Abstract
Remdesivir, a prodrug of the nucleoside analog GS‐441524, plays a key role in the treatment of coronavirus disease 2019 (COVID‐19). However, owing to limited information on clinical trials and inexperienced clinical use, there is a lack of pharmacokinetic (PK) data in patients with COVID‐19 with special characteristics. In this study, we aimed to measure serum GS‐441524 concentrations and develop a population PK (PopPK) model. Remdesivir was administered at a 200 mg loading dose on the first day followed by 100 mg from day 2, based on the package insert, in patients with an estimated glomerular filtration rate (eGFR) greater than or equal to 30 ml/min. In total, 190 concentrations from 37 Japanese patients were used in the analysis. The GS‐441524 trough concentrations were significantly higher in the eGFR less than 60 ml/min group than in the eGFR greater than or equal to 60 ml/min group. Extracorporeal membrane oxygenation in four patients hardly affected the total body clearance (CL) and volume of distribution (V d) of GS‐441524. A one‐compartment model described serum GS‐441524 concentration data. The CL and V d of GS‐441524 were significantly affected by eGFR readjusted by individual body surface area and age, respectively. Simulations proposed a dose regimen of 200 mg on day 1 followed by 100 mg once every 2 days from day 2 in patients with an eGFR of 30 ml/min or less. In conclusion, we successfully established a PopPK model of GS‐441524 using retrospectively obtained serum GS‐441524 concentrations in Japanese patients with COVID‐19, which would be helpful for optimal individualized therapy of remdesivir.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Remdesivir plays a key role in the treatment of coronavirus disease 2019 (COVID‐19). However, limited information on clinical trials and inexperienced clinical use has resulted in a lack of pharmacokinetic (PK) data in patients with COVID‐19 with special characteristics.
WHAT QUESTION DID THIS STUDY ADDRESS?
This study addressed potential covariates that affect interindividual PK variations in clinical practice.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
We successfully established a population PK (PopPK) model of GS‐441524 using retrospectively obtained serum samples from Japanese patients with COVID‐19. PopPK analysis indicated that the renal function of patients affects the total body clearance of GS‐441524, whereas extracorporeal membrane oxygenation hardly affects the same.
HOW MIGHT THIS CHANGE DRUG DISCOVERY, DEVELOPMENT, AND/OR THERAPEUTICS?
Dosage adjustments based on this study will be helpful for ensuring the safety of remdesivir therapy, if remdesivir should be used for patients with severe renal dysfunction in clinical practice, regardless of non‐recommendation by the package insert.
INTRODUCTION
Remdesivir (GS‐5734, Veklury) is a diastereomer monophosphoramidate prodrug of the nucleoside analog GS‐441524. It undergoes intracellular metabolic conversion to form the pharmacologically active triphosphate that inhibits viral RNA polymerase. 1 , 2 Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), a positive‐sense, single‐stranded enveloped RNA virus, is the causative agent of the coronavirus disease 2019 (COVID‐19). 3 Although remdesivir was originally developed for Ebola virus infection, it showed antiviral activities against multiple RNA virus families, including SARS‐CoV‐2. 4 , 5 Remdesivir is superior to placebo in shortening the time to recovery in hospitalized patients. 6 , 7 In May 2020, the US Food and Drug Administration (FDA) issued emergency use authorization of remdesivir for the treatment of COVID‐19. In Japan, remdesivir was approved for the treatment of SARS‐CoV‐2 infection under an exceptional approval pathway in May 2020. Thus, remdesivir plays a key role in the treatment of COVID‐19.
There is little information about dosage adjustments for remdesivir in patients with renal failure. As remdesivir and the predominant active metabolite, GS‐441524, are excreted into urine, 8 the pharmacokinetics (PKs) of these drugs in patients with renal failure can be affected. Acute kidney injury (AKI) is a common clinical presentation among patients hospitalized with COVID‐19, 9 and patients with AKI or end‐stage kidney disease have an increased risk of severe COVID‐19. 10 However, patients with an estimated glomerular filtration rate (eGFR) less than 30 ml/min were excluded from clinical trials. 7 , 11 Although the administration of remdesivir is not recommended for patients with eGFR less than 30 ml/min in the package insert owing to limited clinical information, there are many patients with COVID‐19 with eGFR less than 30 ml/min in real clinical situations. Furthermore, it has been reported that the plasma concentrations of some drugs, such as fentanyl and midazolam, can decrease in patients with extracorporeal membrane oxygenation (ECMO). 12 , 13 Remdesivir was considered to have a moderate possibility to be lost via the ECMO circuit owing to calculated logP and protein binding. 14 To the best of our knowledge, there is a lack of PK data in patients with COVID‐19 in specific populations owing to limited information of clinical trials and inexperienced clinical use.
Remdesivir rapidly undergoes metabolic activation with a short half‐life (0.89 h). 15 We measured serum concentrations of GS‐441524, the predominant active metabolite of remdesivir, because intracellular nucleoside triphosphate cannot be measured in the serum. The purpose of this study is to develop a population PK (PopPK) model of GS‐441524 using serum concentration data from patients with COVID‐19 and identify potential covariates. For individualized remdesivir therapy, we propose a dosing regimen for patients with renal failure based on the obtained PopPK model.
METHODS
Patients and data collection
Japanese inpatients with COVID‐19 treated with remdesivir at Kyoto University Hospital from December 2020 to May 2021 were enrolled in this study. For each patient, serum GS‐441524 concentrations at trough were measured using surplus samples from other blood tests, and the following data were retrospectively collected from electronic medical records: age, sex, remdesivir dosage, time of dosing and sampling, body weight, height, body surface area (BSA), presence of ECMO and ventilator, and clinical laboratory data, such as eGFR, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and albumin levels. The eGFRindexed was automatically calculated by the clinical laboratory using the Modification of Diet in Renal Disorder Study equation for Japanese with standardized serum creatinine. 16 BSA was estimated by Du Bois equations, and the eGFRindexed was readjusted to eGFRnon‐indexed for drug dosing recommendations by using the equation eGFRnon‐indexed = eGFRindexed/1.73 m2 × BSA. 17 During dosing, safety and tolerability were assessed through adverse events and clinical laboratory tests according to Common Terminology Criteria for Adverse Events, version 5.0. This study was performed in accordance with the Declaration of Helsinki and its amendments and was approved by the Ethics Committee of Kyoto University Graduate School of Medicine and Kyoto University Hospital (Approval number: R2768, Approval date: November 28, 2020). All patients or proxies, who were eligible for participation, provided written informed consent before registration or participation.
Remdesivir administration
Remdesivir was administered at a loading dose of 200 mg on the first day, followed by 100 mg from day 2 over 1 h, based on the package insert, in patients with eGFRnon‐indexed greater than or equal to 30 ml/min. In patients with eGFRnon‐indexed less than 30 ml/min, remdesivir was administered at 200 mg on the first day, followed by 100 mg once every 2 days from day 3.
Sample preparation
Calibration curves were prepared by spiking pool plasma with appropriate volumes of standard solutions to procedure calibration curve points equivalent to 5, 25, 50, 250, and 500 ng/ml for GS‐441524. Twenty microliters of 75% isopropanol, 50 μl of human plasma, 10 μl of internal standard (0.25 μg/ml for [13C5]‐GS‐441524 in methanol), and 100 μl of acetonitrile were vortexed and centrifuged. The supernatant obtained after centrifugation was subjected to liquid chromatography–tandem mass spectrometry (LC–MS/MS) analysis.
LC–MS/MS conditions
Quantitative analysis was performed using a Nexera X2 UHPLC system coupled with an LCMS‐8060 triple quadrupole mass spectrometer (Shimadzu). Chromatographic separation was achieved using a Shim‐pack Scepter C18‐120 (50 mm × 2.1 mm I.D., 1.9 μm; Shimadzu) maintained at 40°C. The mobile phases consisted of 0.05% formic acid in water (A) and acetonitrile (B). The flow rate was maintained at 0.4 ml/min. The gradient program for analysis was as follows: 5% B from 0 to 0.3 min, 30% B at 0.35 min, 70% B at 1.5 min, 90% B from 1.8 to 2.8 min, and 5% B from 2.9 to 4.5 min. The MS conditions were as follows: interface electrospray ionization (ESI) mode: polarity, positive; nebulizer gas flow rate, 3 L/min; drying gas flow rate, 10 L/min; heating gas flow rate, 10 L/min; interface temperature, 300°C; desolvation line temperature, 200°C; and heat block temperature, 400°C. High‐purity argon and nitrogen were used as the collision and nebulizer gases, respectively. Analytes were detected using the multiple reaction monitoring (MRM) mode. The product ions (m/z), a quantifier and qualifier, were monitored for each compound in the following MRM transitions: m/z 291.90 → 163.05 and 173.05 for GS‐441524, m/z 296.90 → 164.10, and 174.10 for [13C5]‐GS‐441524. The product ions and collision energy were determined by post‐column infusion of each compound’s methanolic solution. For quantitative analysis of all data, LabSolutions LCMS version 5.99 SP2 software (Shimadzu) was used.
PopPK modeling
PopPK analysis was conducted using a nonlinear mixed‐effects modeling program (NONMEM version 7.5.0; ICON) using the first‐order conditional estimation method with interaction. Perl‐speaks‐NONMEM version 5.0.0 and R 4.0.3 (R‐project.org) were used to evaluate the goodness of fit and visualize the output. A one‐compartment open model was examined for the structural PK model. Interindividual variability in PK parameters was compared between the exponential and additive models. Residual variability in serum concentrations was compared between the proportional and additive error models.
To assess the influence of continuous covariates, such as age, height, body weight, BSA, and clinical laboratory data on total body clearance (CL), height, weight and BSA on the volume of distribution (V d), covariate analysis was performed using the following model:
| (1) |
where θ TV is the typical value of PK parameters (such as CL and V d), θp is the mean parameters to be estimated, and θ cov is the factor contributed by the covariate. COV indicates the value of each patient, and COVmedian is the median value of clinical data. To assess the influence of categorical covariates, such as sex, and presence of ECMO and ventilator on CL, and age, existence of ECMO and ventilator on V d, covariate analysis was performed using the following model:
| (2) |
where COV is 1 for patients cotreated with ECMO or ventilator and 0 for others when evaluating the influence of ECMO or ventilator, and COV is 1 in female patients or 0 for male patients when evaluating the influence of sex. In addition, COV is 1 for patients over 75 years old and 0 for others when evaluating the influence of age.
The influence of each covariate on CL and V d was evaluated based on the difference in the objective function value (OBJ) between the previous model and the model including the covariate by forward stepwise inclusion; thereafter, the result was confirmed using the backward stepwise elimination method. Values of p < 0.05 (ΔOBJ >3.84 with freedom of 1 assuming a χ2 distribution) in the forward inclusion and p < 0.01 (ΔOBJ >6.63 with freedom of 1 assuming a χ2 distribution) in the backward elimination were considered statistically significant. The individual predicted CL and concentration were obtained using an empirical Bayesian estimation in the first‐order conditional estimation method.
Model evaluation
The following diagnostic plots were used to evaluate the model fitting: observed concentration (OBS) versus population predicted value (PRED) or individual predicted value (IPRED) and conditional weighted residuals (CWRES) versus PRED, time after dose, or eGFRnon‐indexed to identify bias corresponding to model miss‐specification.
The predictive performance of the final models and their usefulness for describing observations were assessed using prediction‐corrected visual predictive check (pcVPC) plots. 18 Precision of the final model parameter estimates was assessed using both asymptotic standard errors and a nonparametric bootstrap. Bootstrapping was conducted using 500 randomly sampled (with replacement) replicates obtained from the 37 subjects in the original dataset. The model parameters were estimated for each bootstrap replicate using the NONMEM.
Monte Carlo simulation
The GS‐441524 trough concentrations based on the final PopPK model were evaluated using the Monte Carlo simulation to determine the effects of eGFRnon‐indexed and age on GS‐441524 PKs. Five thousand PK profiles were simulated for a patient with various eGFRsnon‐indexed (15, 30, 60, and 90 ml/min) and ages (over or under 75 years) using the NONMEM program. The dose was selected based on the package insert of remdesivir: 200 mg on day 1, followed by 100 mg from day 2.
Statistical analysis
Statistical analysis was performed using the Mann‐Whitney U test for comparisons of groups using GraphPad Prism 9.0 (GraphPad Software), and statistical significance was set at p < 0.05.
RESULTS
Patients’ demographics
Patient characteristics are summarized in Table 1. In total, 190 concentrations from 37 patients were used in the analysis. The median age of the patients was 72 years, and the age range of the enrolled population was 45–97 years. The eGFRnon‐indexed range at each concentration measurement point was 16.4–147.7 ml/min, and its median was 74.7 ml/min. The number of patients treated with ECMO was four, and the number of measurements in ECMO patients was 21 points.
TABLE 1.
Characteristics of patients treated with remdesivir
| Sex, male/female | 27/10 |
| Age, median (range), years | 72 (45–97) |
| Height, median (range), cm | 167.1 (144–182) |
| Body weight, median (range), kg | 66.8 (36.7–96.3) |
| BSA, median (range), m2 | 1.8 (1.24–2.21) |
| eGFRnon‐indexed, median (range), ml/min | 74.7 (16.4–147.7) |
| AST, median (range), IU/L | 31 (12–229) |
| ALT, median (range), IU/L | 30 (4–191) |
| Albumin, median (range), g/dL | 2.5 (1.7–4.1) |
| Total number of blood samples | 190 |
| Observed concentration of GS−441524, median (range), ng/ml | 116.6 (34.6–366.4) |
| Number of measurements with ECMO | 21 |
| Number of measurements with ventilator | 82 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BSA, body surface area; ECMO, extracorporeal membrane oxygenation; eGFRnon‐indexed, estimated glomerular filtration rate readjusted by individual BSA.
Twenty subjects had an eGFRnon‐indexed greater than or equal to 60 ml/min, 15 subjects had an eGFRnon‐indexed greater than or equal to 30 and less than 60 ml/min, and two subjects had an eGFRnon‐indexed less than 30 ml/min at the start of remdesivir administration.
Pharmacokinetics of GS‐441524
As the half‐life of remdesivir is very short and remdesivir rapidly undergoes metabolic activation, we measured the trough serum concentrations of GS‐441524. The median trough serum concentration of GS‐441524 at steady‐state (after day 4) was 116.6 ng/ml, which was nearly equal to the reference concentration. 19 The trough concentration profiles of GS‐441524 each day after intravenous administration of remdesivir are shown in Figure 1a. The trough concentrations of GS‐441524 were significantly higher in the eGFRnon‐indexed less than 60 ml/min group than in the eGFRnon‐indexed greater than or equal to 60 ml/min group (p < 0.001; Figure 1c). The number of patients treated with ECMO was four. The periods during ECMO were days 1–5, 1–10, 4–10, and 1–14 after starting remdesivir administration in these patients. The GS‐441524 trough concentrations in patients with ECMO were not significantly different before and after ECMO, and there was no difference compared with the non‐ECMO groups (Figure 1b,c).
FIGURE 1.
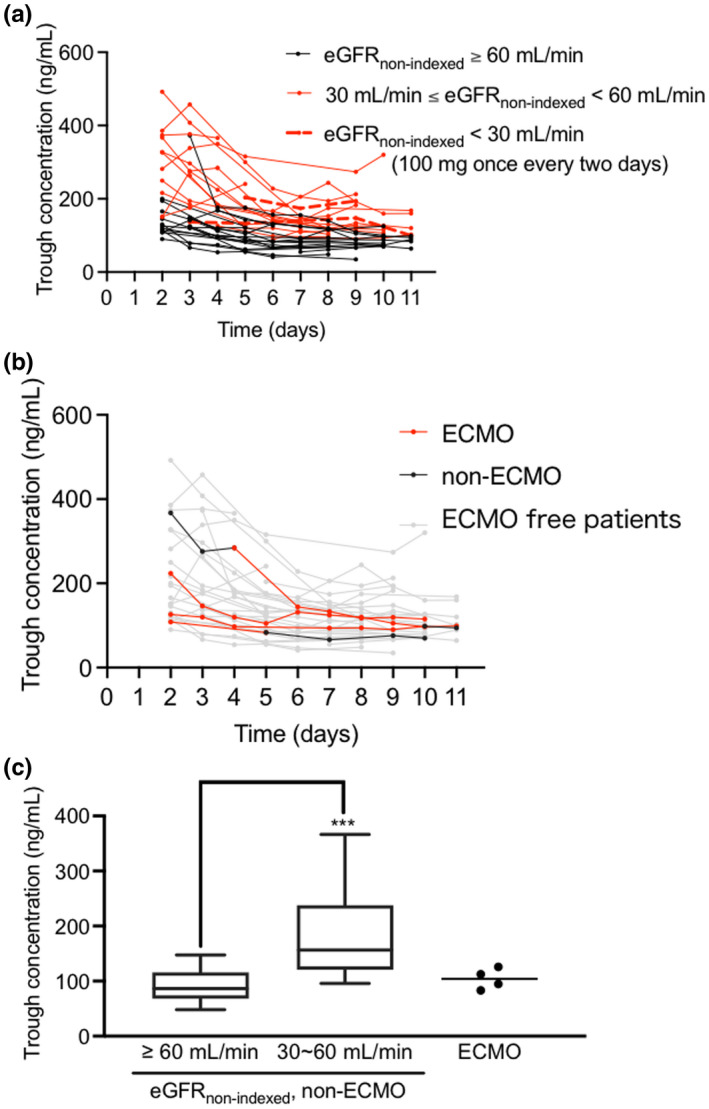
(a, b) Time‐course of trough GS‐441524 concentrations following intravenous administration of remdesivir. (a) The black solid lines denote patients with eGFRnon‐indexed greater than or equal to 60 ml/min (20 patients); the red solid lines denote patients with eGFRnon‐indexed greater than or equal to 30 and less than 60 ml/min (15 patients); the red dotted lines denote patients with eGFRnon‐indexed less than 30 ml/min (2 patients) at the start of remdesivir administration. Most patients received a 200 mg loading dose of remdesivir on the first day, followed by 100 mg from day 2 based on the package insert, and the patients with eGFRnon‐indexed less than 30 ml/min (2 patients) received 200 mg of remdesivir on day 1, followed by 100 mg once every 2 days from day 3. (b) The red solid lines denote the insertion period of ECMO; the black solid lines denote before and after ECMO; the gray solid lines denote ECMO‐free patients. (c) Comparison of GS‐441524 trough concentrations at steady‐state (after day 4) among patients without ECMO with eGFRnon‐indexed greater than or equal to 60 ml/min (20 patients), patients without ECMO with eGFRnon‐indexed = 30–60 ml/min (15 patients), and patients with ECMO (4 patients). The dots denote each mean GS‐441524 steady‐state trough concentration. ***p < 0.001 against eGFRnon‐indexed greater than or equal to 60 ml/min group using Mann‐Whitney U test. eGFRnon‐indexed, estimated glomerular filtration rate readjusted by individual body surface area; ECMO, extracorporeal membrane oxygenation
Fourteen of the 37 subjects showed at least one adverse effect. Eight subjects showed grade 1 ALT elevation, one subject showed grade 1 serum creatinine elevation, and six subjects showed grades 1–3 leukopenia. There was no correlation between the serum GS‐441524 concentration and the adverse effects.
PopPK model and model evaluation
A one‐compartment model described the serum GS‐441524 concentration data, and the exponential error model for interindividual variability and proportional error model for residual variability provided a better model fit than the additive error model for interindividual and residual variability. The estimated PK parameters were CL and V d.
The results of covariate analyses are shown in Table S1. After the forward inclusion and backward elimination steps, the CL and V d of GS‐441524 were significantly affected by eGFRnon‐indexed (ΔOBJ1 = −97.8) and age (ΔOBJ1 = −9.33, ΔOBJ2 = −11.2) of each patient at each concentration measurement point, respectively. For renal function, we assessed creatinine, eGFRnon‐indexed (ml/min), eGFRindexed (ml/min/1.73 m2), and creatinine clearance. The eGFRnon‐indexed (ml/min) provided superior model fitting. To find the best covariate for CL, we used only creatinine as a covariate representing renal function (Table S2). After forward inclusion and backward elimination steps, the CL of GS‐441524 was significantly affected by creatinine (ΔOBJ1 = −65.3), height (ΔOBJ1 = −9.96 and ΔOBJ2 = −26.4) and age (ΔOBJ1 = −14.7, ΔOBJ2 = −23.4, and ΔOBJ3 = −15.7). The V d of GS‐441524 was significantly affected by age (ΔOBJ1 = −9.33, ΔOBJ2 = −10.8, ΔOBJ3 = −10.7, and ΔOBJ4 = −13.5). The values of OBJ in the final model with eGFRnon‐indexed and with creatinine only were 1485 and 1474, respectively. Considering clinical usefulness, the following model with eGFRnon‐indexed was selected as the final PopPK model for CL and V d:
The final estimates of the PopPK parameters of GS‐441524, including relative standard errors, are listed in Table 2. The inclusion of these two covariates improved the model fit, as ΔOBJ was approximately −109, and the interindividual variability for CL also decreased by approximately 21%.
TABLE 2.
Population pharmacokinetic parameters of remdesivir in patients with COVID‐19
| OBJ | Final model | Bootstrap results (N = 500) | |||
|---|---|---|---|---|---|
| 1485 | |||||
| Parameters | Estimates | RSE, % | Median | 95% CI | |
| CL (L/h) = θ CL × (eGFRnon‐indexed/74.7) θ eGFRnon−idexed,CL | |||||
| θ CL (L/h) | 11.8 | 4.9 | 11.8 | 10.6–13.0 | |
| θ eGFRnon‐indexed,CL | 1.09 | 10.8 | 1.08 | 0.833–1.37 | |
| V d (L) = θV × (1 + θAge ≥ 75,V × AGE) | |||||
| θV (L) | 382 | 9.9 | 383 | 303–461 | |
| θ Age ≥ 75,V | −0.429 | 21.1 | −0.427 | −0.578–−0.171 | |
| Interindividual variability, CV% | Variance | RSE, % | Shrinkage | ||
| IIV for CL | 26.0 | 9.6 | 4.22 | 25.2 | 19.9–31.0 |
| IIV for V d | 34.5 | 12.8 | 24.1 | 32.8 | 19.6–40.9 |
| Residual variability, CV% | Variance | RSE, % | Shrinkage | ||
| Proportional error | 15.2 | 11.2 | 14.5 | 15.0 | 12.2–18.7 |
AGE is 1 if a patient is 75 years old or more, or 0 if a patient is under 75 years of age.
Abbreviations: CI, confidence interval; CL, total body clearance; COVID‐19, coronavirus disease 2019; CV, coefficient of variation; eGFRnon‐indexed, estimated glomerular filtration rate readjusted by individual BSA; IIV, interindividual variability; OBJ, objective function value; RSE, relative standard error; V d, volume of distribution.
The median values of the bootstrap replicates and final parameter estimates were similar (Table 2), indicating that the final parameters were properly estimated. The goodness‐of‐fit plots for the final model are shown in Figure 2. The plots of OBS versus PRED and IPRED approached the line of unity. Moreover, CWRES were evenly distributed around zero against PRED, time after dosing, and eGFRnon‐indexed. The pcVPC plot for serum GS‐441524 concentrations versus time showed that the model described the central tendency and variability of data (Figure 3).
FIGURE 2.
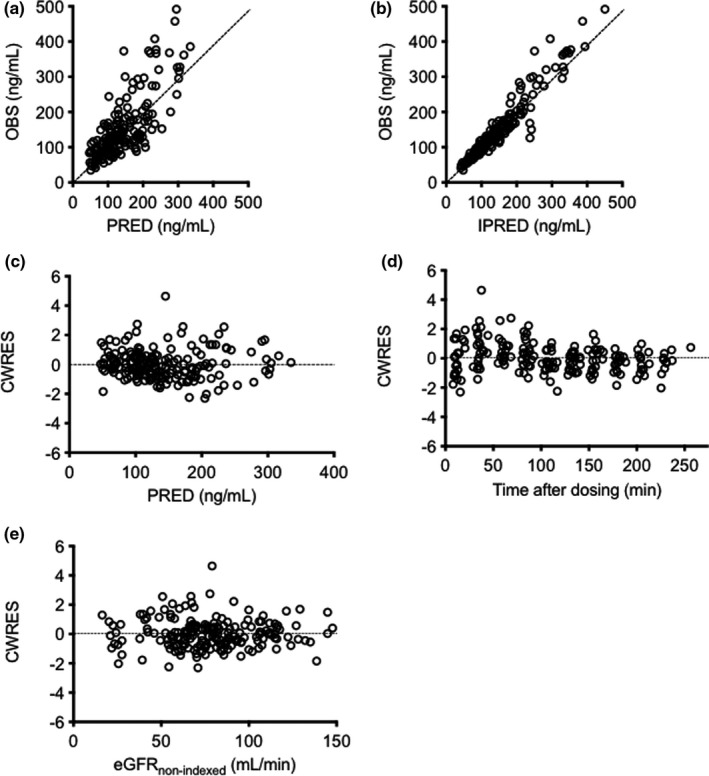
Goodness‐of‐fit plots for the final model of GS‐441524. Observed concentrations (OBS) versus population predictions (PRED) (a) and individual predictions (IPRED) (b). Conditional weighted residuals (CWRES) versus PRED (c), time after dosing (d), and eGFRnon‐indexed (e). Each dotted line in (a) and (b) represents a line of unity. eGFRnon‐indexed, estimated glomerular filtration rate readjusted by individual body surface area
FIGURE 3.
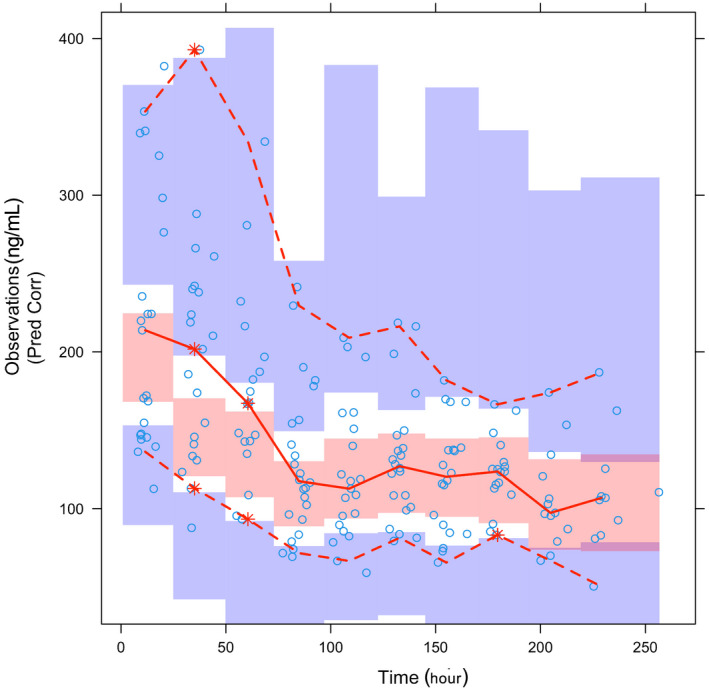
Visual predictive check of GS‐441524 observed data compared with 500‐replication datasets obtained from the final PopPK model. The x‐axis represents time after the first intravenous administration of remdesivir. The circles denote the observed data; the red lines denote the 5th, 50th, and 95th percentiles of the observed data. The shaded areas denote the confidence intervals of the 5th, 50th, and 95th percentiles of the simulated data. PopPK, population pharmacokinetic
Monte Carlo simulation
Figure 4 shows the trough serum concentrations simulated based on the final PopPK model when patients with various renal functions received remdesivir for 10 days. The trough serum concentrations of GS‐441524 were four‐ and two‐fold higher in patients with an eGFRnon‐indexed of 15 and 30 ml/min, respectively, than in those with an eGFRnon‐indexed of 60 ml/min. In contrast, the trough serum concentrations of GS‐441524 were slightly affected by age.
FIGURE 4.
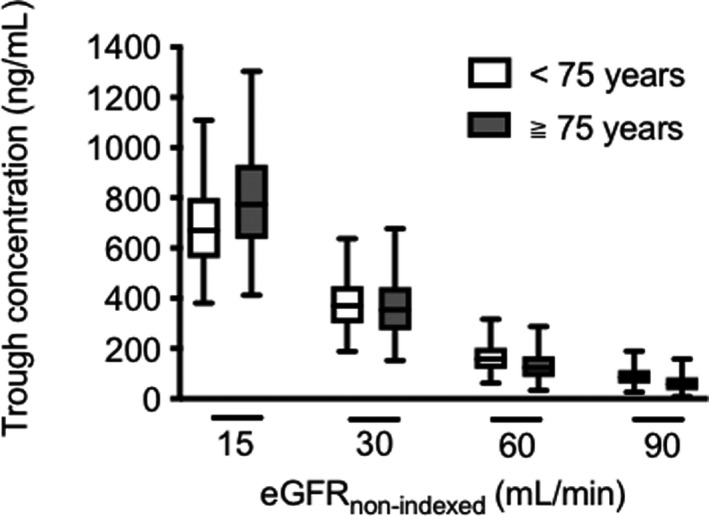
Simulations of trough concentration of GS‐441524 in the 5000‐replication datasets in a typical patient classified by eGFRnon‐indexed and age. The number along the x‐axis represents the patient eGFRnon‐indexed. The white box plot denotes patients under 75 years of age, and the gray box plot denotes patients over 75 years of age. eGFRnon‐indexed, estimated glomerular filtration rate readjusted by individual body surface area
The simulated trough concentrations in patients with renal impairment for each dosing regimen are listed in Table 3. The simulated trough concentration was highly dependent on the eGFRnon‐indexed. In patients aged under 75 years with an eGFRnon‐indexed of 90 ml/min, the median of simulated trough concentration in the dosage according to package insert (regimen 1) was 85.3 ng/ml, which was nearly equal to the observed values in a previous report. 20 In patients with an eGFRnon‐indexed of 60 ml/min, the median of simulated trough concentration in regimen 1 was 158.2 ng/ml. Simulations proposed that regimen 2, 200 mg on day 1 followed by 100 mg once every 2 days from day 2 in patients with an eGFRnon‐indexed of 30 ml/min, showed a similar trough concentration to those in regimen 1 for patients with an eGFRnon‐indexed of 60 ml/min. For patients with an eGFRnon‐indexed of 15 ml/min, 200 mg on day 1 followed by 100 mg once every 4 days from day 2 was comparable with regimen 1 for patients with an eGFRnon‐indexed of 60 ml/min.
TABLE 3.
Simulated trough concentrations (median and 90% prediction intervals) of GS‐441524 at steady‐state by renal function
| Dosing regimen | Trough concentration (ng/ml) | |||
|---|---|---|---|---|
| Age | eGFRnon‐indexed | Regimen 1 | Regimen 2 | Regimen 3 |
| <75 | 15 ml/min | 670.3 (423.1–1030.5) | 351.9 (219.2–542.7) | 184.4 (96.9–296.2) |
| 30 ml/min | 370.5 (211.9–591.9) | 168.3 (83.8–286.8) | 66.0 (21.8–136.9) | |
| 60 ml/min | 158.2 (74.2–284.6) | 56.0 (18.6–119.7) | 13.3 (1.6–44.4) | |
| 90 ml/min | 85.3 (33.3–169.2) | 24.0 (4.8–63.0) | 3.4 (0.1–18.2) | |
Regimen 1: 200 mg on day 1 followed by 100 mg daily from day 2. Regimen 2: 200 mg on day 1 followed by 100 mg once every 2 days from day 2. Regimen 3: 200 mg on day 1 followed by 100 mg once every 4 days from day 2.
eGFRnon‐indexed, estimated glomerular filtration rate readjusted by individual body surface area.
DISCUSSION
In this study, we successfully established a PopPK model of GS‐441524 using retrospectively obtained serum GS‐441524 concentrations in Japanese patients with COVID‐19. To the best of our knowledge, this is the first report to propose a PopPK model of GS‐441524 incorporating eGFRnon‐indexed and age for patients with severe renal dysfunction. The plot of predicted and observed concentrations lay near the line of identity, and the plot of CWRES against population predicted values was evenly distributed, indicating that the model was appropriately unbiased and described the variability adequately. In addition, the bootstrap statistics and pcVPC plots support the precise performance of the final model. The CL and V d of GS‐441524 for a typical patient were calculated to be 11.8 L/h and 382 L, respectively, based on the final PopPK parameters. These values were consistent with the findings of previous studies. 19 , 20 , 21 The PopPK model allometrically related to eGFRnon‐indexed and age can reasonably predict the PK parameters of GS‐441524 in patients with COVID‐19 in clinical practice.
The present PopPK analysis indicated that the eGFRnon‐indexed of patients affected the CL of GS‐441524. The GS‐441524 trough concentrations were approximately two‐fold higher in the eGFRnon‐indexed less than 60 ml/min group than in the eGFRnon‐indexed greater than or equal to 60 ml/min group. Our findings are consistent with the high rate of renal excretion of remdesivir. 8 Although the remdesivir dose is uniformly defined regardless of renal function in the package insert, renal function‐based dosage adjustments should be essential for safe therapy of remdesivir. The present simulations suitably calculated a reduced dosing regimen for patients with an eGFRnon‐indexed of approximately 30 ml/min: 200 mg on day 1, followed by 100 mg once every 2 days from day 2. We administered 100 mg of remdesivir once every 2 days from day 3 for two patients with an eGFRnon‐indexed of 23.3 and 16.4 ml/min, respectively. The trough concentrations in these patients were similar to the concentrations in individuals with normal renal function and maintained above the half‐maximal effective concentration (EC50) value (137 ng/ml) against Vero E6 cells infected with SARS‐CoV‐2 in in vitro studies. 22 Liver dysfunction and renal dysfunction have been reported as adverse events of remdesivir. 7 Because the toxic concentrations of GS‐441524 are largely unknown and we did not measure the concentrations of cyclodextrin, the risk of a two‐fold increase in the trough of GS‐441524 remains unclear. However, high concentrations of GS‐441524 could cause adverse events and should be avoided. Dosage adjustments based on this PopPK model would be helpful for ensuring the safety of remdesivir therapy in clinical practice.
The present study showed that the presence of ECMO hardly affected the CL and V d of GS‐441524, although remdesivir was considered to require increased dosage during ECMO therapy. 14 Significant absorption of drugs in the ECMO circuit is more likely to occur in lipophilic drugs. 23 As the calculated logP and protein binding of GS‐441524 are relatively higher (1.65% and 87.9%, respectively), its plasma concentration is supposed to be low. 14 However, the presence of ECMO did not affect GS‐441524 concentrations in this study. Consequently, we suggest that it is not necessary to increase the dosage of remdesivir even with ECMO, but the drug should be administered according to the package insert.
There are some major limitations to this study that could be addressed in future research. First, GS‐441524 is a metabolite of remdesivir, but a one‐compartment open model was selected for this study. This is because remdesivir rapidly undergoes metabolic activation with a short half‐life, 15 and the generation profile from remdesivir to GS‐441524 could not be precisely predicted owing to insufficient data at early times after intravenous administration. However, the PK parameters were accurately estimated by the final model and were comparable to those in previous reports. 19 , 20 , 21 The one‐compartment open model could be used to accurately predict GS‐441524 trough concentrations. Second, the number of patients with particularly severe renal dysfunction (eGFRnon‐indexed <30 ml/min) or ECMO in this study was insufficient for an accurate analysis. Furthermore, we could only measure GS‐441524 concentrations at the trough, but not for other metabolites of remdesivir and cyclodextrin. However, there was no difference between the simulated and observed concentrations in patients with an eGFRnon‐indexed less than 30 ml/min. For revision of package inserts, further studies involving larger populations should be conducted in the future. Third, it was difficult to evaluate the correlation between serum GS‐441524 concentrations and its therapeutic benefit in COVID‐19 therapy. There are two reasons for this: (1) estimation of the antiviral effect of the drug is difficult because viral RNA titers quantified using real‐time polymerase chain reaction (RT‐PCR) do not correlate with viable RNA viral load 24 ; and (2) it is difficult to evaluate the specific efficacy of the drug because several drugs are often used in combination with it.
In conclusion, we successfully established a PopPK model of GS‐441524 using retrospectively obtained serum GS‐441524 concentrations in Japanese patients with COVID‐19. PopPK analysis indicated that eGFRnon‐indexed affected the CL of GS‐441524 while ECMO did not. According to the FDA’s emergency use authorization and the Japanese package insert, we do not recommend the use of remdesivir in patients with severe renal dysfunction. However, remdesivir should be sometimes administered to patients with COVID‐19 with eGFRnon‐indexed less than 30 ml/min in real clinical situations. Dosage adjustments scaling to the patients’ eGFRnon‐indexed using PopPK model reported in this study would be helpful for optimal individualized therapy of remdesivir.
CONFLICT OF INTEREST
All authors declared no competing interests for this work.
AUTHOR CONTRIBUTIONS
A.S., K.I., A.Y., and I.I. wrote the manuscript. A.S., A.Y., and I.I. designed the research. A.S., K.I., Y.S., K.M., Y.K., T.N., S.H., N.T., S.K., T.H., M.Y., S.O., T.T., and I.I. performed the research. A.S., A.Y., and K.I. analyzed the data. K.I. and E.I. contributed new reagents/analytical tools.
Supporting information
Table S1‐S2
Supplementary Material
ACKNOWLEDGEMENTS
The authors are grateful to all the medical staff of the Department of Respiratory Medicine and Intensive Care Unit at Kyoto University Hospital.
Sukeishi A, Itohara K, Yonezawa A, et al. Population pharmacokinetic modeling of GS‐441524, the active metabolite of remdesivir, in Japanese COVID‐19 patients with renal dysfunction. CPT Pharmacometrics Syst Pharmacol. 2022;11:94–103. doi: 10.1002/psp4.12736
Funding information
This work was supported by the Institutional Funding of Kyoto University and Grants‐in‐Aid for Scientific Research (KAKENHI) from the Japanese Society for the Promotion of Science (Grant‐in‐Aid for Encouragement of Scientists [21H04234] to A.S.).
REFERENCES
- 1. Eastman RT, Roth JS, Brimacombe KR, et al. Remdesivir: a review of its discovery and development leading to emergency use authorization for treatment of COVID‐19. ACS Cent Sci. 2020;6:672‐683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gordon CJ, Tchesnokov EP, Feng JY, Porter DP, Götte M. The antiviral compound remdesivir potently inhibits RNA‐dependent RNA polymerase from Middle East respiratory syndrome coronavirus. J Biol Chem. 2020;295:4773‐4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chan JF, Kok K‐H, Zhu Z, et al. Genomic characterization of the 2019 novel human‐pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect. 2020;9:221‐236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019‐nCoV) in vitro. Cell Res. 2020;30:269‐271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sheahan TP, Sims AC, Leist SR, et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS‐CoV. Nat Commun. 2020;11:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid‐19 ‐ final report. N Engl J Med. 2020;383:1813‐1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Spinner CD, Gottlieb RL, Criner GJ, et al. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID‐19: a randomized clinical trial. JAMA. 2020;324:1048‐1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. European Medicines Agency . Summary on compassionate use: Remdesivir Gilead. https://www.ema.europa.eu/en/documents/other/summary‐compassionate‐use‐remdesivir‐gilead_en.pdf (2020). Accessed May 27, 2021.
- 9. Chan L, Chaudhary K, Saha A, et al. AKI in hospitalized patients with COVID‐19. J Am Soc Nephrol. 2021;32:151‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ng JH, Hirsch JS, Wanchoo R, et al. Outcomes of patients with end‐stage kidney disease hospitalized with COVID‐19. Kidney Int. 2020;98:1530‐1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang Y, Zhang D, Guanhua DU, et al. Remdesivir in adults with severe COVID‐19: a randomised, double‐blind, placebo‐controlled, multicentre trial. Lancet. 2020;395:1569‐1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shekar K, Roberts JA, Mcdonald CI, et al. Sequestration of drugs in the circuit may lead to therapeutic failure during extracorporeal membrane oxygenation. Crit Care. 2012;16:R194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mulla H, Lawson G, von Anrep C, et al. In vitro evaluation of sedative drug losses during extracorporeal membrane oxygenation. Perfusion. 2000;15:21‐26. [DOI] [PubMed] [Google Scholar]
- 14. Chaijamorn W, Rungkitwattanakul D, Nuchtavorn N, et al. Antiviral dosing modification for coronavirus disease 2019‐infected patients receiving extracorporeal therapy. Crit Care Explor. 2020;2:e0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Warren TK, Jordan R, Lo MK, et al. Therapeutic efficacy of the small molecule GS‐5734 against Ebola virus in rhesus monkeys. Nature. 2016;531:381‐385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Imai E, Horio M, Nitta K, et al. Estimation of glomerular filtration rate by the MDRD study equation modified for Japanese patients with chronic kidney disease. Clin Exp Nephrol. 2007;11:41‐50. [DOI] [PubMed] [Google Scholar]
- 17. Seiberth S, Bauer D, Schönermarck U, et al. Correct use of non‐indexed eGFR for drug dosing and renal drug‐related problems at hospital admission. Eur J Clin Pharmacol. 2020;76:1683‐1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. Prediction‐corrected visual predictive checks for diagnosing nonlinear mixed‐effects models. AAPS J. 2011;13:143‐151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Humeniuk R, Mathias A, Cao H, et al. Safety, tolerability, and pharmacokinetics of remdesivir, an antiviral for treatment of COVID‐19, in healthy subjects. Clin Transl Sci. 2020;13:896‐906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Humeniuk R, Mathias A, Kirby BJ, et al. Pharmacokinetic, pharmacodynamic, and drug‐interaction profile of remdesivir, a SARS‐CoV‐2 replication inhibitor. Clin Pharmacokinet. 2021;60:569‐583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lutz JD, Mathias A, German P, Pikora C, Reddy S, Kirby BJ. Physiologically‐based pharmacokinetic modeling of remdesivir and its metabolites to support dose selection for the treatment of pediatric patients with COVID‐19. Clin Pharmacol Ther. 2021;109:1116‐1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pruijssers AK, George AS, Schäfer A, et al. Remdesivir inhibits SARS‐CoV‐2 in human lung cells and chimeric SARS‐CoV expressing the SARS‐CoV‐2 RNA polymerase in mice. Cell Rep. 2020;32:107940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wildschut ED, Ahsman MJ, Allegaert K, Mathot RA, Tibboel D. Determinants of drug absorption in different ECMO circuits. Intensive Care Med. 2010;36:2109‐2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sethuraman N, Jeremiah SS, Ryo A. Interpreting diagnostic tests for SARS‐CoV‐2. JAMA. 2020;323:2249‐2251. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S2
Supplementary Material


