Abstract
Background
The unresolved COVID‐19 pandemic considerably impacts the health services in Iraq and worldwide. Consecutive waves of mutated virus increased virus spread and further constrained health systems. Although molecular identification of the virus by polymerase chain reaction is the only recommended method in diagnosing COVID‐19 infection, radiological, biochemical, and hematological studies are substantially important in risk stratification, patient follow‐up, and outcome prediction.
Aim
This narrative review summarized the hematological changes including the blood indices, coagulative indicators, and other associated biochemical laboratory markers in different stages of COVID‐19 infection, highlighting the diagnostic and prognostic significance.
Methods
Literature search was conducted for multiple combinations of different hematological tests and manifestations with novel COVID‐19 using the following key words: “hematological,” “complete blood count,” “lymphopenia,” “blood indices,” “markers” "platelet" OR "thrombocytopenia" AND "COVID‐19," "coronavirus2019," "2019‐nCoV," OR "SARS‐CoV‐2." Articles written in the English language and conducted on human samples between December 2019 and January 2021 were included.
Results
Hematological changes are not reported in asymptomatic or presymptomatic COVID‐19 patients. In nonsevere cases, hematological changes are subtle, included mainly lymphocytopenia (80.4%). In severe, critically ill patients and those with cytokine storm, neutrophilia, lymphocytopenia, elevated D‐dimer, prolonged PT, and reduced fibrinogen are predictors of disease progression and adverse outcome.
Conclusion
Monitoring hematological changes in patients with COVID‐19 can predict patients needing additional care and stratify the risk for severe course of the disease. More studies are required in Iraq to reflect the hematological changes in COVID‐19 as compared to global data.
Keywords: CBC, coagulopathy, COVID‐19, hematology
Hematological changes appear early in the course of the COVID‐19 infection, however, they are subtle. Monitoring hematological changes in progressive COVID‐19 course predict outcome and guide the management.
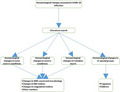
1. INTRODUCTION
The highly contagious novel coronavirus, also known as COVID‐19 and SARS‐COV‐2, was first reported in Wuhan, China, in December 2019. 1 , 2 It has spread worldwide over 110 countries affecting over 118,000 cases in a short period of less than 4 months raising a global concern. Hence, World Health Organization (WHO) declared COVID‐19 a pandemic on March 11.
During 2020, two waves of COVID‐19 hit Europe. 3 The first spike was in April which has been managed with general restrictions and lockdown in the absence of curative treatment and vaccine, followed by the second wave with a soaring number of cases recorded in November. By contrast, the number of the new cases in the United States and Asia continued to increase relatively steadily to peak in November. In Iraq, the first reported case was in February, 4 then the cases slowly increasing until June 20, when the number of new cases first crossed the 1000 mark contrasting the pattern of the neighboring countries such as SA, UAE, Jordan, and Iran which had significant decline in their cases by June. Regression in the reported numbers was noticed until 10th Feb 2021 when numbers escalated again, yet the Ministry of health has not confirmed European mutated strain in Iraq. 5
Novel COVID‐19 is a single‐stranded RNA from Coronaviridae family. It is an enveloped human β‐coronavirus which shares 80% of human SARS‐CoV‐1 genetic sequence and 96.2% of bat coronavirus RaTG13. 6 Virus envelop is coated by spike (S) glycoprotein consisting of S1 and S2 subunits. S1 mediates virus entry by binding host angiotensin‐converting enzyme 2 (ACE2), sialic acid, 7 and CD147. 8 Several strains of COVID‐19 appeared since it was first reported. Phylogenetic tree analysis identified various Asian strains (L, S, V, O) and European strains (G, GH, GR). 9 GR strain has exposed to several mutations in S1 subunit, of which G614 variant linked to increased virus infectivity and transmissibility rather than severity of the illness compared to the original D614 form 9 , 10 via promoting viral replication in human lung epithelial cells and primary human airway tissues. 11
The clinical manifestations of novel COVID‐19 vary from asymptomatic to acute respiratory distress, depending on virus rout of entry, virus load, host immunity, and comorbidity human. 12 Common clinical presentation is summarized in Table 1. Respiratory and gastrointestinal infection represent the bulk of the COVID‐19‐positive patient presentation, yet, asymptomatic/ presymptomatic infection is estimated to be 40% of all cases. 13 Many extrapulmonary neurological, dermatological, and cardiovascular manifestations have been frequently reported 14 concomitant with, after, or less frequently independent of respiratory infection (Table 1). Acute respiratory distress and subsequent respiratory failure are the leading cause of death in COVID‐19‐positive patients. 15 , 16
TABLE 1.
Clinical manifestations of patients with COVID‐19
| Clinical manifestations | % | |
|---|---|---|
| Nonspecific 14 |
Fever Malaise Fatigue Anorexia Myalgia Shivering |
58.6 29.7 28.16 20.26 16.9 5.96 |
| Flu‐like illness 14 |
Sneezing Sore throat Rhinitis Rhinorrhea Nasal congestion |
14.7 14.41 14.29 7.69 5.47 |
| Respiratory 14 |
Dry cough Sputum Dyspnea Hemoptysis |
58.52 25.33 30.82 1.65 |
| Cardiac 14 | Chest pain palpitation | 11.49 |
| Gastrointestinal 14 |
Nausea and/or Vomiting Diarrhea Abdominal pain |
7.33 9.59 5.07 |
| Dermatological 101 |
Viral exanthem‐like maculopapular rash Vesicular Urticarial Acral lesions Chilblain like Thrombotic/ischemic |
5.69 4.15 3.81 1.67 |
| Neurological 102 |
Dizziness Confusion Headache Ageusia Anosmia Stroke Ataxia |
11.3 5.75 12.17 19.6 15.4 3 2.1 |
Although molecular identification of the virus by polymerase chain reaction (PCR) is the only recommended method in diagnosing COVID‐19 infection 17 , 18 radiological, biochemical, biomarkers, and hematological studies are substantially important in risk stratification, patient follow‐up, and outcome prediction. 19 , 20 , 21 Hematological tests including complete blood picture and coagulation studies in PCR‐positive patients depict variable degree of changes according to the patient immune response and infection severity. 22 Many physicians consider early hematological changes as a clue for COVID‐19 infection when the presenting sign and symptoms are unusual particularly when there is constrain of molecular testing. 23 On the other hand, hematological changes provide help when the symptomatic patient receives a negative molecular test, fishing for patients who require PCR test repeat. Taking into consideration the marked heterogeneity of the reference ranges of complete blood cell counts and leukocyte differential cell counts in different racial, ethnic group, 24 in this review, we summarized the hematological changes in different stage of COVID‐19 infection highlighting the diagnostic and prognostic significance.
2. METHODS
Literature search was conducted for multiple combinations of different hematological tests and manifestations with novel COVID‐19 using the following key words: “hematological,” “complete blood count,” “lymphopenia,” “blood indices,” “markers” "platelet" OR "thrombocytopenia" AND "COVID‐19," "coronavirus2019," "2019‐nCoV" OR "SARS‐CoV‐2." Articles written in the English language and conducted on human samples between December 2019 and February 2021 were included. The initial search results were scanned by title and abstract for relevance, then complete texts of the related articles were reviewed. We further included directly relevant studies from reference lists of appropriate records. Non‐English articles were excluded from the study.
3. SEVERITY DEFINITION
According to NIH guidelines reviewed in October 2020, COVID‐19 patients are grouped into five severity categories with slight overlap. 1‐ Asymptomatic or presymptomatic Infection: those are PCR confirmed COVID‐19 patients but exhibiting no symptoms. 2‐Mild Illness: patients signs and symptoms of COVID‐19 (e.g., fever, cough, sore throat, malaise, headache, muscle pain, nausea, vomiting, diarrhea, and loss of taste and smell) with no shortness of breath, dyspnea, or abnormal chest imaging. 3‐Moderate Illness: when lower respiratory disease is evident clinically or radiologically but have saturation of oxygen (SpO2) ≥94%. 4‐Severe Illness: respiratory rate exceeds 30 breaths/min, SpO2 <94%, a ratio of arterial partial pressure of oxygen to fraction of inspired oxygen (PaO2/FiO2) <300 mmHg, or pulmonary involvement >50%. 5‐Critical Illness: when there is respiratory failure, septic shock, and/or multiple organ dysfunction. 25
Most of the hematological changes reported early in the beginning of the pandemic were based on hospital admitted patients. Moreover, severity definition was not consistent in the literature; some studies considered all patient not requiring intensive care unit (ICU) admission as mild cases. 26 , 27 , 28 Subsequent population‐based clinical and laboratory studies increased our understanding of the role of hematology changes in disease prognosis and outcome. In this review, we summarized the hematological changes according to the severity of the presentation. We grouped the asymptomatic, mild, and moderate cases with Po2 >94% as none severe conditions, and patients with severe or critical condition who required assisted ventilation or ICU care as severe conditions. We also reviewed the hematological changes in special groups including children and pregnant women.
4. HEMATOLOGICAL CHANGES IN NONE SEVERE CONDITIONS
4.1. Changes in WBC count and morphology
The majority of early published laboratory findings of none severe COVID‐19 infection are collected from hospital admitted patients at a single time point which limits our understanding of the dynamic hematological changes during the course of the disease. Asymptomatic patients constitute at least 20% of the cases, 29 , 30 however, available literature is more concerned with the prevalence and transmission risk they carry and overlooking hematological changes. This probably because those patients were sent for self‐isolation and further investigation was not recommended particularly with the overwhelmed health systems.
Studies which have looked into the WBC changes in hospitalized COVID‐19 patients with none severe symptoms reported a total WBC count range of 3.1–7.6 × 109/L with a mean of 4.3–5.7 × 109/L. Lymphocytopenia was reported in 80.4% of none sever patients; however, mean lymphocyte counts was more 1.0 × 109/L in most of the cases. 31 , 32 Leukopenia reported in 28.1% of this category with mean neutrophil count reported by different studies to be 0.4–6.6 × 109/L. It is worth noting that both lymphocytopenia and neutropenia in this group of patients were less prominent than those seen in severe cases. 32
A case–control study comparing the complete blood count (CBC) of symptomatic COVID‐19 confirmed patients with those of symptomatic COVID‐19 negative proposed eosinopenia as a potential predicting parameter of COVID‐19 rather than lymphopenia and leukopenia. 33
4.2. Changes in RBC indices
Hemoglobin and to less extent hematocrit are the only RBC indices reported in COVID‐19 studies. Available literature showed no significant alteration in hemoglobin of those with a mild/moderate disease. 34 , 35 , 36
4.3. Changes in coagulation indices
Early studies in Wuhan and Germany showed no significant differences in the platelet count of none severe compared with severe COVID‐19 patients. 35 , 37 A larger study included 926 none severe cases reported a mean platelet count of 172,000 (139,000–212,000), which was significantly higher than that reported in severely ill patients. 38 There was no significant changes in the levels of other blood coagulation parameters in none severe illness.
5. HEMATOLOGICAL CHANGES IN SEVERE CONDITIONS
5.1. Changes in WBC count and morphology
There are consistent data indicating that lymphocytopenia and neutrophilia are features of severe COVID‐19 illness (Table 2). Longitudinal assessment of laboratory parameters of 13 patients with severe illness showed significant and sustained lymphocytopenia [0.6(0.6–0.8)] and neutrophilia [4.7(3.6–5.8)]. 35 Another study of 69 patients reported that severely ill patients developed neutrophilia during hospitalization with a median peak absolute neutrophil count of 11.6 × 109/L. 39 A retrospective study on 201 Wuhan patients, looked into risk factors associating with acute respiratory distress syndrome (ARDS) development and death, concluded that increased neutrophils (p < 0.001) and decreased lymphocytes (p < 0.001) were risk factors for ARDS, and increased neutrophils (p = 0.03) was associated with higher risk of death. 40 , 41
TABLE 2.
Summary of meta‐analysis studies encountering hematological parameters
| Author | Year | No. of studies | Pooled number of patients | Outcome | Hematological parameter | Results | 95%CI | p | Cut‐off |
|---|---|---|---|---|---|---|---|---|---|
| Huang et al. 103 | Jan–20 | 25 | 5350 | IUC or death |
↑CRP ↑D‐dimer |
RR =1.84 RR =2.93 |
1.45, 2.33 2.14, 4.01 |
<0.001 <0.001 |
⩾10 >0.5 |
| Lagunas‐Rangel et al. 104 | Apr–20 | 6 | 828 | Severity |
NP/LYP LYC/CRP |
SMD =2.404 SMD = −0.912 |
0.98, 3.82 −1.27, −0.55 |
0.000 0.005 |
|
| Xu et al. 105 | June–20 | 20 | 4062 | Severity |
↑WBC ↓LYC ↑CRP ↑D‐dimer |
WMD =1.76 WMD =0.42 WMD =2.11 WMD =0.67 |
0.31, 3.22 −0.52, −0.33 0.72, 3.51 0.02, 1.32 |
Not specified | |
| Henry et al. 48 | June–20 | 18 | 2984 | Severity |
↑WBC ↑NP ↓LYC ↓CD4 ↓CD8 ↓Monocyte ↓Eosinophil ↓PLC ↓Hb ↑D‐dimer ↓aPTT |
WMD =0.41 WMD =1.7 WMD = −0.28 WMD = −3.94 WMD = −2.22 WMD = −0.03 WMD = −0.01 WMD = −23.4 WMD = −6.52 WMD =0.71 WMD = −1.11 |
0.16, 0.66 1.57, 1.85 −0.3, −0.25 −8.02, 0.13 −5.01, 0.57 0.07, −0.01 −0.02, −0.01 −30.8, −15.9 −9.2, −3.85 1.48, 0.94 −2.33, 0.10 |
0.00 0.00 0.00 0.12 0.41 0.82 0.01 0.02 0.80 0.05 0.72 |
|
| 3 | 393 | IUC |
↑WBC ↓LYC ↓PLC ↓Hb |
WMD =4.15 WMD = −0.44 WMD = −48.3 WMD = −1.34 |
3.15, 5.15 −0.54, −0.35 −57.7, −38.9 −4.85, 2.1 |
0.53 0.00 0.00 0.64 |
|||
|
Xiong et al. 106 |
Jun–20 |
9 |
1105 |
Severity |
↑PT ↑D‐dimer ↓ PLT ↓ aPTT |
WMD =0·68 WMD =0·53 WMD = −0.08 WMD = −0.3 |
0.43–0.93 0.22, 0.84 0.34, 0.18 0.4, 0.34 |
0.055 0.000 0.039 0.000 |
|
| Lima et al. 107 | Jul–20 | 3 | 648 | Mortality | ↑D‐dimer | 11‐fold higher | ‐ | – | |
| Lippi et al. 108 | Jul–20 | 9 | 1779 | Severity | ↓PLC |
WMD = −31 OR =5.1 |
−35, 29 1.8, 14.6 |
<0.001 | |
| Lapić et al. 66 | Jul–20 | 3 | 819 | Mortality | ↑ESR | SMD 0.82 | 0.16–1.47 | <0.000 | |
| Shi et al. 109 | Jul–20 | 10 | 1845 | Mortality | ↑NP | SMD =0.93 | 0.63–1.24 | <0.001 | |
| Soraya et al. 110 | Aug–20 | 7 | Severity |
↓LYC ↓PLC ↑WBC ↑NP ↑D‐dimer |
SMD = −0.56 SMD = −0.32 SMD =0.31 SMD =0.44 SMD =0.53, |
−0.71, 0.40 −0.49, 0.15 0.07, 0.56 0.24, 0.64 0.31, 0.75 |
<0.000 0.0002 0.01 <0.000 <0.000 |
0.83 170.8 5.3 3.65 |
|
| Zheng et al. 111 | Aug–20 |
13 4 |
3027 1286 |
Mortality |
↑D‐dimer ↓WBC |
OR =43.24 OR =0.30 |
9.92, 188.49 0.17, 0.51 |
0.0001 | <4 × 109/L > 0.5 mg/L |
| Severity |
↑WBC ↑D‐dimer |
OR =0.30 OR =43.24 |
0.17, 0.51 9.92, 188.5 |
||||||
| Ghahramani et al. 112 | Aug–20 | 17 | 1099 | Severity |
↓LYC ↓Monocyte ↓Eosinophil ↓Hb ↓PLC ↑NP |
WMD =−0.43 WMD =−0.06 WMD =−0.03 WMD =−5.94 WMD =−27.97 WMD =0.74 |
−0.56, 0.30 −0.12, 0.0 −0.05, 0.001 −8.23, −3.64 −39.6, −16.4 0.16, 1.33 |
<0.001 0.032 0.037 0.001 <0.001 0.013 |
|
| Elshazli et al. 113 | Aug–20 | 16 | 6320 | Severity |
↑WBC ↑NP ↓LYC ↓PLC ↑D‐dimer ↑PT ↑Fibrinogen |
OR =1.75 OR =2.62 OR =0.30 OR =0.56 OR =3.97 OR =1.82 OR =3.14 |
1.21, 2.54 1.72, 3.97 0.19, 0.47 0.42, 0.74 2.62, 6.02 1.00, 3.33 1.64, 6.00 |
0.000 0.000 0.000 0.000 0.000 0.047 0.001 |
|
| 11 | IUC |
↑WBC ↑NP ↓LYC ↓PLC ↑D‐dimer ↑PT |
OR =5.21 OR =6.25 OR =0.21 OR =0.43 OR =4.19 OR =2.18 |
3.00, 9.05 3.00, 9.05 0.10, 0.47 0.28, 0.68 4.72, 8.58 1.58, 6.47 |
0.000 0.001 0.000 0.000 0.000 0.001 |
||||
| Figliozzi et al. 114 | Aug–20 | 49 | 587,790 | Mortality |
↓LYP ↑D‐dimer |
OR =2.86 OR =4.4 |
1.09, 7.6 1.10, 17.58 |
0.037 0.04 |
|
| Bashash et al. 115 | Sep–20 | 13 | 3383 | Severity | ↓PLC | WMD = −21.5 | 31.57, −11.43 | <0.001 | |
| Alnor et al. 116 | Sep–20 | 21 | 1099 | Severity |
↑WBC ↑NP ↓LYC ↓PLC ↓Hb ↑CRP ↑D‐dimer |
MD =1.28 MD =1.49 MD = −0.32 MD = −22.4 MD = −4.1 MD =49.2 SMD =0.58 |
0.37, 2.20 0.64, 2.35 −0.42, −0.21 −35.3, −9.5 −6.42, −1.78 31.2, 67.1 0.37 −0.78 |
<0.001 <0.001 <0.001 <0.001 <0.001 <0.001 <0.001 |
|
| Wu et al. 68 | Oct–20 | 4 | 31100 | Infection |
ABO group A O |
OR =1.249 OR =0.699 |
1.114, 1.440 0.634, 0.770 |
<0.001 <0.001 |
|
|
Severity |
AB O B |
OR =2.422 OR =0.748 OR =1.271 |
0.94, 6.29 0.56, 1.01 0.89, 1.81 |
0.069 0.056 0.181 |
|||||
| Zhang et al. 117 | Oct–20 | 7 | 4663 | Severity |
↓LYC ↑CRP |
OR =4.5 OR =3.0 |
3.3–6.0 2.1–4.4 |
0.686 0.356 |
|
| Del Sole et al. 118 | Oct–20 | 12 | 2794 | Severity |
↑D‐dimer ↓PLC |
OR: 5.67 OR: 3.61 |
1.45–22.16 2.62–4.97 |
0.001 0.001 |
|
| Kiss et al. 119 | Nov–20 | 93 | 25901 | Mortality |
↑WBC ↑WBC ↓ WBC ↓LYC ↑NP ↑D‐dimer ↑D‐dimer |
OR =3.7 OR =6.25 OR =0.38 OR =3.74 OR =2.32 OR =4.30 OR =6.63 |
1.72, 7.69 2.86, 14.29 0.20, 0.72 1.77, 7.92 1.23, 4.37 1.55, 11.98 3.62, 12.14 |
0.001 0.000 0.001 0.001 0.009 0.005 0.000 |
>9.5 > 10.0 <4.0 <0.8 >6.3 >0.50 >1.0 |
| 78 | IUC |
↑WBC ↑WBC ↓LYC |
OR =4.52 OR =2.64 OR =4.54 |
1.95, 10.52 1.22, 5.71 2.58, 7.95 |
0.000 0.014 |
>9.5 >10.0 <1.0 |
|||
| Hariyanto et al. 120 | Dec–20 | 23 | 4848 | Severity |
↑D‐dimer ↑CRP |
MD =0.43 MD =36.88 |
0.31–0.56 29.10–44.65 |
<0.001 <0.001 |
263.5 0.635 |
| Zong et al. 121 | Jan–21 | 24 | 5637 | Mortality | ↓PLC | OR, 7.37 | 2.08–26.14 | 0.001 | |
| Gungor et al. 122 | Jan–21 | 39 | 7813 | Severity |
↑D‐dimer |
WMD: 0.45 RR =1.58 |
0.34–0.56 1.25–2.00 |
<0.001 <0.001 |
>0.5 |
| Mortality |
↑D‐dimer |
WMD: 5.32 RR: 1.82 |
3.90–6.73 1.40–2.37 |
<0.001 <0.001 |
>0.5 | ||||
| Zhu et al. 123 | Feb–21 | 34 | 6492 | Severity |
↓PLC ↓aPTT ↑D‐dimer ↑Fibrinogen ↑PT |
WMD = −16.29 WMD = −0.81 WMD =0.44 WMD =0.51 WMD: 0.65 |
25.34, −7.23 −1.94, 0.33 0.29–0.58 0.33–0.69 0.44–0.86 |
<0.001 <0.001 <0.001 <0.001 <0.001 |
|
| Mortality |
↑D‐dimer ↑PT ↓PLC |
WMD: 6.58 WMD: 1.27 WMD: −39.73 |
3.59–9.57 0.49–2.06 −61.99, −17.5 |
<0.001 <0.001 <0.001 |
|||||
| Lin et al. 124 | Feb–21 | 13 | 1341 | Severity |
↓PLC ↑D‐dimer ↑Fibrinogen ↓aPTT |
WMD = −24.83 WMD =0.19 WMD =1.02 WMD =0.19 |
−34.12, 15.54 0.09, 0.29 0.50, 1.54 −0.13, 0.51 |
<0.001 <0.001 <0.001 0.243 |
Studies addressing peripheral blood film of a COVID‐19 patient described a characteristic neutrophil nuclei with C‐shaped, single‐lobed nuclei, morphologic abnormalities in the granulocytic series and leukoerythroblastic reaction. 42 , 43 Blue–green leukocyte inclusions were reported in the circulating neutrophils and/or monocytes of critically ill patients with liver impairment and lactic acidosis and proposed as a poor prognostic indicator. 44 By contrast, activated monocytes were observed in clinically improved patients. 42 , 43
Flow cytometry performed on peripheral blood lymphocytes of ICU patients showed a significant reduction in the absolute T‐cell count in most cases as well as CD45+, CD3+, CD4+, CD8+, CD19+, and CD16/56+ counts. However, unlike immunodeficiency virus (HIV) and cytomegalovirus, the CD4/CD8 ratio was not inverted in all groups of patients. 39 , 45
Many studies have suggested neutrophil‐to‐lymphocyte ratio (NLR) as an independent risk factor for mortality in severely ill patients with COVID‐19 with a cut‐off value varied between 3.0 and 13, 46 Table 2. Pooled data from 13 studies involving 1579 patients reported NLR sensitivity of 0.78 and 0.78 specificity in predicting disease severity. Ten studies involving 2967 patients reported higher sensitivity 0.83 and specificity 0.83 in predicting mortality. 47
5.2. Changes in RBC indices
Meta‐analysis results regarding hemoglobin level in severely ill COVID‐19 patients are conflicting. A meta‐analysis study including 224 severely ill COVID‐19 patients reported significantly lower hemoglobin than none severe cases with a weighted mean difference (WMD) of −7.1 g/L; 95% CI, −8.3 to −5.9 g/L. 36 Other study found the difference was not significant. 48 The lowest hemoglobin level was seen in patients who reached a composite endpoint (included admission to ICU, requirement of invasive ventilation, and death). 49 The reduction in Hb may indicate disease progression, however, age and comorbidities are confounding factors in this patient group and data should be interpreted cautiously. 50
Elevated red blood cell distribution width (RDW), a component of complete blood counts that reflects RBC variation in volume and size, has been shown to be associated with elevated mortality risk for patients with COVID‐19. 51
5.3. Changes in coagulation indices
Altered coagulability is a poor prognostic indicator in severely and critically ill patients with COVID‐19, Table 2. The laboratory manifestations of coagulopathy are elevated D‐dimer, slight decrease in platelet count, prolonged PT, and reduced fibrinogen level. Thrombotic events and bleeding often occur in subjects with weak constitutions, multiple risk factors, and comorbidities. 52 A meta‐analysis of 22 studies conducted in china involving 4,889 confirmed COVID‐19 patients found that mean D‐dimer was 0.67 µg/ml (95% CI: 0.56–0.78), mean platelet 186.34 × 109/L (95% CI: 175.84–196.85), mean PT 12.20 s (95% CI: 11.52–12.84), and mean fibrinogen 4.24 g/L (95% CI: 3.40–5.15). 53 Patient with severe illness had longer PT (MD = 0.65 s, 95% CI: 0.36–0.95, p < 0.05), shorter aPTT (MD = −0.01 s, 95% CI: −2.58–2.56, p = 0.99), higher D‐dimer (MD = 0.44 µg/ml, 95% CI: 0.23–0.66, p < 0.05), and lower platelet count (MD = −14.47 × 109/L, 95% CI: −33.0–4.06, p = 0.126). 53
Coagulopathy in COVID‐19 initiated by endothelial injury results in thrombin generation and fibrinolysis shut down, contributing to a hypercoagulable state, which causes a prolonged PT and aPTT. In the late stages of the DIC, PT, aPTT, fibrinogen, and platelets are decreased due to consumptive coagulopathy as observed in none survivors. 54 Moreover, COVID‐19 patients presenting with cardiac injury and elevated troponin‐T levels were more prone to coagulation disorders compared with those without cardiac involvement. 55 Studies have also detected lupus anticoagulant and antiphospholipid antibodies in COVID‐19 patients, which could contribute to the hypercoagulable state. 1
Altered coagulability is complicated by venous thromboembolism in 24%, deep venous thrombosis 7%, and pulmonary embolism 19% of severely ill COVID‐19 patients. 56 Independent predictive parameters for thromboembolism were pneumonia, old age, spontaneous prolongation of PT >3 s, and aPTT >5 s. 57 Arterial embolism in the form of acute MI, acute limb ischemia, and storks was also reported in COVID‐19 patients. 58 , 59
The contribution of von Willebrand factor (vWF) in thromboinflammation is well established. Several mechanisms are involved. These mechanisms starts with endothelial dysfunction. 60 In severely ill COVID‐19 patient, marked increase in vWF and factor VIIIc level was observed similar to that seen in severely septic non‐COVID‐19 ICU patients. 52 , 61 , 62 With disease progression, and in the absence of anticoagulant treatment, VWF and fibrinogen levels decline with persistent high D‐dimer levels and even higher P‐selectin levels indicating poor prognosis. 52
5.4. Other markers
Several biochemical tests are described as potential prognostic indicators in progressive COVID‐19 infection. Serum ferritin level is elevated in 74.2% complicated viral infection. 63 COVID‐19 patients with elevated serum ferritin were at higher risk of developing ARDS (HR = 3.53, 95%CI: 1.52–8.16, p = 0.003). A meta‐analysis of 18 studies concluded that the ferritin level was significantly higher in severely ill patients [WMD 397.77 (95% CI 306.51–489.02)] and non‐survivors [WMD 677.17 (95% CI 391.01–963.33)]. Higher level of ferritin was observed in patients with comorbidities such as diabetes, thrombotic complication, liver dysfunction, and cancer. 64
Elevated C‐reactive protein (CRP) was reported in 81.5%, pro‐calcitonin in 13.7%, and LDH in 58.1% of severe COVID‐19 infection and they were linked to secondary bacterial infection. 38 , 65 Additionally, erythrocyte sedimentation rate (ESR) was reported by several studies to be significantly elevated and was considered as a predictor of infection severity. 66 , 67
ABO blood group was described in many studies to be associated with COVID‐19. A study of pooled 31100 COVID‐19 patients suggested that people with blood type A might be more susceptible to infect COVID‐19 while blood type O might be less susceptible to infect COVID‐19, yet no correlation between ABO blood group and severity or mortality of the infection. 68
6. HEMATOLOGICAL CHANGES IN CYTOKINE STORM
Cytokine storm is a serious clinical state that occurs approximately 7–14 days in the course of COVID‐19 infection; presented clinically as nonspecific constitutional symptoms such as persistent fever, weight loss, joint and muscle pain, fatigue, and headache. Cytokine storm characterized by a hyperinflammatory status as a consequence of proinflammatory cytokines 49 and chemokines overproduction, 69 resulting in pulmonary, cardio‐circulatory, or combined disturbances. Extensive local edema due to vasodilatation and membrane leakage may result in multiorgan failure and uncontrollable shock. 70 , 71 , 72 Nonsurvivor of COVID‐19 as well as severe refractory patients exhibited high levels of IL–6, IL–2R, IL–8, IL–10, and TNF. Sustained high level of IL6 in particular was shown by two meta‐analyses to associate with severe COVID‐19 infection and high mortality. 73
Clinically, cytokine storm is considered when patients show clinical deterioration in the form of reduced rest oxygen saturation below 94% or tachypnea more than 30/min with at least two of three biomarker (in the absence of bacterial infection): high CRP (>100 mg/L), high serum‐ferritin (>900 μg/L at one occasion, or twofold increase in the level at admission within 48 h), and high D‐Dimer level (>1,500 μg/L). 74 , 75
6.1. Changes in WBC count and morphology
Monocytes and macrophages are activated by binding the virus via ACE2 receptors on their surface producing IL‐6, IL‐10, and TNF 76 causing the nonspecific constitutional symptoms in COVID‐19 patients. T‐cell overactivation occurring in severe COVID‐19 infection leads to the production of granulocyte‐macrophage colony‐stimulating factor (GM‐CSF), which stimulates CD14+ CD16 + mononuclear cell to produce IL‐6 and a gush of other inflammatory mediators. 72 Markedly lower absolute lymphocyte count OR 0.41 [0.26–0.62] was observed in 169 COVID‐19 patients with cytokine storm compared with those without. They also showed altered flow cytometric phenotype at the time of presentation in addition to lower eosinophils (OR 0.34 [0.20–0.57], basophils (OR 0.39 [0.20–0.74]), and monocytes (OR 0.52 [0.33–0.79]). 75 Lymphocytopenia seen in patients with cytokine storm may be the results of direct lysis of lymphocytes by the virus as the lymphocytes do express ACE2 on its surface. Lymphocytes apoptosis could be promoted by the plethora of cytokine in this stage, which may also lead to lymphoreticular organs dysfunction impeding lymphocyte turnover. 77 , 78
Neutrophilia is another sign of cytokine storm and considered as a predictor of disease severity. 79 Neutrophils extravasate in the interstitial tissue form extracellular mesh of DNA/histones to control the infection, this exacerbate the inflammation and cause tissue damage. 80
Data regarding NK cells are contradicting. One study reported a reduction in the number of circulating NK cells in COVID‐19 patients, 81 whereas others showed no difference in the number of CD16+CD56+ in severe compared to none severe cases. 82
6.2. Changes in RBC indices
Anemia is reported in some of the critically ill COVID‐19 patients; however, detailed RBC indices changes in response to secondary adult hemophagocytic lymphohistiocytosis associated with this viral infection are not well characterized and require further studies.
6.3. Changes in coagulation indices
During cytokine storm, immune response to infection results in overproduction of proinflammatory cytokines which activate coagulation pathway. At later stage, the tightly controlled, thrombin regulating mechanisms are impaired. Reduced anticoagulant concentrations due to reduced production and increasing consumption results in microthrombosis, disseminated intravascular coagulation, and multiorgan failure. Raised D‐dimer concentrations being a poor prognostic feature and disseminated intravascular coagulation are common in nonsurvivors. 83 A single‐center study in china followed the dynamic platelet‐to‐lymphocyte ratio (PLR) changes during the period of hospitalization, they suggested a possible correlation between high PLR and cytokine storm associating with prolonged hospitalization days. 84
6.4. Other markers
The cytokine profile in COVID‐19‐associated cytokine storm is similar to secondary hemophagocytic lymphohistiocytosis, which is commonly triggered by sepsis and viral infections. In such conditions, elevated ferritin, triglycerides, uric acid, LDH, lactate, and acute kidney injury are observed along with elevated CRP, procalcitonin, D‐dimer, troponin, and alanine aminotransferase. 85
7. HEMATOLOGICAL CHANGES IN SPECIAL GROUPS
7.1. Children
COVID‐19 incidence in children is lower than that seen in adults. Recent reports from the USA Centers for Disease Control and Prevention (CDC) indicates that children younger than 19 years constituted 5% of the all cases. The course of the disease seems to be milder than adults; nonetheless, severe cases have been recorded in 3–5% and mortality 0.3%.
Four systematic reviews and meta‐analyses concluded that the majority of children with COVID‐19 had a normal leukocyte count. Lymphopenia was reported in relatively few number of cases (15%) and was not associated with severe course. 86 , 87 , 88 By contrast, pooled data from 486 hospitalized children reported lymphocytosis in 22% and leukopenia in 21%. Higher incidence of lymphocytosis up to 61% was seen in neonates, yet severe cases in these neonates were relatively higher 7%. 89 , 90
Studies rarely reported changes in hemoglobin or RBC indices and coagulative system, the available literatures showed no significant change in the Hb level in children with COVID‐19 whether asymptomatic, mild, severe, or critical. 90 , 91 , 92 , 93 The infrequent reporting of thrombotic events in children may indicate its rarity in the clinical practice.
Other laboratory findings reported in children were elevated inflammatory markers including ferritin (26% [16–40]), procalcitonin (25% [21–29]), and CRP (19% [16–22]). 94
7.2. Pregnant women
The incidence and clinical manifestations of COVID‐19 in pregnant women have been reported to be similar to general population with approximately 80% having mild course of the disease or asymptomatic and good perinatal outcomes. 38 Sever cases of COVID‐19 in pregnant women were reported in 15% and critical in 5%, the majority of which were associated with risk factor such as comorbidity and obesity. 76 The mortality of pregnant women with COVID‐19 was relatively low (0.43%) and close to the overall maternal mortality rate worldwide (1 in 180). 95 Vertical transmission of the infection is an area of debate. Earlier studies showed absent placental infection and negative PCR of amniotic fluid. 96 More recent studies identified placental infection associated with increased fibrin deposition resulting in fetal distress and subsequent premature delivery. 97
Hematological findings in pregnant women with COVID‐19 were infrequently reported in the published studies but generally, were similar to that of nonpregnant women. A systematic review of 20 related studies included 230 pregnant or women in labor showed that 40.7% had lymphopenia, 16.7% neutropenia, and 4% thrombocytopenia. Hypercoagulability have been reported in pregnant women with severe disease. 98 Another meta‐analysis based on 11 studies with 173 severely to critically ill pregnant patients from the first trimester to the third trimester suggested that elevated D‐dimer (82%), elevated neutrophil count (81%), elevated C‐reactive protein (69%), and decreased lymphocyte count (59%) were the most frequent abnormalities. 99 In a pilot study that included 21 pregnant women in the second and third trimester with COVID‐19 and 48 without, a higher aPTT level, platelet count and lower fibrinogen, D‐dimer levels, and antithrombin time were observed in patient COVID‐19 positive pregnant women as compared to COVID‐19 negative. However, there was no difference observed in CRP and FVIII levels 100 . Pregnancy is already a known risk for venous thromboembolism characterizing by a procoagulant imbalance. Prophylactic anticoagulant has been provided for women undergoing C sections to reduce the risk of venous thromboembolism; however, larger studies addressing the coagulopathy in this group are required.
8. CONCLUSION
Subtle hematological changes might appear early in the course of COVID‐19 infection; progressive disease associates with significant hematological changes that may lead the management plan and predict patient outcome. Research all over the world characterized the clinical, radiological, and laboratorial manifestation associated with the pandemic; however, further Iraqi studies are essential to report the clinical and hematological profile in Iraqi population over the three waves hit the country.
Al‐Saadi EAKD, Abdulnabi MA. Hematological changes associated with COVID‐19 infection. J Clin Lab Anal.2022;36:e24064. 10.1002/jcla.24064
REFERENCES
- 1. Khandait H, Gandotra G, Sachdeva S, et al. COVID‐19 and hematology—what do we know so far? SN Compr Clin Med. 2020;2(12):2631‐2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus‐infected pneumonia. N Engl J Med. 2020;382:1199‐1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. European Centre for Disease Prevention and Control COVID‐19 situation update worldwide: European Centre for Disease Prevention and Control; 2020. https://www.ecdc.europa.eu/en/about‐ecdc. Accessed November 11, 2020.
- 4. Dawood AA, Dawood ZAJV. How will the second wave of the dreadful COVID‐19 be with the increasing number of the infected cases and mortality in Iraq? Vacunas. 2021;22(2):114‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. MEDBOX worldometer‐COVID19: MEDBOX 2020 [cited 2021 April]. https://www.worldometers.info/coronavirus/country/iraq/. Accessed November 11, 2020.
- 6. Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou QJS. Structural basis for the recognition of SARS‐CoV‐2 by full‐length human. ACE2. 2020;367:1444‐1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Morniroli D, Giannì ML, Consales A, Pietrasanta C, Mosca F. Human Sialome and coronavirus disease‐2019 (COVID‐19) pandemic: an understated correlation? Front Immunol. 2020;11:1480‐1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ulrich H, Pillat MM. CD147 as a target for COVID‐19 treatment: suggested effects of azithromycin and stem cell engagement. Stem Cell Rev Rep. 2020;16:434‐440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ko KO, Nagashima S, E. B, et al. Molecular characterization and the mutation pattern of SARS‐CoV‐2 during first and second wave outbreaks in Hiroshima, Japan. PLoS One. 2021;16:e0246383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Korber B, Fischer WM, Gnanakaran S, et al. Tracking changes in SARS‐CoV‐2 spike: evidence that D614G increases infectivity of the COVID‐19 virus. Cell. 2020;182:812‐27.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Plante JA, Liu Y, Liu J, et al. Spike mutation D614G alters SARS‐CoV‐2 fitness. Nature. 2020;592:116‐121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. WHO Clinical managment of COVID‐19: WHO; 2020 [updated May 2020]. https://apps.WHO.int/iris/bitstream/handle/10665/332196/WHO‐2019‐nCoV‐clinical‐2020.5‐eng.pdf. Accessed November 11, 2020.
- 13. Centers for Disease Control and Prevention COVID‐19 Pandamic Planning Scenarios USA: Centers for Disease Control and Prevention; 2020 [updated Sept 2020]. https://www.cdc.gov/coronavirus/2019‐ncov/hcp/planning‐scenarios.html#box1. Accessed November 11, 2020.
- 14. da Rosa Mesquita R, Francelino Silva Junior LC, Santos Santana FM, et al. Clinical manifestations of COVID‐19 in the general population: systematic review. Wien Klin Wochenschr. 2020;133(7‐8):377‐382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grippo F, Navarra S, Orsi C, et al. The Role of COVID‐19 in the Death of SARS‐CoV‐2–Positive Patients: A Study Based on Death Certificates. Journal of Clinical Medicine. 2020;9(11):3459. 10.3390/jcm9113459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vincent JL, Taccone FS. Understanding pathways to death in patients with COVID‐19. Lancet Respir Med. 2020;8:430‐432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019‐nCoV) by real‐time RT‐PCR. Euro Surveill. 2020;25:2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. World Health Organization Laboratory testing for 2019 novel coronavirus (2019‐nCoV) in suspected human cases: interim guidance. 2020; WHO. [cited 2021 20 May]. https://www.who.int/publications‐detail/laboratory‐testing‐for‐2019‐novel‐coronavirus‐in‐suspected‐human‐cases‐20200117. Accessed November 11, 2020. [Google Scholar]
- 19. ACR ACR Recommendations for the Use of Chest Radiography and Computed Tomography (CT) for Suspected COVID‐19 Infection 2020 [cited 2021 May 20]. https://www.ACR.org/Advocacy‐and‐Economics/ACR‐Position‐Statements/Recommendations‐for‐Chest‐Radiography‐and‐CT‐for‐Suspected‐COVID19‐Infection. Accessed November 11, 2020.
- 20. Guo LI, Ren L, Yang S, et al. Profiling early humoral response to diagnose novel coronavirus disease (COVID‐19). Clin Infect Dis. 2020;71:778‐785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Horvath A, Lind T, Frece N, Wurzer H, Stadlbauer V. Risk stratification in hospitalized COVID‐19 patients. J Hepatol. 2021;75(3):740‐742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stegeman I, Ochodo EA, Guleid F, et al. Routine laboratory testing to determine if a patient has COVID‐19. Cochrane Database Syst Rev. 2020;11:Cd013787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Letícia de Oliveira Toledo S, Sousa Nogueira L, das Graças Carvalho M, Romana Alves Rios D, de Barros Pinheiro M. COVID‐19: Review and hematologic impact. Clin Chim Acta. 2020;510:170‐176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Saxena S, Wong ET. Heterogeneity of common hematologic parameters among racial, ethnic, and gender subgroups. Arch Pathol Lab Med. 1990;114:715‐719. [PubMed] [Google Scholar]
- 25. COVID‐19 Treatment Guidelines Panel Coronavirus Disease 2019 (COVID‐19) Treatment Guidelines: National Institutes of Health; 2020. https://www.covid19treatmentguidelines.nih.gov/. Accessed November 11, 2020. [PubMed]
- 26. Xu X‐W, Wu X‐X, Jiang X‐G, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS‐Cov‐2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368: m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lo IL, Lio CF, Cheong HH, et al. Evaluation of SARS‐CoV‐2 RNA shedding in clinical specimens and clinical characteristics of 10 patients with COVID‐19 in Macau. Int J Biol Sci. 2020;16(10):1698‐1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Young BE, Ong SWX, Kalimuddin S, et al. Epidemiologic features and clinical course of patients infected with SARS‐CoV‐2 in Singapore. JAMA. 2020;323:1488‐1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim G‐U, Kim M‐J, Ra SH, et al. Clinical characteristics of asymptomatic and symptomatic patients with mild COVID‐19. Clin Microbiol Infect. 2020;26:948.e1‐48.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Byambasuren O, Cardona M, Bell K, Clark J, McLaws M‐L, Glasziou P. Estimating the extent of asymptomatic COVID‐19 and its potential for community transmission: systematic review and meta‐analysis. Official J Assoc Med Microbiol Infect Dis Canada. 2020;5:223‐234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu X, Zhang R, He G. Hematological findings in coronavirus disease 2019: indications of progression of disease. Ann Hematol. 2020;99:1421‐1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Terpos E, Ntanasis‐Stathopoulos I, Elalamy I, et al. Hematological findings and complications of. COVID‐19. 2020;95:834‐847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li Q, Ding X, Xia G, et al. A simple laboratory parameter facilitates early identification of COVID‐19 patients. 2020.
- 34. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu J, Li S, Liu J, et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS‐CoV‐2 infected patients. EBioMedicine. 2020;55:102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lippi G, Mattiuzzi C. Hemoglobin value may be decreased in patients with severe coronavirus disease 2019. Hematol Transfus Cell Ther. 2020;42:116‐117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen G, Wu DI, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Investig. 2020;130:2620‐2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Guan W‐J, Ni Z‐Y, Hu YU, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2019;2020(382):1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fan BE, Chong VCL, Chan SSW, et al. Hematologic parameters in patients with COVID‐19 infection. Am J Hematol. 2020;95:E131‐E134. [DOI] [PubMed] [Google Scholar]
- 40. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934‐943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yuan X, Huang W, Ye B, et al. Changes of hematological and immunological parameters in COVID‐19 patients. Int J Hematol. 2020;112:553‐559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nazarullah A, Liang C, Villarreal A, Higgins RA, Mais DD. Peripheral blood examination findings in SARS‐CoV‐2 infection. Am J Clin Pathol. 2020;154:319‐329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Singh A, Sood N, Narang V, Goyal A. Morphology of COVID‐19‐affected cells in peripheral blood film. BMJ Case Rep. 2020;13:e236117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cantu MD, Towne WS, Emmons FN, et al. Clinical significance of blue‐green neutrophil and monocyte cytoplasmic inclusions in SARS‐CoV‐2 positive critically ill patients. Br J Haematol. 2020;190:e89‐e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang F, Nie J, Wang H, et al. Characteristics of peripheral lymphocyte subset alteration in COVID‐19 pneumonia. J Infect Dis. 2020;221:1762‐1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Xu J‐B, Xu C, Zhang R‐B, et al. Associations of procalcitonin, C‐reaction protein and neutrophil‐to‐lymphocyte ratio with mortality in hospitalized COVID‐19 patients in China. Sci Rep. 2020;10:15058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li X, Liu C, Mao Z, et al. Predictive values of neutrophil‐to‐lymphocyte ratio on disease severity and mortality in COVID‐19 patients: a systematic review and meta‐analysis. Crit Care. 2020;24:647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Henry BM, de Oliveira MHS, Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID‐19): a meta‐analysis. Clin Chem Lab Med. 2020;58:1021‐1028. [DOI] [PubMed] [Google Scholar]
- 49. Maclean A, Kamal A, Adishesh M, Alnafakh R, Tempest N, Hapangama DK. Human uterine biopsy: research value and common pitfalls. Int J Reprod Med. 2020;2020:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Taneri PE, Gómez‐Ochoa SA, Llanaj E, et al. Anemia and iron metabolism in COVID‐19: a systematic review and meta‐analysis. Eur J Epidemiol. 2020;35:763‐773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Foy BH, Carlson JCT, Reinertsen E, et al. Association of red blood cell distribution width with mortality risk in hospitalized adults with SARS‐CoV‐2 infection. JAMA Netw Open. 2020;3:e2022058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Grobler C, Maphumulo SC, Grobbelaar LM, Bredenkamp JC, Laubscher GJ, Lourens PJ, Steenkamp J, Kell DB, Pretorius E. Covid‐19: The Rollercoaster of Fibrin(Ogen), D‐Dimer, Von Willebrand Factor, P‐Selectin and Their Interactions with Endothelial Cells, Platelets and Erythrocytes. International Journal of Molecular Sciences. 2020;21(14):5168. 10.3390/ijms21145168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jin S, Jin Y, Xu B, Hong J, Yang X. Prevalence and impact of coagulation dysfunction in COVID‐19 in China: a meta‐analysis. Thromb Haemost. 2020;120:1524‐1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID‐19. J Thromb Haemost. 2020;18:1023‐1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shi S, Qin MU, Shen BO, et al. Association of cardiac injury with mortality in hospitalized patients with COVID‐19 in Wuhan. China. 2020;5:802‐810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Porfidia A, Valeriani E, Pola R, Porreca E, Rutjes AWS, Di Nisio M. Venous thromboembolism in patients with COVID‐19: systematic review and meta‐analysis. Thromb Res. 2020;196:67‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Klok FA, Kruip M, van der Meer N, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID‐19. Thromb Res. 2020;191:145‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS‐CoV‐2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089‐1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lodigiani C, Iapichino G, Carenzo L, et al. Venous and arterial thromboembolic complications in COVID‐19 patients admitted to an academic hospital in Milan, Italy. Thrombosis Res. 2020;191:9‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Delrue M, Siguret V, Neuwirth M, et al. von Willebrand factor/ADAMTS13 axis and venous thromboembolism in moderate‐to‐severe COVID‐19 patients. Br J Haematol. 2020;192(6):1097‐1100. [DOI] [PubMed] [Google Scholar]
- 61. Ladikou EE, Sivaloganathan H, Milne KM, et al. Von Willebrand factor (vWF): marker of endothelial damage and thrombotic risk in COVID‐19? Clin Med (Lond). 2020;20:e178‐e182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Panigada M, Bottino N, Tagliabue P, et al. Hypercoagulability of COVID‐19 patients in intensive care unit: a report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020;18:1738‐1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Xie J, Wang Q, Xu Y, et al. Clinical characteristics, laboratory abnormalities and CT findings of COVID‐19 patients and risk factors of severe disease: a systematic review and meta‐analysis. Ann Palliat Med. 2021;10:1928‐1949. [DOI] [PubMed] [Google Scholar]
- 64. Cheng L, Li H, Li L, et al. Ferritin in the coronavirus disease 2019 (COVID‐19): a systematic review and meta‐analysis. J Clin Lab Anal. 2020;34:e23618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lapić I, Rogić D, Plebani M. Erythrocyte sedimentation rate is associated with severe coronavirus disease 2019 (COVID‐19): a pooled analysis. Clin Chem Lab Med. 2020;58:1146‐1148. [DOI] [PubMed] [Google Scholar]
- 67. Chidambaram V, Tun NL, Haque WZ, et al. Factors associated with disease severity and mortality among patients with COVID‐19: A systematic review and meta‐analysis. PLOS ONE. 2020;15(11):e0241541. 10.1371/journal.pone.0241541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wu BB, Gu DZ, Yu JN, Yang J, Shen WQ. Association between ABO blood groups and COVID‐19 infection, severity and demise: a systematic review and meta‐analysis. Infect Genet Evol. 2020;84:104485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kowalewski M, Fina D, Słomka A, et al. COVID‐19 and ECMO: the interplay between coagulation and inflammation—a narrative review. Crit Care. 2020;24:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Coperchini F, Chiovato L, Croce L, Magri F, Rotondi M. The cytokine storm in COVID‐19: An overview of the involvement of the chemokine/chemokine‐receptor system. Cytokine Growth Factor Rev. 2020;53:25‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ulhaq ZS, Soraya GV. Interleukin‐6 as a potential biomarker of COVID‐19 progression. Med Mal Infect. 2020;50:382‐383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Aziz M, Fatima R, Assaly R. Elevated interleukin‐6 and severe COVID‐19: a meta‐analysis. J Med Virol. 2020;92:2283‐2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Chen T, Wu DI, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ramiro S, Mostard RLM, Magro‐Checa C, et al. Historically controlled comparison of glucocorticoids with or without tocilizumab versus supportive care only in patients with COVID‐19‐associated cytokine storm syndrome: results of the CHIC study. Ann Rheum Dis. 2020;79:1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Martens RJH, van Adrichem AJ, Mattheij NJA, et al. Hemocytometric characteristics of COVID‐19 patients with and without cytokine Storm syndrome on the Sysmex XN‐10 hematology analyzer. Clin Chem Lab Med. 2021;59(4):783‐793. [DOI] [PubMed] [Google Scholar]
- 76. Zhang D, Guo R, Lei L, et al. Frontline Science: COVID‐19 infection induces readily detectable morphologic and inflammation‐related phenotypic changes in peripheral blood monocytes. Journal of Leukocyte Biology. 2021;109(1):13‐22. 10.1002/jlb.4hi0720-470r [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Fischer K, Hoffmann P, Voelkl S, et al. Inhibitory effect of tumor cell–derived lactic acid on human T cells. Blood. 2007;109:3812‐3819. [DOI] [PubMed] [Google Scholar]
- 78. Chan JF, Zhang AJ, Yuan S, et al. Simulation of the clinical and pathological manifestations of coronavirus disease 2019 (COVID‐19) in a golden syrian hamster model: implications for disease pathogenesis and transmissibility. Clin Infect Dis. 2020;71:2428‐2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zhang L, Huang B, Xia H, et al. Retrospective analysis of clinical features in 134 coronavirus disease 2019 cases. Epidemiology and Infection. 2020;148: 10.1017/s0950268820002010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Narasaraju T, Yang E, Samy RP, et al. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am J Pathol. 2011;179:199‐210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID‐19) in Wuhan, China. Clin Infect Dis. 2020;71:762‐768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Zheng Y, Huang Z, Yin G, et al. Study of the Lymphocyte Change Between COVID‐19 and Non‐COVID‐19 Pneumonia Cases Suggesting Other Factors Besides Uncontrolled Inflammation Contributed to Multi‐Organ Injury. SSRN Electronic Journal. 10.2139/ssrn.3555267 [DOI] [Google Scholar]
- 83. Jose RJ, Manuel A. COVID‐19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir Med. 2020;8:e46‐e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Qu R, Ling Y, Zhang Y‐H‐Z, et al. Platelet‐to‐lymphocyte ratio is associated with prognosis in patients with coronavirus disease‐19. J Med Virol. 2020;92:1533‐1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sultan S, Sultan M. COVID‐19 cytokine storm and novel truth. Med Hypotheses. 2020;144:109875‐109975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Henry BM, Lippi G, Plebani M. Laboratory abnormalities in children with novel coronavirus disease. Clin Chem Lab Med. 2019;2020(58):1135‐1138. [DOI] [PubMed] [Google Scholar]
- 87. Patel NA. Pediatric COVID‐19: systematic review of the literature. Am J Otolaryngol. 2020;41:102573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Meena J, Yadav J, Saini L, Yadav A, Kumar J. Clinical features and outcome of SARS‐CoV‐2 infection in children: a systematic review and meta‐analysis. Indian Pediatr. 2020;57:820‐826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ma X, Liu S, Chen L, Zhuang L, Zhang J, Xin Y. The clinical characteristics of pediatric inpatients with SARS‐CoV‐2 infection: a meta‐analysis and systematic review. J Med Virol. 2021;93:234‐240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Kosmeri C, Koumpis E, Tsabouri S, Siomou E, Makis A. Hematological manifestations of SARS‐CoV‐2 in children. Pediatr Blood Cancer. 2020;67:e28745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lu X, Zhang L, Du H, et al. SARS‐CoV‐2 infection in children. N Engl J Med. 2020;382:1663‐1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Parri N, Lenge M, Buonsenso D. Children with Covid‐19 in pediatric emergency departments in Italy. N Engl J Med. 2020;383:187‐190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Sun D, Li H, Lu X‐X, et al. Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center's observational study. World J Pediatr. 2020;16:251‐259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Badal S, Thapa Bajgain K, Badal S, Thapa R, Bajgain BB, Santana MJ. Prevalence, clinical characteristics, and outcomes of pediatric COVID‐19: a systematic review and meta‐analysis. J Clin Virol. 2021;135:104715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239‐1242. [DOI] [PubMed] [Google Scholar]
- 96. Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID‐19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809‐815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Schoenmakers S, Snijder P, Verdijk RM, et al. Severe acute respiratory syndrome coronavirus 2 placental infection and inflammation leading to fetal distress and neonatal multi‐organ failure in an asymptomatic woman. J Pediatric Infect Dis Soc. 2021;10(5):556‐561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Chi J, Gong W, Gao Q. Clinical characteristics and outcomes of pregnant women with COVID‐19 and the risk of vertical transmission: a systematic review. Arch Gynecol Obstet. 2020;303(2):337‐345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Shi L, Wang Y, Yang H, Duan G, Wang Y. Laboratory abnormalities in pregnant women with novel coronavirus disease 2019. Am J Perinatol. 2020;37:1070‐1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ciavarella A, Erra R, Abbattista M, et al. Hemostasis in pregnant women with COVID‐19. Int J Gynaecol Obstet. 2021;152(2):268‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Rajan MB, Kumar‐M P, Bhardwaj A. The trend of cutaneous lesions during COVID‐19 pandemic: lessons from a meta‐analysis and systematic review. Int J Dermatol. 2020;59:1358‐1370. [DOI] [PubMed] [Google Scholar]
- 102. Yassin A, Nawaiseh M, Shaban A, et al. Neurological manifestations and complications of coronavirus disease 2019 (COVID‐19): a systematic review and meta‐analysis. BMC Neurol. 2021;21:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Huang I, Pranata R, Lim MA, Oehadian A, Alisjahbana B. C‐reactive protein, procalcitonin, D‐dimer, and ferritin in severe coronavirus disease‐2019: a meta‐analysis. Ther Adv Respir Dis. 2020;14:1753466620937175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Lagunas‐Rangel FA. Neutrophil‐to‐lymphocyte ratio and lymphocyte‐to‐C‐reactive protein ratio in patients with severe coronavirus disease 2019 (COVID‐19): a meta‐analysis. J Med Virol. 2020;92:1733‐1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Xu L, Mao Y, Chen G. Risk factors for 2019 novel coronavirus disease (COVID‐19) patients progressing to critical illness: a systematic review and meta‐analysis. Aging. 2020;12:12410‐12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Xiong M, Liang X, Wei YD. Changes in blood coagulation in patients with severe coronavirus disease 2019 (COVID‐19): a meta‐analysis. Br J Haematol. 2020;189:1050‐1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Lima WG, Barra A, Brito JCM, Nizer WSC. D‐Dimer serum levels as a biomarker associated for the lethality in patients with coronavirus disease 2019: a meta‐analysis. Blood Coagul Fibrinolysis. 2020;31:335‐338. [DOI] [PubMed] [Google Scholar]
- 108. Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID‐19) infections: a meta‐analysis. Clin Chim Acta. 2020;506:145‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Shi L, Wang Y, Liang X, et al. Is neutrophilia associated with mortality in COVID‐19 patients? A meta‐analysis and meta‐regression. Int J Lab Hematol. 2020;42:e244‐e247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Soraya GV, Ulhaq ZS. Crucial laboratory parameters in COVID‐19 diagnosis and prognosis: an updated meta‐analysis. Med Clin (Barc). 2020;155:143‐151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Zheng Z, Peng F, Xu B, et al. Risk factors of critical & mortal COVID‐19 cases: a systematic literature review and meta‐analysis. J Infect. 2020;81:e16‐e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Ghahramani S, Tabrizi R, Lankarani KB, et al. Laboratory features of severe vs. non‐severe COVID‐19 patients in Asian populations: a systematic review and meta‐analysis. Eur J Med Res. 2020;25:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Elshazli RM, Toraih EA, Elgaml A, et al. Diagnostic and prognostic value of hematological and immunological markers in COVID‐19 infection: A meta‐analysis of 6320 patients. PLOS ONE. 2020;15(8):e0238160. 10.1371/journal.pone.0238160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Figliozzi S, Masci PG, Ahmadi N, et al. Predictors of adverse prognosis in COVID‐19: a systematic review and meta‐analysis. Eur J Clin Invest. 2020;50:e13362. [DOI] [PubMed] [Google Scholar]
- 115. Bashash D, Hosseini‐Baharanchi FS, Rezaie‐Tavirani M, et al. The prognostic value of thrombocytopenia in COVID‐19 patients; a systematic review and meta‐analysis. Arch Acad Emerg Med. 2020;8:e75. [PMC free article] [PubMed] [Google Scholar]
- 116. Alnor A, Sandberg MB, Gils C, Vinholt PJ. Laboratory tests and outcome for patients with coronavirus disease 2019: a systematic review and meta‐analysis. J Appl Lab Med. 2020;5:1038‐1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Zhang ZL, Hou YL, Li DT, Li FZ. Laboratory findings of COVID‐19: a systematic review and meta‐analysis. Scand J Clin Lab Invest. 2020;80:441‐447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Del Sole F, Farcomeni A, Loffredo L, et al. Features of severe COVID‐19: a systematic review and meta‐analysis. Eur J Clin Invest. 2020;50:e13378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Kiss S, Gede N, Hegyi P, et al. Early changes in laboratory parameters are predictors of mortality and ICU admission in patients with COVID‐19: a systematic review and meta‐analysis. Med Microbiol Immunol. 2021;210:33‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Hariyanto TI, Japar KV, Kwenandar F, et al. Inflammatory and hematologic markers as predictors of severe outcomes in COVID‐19 infection: a systematic review and meta‐analysis. Am J Emerg Med. 2021;41:110‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Zong X, Gu Y, Yu H, Li Z, Wang Y. Thrombocytopenia Is associated with COVID‐19 severity and outcome: an updated meta‐analysis of 5637 patients with multiple outcomes. Lab Med. 2021;52:10‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Gungor B, Atici A, Baycan OF, et al. Elevated D‐dimer levels on admission are associated with severity and increased risk of mortality in COVID‐19: a systematic review and meta‐analysis. Am J Emerg Med. 2021;39:173‐179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Zhu J, Pang J, Ji P, et al. Coagulation dysfunction is associated with severity of COVID‐19: a meta‐analysis. J Med Virol. 2021;93:962‐972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Lin J, Yan H, Chen H, et al. COVID‐19 and coagulation dysfunction in adults: a systematic review and meta‐analysis. J Med Virol. 2021;93:934‐944. [DOI] [PMC free article] [PubMed] [Google Scholar]


