Abstract
Background
Nutritional status of patients with COVID‐19 can affect the recovery process of patients; however, no nutritional scale was introduced to evaluate the nutritional status of the patients. Thus, the main objective of this study was to examine the usefulness of Nutritional status‐2002 (NRS‐2002) among COVID‐19 patients admitted to the intensive care unit (ICU).
Material and Methods
In this cross‐sectional study, 73 patients with definitive corona diagnosis admitted to the ICUs of Al‐Zahra hospital, Isfahan, Iran in October 2020 to January 2021 were recruited. Dietary intake, NRS‐2002, demographic, anthropometric and biochemical indices of patients were recorded.
Results
The majority of patients were at risk for moderate (69.9%) to severe (12.3%) malnutrition. Daily calorie intake (P = .001) and albumin (P = .001) levels in deceased patients were significantly lower than the recovered group. A direct correlation between NRS‐2002 and age (P < .001) and an inverse correlation with daily calorie intake (P = .002), albumin (P = .05) and PaO2 (P = .034) was found. Moreover, there is a strong correlation between NRS‐2002 score and chance of death among COVID‐19 patients (OR=34.5, 95%CI:(5.2 ‐ 228.93), P‐value<0.001). Likewise, the levels of bilirubin direct (OR=8, 95%CI:(1.30 ‐ 49.38), P‐value=0.025) and creatine‐phosphokinase (OR=0.9, 95%CI:(0.99 ‐ 1.00), P‐value=0.035) have a significant direct association with chance of death.
Conclusion
Results showed patients with COVID‐19 admitted to the ICU did not have appropriate nutritional status and mortality was higher among patients with lower amounts of the serum albumin and daily calorie intakes. Furthermore, there is a strong association between the NRS‐2002 index and the chance of mortality in these patients.
What's known
NRS‐2002 is a useful scale to prognosis nutritional status of critically ill patients. We investigated the usefulness of the NRS‐2002 for COVID‐19 patients who are admitted to the intensive care unit (ICU).
What's new
NRS‐2002 can be used among COVID‐19 ICU patients to evaluate their nutritional status and obtain a better perspective before the beginning of the nutritional intervention of the patients.
1. INTRODUCTION
COVID‐19 is a highly contagious infectious disease caused by a new coronavirus (SARS‐CoV‐2) that can be transmitted from person to person through close contact, 1 , 2 , 3 and each person infected can contaminate an average of about three other people with the virus. 4 SARS‐CoV‐2 is a single‐stranded positive‐sense RNA and, like SARS and Merc, belongs to the beta‐coronavirus cluster. 5 This new disease has several complications including coagulopathy, electrolyte imbalance and involvement of respiratory system, cardiovascular, kidney, hematologic, liver, endocrine, gastrointestinal tract, skin, ocular, central nervous system, neuromuscular system and can finally lead to multi‐organ failure and death. 6 Unfortunately, there is still no effective and approved medication treatment for this disease. 7 Therefore, it is important to find alternative methods to prevent and control the spread of the virus.
In the meantime, maintaining and achieving the desired nutritional status to fight the virus is of great importance. 8 Eating a balanced and healthy diet that contains all the essential nutrients is vital to staying healthy. Balance in micronutrients is a key factor in maintaining a healthy immune system. 9 Today, malnutrition is a common and often unknown problem in hospitalised patients. 10 The global prevalence of hospital malnutrition is between 20% and 50%, which is close to 43% in Iran. 11 , 12 , 13 , 14 Malnutrition can lead to decreased body mass, respiratory muscle mass and strength, cardiac efficiency, nutrient uptake, bed sores, delayed wound healing, increased risk of venous thromboembolism and renal disorders. Taken together, these complications can lead to disease progression in patients with COVID‐19. 15 This is especially true in critically ill patients, as the catabolic state induced by the systemic inflammatory response to critical illness or trauma markedly increases metabolic demands, thereby accelerating the development of malnutrition and further increasing the risk of infectious complications, multiorgan dysfunction and mortality. 16 , 17
Hence, maintaining homeostasis and appropriate nutritional status is one of the main pillars of scientific and care management. 18 Consequently, nutritional assessment tools have been developed to assess the nutritional status of patients, one of the most important of them is the nutritional risk screening 2002 (NRS‐2002). 19 This tool assesses nutritional risk by using the following three components: nutritional status, severity of disease and patient age. Compared to other screening tools that only based on patients age and underlying disease severity, the NRS‐2002 is based on three other variables too: weight loss, BMI and food intake in the past week. 20 , 21
Therefore, since COVID‐19 disease is a viral disease with a high prevalence and has side effects such as anorexia and weight loss, 22 it is substantial to evaluate the nutritional status of these patients. Thus, the use of an appropriate and efficient monitoring and screening system is essential for the management and treatment of patients with COVID‐19. For that reason, this study was performed to determine the effectiveness of the NRS‐2002 in patients with cardiorespiratory problems due to COVID‐19 admitted to the intensive care unit (ICU).
2. MATERIALS AND METHODS
2.1. Study design
This cross‐sectional study was conducted on COVID‐19 patients admitted to the ICU of Al‐Zahra hospital in Isfahan city in January 2021. Patients with definitive diagnosis of COVID‐19 based on reverse transcription polymerase chain reaction (RT‐PCR), and hospitalised in the ICU were recruited to this study. Nevertheless, patients who were unable to stand on the weight and height scales during hospitalisation were excluded from the study. Patients who received parenteral nutrition, and death of patients before nutritional assessments were also considered as exclusion criteria. All the participants filled the written consent before entering the study. The whole study protocol was approved by the ethics committee of Isfahan University of Medical Sciences (ethical code: IR.MUI.MED.REC.1399.243).
2.2. Data collection and variables
We used the Nutrition Risk Screening‐2002 (NRS‐2002) tool to assess the nutritional status of patients. NRS‐2002 is a simple and valid tool using for initial screening includes four questions about body mass index (BMI), weight loss in the last 3 months, reduction of dietary intake in the last week and the severity of the disease. If all the questions get a negative score, patients will be re‐screened at weekly intervals; otherwise, the final screening, which includes substitute measures of nutritional status and data on disease severity, will be performed. The score of each parameter can be between 0 and 3. Also, age over 70 is also included in screening scores as a risk factor and with a score of one. Finally, if the total score is greater than or equal to three, it means that the patient is malnourished or at risk of malnutrition and needs nutritional care. 23
In this study, after admitting patients to the ICU and reviewing inclusion criteria, data including demographic information, acute physiology and chronic health evaluation II (APACHE II) criteria measurements, weight loss in the last 3 months, last week's food intake, previous illness and the severity of current disease was completed and recorded in the appropriate form. Also, initially, nutritional status of patients was evaluated by an experienced nutrition expert. Daily calorie intake of patients during admission in the ICU were also recorded. Patients' weight was recorded with 0.1 kg accuracy and height was measured in 0.1 cm accuracy with Seca scales and BMI was calculated using the method: weight(kg)/height2 (height in metres squared). Due to the critical illness of some patients, if patients who admitted to the ICU were unable to stand on the weight scale, we used the weight and height which were recorded in the hospital admission in the previous week.
To determine the percentage of weight loss in the last 3 months, data about usual weight (weight of the last 3 months) and the patient's current weight were collected and recorded.
Mid‐arm circumference (MAC) was measured using an inelastic metre. To assess the reduction of dietary intake, initially, a 24‐hour dietary recall was completed for each patient for a day before being admitted to the ICU by retrospective self‐report. Medical records, which contained dietary records of patients were also used for individuals who have been hospitalised before the ICU.
Based on the above information and the description of the Supplementary Table, patients were classified into three groups: high‐risk, moderate‐risk and low‐risk malnutrition. 20
To evaluate the severity of the disease (Supplementary Table), score one is awarded to chronic hospitalised patients due to complications of the disease, weak patients but out of bed and patients with increased protein requirements that can be provided through oral diet and supplements. Score two is awarded to patients lying in bed due to the severity of the disease and the patient with increased protein levels, which in most cases can be provided through complementary feeding. Moreover, score three is given to patients admitted to the ICU and dependent on the ventilator and patients with increased protein levels that cannot be provided by artificial feeding. 20
Finally, a nutritional care programme for all patients with the following conditions will be considered: (1) patients with severe malnutrition (score=3), or (2) severe ill patients (score=3), or (3) patients with moderate malnutrition +mild illness (score 2 + 1), or (4) patients with mild malnutrition +moderate illness (score 1 + 2). 20
Blood samples (5 ml) were obtained early in morning after about 6 hours fast in the first day of hospitalisation in the ICU, and were centrifuged at room temperature for 10 min to isolate serum, and stored at −80°C. Enzymatic methods and auto‐analyser were used to measure serum albumin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), bilirubin, lactate dehydrogenase (LDH), Creatine phosphokinase (CPK) and C reactive protein (CRP) using commercial kits (Pars Azmun, Karaj, Iran). Arterial blood gas (ABG) was also taken while the patient was breathing room air.
2.3. Statistical analysis
Q‐Q plot, skewness statistics and Kolmogorov‐Smirnov test were used for determining the normal distribution of variables. The logarithmic transformation approach was applied for those variables with an abnormal distribution. Data were reported as mean and standard deviation (SD) or median [IQR] (for continuous data) and frequency and percentage (for categorical data). The distribution of patients across death status in terms of demographic characteristics, anthropometric and biochemical indices, nutritional status and Illness severity of the COVID‐19 was examined using the Chi‐square test or independent sample t‐test. Correlation analysis was used for assessing the relationship between different parameters with nutritional status index (NRS). Analysis of variance (ANOVA) was used to compare continuous variables across different NRS index scores. To examine the relationship between different parameters and death status, we used binary logistic regression analysis. Data analyses were performed using SPSS version 21 (IBM Corp, Armonk, NY, USA). P‐values <0.05 were considered statistically significant.
3. RESULTS
A total of 82 patients admitted to the ICU of Al‐Zahra hospital were included in the present study. Four patients were excluded from the study due to death before nutritional screening and five patients were unable to stand on the scales. Thus, data analysis was performed on 73 patients (Figure 1). Demographic characteristics, anthropometric and biochemical indices of patients are shown in Table 1.
FIGURE 1.
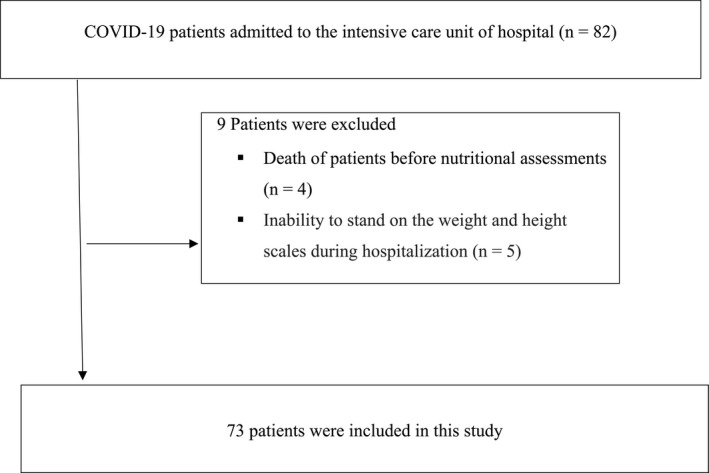
Patients process flowchart
TABLE 1.
Demographic characteristics, anthropometric and biochemical indices, nutritional status and Illness severity of the COVID‐19 patients in intensive care unit
| Variable |
All patients N = 73 |
Dead patients N = 27 |
Recovered patients N = 46 |
p‐value |
|---|---|---|---|---|
|
Age (year) (Mean ±SD) |
58.9 ± 18.8 | 67.7 ± 16.6 | 54.1 ± 18.6 | 0.004 |
|
Sex (frequency(percent)) |
||||
| Male | 46(63%) | 15(55.6%) | 31(67.4%) | 0.312 |
| Female | 27(37%) | 12(44.4%) | 15(32.6%) | |
| Duration of hospitalisation(day) a |
28.3 ± 22.4 23 [12.5‐34.5] |
26.6 ± 22.7 20 [12‐31] |
29.3 ± 22.4 24 [13‐41.5] |
0.625 |
| Duration of hospitalisation in ICU(day) a |
17.2 ± 14.8 12 [7‐26] |
15.9 ± 11 12 [9‐22] |
18 ± 16.7 11 [6‐27] |
0.981 |
| Daily calorie intake (kcal) a | 1992.7 ± 378.8 | 1821.1 ± 274.7 | 2093.4 ± 397.4 | 0.001 |
|
Mid arm circumference (cm) a (Mean ±SD) |
27.9 ± 3.9 | 27.7 ± 4.4 | 28 ± 3.7 | 0.819 |
| Albumin(g/dl) a | 3.2 ± 0.5 | 3 ± 0.4 | 3.4 ± 0.6 | 0.001 |
| ALT(IU/L) a | 41.5 ± 33.2 | 43 ± 30.5 | 40.6 ± 34.9 | 0.761 |
| AST(IU/L) a | 61 ± 42.4 | 62.8 ± 34.5 | 60 ± 46.8 | 0.783 |
| Bilirubin total(mg/dl) a |
1.1 ± 1.2 0.8 [0.5‐1.1] |
1.2 ± 1 0.9 [0.6‐1.5] |
1 ± 1.3 0.8 [0.5‐1] |
0.131 |
| Bilirubin direct(mg/dl) a |
0.3 ± 0.4 0.2 [0.2‐0.4] |
0.5 ± 0.5 0.3 [0.2‐0.7] |
0.3 ± 0.2 0.2 [0.2‐0.3] |
0.027 |
| LDH(U/L) a | 827.3 ± 498.3 | 814.6 ± 324.4 | 835 ± 582.4 | 0.871 |
| CPK(U/L) a | 234.5 ± 144.8 | 176.4 ± 115.2 | 267.3 ± 150.5 | 0.006 |
| CRP(mg/L) a | 51.5 ± 37.7 | 58.5 ± 36.8 | 47.3 ± 38 | 0.224 |
| PaO2(mmHg) a | 56.4 ± 25.4 | 50.7 ± 21.3 | 59.9 ± 27.3 | 0.140 |
|
Nutrition status (frequency(percent)) |
||||
| Low risk | 13(17.8%) | 2(7.4%) | 11(23.9%) | 0.057 |
| Moderate risk | 51(69.9%) | 19(70.4%) | 32(69.6%) | |
| High risk | 9(12.3%) | 6(22.2%) | 3(6.5%) | |
|
Illness severity (frequency(percent)) |
||||
| Light | 0(0%) | 0(0%) | 0(0%) | <0.001 |
| Moderate | 49(67.1%) | 8(29.6%) | 41(89.1%) | |
| Severe | 24(32.9%) | 19 (70.4%) | 5(10.9%) |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CRP, C reactive protein; LDH, lactate dehydrogenase, CPK, creatine phosphokinase; PaO2, Partial pressure of oxygen.
Mean (SD) or Median[Q1‐Q3]; p‐values were calculated based on independent sample t‐test or Chi‐square test.
The mean age of the patients was 58.9 ± 18.8 years with a range of 20‐94 years. The mean age of deceased patients (67.7 ± 16.6) was significantly higher than recovered patients (54.1 ± 18.6) (P =.004). Forty‐six patients (63%) were male and 27 patients (37%) were female. The mean duration of total and ICU hospitalisation was 28.3 ± 22.4 and 17.2 ± 14.8 days respectively.
Regarding daily caloric intake, the average intake of all patients during admission in the ICU, deceased and recovered patients was 1992.7 ± 378.8, 1821.1 ± 274.7 and 2093.4 ± 397.4 kcal, respectively, which was significantly less in deceased group than the other (P =.001). The mean of MAC in total patients was 27.9 ± 3.9 cm, which did not show a significant difference between the two groups (P =.819) (Table 1).
The average of albumin, ALT, AST, bilirubin total, bilirubin direct, LDH, CPK, CRP and partial pressure of oxygen (PaO2) were 3.2 ± 0.5 g/dl, 41.5 ± 33.2 IU/L, 61 ± 42.4 IU/L, 1.1 ± 1.2 mg/dl, 0.3 ± 0.4 mg/dl, 827.3 ± 498.3 U/L, 234.5 ± 144.8 U/L, 51.5 ± 37.7 mg/L and 56.4 ± 25.4 mmHg, respectively, which only in albumin (P =.001), CPK (P =.006) and bilirubin direct (P =.027) indices, a significant difference was observed between the deceased and recovered groups (Table 1).
Overall, 17.8%, 69.9% and 12.3% of the patients were at risk for mild, moderate and severe malnutrition respectively. About severity of illness, 67.1% and 32.9% of the patients had moderate and severe illness respectively. Also, this index was significantly different in the two groups (P <.001) (Table 1).
Table 2 shows the mean of patients' demographic, anthropometrics and laboratory findings and the correlation of these parameters with the NRS. According to the results, age (P <.001), daily calorie intake (P =.002) had significant direct and PaO2 (P =.034) had an inverse correlation with NRS. No significant correlation was observed for other indices.
TABLE 2.
The mean of patients' demographic, anthropometrics and laboratory findings and the correlation of these parameters with nutritional status index (NRS)
| Parameter | Mean ±SD | Correlation with NRS | p‐value |
|---|---|---|---|
| Age(year) | 58.9 ± 18.8 | 0.455 | <0.001 |
| Duration of hospitalisation(day) | 28.3 ± 22.4 | 0.108 | 0.361 |
| Duration of hospitalisation in ICU(day) | 17.2 ± 14.8 | 0.126 | 0.291 |
| Daily calorie intake (kcal) | 1992.7 ± 378.8 | ‐0.352 | 0.002 |
| Mid arm circumference(cm) | 27.9 ± 3.9 | ‐0.072 | 0.547 |
| Albumin(g/dl) | 3.2 ± 0.5 | ‐0.230 | 0.050 |
| ALT(IU/L) | 41.5 ± 33.2 | ‐0.184 | 0.120 |
| AST(IU/L) | 61 ± 42.4 | ‐0.155 | 0.191 |
| Bilirubin total(mg/dl) | 1.1 ± 1.2 | 0.024 | 0.842 |
| Bilirubin direct(mg/dl) | 0.3 ± 0.4 | 0.075 | 0.530 |
| LDH(U/L) | 827.3 ± 498.3 | 0.016 | 0.897 |
| CPK(U/L) | 234.5 ± 144.8 | ‐0.131 | 0.274 |
| CRP(mg/L) | 51.5 ± 37.7 | 0.041 | 0.731 |
| PaO2(mmHg) | 56.4 ± 25.4 | ‐0.252 | 0.034 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CRP, C reactive protein; LDH, lactate dehydrogenase, CPK, creatine phosphokinase; PaO2, Partial pressure of oxygen.
Table 3 presents the mean of demographic, anthropometric and laboratory indices in the groups with different NRS index scores. As a whole, 12, 31 and 30 patients were in the group with NRS scores of 3, 4 and 5≤ respectively. According to the results, the mean of age showed an incremental trend with increase in the NRS score (P =.001), while daily caloric intake (P =.003), albumin (P =.010) and AST (P =.016) showed a decremental trend in different NRS score groups. Also, in the case of ALT, a marginal difference was observed between different NRS scoring groups (P =.051). No significant differences were observed for other indices.
TABLE 3.
The mean of demographic, anthropometric and laboratory indices in the groups with different NRS index scores
| NRS score |
Score 3 N = 12 |
Score 4 N = 31 |
Score 5≤ N = 30 |
p‐value |
|---|---|---|---|---|
|
Parameters (Mean ±SD) or Median[Q1‐Q3] | ||||
| Age(year) | 45 ± 16.2* | 55.8 ± 16.8 | 67.7 ± 17.9* | 0.001 |
| Duration of hospitalisation(day) |
29.7 ± 31.7 17 [10.2‐41.5] |
26.1 ± 18.1 22 [12‐32] |
30.1 ± 22.6 24.5 [13‐38.2] |
0.624 |
| Duration of hospitalisation in ICU(day) |
14.1 ± 12.9 10.5 [6.7‐13.7] |
18 ± 18.1 10.5 [6‐24] |
17.7 ± 11.7 13 [8.7‐28.5] |
0.494 |
| Daily calorie intake (kcal) | 2275 ± 292.7* | 2019.3 ± 492.2 | 1852.3 ± 281.1* | 0.003 |
| Mid arm circumference (cm) | 27.9 ± 4 | 28 ± 3.6 | 27.7 ± 4.3 | 0.959 |
| Albumin (g/dl) | 3.2 ± 0.6 | 3.5 ± 0.6* | 3 ± 0.5* | 0.010 |
| ALT (IU/L) | 61.5 ± 38.6* | 34.2 ± 22.3* | 41 ± 37.8 | 0.051 |
| AST (IU/L) | 92.3 ± 57.7*(a, b) | 52.4 ± 36.1*(a) | 57.4 ± 36.9*(b) | 0.016 |
| Bilirubin total (mg/dl) |
1.1 ± 1.2 1 [0.6‐1] |
1.2 ± 1.6 0.8 [0.5‐1.1] |
1 ± 0.6 0.9 [0.5‐1.2] |
0.943 |
| Bilirubin direct (mg/dl) |
0.4 ± 0.7 0.3 [0.2‐0.3] |
0.3 ± 0.3 0.2 [0.2‐0.3] |
0.3 ± 0.3 0.3 [0.2‐0.5] |
0.597 |
| LDH (U/L) | 855.9 ± 285.5 | 818.1 ± 683.5 | 825.7 ± 326.1 | 0.978 |
| CPK (U/L) | 291.6 ± 176.7 | 221 ± 125.7 | 225.2 ± 149.1 | 0.328 |
| CRP (mg/L) | 54.6 ± 39.5 | 46.2 ± 39.5 | 55.8 ± 35.5 | 0.593 |
| PaO2 (mmHg) | 69.2 ± 29.5 | 58.4 ± 26.2 | 49.2 ± 21 | 0.59 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CRP, C reactive protein; LDH, lactate dehydrogenase, CPK, creatine phosphokinase; PaO2, Partial pressure of oxygen.
represent the significance between scores 3 and 4.
represent the significance between scores 4 and 5≤.
show significant difference between groups; p‐values were calculated based on analysis of variance (ANOVA).
As shown in Table 4, there is a strong significant correlation between NRS score and mortality (OR=34.5, 95%CI:(5.2 ‐ 228.93), P‐value<0.001). Likewise, the levels of bilirubin direct (OR=8, 95%CI:(1.30 ‐ 49.38), P‐value=0.025) and creatine‐phosphokinase (OR=0.9, 95%CI:(0.99 ‐ 1.00), P‐value=0.035) have a significant direct association with chance of death among these patients.
TABLE 4.
The effective of NRS index, age, calorie intake, albumin, bilirubin, creatine phosphokinase on mortality of COVID‐19 patients
| Variable | Odds ratio | 95% confidence interval |
|---|---|---|
| NRS | 34.5 | [5.2‐228.93]** |
| Age | 0.9 | [0.93‐1.03] |
| Calorie intake | 0.9 | [0.99‐1.00] |
| Albumin | 0.2 | [0.04‐1.57] |
| Bilirubin direct | 8 | [1.30‐49.38]* |
| Creatine phosphokinase | 0.9 | [0.99‐1.00]* |
| Nutrition status | 0.3 | [0.05‐2.71] |
P <.05
P <.001.
4. DISCUSSION
Malnutrition is a common and serious problem of patients admitted to ICUs, and this problem is especially important for patients with COVID‐19 who suffer from anorexia, nausea and vomiting. 4 Therefore, it needs special attention. The nutritional status of these patients will have a significant impact on the course of the disease, but so far the nutritional problems of patients with COVID‐19 admitted to the ICU have received less attention. Previous studies have shown that NRS has several advantages to evaluate the status of the patients. For example NRS‐2002 considers the stage of disease effect and would not miss patients who are at nutritional risk because of specific diseases. Another advantage of this tool is that it does not take time and requires less training, in addition to the factors used in other tools such as weight loss, dietary intake, body mass index and underlying diseases, this tool is also adjusted for the age of over 70 years. 21 Also, a review of various nutritional screening methods in patients with COVID‐19 has shown that NRS‐2002 is more sensitive than other screening tools in the past. 24 Therefore, this study was performed to determine the effectiveness of the Nutritional Risk Screening System (NRS‐2002) in patients with cardiorespiratory problems due to COVID‐19 admitted to the ICU.
We found that the mean age of the deceased patients was significantly higher than the recovered patients. Other studies confirmed the results of the present study and reported an increase in mortality with age. 25 , 26 Some other studies have also mentioned old age as a significant risk factor for COVID‐19 mortality. 25 , 27 , 28
About daily caloric intake, the average energy intake of recovered patients was approximately 270 kcal more than that of deceased patients. Less energy intake and increased need to activate and maintain the activity of the immune system in infection can lead to malnutrition, which itself is a risk factor for mortality in patients with COVID‐19. 29 , 30
In terms of albumin, evidence of the present study showed that the mean albumin level in the deceased group was lower than the recovered group. A very recent meta‐analysis published in 2021 showed that low albumin levels in patients with COVID‐19 were associated with higher disease severity and adverse outcomes. 31 Although the exact mechanism of hypoalbuminaemia in COVID‐19 has not yet been determined, one of the proposed mechanisms is that inflammation can reduce serum levels of albumin by increasing capillary permeability and increasing the secretion of serum albumin into the interstitial space and increasing its distribution volume. 31 , 32 , 33
In the case of bilirubin direct, according to our findings, the mean of this index in the deceased group was significantly higher than the improved group. In this regard, results of a pooled analysis study conducted in 2020 showed that elevated bilirubin levels were directly related to disease severity. 34 Some studies believe that the cause of liver test disorders is liver injury caused by COVID‐19 disease or injury induced by medicines used for this disease. 35
The present study showed that the mean CPK level in the patients included in the study was higher than the normal range (20‐200 U/L). 36 It is proposed that increasing in CPK may have occurred as a result of rhabdomyolysis due to COVID‐19 disease. 37 , 38
The study of nutritional status and disease severity in the patients included in the study showed that more than two‐thirds of the patients were at moderate risk of malnutrition (69.9%) and illness severity (67.1%). On the other hand, more than 70% of deceased patients have experienced high‐grade disease. Various aspects such as socioeconomic status, diet, lifestyle, environmental differences, exposed viral load, time of treatment initiation, etc, can affect the nutritional status of the disease in these patients. 39
Investigation of the relationship between different factors and the NRS index has shown that age and NRS had a direct relationship and with increasing age, patients were in a poorer nutritional status. In the current study, it was found that NRS had an inverse significant correlation with daily calorie intake, albumin and PaO2. It is reported that there is an association between malnutrition, age, energy intake and albumin. 40 , 41 , 42 In the case of PaO2, the results of the present study can be reasonable, considering that with increasing malnutrition, the severity of the disease increases and consequently PaO2 decreases was occurred. 15 , 43
According to the results of our study on comparing the age of patients in different NRS score groups, a significant positive trend was observed between the age and NRS score. Thus, the mean age of patients with 5≤NRS score was higher than the other group. Since age is one of the factors involved in calculating NRS score, and older people are at greater risk for malnutrition, the results have been consistent with scientific evidence. 17 , 41
Consideration of daily calorie intake and comparison between different categories of NRS score, it is found that the amount of energy intake of patients with 5≤NRS score is significantly less than the energy intake of the group with three points. Given that the received energy is one of the components of the NRS index, the result is justifiable. 17
Examination of AST levels in groups with different NRS scores also showed a significant difference between groups with NRS score 3 and groups with 4 and 5≤ scores, so that the level of this enzyme in the group with NRS score 3 was higher than the level in the other two groups. On the subject of ALT, a marginal significance was observed between the group with NRS score 3 and the group with score 4. The level of this enzyme was higher in the group with a lower score than the other group. In general, the available evidence has shown that with increasing NRS score, the severity of malnutrition in patients increases. 17 On the other hand, increasing the severity of malnutrition leads to increased levels of hepatic aminotransferases. 44 , 45 However, the findings of our study contradict other evidence, and patients with more severe malnutrition showed lower levels of hepatic aminotransferases. This increase in levels in group with milder malnutrition may be due to previous underlying diseases or may be due to the small sample size of this group compared to other groups. 46
We found a strong significant correlation between NRS score and the chance of death among COVID‐19 patients. In a recent systematic review and meta‐analysis, which was published in 2021, it was found that malnutrition and poor nutritional status with a negative impact on the gastrointestinal, immune and metabolic systems have led to poorer outcomes and ultimately increased mortality risk among patients with COVID‐19. 47 In fact, since the NRS index includes items such as age, nutritional status, comorbidities and disease severity, as well as poor nutritional status predict the severe condition of COVID‐19 disease. Therefore, NRS index can be used as a suitable and practical indicator for prognosis of patients with COVID‐19. 17 , 48 , 49 , 50 , 51 , 52 , 53 Likewise, previous studies have shown the usefulness and applicability of the NRS index in patients with COVID‐19. 54 , 55
4.1. Limitations
The strength of the present study is the evaluation of the nutritional status of patients with COVID‐19 hospitalised in ICU, which can be helpful in future interventional studies and selection of appropriate assessment tools. However, the notable limitations of the present study are as follows: (1) small sample size, (2) impossibility of following up, (3) lack of other clinical tests other than hospital routine tests and (4) Failure to perform sampling from multi‐centres (being single‐centre study).
5. CONCLUSION
The results of present study indicated that a higher score of NRS index significantly increased the odds of mortality in patients with COVID‐19. Likewise, the levels of bilirubin direct and creatine‐phosphokinase have a significant direct correlation with chance of death among these patients. Also, a significant percentage of patients with COVID‐19 in ICUs were in moderate or high‐risk nutritional status that can lead to the development of the disease and increase morbidity and mortality by negatively affecting various indices such as albumin.
DISCLOSURE
The authors declare that they have no competing interests.
Supporting information
Table S1
ACKNOWLEDGEMENT
This study was approved and funded by the Isfahan University of Medical Sciences with grant number 299033.
Alikiaii B, Heidari Z, Fazeli A, et al. Evaluation of the effectiveness of the Nutritional Risk Screening System 2002 (NRS‐2002) in COVID‐19 patients admitted to the intensive care unit. Int J Clin Pract. 2021;75:e14934. doi: 10.1111/ijcp.14934
Sahar Rafiee and Mohammad Bagherniya are equally corresponding author.
Contributor Information
Sahar Rafiee, Email: Rafiee.nut@gmail.com.
Mohammad Bagherniya, Email: Bagherniya@nutr.mui.ac.ir, Email: Bagherniya@yahoo.com.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Banerjee A, Kulcsar K, Misra V, Frieman M, Mossman K. Bats and coronaviruses. Viruses. 2019;11:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Farnoosh G, Alishiri G, Hosseini Zijoud SR, Dorostkar R, Jalali FA. Understanding the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) and coronavirus disease (COVID‐19) based on available evidence‐a narrative review. J Mil Med. 2020;22:1‐11. [Google Scholar]
- 3. Schoeman D, Fielding BC. Coronavirus envelope protein: current knowledge. Virol J. 2019;16:1‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cohen J, Normile D. New SARS‐like virus in China triggers alarm. Science. 2020;367(6475):234‐235. https://www.science.org/doi/abs/10.1126/science.367.6475.234 [DOI] [PubMed] [Google Scholar]
- 5. Chen Y, Liu Q, Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J Med Virol. 2020;92:418‐423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kordzadeh‐Kermani E, Khalili H, Karimzadeh I. Pathogenesis, clinical manifestations and complications of coronavirus disease 2019 (COVID‐19). Future Microbiol. 2020;15:1287‐1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ali MJ, Hanif M, Haider MA, et al. Treatment options for COVID‐19: a review. Front Med. 2020;7:480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aman F, Masood S. How Nutrition can help to fight against COVID‐19 Pandemic. Pak. J Med Sci. 2020;36(COVID19‐S4):S121–S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maciel LRMDA, Franzosi OS, Nunes DSL, et al. Nutritional risk screening 2002 cut‐off to identify high‐risk is a good predictor of ICU mortality in critically ill patients. Nutrition in Clinical Practice. 2019;34(1):137‐141. [DOI] [PubMed] [Google Scholar]
- 10. Rafiee S, Safari Z, Shokri‐Mashhadi N. Current Nutritional Statuses and Gastrointestinal Complications in Critically Ill Patients Admitted to ICUs in Iran: A Cross‐Sectional Study. Nutrition and Food Sciences Research. 2020;7(3):9‐14. [Google Scholar]
- 11. Banks M, Ash S, Bauer J, Gaskill D. Prevalence of malnutrition in adults in Queensland public hospitals and residential aged care facilities. Nutrition & Dietetics. 2007;64(3):172‐178. [Google Scholar]
- 12. Barker LA, Gout BS, Crowe TC. Hospital malnutrition: prevalence, identification and impact on patients and the healthcare system. International Journal of Environmental Research and Public Health. 2011;8(2):514‐527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moghadam AD, Chabok SY, Ramezani F, Leili EK, Rahimi V. Evaluation of nutritional quality and microbial contamination of enteral feeding solutions in hospitalized patients referred to neurosurgical ICU of Poursina Hospital in Rasht. Pejouhandeh. 2010;15(5). [Google Scholar]
- 14. Soeters PB, Reijven PLM, van Bokhorst‐de van der Schueren MAE, et al. A rational approach to nutritional assessment. Clin Nutr. 2008;27(5):706‐716. [DOI] [PubMed] [Google Scholar]
- 15. Constans T. Malnutrition in the elderly. La Revue Du Praticien. 2003;53(3):275‐279. [PubMed] [Google Scholar]
- 16. McClave SA, Taylor BE, Martindale RG, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (ASPEN). J Parenter Enteral Nutr. 2016;40(2):159‐211. [DOI] [PubMed] [Google Scholar]
- 17. Singer P, Berger MM, Van den Berghe G, et al. ESPEN guidelines on parenteral nutrition: intensive care. Clin Nutr. 2009;28(4):387‐400. [DOI] [PubMed] [Google Scholar]
- 18. Wojzischke J, Flerchinger C, Marienfeld S, Bojunga J. Malnutrition in the hospital. Identifying Risk Patients. Pflege Zeitschrift. 2014;67(3):166‐169. [PubMed] [Google Scholar]
- 19. Khalili H, Mojtahedzadeh M, Oveysi M, Tavakoli F. Do critically ill patients receive adequate nutritional support? 2004.
- 20. Kondrup J, Rasmussen HH, Hamberg O, Stanga Z, Group AahEW . Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr. 2003;22(3):321‐336. [DOI] [PubMed] [Google Scholar]
- 21. Sun Z, Kong X‐J, Jing X, Deng R‐J, Tian Z‐B. Nutritional Risk Screening 2002 as a Predictor of Postoperative Outcomes in Patients Undergoing Abdominal Surgery: A Systematic Review and Meta‐Analysis of Prospective Cohort Studies. PLoS One. 2015;10(7):e0132857‐e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Morley JE, Kalantar‐Zadeh K, Anker SD. COVID‐19: a major cause of cachexia and sarcopenia? Journal of Cachexia, Sarcopenia and Muscle. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Reber E, Gomes F, Vasiloglou MF, Schuetz P, Stanga Z. Nutritional Risk Screening and Assessment. J Clin Med. 2019;8(7):1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ali AM, Kunugi H. Approaches to Nutritional Screening in Patients with Coronavirus Disease 2019 (COVID‐19). International Journal of Environmental Research and Public Health. 2021;18(5):2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kang SJ, Jung SI. Age‐Related Morbidity and Mortality among Patients with COVID‐19. Infect Chemother. 2020;52(2):154‐164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Surveillances V. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID‐19)—China, 2020. China CDC Weekly. 2020;2(8):113‐122. [PMC free article] [PubMed] [Google Scholar]
- 27. Leung C. Risk factors for predicting mortality in elderly patients with COVID‐19: A review of clinical data in China. Mech Ageing Dev. 2020;188:111255‐. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet (London, England). 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schaible UE, Stefan H. Malnutrition and infection: complex mechanisms and global impacts. PLoS Medicine. 2007;4(5):e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mehta S. Nutritional status and COVID‐19: an opportunity for lasting change? Clin Med (Lond). 2020;20(3):270‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Paliogiannis P, Mangoni AA, Cangemi M, Fois AG, Carru C, Zinellu A. Serum albumin concentrations are associated with disease severity and outcomes in coronavirus 19 disease (COVID‐19): a systematic review and meta‐analysis. Clinical and Experimental Medicine. 2021;1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Soeters PB, Wolfe RR, Shenkin A. Hypoalbuminemia: Pathogenesis and Clinical Significance. JPEN J Parenter Enteral Nutr. 2019;43(2):181‐193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Huang J, Cheng A, Kumar R, et al. Hypoalbuminemia predicts the outcome of COVID‐19 independent of age and co‐morbidity. J Med Virol. 2020;92(10):2152‐2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Paliogiannis P, Zinellu A. Bilirubin levels in patients with mild and severe Covid‐19: A pooled analysis. Liver International. 2020;40(7):1787‐1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yu D, Du Q, Yan S, et al. Liver injury in COVID‐19: clinical features and treatment management. Virol J. 2021;18(1):121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Aujla RS, Patel R. Creatine phosphokinase. StatPearls [internet]. 2020. [PubMed] [Google Scholar]
- 37. Chan KH, Farouji I, Abu Hanoud A, Slim J. Weakness and elevated creatinine kinase as the initial presentation of coronavirus disease 2019 (COVID‐19). The American Journal of Emergency Medicine. 2020;38:1548.e1–1548.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rivas‐García S, Bernal J, Bachiller‐Corral J. Rhabdomyolysis as the main manifestation of coronavirus disease 2019. Rheumatology (Oxford). 2020;59(8):2174‐2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gao Y‐D, Ding M, Dong X, et al. Risk factors for severe and critically ill COVID‐19 patients: A review. Allergy. 2021;76(2):428‐455. [DOI] [PubMed] [Google Scholar]
- 40. Eckart A, Struja T, Kutz A, et al. Relationship of nutritional status, inflammation, and serum albumin levels during acute illness: a prospective study. The American Journal of Medicine. 2020;133(6):pp. 713–22. e7. [DOI] [PubMed] [Google Scholar]
- 41. Hickson M. Malnutrition and ageing. Postgrad Med J. 2006;82(963):2‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Saunders J, Smith T. Malnutrition: causes and consequences. Clin Med (Lond). 2010;10(6):624‐627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gong J, Dong H, Xia Q‐S, et al. Correlation analysis between disease severity and inflammation‐related parameters in patients with COVID‐19: a retrospective study. BMC Infect Dis. 2020;20(1):963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hanachi M, Melchior JC, Crenn P. Hypertransaminasemia in severely malnourished adult anorexia nervosa patients: risk factors and evolution under enteral nutrition. Clinical Nutrition (Edinburgh, Scotland). 2013;32(3):391‐395. [DOI] [PubMed] [Google Scholar]
- 45. Karajibani M, Montazerifar F, Hosseini R, Suni F, Dashipour AR, Fadaaeimokhtarkanlo M. The Relationship Between Malnutrition and Liver Enzymes in Hospitalized Children in Zahedan: A Case‐control Study. Zahedan J Res Med Sci. 2021;23(1):e102994. [Google Scholar]
- 46. Omrani‐Nava V, Maleki I, Ahmadi A, et al. Evaluation of Hepatic Enzymes Changes and Association with Prognosis in COVID‐19 Patients. Hepat Mon. 2020;20(4):e103179. [Google Scholar]
- 47. Abate SM, Chekole YA, Estifanos MB, Abate KH, Kabtyimer RH. Prevalence and Outcomes of Malnutrition among Hospitalized COVID‐19 Patients. A Systematic Review and Meta‐Analysis. Clinical Nutrition ESPEN. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chávez‐Tostado M, Cervantes‐Guevara G, López‐Alvarado SE, et al. Comparison of nutritional screening tools to assess nutritional risk and predict clinical outcomes in Mexican patients with digestive diseases. BMC Gastroenterol. 2020;20(1):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Del Giorno R, Quarenghi M, Stefanelli K, et al. Nutritional Risk Screening and Body Composition in COVID‐19 Patients Hospitalized in an Internal Medicine Ward. Int J Gen Med. 2020;13:1643‐1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bonanad C, García‐Blas S, Tarazona‐Santabalbina F, et al. The Effect of Age on Mortality in Patients With COVID‐19: A Meta‐Analysis With 611,583 Subjects. J Am Med Dir Assoc. 2020;21(7):915‐918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhou J, Ma YI, Liu Y, et al. A Correlation Analysis between the Nutritional Status and Prognosis of COVID‐19 Patients. The Journal of Nutrition, Health & Aging. 2021;25(1):84‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fang X, Li S, Yu H, et al. Epidemiological, comorbidity factors with severity and prognosis of COVID‐19: a systematic review and meta‐analysis. Aging. 2020;12(13):12493‐12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ng WH, Tipih T, Makoah NA, et al. Comorbidities in SARS‐CoV‐2 Patients. A Systematic Review and Meta‐Analysis. MBio. 2021;12(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Silva DFO, Lima S, Sena‐Evangelista KCM, Marchioni DM, Cobucci RN, Andrade FB. Nutritional Risk Screening Tools for Older Adults with COVID‐19: A Systematic Review. Nutrients. 2020;12(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Liu G, Zhang S, Mao Z, Wang W, Hu H. Clinical significance of nutritional risk screening for older adult patients with COVID‐19. Eur J Clin Nutr. 2020;74(6):876‐883. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


