Abstract
Wearable sensors are emerging as a new technology to detect physiological and biochemical markers for remote health monitoring. By measuring vital signs such as respiratory rate, body temperature, and blood oxygen level, wearable sensors offer tremendous potential for the noninvasive and early diagnosis of numerous diseases such as Covid‐19. Over the past decade, significant progress has been made to develop wearable sensors with high sensitivity, accuracy, flexibility, and stretchability, bringing to reality a new paradigm of remote health monitoring. In this review paper, the latest advances in wearable sensor systems that can measure vital signs at an accuracy level matching those of point‐of‐care tests are presented. In particular, the focus of this review is placed on wearable sensors for measuring respiratory behavior, body temperature, and blood oxygen level, which are identified as the critical signals for diagnosing and monitoring Covid‐19. Various designs based on different materials and working mechanisms are summarized. This review is concluded by identifying the remaining challenges and future opportunities for this emerging field.
Keywords: blood oxygen level, body temperature, Covid‐19, health monitoring, respiratory rate, wearable sensors
Wearable sensors have demonstrated grand potential for remote health monitoring recently, particularly after the Covid‐19 pandemic. The need for wearable telehealth systems capable of monitoring vital signs, such as respiratory rate, body temperature, and blood oxygen level has surged. In this review, the recent advances in creating these sensors and remaining challenges as well as prospects are presented.
1. Introduction
Existing methods for diagnosing infections such as coronavirus rely on the detection of viral RNA in samples collected using nasopharyngeal swabs by trained staff. Such molecular diagnostics methods require dedicated pathology laboratories and a standard 24–48 h turnaround time.[ 1 ] Coronavirus, which causes infectious respiratory disease, has wreaked havoc across the globe and caused significant economic and societal damage.[ 2 ] As of mid‐April 2021, Covid‐19 has caused more than 2.9 million deaths and near 137 million infections worldwide.[ 3 ] Some patients who are infected with this virus may not show any symptoms, while in most cases, there are fast‐developing symptoms that may eventually lead to death.[ 2 , 4 ] Studies on Covid‐19 patients show that fever, shortness of breath, cough, and fatigue are the most reported symptoms.[ 2 , 4 , 5 , 6 , 7 ] Instability in respiration is also an indirect indicator of the elevated probability of fatality.[ 8 ] Since Covid‐19 is a respiratory disease, the blood oxygen level of an infected patient is generally lower. For example, the National Institutes of Health of USA found that oxygen saturation for Covid‐19 patients is ≈92–96%[ 9 ] while a healthy adult normally has an oxygen saturation level between 95% and 100%.[ 10 , 11 ] So, low oxygen saturation in adults below 95% is considered good indirect evidence of infection or illness. Therefore, measuring vital signs, such as respiratory rate, body temperature, and blood oxygen level noninvasively by wearable sensors holds great promise as an early diagnostic technique.[ 12 ] This is particularly useful for people in quarantine, self‐isolation, and outside hospital settings.
Vital signs such as respiratory rate, body temperature, and blood oxygen level are currently monitored by health care providers for ascertaining the health status.[ 13 , 14 ] Various methods are used for measuring these parameters in clinical practices. While manual counting the breath per minute is one of the most commonly used methods for measuring respiratory rate, techniques such as electrical chest impedance, pneumotachograph are also popularly applied. Thermometers such as electronic contact thermometers and infrared thermometers are commonly utilized for measuring body temperature with an accuracy of 0.1 °C.[ 15 ] Blood oxygen level is mostly tested by using a pulse oximeter. All of these techniques have to be used in hospitals/clinics by trained staff, although portable pulse oximeters have been trailed by local health districts for monitoring patients.[ 16 ] One major issue with these devices is that they do not provide continuous monitoring without disturbing the patients’ daily activities. Continuous monitoring enables early detection of the disease so that preventative measures can be taken earlier. Also, techniques that are noninvasive, reliable, as well as comfortable to use are in high demand by users.
Over the past decade, significant progress has been made to develop wearable sensors for measuring physiological and biochemical markers. Wearable technologies offer a convenient means to monitor many physiological signals and a more comfortable as well as cost‐effective alternative for users and healthcare professionals. Intelligent sensor systems that can monitor in real‐time vital biophysical signals and assist healthcare workers to remotely monitor and manage health represent a transformation technology to achieve better patient outcomes. The emergence and wide adoption of wearable sensors provide an unparalleled opportunity to integrate individual health data to tailor the best solution for the person.
Wearable devices usually refer to devices that can be worn as accessories, embedded in clothing, skin‐mountable, or implanted in one's body. Sensing elements are commonly integrated with a wireless data transmission system and energy storage device so that data can be collected and transmitted to smartphones or computers for processing, offering a way to continuously monitor an individual's vital signs.[ 17 ] More recently, researchers applied wireless energy transmission in the design of respiratory sensors for remote personal health monitoring.[ 18 , 19 , 20 ] The wireless transmission design greatly enhances the stability and robustness of the self‐powered sensors. However, it should be noted that the remote sensing distance can significantly affect the performance of wireless power transmission, and its performance is remarkably reduced upon increasing the sensing distance. Also, environmental conditions such as moisture in communication systems during connection to an electronic board can affect their operation and tolerance.[ 20 ]
There are some wearable sensors available in the market. For example, Masimo has developed a type of respiratory sensor, named MightSat Rx[ 21 ] that is designed to be attached to the fingertip. It can monitor the respiratory rate continuously while the data is transmitted to health professionals for analysis. On the other hand, Everon and Empatica have developed wearable multimodal temperature sensors that are worn on the wrist.[ 22 ] Also, the Max3010 series from Maxim Integrated is commonly used as pulse oximeter sensors,[ 23 , 24 , 25 ] which are usually made from rigid materials and are placed on the fingertip. Despite these advances, most of these sensors are made of rigid materials that not only are uncomfortable to wear but also lack conformal contact with our bodies’ curved surfaces and fine topology, making real‐time diagnosis difficult to achieve.
Since the outbreak of the pandemic, several research groups have discussed the potential application of the recently developed wearable sensors for detecting and monitoring the health status of Covid‐19 infected patients.[ 4 , 26 , 27 , 28 , 29 , 30 ] For instance, Seshadri et al.[ 28 ] examined the capabilities of commercial devices in measuring clinically relevant physiological metrics and discussed their potential applications in tracking the health status of Covid‐19 patients and front‐line workers. The importance of developing digital health platforms in managing the Covid‐19 outbreak was also discussed. Ding et al.[ 29 ] reviewed some commercially available wearable devices and telehealth technologies that may be suitable for evaluating the health status of Covid‐19 patients. Moreover, Channa et al.[ 30 ] discussed the use of wearable sensors as well as detection algorithms and chatbots for monitoring the viral response phase of Covid‐19. These recent advances have highlighted that wearable health sensors offer tremendous potential and opportunity for better monitoring and management of viral infections such as Covid‐19.
Herein, we present a critical review of the latest advances in wearable sensors for diagnosing and monitoring the symptoms of Covid‐19, with a particular focus on material design and engineering, sensor fabrication, and working mechanisms. In contrast to some recent reviews concentrating on stretchable respiration sensors,[ 18 ] this review thoroughly examines the current state‐of‐the‐art wearable sensors for measuring vital biophysical signals such as respiration behavior, body temperature, and blood oxygen level that have shown great potential for the early diagnosis of Covid‐19.[ 4 , 26 , 27 , 28 , 29 , 30 ] This paper is organized into two main sections. The first main section describes the recent advances of the three types of wearable sensors in detecting respiration, body temperature, and blood oxygen level. First, various kinds of respiratory sensors are discussed and categorized into four main groups based on the stimuli, including i) electromechanical sensors detecting the localized strain either on chest/abdomen or the airflow of the inhaled/exhaled breath, ii) humidity sensors detecting humidity changes in the breath, iii) temperature sensors that can sense the change in temperature of the inhaled/exhaled breath, and iv) sensors for detecting diaphragm displacement during breathing using ultrasonic waves. Second, the sensors that can be used for body temperature detection and monitoring are presented based on the different sensing materials, which are the main component of the sensing device. The last part of this section covers the recently developed wearable oxygen sensors that combine pulse oximetry with flexible materials. In the second main section, the present difficulties and prospects are addressed, regarding the challenges in selecting most suitable materials and design, being multi‐modal, being reliable, and the integration with data transmission to create intelligent wearable devices.
2. Wearable Sensors for Remote Health Monitoring
For the early diagnosis of Covid‐19 infections remotely, it is necessary to continuously monitor users’ health status.[ 31 , 32 ] Key requirements for wearable sensors include being comfortable to wear, low cost, and be able to be applied noninvasively on the body. Being durable, accurate, sensitive, and having quick responses is also critical.[ 33 , 34 ] Early efforts to design and manufacturing wearable sensors resorted to reducing the thickness of the sensors and associated components. While thin sensors based on silicon or metallic materials can be made to be flexible and of low stiffness, their stretchability remains poor. More recently, new classes of intrinsically stretchable sensing materials have been developed by creating conductive networks in highly stretchable polymers.[ 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 ] Furthermore, self‐powered and low‐powered sensors have recently been created based on piezoelectric or triboelectric materials.[ 48 , 49 ] Below we summarize the state‐of‐the‐art wearable sensors capable of measuring respiratory behavior, body temperature, and blood oxygen saturation to the comparable performance as existing point‐of‐care diagnostic equipment, with a focus on material design and fabrication methods.
2.1. Sensors for Measuring Respiratory Behavior
Some recent significant progress in the development of wearable sensors for breathing monitoring include sensors for breathing pattern detection, respiratory rate detection, as well as gas/chemical sensors for breath analysis. Profilling the body chemistry through volatile organic compounds (VOCs) in the breath offers new avenues in the diagnosis of numerous diseases, having the advantages of being noninvasive and inexpensive.[ 50 ] Wearable gas sensors provide an ideal platform, as a noninvasive means to analyze exhaled breath continuously.[ 20 ] This method can be used to determine the concentration of chemicals such as nitrogen dioxide (NO2), ammonia, and acetone in the breath as biomarkers of diseases like lung cancer, liver disease, myocardial infarction, and diabetes.[ 50 , 51 , 52 , 53 ] For instance, Su et al. designed an alveolus‐inspired membrane sensor for detecting NO2 that showed excellent sensitivity, good linearity, and superior selectivity toward NO2 down to concentrations as low as 50 ppm.[ 52 ] Another example is the porous nickel oxide–zinc oxide‐based gas sensors developed by Chen et al.[ 54 ] The ultraporous nanostructures demonstrate excellent room‐temperature sensitivity to detect acetone and ethanol gas (≈10 ppb), which are two common medical VOCs recognized as biomarkers for nonalcoholic fatty liver disease and diabetes. Therefore, it can be seen that these gas sensing devices can effectively monitor our health status by analyzing VOCs in exhaled breath. However, challenges remain as it is required that the sensors should be able to detect trace concentration of VOCs sensitively and selectively with quick response to variations in VOC concentrations and rapid recover to the original state after detection.
Since the emergence of Covid‐19, researchers started researching more efficient and convenient methods to detect the virus, e.g., via detecting the breath to eliminate the use of oral and nasal swabs. For example, Leung et al.[ 55 ] demonstrated the plausibility of direct detection of the Coronavirus from the droplets of the exhaled breath. Grassin‐Delyle et al.[ 56 ] studied the possibility of determination of the Coronavirus from patients’ exhaled breath. In this study, 303 samples were invasively collected from 40 adult patients (28 of them had confirmed Covid‐19) in ICUs under ventilation systems, via a heated transfer line connected directly to the end of the endotracheal tube. They discovered a specific volatile organic compounds breathprint in the exhaled breath of the Covid‐19 patients. However, no direct link has been established between this breathprint and the severity of the illness. Moreover, Giovannini et al.[ 57 ] proposed that, via converting the breath into liquid (called exhaled breath condensate or aerosol) and analyzing the condensate or aerosol, it is feasible to detect nonvolatile molecules like viruses, RNA, DNA, and microorganisms through regular PCR‐based techniques. However, samples need to be gathered over an extended period of time and the much lower concentration of viral RNA in the breath than that in nasopharyngeal swabs makes this method difficult for use in practice.
Therefore, the focus of this review is placed on wearable sensors capable of detecting other respiratory characteristics (primarily respiratory rate). Respiratory rate (RR) is the number of breathing cycles in 1 min, i.e., breath per minute (bpm). Respiratory frequency (f R, in Hz) is also often used as an indicator for the respiratory rate (Note RR = f R/60).[ 58 , 59 , 60 ] The normal RR for an adult human is 12–22 bpm.[ 61 ] The respiratory rate is very sensitive to a variety of physiological, psychological, and environmental stressors. Measuring respiratory rate is of great importance as it is related to the human health status,[ 58 , 62 , 63 ] from physical activity to mental status.[ 64 , 65 , 66 ] The respiratory rate provides critical information for patients suffering from asthma, sleep apnea, Covid‐19, postoperative respiratory instability, sport science, etc.[ 60 , 67 , 68 , 69 ] Manual breath counting by trained medical staff is one of the most commonly used methods. The medical staff counts the number of breaths by looking at the patients or by feeling the expansion and contraction of their chest. Despite not requiring any special instrument, the method is not considered reliable due to the bias and errors of the staff.[ 58 , 63 , 70 , 71 ] Besides, this method is not capable of continuous monitoring of respiratory rate. Electrical chest impedance, pneumotachograph, arterial blood sampling, diffusion capacity, and optoelectronic plethysmography are some other popular methods for respiratory measurement.[ 72 , 73 , 74 ] Although these methods can provide helpful characteristics about pulmonary function, they are uncomfortable, costly, heavy, and bulky.[ 75 ] They require additional equipment, i.e., a mouthpiece or facemask.[ 74 ] Moreover, the procedure is complicated and needs to be done by trained staff[ 73 ] and patients are forced to conduct a breathing maneuver.[ 71 ] Also, some of these devices can cause skin irritation[ 72 ] and there are some periodic chronic respiratory diseases like asthma that may not show any abnormal functionality during the respiratory test.[ 75 ] Hence, they are not suitable for continuous or long‐term monitoring, or to be used in daily life. Finding a new solution for monitoring respiratory behavior continuously is thus essential.
Wearable sensors for measuring respiratory rate can be categorized based on the detected stimulus. Exhalation of a breath causes an airflow, as well as higher humidity[ 64 , 76 , 77 ] and temperature[ 66 ] than inhaling breath around the nose and mouth. On the other hand, during breathing cycles, the abdomen, chest,[ 78 , 79 , 80 ] as well as diaphragm[ 59 , 81 ] expand and contract regularly. Also, filled lungs cause a different impedance than exhaled lungs. Therefore, respiratory behavior can be measured by various stimuli including humidity, airflow, temperature, sound, and localized skin deformation in the chest and abdomen. Materials that are sensitive to humidity, temperature, and mechanical deformation can be good candidates. For instance, materials in which the capacitance or resistance changes upon the change of humidity, temperature, or airflow can be a good candidate for making these sensors.[ 59 , 76 , 78 ] Microphones can be applied to detect breathing sounds.[ 68 ] Moreover, to analyze the respiratory behavior from expansion/contraction of the chest and abdomen, accelerometers, and pressure/strain sensors have demonstrated great potential.[ 78 , 79 , 80 , 82 ] However, these sensors may output some false readings due to body movements. Hence, sensors capable of detecting diaphragm movement through acoustic waves have been developed, which are independent of the external chest and abdomen expansions.[ 59 , 81 ] Besides, some research has focused on making respiratory sensors more comfortable to wear, e.g., more flexible and breathable. For this purpose, sensors based on conductive textiles have been developed.[ 67 ] Herein, wearable sensors for measuring respiratory behavior will be reviewed according to the measured stimuli, i.e., mechanical deformation, humidity, and temperature.
2.1.1. Electromechanical Sensors (Sense Localized Strain Associated with Respiration)
Electromechanical sensors can be attached to the chest or abdomen to test the breath‐induced expansion/contraction. Sensors are, in many cases, either integrated with an elastic belt that can be worn by patients or directly mounted onto the skin. Moreover, the respiratory rate can be measured by using pressure sensors attached to a face mask to test the exhalation/inhalation frequency.
To ensure the sensors can follow the nonflat contour of our body, significant progress has been made in the design and manufacturing of flexible sensors by utilizing elastic polymers as the supporting substrate, with focuses on improving the sensitivity, reproducibility, and response time of the sensors. Although respiration typically induces a localized strain of less than ≈5%,[ 83 ] human skin can undergo a stretch of 50% or more.[ 84 ] Therefore, sensors that are to be directly attached to the skin need to be stretchable with a maximum workable strain similar to skin's stretchability or higher. Such a high stretchability ensures that the sensors can be safely used without failure.[ 37 , 85 , 86 ] Sensors based on piezoresistive,[ 86 ] piezoelectric,[ 49 ] capacitive,[ 87 ] and triboelectric[ 88 ] effects have been demonstrated promises for this purpose. In contrast to rigid and nonconformable semiconductor‐based or silicon‐based sensors, these sensors employ flexible polymers coupled with various functional materials such as electrically conductive particles or piezoelectric nanomaterials or nanofibers.[ 89 ]
Piezoresistive Sensors
Piezoresistive sensors measure strain/stress based on their resistance changes induced by mechanical deformation. They represent one of the most important and versatile wearable sensors with wide frequency bandwidth and simple construction. Piezoresistive sensors are popular mostly due to the relatively simple read‐out systems and high sensitivity.[ 86 ] The main mechanisms of piezoresistive sensors include geometric effects, the disconnection between overlapped nanomaterials, tunneling effects, or microcracking in the conductive thin films/coating.[ 37 , 43 , 45 , 86 ] A piezoresistive sensor is typically made by combining a conductive network formed by one or multiple electrically conductive materials (such as carbon‐based nanomaterials[ 46 ] including graphene,[ 47 ] carbon black, carbon nanotubes, and carbon nanofibers[ 44 ]), as well as metal nanomaterials (such as copper, gold, and silver nanowires or nanoparticles[ 42 ]) with an elastic polymer (such as silicone‐based elastomers and rubbers). For instance, polymer nanocomposites with electrically conductive networks, such as percolation network and segregation network,[ 90 ] and hydrogels[ 41 ] have been utilized in the design of flexible and stretchable sensors. The sensing elements, i.e., conductive materials, can be integrated with flexible materials by direct mixing,[ 40 ] surface coating/deposition/printing,[ 37 ] filtration method, and micromolding method.[ 86 ] In addition, conductive sensing materials have been created through in situ processes on flexible substrates, e.g., via laser direct writing.[ 91 , 92 , 93 , 94 ] Piezoresistive sensors based on graphitic carbon structures[ 91 , 92 ] and silver or copper nanoparticles[ 93 , 94 ] have been developed by the laser direct writing method. By carefully selecting the laser power and scanning speed with a proper substrate, the metal nanomaterials can be reduced from the corresponding salt solutions while graphitic carbon could be generated by carbonization of the substrate. The one‐step laser direct writing has been widely recognized as a highly valuable processing technique suitable for the large‐scale fabrication of sensing elements.
Stretchability, electrical performance under mechanical strain, reliability under repeated loading cycles, linearity, hysteresis, response time, and recovery time are among critical parameters that contribute to the performance of stretchable strain sensors. When pressure and/or strain is applied to the sensor, microstructural changes in the conductive network cause the electrical resistance to change.[ 37 , 95 ] The sensitivity, k p, commonly called gauge factor, is defined as below[ 37 ]
| (1) |
where ε, R 0, ν, ρ0, ΔR, and Δρ denote the applied strain, initial resistance, Poisson ratio, initial resistivity, change in resistance and resistivity due to strain ε, respectively. The accuracy of piezoresistive sensors, or the smallest change in the strain that can be reliably detected, depends on both the sensitivity and the noise or uncertainties of the sensors under repeated deformation. Sensors of high strain sensitivity often exhibit a higher level of noise than sensors of lower sensitivity, as large physical changes in the conductive networks are often accompanied by high statistical variations. In practical applications, the detection limit is typically set at a signal‐to‐noise ratio greater than 6 dB, i.e., the magnitude of the signal is twice that noise or uncertainty. Using this approach, the smallest change in strain is related to the gauge factor and noise σ is
| (2) |
where the noise σ denotes the maximum variation of the sensor's resistance change, δ(ΔR/R 0), under cyclic stretch (ε).
Stretchability relies upon several factors, including the elasticity of the substrates or matrix, the type of nanomaterials, and the structure of the conductive networks.[ 37 ] One example is a wrinkled platinum (Pt) strain sensor used for real‐time respiratory monitoring, where the micro/nanowrinkles were to increase the sensor's stretchability. The sensor was laser machined into a single‐sided adhesive polymer film to make a mask. The mask was adhered to a thin layer of polystyrene film. A timed magnetron sputter deposition technique was used to deposit Pt thin film onto the masked polystyrene. The second layer of Au is subsequently sputter deposited onto the Pt thin film. Finally, the polystyrene film was removed from the mask to create the metal thin film sensor design. Figure 1a shows the production process of the sensor while the sensing element and the sensor assembly are shown in Figure 1b,c. The sensor mounted on the chest wall as shown in Figure 1d can measure strain as high as 185% (Figure 1e) with a high gauge factor of k p = 42, which is among the highest reported gauge factor of any metal thin film strain sensor. Figure 1e shows the relative resistance change as a function of strain, which demonstrates that the sensor made of thinner Pt has higher stretchability.[ 96 ] At small strains, all samples have similar behavior. Although, in a higher strain, such as 95%, the thickest sample with 50 nm thickness, showed the gauge factor of 27. By comparison, under the same elongation, the gauge factor for the 5 mm thickness sensor was only 9. The sensitivity of the thinnest sensor dramatically increased up to 42 at the strain of 185%. However, the strong nonlinearity of the sensors shown in Figure 1e presents a major challenge to the accurate determination of the strain.
Figure 1.
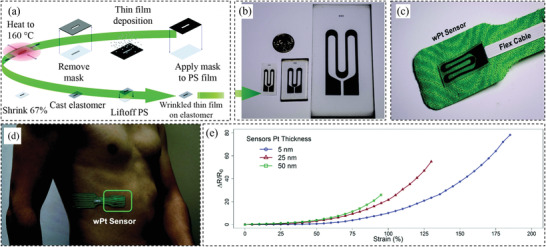
a) Illustration of the production process of the strain sensor. b) As‐fabricated strain sensing element. c) Strain sensor assembly for measuring respiratory behavior. d) Application method of the respiratory sensor. e) Relative resistance change versus strain for the samples consist of different Pt thicknesses. Reproduced with permission.[ 96 ] Copyright 2016, The Royal Society of Chemistry.
Capacitive Sensors
Capacitive sensors are another type of widely reported sensor that a relatively simple read‐out system just like piezoresistive sensors. The sensitivity or gauge factor of a capacitive sensor, k c, is defined in terms of the relative change in capacitance to strain as below[ 37 ]
| (3) |
where ε, C 0, and ΔC assign the applied strain, initial capacitance, and capacitance change, respectively. Parameters νe and νd denote the Poisson's ratios of the electrodes and the dielectric core, respectively. If the electrodes and dielectric layer have the same Poisson's ratio (νe = ν d), the strain sensitivity of a capacitive sensor is approximately equal to 1, independent of the size, thickness, and dielectric constant of the core. But these parameters affect the initial capacitance of the parallel‐plate capacitive sensor.[ 97 ]
Capacitive sensors have been developed to record torso movement to calculate respiration rate.[ 98 ] An example is the interdigitated capacitive sensor made of polydimethylsiloxane (PDMS) microchannels filled with liquid metal.[ 87 ] The use of liquid metal enables high flexibility and stretchability without breaking the electric connections. The sensor can be attached to the chest to detect its motion during a breath and hence detect respiration rate. Capacitive‐type strain sensors show a faster response time comparing to resistive type sensors. In addition, as compared to piezoresistive sensors, capacitive‐type sensors exhibit high linearity, low hysteresis, and little overshooting behavior. However, one limitation is the relatively low sensitivity, which is one of the most important drawbacks for capacitive‐type sensors (typically gauge factor ≤ 1). A recent work reported by Nur et al.[ 97 ] demonstrated surprisingly high sensitivity with the gauge factor being slightly above 3 (Figure 2a). The sensor was made of wrinkled ultrathin gold film electrodes as illustrated in Figure 2b,c. The wrinkled microstructure not only endowed the sensor with high stretchability but also increased the sensitivity. By structure engineering of the soft dielectric layer between the two wrinkled metal thin films, the structural out‐of‐plane deformation results in a larger dielectric thickness reduction and a minimized reduction of the capacitor's width, which leads to the increased sensitivity of the capacitive strain sensor. Another important disadvantage of capacitive type sensors is the strong cross‐sensitivity or interference by its capacitive interaction with the human body which limits direct epidermal applications.[ 86 , 96 ] The results shown in Figure 2a indicate that the capacitive sensor with higher sensitivity exhibits a nonlinear behavior, making the calibration more challenging.
Figure 2.
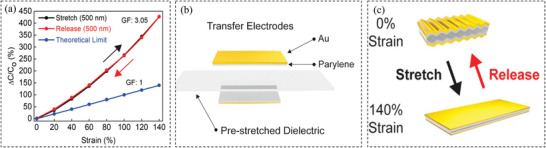
a) Relative capacitance change versus elongation during the stretch and the releasing. b) Strain sensor elements for respiratory monitoring fabricated with a wrinkled Au film. c) The state of the wrinkled film during loading and unloading. Reproduced with permission.[ 97 ] Copyright 2018, The American Chemical Society.
Fiber Optic Sensors
Recently, fiber optic sensors have gained great attention for the development of flexible sensors. Fiber optic sensors are typically polymer optical fibers and optical gratings packaged into polymeric matrices. Among them, fiber Bragg grating (FBG)‐based wearable systems have been developed for biomedical applications and integrated easily into textiles/polymers thanks to their small size, lightweight, flexibility, good dynamic response, and high sensitivity to detect small changes of the input signal. FBG embedded into an elastomeric substrate has been proposed as a wearable system for respiratory measurement from the upper body such as chest and abdomen movements.[ 79 , 99 , 100 ] The change in FBG output gives indirect information about the breathing pattern and respiratory rate. For instance, Presti et al.[ 79 ] demonstrated a wearable system based on FBG to monitor respiratory and cardiac activities. FBG was encapsulated into a Dragon skin20 silicone rubber. The proposed wearable system has a sensitivity of 0.125. The sensor can respond to environmental factors, such as temperature, relative humidity, and water immersion (to mimic the sweating condition). It was found that the effect of the humidity and water immersion was neglectable, while the temperature had a slight influence which needs further investigation.
Self‐Powered Sensors
All the sensors discussed above require a power source to operate. By contrast, self‐powered piezoelectric and triboelectric sensors have attracted considerable interests in the past decade and have demonstrated the potential of future wearable electronics. By utilizing the direct piezoelectric effect of piezoelectric materials, piezoelectric sensors can convert mechanical energy into electricity, which can be employed to detect expansion/contraction in the chest or abdomen associated with breathing cycles. Piezoelectric materials including piezoelectric polymers (e.g., polyvinylidene fluoride (PVDF)[ 101 ]) and ceramics (e.g., PZT,[ 102 ] BaTiO3,[ 103 ] ZnO,[ 104 ] etc.) are commonly used. The piezoelectric polymers are flexible and relatively more stretchable (e.g, PVDF can sustain up to ≈3% strain[ 105 ]) as compared to piezoelectric ceramics. Nevertheless, a much lower piezoelectric coefficient (20–30 pC N−1 for PVDF) is demonstrated, less than one‐tenth that of piezoelectric ceramics (e.g., PZT shows a coefficient of 250–700 pC N−1).[ 105 ] Therefore, a research focus is to develop piezoelectric sensors showing high piezoelectricity in combination with large mechanical compliance. Combining nonstretchy piezoelectric ceramics with stretchable elastic materials or introducing stretchable structures into piezoelectric ceramics are two commonly used solutions. For instance, Jin et al.[ 49 ] integrated silver nanoparticles into oriented PVDF nanofibers to design a respiratory sensor that bent in response toward the airflow. Figure 3a illustrates the fabrication process of the sensor, while Figure 3b shows the working mechanism, i.e., the airflow causes the deflection of the sensor, leading to the generation of electric output. The silver integrated sensor showed increased V p–p compared to the conventional randomly oriented PDVF nanofiber web as shown in Figure 3c. The sensor was tested for up to 3000 cycles to examine the fatigue performance and as indicated by Figure 3d, the sensor shows excellent durability. Figure 3e demonstrates that the sensor was capable of detecting different breathing patterns, e.g., coughing, slow and rapid breathing.
Figure 3.
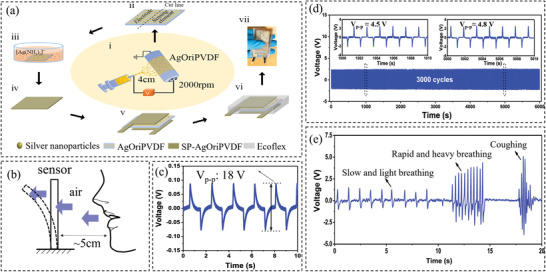
a) Fabrication process of the sensor. b) Illustration of the sensor mechanism. c) Sensor's bending response regarding the V p–p for silver integrated oriented nanofibers. d) The stability test result of the senor after repeated 3000 cycles. e) The sensor's performance in monitoring different breathing patterns. Reproduced with permission.[ 49 ] Copyright 2020, The American Chemical Society.
In addition to piezoelectric sensors, sensors based on triboelectric effect have recently emerged as another type of self‐powered sensors that do not need an external power supply. Triboelectric nanogenerator (TENG) is a new energy‐harvesting technology that can transform mechanical energy into electric energy based on triboelectricity and electrostatic induction. TENG can be employed as self‐powered strain or pressure sensors to measure respiration rate.[ 106 ] Wang et al.[ 88 ] fabricated a sensing system to detect respiratory rate based on a balloon in contact with TENG made of a composite film consisting of Ce‐doped ZnO‐PANI. Figure 4a illustrates the working mechanism of the new sensors: as the patient breathes, the airflow causes the balloon to expand or to shrink, pushing the upper film to approach or separate from the lower film, which results in contact–separation motion. This leads to the generation of electric signals that can be used to extract information about respiratory frequency. Figure 4b shows the response of the sensor to the fixed air volume in different frequencies, while Figure 4c shows the sensor's response to the different air volumes with the same frequency. It was shown that respiratory frequency has a minor effect on the output voltage, while the output voltage increased by increasing the respiratory volume. Moreover, as shown in Figure 4d, the sensor can distinguish different breath patterns including slow, rapid, and deep breaths.
Figure 4.
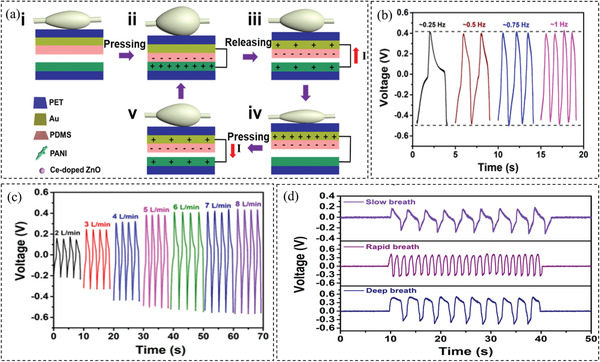
a) The mechanism of the triboelectric sensor. b) The response of the sensor to the fixed breath volume and different frequencies. c) The response of the sensor to the fixed frequency and different breath volumes. d) The performance of the sensor in monitoring different breathing patterns. Reproduced with permission.[ 88 ] Copyright 2019, Elsevier.
Intrinsic properties of the triboelectric materials are one of the most important factors contributing to the TENGs’ output. Among the different tribomaterials, PDMS is most used due to its high electronegativity, flexibility, transparency, and cost‐effectiveness. It can also be easily mixed with nanoparticles to form composite films. For instance, Chen et al.[ 90 ] reported a new way to enhance the performance of TENG by filling PDMS with high permittivity nanoparticles and creating pores. SiO2, TiO2, BaTiO3, and SrTiO3 nanoparticles with high dielectric permittivity were employed while the pore structures were created by using sacrificial NaCl templates. The experimental results show that both the surface charge density and the charge transfer quantity have a close relationship to the relative permittivity and porosity of the tribocomposites. The film consisting of 10% SrTiO3 nanoparticles and 15% pores in volume showed the best performance. Researchers have also demonstrated that the TENG performance is proportional to the charge density of the contact surface. Therefore, strategies such as increasing the contact area by introducing micro/nanoscale surface structures[ 107 ] and functionalizing the surface property by ion doping, electrical poling, or doping[ 108 ] have been demonstrated to be effective to improve the TENG performance.
2.1.2. Humidity Sensors (Sense Humidity Changes in Breath)
The humidity of the exhaled breath is usually higher than that of the inhaled. Therefore, researchers have demonstrated the use of humidity sensors to detect the respiratory rate by measuring the humidity of the breath.[ 64 , 70 , 74 , 76 , 77 , 109 , 111 ] For some materials, such as silica nanoparticles,[ 70 ] carbon‐based nanomaterials (carbon black, graphene, carbon nanotubes),[ 109 , 110 , 112 ] their electrical impedance/resistance change with humidity. When absorbing water molecules, the electronic structure of the sensing materials is changed, resulting in changes in electrical properties. The nanoscale materials with large surface areas, such as graphene, are promising candidates for sensing humidity. For instance, Pang et al.[ 112 ] reported a graphene humidity sensor, which is flexible and highly sensitive. The sensor consists of a porous graphene network coated with different solutions, such as graphene oxide (GO), poly(3,4‐ethylenedioxythiophene) polystyrene sulfonate (PEDOT:PSS), and silver (Ag) colloids on a polyethylene terephthalate substrate mounted on a mask as shown in Figure 5a. The results showed that the graphene network coated with Ag colloids has the best linearity and sensitivity compared to those coated with PEDOT:PSS or graphene oxide (Figure 5b). To improve the sensitivity, researchers have devoted efforts to improving the surface area or introducing functional groups that offer abundant sites for water molecules. GO or reduced graphene oxide (rGO), as compared to pristine graphene, has demonstrated ultrafast humidity sensing response, because of the abundant functional groups on the surfaces as well as porous structures that can provide more active sites for water molecules. Therefore, they can induce a simultaneous resistance and capacitance change of the graphene materials, i.e., comprehensive impedance change. GO or rGO can be composited with other active components to improve the sensitivity.[ 113 ] Borini et al.[ 114 ] fabricated a humidity sensor with an ultrafast response (30 ms) by initially spray‐coating a GO film on polyethylene naphthalate substrate and studied the speed as a function of the GO film thickness. Their results showed that by decreasing the thickness of the film to 15 nm, the response time of the sensor to a humid flow becomes ultrafast.
Figure 5.
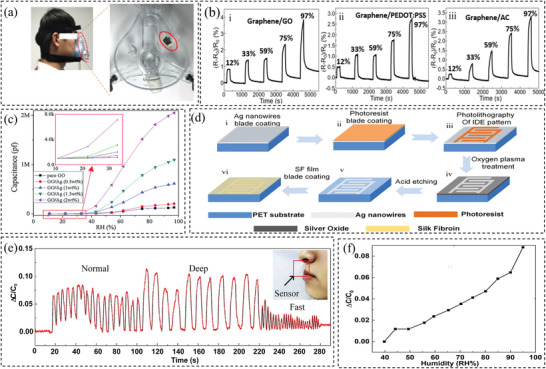
a) Image of the fabricated sensor on the mask and its application. b) Changes in the resistance response under different relative humidity (from 12% to 97%). Reproduced with permission.[ 112 ] Copyright 2018, Elsevier. c) Changes in the capacitance under different relative humidity for a set of samples. Reproduced with permission.[ 115 ] Copyright 2017, The Royal Society of Chemistry. d) The fabrication process of the humidity sensor. e) Sensor performance in detecting different breathing patterns. f) Sensitivity of the sensor (changes in capacitance under different relative humidity). Reproduced with permission.[ 76 ] Copyright 2020, Elsevier.
Humidity can also change the dielectric properties of some materials, which can be used to design capacitance‐based humidity sensors.[ 76 , 111 , 115 ] For example, Li et al.[ 115 ] developed capacitive‐type humidity sensors with ultrahigh sensitivity based on GO combined with Ag nanoparticles with various concentrations. As can be seen from Figure 5c, the sensitivity of GO/Ag (2 wt%) was 22 times higher than pure GO because the wrinkles of the GO/Ag composite increase the surface‐to‐volume ratio of the material and enhance the water absorption capability. Besides, Ag nanoparticles serve as absorption centers in the GO surface, so they can form bulk water layers and strengthen the ion conduction for humidity sensing. In another work done by Lou et al.,[ 76 ] a capacitive‐type humidity sensor containing a silk fibroin dielectric sensing film was proposed. The production process is shown in Figure 5d. This sensor can not only reach a fast response of 4 Hz which is reported to be the highest among the similarly reported sensors but also can demonstrate great mechanical stability in bending. The transmittance of the sensor was also shown to be more than 85%. The sensor has been mounted on a mask for sensing purposes and Figure 5e demonstrates the sensor's ability to detect breath of different patterns. Figure 5f shows the sensitivity of the capacitor sensor as a function of the relative humidity, showing relatively good linearity. In addition, Li et al.[ 111 ] made a capacitive‐type In2O3/GO hybrid sensor to measure breathing rate by measuring the change of air humidity under the nose. The resultant sensor demonstrated excellent performance in detecting human respiration. The sensor was accurate with a fast response and recovery time of 15 and 2.5 s, respectively.
The light intensity of the emitted light of optical fibers is also a function of humidity. Therefore, humidity sensors have been developed basing on optical fibers. For example, Presti et al.[ 74 ] developed a sensor by casting a Fiber Bragg grating into an agar substrate to measure humidity in the nasal exhaled breath for monitoring the respiratory rate. The sensor showed a reliable performance and a sensitivity to relative humidity change of 0.016 nm%−1.
2.1.3. Temperature Sensors (Sense Temperature Changes in Breath)
In addition to humidity and mechanical deformation, temperature changes are also potential stimuli for respiratory detection, due to the difference in the temperature of the exhaled and inhaled breath. For such sensors, temperature‐sensitive materials like a thermistor or pyroelectric materials are good candidates. The sensing materials should also have a faster response time compared to body‐mount temperature sensors. The temperature of the exhaled breath is near the core body temperature which is about 35 °C.[ 116 ] Chen et al.[ 66 ] developed a skin‐like circuit consisting of a stretchable and breathable temperature sensor (made of gold on patterned polyimide) and commercial chips for respiratory monitoring. The configuration and working mechanism in detecting the respiration behavior is illustrated in Figure 6a,b. Serpentine‐shaped conductive film cables were used to fabricate the stretchable circuit. The working principle was based on the temperature change of the inhaled and exhaled air by mounting on the nose (Figure 6b) The resistance increases upon increasing the temperature with a sensitivity of around 0.5 °C. The sensors have been successfully used to detect respiration, as indicated in Figure 6c. Roy et al.[ 117 ] developed a flexible bimodal sensor out of electrospun graphene oxide nanofibers and PVDF. The electrospun PVDF/GO nanofibers, as the sensing component, were sandwiched by metal electrodes and then encapsulated by PDMS. The fabrication process is illustrated in Figure 6d. The PVDF/GO nanofibers demonstrated pyroelectricity with a maximum output power density of 1.2 n W m−2 and can sense the changes in temperature during respiration (Figure 6e). Recently, conductive polymer nanocomposites with a conductive network capable of changes upon temperature variations have demonstrated great potential for temperature sensor applications. This enables the design of flexible and stretchable wearable temperature sensors and more discussion can be seen in Section 2.2. For the detection of respiration rate, the sensors are mostly attached to a mask, it is thus not required for the sensors to withstand high mechanical deformation.
Figure 6.
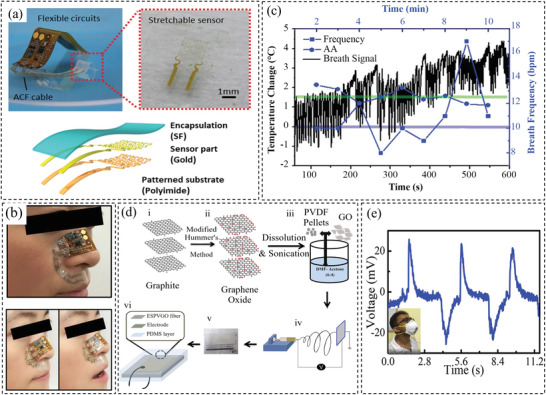
a) Up: the photo of the sensor and the sensing element. Bottom: the sensing element configuration. b) Photo and the illustration (up and bottom) of the sensing principle. c) Performance of the sensor in measuring respiratory behavior. Reproduced with permission.[ 66 ] Copyright 2020, Wiley‐VCH. d) Schematic illustration of the fabrication process of the graphene‐based sensor. e) Performance of the sensor attached to an N95 mask and open‐circuit voltage records of the breathing at room temperature. Reproduced with permission.[ 117 ] Copyright 2019, The American Chemical Society.
2.1.4. Ultrasound‐Based Sensors
Despite the excellent performance of the above‐mentioned sensors, the system is likely to be influenced by the movement of the body, which limits their applications during normal daily life. Therefore, Shahshahani et al.[ 59 , 81 ] introduced the use of ultrasound for human respiratory monitoring. The sensors consist of a PZT piezoelectric transducer and measure the respiration rate by acquiring ultrasound reflections from motions of the heart and surrounding organs during a breath. The sensors were placed either on the chest or zone of apposition. Reflected ultrasound signals were generated by the piezosensors at 1 MHz, which were used to measure the respiratory signals. An accuracy of 89% was reported.
2.2. Sensors for Measuring Body Temperature
Another important vital sign is body temperature which is a health indicator that can reflect some illnesses such as fever, inflammation, or heart stroke.[ 85 , 118 , 119 , 120 , 121 , 122 ] The normal range of body temperature for an adult is between 36.5 and 37.5 °C; lower or higher temperatures indicate hypothermia and hyperthermia, respectively.[ 14 ] Body temperature can be influenced by severe ambient temperature or personal physical activity. Also, it is related to the number of heartbeats. Therefore, higher body temperature causes faster heartbeats.[ 119 , 121 ] As a consequence of heat transfer between organs and the environment, body temperature can also reflect the metabolic rate of the body,[ 120 , 123 ] as well as patients’ recovery rate.[ 123 ] Hence, monitoring body temperature continuously is of great importance.
Thermometers are very widely used to measure body temperature and mercury thermometers are the most commonly used ones. Practices measure the temperature of the body by placing the mercury thermometer under the arm (axilla), in the rectum, or the mouth. One main problem is that such thermometers are not environmentally friendly. Also, the response time is long, e.g., it is suggested to wait a few minutes before reading for a mercury thermometer placed in a mouth.[ 124 , 125 ] Other thermometers used in clinical practices include electronic contact thermometers, infrared thermometers, and temporal artery thermometers. Electronic contact thermometers are much more expensive than mercury thermometers and they need to be in contact with the object for measurements. Hence, they have to be handled under specified protocols to avoid pathogen transmission.[ 125 , 126 ] By contrast, noncontact infrared thermometers are relatively inaccurate in fever mass screening due to the influences of environmental humidity and temperature.[ 127 , 128 , 129 ] Furthermore, thermometers will be used only when the patient already feels unwell.[ 95 ] Therefore, devices that can continuously monitor temperature are needed.
Wearable temperature sensors have been developed based on different materials whose properties are sensitive to temperature.[ 122 , 130 ] Different sensing mechanisms have been demonstrated including resistive, capacitive, optical fiber‐based, and pyroelectric sensors.[ 119 , 131 ] The resistive sensors are made of materials (usually named thermistors), whose electrical resistance varies with the temperature.[ 130 ] Capacitive temperature sensors are usually designed to have temperature‐sensitive materials as the dielectric layer. The relative permittivity of the dielectric layer is dependent on the temperature.[ 132 ] For optical fiber‐based sensors, the working principle is based on the temperature dependence of the light emission intensity of the optical fibers.[ 120 ] Additionally, semiconductors used in transistors or PN‐junction diodes are also sensitive to temperature.[ 133 , 134 ] Pyroelectric materials that have a polar point symmetry can also be applied in fabricating temperature sensors.[ 135 ] Among these sensors, resistive temperature sensors have gained considerable attention because of relatively simple read‐out systems and versatile material designs.[ 131 ] The sensitivity of these sensors is defined as the temperature coefficient of resistance (TCR) as follows[ 62 , 131 , 136 ]
| (4) |
where R(T) is the resistance of the material at the temperature T, while R(T 0) is the resistance of the material at the temperature (T 0).
Different materials have been investigated for making resistive‐type temperature sensors. One of these materials is hydrogels. Temperature can change the internal structure of hydrogels, which leads to a change in the electrical conductivity of the hydrogels.[ 85 , 137 , 138 ] For instance, Wu et al.[ 85 ] used a solvent‐replacement strategy to improve the thermal sensitivity of the hydrogels by introducing ethylene glycol/glycerol, increasing the sensitivity from 0.0295 to 0.131 °C−1. The percolation of hygroscopic ethylene glycol/glycerol in the solvent accounts for the improved sensitivity due to the synergic effect of ions transportation, the bond between water and polyol, and the elevated initial resistance. Another example is the flexible temperature sensors developed by Feng et al.[ 137 ] They used N‐isopropylacrylamide as a thermal‐sensitive component and integrated it into another conductive hydrogel with a dual network that was based on polyvinyl alcohol–graphene oxide and polyacrylic acid–Fe3+. Figure 7a shows the obtained triple‐network structure. The fabricated sensor showed excellent electrical (conductivity of ≈170 Ω mm−1) and mechanical (tolerance of 1.1 MPa) performance, as well as thermal‐sensitive performance for measuring body temperature. Figure 7b shows the sensitivity of the sensor to the temperature. There are two linear regions and by linear fitting, the sensitivity was determined to be 0.04294 and 0.0082 °C−1 in the temperature range of 30–35 and 35–50 °C, respectively. Another example would be the multifunctional temperature and strain sensor using self‐healing hydrogels developed by Ge et al.[ 138 ] The hydrogel‐based temperature sensor has high sensitivity (−0.016 °C−1) with a high gauge factor of 18.28 and a highly discernible temperature resolution of 2.7 °C. The thermal response time of the sensor was satisfactory. The reported sensor has the advantages of being self‐healing and highly stretchable. The accuracy of the temperature sensors depends on the sensitivity and the noise (σ)
| (5) |
where the noise σ denotes the maximum variation of the sensor's resistance change, δ(ΔR/R 0), within the temperature range of the sensor.[ 139 ]
Figure 7.
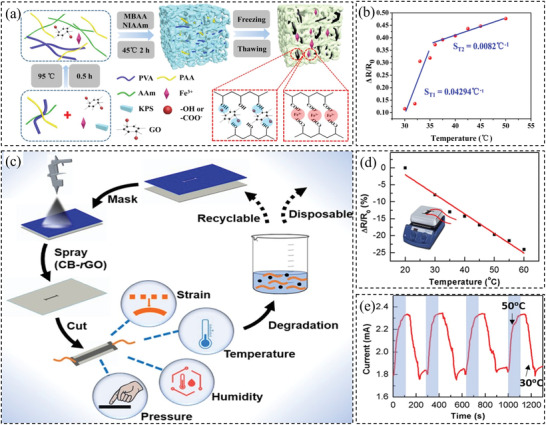
a) Schematic of conductive hydrogels preparation. b) Temperature sensitivity of the sensor. Reproduced with permission.[ 137 ] Copyright 2019, The American Chemical Society. c) Fabrication procedure of the carbon‐based sensor. d) Performance of the sensor in detecting temperature. e) Performance of the sensor in response to the temperature change. Reproduced with permission.[ 109 ] Copyright 2019, The American Chemical Society.
Nanocarbon‐based materials are also suitable candidates for temperature sensors.[ 109 , 130 , 136 , 140 , 141 , 142 ] For example, Pan et al.[ 130 ] embedded polyaniline/graphene in polyvinyl butyral thin film, which was able to detect both temperature and pressure stimuli. Although the sensor has not been tested on the human body, the resultant bimodal sensor material showed a wide detection range (25–80 °C) and good sensitivity of 0.012 °C−1. In another work by Wang et al.,[ 136 ] a flexible temperature sensor was fabricated using a wet‐spun nonwoven structure dip‐coated into the graphene nanoplates (GnPs) and sodium alginate dispersion. The high sensitivity of −0.015 °C−1, high accuracy of 0.1 °C−1, and fast response time of 26.3 s in the temperature range of 20–37 °C−1 were demonstrated by the yield flexible temperature sensor. Another example is the multimodal sensor developed by Liu et al.[ 109 ] and it is based on a flexible paper spray‐coated with carbon black and reduced graphene oxide as illustrated in Figure 7c. The sensors are capable of measuring temperature with a sensitivity of 0.006 °C−1 in the studied temperature range of 20–60 °C (Figure 7d). However, the response time was ≈ 100 s (Figure 7e), indicating it needs a relatively long time to reach a balance when the temperature was changed. Furthermore, a stretchable strain‐insensitive temperature sensor was introduced by Trung et al.[ 140 ] based on rGO/PU (flexible reduced graphene oxide fibers and polyurethane) composite. The temperature sensor can be sewn on the stretchable fabric and bandage using the stretchable nylon thread. The sensing resolution of the sensor is 0.1 °C. Apart from graphene, other carbon‐based materials such as carbon nanotube or carbon nanofibers can be employed to fabricate temperature sensors.[ 142 ] For example, Wang et al.[ 143 ] synthesized poly(octadecyl acrylate)‐grafted‐multiwall carbon nanotubes. Then it was drop‐casted between two Au electrodes that were thermally evaporated on a glass substrate. The fabricated sensor demonstrated an excellent large positive temperature coefficient of resistance values of 72.23 ± 36.77 °C−1 at 42.0 °C.
Other materials have also been used to fabricate such sensors, including silver nanocrystals,[ 131 ] silver nanowires,[ 144 ] and PEDOT:PSS.[ 145 ] For example, Bang et al.[ 131 ] spin‐coated silver nanocrystals onto the PDMS thin film to obtain a temperature sensor with TCR up to 4.84 × 10−1 ± 3.95 × 10−2 °C−1. Li et al.[ 144 ] combined AgNWs into the PDMS substrate to reduce the influence of humidity on the sensor performance. It is shown that the temperature sensitivity of the sensor reaches 16 Ω °C−1 at the temperature range of 30–80 °C. In addition, the present authors developed temperature sensors based on PEDOT:PSS/GO aerogels infiltrated with PDMS[ 146 ] and PEDOT:PSS thin film sandwiched by PDMS.[ 139 ] The sensor made of PEDOT:PSS/GO aerogel showed a sensitivity of −0.0169 °C−1 in the range of 30–50 °C. However, the temperature accuracy of the sensor was not examined. In more recent work,[ 139 ] we employed a novel method to increase the temperature sensitivity up to 0.042 °C−1 by creating microcracks via stretching the PEDOT:PSS thin film sandwiched by PDMS. Figure 8a demonstrates the procedure for fabricating this type of sensor. It is shown in Figure 8b that higher temperature sensitivity can be achieved by both larger length and higher density of the microcracks. The performance of the sensor was shown when worn while running in Figure 8c. The sensor showed an accuracy of less than 0.1 °C comparable to a commercial thermometer (Figure 8d).
Figure 8.
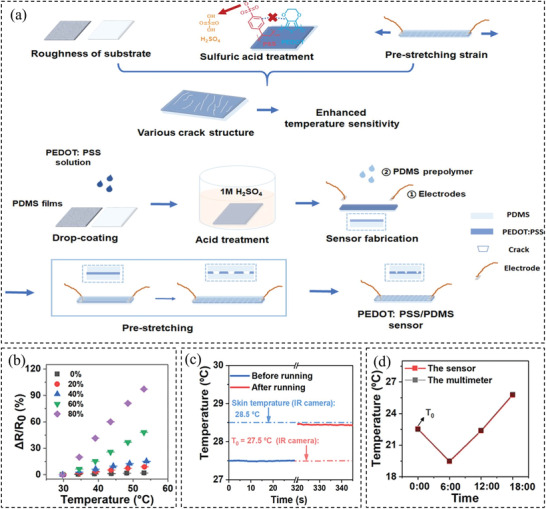
a) Illustration of the design and production procedure of the temperature sensor. b) The influence of the microcracks on the temperature sensitivity of the sensor. c) The performance of the sensor when it is worn before and after running. d) A comparison between temperature sensing of the sensor and a commercial thermometer to show the accuracy. Reproduced with permission.[ 139 ] Copyright 2020, The American Chemical Society.
Some materials show temperature‐dependent dielectric properties. These materials can be utilized for the design of capacitive sensors. For instance, a capacitive temperature sensor with a parallel plate capacitor design was made by Salmaz et al.[ 147 ] with a mean sensitivity of 156 fF °C−1, and a resolution of 0.08 °C. In this study, they have chosen PDMS as the dielectric material. The advantage of using PDMS is that it is a chemically inert, nonflammable, and stable material. Therefore, the resultant sensor is highly reliable and durable. Temperature can affect the density of the dielectric material, which leads to a change in both the contact area and thickness of the layer and hence the change of capacitance. It was shown that the sensor which was made of biocompatible material is suitable for biomedical applications. In another study,[ 148 ] a novel capacitive temperature sensor was made by microfluidic techniques. To produce this sensor, CNT/PDMS composite was used as a dielectric part and ionic liquids were used as electrodes with different concentrations of CNF. The schematic of the fabricated sensor is illustrated in Figure 9a. Figure 9b shows capacitance changes when subjected to temperature variations. It was found that the sensor with the highest concentration of CNF (6 wt%) demonstrates the highest temperature sensitivity of 1.517 pF °C−1. In addition, since the dielectric constant of polyethylene glycol has a strong relationship with temperature in the near body temperature range (34–42 °C), a new type of capacitive temperature sensor was developed by Lu et al.[ 149 ]
Figure 9.
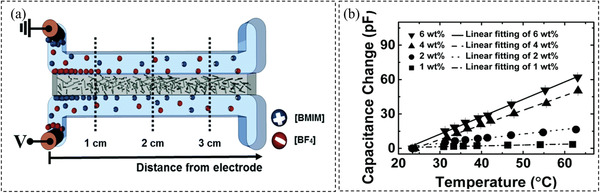
a) Illustration of the microfluidic capacitive sensor. b) Performance of the different temperature sensors with the variety of the CNF content. Reproduced with permission.[ 148 ] Copyright 2017, The Royal Society of Chemistry.
Apart from the materials discussed above, functionalized optical waveguides offer new opportunities to create wearable temperature sensors. Conventional inorganic (silicon or glass) optical fibers are highly stiff. Therefore, researchers have spent efforts on seeking other stretchable and soft alternatives. Elastic polymers such as hydrogels[ 150 , 151 ] or elastomers[ 120 , 152 ] have demonstrated great potentials in developing optical waveguides capable of large mechanical deformation. For example, Guo et al.[ 120 ] designed a stretchable temperature sensor based on PDMS‐based optical fibers incorporated with temperature‐sensitive upconversion nanoparticles. The PDMS optical fibers were made by injecting an elastomer into a thin tube and were designed with a core‐cladding structure (core material has a larger reflective index than the cladding) to achieve effective light confinements. The setup is illustrated in Figure 10a and the sensors with high flexibility are shown in Figure 10b,c. The sensor has demonstrated the ability to accurately detect temperatures in the range of 25 to 70 °C and excellent linearity and sensitivity (0.018 °C−1) with a detection limit of ± 0.3 °C. Particularly, its sensing performance was found to be insensitive to strain up to 80% (Figure 10d). The sensor has demonstrated great potential in detecting human body temperature (Figure 10e). P(VDF‐TrFE) can also be used to fabricate temperature sensors, as reported by Marchiori et al.[ 153 ] The P(VDF‐TrFE) was used as an optical material and sandwiched between two electrodes. To minimize the influence of mechanical deformation, the nonstretchable P(VDF‐TrFE) was placed on metallic interconnections with island design. Without poling, a sensitivity of ≈ 7 pF °C−1 was achieved up to a strain of 35%.
Figure 10.
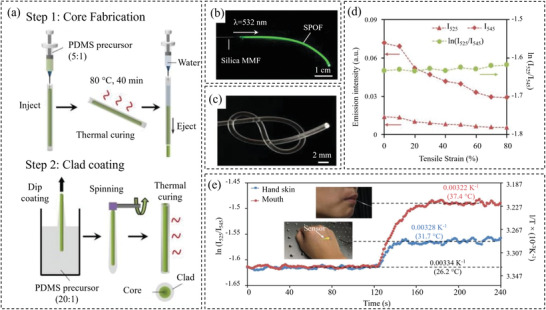
a) Fabrication process of the stretchable temperature sensor. b) Incorporation of the core–shell upconversion nanoparticles in the optical fiber. c) Flexibility of the optical fiber‐based sensor. d) Sensor's respond to different strains. e) Performance of the sensor in detecting body temperature. Reproduced with permission.[ 120 ] Copyright 2019, Wiley‐VCH.
2.3. Sensors for Measuring Blood Oxygen Level
It is well known that oxygen is key for human life, which produces energy in the body and helps to regenerate tissues[ 154 ] Shortage of oxygen for the body can lead to severe problems or even mortality.[ 155 , 156 ] Measuring the level of oxygen saturation within the blood, termed SpO2, is the way to measure whether the oxygen level is sufficient or not.[ 10 ] Low oxygen level is often observed in Covid‐19 patients, even when they are feeling well. The low oxygen level can be treated as an early warning sign and helps with timely prevention and treatment. Monitoring SpO2 is necessary not only for Covid‐19 patients but also for patients undergoing surgery or afflicted with pulmonary disorders. Oxygen is carried and circulated in the body via hemoglobin cells in the blood known as red blood cells.[ 157 ] SpO2 which indicates the efficiency of the patient breathing is defined as the ratio of the hemoglobin cells saturated with oxygen (N HbO) to the total hemoglobin cells (saturated (N HbO) and unsaturated (N Hb)), in percentages, as follows[ 10 , 157 , 158 , 159 , 160 ]
| (6) |
The most popular method for measuring blood oxygen level is a pulse oximeter which is a small clamp‐like device that can be mounted on a fingertip, toe, or earlobe. This is a noninvasive approach, based on measuring the intensity of a light beam.[ 95 ] Oxygen‐saturated hemoglobin cells have a unique shade of red color compare to cells without oxygen. According to this, oxygen saturation of the blood can be indirectly calculated by detecting absorbed light in oxygenated or deoxygenated blood.[ 161 , 162 ] However, this method is limited to only certain sensing locations on the body[ 23 , 163 ] and they need to be perfectly mounted to prevent noises during measurement. This is challenging to use especially when the patient is moving. Moreover, they are bulky and made from a rigid material. Therefore, they are not suitable for continuous monitoring and remote monitoring.[ 164 , 165 ] Other less common ways include the use of Laser Doppler velocimetry or Clark Electrode.[ 154 ] The Laser Doppler velocimetry can measure the blood flow rate for calculating oxygen saturation, which is not straightforward and requires trained staff to apply.[ 166 ] By contrast, the Clark Electrode method is an invasive approach that causes piercing pain for the patient.[ 167 ]
Therefore, researchers attempt to develop new wearable oxygen sensors by combining pulse oximetry with flexible materials.[ 23 , 82 , 154 , 161 , 168 ] For instance, Bae et al. fabricated a complex device consisting of µ‐LEDs, a photodetector, and a metal heater. The stretchable metal heater was made of AgNWs spin‐coated on a PDMS substrate and was used to expand the blood vessels. The µ‐LEDs were embedded into PDMS while a photodetector consisting of PEDOT:PSS spin‐coated on an ITO layer was employed to read the emitted photo from the µ‐LEDs. The device can successfully measure oxygen saturation, heart rate, respiratory rate, coughs, and sighs. Another example is a transcutaneous oximetry sensor developed by Lim et al.[ 154 ] The sensor consisted of µ‐LEDs placed into a PDMS layer as the light source and phosphorescent material (PtOEP) with TiO2 nanoparticles as well as polystyrene as the main component. ITO deposited on polyimide substrate was used as the heater component (Figure 11a). The heater increases the skin temperature to reduce its effect on sensitivity. This sensor is capable of measuring the oxygen level in the underskin tissue. The advantage is that it can be used in any part of the body. Other examples include sensors developed by Khan et al.,[ 161 ] which consisted of OLEDs and organic photodiodes printed on a plastic substrate as shown in Figure 11b and those created by Han et al.,[ 168 ] which are composed of two organic photodiodes printed on a planarized polyethylene naphthalate substrates. The sensors show comparable performance to a commercial oximeter. As can be seen from Figure 11c, the fabricated sensor is well capable of measuring SpO2.
Figure 11.
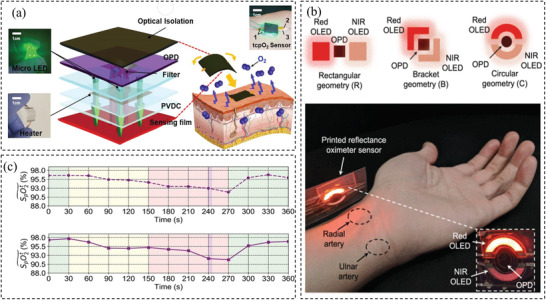
a) Configuration of the sensor. Reproduced with permission.[ 154 ] Copyright 2019, Elsevier. b) OLED geometrics with the same active areas and the application of the fabricated sensor on the wrist. Reproduced with permission.[ 161 ] Copyright 2019, IEEE Access. c) Bottom: performance of the sensor. Up: for measuring SpO2 compared to a commercial sensor. Reproduced with permission.[ 168 ] Copyright 2020, Wiley‐VCH.
3. Current Challenges and Future Opportunities
Intending to examine the newest development and emerging trends of wearable sensors for early and remote detection of Covid‐19 infections, we have summarized various wearable sensors created recently capable of detecting vital signs. For detecting respiration behaviors, four different groups of sensors have been identified based on the stimulus including respiratory sensors based on measuring localized mechanical deformation in chest/abdomen associated with breath, respiratory sensors based on detecting diaphragm motion, humidity changes, and temperature changes in the breath. Sensors for measuring body temperature have been discussed based on different working mechanisms including resistive, capacitive, optical fiber‐based, and pyroelectric sensors. By contrast, fewer reports can be found in the literature concerning sensors for measuring blood oxygen levels and most of the reported sensors are based on optical techniques. Despite these recent advances in the development of wearable sensors, their adoption for daily life health monitoring has been limited. Below we consider the future perspective of flexible wearable electronic devices and their continual challenges and the opportunities in healthcare monitoring.
Material selection: As can be seen from the previous sections, various materials have proven to be promising in developing wearable sensors. The material used for the sensors plays a crucial role in determining a sensor's performance. It is therefore of great importance to select suitable materials when designing a sensor. The material selection is largely dependent on the objective and sensing principles of the sensors. Diverse performances can be obtained when different materials are used while the same material may result in variations in performance if they are designed to have different microstructures. For instance, carbon nanomaterials such as carbon black, graphene, CNTs, and CNFs have been widely used as the sensing elements to develop various resistive or capacitive wearable sensors. For nanocomposite‐based piezoresistive sensors containing the 0D carbon blacks, 1D nanotubes/fibers, or 2D graphene, it has been shown that those with 0D nanoparticles generally demonstrated higher sensitivity but lower stretchability.[ 37 ] This is due to the low aspect ratio of the nanoparticles, making it easier for them to be disconnected from each other by the applied strain. When graphene is used to create sensors, it can be directly dispersed into a polymer matrix (e.g., PDMS) or organized into 3D interconnected networks (e.g., porous vertical graphene or graphene aerogel/foam) which are then backfilled with polymers (e.g. PDMS). It is found that the latter design resulted in much higher sensitivity,[ 47 ] demonstrating the significance of the structural design of the sensors.
Moreover, hybridization of two or more different materials has demonstrated great potential in enhancing sensor performance. For example, Zhang et al.[ 46 ] reported that incorporation of CNFs and graphene nanoplates hybrids in PDMS resulted in a greater linear range up to ≈50% of strain and much‐improved stability (less drift) under repeated loading compared with equivalent sensors containing only CNFs or GNPs. Li et al.[ 115 ] found that capacitive‐type humidity sensors based on GO/Ag hybrid performed much better than those made of pure GO with 22 times higher sensitivity.
Therefore, it is important to consider multiple objectives of the sensors when selecting the sensing materials, such as stretchability, sensitivity, durability, etc., to take advantage of their specific attributes in the design and fabrication process.
Multimodal sensing capability: The need for wearable sensor applications is clearly recognized and the interest is growing day by day, especially during the pandemic. Developing integrated sensors that can remotely measure multiple physiological parameters as indirect evidence of the infection will bring huge benefits to people in quarantine or treatment, especially for people away from major healthcare facilities. Significant efforts are being devoted to design and manufacture wearable sensors that can meet users’ different needs, from Covid‐19 to other respiratory diseases, and aged care. Although the use of sensors is becoming increasingly popular, users prefer not to use more than one wearable device simultaneously. Therefore, it is important to design multimodal sensors capable of measuring different physiological parameters. In this regard, significant efforts have been reported in the recent literature on the development of sensor arrays, e‐tongue, e‐nose, or e‐skin capable of sensing multiple stimuli.[ 169 , 170 , 171 , 172 ] For instance, Yu et al.[ 169 ] designed and fabricated a six‐unit sensing array system for multiflavor detection. The sensor array system was fabricated by laser direct writing on a polymer substrate, leading to a six‐unit sensing array that could perform multiple measurements simultaneously. Using the data from the different units, the system was able to respond to a range of different analytes in solution and predict analyte concentration ratios. Besides, the sensing system shows high sensitivity and selectivity for mixed elements. Another example is the wearable noninvasive bimodal glucose and strain sensors developed by Zhai et al.[ 173 ] Enokitake mushroom‐like gold with nanoparticles as the “head” and nanowires as the “tail” grown on elastomeric substrates was used as the stretchable electrodes to develop glucose sensors that capable of simultaneous strain detection. Despite these recent developments, the integration of multiple sensors capable of measuring multiple signals remains a major challenge due to the complex design and possible crosstalk between different sensors. Moreover, the data analysis is also challenging, by which we should distinguish the sensor response to a certain stimulus.
Reliable sensor performance: Despite many commercial wearable sensors available on the market and the tremendous work reported by researchers around the world, user acceptance and adoption remain challenging in addition to the lack of comfortable, affordable, and durable wearable sensors that give reliable sensing data. As sensors are expected to be used continuously over a reasonably long time, they need to be comfortable enough to be worn without interfering with daily life. Body movements and other types of noises should be avoidable for wearable sensors to provide an accurate measurement. Incorporating sensors with a lightweight elastic membrane‐like element makes it possible to fabricate a flexible sensor that interacts with the human body on curved surfaces to increase the comfort of the devices. Also, textile‐based sensors are becoming more attractive due to their comfortability. They must also be stylish with excellent aesthetic appeal.
Data transmission and analysis: Another important factor for wearable sensors needing improvement is data communication, processing, and storage (privacy). An ideal wearable sensor needs to be able to transmit data to a processor unit actively and passively. There are opportunities to integrate wearable sensors with smartphones and Internet of Things protocols. This can facilitate remote health monitoring for people in need, like Covid‐19 patients in self‐isolation, or the elderly in their home. Early detection of any abnormality in the vital signs can greatly improve patient outcomes. It is worth mentioning that the issue of data privacy, data sharing, and data reporting is one of the biggest challenges for the popular use of wearable sensors. The technology must ensure that only users can share their data and they should be anonymized.
Intelligent wearable sensing systems: With the increasing demand for wearable devices, intelligent systems capable of analyzing, transmitting, and deciding, supported by continuous and sustainable power supply are an emerging area of research. Although the concept of self‐powered sensors has recently been extensively studied, their practical applications are limited, partly because power is still needed for storing and transmitting data. Self‐powered sensors are now being coupled with other sensing interfaces to develop fully self‐powered sensing systems. One fruitful area of future research is in the integration strategy to develop intelligent self‐powered wearable devices with low power consumption and high energy storage capacity. Next generation of advanced intelligent wearable sensing systems will need to be able to communicate directly with smartphones or cloud computing platforms for better health management. Systematic and intelligent wearable sensing systems that are integrated with auxiliary components such as signal processing and display modules are essential to providing users with timely feedbacks.
Despite the aforementioned problems and challenges in the current commercial and clinical sensors, there is a great opportunity to address these problems and bring to market new sensors with better functionality and user acceptance by creating highly robust, accurate, and reliable sensors for the health sectors.
4. Conclusion
Newly developed and commercial wearable sensors have shown great potential for early detection of multiple symptoms indicating viral infections such as Covid‐19. Sensors sensitive to mechanical, chemical, and biological signals can be used for these purposes. In this paper, we have reviewed recent advances in wearable sensors that are capable of accurately detecting respiratory rate, body temperature, and blood oxygen level remotely, e.g., at home, or in hotel quarantine settings. For respiration detection, different types of new sensors featuring various working mechanisms have recently been developed. These sensors are able to measure respiratory rate via sensing either the humidity change or temperature change in the breath, or through measuring the airflow, ultrasound reflection, and localized skin deformation associated with respiration. These sensors are mostly designed to be flexible and stretchable so that they can be comfortably worn or directly attached to human skin. Compared to conventional methods for respiration measurement (such as manual breath counting by trained medical staff), wearable sensors provide much more reliable measurements.
Excellent progress has been made to advance wearable temperature sensors that offer continuous measurement of body temperature to the same precision as thermometers (mercury, contact, or noncontact electronic thermometers) commonly used for point‐of‐care testing. Soft, flexible temperature‐sensitive materials (such as hydrogels, elastomers composites) have been designed and used to create temperature sensors that can be attached to the skin for continuous body temperature measurement. A few newly developed sensors demonstrated an accuracy of ≈0.1 °C comparable to the commercial thermometers and can be worn without interfering too much with a person's daily activities.
Finally, wearable sensors have been developed that can more conveniently measure blood oxygen level by combining pulse oximetry with flexible materials such as PDMS to create sensors that can detect the oxygen level in the underskin tissue. The advantage is that the sensors can be used at any part of the body and capable of measuring oxygen level, similar to the common pulse oximeter.
In summary, a wide range of wearable sensors have demonstrated high potential for noninvasive and early diagnosis of numerous diseases such as Covid‐19. Further development of advanced intelligent wearable sensors represents a great opportunity to accelerate the transformation of connected health technology.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgements
The authors are thankful for the financial support received from the Australian Research Council (ARC) via Discovery Grant Program (DP190102790). S.P. would also like to acknowledge the financial support from ARC via Discovery Early Career Researcher Award (DE190100311).
Biographies
Sheyda Mirjalali is currently a Ph.D. student under the supervision of Dr. Shuying Wu at Macquarie University, Sydney, Australia. She obtained her B.Sc. and M.Sc. degrees in textile engineering from the Amirkabir University of Technology, Tehran, Iran. She worked on pressure garments in her Master's course. Currently, her main research interest concerns the design and fabrication of stretchable and flexible sensors.

Shuhua Peng obtained his Ph.D. in polymer science and engineering from Deakin University in 2013. He is currently an ARC DECRA Fellow at the University of New South Wales Sydney. His research interests are to understand and design advanced functional materials that possess unique micro‐/nanostructures and explore their extraordinary functions in technologies such as wearable electronics and sensors, energy harvesting and storage, soft robotics, and surface treatments.

Chun‐Hui Wang obtained his Ph.D. from the University of Sheffield in 1991. He was the head of Advanced Composites Technologies at the Defence Science and Technology Organisation between 1995 and 2009. After that, from 2009 until 2016, he became the Director of the Sir Lawrence Wackett Aerospace Research Centre at RMIT University. Currently, he is the Head of the School of Mechanical and Manufacturing Engineering, University of New South Wales Sydney. Regarding his research interests, he has been focusing on lightweight advanced functional composites design and manufacturing, new energy vehicles, structural health monitoring, structural batteries, and multifunctional polymer nanocomposites.

Shuying Wu received her Ph.D. in polymer science and engineering from Deakin University in 2013. After working as a Research Fellow for approximately 5 years at Royal Melbourne Institute of Technology (RMIT University), University of New South Wales Sydney, she has joined the School of Engineering, Macquarie University as a Senior Lecturer, since 2018. Her research interests involve multifunctional polymer nanocomposites, flexible and stretchable sensors, conductors and actuators, nanocarbon‐based materials, fiber‐reinforced polymer composites, and polymer composites/blends.

Mirjalali S., Peng S., Fang Z., Wang C.‐H., Wu S., Wearable Sensors for Remote Health Monitoring: Potential Applications for Early Diagnosis of Covid‐19. Adv. Mater. Technol. 2022, 7, 2100545. 10.1002/admt.202100545
References
- 1.NSW Health, NSW Health Pathology – diagnostic COVID‐19 testing, https://www.health.nsw.gov.au/Infectious/covid-19/Pages/pathology.aspx#:~:text=NSW%20Health%20Pathology%20continues%20to,health%20staff%20as%20a%20priority (accessed: November 2020).
- 2. Ajibade A., Younas H., Pullan M., Harky A., J. Cardiovasc. Surg. 2020, 35, 2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WorldoMeters, Coronavirus cases update (live), https://www.worldometers.info/coronavirus/ (accessed: April 2021).
- 4. Hyoyoung Jeong J. A. R., Xu S., Sci. Adv. 2020, 6, 4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., Xie C., Ma K., Shang K., Wang W., Clin. Infect. Dis. 2020, 71, 762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Du Y., Tu L., Zhu P., Mu M., Wang R., Yang P., Wang X., Hu C., Ping R., Hu P., Am. J. Respir. Crit. Care Med. 2020, 201, 1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bhatraju P. K., Ghassemieh B. J., Nichols M., Kim R., Jerome K. R., Nalla A. K., Greninger A. L., Pipavath S., Wurfel M. M., Evans L., N. Engl. J. Med. 2020, 382, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen L., Dubrawski A., Clermont G., Hravnak M., Pinsky M. R., AMIA Annu. Symp. Proc. 2015, 2015, 1841. [PMC free article] [PubMed] [Google Scholar]
- 9. Shenoy N., Luchtel R., Gulani P., BMC Med. 2020, 18, 260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ali M. M., Haxha S., Alam M. M., Nwibor C., Sakel M., Wirel. Pers. Commun. 2019, 111, 1. [Google Scholar]
- 11. Lathiya N., Ruqaya P. R., Rathore P., J. Liaquat Uni. Med. Health Sci. 2016, 15, 199. [Google Scholar]
- 12. Suresh Kumar V., Krishnamoorthi C., Sens. Actuators, A 2021, 321, 112582. [Google Scholar]
- 13. Park C., Lee B., IEEE Access. 2019, 7, 71072. [Google Scholar]
- 14. Wijaya N. H., Alvian A. G., Arfianto A. Z., Poetro J. E., Waseel F., J. Rob. Control 2019, 1, 11. [Google Scholar]
- 15. Crawford D. C., Hicks B., Thompson M. J., J. Med. Eng. Technol. 2006, 30, 199. [DOI] [PubMed] [Google Scholar]
- 16.NSW Health, TeleClinical Care – COVID‐19, https://www.seslhd.health.nsw.gov.au/tcc-covid (accessed: March 2021).
- 17. Aqueveque P., Gutierrez C., Rodríguez F. S., Pino E. J., Morales A. S., Wiechmann E. P., IEEE Trans. Ind. Appl. 2017, 53, 2628. [Google Scholar]
- 18. Dinh T., Nguyen T., Phan H.‐P., Nguyen N.‐T., Dao D. V., Bell J. J. B., Biosens. Bioelectron. 2020, 166, 112460. [DOI] [PubMed] [Google Scholar]
- 19. Su Y., Yang T., Zhao X., Cai Z., Chen G., Yao M., Chen K., Bick M., Wang J., Li S., Xie G., Tai H., Du X., Jiang Y., Chen J., Nano Energy 2020, 74, 104941. [Google Scholar]
- 20. Roudjane M., Messaddeq Y., Wireless Sensor Networks—Design, Deployment and Applications, IntechOpen, London 2020. [Google Scholar]
- 21. Yeotkar H. S., Gaikwad V., Int. Res. J. Eng. Technol. 2019, 6, 2458. [Google Scholar]
- 22. Tyler J., Choi S. W., Tewari M., Curr. Opin. Syst. Biol. 2020, 20, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Abdollahi S., Markvicka E. J., Majidi C., Feinberg A. W., Adv. Healthcare Mater. 2020, 9, 1901735. [DOI] [PubMed] [Google Scholar]
- 24. Jansi R., Amutha R., Radha S., in Telemedicine Technology (Eds: Jude H. D., Balas V. E.), Academic Press, San Diego, CA, USA: 2019, Ch. 8, p. 121. [Google Scholar]
- 25. Pedrana A., Comotti D., Locatelli P., Re V., Traversi G., presented at IEEE Int. Wksh. Met. Ind. IoT., Rome, Italy, June 2020. [Google Scholar]
- 26. Tanweer Alam S. Q., J. Inform. Univ. Pamulang. 2020, 5, 213. [Google Scholar]
- 27. Nasajpour M., Pouriyeh S., Parizi R. M., Dorodchi M., Valero M., Arabnia H. R., J. Healthc. Inform. Res. 2020, 4, 325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Seshadri D. R., Davies E. V., Harlow E. R., Hsu J. J., Knighton S. C., Walker T. A., Voos J. E., Drummond C. K., Front. Digit. Health 2020, 2, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ding X.‐R., Clifton D., Nan J., Lovell N. H., Bonato P., Chen W., Yu X., Xue Z., Xiang T., Long X., IEEE. Rev. Biomed. Eng. 2020, 14, 48. [DOI] [PubMed] [Google Scholar]
- 30. Channa A., Popescu N., presented at Int. Cong. Ultra Mod. Telecom. Ctrl. Sys. Wksh., October 2020.
- 31. Massaroni C., Di Tocco J., Presti D. L., Schena E., Bressi F., Bravi M., Miccinilli S., Sterzi S., Longo U. G., A. Berton, presented at IEEE Int. Symp. on Medical Measurments and Applications (MeMeA), Istanbul, Turkey, June 2019.
- 32. Lee W. S., Jeon S., Oh S. J., Nano Convergence 2019, 6, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shrestha K., Alsadoon A., Prasad P., Maag A., Hang P. D. T., Elchouemi A., presented at Int. Conf. on Knowledge and Systems Engineering (KSE), Da Nang, Vietnam, October 2019.
- 34. Gao Q., Zhang J., Xie Z., Omisore O., Zhang J., Wang L., Li H., J. Mater. Sci. 2019, 54, 5187. [Google Scholar]
- 35. Wu S., Peng S., Wang C. H. J. S., Physical A. A., Sens. Actuators, A 2018, 279, 90. [Google Scholar]
- 36. Azadi S., Peng S., Moshizi S. A., Asadnia M., Xu J., Park I., Wang C. H., Wu S., Adv. Mater. Technol. 2020, 5, 2000426. [Google Scholar]
- 37. Wu S., Peng S., Yu Y., Wang C.‐H., Adv. Mater. Technol. 2020, 5, 1900908. [Google Scholar]
- 38. Moshizi S. A., Azadi S., Belford A., Razmjou A., Wu S., Han Z., Asadnia M., Nanomicro. Lett. 2020, 12, 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hageman D. J., Wu S., Latimer D., Virdi K., Wang C. H., Knothe Tate M. L., Sens. Actuators, A 2019, 289, 100. [Google Scholar]
- 40. Zhang F., Wu S., Peng S., Wang C. H., Compos. Sci. Technol. 2018, 165, 131. [Google Scholar]
- 41. Pan K., Peng S., Chu Y., Liang K., Wang C. H., Wu S., Xu J., Int. J. Polym. Sci. 2020, 58, 3069. [Google Scholar]
- 42. Peng S., Wu S., Yu Y., Xia B., Lovell N. H., Wang C. H., ACS Appl. Mater. Interfaces 2020, 12, 22179. [DOI] [PubMed] [Google Scholar]
- 43. Peng S., Wu S., Yu Y., Blanloeuil P., Wang C. H., J. Mater. Chem. A 2020, 8, 20531. [Google Scholar]
- 44. Wu S., Zhang J., Ladani R. B., Ravindran A. R., Mouritz A. P., Kinloch A. J., Wang C. H., ACS Appl. Mater. Interfaces 2017, 9, 14207. [DOI] [PubMed] [Google Scholar]
- 45. Peng S., Blanloeuil P., Wu S., Wang C. H., Adv. Mater. Interfaces 2018, 5, 1800403. [Google Scholar]
- 46. Zhang F., Wu S., Peng S., Sha Z., Wang C. H., Compos. Sci. Technol. 2019, 172, 7. [Google Scholar]
- 47. Wu S., Peng S., Han Z., Zhu H., Wang C.‐H., ACS Appl. Mater. Interfaces 2018, 10, 36312. [DOI] [PubMed] [Google Scholar]
- 48. Ming D. K., Sangkaew S., Chanh H. Q., Nhat P. T., Yacoub S., Georgiou P., Holmes A. H., Int. J. Infect. Dis. 2020, 96, 648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jin L., Zheng Y., Liu Z., Li J., Zhai H., Chen Z., Li Y., ACS Appl. Mater. Interfaces 2019, 12, 1359. [DOI] [PubMed] [Google Scholar]
- 50. Zhou X., Xue Z., Chen X., Huang C., Bai W., Lu Z., Wang T., J. Mater. Chem. B 2020, 8, 3231. [DOI] [PubMed] [Google Scholar]
- 51. Wang S., Jiang Y., Tai H., Liu B., Duan Z., Yuan Z., Pan H., Xie G., Du X., Su Y. J. N. E., Nano Energy 2019, 63, 103829. [Google Scholar]
- 52. Su Y., Wang J., Wang B., Yang T., Yang B., Xie G., Zhou Y., Zhang S., Tai H., Cai Z., Chen G., Jiang Y., Chen L.‐Q., Chen J., ACS Nano 2020, 14, 6067. [DOI] [PubMed] [Google Scholar]
- 53. Kang K., Yang D., Park J., Kim S., Cho I., Yang H.‐H., Cho M., Mousavi S., Choi K. H., Park I., Sens. Actuators, B 2017, 250, 574. [Google Scholar]
- 54. Chen H., Bo R., Shrestha A., Xin B., Nasiri N., Zhou J., Di Bernardo I., Dodd A., Saunders M., Lipton‐Duffin J., White T., Tsuzuki T., Tricoli A., Adv. Opt. Mater. 2018, 6, 1800677. [Google Scholar]
- 55. Leung N. H. L., Chu D. K. W., Shiu E. Y. C., Chan K.‐H., McDevitt J. J., Hau B. J. P., Yen H.‐L., Li Y., Ip D. K. M., Peiris J. S. M., Seto W.‐H., Leung G. M., Milton D. K., Cowling B. J., Nat. Med. 2020, 26, 676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Grassin‐Delyle S., Roquencourt C., Moine P., Saffroy G., Carn S., Heming N., Fleuriet J., Salvator H., Naline E., Couderc L.‐J., Devillier P., Thévenot E. A., Annane D., EBioMedicine 2021, 63, 103154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Giovannini G., Haick H., Garoli D., ACS Sens. 2021, 6, 1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hughes S., Liu H., Zheng D., Front. Physiol. 2020, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Shahshahani A., Zilic Z., Bhadra S., IEEE Sens. J. 2019, 20, 6872. [Google Scholar]
- 60. Alam R., Peden D. B., Lach J. C., IEEE J. Biomed. Health Inform. 2020, 25, 1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Jun Z., Chun‐na L., Wen‐liang Z., Hong Z., Yong‐feng L., Xue‐feng H., J. Phys.: Conf. Ser. 2020, 1570, 012033. [Google Scholar]
- 62. Yang H., Xue T., Li F., Liu W., Song Y., Adv. Mater. Technol. 2019, 4, 1800574. [Google Scholar]
- 63. Singh G., Tee A., Trakoolwilaiwan T., Taha A., Olivo M., Intensive Care Med. Exp. 2020, 8, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Carotenuto G., Camerlingo C., Proceedings 2019, 42, 9. [Google Scholar]
- 65. Zaltieri M., Di Tocco J., Presti D. L., Massaroni C., Formica D., Schena E., D'Alesio G., Filosa M., D'Abbraccio J., Cesini I., presented at IEEE Int. Wksh. Met. Ind. IoT., Rome, Italy, June 2020.
- 66. Chen Y., Liu F., Lu B., Zhang Y., Feng X., Adv. Electron. Mater. 2020, 6, 2000145. [Google Scholar]
- 67. Massaroni C., Di Tocco J., Presti D. L., Longo U. G., Miccinilli S., Sterzi S., Formica D., Saccomandi P., Schena E., IEEE Sens. J. 2019, 19, 7718. [Google Scholar]
- 68. Yuasa Y., Suzuki K., Adv. Biomed. Eng. 2019, 8, 85. [Google Scholar]
- 69. Klum M., Urban M., Pielmus A.‐G., Orglmeister R., Curr. Dir. Biomed. Eng. 2020, 6, 233. [Google Scholar]
- 70. Kano S., Yamamoto A., Ishikawa A., Fujii M., presented at Conf. Proc. IEEE Eng. Med. Biol. Soc., Berlin, Germany, July 2019. [DOI] [PubMed]
- 71. Sharma P., Hui X., Zhou J., Conroy T. B., Kan E. C., NPJ Digit. Med. 2020, 3, 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Chen H., Bao S., Ma J., Wang P., Lu H., Oetomo S. B., Chen W., presented at Conf. Proc. IEEE Eng. Med. Biol. Soc., Berlin, Germany, July 2019.
- 73. Alam R., Peden D., Ghaemmaghami B., Lach J., presented at Conf. Proc. IEEE Eng. Med. Biol. Soc., Berlin, Germany, July 2019. [DOI] [PubMed]
- 74. Presti D. L., Massaroni C., Zaltieri M., Sabbadini R., Carnevale A., Di Tocco J., Longo U. G., Caponero M. A., D'Amato R., Schena E., IEEE Sens. J. 2020. [Google Scholar]
- 75. Chen A., Halton A. J., Rhoades R. D., Booth J. C., Shi X., Bu X., Wu N., Chae J., ACS Sens. 2019, 4, 944. [DOI] [PubMed] [Google Scholar]
- 76. Luo Y., Pei Y., Feng X., Zhang H., Lu B., Wang L., Mater. Lett. 2020, 260, 126945. [Google Scholar]
- 77. Guan Y., Le X., Xie J., presented at IEEE Int. Conf. on Micro Electro Mechanical Systems (MEMS), Seoul, Korea, January 2019.
- 78. Di Tocco J., Massaroni C., Raiano L., Formica D., Schena E., presented at IEEE Int. Wksh. Met. Ind. IoT., Rome, Italy, June 2020.
- 79. Presti D. L., Massaroni C., D'Abbraccio J., Massari L., Caponero M., Longo U. G., Formica D., Oddo C. M., Schena E., IEEE Sens. J. 2019, 19, 7391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Xin Y., Liu T., Xu Y., Zhu J., Lin T., Zhou X., Sens. Actuators, A 2019, 296, 357. [Google Scholar]
- 81. Shahshahani A., Bhadra S., Zilic Z., presented at IEEE Int. Symp. on Circuits and Systems (ISCAS), Florance, Italy, May 2018.
- 82. Bae S.‐H., Kim D., Chang S.‐Y., Hur J., Kim H., Lee J.‐W., Zhu B., Han T.‐H., Choi C., Huffaker D. L., ACS Sens. 2020, 5, 1582. [DOI] [PubMed] [Google Scholar]
- 83. Liu H., Guo S., Zheng K., Guo X., Kuramoto‐Ahuja T., Sato T., Onoda K., Maruyama H., J. Phys. Ther. Sci. 2017, 29, 1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Wu S., Ladani R. B., Zhang J., Ghorbani K., Zhang X., Mouritz A. P., Kinloch A. J., Wang C. H., ACS Appl. Mater. Interfaces 2016, 8, 24853. [DOI] [PubMed] [Google Scholar]
- 85. Wu J., Wu Z., Wei Y., Ding H., Huang W., Gui X., Shi W., Shen Y., Tao K., Xie X., ACS Appl. Mater. Interfaces 2020, 12, 19069. [DOI] [PubMed] [Google Scholar]
- 86. Amjadi M., Kyung K. U., Park I., Sitti M., Adv. Funct. Mater. 2016, 26, 1678. [Google Scholar]
- 87. Li Y., Nayak S., Luo Y., Liu Y., Salila Vijayalal Mohan H. K., Pan J., Liu Z., Heng C. H., Thean A. V.‐Y., Materials 2019, 12, 1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Wang S., Tai H., Liu B., Duan Z., Yuan Z., Pan H., Su Y., Xie G., Du X., Jiang Y. J. N. E., Nano Energy 2019, 58, 312. [Google Scholar]
- 89. Hageman D. J., Wu S., Kilbreath S., Rockson S. G., Wang C., Tate M. L. K., Trends Biotechnol. 2018, 36, 537. [DOI] [PubMed] [Google Scholar]
- 90. Chen J., Guo H., He X., Liu G., Xi Y., Shi H., Hu C., ACS Appl. Mater. Interfaces 2016, 8, 736. [DOI] [PubMed] [Google Scholar]
- 91. Rahimi R., Ochoa M., Yu W., Ziaie B., ACS Appl. Mater. Interfaces 2015, 7, 4463. [DOI] [PubMed] [Google Scholar]
- 92. Luo S., Hoang P. T., Liu T., Carbon 2016, 96, 522. [Google Scholar]
- 93. Bai S., Zhang S., Zhou W., Ma D., Ma Y., Joshi P., Hu A., Nanomicro. Lett. 2017, 9, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Zhou W., Yu Y., Bai S., Hu A., Opt. Laser Technol. 2021, 135, 106694. [Google Scholar]
- 95. Mukhopadhyay S. C., Wearable Electronics Sensors: For Safe and Healthy Living, Springer, Palmerston North, New Zealand: 2015. [Google Scholar]
- 96. Pegan J. D., Zhang J., Chu M., Nguyen T., Park S.‐J., Paul A., Kim J., Bachmand M., Khine M., Nanoscale Adv. 2016, 8, 17295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Nur R., Matsuhisa N., Jiang Z., Nayeem M. O. G., Yokota T., Someya T., Nano Lett. 2018, 18, 5610. [DOI] [PubMed] [Google Scholar]
- 98. Chu M., Nguyen T., Pandey V., Zhou Y., Pham H. N., Bar‐Yoseph R., Radom‐Aizik S., Jain R., Cooper D. M., Khine M., NPJ Digit. Med. 2019, 2, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Romano C., Presti D. L., Schena E., Massaroni C., Formica D., Caponero M. A., Oddo C. M., D'Abbraccio J., Massari L., Longo U. G., presented at IEEE Int. Symp. on Medical Measurments and Applications (MeMeA), Istanbul, Turkey, June 2019.
- 100. Kam W., Mohammed W. S., O'Keeffe S., Lewis E., presented at IEEE World Forum on Internet of Things (WF‐IoT), Limerick, Ireland, April 2019.
- 101. Gu H., Zhao Y., Wang M. L., Struct. Control Health Monit. 2005, 12, 329. [Google Scholar]
- 102. Hwang G.‐T., Annapureddy V., Han J. H., Joe D. J., Baek C., Park D. Y., Kim D. H., Park J. H., Jeong C. K., Park K.‐I., Choi J.‐J., Kim D. K., Ryu J., Lee K. J., Adv. Energy Mater. 2016, 6, 1600237. [Google Scholar]
- 103. Alluri N. R., Selvarajan S., Chandrasekhar A., Saravanakumar B., Jeong J. H., Kim S.‐J., Compos. Sci. Technol. 2017, 142, 65. [Google Scholar]
- 104. Han W., He H., Zhang L., Dong C., Zeng H., Dai Y., Xing L., Zhang Y., Xue X., ACS Appl. Mater. Interfaces 2017, 9, 29526. [DOI] [PubMed] [Google Scholar]
- 105. Zhou H., Zhang Y., Qiu Y., Wu H., Qin W., Liao Y., Yu Q., Cheng H., Biosens. Bioelectron. 2020, 168, 112569. [DOI] [PubMed] [Google Scholar]
- 106. Dong K., Peng X., An J., Wang A. C., Luo J., Sun B., Wang J., Wang Z. L., Nat. Commun. 2020, 11, 2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Lee K. Y., Chun J., Lee J. H., Kim K. N., Kang N. R., Kim J. Y., Kim M. H., Shin K. S., Gupta M. K., Baik J. M., Adv. Mater. 2014, 26, 5037. [DOI] [PubMed] [Google Scholar]
- 108. Wang S., Xie Y., Niu S., Lin L., Liu C., Zhou Y. S., Wang Z. L., Adv. Mater. 2014, 26, 6720. [DOI] [PubMed] [Google Scholar]
- 109. Liu H., Xiang H., Wang Y., Li Z., Qian L., Li P., Ma Y., Zhou H., Huang W., ACS Appl. Mater. Interfaces 2019, 11, 40613. [DOI] [PubMed] [Google Scholar]
- 110. Lou C., Hou K., Zhu W., Wang X., Yang X., Dong R., Chen H., Guo L., Liu X., Materials 2020, 13, 430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Li B., Tian Q., Su H., Wang X., Wang T., Zhang D., Sens. Actuators, B 2019, 299, 126973. [Google Scholar]
- 112. Pang Y., Jian J., Tu T., Yang Z., Ling J., Li Y., Wang X., Qiao Y., Tian H., Yang Y., Biosens. Bioelectron. 2018, 116, 123. [DOI] [PubMed] [Google Scholar]
- 113. Lv C., Hu C., Luo J., Liu S., Qiao Y., Zhang Z., Song J., Shi Y., Cai J., Watanabe A., Nanomaterials 2019, 9, 422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Borini S., White R., Wei D., Astley M., Haque S., Spigone E., Harris N., Kivioja J., Ryhänen T., ACS Nano 2013, 7, 11166. [DOI] [PubMed] [Google Scholar]
- 115. Li N., Chen X., Chen X., Ding X., Zhao X., RSC Adv. 2017, 7, 45988. [Google Scholar]
- 116. Popov T. A., Dunev S., Kralimarkova T. Z., Kraeva S., DuBuske L. M., Respir. Med. 2007, 101, 2044. [DOI] [PubMed] [Google Scholar]
- 117. Roy K., Ghosh S. K., Sultana A., Garain S., Xie M., Bowen C. R., Henkel K., Schmeiβer D., Mandal D., ACS Appl. Nano Mater. 2019, 2, 2013. [Google Scholar]
- 118. Saurabh K., Rao H., Amrutur B., Sundarrajan A., presented at Int. Conf. Biomed. Elec. Dev., Loire Valley, France, March 2014.
- 119. Jayathilaka W. A. D. M., Qi K., Qin Y., Chinnappan A., Serrano‐García W., Baskar C., Wang H., He J., Cui S., Thomas S. W., Adv. Mater. 2019, 31, 1805921. [DOI] [PubMed] [Google Scholar]
- 120. Guo J., Zhou B., Yang C., Dai Q., Kong L., Adv. Funct. Mater. 2019, 29, 1902898. [Google Scholar]
- 121. Wang L., Lou Z., Jiang K., Shen G., Adv. Intell. Syst. 2019, 1, 1900040. [Google Scholar]
- 122. Xu K., Lu Y., Takei K., Adv. Mater. Technol. 2019, 4, 1800628. [Google Scholar]
- 123. Huang H., Su S., Wu N., Wan H., Wan S., Bi H., Sun L., Front. Chem. 2019, 7, 399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Davie A., Amoore J., Nurs. Stand. 2010, 24, 42. [DOI] [PubMed] [Google Scholar]
- 125. Periasami V., Int. J. Contemp. Pediatr. 2017, 4, 1476. [Google Scholar]
- 126. John A. R., Alhmidi H., Cadnum J. L., Jencson A. L., Gestrich S., Donskey C. J., Am. J. Infect. Control 2018, 46, 708. [DOI] [PubMed] [Google Scholar]
- 127. Fletcher T., Whittam A., Simpson R., Machin G., J. Med. Eng. Technol. 2018, 42, 65. [DOI] [PubMed] [Google Scholar]
- 128. Sollai S., Dani C., Berti E., Fancelli C., Galli L., De Martino M., Chiappini E., BMJ Open 2016, 6, 008695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Khan S., Saultry M. B., Adams S., Kouzani A. Z., Decker M. K., Digby R., Bucknall A. D. P. T., Am. J. Infect. Control 2021, 49, 597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Pan J., Liu S., Zhang H., Lu J., Sensors 2019, 19, 4105. [Google Scholar]
- 131. Bang J., Lee W. S., Park B., Joh H., Woo H. K., Jeon S., Ahn J., Jeong C., Kim T. i., Oh S. J., Adv. Funct. Mater. 2019, 29, 1903047. [Google Scholar]
- 132. Sadasivuni K. K., Kafy A., Kim H.‐C., Ko H.‐U., Mun S., Kim J., Synth. Met. 2015, 206, 154. [Google Scholar]
- 133. Ren X., Pei K., Peng B., Zhang Z., Wang Z., Wang X., Chan P. K., Adv. Mater. 2016, 28, 4832. [DOI] [PubMed] [Google Scholar]
- 134. Kimura M., Toshima K., Sens. Actuators, A 2003, 108, 239. [Google Scholar]
- 135. Whatmore R., Rep. Prog. Phys. 1986, 49, 1335. [Google Scholar]
- 136. Wang F., Jiang J., Sun F., Sun L., Wang T., Liu Y., Li M., Cellulose 2020, 27, 2369. [Google Scholar]
- 137. Feng S., Li Q., Wang S., Wang B., Hou Y., Zhang T., ACS Appl. Mater. Interfaces 2019, 11, 21049. [DOI] [PubMed] [Google Scholar]
- 138. Ge G., Lu Y., Qu X., Zhao W., Ren Y., Wang W., Wang Q., Huang W., Dong X., ACS Nano 2019, 14, 218. [DOI] [PubMed] [Google Scholar]
- 139. Yu Y., Peng S., Blanloeuil P., Wu S., Wang C. H., ACS Appl. Mater. Interfaces 2020, 12, 36578. [DOI] [PubMed] [Google Scholar]
- 140. Trung T. Q., Dang T. M. L., Ramasundaram S., Toi P. T., Park S. Y., Lee N.‐E., ACS Appl. Mater. Interfaces 2018, 11, 2317. [DOI] [PubMed] [Google Scholar]
- 141. Vuorinen T., Niittynen J., Kankkunen T., Kraft T. M., Mäntysalo M., Sci. Rep. 2016, 6, 35289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Wang C., Xia K., Zhang M., Jian M., Zhang Y., ACS Appl. Mater. Interfaces 2017, 9, 39484. [DOI] [PubMed] [Google Scholar]
- 143. Wang A. J., Maharjan S., Liao K.‐S., McElhenny B. P., Wright K. D., Dillon E. P., Neupane R., Zhu Z., Chen S., Barron A. R., ACS Appl. Nano Mater. 2020, 3, 2288. [Google Scholar]
- 144. Li S., Liu D., Tian N., Liang Y., Gao C., Wang S., Zhang Y., Mater. Today Commun. 2019, 20, 100546. [Google Scholar]
- 145. Zhang Y., Cui Y., IEEE Trans. Electron Devices 2019, 66, 3129. [Google Scholar]
- 146. Zhang F., Hu H., Islam M., Peng S., Wu S., Lim S., Zhou Y., Wang C.‐H., Compos. Sci. Technol. 2020, 187, 107959. [Google Scholar]
- 147. Salmaz U., Islam T., Sohail S., IEEE Trans. Instrum. Meas. 2020, 69, 7887. [Google Scholar]
- 148. Yoon S. G., Chang S. T., J. Mater. Chem. C 2017, 5, 1910. [Google Scholar]
- 149. Lu D., Yan Y., Avila R., Kandela I., Stepien I., Seo M. H., Bai W., Yang Q., Li C., Haney C. R., Adv. Healthcare Mater. 2020, 9, 2000942. [DOI] [PubMed] [Google Scholar]
- 150. Yetisen A. K., Jiang N., Fallahi A., Montelongo Y., Ruiz‐Esparza G. U., Tamayol A., Zhang Y. S., Mahmood I., Yang S. A., Kim K. S., Adv. Mater. 2017, 29, 1606380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Guo J., Liu X., Jiang N., Yetisen A. K., Yuk H., Yang C., Khademhosseini A., Zhao X., Yun S. H., Adv. Mater. 2016, 28, 10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Leber A., Cholst B., Sandt J., Vogel N., Kolle M., Adv. Funct. Mater. 2019, 29, 1802629. [Google Scholar]
- 153. Marchiori B., Regal S., Arango Y., Delattre R., Blayac S., Ramuz M., Sensors 2020, 20, 623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Lim C.‐J., Park J.‐W., Sens. Actuators, A 2019, 298, 111607. [Google Scholar]
- 155. Datcu M., Luca C., Corciova C., presented at Conf. on Advanced Medical Healthcare Techology, Cluj‐Napoca, Romania, October 2018.
- 156. Cugmas B., Plavšić A., Štruc E., Spīgulis J., Photoplethysmography for Bovine Heat Detection: The Preliminary Results, SPIE, San Francisco, CA: 2020. [Google Scholar]
- 157. Sasidharan P., Rajalakshmi T., Snekhalatha U., presented at Int. Conf. on Communications: Signal Processing (ICCSP), India, April 2019.
- 158. Preejith S., Ravindran A. S., Hajare R., Joseph J., Sivaprakasam M., presented at Int. Conf. of the IEEE Engneering in Medicine and Biology Society (EMBC), Florida, USA, August 2016. [DOI] [PubMed]
- 159. Evangeline C. S., Lenin A., Sens. Rev. 2019, 39, 364. [Google Scholar]
- 160. Kavassalis F. I., Chieng B., Baudino F. A., Flexible Wearable Sensor, Worcester Polytechnic Institute, Worcester, MA, USA: 2020. [Google Scholar]
- 161. Khan Y., Han D., Ting J., Ahmed M., Nagisetty R., Arias A. C., IEEE Access 2019, 7, 128114. [Google Scholar]
- 162. Lopez S., Americas R., Pulse Oximeter Fundamentals and Design, Austin, Texas, USA: 2012. [Google Scholar]
- 163. Ribeiro M. M. M. S. Á., M.Sc., University of Porto (Universidade do Porto), Portugal: 2020. [Google Scholar]
- 164. Yang Y., Gao W., Chem. Soc. Rev. 2019, 48, 1465. [DOI] [PubMed] [Google Scholar]
- 165. Delia D. A. (APN INTELECTUAL PROPERTY LLC) US20160310085A1, 2019.
- 166. Yuen J. C., Feng Z., Plast. Reconstr. Surg. 2000, 105, 55. [DOI] [PubMed] [Google Scholar]
- 167. Strovas T. J., McQuaide S. C., Anderson J. B., Nandakumar V., Kalyuzhnaya M. G., Burgess L. W., Holl M. R., Meldrum D. R., Lidstrom M. E., Adv. Biosci. Biotechnol. 2010, 5, 398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Han D., Khan Y., Ting J., Zhu J., Combe C., Wadsworth A., McCulloch I., Arias A. C., Adv. Mater. Technol. 2020, 5, 1901122. [Google Scholar]
- 169. Yu Y., Joshi P. C., Wu J., Hu A., ACS Appl. Mater. Interfaces 2018, 10, 34005. [DOI] [PubMed] [Google Scholar]
- 170. Tian X., Wang J., Ma Z., Li M., Wei Z., J. Food Qual. 2019, 2019, 4342509. [Google Scholar]
- 171. Tran T.‐T., Park H., To D., Gangadhara K., Brady J., Hart J., Jung S., Myung N. V., ECS Meet. Abstr. 2020, MA2020–02, 1471. [Google Scholar]
- 172. Jeon S., Lim S. C., Trung T. Q., Jung M., Lee N. E., Proc. IEEE 2019, 107, 2065. [Google Scholar]
- 173. Zhai Q., Gong S., Wang Y., Lyu Q., Liu Y., Ling Y., Wang J., Simon G. P., Cheng W., ACS Appl. Mater. Interfaces 2019, 11, 9724. [DOI] [PubMed] [Google Scholar]


