Abstract
Purpose
Coronavirus disease‐2019 (COVID‐19) may predispose to venous thromboembolism (VTE) and arterial thromboembolism because of excessive inflammation, hypoxia, immobilisation and diffuse intravascular coagulation. The understanding of the association might be helpful in early vigilant monitoring and better management of COVID‐19 patients at high risk. Thus, in this meta‐analysis, we aim to assess the association of VTE with the severity of COVID‐19 disease.
Methods
A literature search was conducted on PubMed and Cochrane Central Register of Controlled Trials using the keywords “COVID‐19 and thromboembolism” and “COVID‐19 and embolism,” till 20 February 2021. Thirteen studies including 6648 COVID‐19 patients were incorporated in this systematic review and exploratory meta‐analysis.
Results
The analysis revealed nearly three times more risk than intensive care unit (ICU) care in patients with VTE compared to non‐VTE patients (RR: 2.78; 95% CI: 1.75‐4.39; P < .001; I 2: 65.1%). Patients with pulmonary embolism and deep vein thrombosis are at increased risk of being admitted to ICU (RR: 2.21; 95% CI: 1.86‐2.61; P < .001; I 2: 41.2%) and (RR: 2.69; 95% CI: 2.37‐3.06; P < .001; I 2: 0.0%), respectively. The quality assessment indicated that the included studies were of fair quality.
Conclusions
Our findings suggest that VTE either deep vein thrombosis or pulmonary embolism may have a negative effect on the health status of COVID‐19 patients. This study highlights the need to consider measures for reducing thromboembolism risk amongst COVID‐19 patients.
Review criteria
We conducted a systematic review and meta‐analysis of the studies describing the incidence of venous thromboembolism in COVID‐19 patients requiring intensive care unit care.
We searched PUBMED and Cochrane Central Register of Controlled Trials using the keywords “COVID‐19 and thromboembolism” and “COVID‐19 and embolism” till 20 February 2021.
We pooled dichotomous outcomes as risk ratios and continuous outcomes as mean differences with 95% confidence intervals, both under the random or fixed effects model.
Message for the clinic
This study highlights the need to consider measures for reducing thromboembolism risk amongst COVID‐19 patients.
Precise knowledge of the incidence of thrombotic complications in COVID‐19 patients is important for decision‐making with regards to the intensity of thromboprophylaxis.
The understanding of the association might be helpful in early vigilant monitoring and better management of COVID‐19 patients at high risk.
1. INTRODUCTION
Coronavirus disease‐2019 (COVID‐19), a viral illness caused by severe acute respiratory syndrome‐coronavirus‐2 (SARS‐CoV‐2), can lead to coagulation dysfunction in patients with severe COVID‐19 infection. 1 Venous thromboembolism (VTE) is a recurrent complication in COVID‐19 patients 2 and results in a poor prognosis. 1 COVID‐19 may predispose to both venous and arterial thromboembolic disease because of excessive inflammation, hypoxia, immobilisation and diffuse intravascular coagulation. 3 , 4 , 5 , 6 Many of the hospitalised COVID‐19 patients are elderly people, suffering from severe infectious illness and are immobile in an intensive care unit (ICU) setting. Therefore, a relatively high incidence of VTE amongst patients diagnosed with COVID‐19 is expected because of the severity of their disease and distinctive risk factors. 7
However, so far, there have been only a few studies describing VTE either deep vein thrombosis or pulmonary embolism in patients with COVID‐19 infection. 8 , 9 , 10 , 11 A high incidence of VTE in COVID‐19 patients has been reported in various studies. 1 , 12 , 13 , 14 A retrospective study reported a high prevalence of deep vein thrombosis and associated adverse outcomes in COVID‐19 patients. 15 Another prospective study demonstrated a very high incidence of deep vein thrombosis in COVID‐19 patients requiring ICU admission. 16 A cohort study observed a high risk for VTE in COVID‐19 patients requiring ICU care. 14
Several studies have demonstrated a higher incidence of VTE in COVID‐19 patients, 1 , 14 , 17 , 18 however, the effect of the incidence of VTE on the prognosis of the disease needs further exploration. Precise knowledge of the incidence of thrombotic complications in COVID‐19 patients is important for decision‐making with regards to the intensity of thromboprophylaxis. 13 The understanding of the association might be helpful for clinicians in early vigilant monitoring and better management of COVID‐19 patients at high risk. Thus, in the present exploratory meta‐analysis, we aimed to assess the association of VTE with the severity of disease in COVID‐19 patients.
2. MATERIALS AND METHODS
Preferred Reporting Items for Systematic Review and Meta‐Analysis (PRISMA) guidelines extension for scoping reviews 19 was followed for designing and reporting this systematic literature review.
2.1. Data sources and searches
We searched PubMed and Cochrane Central Register of Controlled Trials until 20 February 2021 using the keywords “COVID‐19 and thromboembolism” and “COVID‐19 and embolism.” Grey literature was searched on Clinical Trial Registry of India, Clinicaltrials.gov, Google Scholar and reference list of eligible articles.
2.2. Inclusion and exclusion
Studies describing the incidence of thromboembolism according to disease severity were included. We excluded duplicate publications, reviews, editorials, case reports, letters, meta‐analyses, protocols, studies in a language other than English and studies not reporting the required data. The first author (RS) searched data and screened articles for eligibility. Senior author (RP) double checked all the included articles and any dispute was resolved by consensus.
2.3. Quality assessment
Two reviewers (RS and RP) assessed the quality of data included in this study using the National Institute of Health (NIH) quality assessment tools developed by the National Heart, Lung, and Blood Institute. 20 We preferred the NIH tool because it is comprehensive and widely accepted for an exhaustive assessment of data quality. We rated the general quality of included studies nearly as good, fair and poor and incorporated them within the result of the meta‐analysis.
2.4. Data extraction
Data were inputted into a standardised data extraction table (Excel) and independently checked by a second reviewer (RP) for accuracy. The following variables were extracted: name of the first author, year of publication, study design, age, gender, number of patients in ICU and non‐ICU care with comorbidities as well as prognosis. The disease was considered severe if the patient with COVID‐19 required ICU care.
2.5. Data synthesis
We performed an exploratory meta‐analysis to understand the magnitude and direction of the effect estimate. Relative risk (RR) was calculated and presented with respective 95% confidence intervals (CIs). Mantel‐Haenszel random effects meta‐analysis using DerSimonian and Laird method was used to pool RR. 21 Heterogeneity between studies was assessed using the χ 2‐based Cochran’s Q statistic (P < .1 considered as the presence of heterogeneity) and I 2 statistics (>50% representing moderate heterogeneity). 21 Forest plot was produced, and subgroup analysis was conducted according to the study design. The 95% prediction interval (PI) was calculated, which estimates the uncertainty bounds for a new study evaluating that the same association by considering between‐study heterogeneity. Publication bias was not assessed as a total number of studies were <10 for a given outcome. 21 P value < .05 was set as statistical significance for comparing study level effects.
3. RESULTS
3.1. Search results
The systematic search yielded a total of 1607 publications. Out of 1607 studies, 920 studies were found using the keywords “COVID‐19 and thromboembolism,” and 686 studies with keywords “COVID‐19 and embolism.” One study was found from another source. After removing duplicates, 1200 articles were found to be potential publications for screening. After the application of predefined inclusion and exclusion criteria, a total of 13 studies were included for the meta‐analysis (Figure 1).
FIGURE 1.
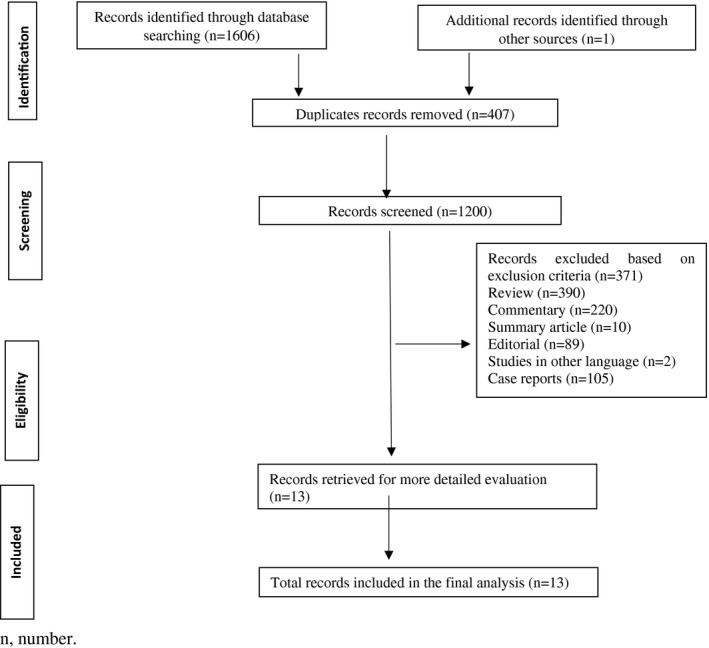
PRISMA flow diagram of study selection
3.2. Study characteristics
The incidence of VTE either deep vein thrombosis or pulmonary embolism was reported in ICU care and non‐ICU care in 12 studies, 12 , 14 , 15 , 16 , 17 , 22 , 23 , 24 , 25 , 26 , 27 , 28 and one study reported the incidence of VTE either deep vein thrombosis or pulmonary embolism in survivors and non‐survivors group. 1 Among the 13 included studies, a total of 6648 patients were enrolled, including 3973 males and 2675 females. The baseline characteristics of the subjects included in these studies are provided in Table 1.
TABLE 1.
Baseline characteristics
| Author, year | Study design | Age | No. of patients | Sex N (%) | Prognosis | Comorbidities N (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Males | Females | Discharged | Continued hospitalisation | Transferred to another hospital | Death | ||||||
| Middeldorp, 2020 | Cohort | 61 (14) | 198 | 130 (66) | 68 (34) | 136 (69) | 16 (8) | 8 (4) | 38 (19) | Active cancer | 7 (3.5) |
| Grillet, 2020 | Retrospective | 66 (13) | 100 | 70 | 30 | NA | NA | NA | NA | Cardiovascular Disease | 39 |
| Chronic Respiratory Insufficiency | 15 | ||||||||||
| T2DM | 20 | ||||||||||
| Malignancy | 20 | ||||||||||
| Zhang, 2020 | Retrospective | 63 (14) | 143 | 74 (51.71) | 69 (48.25) | 92 (64.3) | 19 (13.3) | NA | 32 (22.4) | HTN | 56 (39.2) |
| DM | 26 (18.2) | ||||||||||
| CAD | 17 (11.9) | ||||||||||
| Malignancy | 7 (4.9) | ||||||||||
| Cerebal Infarction | 5 (3.5) | ||||||||||
| Chronic Liver Disease | 5 (3.5) | ||||||||||
| CKD | 4 (2.8) | ||||||||||
| Lorant, 2020 | Retrospective | 64 (22) | 106 | 70 (66) | 36 (34) | NA | NA | NA | NA | NA | |
| Demelo, 2020 | Cohort | 68.1 (14.5) | 156 | 102 (66) | 54 (34) | NA | NA | NA | NA | Active Cancer | 16 (10.3) |
| Lodigiani, 2020 | Cohort | 66 | 388 | 264 (68) | 124 (32) | NA | NA | NA | NA | HTN | 183 (47.2) |
| DM | 88 (22.7) | ||||||||||
| Dyslipidemia | 76 (19.6) | ||||||||||
| Chronic Renal Dysfunction | 61 (15.7) | ||||||||||
| Active Cancer | 25 (6.4) | ||||||||||
| Poyiadi, 2020 | Retrospective | 62 (16) | 328 | 148 (45) | 180 (55) | NA | NA | NA | NA | Cancer History | 14 |
| DM | 38 | ||||||||||
| HTN | 61 | ||||||||||
| COPD | 13 | ||||||||||
| CHF | 9 | ||||||||||
| Cui, 2020 | Retrospective | 59.9 | 81 | 37 (46) | 44 (54) | 9 (11) | 64 (79) | 8 (10) | HTN | 20 (25) | |
| DM | 8 (10) | ||||||||||
| CHD | 10 (12) | ||||||||||
| Coronary Heart Disease | 10 (12) | ||||||||||
| Bilalogu et al, 2020 | Retrospective Study | 64 | 3334 | 2014 (60.4) | 1320 (39.6) | NA | NA | NA | NA | Myocadial Infarction | 195 |
| Congestive Heart failure | 279 | ||||||||||
| HTN | 1676 | ||||||||||
| Diabetes | 1246 | ||||||||||
| Hyperlipidemia | 1285 | ||||||||||
| Coronary artery disease | 617 | ||||||||||
| Fauvel et al, 2020 | Cohort Study | 64 | 1240 | 721 (58.1) | 519 (41.9) | NA | NA | NA | 151 (12.2) | HTN | 559 (45.4) |
| Diabetes | 268 (21.7) | ||||||||||
| Dyslipidemia | 316 (25.6) | ||||||||||
| Cardiovascular disease | 19 (1.6) | ||||||||||
| COPD | 77 (6.2) | ||||||||||
| CKD | 126 (10.3) | ||||||||||
| Stroke | 94 (7.7) | ||||||||||
| Peripheral arterial disease | 60 (4.9) | ||||||||||
| Atrial fibrillation | 117 (9.5) | ||||||||||
| CHF | 117 (9.5) | ||||||||||
| CAD | 133 (10.7) | ||||||||||
| Malignancy | 167 (13.7) | ||||||||||
| Trimaille et al, 2020 | Cohort Study | 62.2 | 289 | 171 (59.2) | 118 (40.8) | 236 (88.7) | NA | NA | 24 (8.3) | NA | NA |
| Whyte et al, 2020 | Cohort Study | 63.5 | 214 | 129 | 85 | NA | 36 | NA | 31 | Malignancy | 16 |
| Haemoptysis | 12 | ||||||||||
| Artifoni et al, 2020 | Cohort Study | 64 | 71 | 43 (60.6) | 28 (39.4) | NA | NA | NA | NA | HTN | 32 (60) |
| Diabetes | 14 (20) | ||||||||||
| Cancer | 4 (6) | ||||||||||
Data are presented as median (IQR) or number (%).
Abbreviations: CAD, coronary artery disease; CHD, coronary heart disease; CKD, chronic kidney disease; CHF, coronary heart failure; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; HTN, hypertension; IQR, interquartile range; NA, not available; No., number; T2DM, diabetes mellitus type 2.
3.3. Quality assessment
We assessed the quality of data in the included studies using the NIH quality assessment tools. The quality assessment indicated that most of the included studies were of fair quality. All the studies clearly stated the research question or the objective, the study population was clearly specified and defined, all the subjects were selected from similar populations. The detailed result of the quality assessment is provided in Supplementary File 1.
3.4. Association between VTE and disease severity
In order to assess the association between VTE and disease severity, four cohort studies qualified for inclusion in quantitative analysis. The pooled estimate of four cohort studies with substantial heterogeneity revealed nearly three times more risk of ICU care requirement in patients with VTE compared to non‐VTE patients (RR: 2.78; 95% CI: 1.75‐4.39; P < .001; I 2: 65.1%) (Figures 2 and S2). The mortality outcome was assessed in one retrospective study indicative of a higher risk of VTE in ICU patients (RR: 50.19; 95% CI: 3.05‐832.75; P < .001).
FIGURE 2.
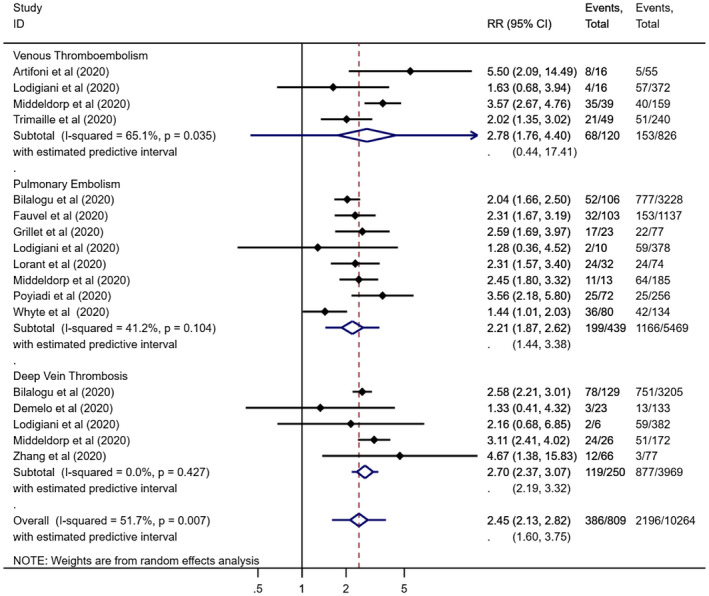
Relative risk of ICU admission in venous thromboembolism (A), pulmonary embolism (B) and deep vein thrombosis subgroups (C)
3.5. Association between pulmonary embolism and disease severity
To assess the association between pulmonary embolism and disease severity, eight studies (four cohort and four retrospective studies) qualified for inclusion in quantitative analysis. The pooled estimate of four cohort and four retrospective studies with substantial heterogeneity study showed that patients with pulmonary embolism are at nearly three times increased risk of being admitted to ICU (RR: 2.21; 95% CI: 1.86‐2.61; P < .001; I 2: 41.2%) (Figure 2). The subgroup pooled analysis of four cohort studies demonstrated nearly two times higher risk of ICU care in patients with pulmonary embolism (RR: 1.97; 95% CI: 1.44‐2.70; P < .001; I 2: 57.6%) (Figure S2). The subgroup pooled estimate of four retrospective studies highlighted that patients with pulmonary embolism are around three times higher risk of being admitted to ICU (RR: 2.39; 95% CI: 1.91‐2.99; P < .001; I 2: 37.8%) (Figure S2).
3.6. Association between deep vein thrombosis and disease severity
For the outcome, in order to assess the association between deep vein thrombosis and disease severity, five studies (three cohort studies and two retrospective studies) qualified for inclusion in quantitative analysis. The pooled estimate of three cohort and two retrospective studies demonstrated around three times higher risk of deep vein thrombosis in ICU patients (RR: 2.69; 95% CI: 2.37‐3.06; P < .001; I 2: 0.0%) (Figure 2). The subgroup pooled analysis of three cohort studies highlighted a higher risk of requiring ICU care in patients with deep vein thrombosis (RR: 2.57; 95% CI: 1.53‐4.30; P < .001; I 2: 30.7%) (Figure S2). The subgroup pooled estimate of two retrospective studies showed that the need for ICU care was higher in patients with deep vein thrombosis (RR: 2.61; 95% CI: 2.19‐3.11; P < .001; I 2: 1.1%) (Figure S2).
4. DISCUSSION
Recent evidence on COVID‐19 postulated that the high mortality observed among COVID‐19 patients may be partly because of unrecognised pulmonary embolism and pulmonary in situ thrombosis. 1 , 13 Several studies have demonstrated a higher incidence of VTE in COVID‐19 patients, 17 , 18 however, the effect of the incidence of VTE on the severity of disease needs further exploration. Thus, the present meta‐analysis was conducted to assess the association of VTE either pulmonary embolism or deep vein thrombosis with disease severity in COVID‐19 patients. The present meta‐analysis demonstrated a high incidence of pulmonary embolism, deep vein thrombosis and VTE in patients requiring ICU care.
The present meta‐analysis demonstrated a positive association between COVID‐19 and the incidence of pulmonary embolism in ICU patients. Whyte et al performed a cohort study on 214 COVID‐19 patients and revealed that the overall proportion of patients with pulmonary embolism was 5.4%, increasing to 16.2% in ICU patients. Pulmonary embolism was diagnosed in 3.5% patients receiving ward‐based care. The higher incidence of pulmonary embolism in ICU patients is consistent with previous studies (16.7%‐47%). 28 A cohort study reported a high incidence of pulmonary embolism in the critically ill COVID‐19 patients, also it was found to be one of the major thrombotic complications in this study. 13 Therefore, pulmonary thromboembolism may be considered in COVID‐19 patients with sudden onset of oxygenation deterioration, respiratory distress and reduced blood pressure as these patients are often immobile and present with an acute inflammatory state. 29
The present meta‐analysis demonstrated the positive association between COVID‐19 ICU patients and deep vein thrombosis. A systematic literature review of four prospective studies conducted on patients requiring critical care reported the rate of objectively confirmed deep vein thrombosis which ranged from 13% to 31% and suggested a potential role for thromboprophylaxis in patients requiring critical care. 30 A retrospective study reported an 18.2% incidence of deep vein thrombosis in COVID‐19 ICU patients. 15 Various cohort studies have reported 32%, 14 13% 16 and 4.1% 25 incidence of deep vein thrombosis in COVID‐19 ICU patients.
The present meta‐analysis demonstrated the positive association between COVID‐19 ICU patients and VTE. Malas et al conducted a systematic review involving 8271 SARS‐CoV‐2 patients determining the overall incidence of VTE to be 21%. Amongst ICU patients, the VTE rate was as high as 31%. Patients who developed VTE were at 74% increased odds of death compared to those who did not. 18 Collectively, several cohort studies have reported 47% 14 and 8.3% 25 incidence of VTE in COVID‐19 ICU patients. A retrospective study reported an incidence of 25% of VTE in COVID‐19 hospitalised patients. 1
There are various mechanisms explaining the risk of VTE either deep vein thrombosis or pulmonary embolism or both in COVID‐19 patients. One such mechanism explains that the virus can bind to the endothelial cells via angiotensin 2 receptors, which are most commonly found in the alveolar epithelial cells, followed by endothelial cells—a process that may ultimately damage blood vessels and increase the risk of thrombogenicity. 2 However, apart from the severe COVID‐19 patients in ICUs, those hospitalised in hospital wards share common predisposing factors for VTE, namely strict and long isolation and subsequently immobilisation. Any severe infection can predispose to VTE. However, it appears that in COVID‐19 additional mechanisms might contribute to increased VTE risk, including endothelial damage, microvascular thrombosis and occlusion, or even autoimmune mechanisms. 31 Another mechanism explains that the abnormal expression of T cell‐associated mRNA can lead to VTE. 32 This means that older patients with more underlying diseases were more likely to develop immune dysfunction and have a higher risk of VTE because of their poor immunity. 1
There are several limitations worth mentioning. First, most studies included a relatively small number of cases with a poor description of patient characteristics, which limited the possibility to explore the effects of concomitant risk factors on the incidence of VTE. Second, the lack of randomisation and the little data provided on the incidence of VTE stratified by use, type, dose and duration of anticoagulation (ie, prophylactic, intermediate or therapeutic dose) precluded subgroup analysis on the effects of pharmacological prophylaxis. Third, the nature of the data available in the individual studies did not allow the meta‐analysis to be stratified by some clinically relevant variables such as thromboprophylaxis status, race and healthcare access/quality to assess their effect on the incidence of VTE and mortality.
To conclude, our findings suggest that VTE either deep vein thrombosis or pulmonary embolism may have a negative effect on the health status of COVID‐19 patients. However, large incidence studies demonstrating the consequences of VTE are urgently needed for decision‐making with regards to the intensity of thromboprophylaxis.
DISCLOSURES
No potential conflict of interest to declare in relation to this publication.
AUTHOR CONTRIBUTIONS
Conception and design: Rashmi and Rizwana; Acquisition of data: Rashmi and Rizwana; Analysis and interpretation of data: Pinki and Ram; Drafting the manuscript: Rashmi and Pinki; Revised manuscript critically for important intellectual content: Nilanjan and Nidhi; Final approval of the version to be published: Nidhi and Rizwana; Agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: Rashmi and Nidhi.
Supporting information
File S1
Fig S2
Srivastava R, Parveen R, Mishra P, Saha N, Bajpai R, Agarwal NB. Venous thromboembolism is linked to severity of disease in COVID‐19 patients: A systematic literature review and exploratory meta‐analysis. Int J Clin Pract. 2021;75:e14910. doi: 10.1111/ijcp.14910
REFERENCES
- 1. Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18:1421‐1424. 10.1111/jth.14830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tal S, Spectre G, Kornowski R, Perl L. Venous thromboembolism complicated with COVID‐19: what do we know so far? Acta Haematol. 2020;143:417‐424. 10.1159/000508233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guan W‐J, Ni Z‐Y, Hu YU, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708‐1720. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang D, Hu BO, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323:1061. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054‐1062. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kollias A, Kyriakoulis KG, Dimakakos E, Poulakou G, Stergiou GS, Syrigos K. Thromboembolic risk and anticoagulant therapy in COVID‐19 patients: emerging evidence and call for action. Br J Haematol. 2020;189:846‐847. 10.1111/bjh.16727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Danzi GB, Loffi M, Galeazzi G, Gherbesi E. Acute pulmonary embolism and COVID‐19 pneumonia: a random association? Eur Heart J. 2020;41:1858. 10.1093/eurheartj/ehaa254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS‐CoV‐2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089‐1098. 10.1007/s00134-020-06062-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wichmann D, Sperhake J‐P, Lütgehetmann M, et al. Autopsy findings and venous thromboembolism in patients with COVID‐19. Ann Int Med. 2020;173:268‐277. 10.7326/M20-2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xie Y, Wang X, Yang P, Zhang S. COVID‐19 complicated by acute pulmonary embolism. Radiol Cardiothorac Imaging. 2020;2:e200067. 10.1148/ryct.2020200067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grillet F, Behr J, Calame P, Aubry S, Delabrousse E. Acute pulmonary embolism associated with COVID‐19 pneumonia detected by pulmonary CT angiography. Radiology. 2020;296:E186‐E188. 10.1148/radiol.2020201544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Klok FA, Kruip M, van der Meer N, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID‐19. Thromb Res. 2020;191:145‐147. 10.1016/j.thromres.2020.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Middeldorp S, Coppens M, van Haaps TF, et al. Incidence of venous thromboembolism in hospitalized patients with COVID‐19. J Thromb Haemost. 2020;18:1995‐2002. 10.1111/jth.14888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang L, Feng X, Zhang D, et al. Deep vein thrombosis in hospitalized patients with coronavirus disease 2019 (COVID‐19) in Wuhan, China: prevalence, risk factors, and outcome. Circulation. 2020;142:114‐128. 10.1161/CIRCULATIONAHA.120.046702 [DOI] [PubMed] [Google Scholar]
- 16. Demelo‐Rodríguez P, Cervilla‐Muñoz E, Ordieres‐Ortega L, et al. Incidence of asymptomatic deep vein thrombosis in patients with COVID‐19 pneumonia and elevated D‐dimer levels. Thromb Res. 2020;192:23‐26. 10.1016/j.thromres.2020.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Artifoni M, Danic G, Gautier G, et al. Systematic assessment of venous thromboembolism in COVID‐19 patients receiving thromboprophylaxis: incidence and role of D‐dimer as predictive factors. J Thromb Thrombolysis. 2020;50:211‐216. 10.1007/s11239-020-02146-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Malas MB, Naazie IN, Elsayed N, Mathlouthi A, Marmor R, Clary B. Thromboembolism risk of COVID‐19 is high and associated with a higher risk of mortality: a systematic review and meta‐analysis. EClinicalMedicine. 2020;29:100639. 10.1016/j.eclinm.2020.100639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA‐ScR): checklist and explanation. Ann Intern Med. 2018;169:467. 10.7326/M18-0850 [DOI] [PubMed] [Google Scholar]
- 20. NIH . Study Quality Assessment Tools. National Heart, Lung and Blood Institute. https://www.nhlbi.nih.gov/health‐topics/study‐quality‐assessment‐tools. Accessed June 9, 2020. [Google Scholar]
- 21. Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration. https://training.cochrane.org/handbook. Accessed June 9, 2020. [Google Scholar]
- 22. Bilaloglu S, Aphinyanaphongs Y, Jones S, Iturrate E, Hochman J, Berger JS. Thrombosis in hospitalized patients with COVID‐19 in a New York City health system. JAMA. 2020;324:799‐801. 10.1001/jama.2020.13372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fauvel C, Weizman O, Trimaille A, et al. Pulmonary embolism in COVID‐19 patients: a French multicentre cohort study. Eur Heart J. 2020;41:3058‐3068. 10.1093/eurheartj/ehaa500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leonard‐Lorant I, Delabranche X, Severac F, et al. Acute pulmonary embolism in COVID‐19 patients on CT angiography and relationship to D‐Dimer Levels. Radiology. 2020;296:E189‐E191. 10.1148/radiol.2020201561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lodigiani C, Iapichino G, Carenzo L, et al. Venous and arterial thromboembolic complications in COVID‐19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9‐14. 10.1016/j.thromres.2020.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Poyiadi N, Cormier P, Patel PY, et al. Acute pulmonary embolism and COVID‐19. Radiology. 2020;297:E335‐E338. 10.1148/radiol.2020201955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Trimaille A, Curtiaud A, Marchandot B, et al. Venous thromboembolism in non‐critically ill patients with COVID‐19 infection. Thromb Res. 2020;193:166‐169. 10.1016/j.thromres.2020.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Whyte MB, Kelly PA, Gonzalez E, Arya R, Roberts LN. Pulmonary embolism in hospitalised patients with COVID‐19. Thromb Res. 2020;195:95‐99. 10.1016/j.thromres.2020.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Scialpi M, Scialpi S, Piscioli I, Battista Scalera G, Longo F. Pulmonary thromboembolism in critical ill COVID‐19 patients. Int J Infect Dis. 2020;95:361‐362. 10.1016/j.ijid.2020.04.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Geerts W, Cook D, Selby R, Etchells E. Venous thromboembolism and its prevention in critical care. J Crit Care. 2002;17:95‐104. 10.1053/jcrc.2002.33941 [DOI] [PubMed] [Google Scholar]
- 31. Zhang Y, Xiao M, Zhang S, et al. Coagulopathy and antiphospholipid antibodies in patients with Covid‐19. N Engl J Med. 2020;382:e38. 10.1056/NEJMc2007575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Duan Q, Gong Z, Song H, et al. Symptomatic venous thromboembolism is a disease related to infection and immune dysfunction. Int J Med Sci. 2012;9:453‐461. 10.7150/ijms.4453 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
File S1
Fig S2


