Abstract
Objective
COVID‐19 may yield a variety of clinical pictures, differing from pneumonitis to Acute Respiratory Distress Syndrome along with vascular damage in the lung tissue, named endotheliitis. To date, no specific treatment strategy was approved for the prevention or treatment of COVID‐19 in terms of endotheliitis‐related comorbidities. Here, we presented our treatment strategies for 11 190 COVID‐19 patients depending on categorisation by the severity of both the respiratory and vascular distress and presented the manifestations of endotheliitis in skin, lung and brain tissues according to the different phases of COVID‐19.
Methods
After a retrospective examination, patients were divided into three groups according to their repercussions of vascular distress, which were represented by radiological, histopathological and clinical findings. We presented the characteristics and courses of seven representative and complicated cases which demonstrate different phases of the disease and discussed the treatment strategies in each group.
Results
Among 11 190 patients, 9294 patients met the criteria for Group A, and 1376 patients were presented to our clinics with Group B characteristics. Among these patients, 1896 individuals (Group B and Group C) were hospitalised. While 1220 inpatients were hospitalised within the first 10 days after the diagnosis, 676 of them were worsened and hospitalised 10 days after their diagnosis. Among hospitalised patients, 520 of them did not respond to group A and B treatments and developed hypoxemic respiratory failure (Group C) and 146 individuals needed ventilator support and were followed in the intensive care unit, and 43 (2.2%) patients died.
Conclusion
Distinctive manifestations in each COVID‐19 patient, including non‐respiratory conditions in the acute phase and the emerging risk of long‐lasting complications, suggest that COVID‐19 has endotheliitis‐centred thrombo‐inflammatory pathophysiology. Daily evaluation of clinical, laboratory and radiological findings of patients and deciding appropriate pathophysiological treatment would help to reduce the mortality rate of COVID‐19.
What’s known
Novel coronavirus disease 2019 (COVID‐19) has resulted in a dramatic pandemic crisis by causing mainly a respiratory disease that can rapidly progress to pneumonia and, in severe cases, to acute respiratory distress syndrome (ARDS).
SARS‐CoV‐2 infection is a multisystemic disease which courses rapidly with respiratory failure and complications secondary to vascular alterations (ie, microvascular thrombosis, endotheliitis and cytokine‐induced plasma toxicity).
What’s new
Distinctive manifestations in each COVID‐19 patient, including non‐respiratory conditions in the acute phase and the emerging risk of long‐lasting complications, suggest that COVID‐19 has an endotheliitis‐centred thrombo‐inflammatory pathophysiology.
Potential pathophysiological mechanisms contributing to endotheliitis includes cytokine storm and toxic plasma, thromboinflammation and systemic microangiopathy that could be used as a target to provide appropriate treatment agents.
Endotheliitis can explain the mechanism behind the respiratory failure in COVID‐19, and the difference of COVID‐19 related ARDS from ARDS seen in other critical conditions.
The vascular distress phenomenon is a clinical parameter that can be used practically in the clinics for COVID‐19 patients, both by recognising radiological images and clinical findings of the patients, to provide an objective approach for treatment.
Endotheliitis‐based pathophysiological mechanisms are known to be momentarily changing and difficult to manage because of their risk of sudden aggravation. Hence, daily evaluation of patients and deciding appropriate pathophysiological treatment for the momentary changes in clinical, laboratory and radiological findings would help to reduce the mortality rate of this novel virus.
1. INTRODUCTION
Novel coronavirus disease 2019 (COVID‐19) has resulted in a dramatic pandemic crisis by causing mainly a respiratory disease that can rapidly progress to pneumonia and, in severe cases, to acute respiratory distress syndrome (ARDS). 1 Globally, as of 15 July 2021, there have been 187 519 798 confirmed cases of COVID‐19, including 4 049 372 deaths, reported to World Health Organization (WHO). 2 The heterogeneity of the disease serves a spectrum from asymptomatic cases to respiratory failure. With the experience of 11 190 COVID‐19 patients since March 2020, we observed that distinctive clinical, radiological and histopathological manifestations of each COVID‐19 patient, including non‐respiratory conditions in the acute phase and the emerging risk of long‐lasting complications, suggest endotheliitis‐centred thrombo‐inflammatory pathophysiology for COVID‐19. 3 Therefore, understanding the pathophysiology of SARS‐CoV‐2 infection, and more significantly the host response against it, is a fundamental tool to develop a personalised treatment for the patient's need and momentary response. Accordingly, we adopted our treatment strategies depending on the categorisation of patient groups by severity of both the respiratory and vascular distress. In this paper, we aimed to present the clinical, radiological and histopathological manifestations of COVID‐19‐related endotheliitis as well as present our treatment categories which focused on preventing endotheliitis‐related consequences in different phases of the disease.
2. METHODS
2.1. Patient analysis and classification
All COVID‐19 patients who were diagnosed and treated in Samsun VM Medicalpark Hospital, Turkey, between March 2020 and April 2021, were retrospectively evaluated. Patients who were suspected to be infected by SARS‐CoV‐2 were confirmed with clinical, laboratory (positive reverse‐transcriptase‐polymerase chain reaction assay of nasopharyngeal swabs or serological IgM/IgG rapid antibody test against SARS‐CoV‐2) and radiological (consistent HRCT findings) results included in the study. Demographic characteristics, presenting symptoms of the patients at the time of admission, radiological images, hospitalisation status and the presence of the need for respiratory support were retrieved from patient records at the time of admission (Table 1). Confidentiality of the study participants’ information was maintained throughout the study. The study was performed in accordance with the Helsinki Declaration and approval for this study procedure was obtained from the Istinye University Institutional Review Boards/ethical committees with respect to its scientific content.
TABLE 1.
The characteristics of patients with COVID‐19
| Characteristics | N (%) |
|---|---|
| Age (mean ± SD) | 59.2 ± 17.3 |
| Gender | |
| Male/female | 5507/5683 |
| Symptoms | |
| Asymptomatic | 2460 (21.9%) |
| Cough | 6870 (54.5%) |
| Pain | 5370 (47.9%) |
| Dyspnea | 4140 (36.9%) |
| Fever | 3540 (31.6%) |
| Pneumonitis | 5061 (45.2%) |
| Patient groups | |
| Group A | 9294 (83.05%) |
| Group B | 1376 (12.29%) |
| Group C | 520 (4.64%) |
| Hospitalisated patients | 1896 (16.9%) |
| In first 10 days | 1220 (64.3%) |
| After 10 days | 676 (35.7%) |
| Respiratory failure | 520 (27.4%) |
| NIMV + IMV | 146 (7.7%) |
| Mortality | 43 (2.2%) |
Abbreviations: IMV, invasive mechanical ventilation;NIMV, non‐invasive mechanical ventilation.
2.2. Division into three main groups for different phases SARS‐CoV‐2 infection
This heterogeneous population of patients were divided into three groups (A, B and C) according to their repercussions of vascular and respiratory distress, which were represented by radiological, histopathological and clinical findings. Divisions into three groups were made according to combined criteria that were adopted from WHO severity classification and the extent of endotheliitis, which were represented by the clinical symptoms, baseline oxygenations status, radiological findings (chest X‐ray/CT findings) and haemodynamic differences. 4 Accordingly, three escalating phases of COVID‐19 disease progression with associated signs, symptoms and potential phase‐specific treatments were described as early infection phase (Group A), progressive phase (Group B), and severe and dissipative phase (Group C).
The treatment modalities for each group were adopted from current local guidelines, as well as interventions that were later standardised according to clinical observation of momentary changes by two different pulmonologists. 5 , 6 , 7
2.2.1. Group A
Group A included patients who had any of the various signs and symptoms of COVID‐19 (eg, fever, cough, sore throat, headache, muscle pain, diarrhoea, loss of taste and smell) but did not have shortness of breath. Group A patients either presented no imaging findings of pneumonia or they presented with the typical radiological representation of minimal patchy, subpleural, peripheral, perivascular ground‐glass opacities (GGO) (Figure 1I,II). The placement of GGOs in the early presentation of the disease is compatible with the distribution of microvascular capillaries of the lung.
FIGURE 1.
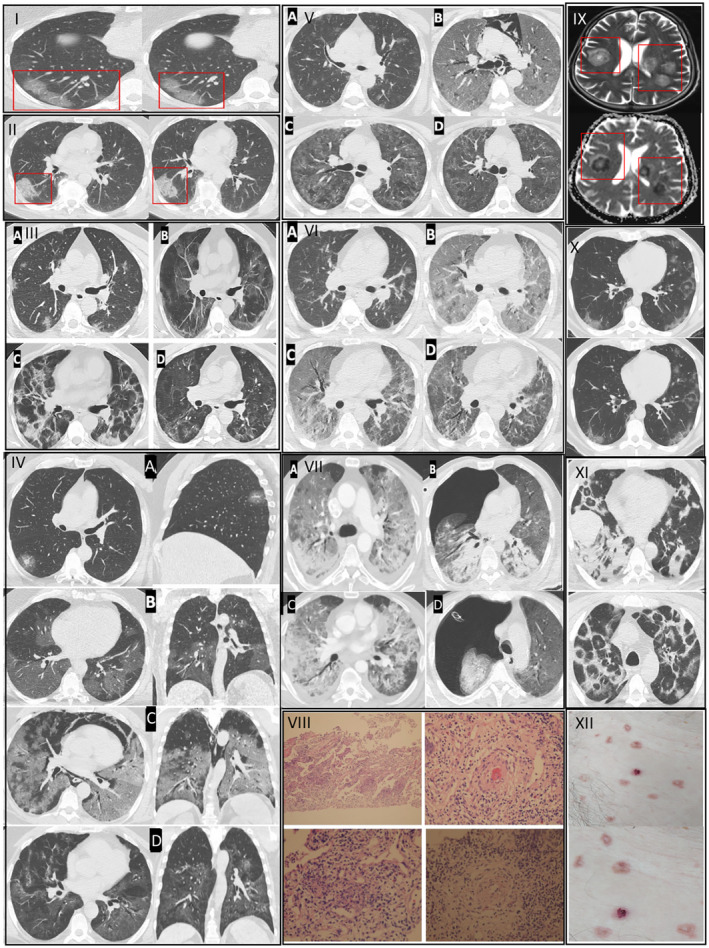
Consists of seven cases with variable stages of COVID‐19 and examples of early and severe endotheliitis in the brain, pulmonary and skin tissues. Histopathological and radiological findings of pneumonitis changed depending on the phase of the disease (early, progressive, severe and dissipative, respectively), which leads to a divergence in treatment groups. Examples of patients who were convenient for Group A treatment are depicted in Sections I and II. I. Case 1. HRCT images showed patchy, subpleural and peripheral perivascular ground‐glass opacities, corresponding to the early phase of pneumonitis. She received Group A treatment. GGOs in transaxial images were located in the subpleural area, where the microvascular endoteheliitis and endothelial destructions mostly likely occur because of the interaction with toxic plasma in the capillaries of pulmonary interstitial space. II. Case 2. HRCT images showed characteristics of an early phase COVID‐19 pneumonitis (end‐capillary microangiopathy, explained in 1I) and perivascular consolidation. It appeared as the continuation of the crazy‐paving pattern, which was demonstrated by the thickening in inter‐lobular and intra‐lobular septa. Lung involvement was limited and monofocal. Hence, the patient received Group A treatment. An example of a patient who was convenient for Group B treatment is depicted in Section III. III. Case 3. Transaxial HRCT image in the first day of positivity shows bilateral and patchy nodulary GGOs as expected in early phase (IIIa). On the 5th day of positivity, affected pulmonary areas were advanced into scattered consolidations (IIIb). This appearance was noted as the progressive phase of pneumonitis and was considered as the representation of clinical deterioration clinically (ie, dyspnoea, respiratory failure). On the 15th day of positivity, fibroreticular consolidations were conspicuous (IIIc). The dissipative phase was the healing process, characterised by the resolution in lung parenchyma and residual GGO, observed after 35 days of positivity (IIId). Parenchymal bands, originated from previous fibroreticular proliferation, were also visible (IIId). If the patients have a tendency of severe phase and/or unresponsiveness to Group B therapy, Group C treatment was used. Representative cases of this group are shown in Sections IV–VII This group of patients also undergo early and progressive phases of COVID‐19 but they also showed further deterioration of lung involvement and endothelial damage, therefore, they received the last group of treatment. For the description of early and progressive phases, Sections I, II and can be referred. IV. Case 4. Coronal, sagittal and axial planes of HRCT images initially showed small GGO with a local subpleural sparing, particularly around the microvascular area on the first day of positivity (IVa). On the fifth day of treatment, increased GGOs were visible in the progressive phase (IVb). After 18 days of positivity, pneumomediastinum, Diffuse Alveolar Damage (DAD) and ARDS were seen in a severe phase (IVc). Lastly, the dissipative phase was seen, after 30 days of positivity, with residual fibrotic parenchyma (IVd). V. Case 5. The first HRCT image, on the second day of positivity, showed COVID‐19‐related bilateral and multifocal nodular GGOs (Va). After 10 days of positivity, DAD developed along with ARDS and pneumomediastinum characterised with the severe phase (Vb). She recovered and discharged after 30 days of positivity with the dissipative phase (Vc). The regression in pulmonary lesions was visible on the HRCT image 2 months after diagnosis (Vd). VI. Case 6. HRCT image showed moderate pneumonia in the early phase (VIa). After 12 days of positivity, the severe phase develops with DAD and ARDS (VIb). On the 25th day of positivity, recovery was observed in the dissipative phase (VIc). The extent of improvement in pulmonary lesions can be noticed in VId, which was 40 days after the diagnosis. VII. Case 7. First HRCT showed ARDS pattern with dense consolidations (VIIa). Pneumothorax developed after 2 weeks from diagnosis (VIIb). In addition to the respiratory failure, haemorrhagical intracranial areas were seen in the T2‐weighted MRI. (VIIc). He was lost on the 20th day of positivity. Representative histopathological and radiological images of endotheliitis are seen in Sections VIII, IX, X, XI. VIII Haematoxylin&Eosin‐stained sections from representative areas of lung parenchyma were seen with the mixt‐type inflammatory‐cell infiltration of lung tissue and exudative capillaritis with thickened microvascular walls. Interstitial and intra‐alveolar proliferation of fibroblasts is noted. IX. Diffusion MRI of the head image of COVID‐19 positive patient showing characteristic COVID‐19 related endotheliitis causing characteristic lesions and intracranial haemorrhage. X. Early endotheliitis. XI. Late endotheliitis. XII. Papulovesicular eruptions as the skin manifestations of COVID‐19‐related endotheliitis
If the pulmonary involvement was absent or mild‐to‐moderate and the patient was suitable for ambulatory treatment, Favipiravir tablet (1600 mg BID for the first day, followed by 600 mg BID for 4 days, making 5 days in total), Dexamethasone (0.1‐0.2 mg/kg/day), Azithromycin tablet, low‐molecular weight heparin (LMWH) and acetylsalicylic acid (ASA) therapies were applied for 1 week.
2.2.2. Group B
Patients who showed evidence of lower respiratory disease during clinical assessment (respiratory symptoms) or imaging (findings of pneumonia) but an oxygen saturation (SpO2) ≥94% on room air at sea level, were hospitalised for close follow‐up. Patients with comorbidities or special conditions (ie, age >65, diabetes, cancer, obesity, cardiovascular disease, chronic lung disease, sickle cell disease, chronic kidney disease, being pregnant, cigarette smoking, transplant or immunosuppression recipient) were also hospitalised because of their high risk of severity. 4 Patients who had moderate pulmonary involvement or unresponsive to the Group A treatment in terms of the clinical symptoms, with no evident respiratory failure, but had been indicated for hospitalisation were classified as Group B. Group B corresponds to the progressive phase of the disease, which is characterised by multifocal, bilaterally diffused GGOs with poorly circumscribed consolidations scattered in peripheral zones of lungs, along with vascular and intra‐lobular septal thickenings called “crazy paving pattern” (Figure 1‐IIIb). 7
For this group, Favipiravir tablet (1600 mg BID for the first day, followed by 600 mg BID for 4 days, making 5 days in total), Dexamethasone (0.1‐0.2 mg/kg/day) or methylprednisolone (1‐2 mg/kg/day), Azithromycin tablet or fluoroquinolone, LMWH, ASA and Famotidine tablet therapies were prescribed. Although the treatment takes 1 week, we observed that this phase has the peak stage in 10‐13 days and may include potential secondary complications. Thus, patients require a close follow‐up by serial chest X‐rays to establish a baseline to assess the improvement of aeration.
2.2.3. Group C
Patients with moderate to severe pulmonary involvement (lung infiltrates >50%), accompanied by respiratory failure (SpO2 <94% on room air at sea level and, a ratio of arterial partial pressure of oxygen to fraction of inspired oxygen (PaO2/FiO2) <300 mm Hg, respiratory frequency >30 bpm) or to the patients who were recalcitrant to standard therapy and had deteriorating clinical status with laboratory (particularly increasing ferritin levels) and radiological findings were classified as Group C. In addition, patients who showed rapid progression (>50%) on CT imaging and who presented with respiratory failure or shock within 24‐48 hours were also included in this group. This group represents the progressive, peak, dissipative and severe phases of the disease. The radiological findings of Group C were microscopic lacerations and infiltrations in perialveolar vessels which were radiologically appeared as developing pulmonary GGOs, consolidation and diffuse alveolar damage (DAD), which may be accompanied by pneumothorax, pneumomediastinum or intracranial haemorrhage at this stage (Figure 1IV‐VII).
For this severe group of patients, Favipiravir tablet (1600 mg BID for the first day, followed by 600 mg BID for 4 days, continued for 5 or 10 days in total), methylprednisolone (250‐1000 mg/d for at least 3 days), convalescent plasma (once in a day during the first and second days of radiological detoriation, applied for maximum 3 days), tocilizumab (400 mg QD/daily, applied two times), supported by Piperacilin/Tazobactam, LMWH and famotidine therapies were administered. The effectiveness of this group of treatments was measured by the overall mortality ratio.
3. RESULTS
A total of 11 190 patients were evaluated. The mean age was 59.2 ± 17.3 years and the male to female ratio was 5507/5683. Among 11 190 patients, 9294 (83.05%) patients met the criteria for Group A, and 1376 (12.29) patients were presented to our clinics with Group B characteristics. Among these patients, 1896 (16.9%) individuals (Groups B and C) were hospitalised. While 1220 inpatients were hospitalised within the first 10 d after the diagnosis, 676 of them were worsened and hospitalised 10 days after their diagnosis. Among hospitalised patients, 520 (4.64%) of them did not respond to groups A and B treatments and developed hypoxemic respiratory failure (Group C). Among patients who developed hypoxemic respiratory failure, 146 (7.7%) individuals needed ventilator support (non‐invasive/invasive mechanical ventilation) and were followed in the intensive care unit, and 43 (2.2%) patients died. As a complication, among 11 190 patients, 30 patients developed either spontaneous pneumothorax, pneumomediastinum or subcutaneous emphysema, and 4 patients died with a 13.3% mortality rate.
Radiological images and histopathological samples of representative groups are presented in Figure 1 which consists of seven representative and complicated cases with variable stages of COVID‐19, together with additional examples of findings of early and severe endotheliitis in the brain, pulmonary and skin tissues. Representative cases for early phase: Group A (Figure 1I,II), progressive phase: Group B (Figure 1III), severe and dissipative phase: Group C (Figure 1IV‐VII) were presented and cases were presented in Figure 1.
Case 1 (Figure 1I) was a 51‐year‐old female patient who was admitted to the hospital with complaints of cough and fever. She was diagnosed with COVID‐19 due to PCR positivity. HRCT images showed patchy, subpleural and peripheral perivascular ground‐glass opacities, corresponding to the early phase of pneumonitis. She received Group A treatment. GGOs in transaxial images were located in the subpleural area, where the microvascular endoteheliitis and endothelial destructions mostly likely occur, due to the interaction with toxic plasma in the capillaries of pulmonary interstitial space. She was completely recovered on the 14th days at control appointment, after 5 days of treatment with favipiravir.
Case 2 (Figure 1II) was a 51‐year‐old male patient who was admitted to the hospital with complaints of fever and chest pain. His COVID‐19 PCR test was positive. In addition to the characteristics of an early phase COVID‐19 pneumonitis (end‐capillary microangiopathy, explained in Figure 1I) his HRCT images showed perivascular consolidation. It appeared as the continuation of the crazy‐paving pattern, which was demonstrated by the thickening in inter‐lobular and intra‐lobular septa. Lung involvement was limited and monofocal. Hence, the patient received Group A treatment. He was completely recovered on the 14th days at the control appointment, after 5 days of treatment with favipiravir.
Case 3 (Figure 1‐III) was a 65‐year‐old male patient admitted to the hospital with complaints of fever and cough. His PCR test resulted positive for SARS‐CoV‐2. Transaxial HRCT image in the first day of positivity shows bilateral and patchy nodular GGOs as expected in the early phase (IIIa). He received group B treatment and was hospitalised for close follow‐up. On the fifth day of positivity, affected pulmonary areas were advanced into scattered consolidations (IIIb). This appearance was noted as the progressive phase of pneumonitis and was considered as the representation of clinical deterioation clinically (ie, dyspnea, respiratory failure). On the 15th day of positivity, fibroreticular consolidations were conspicuous (IIIc). The dissipative phase was the healing process, characterised by the resolution in lung parenchyma and residual GGO, observed after 35 days of positivity (IIId). Parenchymal bands, originated from previous fibroreticular proliferation, were also visible (IIId).
If the patients have a tendency of severe phase and/or unresponsiveness to Group B therapy, Group C treatment was used. Representative cases of this group are shown in sections IV–VII. This group of patients also underwent early and progressive phases of COVID‐19 but they showed further deterioration of lung involvement and endothelial damage, therefore, they received the last group of treatment.
Case 4 (Figure 1IV) was a 55‐year‐old male patient admitted to the hospital with complaints of fever. His PCR test was positive results positive for SARS‐CoV‐2. Coronal, sagittal and axial planes of HRCT images initially showed small GGO with a local subpleural sparing, particularly around the microvascular area on the first day of positivity (IVa). On the fifth day of treatment, increased GGOs were visible in the progressive phase (IVb). After 18 days of positivity, pneumomediastinum, Diffuse Alveolar Damage (DAD) and ARDS were seen in a severe phase (IVc). Lastly, the dissipative phase was seen, after 30 days of positivity, with residual fibrotic parenchyma (IVd).
Case 5 (Figure 1V) was a 50‐year‐old female patient admitted to the hospital with complaints of fever and cough. Her COVID‐19 PCR test was positive. Her first HRCT, on the second day of positivity, showed COVID‐19‐related bilateral and multifocal nodular GGOs (Va). After 10 days of positivity, DAD developed along with ARDS and pneumomediastinum characterised with the severe phase (Vb). She recovered and discharged after 30 days of positivity with the dissipative phase (Vc). The regression in pulmonary lesions was visible on the HRCT image 2 months after diagnosis (Vd).
Case 6 (Figure 1VI) was a 53‐year‐old male patient admitted to the hospital with complaints of fever and cough. His COVID‐19 PCR test was positive. His HRCT showed moderate pneumonia in the early phase (VIa). After 12 days of positivity, the severe phase develops with DAD and ARDS (VIb). On the 25th day of positivity, recovery was observed in the dissipative phase (VIc). The extent of improvement in pulmonary lesions can be noticed in VId, which was 40 days after the diagnosis.
Case 7 (Figure 1VII) was a 51‐year‐old male patient admitted to the hospital with a complaint of dyspnoea. His COVID‐19 PCR test was positive. His first HRCT showed an ARDS pattern with dense consolidations (VIIa). Pneumothorax developed after 2 weeks from diagnosis (VIIb). In addition to the respiratory failure, hemorrhagical intracranial areas were seen in the T2‐weighted MRI (VIIc). He was lost on the 20th day of positivity.
The demonstration of endotheliitis in the biopsy section of lung parenchyma (Figure 1VIII), radiological images of the brain (Figure 1IX) and lung (Figure 1X,XI) and skin manifestation (Figure 1XII) of endotheliitis are also presented in Figure 1.
4. DISCUSSION
4.1. The rationale behind the clinical categorisation and management
Our classification of patient population and treatment groups was primarily based on the clinical and radiological findings rather than the laboratory findings. During a year of the pandemic, in our clinics, we observed that even though the laboratory findings may show the degree of pathophysiology during the disease course, it may mislead the clinician when it comes to clinical practice. While C‐reactive protein, ferritin, lactate dehydrogenase, erythrocyte sedimentation rate, IL‐6 elevation and TNF‐alpha represent inflammatory changes, D‐dimer, prolonged PTT and troponin elevation show the predisposition to thrombosis and myocardial damage. 8 , 9 Cheng et al 10 point out that severely affected patients, along with the ones who could not survive the disease, are subject to significantly higher ferritin levels in comparison to the non‐severe and survivor groups of patients. However, COVID‐19 is an acute syndrome of different age and comorbidity groups. When the clinician fails to detect the pre‐infectious baseline values of the patient, overtreatment may worsen the situation. In our study group, a patient with 250 mg/L ferritin received Group C treatment, whereas another with 2550 mg/L received Group A because of the difference in their previous normal values (ferritin increased 10 and <1 time, respectively). The change in the concentrations of inflammatory markers seems to be significantly different in COVID‐19 than in typical non‐COVID‐19‐related ARDS, suggesting that COVID‐19 features its own unique, poorly understood, yet detrimental, inflammatory profile. 11 Therefore, we advise the categorisation and treatment strategies to be selected by monitoring radiological findings 12 and the severity of clinical features (such as dyspnoea and oxygen saturation).
4.2. Farsighted evaluation: What are the causes in pathogenic scene after infection with SARS‐CoV‐2?
4.2.1. Cytokine storm and emergence of toxic plasma
Pathophysiology of COVID‐19 is closely related to cytokine storm, which arises from the consecutive and intricate activation of numerous inflammatory cells that cause excessive and/or unregulated, proinflammatory cytokine release. 13 Cytokines force blood plasma to undergo a chemical alteration, revealing toxic and irritant characteristics. Cytokine storm comprises the systemic activation of unstimulated tissue cells, epithelial and endothelial cells in addition to hyperactivation of hematopoietic cells, including B lymphocytes, natural killer (NK) cells, macrophages, dendritic cells, neutrophils and monocytes which provoke the excessive release of pro‐inflammatory cytokines. 14 This toxic setting not only causes inflammation but also damages various systemic tissues via the signals of pro‐apoptosis. 15 Main clinical manifestations of cytokine storm appear as fever, progressive dyspnoea, tachypnoea and elevated inflammatory markers such as IL‐6, CRP and ferritin as is observed in COVID‐19 patients. 14 , 15 The uncontrolled production of pro‐inflammatory factors (IL‐6, IL‐8, IL‐1β and GM‐CSF) and chemokines (CCL2, CCL3, CCL‐5) together with reactive oxygen species (ROS) cause ARDS which leads to pulmonary fibrosis and death. Abnormal nitric oxide metabolism, upregulation of ROS and proteases, downregulation of endothelium‐associated anti‐oxidant defence mechanisms and induction of tissue factor altogether provide a basis for vascular pathology in COVID‐19. 3
Additionally, the systemic inflammatory response against SARS‐CoV‐2 infection is supported by the circulating mediators found in different organ systems, which are demonstrated in postmortem histopathological analysis to indicate the organ‐dependent cytokines. 16 , 17 Figure 1VIII represents the areas of lung parenchyma with the mixt‐type inflammatory‐cell infiltration and exudative capillaritis with thickened microvascular walls, in addition to the interstitial and intra‐alveolar proliferation of fibroblasts.
4.2.2. Vascular effects: Endotheliitis, thromboinflammation and systemic microangiopathy
Endotheliitis, hypercoagulability and thrombotic microangiopathy are namely the vascular hallmarks of COVID‐19. 18 , 19 These vascular complications should be evaluated separately from ARDS. As a matter of fact, the histopathological changes observed in several tissue samples might be primarily the result of C3‐mediated pathways in thromboinflammation. 18 Beigee et al 16 stated that vascular widespread platelet–fibrin microthrombi was the main pathological finding in the lung samples of critical COVID‐19 patients with severe hypoxemia and minor radiological abnormalities on imaging. They also indicated that clinically not all patients with ARDS present DAD. However, the presence of DAD with ARDS contributes to worsening of clinical outcomes compared with those without DAD. Early and late endotheliitis lesions on pulmonary HRCT are seen in Figure 1X‐XI.
Varga et al 20 were first to demonstrate that irritant plasma, together with the SARS‐CoV‐2 infection, cause endotheliitis in microvascular capillary endothelium, which is the primary pathology seen under non‐immune, corrosive and irritant conditions. Similarly, Zhang et al 21 demonstrated that COVID‐19 infection resembles more of the pathophysiology and phenotype of complement‐mediated thrombotic microangiopathies(TMA); rather than sepsis‐induced coagulopathy or disseminated intravascular coagulation. A common denominator of complement‐mediated effects in TMAs is angiocentric inflammation‐causing endothelial dysfunction; mononuclear and neutrophilic inflammation of microvessels and as a result, microvascular thrombosis, which brings poor prognosis, multiple organ dysfunction syndromes and ARDS. 22 Microangiopathies in COVID‐19 patients are characterised by anaemia, increased lactate dehydrogenase, thrombocytopenia and organ damage (eg, skin lesions, neurological, renal, cardiac dysfunction). 22 , 23 In our cohort of patients, skin lesions were seen as COVID‐19‐associated papulovesicular exanthema scattered in the trunk and mild pruritus (Figure 1XII). Trellu et al 24 indicated that histopathological findings of papulovesicular eruption reveal the signs of endotheliitis and microthrombosis in the dermal vessels. Unfortunately, patients may die, not from respiratory failure, but because of the vascular coagulopathies (ie, haemorrhage) in the brain, kidneys and heart (Figure 1IX).
4.3. The relationship between endotheliitis and COVID‐19‐related respiratory failure
Endothelial dysfunction plays a key role in understanding the multisystemic attack of SARS‐CoV‐2 infection. Microvascular capillary endotheliitis is the primary mechanism that causes clinical detoriation, particularly in those patients with advanced pulmonary involvement. 25 The critical point in this manner is to differentiate non‐immune endotheliitis from immune complex endotheliitis in lungs and to consider it as the main pathology of COVID‐19 since it is not directly mediated by the active antigen‐antibody complexes or the virulence of SARS‐CoV‐2 itself. 26 , 27
Initially, the irritant action of plasma leads to the thickening of the vessel wall and deceleration in blood flow, which is responsible for the microthrombosis in capillary beds in the lungs, which may lead to respiratory failure. Fox et al 28 demonstrated the microvascular thrombosis and haemorrhage in the lungs, as a remarkable contributor to death, in the autopsies of COVID‐19 non‐survivors. They also proved that the cardiovascular damage is “non‐immune” by demonstrating cardiac cell necrosis without lymphocytic myocarditis in deceased patients.
The concept of virus‐induced pulmonary vasculitis is consistent with a substantial ventilation/perfusion mismatch in COVID‐19 based on a right‐to‐left pulmonary shunt because of a vicious cycle beginning with an increase in respiratory effort and oxygen consumption in inflamed and hyperperfused lungs, failure of hypoxic vasoconstriction and resulting in fatal outcome. 3 Therefore, the failure of simple ventilatory support in COVID‐19 is commonly observed in patients who are unable to satisfy the oxygen demand as a result of reduced lung capacity (such as older patients and patients with obesity) and cardiovascular comorbidities.
In the clinical presentation, aggravation of dyspnoea and hypoxaemia symptoms were attributed to dysfunctional crosstalk between leucocytes and endothelial cells that manifest as vascular immunopathology predominantly confined to the lungs. Eventually, since microvascular walls are prone to damage, the destruction most conveniently occurs as endotheliitis at the site of pulmonary interstitial capillaries, with the help of lung elasticity and thin vascular walls, conveys into the perivascular space. 29 Remarkably, because of SARS‐CoV‐2’s endotheliophilic nature, endothelial and epithelial infections appear to be the predominating factors during the course of the disease. 3 On the other hand, alveolar‐centred infection and the disruption of alveolar epithelial–endothelial barriers contribute to the development of DAD and pneumonitis, which manifest as GGOs in alveolar spaces 30 , 31 (Figure 1III,VIII). The aforementioned endothelial damage may spread to different systems and become lethal (Figure 1VII).
Ekanem et al 32 stated that higher inflammatory markers (ferritin, CRP and fibrinogen), increased fibrosis in HRCT images, and absence of receiving an interleukin‐6 inhibitor or convalescent plasma are associated with a higher probability of severity and mortality via the spontaneous pneumothorax (SPT). They also suggested that there must be factors uniquely associated with COVID‐19 that contribute to the incidence of SPT since half of the patients were not on a ventilator when the pneumothorax was diagnosed. In Group C (Figure 1IV‐VII), we also observed that endothelial damage, along with thromboinflammation, brought increased incidence of pneumothorax secondary to DAD in patients with ARDS. Here, among 11 190 patients, 30 patients developed either SPT, pneumomediastinum or subcutaneous emphysema with a 13.3% mortality rate. The most important reason behind these complications was most likely DAD, which stems from the high transpulmonary pressure and alveolar wall vulnerability, with decreased compliance and increased frailty, resulting in an air leakage into the chest compartments. 33 SPT that is observed in severe COVID‐19 patients is thought to be derived from reduced alveolar vessel calibre because of the virus‐induced cytolysis, mononuclear immunological response to injury and the small vessel thrombosis at the site of the perialveolar area, which should be differentiated from iatrogenic pneumothorax related to mechanical ventilation. 34
4.4. Prevention strategies for microvascular pathology and mortality
Planning effective therapy for COVID‐19 infection is a complex process. According to Mastellos et al 35 broader pathogenic involvement of C3‐mediated pathways in thromboinflammation supports the utilisation of complement inhibitors in COVID‐19, which result in diminished hyper‐inflammation and marked lung function improvement. Teuwen et al 36 suggested that normalisation of vascular walls through metabolic interventions might be considered as an additional potential target for the therapy. Therefore, until a specific antiviral is discovered against SARS‐CoV‐2, convalescent plasma therapy and immunomodulators play a significant role to control the consequences of SARS‐CoV‐2 infection (ie, cytokine storm), to reduce inflammatory cell infiltration in lungs and to prevent fatal course in severe patients by reducing the likelihood of the Systemic Inflammatory Response Syndrome. 37
The latest COVID‐19 treatment guideline published by the Turkish Ministry of Health included the favipiravir, ASA, famotidine, LMWH, dexamethasone, tocilizumab, which were shown to be safe and in vitro effective against SARS‐CoV‐2. 5 , 6 Based on indirect evidence from different clinical trials, convalescent plasma therapy and pulse steroid therapy were also suggested for severe patients. 38 The required interventions were made according to clinical severity of patients, vitals, time from symptom onset and radiological images. Additionally, treatment strategies mentioned in this paper were adapted for each patient according to the presence of contraindications for certain drugs, for instance, pregnant patients did not receive favipiravir because of its teratogenic effects.
Convalescent plasma therapy plays a critical role in neutralising the plasma and diminishing its corrosiveness. Convalescent plasma not only demonstrates an antibody response but also denotes immunomodulatory, anticytokine and pro‐inflammatory effects, which appear as key factors to minimise disease severity and mortality in COVID‐19 cases. 38 Gomez‐Pastora et al 39 explain this correlation by the phenomena of pro‐inflammatory and anti‐inflammatory cytokine activation as a result of macrophage‐associated hyperferritinemia. They stated that ferritin plays an active role as a pathogenic mediator in COVID‐19 and the therapeutic use of plasma is beneficial to reduce ferritin and cytokine levels in the body. Our experience with convalescent plasma showed rapid and positive results against the symptoms of dyspnea, hypoxemia, fever and radiologically seen infiltrations, which was demonstrated in Cases 4‐7.
Antiviral drugs are being used to decrease the viral load. Correspondingly, favipiravir was the drug of choice that is recommended by the Turkish Republic of Health Ministry Guidelines. 5 , 6 However, it should be noted that antiviral therapy fails to prevent pulmonary involvement, which is the result of the inflammatory process rather than the effect of SARS‐CoV‐2 infection itself. 40
Systemic corticosteroid drugs (dexamethasone and methylprednisolone) are the only effective therapeutic agents to repair non‐immune capillary microvascular endotheliitis, hence, advised to be used even in the presence of minimal ground‐glass opacities. 41 In our clinical experience, to benefit the best of steroids, steroids should be utilised in the early phase rather than the progressive phase (approximately the second week of infection). Minimally distributed GGOs may easily progress to severe ARDS in the absence of steroid treatment (Figure 1VIIa‐VIb). In a multicentred study conducted by RECOVERY Collaborative Group, the mortality ratio in patients, who receive oxygen support and dexamethasone, found to be lower than the control group, especially if the patients are receiving invasive mechanical ventilation (29.3% vs 41.4%; rate ratio, 0.64; 95% CI, 0.51‐0.81). 42 Although the role of corticosteroids in COVID‐19 has been well recognized in the therapeutic algorithm, the right timing, dosage and duration of corticosteroid use is still unknown. Pinna et al 43 suggested that although the early use of corticosteroids might facilitate the viral replication in the upper airways, late administration fails to prevent the alveolar damage. It has been suggested that early initiation of low‐dose methylprednisolone therapy (with up to 40 mg/day for <10 days) provides clinical, radiological improvement in patients with an active disease state of COVID‐19, by reducing the immune cascade and progression into cytokine without any adverse effect. 44 , 45 , 46
Immunomodulatory therapies help to diminish the cytokine response of the body. Tocilizumab therapy, a monoclonal IL‐6 antagonist, reduced the likelihood of progression to the composite outcome of mechanical ventilation or death. 47 Capra et al 48 have demonstrated that tocilizumab can be used as an immunomodulatory drug of choice in case of severe COVID‐19 to reduce mortality, diminish oxygen intake and treat lung opacities as well. Findings by Gupta 49 et al also supported the early use of (within the first 2 days of ICU) tocilizumab to reduce the in‐hospital mortality among critically ill patients with COVID‐19.
LMWH and ASA are well known to prevent the formation of microvascular thrombosis and cure hypoxemia, thus can be used as supportive treatment against cardiovascular complications of COVID‐19. 50 However, before the use of ASA, patient history should be questioned in terms of renal failure, gastrointestinal system disease, cardiovascular disease. Local current guidelines recommended the use of NSAIDs, especially in the first 5‐10 days; and steroid treatment to be started in the early period in patients who worsen after theoretical viral clearance is completed in the first 5‐10 days. 5
4.5. Limitation
We acknowledge that there are confounding factors related to the management of COVID‐19 patients because of the lack of a standardised guideline for the treatment of each and every patient. However, while the in‐hospital mortality was reported to be up to 25% in different prospective trials, we believe that our standardised treatment approach, which has a result of 2.2% in‐hospital mortality, represent the success of the personalised management of each COVID‐19 patient in a single centre. 50 , 51 As the COVID‐19 pandemic continues, our current strategy represents a snapshot that would most probably change drastically over time. Our categorisation strategy has enabled us to implement systematic practices that we saw as beneficial instead of following a “random” approach for each new patient.
As a limitation, the design of the current study does not allow for further analysis of the COVID‐19 patients in terms of the change in their laboratory, clinical and radiological parameters and investigation of the effects of different SARS‐CoV‐2 variants (such as Alpha, B.1.1.7, Beta B.1.351 or Delta B.1.617.2) in the clinical presentation of the patients. The observational methodology suggested may be a source of bias that could lead to wrong conclusions on the effectiveness of treatments and the clinical representation of the underlying pathophysiology. Provided treatment strategies are implemented to all patients regardless of their SARS‐CoV‐2 variant types. No corruptions from Turkey's Ministry of Health guidelines are enforced on the patients in terms of new experimental drugs and/or biological agents.
We would like to declare that our study did not interfere with any patient's right to receive treatment by addressing a control group in a pandemic situation. Here, we also did not aim to demonstrate the effectiveness of the therapy in the means of laboratory data, however, we aimed to present the representations of the required interventions in each group of patients by preventing the progression of endotheliitis. Therefore, we used the current literature to support our clinical observation and own perspective. In the end of the study, our aim was to declare our own point of view by using our clinical experience, clinical representation of endotheliitis and the current literature. We would like to approach the vascular distress phenomenon as a clinical parameter that can be used practically in the clinics, both by recognising radiological images and clinical findings of the patients.
5. CONCLUSION
SARS‐CoV‐2 infection is a multisystemic disease which courses rapidly with respiratory failure and complications secondary to vascular alterations (ie, microvascular thrombosis, endotheliitis and cytokine‐induced plasma toxicity). Early detection of radiological detoriation before laboratory findings, via monitoring chest X‐rays daily, and planning personalised treatments constitute a crucial and life‐saving manoeuver in the treatment of COVID‐19. Our group suggests that an important key to success relies on how closely the clinicians follow patients from diagnosis to treatment, including the whole course of the disease from outpatient clinic to ICU, in order to differentiate instant clinical deviations from previous general status.
Distinctive manifestations in each COVID‐19 patient, including non‐respiratory conditions in the acute phase and the emerging risk of long‐lasting complications, suggest that COVID‐19 has an endotheliitis‐centred thrombo‐inflammatory pathophysiology. Potential pathophysiological mechanisms contributing to endotheliitis includes cytokine storm and toxic plasma, thromboinflammation and systemic microangiopathy. Endotheliitis can also explain the mechanism behind the respiratory failure in COVID‐19, and the difference of COVID‐19‐related ARDS from ARDS seen in other critical conditions. In our observations, utilisation of early dexamethasone in Group A prevented the progression of the COVID‐19 into a more severe form. In addition, the use of early steroids in Group A and early tocilizumab in group C helps to reduce mortality and progression of the disease. Endotheliitis‐based pathophysiological mechanisms are known to be momentarily changing and difficult to manage due to their risk of sudden aggravation. Hence, daily evaluation of patients and deciding appropriate pathophysiological treatment for the mmentary changes in clinical, laboratory and radiological findings would help to reduce the mortality rate of this novel virus. The collaboration of scientists and clinicians around the world is required to develop novel prognostic biomarkers and establish precise predictive thresholds for known biomarkers to foresee the severity for COVID‐19 pneumonitis that is characterised by vasculopathy and a wide range of immune derangements.
ETHICS STATEMENT
The patients signed written informed consents to be able to provide data in this study. The study was approved by the Republic of Turkey Ministry of Health and concerned tertiary institutions and performed in accordance with the principles of the Declaration of Helsinki.
DISCLOSURES
The authors have declared no disclosures.
AUTHOR CONTRIBUTION
AD and SO diagnosed, treated the patients and designed the analysis, SI, TU and IK collected the data, analysis tools and wrote the paper.
Dirican A, Ildir S, Uzar T, Karaman I, Ozkaya S. The role of endotheliitis in COVID‐19: Real‐world experience of 11 190 patients and literature review for a pathophysiological map to clinical categorisation. Int J Clin Pract. 2021;75:e14843. 10.1111/ijcp.14843
DATA AVAILABILITY STATEMENT
The research article data used to support the findings of this study are available from the corresponding author upon request.
REFERENCES
- 1. Li X, Ma X. Acute respiratory failure in COVID‐19: is it “typical” ARDS? Crit Care. 2020;24(1):1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Coronavirus update (live)‐worldmeter. www.worldometers.info. Accessed May 11, 2021
- 3. Osuchowski MF, Winkler MS, Skirecki T, et al. The COVID‐19 puzzle: deciphering pathophysiology and phenotypes of a new disease entity. Lancet Res Med. 2021;9(6):622‐642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zu ZY, Jiang MD, Xu PP, et al. Coronavirus disease 2019 (COVID‐19): a perspective from China. Radiology. 2020;296(2):E15‐E25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Republic of Turkey Ministry of Health . COVID‐19 Interim Guidance (T.C. Sağlık Bakanlığı COVID‐19 (SARS‐CoV‐2 Enfeksiyonu) Rehberi). 2020. https://covid19bilgi.saglik.gov.tr/depo/rehberler/COVID‐19_Rehberi.pdf?type=file. Accessed Apr 14, 2020
- 6. Köktürk N, İtil BO, Altınışık G, et al. COVID‐19 pandemic and the global perspective of Turkish thoracic society. Turkish Thorac J. 2020;21(6):419‐432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. COVID‐19 Treatment Guidelines Panel . Coronavirus Disease 2019 (COVID‐19) Treatment Guidelines. National Institutes of Health. 2021. https://www.covid19treatmentguidelines.nih.gov/. Accessed Mar 28, 2021 [PubMed]
- 8. Merad M, Martin JC. Pathological inflammation in patients with COVID‐19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020;20(6):355‐362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Grasselli G, Tonetti T, Protti A, et al. Pathophysiology of COVID‐19‐associated acute respiratory distress syndrome: a multicentre prospective observational study. Lancet Resp Med. 2020;8(12):1201‐1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cheng L, Li H, Li L, et al. Ferritin in the coronavirus disease 2019 (COVID‐19): a systematic review and meta‐analysis. J Clin Lab Anal. 2020;34(10):e23618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sinha P, Calfee CS, Cherian S, et al. Prevalence of phenotypes of acute respiratory distress syndrome in critically ill patients with COVID‐19: a prospective observational study. Lancet Resp Med. 2020;8(12):1209‐1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ufuk F, Savaş R. Chest CT features of the novel coronavirus disease (COVID‐19). Turkish J Med Sci. 2020;50(4):664‐678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Coperchini F, Chiovato L, Croce L, Magri F, Rotondi M. The cytokine storm in COVID‐19: An overview of the involvement of the chemokine/chemokine‐receptor system. Cytokine Growth Factor Rev. 2020;53:25‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ye Q, Wang B, Mao J. The pathogenesis and treatment of the Cytokine Storm in COVID‐19. J Infect. 2020;80(6):607‐613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Becker RC. COVID‐19‐associated vasculitis and vasculopathy. J Thromb Thrombol. 2020;50(3):499–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Beigee FS, Toutkaboni MP, Khalili N, et al. Diffuse alveolar damage and thrombotic microangiopathy are the main histopathological findings in lung tissue biopsy samples of COVID‐19 patients. Pathol Res Pract. 2020;216(10):153228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Elsoukkary SS, Mostyka M, Dillard A, et al. Autopsy findings in 32 patients with COVID‐19: a single‐institution experience. Pathobiology. 2021;88(1):55‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mosleh W, Chen K, Pfau SE, Vashist A. Endotheliitis and endothelial dysfunction in patients with COVID‐19: its role in thrombosis and adverse outcomes. J Clin Med. 2020;9(6):1862. 10.3390/jcm9061862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Calabretta E, Moraleda JM, Iacobelli M, et al. COVID‐19‐induced endotheliitis: emerging evidence and possible therapeutic strategies. Br J Haematol. 2021;193(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID‐19. Lancet. 2020;395(10234):1417‐1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang Y, Xiao M, Zhang S, et al. Coagulopathy and antiphospholipid antibodies in patients with Covid‐19. N Engl J Med. 2020;382(17):e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang X, Sahu KK, Cerny J. Coagulopathy, endothelial dysfunction, thrombotic microangiopathy and complement activation: potential role of complement system inhibition in COVID‐19. J Thromb Thrombolysis. 2021;51(3):657‐662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Thakur V, Ratho RK, Kumar P, et al. Multi‐organ involvement in COVID‐19: beyond pulmonary manifestations. J Clin Med. 2021;10(3):446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Trellu LT, Kaya G, Alberto C, Calame A, McKee T, Calmy A. Clinicopathologic aspects of a papulovesicular eruption in a patient with COVID‐19. JAMA Dermatol. 2020;156(8):922‐924. [DOI] [PubMed] [Google Scholar]
- 25. Rovas A, Osiaevi I, Buscher K, et al. Microvascular dysfunction in COVID‐19: the MYSTIC study. Angiogenesis. 2021;24(1):145‐157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Azkur AK, Akdis M, Azkur D, et al. Immune response to SARS‐CoV‐2 and mechanisms of immunopathological changes in COVID‐19. Allergy. 2020;75(7):1564‐1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gavriilaki E, Anyfanti P, Gavriilaki M, Lazaridis A, Douma S, Gkaliagkousi E. Endothelial dysfunction in COVID‐19: lessons learned from coronaviruses. Curr Hypertens Rep. 2020;22(9):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fox SE, Akmatbekov A, Harbert JL, Li G, Brown JQ, Vander Heide RS. Pulmonary and cardiac pathology in African American patients with COVID‐19: an autopsy series from New Orleans. Lancet Resp Med. 2020;8(7):681‐686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Arachchillage DJ, Stacey A, Akor F, Scotz M, Laffan M. Thrombolysis restores perfusion in COVID‐19 hypoxia. Br J Haematol. 2020;190(5):e270‐e274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Doglioni C, Ravaglia C, Chilosi M, et al. Covid‐19 interstitial pneumonia: histological and immunohistochemical features on cryobiopsies. Respiration. 2021;100(5):369‐379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dumoulin DW, Gietema HA, Paats MS, Hendriks LE, Cornelissen R. Differentiation of COVID‐19 pneumonitis and ICI induced pneumonitis. Front Oncol. 2020;10:577696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ekanem E, Podder S, Donthi N, et al. Spontaneous pneumothorax: An emerging complication of COVID‐19 pneumonia. Heart Lung. 2021;50(3):437‐440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Elhakim TS, Abdul HS, Romero CP, Rodriguez‐Fuentes Y. Spontaneous pneumomediastinum, pneumothorax and subcutaneous emphysema in COVID‐19 pneumonia: a rare case and literature review. BMJ Case Reports CP. 2020;13(12):e239489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zantah M, Castillo ED, Townsend R, Dikengil F, Criner GJ. Pneumothorax in COVID‐19 disease‐incidence and clinical characteristics. Respir Res. 2020;21(1):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mastellos DC, da Silva BGP, Fonseca BA, et al. Complement C3 vs C5 inhibition in severe COVID‐19: early clinical findings reveal differential biological efficacy. Clin Immunol. 2020;220:108598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Teuwen L‐A, Geldhof V, Pasut A, Carmeliet P. COVID‐19: the vasculature unleashed. Nat Rev Immunol. 2020;20(7):389‐391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sun X, Wang T, Cai D, et al. Cytokine storm intervention in the early stages of COVID‐19 pneumonia. Cytokine Growth Factor Rev. 2020;53:38‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Altuntas F, Ata N, Yigenoglu TN, et al. Convalescent plasma therapy in patients with COVID‐19. Transfus Apheres Sci. 2021;60(1):102955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gómez‐Pastora J, Weigand M, Kim J, et al. Hyperferritinemia in critically ill COVID‐19 patients–Is ferritin the product of inflammation or a pathogenic mediator? Clinica Chimica Acta. 2020;509:249–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Solaymani‐Dodaran M, Ghanei M, Bagheri M, et al. Safety and efficacy of Favipiravir in moderate to severe SARS‐CoV‐2 pneumonia. Int Immunopharmacol. 2021;95:107522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Raju R, Prajith V, Biatris PS. Therapeutic role of corticosteroids in COVID‐19: a systematic review of registered clinical trials. Future J Pharm Sci. 2021;7(1):1‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Group RC . Dexamethasone in hospitalized patients with Covid‐19. N Engl J Med. 2021;384(8):693‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pinna SM, Scabini S, Corcione S, Lupia T, De Rosa FG. COVID‐19 pneumonia: do not leave the corticosteroids behind! Future Microbiol. 2021;16(5):317‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Diken E, Yıldırım F, Yıldız Gülhan P, et al. Corticosteroid use in COVID‐19 pneumonia. Tuberk Toraks. 2021;69(2):217‐226. [DOI] [PubMed] [Google Scholar]
- 45. Chrousos GP, Meduri GU. Critical COVID‐19 disease, homeostasis, and the “surprise” of effective glucocorticoid therapy. Clin Immunol. 2020;219:108550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang Y, Jiang W, He Q, et al. A retrospective cohort study of methylprednisolone therapy in severe patients with COVID‐19 pneumonia. Signal Transduct Targeted Ther. 2020;5(1):1‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Salama C, Han J, Yau L, et al. Tocilizumab in patients hospitalized with Covid‐19 pneumonia. N Engl J Med. 2021;384(1):20‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Capra R, De Rossi N, Mattioli F, et al. Impact of low dose tocilizumab on mortality rate in patients with COVID‐19 related pneumonia. Eur J Intern Med. 2020;76:31‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gupta S, Wang W, Hayek SS, et al. Association between early treatment with tocilizumab and mortality among critically ill patients with COVID‐19. JAMA Intern Med. 2021;181(1):41‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bikdeli B, Madhavan MV, Jimenez D, et al. COVID‐19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow‐up: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2020;75(23):2950‐2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, et al. Features of 20 133 UK patients in hospital with covid‐19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The research article data used to support the findings of this study are available from the corresponding author upon request.


