We describe a 70‐year‐old woman with stage IV diffuse large B‐cell lymphoma, not otherwise specified (activated B‐cell subtype, non‐BCL2/MYC double expressor) treated from May to October 2019 on the phase III POLARIX trial (polatuzumab vedotin plus R‐CHP versus R‐CHOP), who was subsequently in remission.
In December 2020, she developed dyspnea, fever, and cough, eventually testing positive for SARS‐CoV‐2. Given progressive persistent symptoms, she was hospitalized for seven days in January 2021 and treated with broad spectrum antibiotics and dexamethasone with improvement in her symptoms. Following discharge, the patient endorsed unabating low‐grade fevers and fatigue into February 2021. PET/CT imaging revealed new FDG‐avid para‐aortic, iliac, and inguinal lymphadenopathy concerning for recurrent lymphoma. The largest right external iliac lymph node measured 2.7 × 1.3 cm with an SUVmax of 8.7, and the largest right inguinal lymph node measured 1.9 × 1.1 cm with an SUVmax of 9.5. A bone marrow biopsy was negative for B cell lymphoma and featured mildly increased polytypic plasma cells and a prominent population of large granular lymphocytes (T‐LGL), as well as pathogenic genetic variants in DNMT3A, TET2, and STAT3.
An excisional inguinal lymph node biopsy was performed, which showed interfollicular expansion by numerous small lymphocytes, epithelioid histiocytes, and focal sheets of plasmacytoid cells with mostly round nuclei, clumped to moderately condensed chromatin, variably distinct nucleoli, and moderate to abundant amounts of amphophilic cytoplasm. High endothelial venules were prominent. Large lymphoid cells were not seen. Flow cytometric analysis did not show involvement by a B‐ or T‐cell lymphoproliferative disorder, and there was no increase in T‐LGLs.
Immunoperoxidase and in situ hybridization studies revealed that the plasmacytoid cells were positive for CD38, MUM1, and CD79a, and were negative for CD138, CD56, cyclin D1, CD117, and Epstein–Barr virus‐encoded small RNAs (EBER). The cells showed excess kappa surface light chain expression compared to lambda, and most of them expressed IgG heavy chain, and were negative for IgA, IgD, and IgM. The Ki67 proliferation index was overall 5%. CD3 stain highlighted small T cells in interfollicular areas, and CD20 stain marked subcortical aggregates of small B cells that co‐expressed PAX5 and CD79a. A small germinal center containing BCL6 and CD10 positive cells was seen.
Cytogenetic analysis demonstrated a normal karyotype. Molecular analysis did not identify a clonal immunoglobulin heavy chain (IGH) gene rearrangement. Serologic studies were notable for a preserved kappa:lambda ratio, and serum protein electrophoresis showed a biclonal gammopathy with IgG lambda and IgG kappa paraproteins migrating together by immunofixation (Image 1).
IMAGE 1.
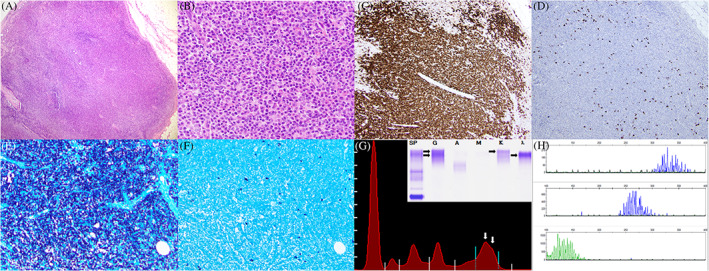
Excisional biopsy of an enlarged inguinal lymph node (A; ×40) with architectural effacement by an atypical proliferation of plasmacytoid cells (B; ×400). Immunoperoxidase studies reveal that the cells are strongly positive for CD38 (C; ×100) with virtually absent staining for CD138 (D; ×100). In situ hybridization reveals monotypic expression of kappa light chains (E; ×100) compared to lambda light chains (F; ×100). Serum protein electrophoresis (G) reveals a biclonal gammopathy (white arrows; M‐spike of 1.23 g/dL) with IgG lambda and IgG kappa paraproteins by immunofixation (inset; black arrows). Molecular analysis demonstrates a polyclonal pattern of IGH gene rearrangement in frameworks I, II, and III (H)
During follow‐up, PET/CT imaging after 5 months showed resolving lymphadenopathy and no evidence of recurrent malignancy. While the patient's large B‐cell lymphoma remains in remission, the population of monotypic plasmacytoid cells in her inguinal lymph node is very unusual. It demonstrates expression of many plasma cell antigens, with the notable exception of CD138. The monotypic kappa light chain expression is particularly concerning for a clonal neoplasm with plasmacytic differentiation; however, an IGH gene rearrangement was not detected. Instead, the morphologic and immunophenotypic findings favor an atypical reactive response, possibly to SARS‐CoV‐2. Recent literature has documented the presence of plasmacytoid cells in the peripheral blood of COVID‐19 patients 1 , 2 and interfollicular expansion of plasmablasts has also been previously identified within COVID‐19‐infected lymph nodes. 3 This case demonstrates that new lymphadenopathy in the setting of COVID‐19 infection or recovery may not always represent disease recurrence in patients with a prior malignancy, and a “wait‐and‐see” approach may be warranted for management. Moreover, pathologists should carefully and thoroughly exclude reactive changes before diagnosing neoplasia in the setting of recent SARS‐CoV‐2 infection.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
Evans MG, Crymes A, Crombie JL, Basu SS, Dillon DA, Wong WJ. Monotypic plasmacytoid cells mimicking lymph node malignancy in the setting of COVID‐19 recovery. Am J Hematol. 2022;97(5):666-667. doi: 10.1002/ajh.26408
REFERENCES
- 1. Harris CK, Hung YP, Nielsen GP, Stone JR, Ferry JA. Bone marrow and peripheral blood findings in patients infected by SARS‐CoV‐2. Am J Clin Pathol. 2021;155(5):627‐637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pozdnyakova O, Connell NT, Battinelli EM, Connors JM, Fell G, Kim AS. Clinical significance of CBC and WBC morphology in the diagnosis and clinical course of COVID‐19 infection. Am J Clin Pathol. 2021;155(3):364‐375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kaneko N, Kuo HH, Boucau J, et al. Loss of Bcl‐6‐expressing T follicular helper cells and germinal centers in COVID‐19. Cell. 2020;183(1):143‐157.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]


