Abstract
Background
Most pediatric studies of asthma and COVID‐19 to date have been ecological, which offer limited insight. We evaluated the association between asthma and COVID‐19 at an individual level.
Methods
Using data from prospective clinical registries, we conducted a nested case‐control study comparing three groups: children with COVID‐19 and underlying asthma (“A+C” cases); children with COVID‐19 without underlying disease (“C+” controls); and children with asthma without COVID‐19 (“A+” controls).
Results
The cohort included 142 A+C cases, 1110 C+ controls, and 140 A+ controls. A+C cases were more likely than C+ controls to present with dyspnea and wheezing, to receive pharmacologic treatment including systemic steroids (all p < .01), and to be hospitalized (4.9% vs. 1.7%, p = .01). In the adjusted analysis, A+C cases were nearly 4 times more likely to be hospitalized than C+ controls (adjusted OR = 3.95 [95%CI = 1.4–10.9]); however, length of stay and respiratory support level did not differ between groups. Among A+C cases, 8.5% presented with an asthma exacerbation and another 6.3% developed acute exacerbation symptoms shortly after testing positive for SARS‐CoV‐2. Compared to historic A+ controls, A+C cases had less severe asthma, were less likely to be on controller medications, and had better asthma symptom control (all p < .01).
Conclusions
In our cohort, asthma was a risk factor for hospitalization in children with COVID‐19, but not for worse COVID‐19 outcomes. SARS‐CoV‐2 does not seem to be a strong trigger for pediatric asthma exacerbations. Asthma severity was not associated with higher risk of COVID‐19.
Keywords: asthma, COVID‐19, SARS‐CoV‐2
Key Message.
Asthma severity was not associated with higher risk of COVID‐19 in children, and SARS‐CoV‐2 was not a strong trigger for asthma exacerbations. Asthma was a risk factor for hospitalization in children with COVID‐19, but not for worse COVID‐19 outcomes.
1. INTRODUCTION
There have been conflicting reports on whether asthma increases COVID‐19 risk or severity, 1 , 2 , 3 , 4 , 5 , 6 with scarce data in children. 7 While the majority of COVID‐19 cases have occurred in adults, 8 pediatric COVID‐19 cases in the United States have been steadily increasing since July 2021. 9 The rise of the highly infectious Delta variant coincides with the return of millions of unimmunized students to in‐person classrooms this fall with highly variable mitigation strategies, making it critically important that we better elucidate the impact of COVID‐19 on children, particularly those with common chronic diseases such as asthma.
Most pediatric studies on COVID‐19 and asthma to date have been epidemiological or ecological, which examine associations at a large scale but provide limited inferences into the individual characteristics driving the findings. For instance, there have been ecological reports describing reduced pediatric asthma exacerbations and morbidity during the pandemic, 10 , 11 , 12 , 13 likely as a result of physical distancing, masks, and perhaps other factors such as decreases in air pollution. Yet, those have focused on the concurrent effects of the pandemic on population‐level asthma morbidity and healthcare utilization, rather than the direct potential associations between asthma and incident COVID‐19 characteristics in children.
In this study, we examined the association between asthma and COVID‐19 in children using nested case‐control analyses. We hypothesized that (1) there is an association between asthma and COVID‐19 presentation and outcomes and (2) that asthma severity is correlated with COVID‐19 risk.
2. METHODS
2.1. Study population and data collection
The Western Pennsylvania COVID‐19 Registry (WPACR) is a secure database established in March 2020 to record baseline characteristics, acute presentation, and initial outcomes of pediatric patients (ages 0–21 years) presenting with a SARS‐CoV‐2 infection to UPMC Children's Hospital of Pittsburgh (CHP, the largest pediatric referral center in the region) and Children's Community Pediatrics (CCP, the associated primary care network) 14 For this analysis, we extracted data from children with pre‐existing asthma who presented with COVID‐19 between March and December 2020 (“A+C” cases). As disease controls, we selected from the WPACR children without pre‐existing conditions who presented with COVID‐19 (“C+” controls) during the same period, as well as non‐overlapping children with asthma (“A+” controls) recruited to the CHP Asthma Registry during the same period the year prior to the pandemic (March to December 2019). The Asthma Registry includes children seen for asthma in the CHP Pulmonary or Allergy clinics, the Emergency Department, who are hospitalized for asthma, or who participate in asthma research studies at our center.
We have previously described details of the WPACR 14 in brief, we included subjects if they had a positive SARS‐CoV‐2 RT‐PCR or if they met criteria for the multisystem inflammatory syndrome in children (MIS‐C), and a multidisciplinary team representing the different pediatric specialties involved in the care of patients with COVID‐19 at our institution (including primary care, pulmonology, hospital medicine, adolescent medicine, infectious disease, and critical care medicine) extracted relevant clinical data from the EHR. Data abstracted included patient demographics, symptoms and initial presentation, healthcare utilization data, laboratory results, and acute disease outcomes.
For all patients with asthma (A+C cases and A+ controls), we also directly abstracted EHR data on baseline asthma severity, asthma controller medications, symptom control (Asthma Control Test [ACT] 15 scores for adolescents ≥12 years old or Childhood Asthma Control Test [C‐ACT] 16 scores for children 4–11 years old), lung function (FEV1 and FVC as percent of predicted [%pred], as well as FEV1/FVC ratio), and atopy biomarkers (total and specific IgE, allergy skin testing, and peripheral blood eosinophil counts). We defined asthma severity based on National Asthma Education and Prevention Program (NAEPP) guidelines 17 and poor control as an ACT or C‐ACT ≤19. We evaluated asthma exacerbations in both the A+C and A+ groups from January 1, 2018, to December 31, 2019, to avoid any potential impact of the pandemic on asthma control, management, or healthcare utilization.
The WPACR and the CHP Asthma Registry are both approved by the Institutional Review Board at the University of Pittsburgh (protocols STUDY20110072 and STUDY19020359, respectively).
2.2. Statistical analysis
For the analysis of A+C cases vs C+ controls (i.e., the differences in COVID‐19 between children with and without asthma), we compared initial symptoms at presentation, history of recent travel, and known exposures. Our primary outcomes of interest were hospitalization, hospital length of stay (LOS), PICU admission, and the maximal respiratory support required during hospitalization. For the analysis between A+C cases and A+ controls (i.e., to assess whether patients presenting with COVID‐19 had more severe pre‐existing asthma than expected from our usual hospital population), our primary variables were asthma severity and symptom control, and secondary characteristics were asthma exacerbations, lung function, and atopy biomarkers. We conducted bivariate analyses using 2‐tailed t tests or Wilcoxon rank‐sum (Mann‐Whitney U) tests for continuous variables, and chi‐squared or Fisher's exact tests for categorical variables. We conducted adjusted analyses using logistic regression for categorical variables (e.g., hospitalization), Poisson regression for count data (e.g., number of severe asthma exacerbations), or linear regression for continuous variables. We adjusted models for hospitalization for age, sex, and covariates that were significant in the bivariate analyses of asthma and hospitalization: race, zip code median household income, BMI, days from symptom onset to presentation, and non‐asthma symptoms (fever, fatigue, and vomiting). In addition, we performed a sensitivity analysis using propensity score matching (PSM) to account for differences in relevant potential confounders such as age and race (see Online Supplement). We performed all analyses using STATA v16.1 (StataCorp, College Station, TX) or SAS v9.4 (SAS Institute, Cary, NC).
3. RESULTS
From March 11, 2020, to December 21, 2020, there were 1,802 cases with SARS‐CoV‐2 RT‐PCR+in the WPACR (Figure 1). Of those, 142 had asthma and were extracted as A+C cases; and 1,110 had no reported pre‐existing conditions and were selected as C+ controls. Distribution of COVID‐19 cases over time showed a significant peak in the summer and a larger peak in the late fall/early winter (Figure 2). We also extracted 140 non‐overlapping asthma controls (A+ controls) recruited to the CHP Asthma Registry during the same period in 2019.
FIGURE 1.
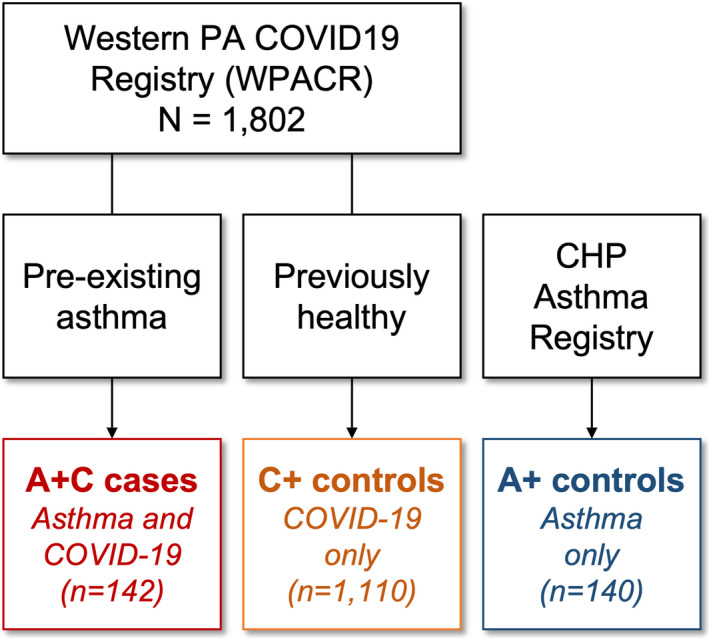
Diagram of study cohort selection. A+C cases and C+ controls were recruited from March to December 2020. A+ controls were selected from patients recruited from March to December 2019. Data on asthma characteristics (for both A+C cases and A+ controls) were collected for 2018–2019 to avoid any potential impact of the pandemic on asthma control, management, or healthcare utilization
FIGURE 2.
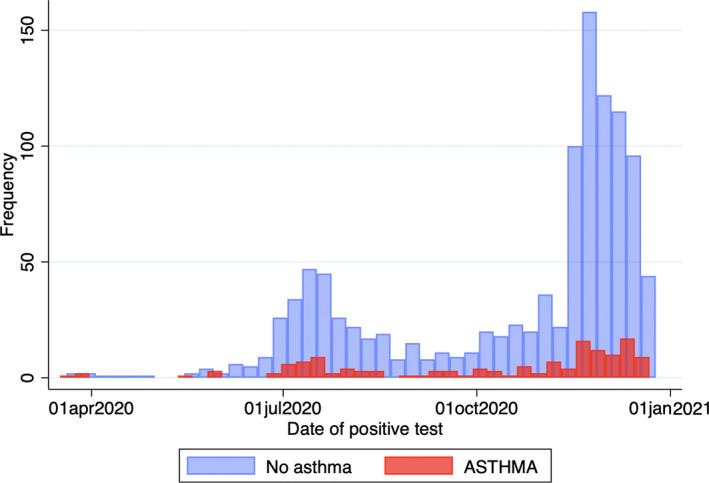
Distribution of cases over time during the study period. Distribution of COVID‐19 (A+C cases and C+ controls) over time
Baseline cohort characteristics are shown in Table 1. A+C cases (COVID‐19 with pre‐existing asthma) were approximately 4 years older than both C+ and A+ controls (median ages 14.6, 12.0, and 10.2 years, respectively), but there were no differences in sex distributions between the three groups. Compared to children without asthma, those with asthma (with or without COVID‐19) were more likely to be Black (A+C cases 25.4% and A+ controls 23.6%, vs. C+ controls 10.2%), had a higher BMI (median BMI percentiles 79.0 and 78.6, respectively, vs. 63.0), and were more likely to live in zip codes with lower household income (Table 1).
TABLE 1.
Sociodemographic characteristics of the participants in the study
| A+C Cases (Asthma and COVID−19) | C+ Controls (COVID−19 only) | p‐value | A+ Controls (Asthma only) | p‐value | |
|---|---|---|---|---|---|
| N | 142 | 1,110 | 140 | ||
| Age, years | 14.6 [10.7–17.9] | 12.0 [4.8–16.9] | <.001 | 10.2 [7.8–13.9] | <.001 |
| Male sex | 79 (55.6%) | 560 (50.5%) | .24 | 87 (62.1%) | .27 |
| Race: | <.001 | .88 | |||
| White | 103 (72.5%) | 905 (81.5%) | 103 (73.6%) | ||
| Black | 36 (25.4%) | 113 (10.2%) | 33 (23.6%) | ||
| Other/unknown | 3 (2.1%) | 92 (8.3%) | 4 (2.9%) | ||
| Hispanic | 1 (0.7%) | 8 (0.7%) | .96 | 7 (5%) | .03 |
| BMI, percentile | 79.0 [50.0–95.2] | 63.0 [30.6–87.6] | <.001 | 78.6 [48.1–95.3] | .93 |
| Household income | |||||
| Zip code median a | $66.9 ($24.9) | $68.7 ($22.7) | .38 | $61.7 ($19.0) | .048 |
| Below median b | 80 (57.6%) | 514 (47.6%) | .028 | 81 (58.3%) | .90 |
Numbers represent mean (SD) or median [interquartile range] for continuous variables and n (%) for categorical variables. p‐values for the comparison of each control group vs A+C cases.
Bold indicates statistically significant p‐value (p<.05).
Abbreviations: BMI, Body mass index; LOS, Length of stay (days).
Average of the median household incomes (in thousands of USD) for all participants’ zip codes, based on US Census American Community Survey (ACS) 5‐year data (2015–2019).
Number of participants who live in zip codes with median household income below the median for all zip codes in the study.
3.1. COVID‐19 hospitalizations
Altogether, 26 children (2.1%) in the WPCAR were hospitalized during the study period (Table S1), including 9 PICU admissions and no deaths. Compared to children with COVID‐19 who were not hospitalized, hospitalized patients were more likely to have asthma (26.9% vs. 11.0%). They were also younger (median ages 7.4 vs. 12.6 years) and more likely to be Black (38.5% vs. 12.3%); to live in zip codes with lower median household income; to present with fever, fatigue, wheezing, dyspnea, chest pain, and/or vomiting; and to receive pharmacologic treatment (38.5% vs. 1.2%) (Table S1). There were no clear discernible hospitalization patterns over time (Figure S1). PICU admissions were few but included 1/7 (14.3%) A+C cases and 8/19 (42.1%) C+ controls.
3.2. COVID‐19 in children with asthma vs. no underlying disease
A+C cases were more likely than C+ controls to endorse recent travel; to present with wheezing, dyspnea, chest pain, and loss of taste; and to be hospitalized (4.9% vs. 1.7%) (Table 2). They were also more likely to receive pharmacologic treatment including albuterol (17.6% vs. 0.7%) and systemic steroids (8.5% vs. 0.8%). Among the 142 A+C cases, 12 (8.5%) had an asthma exacerbation upon initial presentation leading to SARS‐CoV‐2 testing, and 9 (6.3%) developed an asthma exacerbation shortly after they tested positive for COVID‐19. In the remaining 118 cases, asthma was either solely a pre‐existing condition with no active symptoms upon COVID‐19 presentation (80.1%) or there was insufficient data to determine asthma symptoms (4.2%). A+C cases presenting with acute asthma symptoms were more likely to be hospitalized than those with no acute asthma symptoms (3/21 [14.3%] vs. 3/118 [2.5%], p = .04).
TABLE 2.
COVID‐19 characteristics in COVID‐19 cases (A+C) and (C+) controls
| A+C Cases | C+ Controls | p‐value | |
|---|---|---|---|
| Initial presentation: | .58 | ||
| ED or urgent care | 19 (13.4%) | 113 (10.2%) | |
| Primary care or telemedicine | 122 (85.9%) | 974 (87.8%) | |
| Other/unknown | 1 (0.7%) | 23 (2.1%) | |
| Recent travel | 10 (7.0%) | 34 (3.1%) | .009 |
| Known exposure | 86 (62.3%) | 746 (68.3%) | .16 |
| Interval, days: | |||
| Symptoms to presentation | 2 [1–3] | 2 [1–3] | .37 |
| Symptoms to test | 2.5 [1–4] | 2 [1–4] | .47 |
| Initial symptoms: | |||
| Asymptomatic | 16 (11.3%) | 124 (11.2%) | .97 |
| Fever | 53 (37.3%) | 443 (39.9%) | .55 |
| Fatigue | 24 (16.9%) | 162 (14.6%) | .47 |
| Cough | 62 (43.7%) | 477 (43.0%) | .88 |
| Wheezing | 7 (4.9%) | 5 (0.5%) | <.001 |
| Dyspnea | 13 (9.2%) | 25 (2.3%) | <.001 |
| Chest pain | 7 (4.9%) | 16 (1.4%) | .004 |
| Loss of smell | 23 (16.2%) | 125 (11.3%) | .09 |
| Loss of taste | 26 (18.3%) | 129 (11.6%) | .02 |
| Abdominal pain | 8 (5.6%) | 52 (4.7%) | .62 |
| Vomiting | 6 (4.2%) | 36 (3.2%) | .54 |
| Initial treatment: | |||
| Any pharmacologic treatment | 10 (7%) | 15 (1.3%) | <.001 |
| Albuterol | 25 (17.6%) | 8 (0.7%) | <.001 |
| Systemic steroids | 12 (8.5%) | 9 (0.8%) | <.001 |
| Main outcomes | |||
| Hospitalized | 7 (4.9%) | 19 (1.7%) | .01 |
| Hospital LOS (days) | 1 [1–4] | 2 [1–4] | .51 |
| Respiratory support a : | .72 | ||
| None (room air) | 6 | 14 | |
| Nasal cannula (NC) | 0 | 2 | |
| High‐flow NC or NPAP | 1 | 2 | |
| Invasive ventilation | 0 | 1 | |
Numbers represent mean (SD) or median [interquartile range] for continuous variables and n (%) for categorical variables.
Abbreviation: LOS, Length of stay (days); NPAP, Non‐invasive positive airway pressure (includes CPAP and BiPAP).
Among hospitalized patients.
In the multivariable analysis (Table 3), asthma was a risk factor for hospitalization with COVID‐19: After adjustment for age, sex, race, recent travel, and known exposure, A+C cases were nearly 4 times more likely to be hospitalized than C+ controls (aOR = 3.95 [95%CI = 1.4–10.9]; Table 3 Model 1). Results were similar after additionally adjusting for zip code household income, BMI percentile, and the number of days between symptom onset and presentation (Table 3 Model 2), or after adjusting for non‐asthma symptoms that were associated with hospitalization (fever, fatigue, and vomiting; Table 3 Model 3). In our sensitivity analysis, PSM adequately balanced for relevant covariates (Figure S2, Table S2) and results were similar to those from the adjusted regression models, with odds ratios ranging from 3.38 to 4.08 (Table S3). Among hospitalized patients, however, LOS and respiratory support level did not significantly differ by asthma status, although sample sizes were very small (Table 2).
TABLE 3.
Association between asthma and hospitalization for COVID‐19
| Model 1 (N = 1,252) | Model 2 (N = 857) | Model 3 (N = 1,252) | |
|---|---|---|---|
| Asthma | 3.95 (1.43–10.9)** | 4.87 (1.44–16.43)** | 3.33 (1.19–9.33)* |
| Age, years | 0.88 (0.82–0.95)** | 0.91 (0.83–1.01) | 0.91 (0.85–0.98)* |
| Male sex | 0.55 (0.24–1.26) | 0.42 (0.14–1.27) | 0.49 (0.21–1.15) |
| Race: | |||
| White | Ref. | Ref. | Ref. |
| Black | 3.00 (1.28–7.07)* | 1.41 (0.35–5.71) | 4.55 (1.87–11.1)* |
| Other/unknown | 0.54 (0.07–4.23) | 2.69 (0.30–24.3) | 0.51 (0.06–4.18) |
| Recent travel | 1.39 (0.52–3.68) | 2.79 (0.29–26.5) | 1.34 (0.40–4.52) |
| Known exposure | 0.27 (0.52–0.62)** | ||
| Low zip code income | 2.54 (0.75–8.58) | ||
| BMI, percentile | 1.01 (0.99–1.03) | ||
| Days from symptom onset to presentation | 1.002 (0.99–1.01) | ||
| Initial symptoms: | |||
| Fever | 1.90 (0.40–4.52) | ||
| Fatigue | 3.15 (1.23–8.09)* | ||
| Vomiting | 8.62 (3.15–23.6)** | ||
Numbers shown are odds ratios (95% confidence intervals) for hospitalization. All models adjusted for age, sex, race, recent travel, and known exposure. Model 2 additionally adjusted for zip code's median household income, BMI percentile, and the time interval (days) between symptom onset and presentation; these were not included in Model 1 because of greater missingness (~30% were missing BMI or time interval data, thus N = 873). Model 3 additionally adjusted for non‐asthma related symptoms that were significant in the unadjusted analysis (fever, fatigue, and vomiting; see Table S1).
*p < .05.
**p < .01.
3.3. Comparison between cases and asthma controls
In our evaluation of whether children with asthma presenting with COVID‐19 differ from children with asthma usually seen at our center (Table 4), A+C cases had less severe asthma (58% vs. 23% had intermittent asthma) and lower eosinophil counts (median 110 vs. 300 cells/µl), and they were less likely to be on controller medications, and less likely to have poor symptom control (13.6% vs. 30.7%). This was driven by adolescent A+C cases who had better ACT scores compared to A+ controls (mean 22.1 vs. 20.3), with no differences in C‐ACT scores among the younger children. There were no differences in lung function (FEV1, FVC, or FEV1/FVC), but A+C cases were less likely to have atopic comorbidities, and they had lower rates of severe exacerbations compared to A+ controls (median 1.0 vs. 1.5 events per year, Mann‐Whitney p = .042); this difference persisted after adjustment for age, sex, and race (difference −0.43 events per year [95%CI −0.77 to −0.10], Poisson p = .01).
TABLE 4.
Asthma characteristics in COVID‐19 cases (A+C) and asthma (A+) controls
| A+ controls | p‐value | ||
|---|---|---|---|
| Asthma severity | |||
| Intermittent | 81 (57.9%) | 32 (22.9%) | <.001 |
| Mild persistent | 40 (28.6%) | 53 (37.9%) | .08 |
| Moderate persistent | 16 (11.4%) | 42 (30.0%) | <.001 |
| Severe persistent | 3 (2.1%) | 13 (9.3%) | .009 |
| Controller medication: | |||
| ICS | 40 (28.2%) | 75 (53.6%) | <.001 |
| ICS/LABA | 10 (7.0%) | 22 (15.7%) | .02 |
| Symptom control: | |||
| C‐ACT score | 23.2 (4.03) | 21.6 (4.01) | .13 |
| ACT score | 22.1 (3.97) | 20.3 (4.62) | .03 |
| Poorly controlled a | 12 (13.6%) | 27 (30.7%) | .007 |
| Lung function b : | |||
| FEV1, %pred | 95.2 (13.3) | 94.3 (17.9) | .85 |
| FVC, %pred | 98.7 (10.7) | 90.9 (37.8) | .28 |
| FEV1/FVC | 84.3 (8.6) | 71.4 (29.4) | .19 |
| History of atopy: | |||
| Eczema | 27 (19.0%) | 64 (45.7%) | <.001 |
| Allergic rhinitis | 72 (50.7%) | 105 (75.0%) | <.001 |
| Food allergies | 11 (7.8%) | 40 (28.6%) | <.001 |
| Any specific IgE+ | 3 (2.1%) | 7 (5.0%) | .19 |
| Any skin test+ | 1 (0.7%) | 6 (4.3%) | .05 |
| None | 53 (37.3%) | 21 (15.0%) | <.001 |
| Atopy biomarkers: | |||
| Eosinophils, percent | 2 [1–4] | 4 [2–11] | <.001 |
| Eosinophils, cells/µL | 110 [50–270] | 300 [200–580] | <.001 |
| Total IgE, UI/mL | 380 [182–1036] | 195 [80–704] | .30 |
| Subspecialist care: | |||
| Pediatric Pulmonology | 20 (14.1%) | 84 (60.0%) | <.001 |
| Allergy / Immunology | 11 (7.8%) | 72 (51.4%) | <.001 |
| Severe exacerbations: | |||
| Any events c | 30 (22.5%) d | 73 (54.5%) e | <.001 |
| Events in the past year f | 1 [0.5–1.5] | 1.5 [1.0–2.0] | .04 |
Numbers represent mean (SD) or median [interquartile range] for continuous variables and n (%) for categorical variables.
Bold indicates statistically significant p‐value (p<.05).
Abbreviations: ACT, Asthma control test (ages ≥12 years, range 5–25); C‐ACT, Childhood asthma control test (ages 4–11 years, range 0–27).
Defined as C‐ACT or ACT ≤19.
For A+C cases, N=17 with spirometry data.
Number of patients with ≥1 asthma‐related ED visit, hospitalization, or systemic steroid course from January 2018 to December 2019.
N = 133 with asthma event data.
N = 134 with asthma event data.
Rate among those who had at least one event.
We then evaluated the subgroup of cases and controls who had been previously seen at one of the asthma subspecialty clinics (17 A+C cases and 86 A+ controls seen in either Pulmonology or Allergy clinics; Table S4). In the analysis restricted to this subgroup, A+C cases and A+ controls were similar in most asthma characteristics, except for persistent differences in eosinophil counts and the proportion of patients with no atopic comorbidities.
4. DISCUSSION
Our study is one of the first individual‐level, case‐control studies on pediatric COVID‐19 and asthma. To our knowledge, it is also one of the first studies to report the incidence of asthma exacerbations among children with asthma and acute SARS‐CoV‐2 infection.
Of the 1,802 patients in our pediatric COVID‐19 registry during the study period, 142 (7.9%) had asthma. In our initial report over the first five months of the pandemic, asthma cases comprised 10.6% of the registry 14 Thus, asthma does not appear to be over‐represented among pediatric cases of COVID‐19 compared to local (8%) or national (7%) asthma prevalence 18 , 19 consistent with previous retrospective studies 20 Other studies have reported asthma prevalence rates among pediatric COVID‐19 cases to vary from 0.5% (Wuhan, China) 21 to 20% (Washington, DC) 22 and from 2% (Lombardy and Liguria, Italy) 23 to 24% (New York City) 24 among hospitalized pediatric COVID‐19 patients. This may be partly explained by variability in local asthma prevalence, though at least one study reported lower asthma prevalence rate among children with COVID‐19 compared to the local pediatric asthma prevalence (13% vs. 20%–25%), suggesting that there may be other contributing factors. 25
In our cohort, children with asthma were nearly 4 times more likely to be hospitalized for COVID‐19 compared to children without asthma, even after adjusting for potential confounders like age, sex, race/ethnicity, zip code household income, BMI, recent travel, known exposures, and the number of days between symptom development and the patient's initial presentation to care. Results remained essentially unchanged after further adjusting for non‐asthma‐related symptoms that could have independently increased likelihood of hospitalization, such as fever, vomiting, and fatigue. Consistent with our results, some studies have reported asthma as a risk factor for hospitalization in children with COVID‐19, 26 , 27 while others have reported conflicting results. 20 , 22 Although our sample size was small and thus results need to be interpreted with caution, hospital LOS and the need for respiratory support or PICU admission did not seem to differ by asthma status. Taken together, these results suggest that the increased hospitalization rate may have been due to a different threshold to admit children with asthma, rather than truly because of a more severe presentation. Given the paucity of data on pediatric asthma and COVID‐19, providers may have been subjectively more concerned about potential severe course or outcomes in a child with asthma compared to one without pre‐existing conditions. A survey of 174 centers in Europe similarly reported that 33/49 (67%) children with asthma and COVID‐19 were hospitalized, but only 19 (39% of the total) required any respiratory support and concluded that this “may suggest the children were admitted for safety reasons and uncertainty about the course of COVID‐19”. 28 While some studies in adults have reported associations between non‐atopic asthma and severe COVID‐19, 3 our results are consistent with a recent meta‐analysis (also in adults) showing no association between asthma and hospital LOS or ICU admission for COVID‐19. 29 More detailed, prospective, multi‐center pediatric studies will be necessary to further examine this issue.
We found that SARS‐CoV‐2 does not seem to be a particularly strong trigger of asthma exacerbations, with only ~15% of children with asthma presenting with significant asthma symptoms during acute COVID‐19. While we cannot rule out that some patients with asthma may have had exacerbations triggered by mild COVID‐19 that were managed at home and who never sought medical attention, our estimates are consistent with a recent study of adolescents and adults with COVID‐19 that similarly reported that only 13% of patients with asthma had acute wheezing. 30 As expected, children with asthma were more likely to receive systemic steroids as part of their management, and those presenting with acute asthma symptoms were more likely to be hospitalized—again suggesting that at least some of those admissions may have been due to asthma rather than COVID‐19 severity.
In our analysis of cases vs historic asthma controls, we found that asthma severity was not associated with higher risk of SARS‐CoV‐2 infection. Due to the nature of our registries, we were unable to compare these patients to children with asthma managed by community providers (i.e., without subspecialty referral or recent admissions to our hospital). However, our findings suggest that children with asthma and COVID‐19 were at least not as severe as those who would have been usually referred to subspecialty asthma care at our center. When we restricted our analyses to children with asthma who had been followed by a specialty clinic in the years prior to the pandemic, there was no significant difference in severity or symptoms compared to controls. While the sample size for this subgroup analysis was small, it again suggests that asthma severity was no different in children with COVID‐19 than what we would have expected from the population our center usually serves.
Several factors contribute to a complex relationship between asthma and COVID‐19. Obesity is a risk factor for asthma 31 and has been identified as a risk factor for COVID‐19 infection and worse outcomes. 32 In our analysis, children with asthma had higher BMI, but this did not alter the association between asthma and hospitalization. Likewise, African American children in our cohort were more likely to have asthma and to be hospitalized for COVID‐19, consistent with prior studies identifying racial disparities in asthma and severe COVID‐19. 33 , 34 , 35 We also found that children with asthma presenting with COVID‐19 had lower eosinophil counts and fewer atopic comorbidities than asthma controls. Eosinopenia has been associated with worse outcomes and mortality in adult COVID‐19. 36 , 37 Eosinophils play an important role in the immune response to respiratory viruses; atopic patients with higher eosinophil counts may be somewhat “protected” from SARS‐CoV‐2 infection, 38 and A+C cases with relatively lower counts may have thus been more susceptible. Gene expression of ACE2, which plays a crucial role in SARS‐CoV‐2 cell entry, is lower in asthma and in younger ages, 39 , 40 and ICS can downregulate ACE2 expression 41 ; however, large epidemiological studies have linked high‐dose ICS to worse risk of death from COVID‐19 in both asthma and COPD. 42 These and other factors may partly explain the conflicting findings on asthma and COVID‐19, which may vary by age, asthma severity and treatment, degree of atopy, and other determinants.
There are several key strengths to this study. It is one of the first studies to compare COVID‐19 presentation and outcomes between children with and without asthma using individual‐level characteristics rather than ecological data and is likely the first such study in a cohort from the United States. There has been a significant knowledge gap regarding COVID‐19 outcomes of children with underlying pulmonary diseases; this study starts to fill some of these gaps, which is of utmost importance for counseling families of children with asthma. Data for this study are based on registries in which information is manually abstracted from the EHR by a multidisciplinary team who is involved in the care of patients with COVID‐19, providing more detailed, complete, and accurate information than reports based on diagnostic or billing codes. Furthermore, all asthma information for both cases and historic controls was abstracted by a single asthma provider, eliminating concerns for inter‐rater bias. Finally, given our sample size, we were able to evaluate subgroup characteristics, such as those patients with asthma referred to subspecialty care at our center.
Our study also has several limitations. Given the nature of the pandemic, follow‐up duration has been short, precluding the ability to track long‐term outcomes. However, it seems unlikely that asthma would have a lasting impact on COVID‐19 outcomes, given the generally mild courses of the children in this study. Yet, future studies should also focus on long‐term consequences such as changes in lung function or asthma severity and control in children who recovered from acute COVID‐19. Another limitation is the small number of hospitalized patients in this study; it is unclear whether this is secondary to regional variation in severity or management, possibly due to local variants, or whether the low proportion of hospitalized children is secondary to our ability to include even mild and asymptomatic cases in the registry. It will be crucial to analyze multi‐center data to evaluate whether findings vary across different settings. In addition, our analysis was limited to data from the EHR, and thus, other relevant covariates were not included. Moreover, the current study period preceded the emergence of the Delta variant of the virus; our registry is ongoing, and we plan to analyze potential differences as we see the already surging wave of pediatric cases across the country.
In summary, we found that asthma severity does not seem to be associated with increased risk of SARS‐CoV‐2 infection in children. Pre‐existing asthma did increase the risk of hospitalization for COVID‐19 in our population, but hospital LOS, need for respiratory support, and disposition did not differ from children without asthma. Furthermore, SARS‐CoV‐2 infection was not commonly a trigger for asthma exacerbations, but children presenting with symptoms of an asthma exacerbation were more likely to be admitted. With the advent of the Delta variant and current rise in COVID‐19 cases, it will be important to conduct multi‐center, individual‐level, case‐control, or cohort studies of COVID‐19 and asthma to better understand this evolving disease and its impact on children with asthma.
CONFLICT OF INTERESTS
The authors declare no conflicts of interest related to the current work.
AUTHOR CONTRIBUTIONS
Kristina Gaietto: Conceptualization (equal); Data curation (lead); Formal analysis (lead); Investigation (equal); Writing‐original draft (lead); Writing‐review & editing (lead). Megan Culler Freeman: Conceptualization (equal); Data curation (lead); Validation (equal); Writing‐review & editing (equal). Leigh Anne DiCicco: Conceptualization (equal); Data curation (equal); Validation (lead); Writing‐review & editing (equal). Sherry Rauenswinter: Data curation (lead); Project administration (equal); Resources (lead); Validation (equal); Writing‐review & editing (equal). Joseph R Squire: Data curation (equal); Validation (supporting); Writing‐review & editing (equal). Zachary Aldewereld: Conceptualization (equal); Data curation (equal); Validation (equal); Writing‐review & editing (equal). Jennifer Iagnemma: Conceptualization (equal); Resources (equal); Writing‐review & editing (equal). Brian T Campfield: Conceptualization (equal); Supervision (equal); Writing‐review & editing (equal). David Wolfson: Conceptualization (equal); Resources (equal); Writing‐review & editing (equal). Traci M Kazmerski: Conceptualization (lead); Data curation (lead); Formal analysis (equal); Investigation (equal); Supervision (lead); Writing‐original draft (equal); Writing‐review & editing (lead). Erick Forno: Conceptualization (lead); Data curation (lead); Formal analysis (equal); Investigation (equal); Project administration (lead); Supervision (lead); Writing‐original draft (equal); Writing‐review & editing (lead).
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/pai.13696.
Supporting information
Supplementary Material
Gaietto K, Freeman MC, DiCicco LA, et al. Asthma as a risk factor for hospitalization in children with COVID‐19: A nested case‐control study. Pediatr Allergy Immunol.2021;33:e13696. doi: 10.1111/pai.13696
Traci M. Kazmerski and Erick Forno are shared senior authors.
Funding information
Dr. Gaietto's contribution was funded in part by grant number T32‐HL129949 from the U.S. National Institutes of Health (NIH). Dr. Forno's contribution was funded in part by grant HL149693 from the U.S. NIH. The funding agencies had no role in the study or the preparation of the manuscript.
Footnotes
Bold indicates statistically significant p‐value (p<.05).
REFERENCES
- 1. Matsumoto K, Saito H. Does asthma affect morbidity or severity of COVID‐19? J Allergy Clin Immunol. 2020;146(1):55‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Morais‐Almeida M, Pite H, Aguiar R, Ansotegui I, Bousquet J. Asthma and the coronavirus disease 2019 pandemic: a literature review. Int Arch Allergy Immunol. 2020;181(9):680‐688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yang JM, Koh HY, Moon SY, et al. Allergic disorders and susceptibility to and severity of COVID‐19: a nationwide cohort study. J Allergy Clin Immunol. 2020;146(4):790‐798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID‐19‐related death using OpenSAFELY. Nature. 2020;584(7821):430‐436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Choi HG, Wee JH, Kim SY, et al. Association between asthma and clinical mortality/morbidity in COVID‐19 patients using clinical epidemiologic data from Korean disease control and prevention. Allergy. 2021;76(3):921‐924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Broadhurst R, Peterson R, Wisnivesky JP, et al. Asthma in COVID‐19 hospitalizations: an overestimated risk factor? Ann Am Thorac Soc. 2020;17(12):1645‐1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Castro‐Rodriguez JA, Forno E. Asthma and COVID‐19 in children: a systematic review and call for data. Pediatr Pulmonol. 2020;55(9):2412‐2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. CDC . CDC COVID‐19 Data Tracker. Available from: https://covid.cdc.gov/covid‐data‐tracker/#demographics. Accessed August 15, 2021.
- 9. AAP Children and COVID‐19: State‐Level Data Report. Available from: https://www.aap.org/en/pages/2019‐novel‐coronavirus‐covid‐19‐infections/children‐and‐covid‐19‐state‐level‐data‐report/. Accessed November 15, 2021.
- 10. Kenyon CC, Hill DA, Henrickson SE, Bryant‐Stephens TC, Zorc JJ. Initial effects of the COVID‐19 pandemic on pediatric asthma emergency department utilization. J Allergy Clin Immunol Pract. 2020;8(8):2774‐2776.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Simoneau T, Greco KF, Hammond A, Nelson K, Gaffin JM. Impact of the COVID‐19 Pandemic on Pediatric Emergency Department Use for Asthma. Annals of the American Thoracic Society. 2021;18(4):717‐719. 10.1513/annalsats.202007-765rl [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hurst JH, Zhao C, Fitzpatrick NS, Goldstein BA, Lang JE. Reduced pediatric urgent asthma utilization and exacerbations during the COVID‐19 pandemic. Pediatr Pulmonol. 2021;56(10):3166‐3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ulrich L, Macias C, George A, Bai S, Allen E. Unexpected decline in pediatric asthma morbidity during the coronavirus pandemic. Pediatr Pulmonol. 2021;56(7):1951‐1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Freeman MC, Gaietto K, DiCicco LA, et al. A comprehensive clinical description of pediatric SARS‐CoV‐2 infection in Western Pennsylvania. medRxiv. 2020. 10.1101/2020.12.14.20248192 [DOI] [Google Scholar]
- 15. Nathan RA, Sorkness CA, Kosinski M, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113(1):59‐65. [DOI] [PubMed] [Google Scholar]
- 16. Liu AH, Zeiger R, Sorkness C, et al. Development and cross‐sectional validation of the childhood asthma control test. J Allergy Clin Immunol. 2007;119(4):817‐825. [DOI] [PubMed] [Google Scholar]
- 17. Expert Panel Working Group of the National Heart L, Blood Institute a, coordinated National Asthma E, Prevention Program Coordinating C, Cloutier MM, Baptist AP , et al. 2020 focused updates to the asthma management guidelines: a report from the national asthma education and prevention program coordinating committee expert panel working group. J Allergy Clin Immunol. 2020;146(6):1217‐1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. CDC . Asthma Data, Statistics, and Surveillance 2019. Most Recent Asthma Data. Available from: https://www.CDC.gov/asthma/most_recent_data.htm. Accessed March 30, 2021.
- 19. Jones L, Feldmiller J, Selker K Allegheny County Asthma Task Force Report 2019 [released February 2020]. Allegheny County Health Department. Available from: https://alleghenycounty.us/uploadedFiles/Allegheny_Home/Health_Department/Programs/Air_Quality/2019‐asthma‐task‐force‐report.pdf
- 20. Floyd GC, Dudley JW, Xiao R, et al. Prevalence of asthma in hospitalized and non‐hospitalized children with COVID‐19. J Allergy Clin Immunol Pract. 2021;9(5):2077‐2079.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Du H, Dong X, Zhang JJ, et al. Clinical characteristics of 182 pediatric COVID‐19 patients with different severities and allergic status. Allergy. 2021;76(2):510‐532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. DeBiasi RL, Song X, Delaney M, et al. Severe coronavirus disease‐2019 in children and young adults in the Washington, DC, metropolitan region. J Pediatr. 2020;223:199‐203.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ciprandi G, Licari A, Filippelli G, Tosca MA, Marseglia GL. Children and adolescents with allergy and/or asthma seem to be protected from coronavirus disease 2019. Ann Allergy Asthma Immunol. 2020;125(3):361‐362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chao JY, Derespina KR, Herold BC, et al. Clinical characteristics and outcomes of hospitalized and critically ill children and adolescents with coronavirus disease 2019 at a tertiary care medical center in New York City. J Pediatr. 2020;223:14‐19.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rabha AC, Oliveira Junior FI, Oliveira TA, et al. Clinical manifestations of children and adolescents with Covid‐19: report of the first 115 cases from Sabara Hospital Infantil. Rev Paul Pediatr. 2020;39:e2020305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Metbulut A, Mustafaoğlu Ö, Şen G, et al. Evaluation of the Clinical and Laboratory Findings of Asthmatic Children with SARS‐CoV‐2 Infection. International Archives of Allergy and Immunology. 2021;1‐8. 10.1159/000517153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Graff K, Smith C, Silveira L, et al. Risk Factors for Severe COVID‐19 in Children. Pediatric Infectious Disease Journal. 2021;40(4):e137‐e145. 10.1097/inf.0000000000003043 [DOI] [PubMed] [Google Scholar]
- 28. Moeller A, Thanikkel L, Duijts L, et al. COVID‐19 in children with underlying chronic respiratory diseases: survey results from 174 centres. ERJ Open Res. 2020;6(4:409‐2020). 10.1183/23120541.00409-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sitek AN, Ade JM, Chiarella SE, et al. Outcomes among patients with COVID‐19 and asthma: a systematic review and meta‐analysis. Allergy Asthma Proc. 2021;42(4):267‐273. [DOI] [PubMed] [Google Scholar]
- 30. Garcia‐Pachon E, Ruiz‐Alcaraz S, Baeza‐Martinez C, et al. Symptoms in patients with asthma infected by SARS‐CoV‐2. Respir Med. 2021;185:106495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Peters U, Dixon AE, Forno E. Obesity and asthma. J Allergy Clin Immunol. 2018;141(4):1169‐1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sattar N, Valabhji J. Obesity as a risk factor for severe COVID‐19: summary of the best evidence and implications for health care. Curr Obes Rep. 2021;10(3):282‐289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Webb Hooper M, Nápoles AM, Pérez‐Stable EJ. COVID‐19 and Racial/Ethnic Disparities. JAMA. 2020;323(24):2466. 10.1001/jama.2020.8598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mackey K, Ayers CK, Kondo KK, et al. Racial and ethnic disparities in COVID‐19‐related infections, hospitalizations, and deaths: a systematic review. Ann Intern Med. 2021;174(3):362‐373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Munoz‐Price LS, Nattinger AB, Rivera F, et al. Racial disparities in incidence and outcomes among patients with COVID‐19. JAMA Netw Open. 2020;3(9):e2021892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Du Y, Tu L, Zhu P, et al. Clinical features of 85 fatal cases of COVID‐19 from Wuhan. A retrospective observational study. Am J Respir Crit Care Med. 2020;201(11):1372‐1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cortes‐Vieyra R, Gutierrez‐Castellanos S, Alvarez‐Aguilar C, et al. Behavior of eosinophil counts in recovered and deceased COVID‐19 patients over the course of the disease. Viruses. 2021;13(9):1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lindsley AW, Schwartz JT, Rothenberg ME. Eosinophil responses during COVID‐19 infections and coronavirus vaccination. J Allergy Clin Immunol. 2020;146(1):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bunyavanich S, Do A, Vicencio A. Nasal gene expression of angiotensin‐converting enzyme 2 in children and adults. JAMA. 2020;323(23):2427‐2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jackson DJ, Busse WW, Bacharier LB, et al. Association of respiratory allergy, asthma, and expression of the SARS‐CoV‐2 receptor ACE2. J Allergy Clin Immunol. 2020;146(1):203‐206.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Finney LJ, Glanville N, Farne H, et al. Inhaled corticosteroids downregulate the SARS‐CoV‐2 receptor ACE2 in COPD through suppression of type I interferon. J Allergy Clin Immunol. 2021;147(2):510‐519.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schultze A, Walker AJ, MacKenna B, et al. Risk of COVID‐19‐related death among patients with chronic obstructive pulmonary disease or asthma prescribed inhaled corticosteroids: an observational cohort study using the OpenSAFELY platform. Lancet Respir Med. 2020;8(11):1106‐1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


