Summary
The data on the predictors and prognosis of acute liver injury (ALI) among patients in coronavirus disease 2019 (COVID‐19) patients are limited. The aim of this study was to determine the prevalence, predictors and outcomes of ALI among patients with COVID‐19. A systematic review was conducted up to 10 June 2021. The relevant papers were searched from PubMed, Embase, Cochrane and Web of Science, and the data were analysed using a Z test. A total of 1331 papers were identified and 16 papers consisting of 1254 COVID‐19 with ALI and 4999 COVID‐19 without ALI were analysed. The cumulative prevalence of ALI among patients with COVID‐19 was 22.8%. Male and having low lymphocyte levels were more likely to be associated with ALI compared with female and having higher lymphocyte level, odds ratio (OR): 2.70; 95% confidence interval (CI): 2.03, 3.60 and mean difference (MD) −125; 95% CI: −207, −43, respectively. COVID‐19 patients with ALI had higher risk of developing severe COVID‐19 compared with those without ALI (OR: 3.61; 95% CI: 2.60, 5.02). Our findings may serve as the additional evaluation for the management of ALI in COVID‐19 patients.
Keywords: acute liver injury, COVID‐19, outcome, predictor, prevalence
Abbreviations
- ACE2
angiotensin‐converting enzyme 2
- ALI
acute liver injury
- BMI
body mass index
- CAD
coronary artery disease
- COVID‐19
coronavirus disease 2019
- DM
diabetes mellitus
- NOS
Newcastle–Ottawa Scale
- PRISMA
Preferred Reporting Items for Systematic Review and Meta‐Analysis
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
1. INTRODUCTION
The coronavirus disease 2019 (COVID‐19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) remains the major global concern. The pathogenesis of COVID‐19 is complicated 1 , 2 and involves multiple organs including lung, kidney, heart, neurologic system, gastrointestinal system and liver. 3 Although the respiratory tract is the primary target of SARS‐CoV‐2, more than 50% of COVID‐19 patients had nausea, vomiting, diarrhoea and loss of appetite 4 suggesting the involvement of gastrointestinal and hepatobiliary system. A recent study also found that moderate microvascular steatosis was prevalent in liver biopsies of COVID‐19 patients, suggesting that liver injury might occur during COVID‐19. 5 The involvement of liver in SARS‐CoV‐2 infection is mystifying, 6 and it was suggested that liver involvement is mediated by several mechanisms, including direct infection of the liver, drug‐induced liver injury, systemic inflammatory response or hypoxic hepatitis. 7
The optimal management of acute liver injury (ALI) in COVID‐19 patients remains controversial. Although one recommendation suggested that ALI in COVID‐19 is reversible and does not require specific treatment, 7 liver involvement was reported to cause poor prognosis of COVID‐19 patients. 8 Moreover, liver involvement has been included in predicting the outcomes of patients with COVID‐19. 9 To date, no information is available regarding predictors of when and who among COVID‐19 patients will suffer from ALI. In addition, data on the outcomes of COVID‐19 patients with ALI are also limited. Therefore, the objective of this study was to determine the prevalence of ALI in COVID‐19 patients, predictors of ALI occurrence and prognosis of COVID‐19 patients with ALI.
2. METHODS
2.1. Study design and eligibility criteria
A systematic review following the Preferred Reporting Items for Systematic Review and Meta‐Analysis 10 was conducted up to 10 June 2021 on four databases including PubMed, Embase, Cochrane and Web of Science. The included papers should: (1) have the design of double‐arm analysis such as randomised controlled trial (RCT) and non‐RCT or observational studies (case–control, cross‐sectional or cohort); (2) report either the prevalence, predictor or the outcome of ALI in COVID‐19 patients; (3) contain information about COVID‐19 cases diagnosed using RT‐PCR from nasopharyngeal or oropharyngeal swab samples; and (4) have sufficient criteria for the diagnosis of ALI. 11
2.2. Search strategy and data extraction
All papers in English were searched using Medical Subjects Heading: (“COVID‐19” OR “SARS‐CoV‐2”) AND (“acute liver injury” OR “liver dysfunction” OR “liver abnormality”) AND (“prevalence” OR “predictor” OR “outcome”). Additional papers from the reference list of the articles were searched and in case of dual duplication, a paper with the higher sample size was included. The following information were collected from each study: (1) first author name and publication year; (2) country and city of origin; (3) study design; (4) study setting; (5) sample size of COVID‐19 patients with and without ALI; (5) the incidence of ALI; (6) the factors associated with ALI; and (7) severity and mortality rate of COVID‐19 patients with and without ALI. The definition of variables and study protocols were defined prior to data collection, and a kappa test was used to assess the understanding among investigators.
2.3. Quality assessment
Potential articles were evaluated for their methodological quality using Newcastle–Ottawa Scale (NOS) that evaluates sample selection, comparison and exposure. 12 The calculation of NOS score was used to classify the quality of articles into low (score 0–3), moderate (score 4–6) and high quality (score 7–9) and only articles with moderate and high quality were included into analysis. All letters to the editor, commentaries, case reports, case series and reviews were excluded.
2.4. Study variables
ALI refers to an acute abnormality of liver blood tests and the development of a coagulopathy, but does not exhibit any alteration of consciousness in an individual without underlying chronic liver disease. 9 The predictor variables included age, gender, body mass index (BMI), the presence of comorbidities [diabetes mellitus (DM), coronary artery disease (CAD) and hypertension], pre‐existing liver disease, as well as the levels of leucocytes, lymphocytes and neutrophils. Those variables were defined after considering the available data.
2.5. Statistical analysis
To assess the publication bias, an Egger test was applied and a p < 0.05 indicated potential publication bias. 13 The heterogeneity among studies was assessed using a Q test and the random effect model was used if the heterogeneity across the studies were observed (p < 0.10). 13 The prevalence of ALI, the associated predictors of ALI, and the association between ALI and the clinical outcomes of patients with COVID‐19 were determined using a Z test. The summary of statistical analysis was presented in forest plot. A Review Manager (Revman Cochrane, London, UK) version 5.3 was used to analyse the data.
3. RESULTS
3.1. Study eligibility results
A total of 1331 papers were identified across the databases of which 1283 papers were excluded due to having irrelevant studies. Full‐text assessment was conducted on 48 papers and additional 32 papers were excluded as they did not meet the eligibility criteria (Figure 1). 16 papers consisting of two cross‐sectional studies, three prospective studies and 11 retrospective studies were finally included into meta‐analysis (Table 1). 5 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28
FIGURE 1.
A flowchart of article selection
TABLE 1.
Baseline characteristics of articles included in our analysis
| Author and years | Country | Study design | Study group comparation | Sample size | Quality (NOS) | |
|---|---|---|---|---|---|---|
| ALI | Non‐ALI | |||||
| Bloom et al. (2021) | US | Prospective cohort | Normal versus hepatocellular injury | 10 | 50 | 6 |
| Cai et al. (2020) | China | Cross‐sectional | Normal liver versus ALI | 22 | 225 | 6 |
| Cai et al. (2020) | China | Retrospective | Normal liver versus ALI | 90 | 327 | 6 |
| Chen et al. (2021) | China | Prospective cohort | Normal liver versus ALI | 32 | 603 | 5 |
| Chen et al. (2020) | China | Retrospective | Normal liver versus ALI | 13 | 261 | 6 |
| Chew et al. (2021) | China | Retrospective | Normal liver versus ALI | 105 | 729 | 6 |
| Fan et al. (2020) | China | Cross‐sectional | Normal liver versus ALI | 55 | 93 | 5 |
| Mishra et al. (2020) | US | Retrospective | Normal liver versus ALI | 166 | 162 | 8 |
| Phipps et al. (2020) | US | Retrospective | Normal liver versus ALI | 145 | 1784 | 6 |
| Piano et al. (2020) | Italy | Retrospective | Normal liver versus ALI | 329 | 236 | 6 |
| Qi et al. (2020) | China | Prospective cohort | Non‐ALI versus ALI | 32 | 38 | 5 |
| Sarin et al. (2020) | India | Retrospective | Non‐ALI versus ALI | 97 | 88 | 6 |
| Wang et al. (2020) | China | Retrospective | Normal liver versus ALI | 96 | 243 | 6 |
| Xie et al. (2020) | China | Retrospective | Non‐ALI versus ALI | 29 | 50 | 6 |
| Yang et al. (2021) | China | Retrospective | Normal liver versus ALI | 15 | 37 | 7 |
| Zhao et al. (2020) | China | Retrospective | Non‐ALI versus ALI | 18 | 73 | 8 |
Abbreviations: ALI, acute liver injury; NOS, Newcastle–Ottawa Scale.
3.2. The global prevalence, predictors and prognosis of ALI among patients with COVID‐19
The included studies comprised 1254 COVID‐19 with ALI and 4999 COVID‐19 without ALI, and the prevalence of ALI was found to be 22.8% [95% confidence interval (CI): 14.1, 34.6] (Figure 2a). A total of 10 potential predictors of ALI (age, gender, BMI; the presence of DM, CAD, and hypertension, liver disease; as well as the level of white blood cells (WBC), neutrophils and lymphocytes) were analysed (Table 2). Male and having high lymphocyte count were associated with ALI with OR: 2.70; 95% CI: 2.03, 3.60 and mean difference (MD): −125; 95% CI: −207, −43, respectively (Figures 2b,c). Our data suggested that COVID‐19 patients with ALI had higher odds of suffering from severe disease compared with those without ALI, OR: 3.61; 95% CI: 2.60, 5.02 (Figure 2d and Table 2).
FIGURE 2.
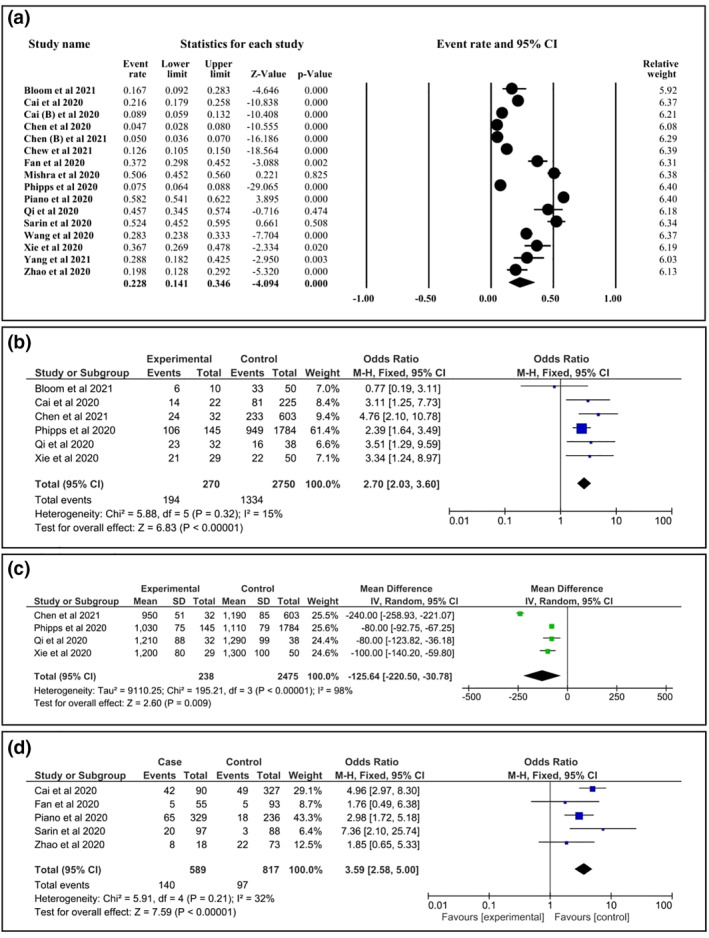
The summary of acute liver injury (ALI) in patients with coronavirus disease 2019 (COVID‐19). (a) The global prevalence of ALI in patients with COVID‐19. (b) Association of gender (male) with ALI in COVID‐19 patients. (c) Association of low level of lymphocyte with ALI in COVID‐19 patients. (d) Association between ALI and the severity of COVID‐19
TABLE 2.
The global prevalence, predictors and prognosis of acute liver injury among patients with COVID‐19
| Variable | NS | Model | Study group | Point estimate | 95%CI | p Egger | p Het | p‐value | |
|---|---|---|---|---|---|---|---|---|---|
| ALI | Non‐ALI | ||||||||
| ALI prevalence | 16 | Random | 1254 (20.05) | 4999 (79.94) | 22.8% a | 14.1, 34.6 | 1.1760 | <0.0001 | <0.0001 |
| ALI predictors | |||||||||
| Age (years), mean ± SD | 5 | Random | 55.2 ± 8.8 | 53.6 ± 10.2 | 1.76 b | −3.26, 6.78 | 0.3260 | 0.0030 | 0.4660 |
| Male, n (%) | 6 | Fixed | 194 (71.9) | 1334 (48.5) | 2.70 c | 2.03, 3.60 | 0.1720 | 0.3180 | <0.0001 |
| BMI (kg/m2), mean ± SD | 2 | Fixed | 28.5 ± 3.5 | 26.5 ± 4.9 | 2.63 b | 0.97, 4.30 | <0.0001 | 0.3630 | 0.0020 |
| Diabetes mellitus, n (%) | 6 | Fixed | 56 (20.7) | 828 (30.1) | 0.72 c | 0.52, 0.99 | <0.0001 | 0.8450 | 0.0450 |
| Coronary artery disease, n (%) | 4 | Fixed | 10 (9.7) | 77 (10.4) | 1.26 c | 0.60, 2.63 | <0.0001 | 0.8520 | 0.5450 |
| Hypertension, n (%) | 5 | Random | 105 (40.4) | 1309 (48.5) | 1.31 c | 0.67, 2.54 | 0.5790 | 0.0220 | 0.4260 |
| Liver disease, n (%) | 4 | Random | 15 (7.2) | 110 (4.2) | 2.98 c | 1.00, 8.88 | 0.8400 | 0.0630 | 0.0500 |
| Leucocyte (cells/μl), mean ± SD | 3 | Random | 7983 ± 3953 | 5553 ± 1700 | 2432 b | −89, 4954 | 0.4550 | <0.0001 | 0.0590 |
| Neutrophils (cells/μl), mean ± SD | 3 | Random | 5556 ± 2833 | 3893 ± 1334 | 166 b | −29, 362 | 0.4400 | <0.0001 | 0.0970 |
| Lymphocytes (cells/μl), mean ± SD | 4 | Random | 1097 ± 128 | 1222 ± 89 | −125 b | −207, −43 | 0.8610 | <0.0001 | 0.0090 |
| Prognosis | |||||||||
| Severe versus non severe | 5 | Fixed | 140 (27.80) | 97 (11.90) | 3.61 b | 2.60, 5.02 | 0.2810 | 0.2060 | <0.0001 |
| Mortality | 8 | Random | 252 (28.77) | 445 (24.07) | 1.38 b | 0.85, 2.25 | 0.5130 | 0.0060 | 0.1940 |
Abbreviations: ALI, acute liver injury; BMI, body mass index; CI, confidence interval; NOS, Newcastle–Ottawa Scale; NS, number of studies; p Het, p heterogeneity; WBC, white blood cells.
Event rate.
Odds ratio.
Mean difference.
3.3. Heterogeneity and potency of bias across the studies
The heterogeneity was identified on data of prevalence of ALI in COVID‐19 patients, mortality of COVID‐19 and data of some predictors of ALI such as age, hypertension, liver disease, WBC, neutrophils and lymphocytes and therefore random effect model was used while other predictors were and the association between ALI and severity of COVID‐19 was assessed using fixed effect model. The potency of publication bias was found in several predictors of ALI including BMI, DM and CAD (Table 2).
4. DISCUSSION
Our study found that the cumulative prevalence of ALI among patients with COVID‐19 was 22.8%. This finding is higher compared with that of a previous meta‐analysis using data of five studies (prevalence 15.7%). 29 Our data suggest that male and having high lymphocyte level were associated with ALI. Although the mechanism of ALI in SARS‐CoV‐2 infection is debatable, it is known that the expression of angiotensin‐converting enzyme 2 (ACE2) receptors, the primary receptor for SARS‐CoV‐2 to enter human cells, was high in the liver. 30 A previous investigation reported that the expression of ACE2 receptors was higher in male than female 31 and ACE2 expression is mediated by androgen. 32 A study revealed that ACE2 receptors were also expressed in lymphocytes, 33 suggesting that SARS‐CoV‐2 may also attack lymphocytes leading to decreased numbers. Interestingly, our study also found that patients with higher BMI and DM had higher risk to develop ALI, although the Egger test is insufficient to support the findings. The liver abnormality in patients with the obesity and DM suggested that the metabolic associated steatohepatitis might also affect the involvement of liver injury, and this circumstance might also contribute to the severity of COVID‐19 infection. 31 Our findings also showed that COVID‐19 patients with ALI had higher risk of developing severe disease, consistent with previous meta‐analyses. 29 , 34 , 35 , 36 Therefore, patients with ALI on initial admission should be strictly monitored since they are at higher risk of developing severe outcomes including death. The involvement of liver could trigger dysregulated immune responses leading to cytokine storm, 37 a pathological state associated with fatal COVID‐19 outcomes. 38 This presumably explains why COVID‐19 patients with ALI possess higher risk of developing severe conditions.
The present study, to the best of our knowledge, is the first to provide comprehensive data on prevalence, predictors and prognosis of ALI in COVID‐19. Robust results indicated that ALI is associated with severe COVID‐19. Therefore, some parameters should be monitored during COVID‐19 management to anticipate the occurrence of ALI and to prevent severe outcomes. The present data might helpfully be used as the reference for the management of COVID‐19 with ALI.
There are some limitations of our study. Potential confounding factors such as previous medication, drug interactions, previous liver disease, status of metabolism and previous history of infectious disease were not reported and therefore could not be controlled. In addition, the heterogeneity of the quality of included articles in our meta‐analysis might contribute to certain degree of bias. Furthermore, the limited studies on the topic led us to include only limited number of papers, and therefore the potential for publication bias should be carefully interpreted.
5. CONCLUSION
The prevalence of ALI among patients with COVID‐19 is 22.8%. Male and having lower lymphocyte level are more likely to be associated with ALI. COVID‐19 patients with ALI have high risk for severe COVID‐19 and therefore should be monitored closely to prevent the development of severe conditions. Nevertheless, further prospective studies are required to provide more robust data and to confirm the findings of the present study.
CONFLICT OF INTEREST
Authors have no conflict of interest.
ETHICS STATEMENT
Not applicable.
AUTHOR CONTRIBUTIONS
Conceptual: Harapan Harapan, Jonny Karunia Fajar, Supriono Supriono. Design: Jonny Karunia Fajar. Control/supervision: Supriono Supriono, Gatot Soegiarto, Laksmi Wulandari. Data collection/processing: Fiha Seratin, Nyoman Gede Prayudi, Dara Puspita Dewi, Maria Theresia Monica Elsina, Lasarus Atamou, Sinta Wiranata, Dhito Pemi Aprianto, Erlin Friska, D. Fitria Sari Firdaus, Makdum Alaidin, Firdha Aprillia Wardhani, Nurdina Wahyu Hidayati, Yeni Hendriyanti, Kristia Wardani, Arde Evatta, Reizal Audi Manugan, Wiryawan Pradipto, Ade Rahmawati, Fredo Tamara, Aditya Indra Mahendra, Budi Santoso, Chandra Adi Irawan Primasatya, Nindy Tjionganata, Hendarto Arif Budiman. Extraction/analysis/interpretation: Jonny Karunia Fajar, Fredo Tamara, Aditya Indra Mahendra. Literature review: Jonny Karunia Fajar, Fredo Tamara, Aditya Indra Mahendra. Writing the article: Harapan Harapan, Jonny Karunia Fajar, Fredo Tamara, Aditya Indra Mahendra. Critical review: Harapan Harapan, Supriono Supriono, Jonny Karunia Fajar, Gatot Soegiarto, Laksmi Wulandari, Milda Husnah. All authors have read and approved the final draft.
CONSENT FOR PUBLICATION
Not applicable.
ACKNOWLEDGEMENTS
Authors would like to thank to Lembaga Pengelola Dana Pendidikan (LPDP) Republic of Indonesia for supporting our project. Harapan is supported by the Indonesian Endowment Fund for Education and the Indonesian Science Fund through the International Collaboration RISPRO Funding Program (No. RISPRO/KI/B1/TKL/5/15448/1/2020) and Universitas Syiah Kuala (Ministry of Education, Culture, Research and Technology) through the H‐Index Research Scheme (No. 169/UN11/SPK/PNBP/2021).
Harapan H, Fajar JK, Supriono S, et al. The prevalence, predictors and outcomes of acute liver injury among patients with COVID‐19: a systematic review and meta‐analysis. Rev Med Virol. 2022;32(3):e2304. 10.1002/rmv.2304
Contributor Information
Harapan Harapan, Email: harapan@unsyiah.ac.id.
Gatot Soegiarto, Email: gatot_soegiarto@fk.unair.ac.id.
DATA AVAILABILITY STATEMENT
Data used in our study were presented in the main text.
REFERENCES
- 1. Keam S, Megawati D, Patel SK, Tiwari R, Dhama K, Harapan H. Immunopathology and immunotherapeutic strategies in severe acute respiratory syndrome coronavirus 2 infection. Rev Med Virol. 2020;30:e2123. doi: 10.1002/rmv.2123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Harapan H, Itoh N, Yufika A, et al. Coronavirus disease 2019 (COVID‐19): a literature review. J Infect Public Health. 2020;13:667‐673. doi: 10.1016/j.jiph.2020.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thakur V, Ratho RK, Kumar P, et al. Multi‐organ involvement in COVID‐19: beyond pulmonary manifestations. J Clin Med. 2021;10:446. doi: 10.3390/jcm10030446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carfi A, Bernabei R, Landi F, et al. Persistent symptoms in patients after acute COVID‐19. J Am Med Assoc. 2020;324:603‐605. doi: 10.1001/jama.2020.12603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen TWD, Chen H, Yan W, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1295. doi: 10.1136/bmj.m1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhou P, Yang X‐L, Wang X‐G, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nguyen‐Contant P, Embong AK, Kanagaiah P, et al. S protein‐reactive IgG and memory B cell production after human SARS‐CoV‐2 infection includes broad reactivity to the S2 subunit. mBio. 2020:11. doi: 10.1128/mBio.01991-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mao R, Qiu Y, He JS, et al. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID‐19: a systematic review and meta‐analysis. Lancet Gastroenterol Hepatol. 2020;5:667‐678. doi: 10.1016/S2468-1253(20)30126-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen Y, Zhou X, Yan H, et al. CANPT Score: a tool to predict severe COVID‐19 on admission. Front Med. 2021;8:608107. doi: 10.3389/fmed.2021.608107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA Statement. Open Med 2009;3:e123‐30. [PMC free article] [PubMed] [Google Scholar]
- 11. European Association for the Study of the Liver . Electronic address easloffice@easloffice.eu, Clinical Practice Guidelines Panel, et al. EASL Clinical Practical Guidelines on the management of acute (fulminant) liver failure. J Hepatol. 2017;66:1047‐1081. doi: 10.1016/j.jhep.2016.12.003 [DOI] [PubMed] [Google Scholar]
- 12. Stang A. Critical evaluation of the Newcastle–Ottawa Scale for the assessment of the quality of nonrandomized studies in meta‐analyses. Eur J Epidemiol. 2010;25:603‐605. doi: 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 13. Fahriani M, Ilmawan M, Fajar JK, et al. Persistence of long COVID symptoms in COVID‐19 survivors worldwide and its potential pathogenesis: a systematic review and meta‐analysis. Narra J. 2021;1:e36. doi: 10.52225/narraj.v1i2.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bloom PP, Meyerowitz EA, Reinus Z, et al. Liver biochemistries in hospitalized patients with COVID‐19. Hepatology. 2021;73:890‐900. doi: 10.1002/hep.31326 [DOI] [PubMed] [Google Scholar]
- 15. Cai Q, Huang D, Yu H, et al. COVID‐19: abnormal liver function tests. J Hepatol. 2020;73:566‐574. doi: 10.1016/j.jhep.2020.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cai Q, Huang D, Ou P, et al. COVID‐19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy. 2020;75:1742‐1752. doi: 10.1111/all.14309 [DOI] [PubMed] [Google Scholar]
- 17. Chen F, Chen W, Chen J, et al. Clinical features and risk factors of COVID‐19‐associated liver injury and function: a retrospective analysis of 830 cases. Ann Hepatol. 2021;21:100267. doi: 10.1016/j.aohep.2020.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chew M, Tang Z, Radcliffe C, et al. Significant liver injury during hospitalization for COVID‐19 is not associated with liver insufficiency or death. Clin Gastroenterol Hepatol. 2021;19:2182‐2191. doi: 10.1016/j.cgh.2021.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fan Z, Chen L, Li J, et al. Clinical features of COVID‐19‐related liver functional abnormality. Clin Gastroenterol Hepatol. 2020;18:1561‐1566. doi: 10.1016/j.cgh.2020.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mishra K, Naffouj S, Gorgis S, et al. Liver injury as a surrogate for inflammation and predictor of outcomes in COVID‐19. Hepatol Commun. 2021;5:24‐32. doi: 10.1002/hep4.1586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Phipps MM, Barraza LH, LaSota ED, et al. Acute liver injury in COVID‐19: prevalence and association with clinical outcomes in a large U.S. cohort. Hepatology. 2020;72:807‐817. doi: 10.1002/hep.31404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Piano S, Dalbeni A, Vettore E, et al. Abnormal liver function tests predict transfer to intensive care unit and death in COVID‐19. Liver Int. 2020;40:2394‐2406. doi: 10.1111/liv.14565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Qi X, Liu C, Jiang Z, et al. Multicenter analysis of clinical characteristics and outcomes in patients with COVID‐19 who develop liver injury. J Hepatol. 2020;73:455‐458. doi: 10.1016/j.jhep.2020.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sarin SK, Choudhury A, Lau GK, et al. Pre‐existing liver disease is associated with poor outcome in patients with SARS CoV2 infection; the APCOLIS Study (APASL COVID‐19 Liver Injury Spectrum Study). Hepatol Int. 2020;14:690‐700. doi: 10.1007/s12072-020-10072-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang L, He W, Yu X, et al. Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4‐week follow‐up. J Infect. 2020;80:639‐645. doi: 10.1016/j.jinf.2020.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xie H, Zhao J, Lian N, Lin S, Xie Q, Zhuo H. Clinical characteristics of non‐ICU hospitalized patients with coronavirus disease 2019 and liver injury: a retrospective study. Liver Int. 2020;40:1321‐1326. doi: 10.1111/liv.14449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gao Q, Bao L, Mao H, et al. Development of an inactivated vaccine candidate for SARS‐CoV‐2. Science. 2020;369:77‐81. doi: 10.1126/science.abc1932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054‐1062. doi: 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ahmed J, Rizwan T, Malik F, et al. COVID‐19 and liver injury: a systematic review and meta‐analysis. Cureus. 2020;12:e9424. doi: 10.7759/cureus.9424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hamming I, Timens W, Bulthuis ML, Lely A, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631‐637. doi: 10.1002/path.1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Patel SK, Velkoska E, Burrell LM. Emerging markers in cardiovascular disease: where does angiotensin‐converting enzyme 2 fit in? Clin Exp Pharmacol Physiol. 2013;40:551‐559. doi: 10.1111/1440-1681.12069 [DOI] [PubMed] [Google Scholar]
- 32. Mjaess G, Karam A, Aoun F, Albisinni S, Roumeguère T. COVID‐19 and the male susceptibility: the role of ACE2, TMPRSS2 and the androgen receptor. Prog Urol. 2020;30:484‐487. doi: 10.1016/j.purol.2020.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xu H, Zhong L, Deng J, et al. High expression of ACE2 receptor of 2019‐nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12:8. doi: 10.1038/s41368-020-0074-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wu ZH, Yang DL. A meta‐analysis of the impact of COVID‐19 on liver dysfunction. Eur J Med Res. 2020;25:54. doi: 10.1186/s40001-020-00454-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wong YJ, Tan M, Zheng Q, et al. A systematic review and meta‐analysis of the COVID‐19 associated liver injury. Ann Hepatol. 2020;19:627‐634. doi: 10.1016/j.aohep.2020.08.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sharma A, Jaiswal P, Kerakhan Y, et al. Liver disease and outcomes among COVID‐19 hospitalized patients—a systematic review and meta‐analysis. Ann Hepatol. 2021;21:100273. doi: 10.1016/j.aohep.2020.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yang Z, Xu M, Yi JQ, et al. Clinical characteristics and mechanism of liver damage in patients with severe acute respiratory syndrome. Hepatobiliary Pancreat Dis Int. 2005;4:60‐63. [PubMed] [Google Scholar]
- 38. Hojyo S, Uchida M, Tanaka K, et al. How COVID‐19 induces cytokine storm with high mortality. Inflamm Regen. 2020;40:37. doi: 10.1186/s41232-020-00146-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data used in our study were presented in the main text.


