‐to‐head post hoc comparisons of each antiplatelet agent were performed to help guide future studies. No differences in mortality were found when comparing aspirin with clopidogrel and when comparing aspirin with dual antiplatelet therapy. However, given the reduced sample size in both the clopidogrel and dual antiplatelet therapy cohorts, subgroup analyses involving these groups may have been underpowered to detect any differences. Future studies are necessary to determine if mortality outcomes are different for P2Y12 antagonists, COX‐1 inhibitors, or phosphodiesterase inhibitors.
6. CONCLUSION
After more than 1 year and 4.6 million lives lost because of the COVID‐19 pandemic, extraordinary global efforts, unprecedented collaboration, and an accelerated pace of scientific research have led to inclusion of dexamethasone, remdesivir, monoclonal antibodies, and vaccines in our repertoire against COVID‐19. Our analysis adds to the existing evidence supporting the use of antiplatelet therapy in COVID‐19. Among patients ≥50 years old, there was a 12.1% relative reduction and 2.6% absolute reduction in mortality observed in our study, resulting in an NNT of 39. Like dexamethasone, antiplatelet agents such as aspirin are inexpensive and widely available throughout the world. Antiplatelet drugs are not a substitute for vaccination, but as the global population continues to experience high infection rates, alarming variants, and overwhelmed health care systems, adjunct therapies are needed. Our study suggests that antiplatelet drugs are associated with a modest reduction in mortality in an older, diverse patient population with relatively high rates of baseline cardiovascular comorbidities.
CONFLICT OF INTERESTS
Dr. Chow has served on the Speaker's Bureau for La Jolla Pharmaceutical Company, outside the scope of the submitted work. The other authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTIONS
Concept, design, analysis or interpretation of data: Jonathan H. Chow, Ying Yin, Michael A. Mazzeffi, and Stuart J. Nelson. Critical writing or revising the intellectual content: Jonathan H. Chow, Ying Yin, David P. Yamane, Danielle Davison, Ryan J. Keneally, Katrina Hawkins, K. Gage Parr, Mustafa Al‐Mashat, Jeffery S. Berger, Reamer L. Bushardt, Michael A. Mazzeffi, and Stuart J. Nelson. Final approval of the manuscript: Jonathan H. Chow, Ying Yin, David P. Yamane, Danielle Davison, Ryan J. Keneally, Katrina Hawkins, K. Gage Parr, Mustafa Al‐Mashat, Jeffery S. Berger, Reamer L. Bushardt, Michael A. Mazzeffi, and Stuart J. Nelson. Jonathan H. Chow has seen the original study data, reviewed the analysis of the data, written and approved the final manuscript, and is the author responsible for correspondence. All authors have seen the original study data, reviewed the analysis of the data, written and approved the final manuscript.
CONSENT TO PARTICIPATE
The requirement for written informed consent was waived by the institutional review board.
Footnotes
Drs. Mazzeffi and Nelson are co‐senior authors who contributed equally to this work.
Manuscript Handled by: Sabine Eichinger
Final decision: Ton Lisman, 26 August 2021
This report describes a cohort observational clinical study. The authors state that the report includes every item in the STROBE checklist for cohort studies.
REFERENCES
- 1.World Health Organization Coronavirus disease (COVID‐19) outbreak. https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019. Published 2020. Accessed Sept 9, 2021.
- 2.New York Times Tracking coronavirus vaccinations around the world. https://www.nytimes.com/interactive/2021/world/covid‐vaccinations‐tracker.html. Published 2021. Accessed Sept 9, 2021.
- 3.Johns Hopkins University Coronavirus Resource Center. https://coronavirus.jhu.edu/. Published 2021. Accessed Sept 9, 2021.
- 4.Jin Y., Ji W., Yang H., Chen S., Zhang W., Duan G. Endothelial activation and dysfunction in COVID‐19: from basic mechanisms to potential therapeutic approaches. Signal Transduct Target Ther. 2020;5(1):293. doi: 10.1038/s41392-020-00454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spiezia L., Boscolo A., Poletto F., et al. COVID‐19‐related severe hypercoagulability in patients admitted to intensive care unit for acute respiratory failure. Thromb Haemost. 2020;120(6):998–1000. doi: 10.1055/s-0040-1710018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wright F.L., Vogler T.O., Moore E.E., et al. Fibrinolysis shutdown correlates to thromboembolic events in severe COVID‐19 infection. J Am Coll Surg. 2020;231(2):193–203. doi: 10.1016/j.jamcollsurg.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ranucci M., Ballotta A., Di Dedda U., et al. The procoagulant pattern of patients with COVID‐19 acute respiratory distress syndrome. J Thromb Haemost. 2020;18(7):1747–1751. doi: 10.1111/jth.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rapkiewicz A.V., Mai X., Carsons S.E., et al. Megakaryocytes and platelet‐fibrin thrombi characterize multi‐organ thrombosis at autopsy in COVID‐19: a case series. EClinicalMedicine. 2020;24:100434. doi: 10.1016/j.eclinm.2020.100434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lodigiani C., Iapichino G., Carenzo L., et al. Venous and arterial thromboembolic complications in COVID‐19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Middeldorp S., Coppens M., van Haaps T.F., et al. Incidence of venous thromboembolism in hospitalized patients with COVID‐19. J Thromb Haemost. 2020;18(8):1995–2002. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Llitjos J.F., Leclerc M., Chochois C., et al. High incidence of venous thromboembolic events in anticoagulated severe COVID‐19 patients. J Thromb Haemost. 2020;18(7):1743–1746. doi: 10.1111/jth.14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paranjpe I., Fuster V., Lala A., et al. Association of treatment dose anticoagulation with in‐hospital survival among hospitalized patients with COVID‐19. J Am Coll Cardiol. 2020;76(1):122–124. doi: 10.1016/j.jacc.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Institutes of Health. Full‐dose blood thinners decreased need for life support and improved outcome in hospitalized COVID‐19 patients. https://www.nih.gov/news‐events/news‐releases/full‐dose‐blood‐thinners‐decreased‐need‐life‐support‐improved‐outcome‐hospitalized‐covid‐19‐patients. Published 2021. Accessed April 20, 2021.
- 14.National Institutes of Health. NIH ACTIV Trial of blood thinners pauses enrollment of critically ill COVID‐19 patients. https://www.nih.gov/news‐events/news‐releases/nih‐activ‐trial‐blood‐thinners‐pauses‐enrollment‐critically‐ill‐covid‐19‐patients. Published 2020. Accessed April 20, 2021.
- 15.Chow J.H., Khanna A.K., Kethireddy S., et al. Aspirin use is associated with decreased mechanical ventilation, intensive care unit admission, and in‐hospital mortality in hospitalized patients with coronavirus disease 2019. Anesth Analg. 2021;132(4):930–941. doi: 10.1213/ANE.0000000000005292. [DOI] [PubMed] [Google Scholar]
- 16.Meizlish M.L., Goshua G., Liu Y., et al. Intermediate‐dose anticoagulation, aspirin, and in‐hospital mortality in COVID‐19: a propensity score‐matched analysis. Am J Hematol. 2021;96(4):471–479. doi: 10.1002/ajh.26102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osborne T.F., Veigulis Z.P., Arreola D.M., Mahajan S.M., Roosli E., Curtin C.M. Association of mortality and aspirin prescription for COVID‐19 patients at the Veterans Health Administration. PLoS One. 2021;16(2) doi: 10.1371/journal.pone.0246825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cerner Corporation. FAQ: COVID‐19 de‐identified data cohort access offer for academic researchers. https://www.cerner.com/‐/media/covid‐19/response/2263471793_covid‐19‐de‐identified‐data‐cohort‐access‐offer‐faq_v1.aspx. Accessed May 10, 2021.
- 19.Centers for Disease Control and Prevention. ICD‐10‐CM Official Guidelines for Coding and Reporting. https://www.cdc.gov/nchs/data/icd/ICD‐10cmguidelines‐FY2021‐COVID‐update‐January‐2021‐508.pdf. Published 2020. Accessed May 14, 2021.
- 20.Kadri S.S., Gundrum J., Warner S., et al. Uptake and accuracy of the diagnosis code for COVID‐19 among US hospitalizations. JAMA. 2020;324(24):2553–2554. doi: 10.1001/jama.2020.20323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pedregosa F., Varoquaux G., Gramfort A., et al. Scikit‐learn: machine learning in {P}ython. J Mach Learn Res. 2011;12:2825–2830. [Google Scholar]
- 22.Zhang Z., Kim H.J., Lonjon G., Zhu Y., AMEB‐DCTCG Balance diagnostics after propensity score matching. Ann Transl Med. 2019;7(1):16. doi: 10.21037/atm.2018.12.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.VanderWeele T.J., Ding P. Sensitivity analysis in observational research: introducing the E‐value. Ann Intern Med. 2017;167(4):268–274. doi: 10.7326/M16-2607. [DOI] [PubMed] [Google Scholar]
- 24.Stone J.H., Frigault M.J., Serling‐Boyd N.J., et al. Efficacy of tocilizumab in patients hospitalized with Covid‐19. N Engl J Med. 2020;383(24):2333–2344. doi: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salvarani C., Dolci G., Massari M., et al. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized With COVID‐19 Pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;181(1):24–31. doi: 10.1001/jamainternmed.2020.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Group RC. Horby P., Lim W.S., et al. Dexamethasone in hospitalized patients with Covid‐19. N Engl J Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beigel J.H., Tomashek K.M., Dodd L.E., et al. Remdesivir for the treatment of Covid‐19 ‐ final report. N Engl J Med. 2020;383(19):1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Warner T.D., Nylander S., Whatling C. Anti‐platelet therapy: cyclo‐oxygenase inhibition and the use of aspirin with particular regard to dual anti‐platelet therapy. Br J Clin Pharmacol. 2011;72(4):619–633. doi: 10.1111/j.1365-2125.2011.03943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Damman P., Woudstra P., Kuijt W.J., de Winter R.J., James S.K. P2Y12 platelet inhibition in clinical practice. J Thromb Thrombolysis. 2012;33(2):143–153. doi: 10.1007/s11239-011-0667-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ackermann M., Verleden S.E., Kuehnel M., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid‐19. N Engl J Med. 2020;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wichmann D., Sperhake J.P., Lutgehetmann M., et al. Autopsy findings and venous thromboembolism in patients with COVID‐19: a prospective cohort study. Ann Intern Med. 2020;173(4):268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Y., Li M., Wang M., et al. Acute cerebrovascular disease following COVID‐19: a single center, retrospective, observational study. Stroke Vasc Neurol. 2020;5(3):279–284. doi: 10.1136/svn-2020-000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheruiyot I., Kipkorir V., Ngure B., Misiani M., Munguti J., Ogeng'o J. Arterial thrombosis in coronavirus disease 2019 patients: a rapid systematic review. Ann Vasc Surg. 2021;70:273–281. doi: 10.1016/j.avsg.2020.08.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horby P.W., Pessoa‐Amorim G., Staplin N., et al. Aspirin in patients admitted to hospital with COVID‐19 (RECOVERY): a randomised, controlled, open‐label, platform trial. medRxiv. 2021 [Google Scholar]
- 35.McNeil J.J., Wolfe R., Woods R.L., et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379(16):1509–1518. doi: 10.1056/NEJMoa1805819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng S.L., Roddick A.J. Association of aspirin use for primary prevention with cardiovascular events and bleeding events: a systematic review and meta‐analysis. JAMA. 2019;321(3):277–287. doi: 10.1001/jama.2018.20578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leisman D.E. Ten pearls and pitfalls of propensity scores in critical care research: a guide for clinicians and researchers. Crit Care Med. 2019;47(2):176–185. doi: 10.1097/CCM.0000000000003567. [DOI] [PubMed] [Google Scholar]