Abstract
The development of effective and safe COVID‐19 vaccines is a major move forward in our global effort to control the SARS‐CoV‐2 pandemic. The aims of this study were (1) to develop an inactivated whole‐virus SARS‐CoV‐2 candidate vaccine named BIV1‐CovIran and (2) to determine the safety and potency of BIV1‐CovIran inactivated vaccine candidate against SARS‐CoV‐2. Infectious virus was isolated from nasopharyngeal swab specimen and propagated in Vero cells with clear cytopathic effects in a biosafety level‐3 facility using the World Health Organization’s laboratory biosafety guidance related to COVID‐19. After characterisation of viral seed stocks, the virus working seed was scaled‐up in Vero cells. After chemical inactivation and purification, it was formulated with alum adjuvant. Finally, different animal species were used to determine the toxicity and immunogenicity of the vaccine candidate. The study showed the safety profile in studied animals including guinea pig, rabbit, mice and monkeys. Immunisation at two different doses (3 or 5 μg per dose) elicited a high level of SARS‐CoV‐2 specific and neutralising antibodies in mice, rabbits and nonhuman primates. Rhesus macaques were immunised with the two‐dose schedule of 5 or 3 μg of the BIV1‐CovIran vaccine and showed highly efficient protection against 104 TCID50 of SARS‐CoV‐2 intratracheal challenge compared with the control group. These results highlight the BIV1‐CovIran vaccine as a potential candidate to induce a strong and potent immune response that may be a promising and feasible vaccine to protect against SARS‐CoV‐2 infection.
Keywords: BIV1‐CovIran, COVID‐19, immunisation, inactivated vaccine, SARS‐CoV‐2
Abbreviations
- ADE
antibody‐dependent enhancement
- ALT
Alanine Aminotransferase
- APC
antigen‐presenting cell
- AST
aspartate aminotransferase
- BPL
β‐propiolactone
- COVID‐19
Coronavirus Disease 2019
- CPE
cytopathic effects
- CRP
C‐reactive Protein
- CTL
cytotoxic t lymphocyte
- cVNT
conventional virus‐neutralising test
- DMEM
Dulbecco's modified eagle medium
- EDTA
ethylenediaminetetraacetic acid
- ELISA
enzyme‐linked immunosorbent assay
- FBS
foetal bovine serum
- FHD
full human dose
- HAV
hepatitis A virus
- Hb
haemoglobin
- HCT
haematocrit
- IEC
ion‐exchange chromatography
- JEV
Japanese encephalitis virus
- LDH
lactate dehydrogenase
- MCH
mean corpuscular haemoglobin
- MCHC
mean corpuscular haemoglobin concentration
- MCV
mean corpuscular volume
- MTD
maximum tolerated dose
- PBS
phosphate‐buffered saline
- PBST
phosphate buffered saline with Tween® 20
- PLT
platelet count
- PRRs
pattern recognition receptors
- PV
poliovirus
- RBD
receptor‐binding domain
- RDW
red cell distribution width
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- SDS
sodium dodecyl sulphate
- SEC
size exclusion chromatography
- TCID50
median tissue culture infectious dose
- TBEV
Tick‐borne encephalitis virus
- TEM
transmission electron microscope
- TMB
3,3′,5,5′‐tetramethylbenzidine
- VAERD
vaccine‐associated enhanced respiratory disease
1. INTRODUCTION
The impact of vaccines on human health and control of infectious diseases is one of the brightest triumphs in the history of science. 1 Candidate platforms for coronavirus disease in 2019 (COVID‐19) vaccines are categorised into five major types: recombinant viral vectors (Oxford/AstraZeneca vaccine, Janssen vaccine and Sputnik V), inactivated viruses (Sinopharm, Sinovac, Bharat Biotech and QazCovid), nucleic acid‐based vaccines (Moderna and Pfizer/BioNTech vaccine), protein subunit (Novavax and Soberana) and live attenuated virus (Codagenix). 2 , 3 Inactivated vaccines are safe and effective since they cannot replicate at all in an immunised individual or there is no risk of reversion to a wild‐type form which is capable of causing diseases. 4
Currently, there are six licenced viral vaccines inactivated through either β‐propiolactone (BPL) or formaldehyde. BPL is commonly used as an inactivating reagent for rabies and influenza virus vaccines whereas formaldehyde is used to inactivate poliovirus (PV), hepatitis A virus (HAV), Japanese encephalitis virus (JEV) and tick‐borne encephalitis virus (TBEV) in vaccine development. 5 Initially, back in 1936, chemical inactivation was successfully applied to manufacture the influenza vaccine. 6 Experience with that vaccine led to a formalin‐inactivated polio vaccine developed by Jonas Salk that came into use in 1955. 7 Provost and coworkers prepared a hepatitis A vaccine based on chemical inactivation with formalin in 1986. 8 The high efficacy of the hepatitis A vaccine testifies to the ability of careful inactivation to retain immunogenicity.
Vaccine development procedures depend on the selection of antigens, vaccine platforms, route of administration and regimen number. Since the whole virus has all viral structural proteins, immune cells can recognise all viral immunogenic proteins present in its structure. An inactive SARS‐CoV‐2 vaccine similar to the native virus has four structural proteins, known as the S (spike), E (envelope), M (membrane) and N (nucleocapsid) proteins. 4 The S protein is the main target for neutralising antibodies in all coronaviruses, which is composed of S1 and S2 subunits. SARS‐CoV‐2 spike protein covers several distinct antigenic sites, comprising several receptor‐binding domain (RBD) epitopes along with non‐RBD epitopes. 9 Although antibodies against nucleoprotein (N), which is the most abundant protein in coronavirus, 10 , 11 are unlikely to neutralise the virus, they confer protection against mouse hepatitis virus and coronavirus in mice. 12
The duration of the antibody response for SARS‐CoV‐2 has not been well defined. However, previous longitudinal studies of patients with SARS‐CoV infection report that neutralising SARS‐CoV antibodies last more than 3 years after the onset of symptoms. 13 Inactivated vaccines are formulated with potent adjuvant and need boosters to maintain satisfactory and long‐term immunity. The most commonly used adjuvants in human vaccines are aluminium salts whose activity was defined in 1926. 14 Due to COVID‐19 cytokine storm, 15 , 16 alum, an adjuvant that promotes Th2‐type immunity, may reduce immunopathology and seems to be a suitable option for vaccine formulation. 17
Inactivated SARS‐CoV‐2 vaccines may have better efficacy against variants that have mutations in the spike protein. In the present study, the efficiency and safety of an inactivated whole‐virus SARS‐CoV‐2 candidate vaccine (BIV1‐CovIran vaccine) were determined through preclinical studies.
2. MATERIALS AND METHODS
2.1. Virus isolation and characterisation
All experiments with live SARS‐CoV‐2 viruses were performed in a biosafety level‐3 facility according to World Health Organisation (WHO) guidelines. 18 The virus was isolated from a nasopharyngeal sample from a COVID‐19 patient. Before infecting monolayer Vero cells (ATCC# CCL81), samples were diluted with a viral transfer medium containing antibiotics and passed through a 0.22 μm filter (Sartorius). Infected Vero cells were maintained in Dulbecco’s modified Eagle’s medium (Bio Idea) supplemented with 10% heat‐inactivated foetal bovine serum (FBS) and incubated for 72 h at 37°C in a 5% CO2 incubator. Virus growth was verified through cytopathic effects (CPE), gene detection and electron microscopy. The SARS‐CoV‐2 virus titre was determined by 50% tissue culture infective dose (TCID50). 19 Vero cells (1–2 × 104 cells/mL) were seeded in 96 well plates and incubated for 16–24 h at 37°C temperature. Serial 10‐fold dilutions of virus‐containing samples were added to the 96‐well culture plate and cultured for 3–7 days in a 5% CO2 incubator at 37°C, and cells were checked under a microscope for the presence of CPE. The virus titre was calculated by the Spearman Karber method. 20
After four passages, viral RNA was extracted from the clarified cell culture supernatant of infected Vero cells by RNA extraction kit (Rojetechnologies), and converted to cDNA. Whole‐genome sequencing was carried out using the Sanger method. For evaluation of viral genome sequence the final passage (P10) was sequenced by Sanger sequencing. The sequences are classified as a B4 lineage by PANGO lineage finder. The list of mutations is shown in Supplementary Data 1 (Excel file). In the S region of SARS‐CoV‐2 three mutations were shown that include amino‐acid change D416G, deletion isoleucine 210 and synonymous mutation in 294 (C > T). 3‐UTR and 5‐UTR of the genome were sequenced partially. The quality of the sequences in each mutated position were evaluated. Low quality reads were sequenced again.
2.2. Vaccine preparation
2.2.1. Virus inactivation and its validation
SARS‐CoV‐2 virus culture supernatant was harvested 72 h post‐infection and clarified. After that, the virus particles were inactivated using BPL (1:1400 dilution) at 2–8°C for 20–24 h. According to the WHO guidelines, the recommended gold standard for detection of the residual live virus during the inactivation process and laboratories are the cell culture systems and the use of three blind passages. 21 Therefore, to confirm the effectiveness of the virus inactivation procedure, the inactivated SARS‐CoV‐2 virus was inoculated onto Vero monolayers and incubated at 37°C in a 5% CO2 incubator. Daily CPE monitoring was performed consecutively for three blind passages under a microscope. No CPE was observed in three passages, while positive controls showed 100% CPE.
2.2.2. Purification
The inactivated virus solution was concentrated via ultrafiltration and buffer was exchanged using diafiltration. Purification of the inactivated virus was performed with two methods: ultracentrifugation and chromatography. In the first method, the concentrated inactivated virus solution was layered on the discontinuous sucrose gradient (20%/60% w/v) and centrifuged in an Optima XL‐90 Beckman rotor Ti‐90 (4°C, 3.5 h, 100,000 g). The purified viral particles were harvested from the boundary of the two sucrose layers (20%/60% w/v), and dialysed against phosphate buffer saline (PBS; Bio Idea) overnight at 4°C to eliminate the residual sucrose. 22 , 23 In the second method, the ÄKTA ready chromatography system (GE Healthcare Life Sciences) together with the cellufine sulphate as an affinity chromatography was applied to purify the inactivated virus. Dialysed and clarified inactivated virus solution was loaded on to the column to capture the viral particles. Subsequently, the column was washed with 10 column volume of load buffer (10 mM Na Phosphate, 150 mM NaCl pH 7.4) to through away faintly linked impurities. The inactivated SARS‐CoV‐2 captured on the column was then eluted via 10 mM phosphate buffer, 2.0 M sodium chloride, pH 7.4. 24 , 25 The protein composition of the purified virus was confirmed through SDS‐PAGE analysis followed by staining with silver nitrate and western blot. The total protein concentration was determined by the Bradford assay. The structural integrity of the isolated virus particles was monitored using the transmission electron microscope (TEM). Viral particles were absorbed on the carbon‐coated grids and stained with 1% uranyl acetate. Grids were analysed using a transmission electron microscope (TEM, EM208S, Philips, 100 kV) at final magnifications of 90,000×.
2.2.3. Formulation
Alhydrogel® adjuvant 2% (Brenntag Biosector), referred to as alum, is an aluminium hydroxide wet gel suspension which used as vaccine adjuvant in the current study. The working concentration of Alhydrogel® adjuvant 2% was 250 μg for vaccine used in monkeys and rabbits and it was 50 μg in mice. For vaccine formulation, the purified inactivated viral particles (antigen) were sterilised using filtration. A mixture of alum‐antigen in two doses, a high dose of 5 µg and a low dose of 3 μg, of inactivated viral antigen was prepared and well mixed. 26
2.3. Animal studies
The animals were used in this study were as follow: 6–8 weeks old rodents (BALB/c mice, Guinea pigs and Wistar Rat), lagomorph (3–6 months New Zealand White rabbits) and non‐human primates (3–4 years old Rhesus Macaque). Rodents and rabbits were purchased from the Laboratory Animal Science Department, Pasteur Institute of Iran, Karaj, Iran. The monkeys were purchased from Royan Institute (Tehran, Iran). Housing, feeding and interventions were carried out in accordance with the approval of the animal ethics committee guidelines of the Ministry of Health and Medical Education (Tehran, Iran; ethical code: IR.ACECR.IBCRC.REC.1399.016). Figure 1 shows the schematic diagram of animal studies on the BIV1‐CovIran vaccine.
FIGURE 1.
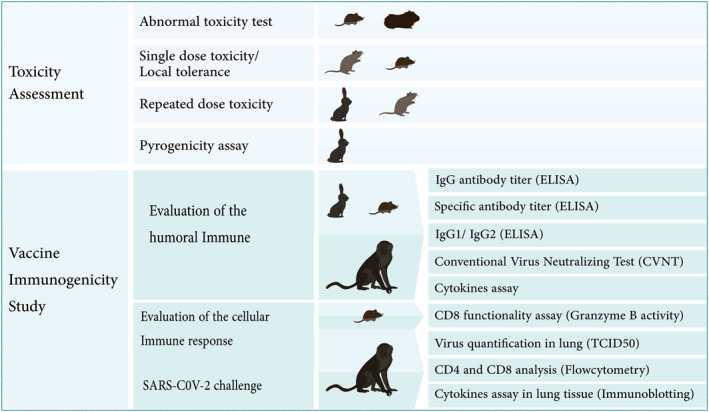
Animal studies for evaluating the safety and efficacy of the BIV1‐CovIran vaccine
2.3.1. Toxicology study
The abnormal toxicity test
To perform the abnormal toxicity test, five SW1 mice 6‐ to 8‐week‐old were injected intraperitoneally with 0.5 mL full human dose vaccine (1 FHD). Additionally, in two guinea pigs, FHD (0.5 mL) was injected intraperitoneally. The injected mice and guinea pigs were weighed daily for 7 days and observed for any evidence of ill‐health. At the end of the 7‐day observation period the mice were sacrificed, and gross examination of organs and tissues were performed by trained veterinary pathologist. The vaccine passes if none of the animal die or show any sign of illness for 7 days. 27 , 28
Single dose toxicity study and local tolerance study
The local tolerance was assessed as part of the single‐dose toxicity by intramuscular administration of full human dose to 6–8 weeks old male and female Wistar rats. This part of toxicity assessment provides preliminary tolerability and safety data to evaluate the acute action of a vaccine. The local tolerance and systemic toxicity of a single intramuscular dose (1 FHD in each 3 and 5 µg formulation and a threefold of 5 µg FHD) of vaccine was assessed in rat (Table 1). In‐life assessment (clinical signs, local reactions and body weight), clinical pathology and histopathology of tissues (brain, liver, kidney, spleen, heart, gonad, site of administration and inguinal lymph node) were evaluated for any adverse alterations. 28 , 29 , 30
TABLE 1.
BIV1‐CovIran inactivated vaccine toxicity assessment in rats
| Treatment | Size | Early sacrifice | Late sacrifice | |
|---|---|---|---|---|
| 1 | Placebo (PBS) | 20 M + 20 F | 10 M + 10 F | 10 M + 10 F |
| 2 | Adjuvant | 20 M + 20 F | 10 M + 10 F | 10 M + 10 F |
| 3 | 3 μg (1 FHD) | 20 M + 20 F | 10 M + 10 F | 10 M + 10 F |
| 4 | 5 μg (1 FHD) | 20 M + 20 F | 10 M + 10 F | 10 M + 10 F |
| 5 | 5 μg (3 FHD) | 20 M + 20 F | 10 M + 10 F | 10 M + 10 F |
| 6 | Vaccine repeated dose | 10 M + 10 F | – | 10 M + 10 F |
Abbreviations: F, female; FHD, full human dose vaccine; M, male.
Repeated dose toxicity study
The primary goal of repeated dose toxicity studies is to characterise the adverse toxicological effects occurring because of repeated boosting with, or exposure, to a vaccine candidate for a specified period up to the expected lifespan of the test species. Male and female Wistar rats (n = 20) were injected 1FHD four times with 2 weeks intervals and 10 New Zealand white rabbits in both sexes administrated by 1FHD vaccine three times (days 0, 14 and 28). In‐life assessment in addition to clinical pathology and histopathology were performed for both rats and rabbits. 27 , 28 , 29 , 30
Rabbit pyrogenicity test
The pyrogen test in rabbits is based on the measurement of the increase in the rabbit’s temperature upon being injected with a product that could contain a contaminant of the pyrogen type. To conduct the pyrogenicity assay, three adult rabbits for three consecutive batches of candidate vaccines were injected. One FHD vaccine substance was injected into the marginal vein of the rabbit’s ear. The post‐injection body temperature of rabbits was measured in the first, second and third hours using a special thermometer. 27
2.3.2. Vaccine immunogenicity analysis
Two doses of the inactivated purified virus (3 and 5 μg) as antigen alone or in combination with alum adjuvant were administrated to different animals to evaluate vaccine immunogenicity. The control group was injected with physiological saline (PBS).
Mice: 1
Ten female BALB/c mice (6–8 weeks) were injected intramuscularly with 100 μL of different doses of the vaccine according to Table 2 on days 0 and 14. About 2, 4 and 5 weeks after the final immunisation, blood samples were collected by puncture of the retro‐orbital vein. The serum samples were taken to evaluate anti‐whole virus particle, anti‐spike glycoprotein (S), anti‐receptor binding domain (RBD) IgG and antibody isotyping profile (IgG1/IgG2a) by Enzyme‐Linked Immunosorbent Assay (ELISA) and also conventional virus‐neutralising test (cVNT) was applied to analyse the vaccine’s immunogenicity.
TABLE 2.
Different formulation used for injection in the mice
| Groups/volume of injection (100 μL) | Abbreviation | |
|---|---|---|
| 1 | 3 μg of inactivated purified virus as antigen without any adjuvant | 3 μg Ag |
| 2 | 5 μg of inactivated purified virus as antigen without any adjuvant | 5 μg Ag |
| 3 | 3 μg of inactivated purified virus as antigen with alum adjuvant | 3 μg Ag + alum |
| 4 | 5 μg of inactivated purified virus as antigen with alum adjuvant | 5 μg Ag + alum |
| 5 | Alum adjuvant | Alum |
| 6 | Control group (PBS) | PBS |
Mice: 2
Ten BALB/c mice were injected with the candidate vaccine using the same protocol presented in Table 2. Two weeks after the final immunisation, serum samples were collected to evaluate the vaccine’s immunogenicity by virus‐neutralising assay. Then, the animals were sacrificed, spleens were isolated and splenocyte culture supernatants were used for evaluating cellular immunity responses by Granzyme B (Gzm B) activity assay.
Rabbit
Fifty New Zealand White rabbits (3–6 months) were divided into two groups: the test and control groups. The test group was injected intramuscularly with 5 μg antigen in combination with alum adjuvant and the control group was injected with PBS. Immunisations were carried out three times (on days 0, 14 and 28) using 5 μg of antigens in combination with alum. After the third injections, the rabbits were bled and individual sera were assayed at 7 and 28 days after the last injection.
Monkey
The Rhesus macaques (Macaca mulatta, 3–4 years old), were divided into three groups (n = 4): in the control group, the animals were injected intramuscularly with physiological saline (PBS) and the two test groups were injected intramuscularly with 3 and 5 μg antigen in combination with alum adjuvant. All macaques were vaccinated on days 0 and 14. A challenge study was carried out 14 days after the second immunisation under anaesthesia through direct intratracheal inoculation of 104 TCID50 of SARS‐CoV‐2. Safety parameters including immunisation evaluation before and after the challenge, daily clinical and general observation, body weight and body temperature were collected and preserved as a database. Peripheral blood was collected on days 0, 14, 21 and 28 post‐immunisations and 10 and 17 days after the challenge.
Analysis of S‐specific and RBD‐specific IgG antibody titre, virus‐neutralising antibody, lymphocyte subset percent (CD4, CD8 and CD20), key cytokines (TNF‐α, IFN‐γ, IL‐4, IL‐6 and IL‐10), biochemical blood test and haematological indices was performed in collected blood samples. Euthanasia was performed by intravenous administration of sodium thiopental (100 mg/kg), followed by complete systematic necropsy and sampling. In addition to macroscopic evaluation of organs and the site of injection, tissue sampling was performed for histopathology. The animals were euthanised on days 7 and 17 post‐challenge. Different organs such as the lung, heart, spleen, liver, kidney and brain were collected. Lung tissue samples were quickly frozen for quantification of the virus load using TCID50, cytokine assay via western blotting, histopathology pattern determination and characterisation of lung immune cell population by immunohistochemistry. 31 , 32
Evaluation of the humoural immune response
SARS‐CoV‐2 specific IgG antibody titre assay by ELISA
In serum samples of the mice groups, the anti‐whole virus particle IgG antibody titre was determined through enzyme‐linked immunosorbent assay (ELISA). Briefly, a 96‐well plates were coated with the purified whole inactivated virus solution (5 μg/mL) in PBS (pH 7.2) by overnight incubation at 4°C. Then the plates were washed three times with PBS containing 0.05% Tween 20 (PBST) and blocked with 5% skim milk in PBS at 37°C for 1.5 h. After the washing step, serial dilutions of sera in PBS were added to the plates and incubated at 37°C for 1.5 h. Horseradish peroxidase‐conjugated goat anti‐mouse IgG antibody (RaziBiotec AP8036) at 1/5000 dilution was added to the wells and after washing with PBST, the plates were incubated for 1.5 h at 37°C. Colourimetric detection was performed using 100 µL 3,3′,5,5′‐Tetramethylbenzidine (TMB, Sigma) as substrate after washing and incubation for 30 min at RT. The reaction was stopped with 50 μL H2SO4 (2 N), and absorbance was measured at 450 nm using an ELISA reader.
Furthermore, in serum samples of the mice, rabbits and monkey groups, S‐specific and RBD‐specific IgG antibody titres were determined by quantitative SARS CoV2 anti‐S IgG and quantitative SARS CoV2 anti‐RBD IgG COVID‐19 IgG ELISA Kit (Pishtazteb) according to manufacturer instructions with slight modification. Briefly, the sera were added to the plates coated with the purified S or RBD proteins and incubated at 37°C for 30 min. The plate was washed five times with washing buffer and then, goat anti‐human IgG HRP conjugated antibody was added to the wells and the plate was incubated for 30 min at 37°C. In this step, HRP‐conjugated goat anti‐mouse IgG antibody (RaziBiotec AP8036) at 1/5000 dilution used in ELISA of mice serums and HRP‐conjugated goat anti‐rabbit (Abcam) at 1/20,000 dilution used in ELISA of rabbit serums. Following the washing step, colourimetric detection was performed using TMB as the substrate for 15 min at RT. The reaction was stopped with 50 μL H2SO4 (2 N), and absorbance was measured at 450 nm using an ELISA reader.
Immunoglobulin (IgG) Subclass (IgG2a/IgG1) assessment
A 96‐well ELISA Maxisorp (96‐well) plates coated overnight at 37°C with 50 μL of SARS‐CoV‐2 (whole inactivated antigen) protein were washed with PBS containing 0.05% Tween 20, retained for 1 h at 37°C with 1% bovine serum albumin (BSA) solution made in 1× PBS/Tween 20, and then washed with (PBS‐0.05% Tween 20). Sera were added to each well and incubated at 37°C for 2 h. The plates were washed with PBS‐Tween 20 (0.1%). After that, horseradish peroxidase‐conjugated goat anti‐mouse IgG and IgG2a (Mouse Monoclonal Antibody Isotyping Reagents ISO2, M5532 and M5657, Sigma) were added to the plate and incubated for 1 h at 37°C. The wells were washed and 100 μL of TMB substrate was added and incubated for 30 min. After stopping the reaction, the absorbance was read at 450 nm by Universal Microplate Reader (Biohit 8000).
SARS‐CoV‐2 virus neutralising test
The conventional virus neutralisation test (cVNT) was performed to evaluate vaccine protectivity and levels of functional antibodies raised against SARS‐CoV‐2. Briefly, 50 μL of twofold serial dilutions of heat‐inactivated sera (at 56°C for 30 min; as triplicates) were mixed with 50 μL of 100 TCID50/mL of SARS‐CoV‐2 in in Dulbecco’s Modified Eagle Medium (DMEM) and incubated for 60 min at 37°C. The virus/serum mixtures were then inoculated onto monolayers of 1–2 × 104 Vero cells in 96‐well plates to attach free viruses for 60 min at 37°C. The supernatant was removed at 1 h post‐infection, the infected cells were washed twice with DMEM and kept in DMEM for 48 h at 37°C in a 5% CO2 incubator. The virus‐specific CPE of each well was recorded under microscopes 72 h post‐infection and the neutralising titre antibody titres are presented as values of the highest dilution inhibiting CPE formation. 33 , 34 , 35 A neutralisation antibody potency <1:4 is negative, while that ≥1:4 is positive.
Blood biochemical and haematological analysis in rhesus macaques
Blood sampling and evaluations were performed weekly. Biochemical markers including glucose, urea, creatinine (Cr), total protein, albumin, C‐reactive proteins (CRP), alanine aminotransferase (ALT), aspartate aminotransferase (AST) and lactate dehydrogenase (LDH) were analysed by an autoanalyser (alpha classic model) using animal specific kits. Haematology including measurement of White blood cell (WBC), Red blood cell (RBC), Neutrophil, Lymphocyte, Monocyte and Eosinophil count, haemoglobin (Hb), haematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH), mean corpuscular haemoglobin concentration (MCHC), red cell distribution width (RDW) and platelet count (PLT) were performed by a cell counter (Nihon Kohden Celltac alpha MEK‐6450 haematology analysers).
Cytokine assay in mice and monkeys
Spleens were isolated from immunised mice 2‐week post‐immunisation, and single cell suspensions were prepared by gentle homogenisation. RBCs were lysed by incubation in RBC lysis buffer (20 mM Tris, 160 mM NH4Cl, pH 7.4) for 5 min at room temperature, and the resulting splenocytes were resuspended at a density of 2 × 106 cells/mL in RPMI 1640 supplemented with 10% FBS, 2 mM glutamine, 100 U/mL penicillin and 100 μg/mL streptomycin after following washing twice with RPMI. Cells were seeded in a flat‐bottom 24‐well plates and stimulated with 10 μg/mL purified inactivated virus and then incubated at 37°C with 5% CO2. Culture supernatants were collected for measurement of different cytokines after 60 h. Cytokine levels were determined by mouse ELISA kits (MABTECH, Inc): IFN‐γ (3321‐1HP‐2), TNF‐α (3511‐1H‐6), IL‐4 (3311‐1H‐20) and IL‐17 (3521‐1H‐20). Values were presented as pg cytokine/mL (mean ± SD). Furthermore, in serum samples of monkey groups, key cytokines including tumour necrosis factor (TNF)‐α, interferon (IFN)‐γ and interleukin (IL)‐1β, IL‐4, IL‐6 and IL‐10 were determined by commercial Quantikine ELISA kits according to manufacturer instructions. Measuring of cytokine levels in serum of monkeys were performed using ELISA kits (R&D system, Inc): IL‐6 (D6050), IL‐10 (D1000B), IL‐4 (DY204), TNF‐α (DTA00) and IFN‐γ (DIF50C). Values were presented as pg cytokine/mL (mean ± SD).
Lung tissue cytokine assessment of rhesus macaques
The lung tissue samples were used for protein extraction with RIPA buffer containing 50 mM Tris–HCl pH 7.4, 150 mM NaCl, 5 mM EDTA, 1% Nonidet P‐40, 1% sodium deoxycholate, 0.1% SDS, 1% aprotinin, 50 mM NaF and 0.1 mM Na3VO4 (CitoMatin Gene) under class II biosafety cabinet. The total protein concentration was determined by the Bradford assay. The assessment of IFN‐γ and IL‐6, IL‐4, IL‐10 in lung extracted proteins was performed through western blot. SDS‐PAGE (Padideh Nojen UV‐80V system with a standard protocol) followed by western blot analysis using anti‐IL‐4 (ab34277), anti‐IL‐6 (ab233706), anti‐IL‐10 (ab133575), anti‐IFN‐γ (ab231036) and anti‐β actin as normalised control (ab170325) according to the standard protocol. Cytokine levels were normalised to the total protein concentration in each lung homogenate. Western blot images were analysed using NIH Image J computer software and the relative intensities of each cytokine bands to β‐actin protein were calculated.
CD4, CD8 and CD20 analysis in the peripheral blood of rhesus macaques
Immune cell subsets in the peripheral blood sample were assessed after challenge. Flow cytometric evaluation by Anti CD4‐PE (1P‐359‐exbio), Anti CD8‐APC (1A‐207‐exbio) and Anti CD20‐PE (1P‐638‐exbio) antibodies was performed in the Bricyte E6‐Mindray cytometer system. The standard flow cytometry process was done for all sample.
Evaluation of the cellular immune response
Gzm B activity assay in mice
The potential of cellular immunity induction was evaluated by Gzm B activity in different vaccine preparations. Spleens were isolated from the immunised mice 2 weeks post‐immunisation, and single‐cell suspensions were prepared by gentle homogenisation. RBCs were lysed by incubation in RBC lysis buffer (20 mM Tris, 160 mM NH4Cl, pH 7.4) for 5 min at RT, and the resulting splenocytes were resuspended at a density of 2 × 106 cells/mL in RPMI 1640 supplemented with 10% FBS, 2 mM L‐glutamine, 100 U/mL penicillin and 100 μg/mL streptomycin after washing twice with RPMI. Cells were seeded in a flat‐bottom 24‐well plate (Nunc) and stimulated with purified inactive whole SARS‐CoV‐2 particles and then incubated at 37°C with 5% CO2. Culture supernatants were collected for measurement of Gzm B activity after 72 h. Gzm B activity was determined using commercial mouse Gzm B ELISA Ready‐SET‐Go kit (88‐8022, eBioscience).
SARS‐CoV‐2 quantification in lungs of challenged rhesus macaques
To remove cell debris, lung homogenates were clarified via centrifugation at 4000 g for 10 min, and the supernatants were then stored at −80°C until the assay was carried out. Vero cells with 80 confluences in 96‐well plates were infected with serial log10 dilutions of clarified lung samples in DMEM. After 72 h of incubation at 37°C in a 5% CO2 atmosphere, the culture supernatants were tested for the presence of SARS‐CoV‐2. The viral titre in each lung specimen was calculated by the Karber method 20 and expressed as the 50% tissue culture infective dose (TCID50).
Histopathology and immunohistochemistry in rhesus macaques
Tissues were fixed in formalin and embedded in paraffin blocks. About 5 μm thick sections were stained with haematoxylin and eosin for histopathological interpretation. About 3 μm thick sections of lung paraffin blocks were used for immunohistochemical staining. Anti‐CD4 (CMGCD4‐S50), Anti‐CD8 (BRB036‐ZYTOMED), Anti‐CD20 (BMS003‐ZYTOMED) and Anti CD68 (MAD‐002097QD) were the markers that were applied for the detection of immune cells in different parts of lung sections. Throughout each slide, 10 randomly chosen fields were evaluated in a high power field (HPF) for counting CD4, CD8, CD20 lymphocytes and CD68 positive macrophages by light microscopy (Nikon Eclipse E600, Japan).
2.4. Statistical analysis
Data were analysed by GraphPad Prism 7 software, using one‐way ANOVA and two‐way ANOVA followed by Tukey’s post hoc test. A P‐value of P ≤ 0.01 was considered statistically significant.
3. RESULTS
3.1. Virus isolation and characterisation
The SARS‐CoV‐2 used in developing the BIV1‐CovIran vaccine was retrieved from a nasopharyngeal sample from a COVID‐19 patient. The sample propagation and virus isolation were performed in the Vero CCL‐81. For efficient growth of viral stock in Vero cells, the isolated virus was first plaque purified and passaged once in Vero cells to generate the P1 stock. After this, three other passages were performed to generate the P2 to P4 stocks. At multiplicities of infection (MOI) of 0.001–0.01, multiplication kinetics analysis of the P4 stock in Vero cells showed that the stock replicated efficiently and yielded a peak titre of 6 log10 TCID50/mL by 3 days after infection (dpi). After four passages, the master and working vaccine seed was prepared. Virus growth and isolation were verified through CPE and gene detection. In terms of the overall divergence of isolated SARS‐CoV‐2, this strain had 99.9% identity to the earliest detected strain, Wuhan Hu‐1. 36 Results showed genetic stability of the isolated virus in multiple passages of Vero cells which is suitable for vaccine development. The flowchart of BIV1‐CovIran vaccine preparation is illustrated in Figure 2.
FIGURE 2.
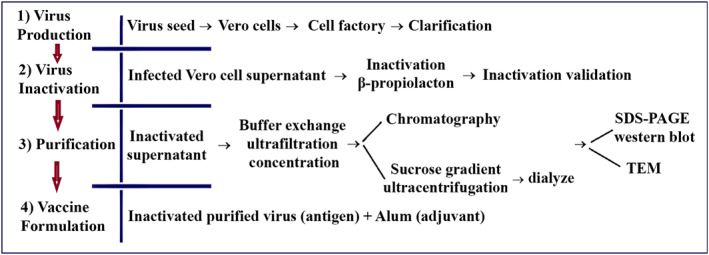
Flowchart of BIV1‐CovIran vaccine preparation
Growth kinetics analysis showed that the virus replicated to 7.0 log10 TCID50 at 72 h post‐infection. The infected Vero cell culture supernatant was harvested 72 h post‐infection and viral particles were inactivated via BPL. To verify the inactivation process, the third blind passage was carried out for three generations from the inactivated virus and did not show any CPE (Figure 3a–c). The inactivated virus was concentrated through ultrafiltration and purified using ion‐exchange and size exclusion chromatography or the discontinuous sucrose gradient (20/60% w/v) ultracentrifugation.
FIGURE 3.
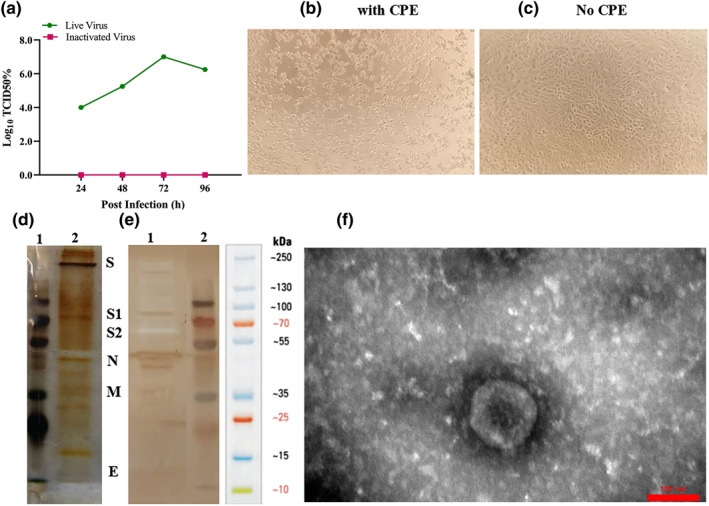
Analysis of the purified inactivated viral particles. (a) Cytopathic effect (CPE) of SARS‐CoV‐2 virus before and after inactivation. Virus titre (104–107) measured by CCID50 at three‐time points: 24, 48 and 72 h, respectively. (b) Image of Vero cell monolayer with CPE before inactivation. (c) No CPE after inactivation. (d) Samples of the purified inactivated viral particles were separated on 10% SDS‐PAGE and stained with silver nitrate: 1, molecular size markers; 2 purified viral particles. (e) Western blot: proteins on SDS‐PAGE gel were transferred onto PVDF membrane and SARS‐CoV‐2 proteins were detected using the anti‐rabbit polyclonal antibody: 1, purified viral particles and 2, molecular size markers. SDS‐PAGE and Western blot patterns show the major structural proteins: spike protein (S), nucleocapsid protein (N), membrane protein (M), and envelope protein (E). (f) Electron micrographs of negatively stained purified viral particles under TEM. Purified viral particles were negatively stained with 1% uranyl acetate and observed under TEM (TEM, EM208S, Philips, 100 kV) at 90,000 × magnification
Four major structural proteins with their corresponding equivalent molecular weights were identified by protein composition analysis of the purified inactivated virus particles using SDS‐PAGE and western blot: the spike (S) protein, nucleocapsid (N) protein, membrane (M) protein and the envelope (E) protein (Figure 3e and f). Transmission electron microscopy (TEM) analysis showed that the purified inactivated viral particles were intact, oval‐shaped and accompanied by a crown‐like structure representing the well‐defined spike on the virus membrane (Figure 3g). These results showed that the final purified inactivated bulk of the candidate vaccine is highly pure and contains the majority of structural proteins.
3.2. Toxicity assessment
Intraperitoneally injection of 5 μg purified inactivated viral antigen with Alum adjuvant (full human dose) to guinea pigs and mice revealed no changes in clinical investigations, weight loss and macroscopic tissue alterations in 2 weeks post‐injection necropsy. Microscopic evaluation of tissue samples (liver, kidney, heart and lung) determined no toxic changes due to vaccine administrations. Three times intramuscular administration of FHD to 10 rabbits and four times of FHD injection to rats with 2‐week intervals, were done to investigate any toxicity changes due to repeated dose administration. In‐life assessment of clinical examination, clinical pathology, macroscopic evaluation of injection site and finally histopathology of some tissues (brain, liver, kidney, spleen, heart, gonad, site of administration and inguinal lymph node) showed no adverse effect of vaccine injection. Weight average in rabbits increased during the 3‐month period of study from 1.9 to 4.5 kg. In blood analysis there was not determined any significant changes in biochemical and haematological factors.
The intramuscular injection of vaccine was clinically well tolerated in the animals with no unscheduled deaths; no adverse local or general clinical signs and no effects on body weight or water and food intake were detected. No treatment‐related or toxicologically significant effects were observed for administration site, body weights, body weight gains, food consumption, body temperatures, clinical pathology and gross organ evaluation. No statistically significant differences were observed in mean body weights during the study for either sex. No significant changes in clinical chemistry were noted in either sex in the immunised group.
The 3FHD (15 μg Ag with adjuvant) designate as the maximum tolerated dose (MTD) per each rat and rabbit. Any significant changes were not observed during study just in some cases pain in injection site and redness was observed 24–48 h after injection which were transient.
Intramuscular injection of adjuvant, 15 μg of antigen adjuvanted with Alum was generally well tolerated in male and female New Zealand White rabbits and Wistar Rats. Three FHD administration caused a mild transient anorexia and pyrexia in rabbits. There were no microscopic lesions associated with injection at the site of injection.
Blood biochemical haematological profiles showed mild changes without statistical significance in Glucose, ALT and WBC count 2 days post‐injection in comparison with PBS administrated animals. All animals survived until scheduled sacrifice and there was no adverse clinical observations or dermal reactions due to 3FHD vaccine administration. Histopathology of tissues did not reveal toxic alterations in early and late sacrifice. Based on the results of pyrogenicity test, none of the three rabbits showed pyrexia and the maximum temperature due to vaccine administration was 0.6°C.
3.3. Immunogenicity assay
BALB/c mice, New Zealand White rabbits, and monkeys were immunised at two times (on days 0 and 14) with two doses of 3 or 5 μg/dose of the candidate vaccine, and the control group was injected with PBS. No inflammation or other adverse effects were observed. To analyse the vaccine’s immunogenicity, the humoural and cellular immune responses were evaluated by different methods including specific IgG antibody titres and IgG2a/IgG1 assessment (ELISA), virus‐neutralising assay and Gzm B activity assay in serum samples, biochemical and haematological analysis in peripheral blood samples, cytokines assay (western blotting), histopathology, immunohistochemistry in lung tissue and flow cytometry.
3.3.1. Evaluation of the humoural immune response:
Antibody assay and VNT
Mice
Increasing serum anti‐whole virus particle IgG antibody titres in vaccinated mice was monitored 2 and 5 weeks post‐immunisation using indirect ELISA. The results showed that SARS‐CoV‐2 specific IgG quickly boosted in the sera of vaccinated mice, and the titre of COV‐2 specific IgG was significantly (P < 0.001) higher in the mice immunised with a combination of the purified inactivated viral particles (antigen) and alum adjuvant compared to the mice administered with antigen alone. Furthermore, in all groups administered with antigen alone or in combination with adjuvants, there was a significant increase of antibody titre (P < 0.0001) in groups that received the 5 μg dose of antigen in comparison with groups immunised with a dose of 3 μg antigen (Figures S2 and 4). It was found that the formulated vaccine (antigen + alum) increased the antibody titre after 42 days due to antigen storage, while antibody titres decreased in the group that received only antigen in the same period. The production of specific antibodies against S and RBD showed a significant increase in the groups receiving 3 and 5 μg of antigen + alum compared to the other groups. Antibody titres also increase over a period of 2–5 weeks. In the second week, the antibody titre in the group receiving 5 μg of antigen + alum was significantly higher than the antibody titre in the group receiving 3 μg of antigen + alum, but the antibody titre in the fourth and fifth weeks in both groups was equal (Figures 4 and S1). In addition, IgG1 and IgG2a antibodies were raised in all mice that received the alum formulated (Figure 5)
FIGURE 4.
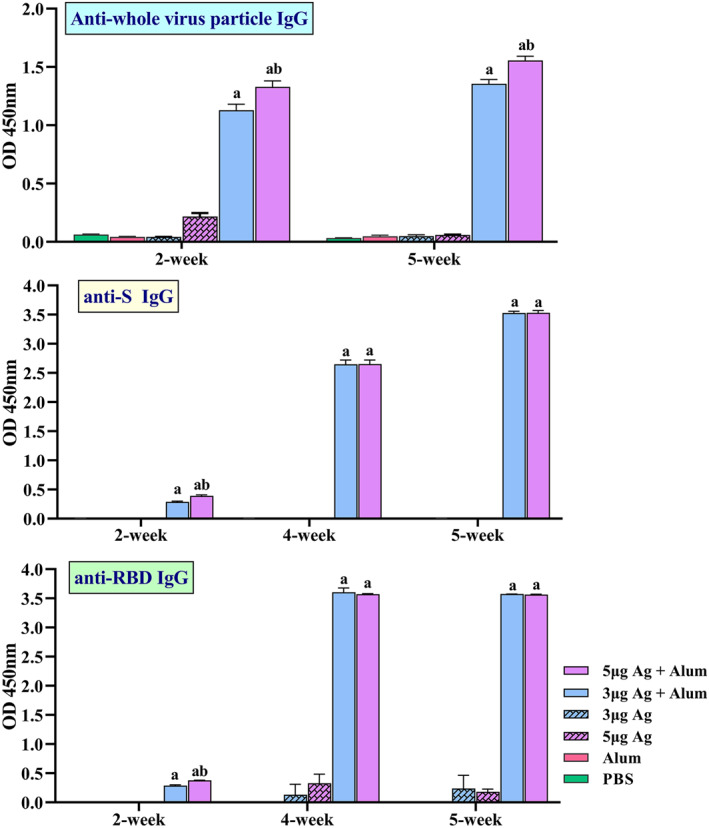
Induction of specific IgG antibodies by different vaccine preparation in mice groups. Immunisations were carried out two times during 2 weeks with 100 μL intramuscular injection of different vaccine preparations according to Table 1. A PBS solution containing three or 5 μg of purified inactivated viruses as antigens, administered alone or in combination with alum. The control groups received alum or PBS. Anti‐whole virus particle IgG, Anti‐spike glycoprotein (S) IgG and Anti‐receptor binding domain (RBD) IgG antibody titres in different dilutions of serum were assayed by ELISA at 2, 4 and 5 weeks post‐immunisation. Data are mean ± SD. The levels of statistical significance for differences between test groups were determined using two‐way ANOVA followed by Tukey’s post hoc test. a: Statistical significance (P < 0.0001) in comparison with groups that received 3 or 5 μg antigen alone and b: Statistical significance (P < 0.0001) between groups that received 3 μg antigens + alum and groups immunised with 5 μg antigens + alum
FIGURE 5.
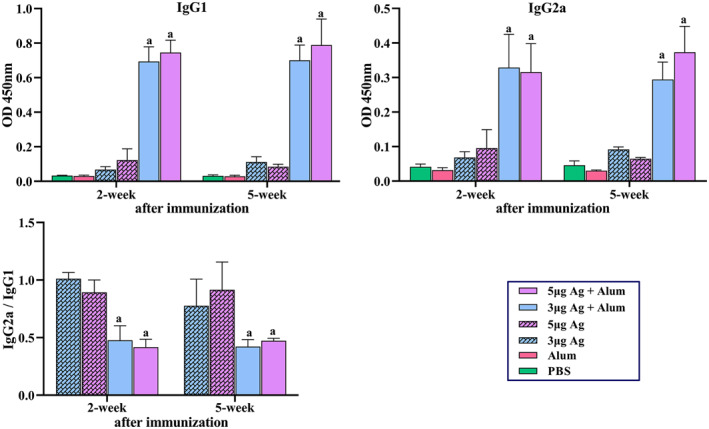
Measurement of specific IgG1, IgG2a antibodies and IgG2a/IgG1 ratio in mice groups that received different vaccine preparations. Immunisations were carried out two times during 2 weeks with 100 μL intramuscular injection of different vaccine preparations according to Table 1. A PBS solution containing 3 or 5 μg of purified inactivated viruses as antigens, administered alone or in combination with alum. The control groups received alum or PBS. The mice were bled at 2 and 5 weeks post‐immunisation, and individual sera were assayed by ELISA. (a) Anti‐COV IgG1. (b) IgG2a antibody titres in 1/1000 dilutions of serum at 2 and 5 weeks post‐immunisation. (c) IgG2a/IgG1 ratio. Data are mean ± SD. The levels of statistical significance for differences between test groups were determined using two‐way ANOVA followed by Tukey’s post hoc test. (a) Statistical significance (P < 0.01) in comparison with groups that received 3 or 5 μg antigen alone
Rabbits
In order to determine the repeated dose toxicity and the amount of antibody production, vaccination of the rabbits was repeated 3 times (on days 0, 14 and 28). On the 28th days after the last injection, the animals were bled, and serum samples (at dilution 1/20,000) were taken for antibody evaluation by indirect ELISA. As illustrated in Figure 6, the titre of S and RBD‐specific IgG was significantly (P < 0.05) higher than the control group.
FIGURE 6.
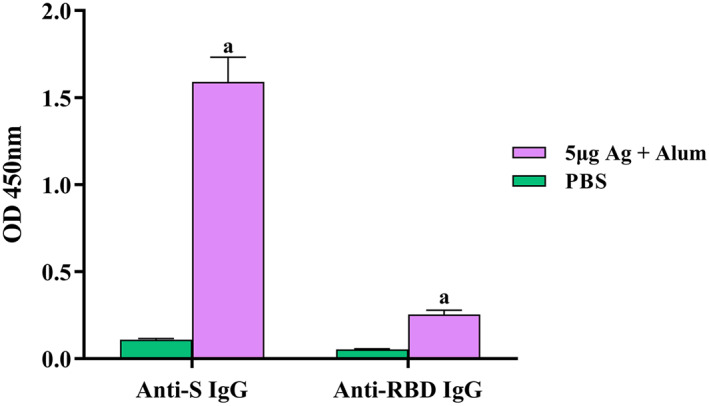
Induction of specific IgG antibodies by candidate vaccine in rabbit groups. Immunisations were carried out three times (on days 0, 14 and 28) using 5 μg antigens in combination with alum. The control groups received PBS. After the third injections, the rabbits were bled at 28 days after last vaccination. S‐specific and RBD‐specific IgG antibody titre in sera were assayed by ELISA kits. Data are reported as mean ± SD. (a) Statistical significance (P < 0.0001) in comparison with groups
Monkeys
Furthermore, the immunogenicity and protective efficacy of the BIV1‐CovIran vaccine were evaluated in rhesus macaques, a nonhuman primate species that shows a COVID‐19‐like disease caused by SARS‐CoV‐2 infection. 31 Macaques were immunised two times intramuscularly with 3 and 5 μg doses of BIV1‐CovIran vaccine on days 0 and 14. S‐specific and RBD‐specific IgG antibody titre was determined over time in serum samples of monkey groups using ELISA. The control group did not show any detectable specific IgG antibody responses. However, S‐specific and RBD‐specific IgG antibody titre emerged at week 1 and surged after 2 weeks in monkey groups that were immunised with the 3 or 5 μg doses of BIV1‐CovIran vaccine (antigen + alum). The titre of S‐specific and RBD‐specific IgG was significantly (P < 0.05) higher than the control group (Figure 7). In the second week after the first injection, the S‐specific and RBD‐specific IgG antibodies titre was significantly higher in the group receiving 5 μg than the group receiving the dose of 3 μg antigen + alum, but at other times there is no significant difference between the two groups. After injection of the booster dose of the vaccine, antibody production significantly increased and antibody stability remained high.
FIGURE 7.
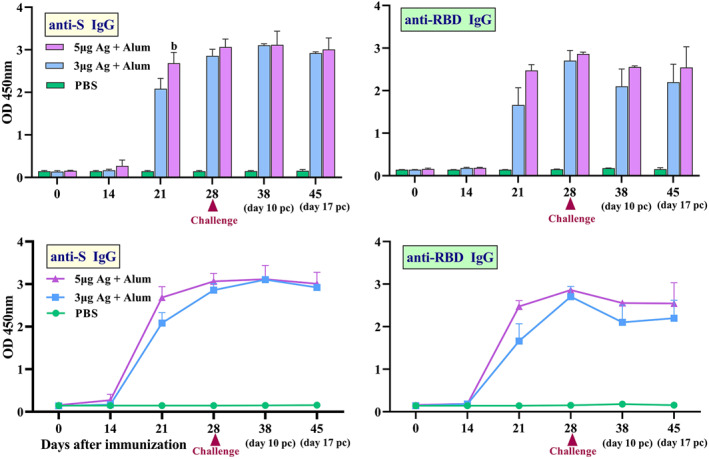
Induction of specific IgG antibodies by candidate vaccine in monkey groups. Rhesus macaques were intramuscularly immunised two times (at days 0 and 14) with 3 or 5 μg doses of candidate vaccine (antigen + alum). The control group was injected with PBS. The challenge study was carried out 14 days after the second immunisation. Anti‐spike glycoprotein (S) and anti‐receptor binding domain (RBD) IgG antibody titre in serum samples of monkey groups was determined by ELISA over time and data are reported as mean ± SD. The statistically significant differences between groups were determined using two‐way ANOVA followed by Tukey’s post hoc test. (b) Statistical significance (P < 0.01) between vaccinated groups
VNT
The results of VNT in mice groups showed that the serum titre up to 1/512 has the ability to neutralise the virus while in rabbit groups, the serum titre up to 1/8192 has the ability to neutralise the virus which resulted after five injections. The virus neutralisation antibody in monkey groups was 1/256 (Figure 8). Subsequently, a challenge study was conducted by direct inoculation of 104 TCID50 of SARS‐CoV‐2 into the vaccinated and control macaques’ lungs intratracheally on day 28 (2 weeks after the second immunisation) to verify the protective efficacy. All vaccinated macaques were highly protected against SARS‐CoV‐2 infection.
FIGURE 8.
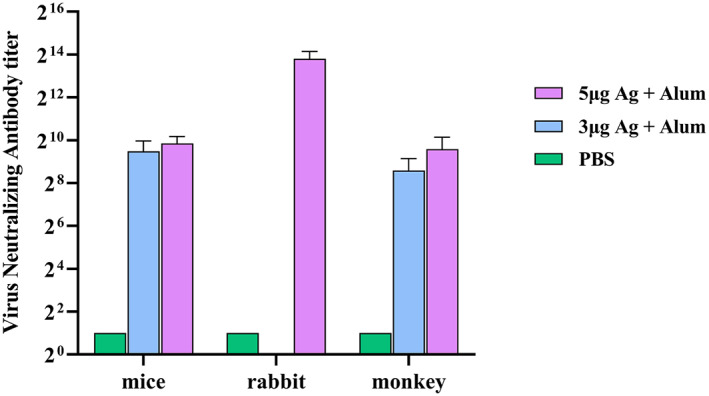
Conventional virus neutralisation test (cVNT) in animal groups that received different vaccine preparations
Blood biochemical and haematological analysis in rhesus macaques
A number of biochemical and haematological indicators were measured at different time points post‐immunisation. There was no significant difference in terms of biochemical markers in the vaccinated animals (Figure S2). Haematological analysis after viral challenge in 3 μg vaccinated and control animals showed lymphocytosis (Figure S3).
Cytokines assay in mice and monkeys
A number of key cytokines in the spleen cell culture supernatant of mice groups were measured 2 weeks after last immunisation by ELISA kits. The results showed that the IFN‐γ and TNF‐α secretion levels significantly increased in groups receiving antigen + alum compared to the groups receiving antigen alone. The highest level of IL‐4 was produced in the group receiving 5 μg of antigen + alum, which shows a significant increase compared to other groups. A significant level of IL‐17 in the groups receiving antigen + alum was induced, while very low levels of IL‐17 were seen in the other groups (Figure 9). Besides, several cytokines in serum samples of monkeys were measured over times after immunisation by ELISA kits. The results showed that IFN‐γ, TNF‐α and IL‐6 (inflammatory cytokines) in vaccinated macaques have been increased in serum after vaccination. But after challenge, TNF‐α and IL‐6 decreased in vaccinated groups, while these main inflammatory cytokines rise in control group. TNF‐α and IL‐6 decreased in vaccinated groups, while these main inflammatory cytokines rise in control group after challenge. Although vaccination has somewhat increased IL‐4 in both vaccinated groups, it has increased in all groups after the challenge (Figure 10).
FIGURE 9.
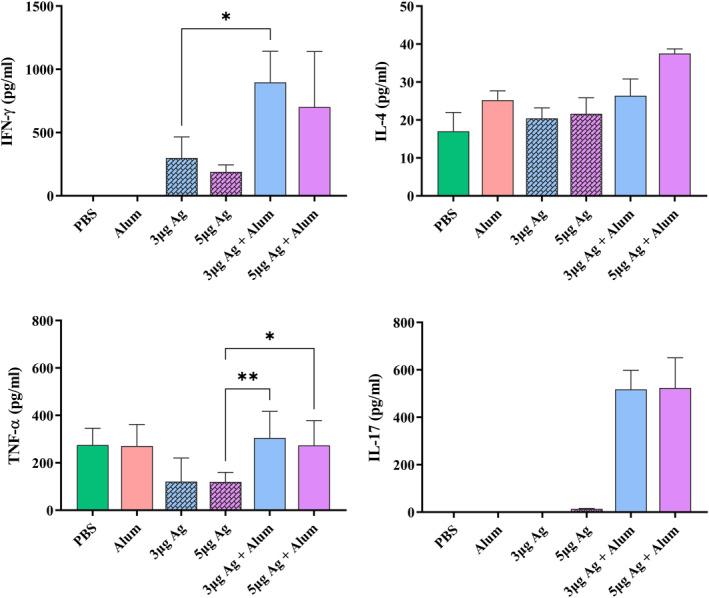
Measurement of cytokine levels secreted by splenocytes of immunised mice. BALB/c mice were immunised two times biweekly, and 2‐week post‐immunisation, spleens were harvested. Splenocytes were isolated and stimulated ex vivo in the presence of the purified inactivated virus 60 h. Cytokines released into culture media were determined by ELISA kit. Data are mean ± SD. The levels of statistical significance for differences between test groups were determined using ANOVA followed by Tukey’s post hoc test. *Statistical significance (P < 0.05)
FIGURE 10.
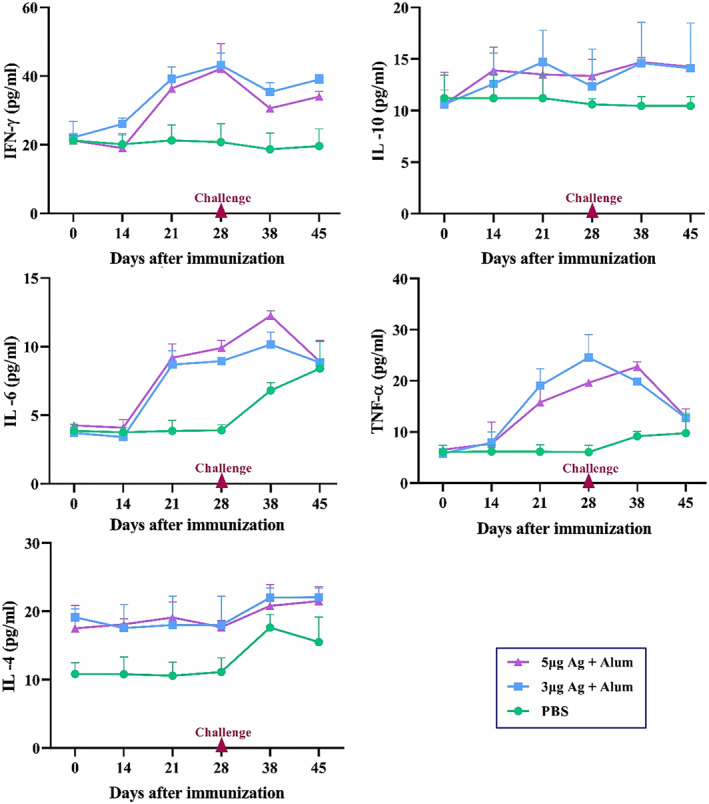
Cytokine level in serum samples of monkeys. Macaques were immunised two times on days 0 and 14 through the intramuscular route with 3 or 5 μg antigen + alum. The control group was injected with PBS. A number of key cytokines in serum samples were measured over times after immunisation by ELISA kits. Data presented as mean ± SD. The statistically significant differences between groups were determined using two‐way ANOVA followed by Tukey’s post hoc test
Cytokines assay in lung tissue of rhesus macaques
The lung tissue samples were used for protein extraction. The total protein concentration was determined by the Bradford assay. The cytokine assay was performed in homogenised tissue samples of cranial, middle and caudal lung lobes by SDS‐PAGE followed by western blot analysis using specific antibody and anti‐β actin as a normalised control. IL‐6 (a pro‐inflammatory cytokine), IL‐4 (a Th2 lymphocytes marker), IL‐10 (an anti‐inflammatory mediator) and IFN‐γ (an amplifier and stimulator cytokine in the immune system) were detected in the homogenised tissue samples of cranial, middle and caudal lung lobes, using western blot. The results showed that IL‐6 decreased significantly in vaccinated animals compared to the control group (P < 0.0001). On the other hand, IL‐4 and IL‐10 increased significantly in 3 and 5 µg vaccinated groups in comparison with the control group (P < 0.05). The level of IFN‐γ was decreased in 5 µg vaccinated macaques in compared to 3 µg vaccine group and the control group (P < 0.05; Figure 11).
FIGURE 11.
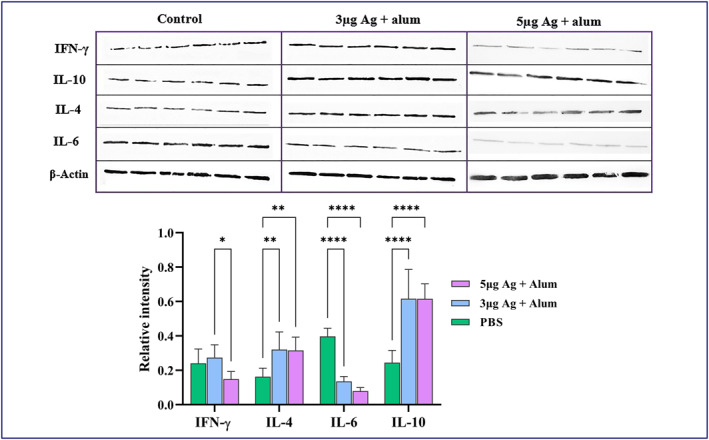
Cytokine level in homogenised lung tissue samples. Macaques were immunised two times on days 0 and 14 through the intramuscular route with 3 or 5 μg antigen + alum. The control group was injected with PBS. A number of key cytokines in lung tissues were measured on day 45 after immunisation by western blot analysis. The lung tissue samples were used for protein extraction. The total protein concentration was determined by the Bradford assay. Assessment of IL‐6, IL‐4, IL‐10 and IFN‐γ in lung extracted proteins was performed by SDS‐PAGE followed by western blot analysis. Data presented as mean ± SD. The statistically significant differences between groups were determined using two‐way ANOVA followed by Tukey’s post hoc test. Asterisks indicate significance: *P < 0.05, **P < 0.001, ***P < 0.0001 and ****P < 0.00001 in comparison with the control group
3.3.2. Evaluation of the cellular immune response
Gzm B activity assay in mice
The potential for induction of cellular immunity at different vaccine concentrations was evaluated by measurement of Gzm B activity using ELISA. The results revealed that Gzm B activity increased in the mice immunised with a mixture of antigen and alum adjuvant in comparison with the animals that received only antigen (P < 0.0001). Furthermore, there was a significant increase in Gzm B activity in the group that received the 5 μg dose of antigen + alum in comparison to the group which was immunised with the 3 μg dose of antigen + alum (Figure 12).
FIGURE 12.
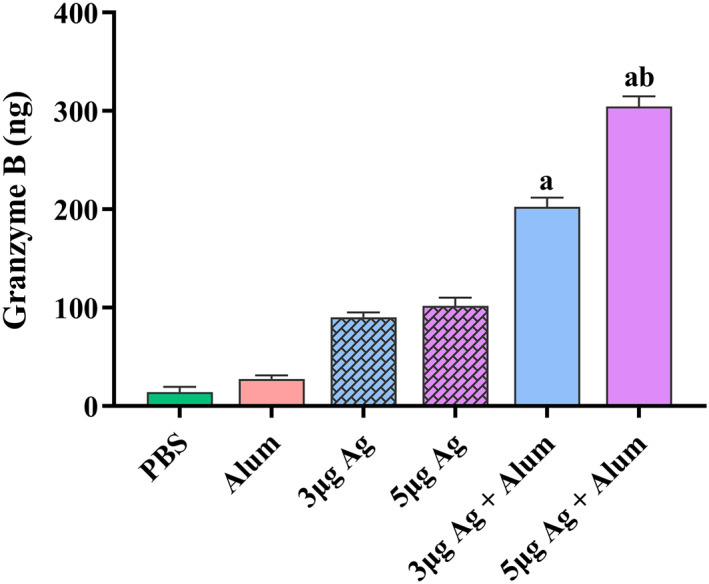
Measurement of Gzm B activity in mice groups that received different vaccine preparations. Immunisations were carried out two times over 2 weeks with 100 μL intramuscular injection of different vaccine preparations. The control groups received alum or PBS. At 2 weeks after the last immunisation, spleens were isolated from immunised mice and Gzm B activity was measured. Data are reported as mean ± SD. The levels of statistical significance for differences between test groups were determined using one‐way ANOVA followed by Tukey’s post hoc test. (a) Statistical significance (P < 0.0001) in comparison with groups that received 3 and 5 μg antigen alone. (b) Statistical significance (P < 0.0001) between groups that received 3 μg antigens with the groups immunised with 5 μg antigens
Viral clearance from the Lungs of Immunised rhesus macaques after challenge
Rhesus macaques injected with 3 μg candidate vaccine showed a significant reduction in lung virus titre titres compared with PBS‐treated controls. The titre of virus was 102 TCID50/mL in the group that received 3 μg while the PBS injected group had 106 TCID50/mL. A higher level of viral clearance was detected in rhesus macaques immunised with 5 μg candidate vaccine and no viruses were detected (Figure 13).
FIGURE 13.
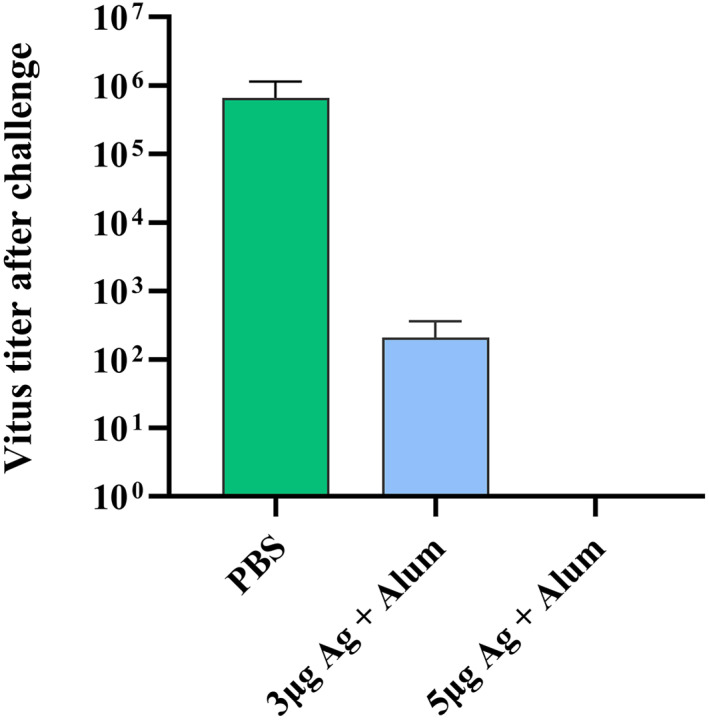
Viral clearance from the lungs of immunised rhesus macaques after challenge
CD4, CD8 and CD20 analysis in the peripheral blood of rhesus macaques
The immune cell subsets in the peripheral blood were assessed in SARS‐CoV‐2 challenged macaques using flow cytometry method after challenge. Lymphocytes population in flow cytometric evaluation of post‐challenged animals showed that the total percentage of lymphocytes significantly increased in the group receiving 3 μg antigen + alum (% 52.6), compared to the group receiving 5 μg antigen + alum (% 27.9) and the control group (29.7%) which is associated with a shift to cellular immunity and the production of cytotoxic CD8+ lymphocytes (Figure 14). In the group receiving 5 μg antigen + alum, although the overall percentage of lymphocytes did not change significantly compared to the control group, the lymphocyte shifts as in the group receiving 3 μg antigen + alum is towards the production of cytotoxic CD8+ lymphocytes. As a consequence, lymphocyte subsets shifted from CD4+ to CD8+ (CD4+: 29% and 22%, CD8+: 56% and 58%) in the vaccinated groups. Also, from the ratio of CD8+ to CD4+ lymphocytes (1.9 and 2.7), This lymphocyte subset pattern in flow cytometry of peripheral blood show that vaccine could shift systemic immune system to cellular acquired immunity. CD20 marker‐positive cells, often known as B lymphocytes becoming antibody‐producing plasma cells, decreased in the 3 μg antigen + alum dose group despite the overall lymphocyte percentage has increased in this group compared to the other two groups, which confirms the shift of immunity to acquired cellular immunity. In the control challenged animals (control group), there was not determined any change, no dominance for a specific cell line and the percentage of different cell types is almost the same as the percentage mentioned in the sub population lymphocyte in Rhesus monkeys. 37
FIGURE 14.
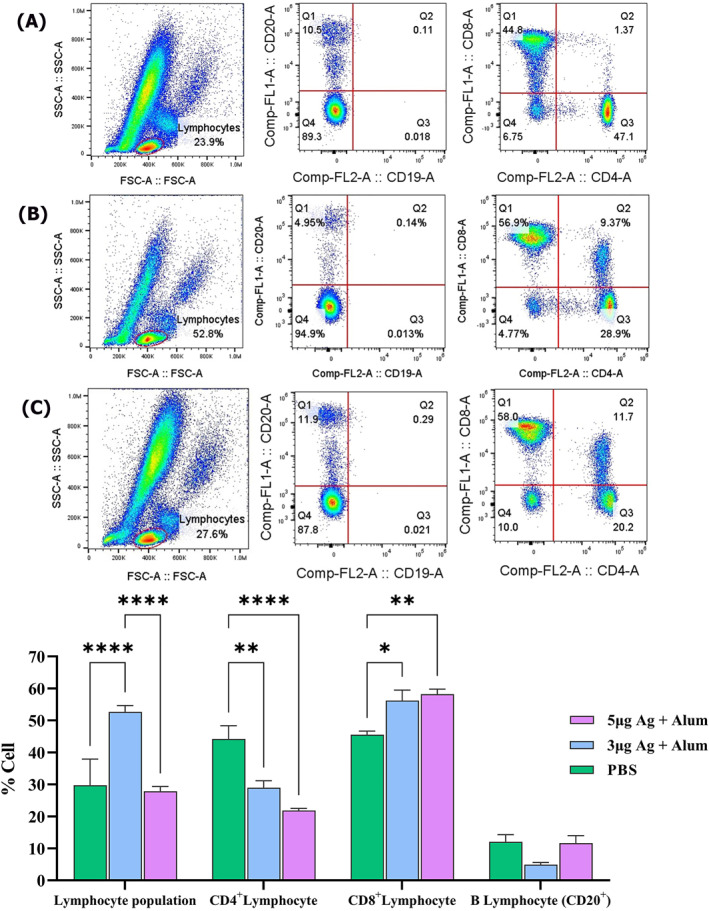
The flow cytometric evaluation of immune cell subsets (CD4+, CD8+ and CD20+) in the peripheral blood of rhesus macaques after the post‐vaccination viral challenge. (a) Control group. (b) Animals vaccinated with 3 µg Ag + alum. (c) Animals vaccinated with 5 µg Ag + alum. (D) Comparison of data in all groups
Histopathology and immunohistochemistry
Histopathological evaluation of various organs, heart, spleen, liver, kidney and brain in the vaccinated monkeys revealed no obvious lesion in the vaccinated animals. In infected control animals, in addition to severe lung lesions, renal tubular epithelium necrosis was found, probably due to SARS‐CoV‐2. Histopathology of lung in infected control animals showed progressive moderate‐to‐severe interstitial pneumonia accompanied by macrophage and lymphocyte infiltration, particularly in the cranial and middle lobes. Moreover, we found moderate‐to‐severe thickening of alveolar septa with type II pneumocyte hyperplasia and fibrin deposition. Vasculitis with accumulation of lymphocytes around small vessels was detected in control monkeys’ lung tissue sections. In the vaccinated animals, the lung tissue showed diffuse mild interstitial pneumonia and mild bronchial epithelial necrosis limited to some areas in the cranial lobes (Figure 15). Immunohistochemically characterisation of immune cell population within the lung tissue sections revealed that the highest presence of CD68 positive M1 macrophages was detected in infected control monkeys. CD4 positive T lymphocytes were observed particularly near the vessels and airways of control monkeys’ lung tissue. More CD20 positive B cells and CD8 positive cytotoxic T cells were identified in the vaccinated animals compared to the control group (Figure 16).
FIGURE 15.
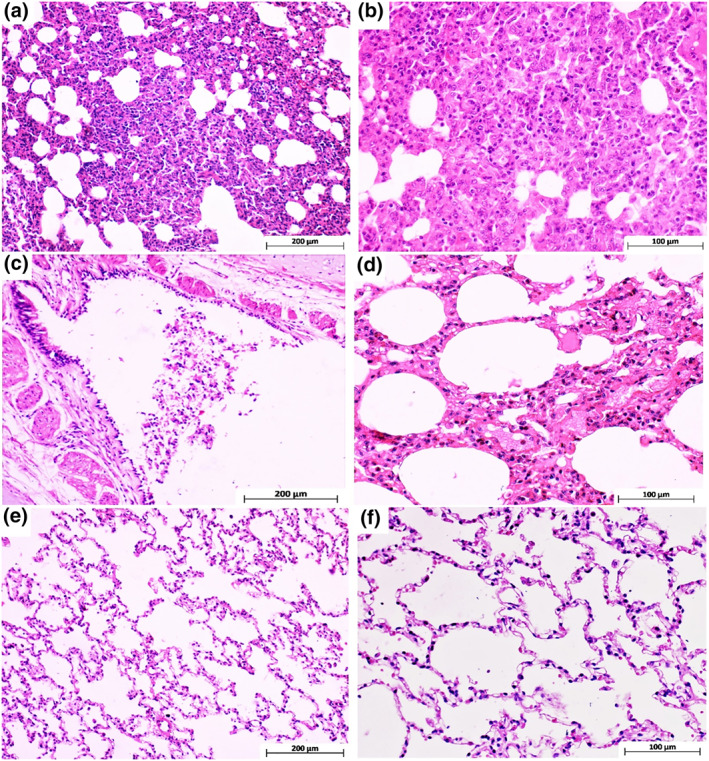
Monkeys’ lung tissue sections stained with haematoxylin and eosin. (a and b) Different magnifications of cranial and middle lung lobes in the control group, indicating severe interstitial pneumonia with marked thickening of alveolar septa and mononuclear inflammatory cell infiltration. (c) Bronchus section from the cranial lung lobe in the animal vaccinated with 5 µg Ag + alum which shows epithelial cell necrosis and sloughing due to SARS‐Cov‐2 infection. (d) Mild interstitial inflammation of only cranial lung lobe tissue in animals vaccinated with 3 µg Ag + alum. (e and f) Show some areas of the cranial and the whole middle and caudal lung lobes in monkeys vaccinated with 3 and 5 µg Ag + alum, respectively
FIGURE 16.
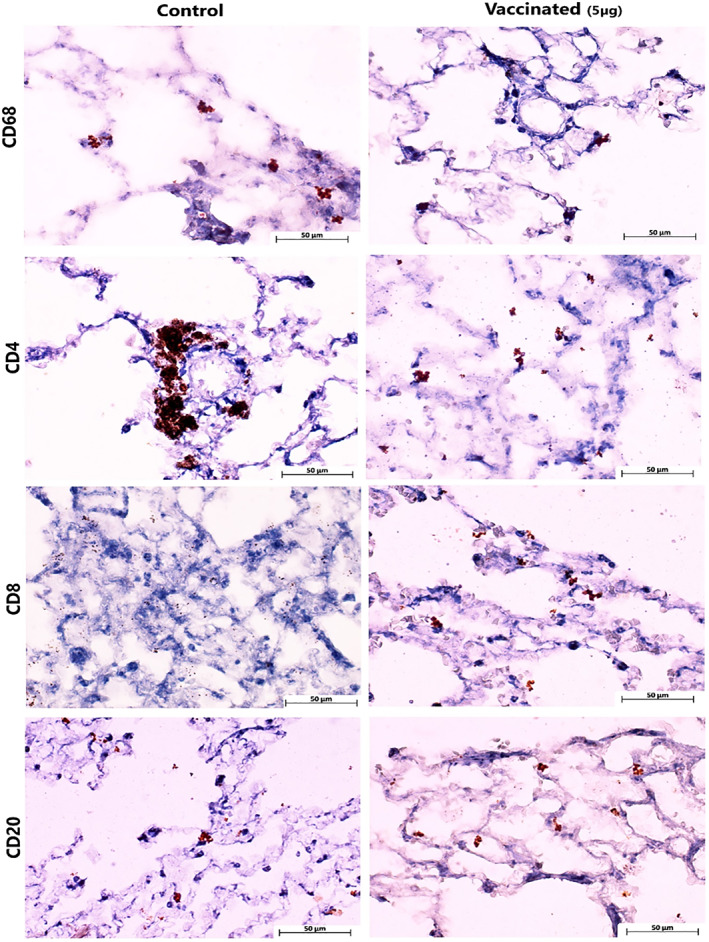
Immunohistochemical staining of immune cells within the lung tissue sections
4. DISCUSSION
Scientists have focused their efforts on the development of vaccines to stop the COVID‐19 pandemic and its quick spread. 38 To achieve this goal, several vaccine trials are being conducted worldwide. For decades, inactivated whole particle vaccines have been broadly administrated to prevent emerging respiratory diseases. 39 The results from preclinical and clinical studies of three inactivated COVID‐19 vaccines have shown promising outcomes against SARS‐CoV‐2. 40 , 41 , 42 On the other hand, the application of adjuvants in vaccine formulation can increase and/or modulate the intrinsic immunogenicity of antigen and elicit strong and long‐lasting immune responses. 43 It may also substantially reduce the amount of antigen and/or the number of boost injections required to achieve the desired immune responses. 44 Meanwhile, alum adjuvant works primarily through the formation of a depot at the injection site, enabling enhanced antigen availability, activation of antigen‐presenting cells (APCs), uptake by immune cells and activation of innate immunity pathways through pattern recognition receptors (PRRs). 45
In the present study after safety assays, the inactivated vaccine was injected into rodent and non‐rodent animal models to evaluate the titre of antibody level. In addition, Rhesus macaques were immunised with two doses of the vaccines and 14 days after the second immunisation, challenged intratracheally with 104 TCID50 of SARS‐CoV‐2. The number of infectious coronavirus particles in humans is usually reported below 103 particles. In the present study, like some other studies, 46 we used 10–20 times the human infectious dose to challenge monkeys, which is scientifically correct for the challenge.
Measurement of anti‐COV‐2 IgG antibody titres in the serum of vaccinated mice showed that the injection of the candidate vaccine (purified inactivated viruses in combination with adjuvant) significantly induced a high level of specific antibodies on day 21 and day 42 after the first injection. The administration of alum adjuvant causes the high antibody response to persist for 42 days after immunisation due to the continuous release of antigen. These results also show that the antibody produced has adequate durability after 42 days of vaccination and can neutralise the wild virus. Therefore, alum adjuvant is a good option for vaccine formulation and enhancement of the immune response. The antibody quantity after vaccination is not very important, because by shifting the immune system only to Th2, a high antibody titre may be produced but in practice, it is not able to neutralise the virus. The T helper 1 (Th1) and T helper 2 (Th2) balance is the most important factor in the antibody function, which in fact indicates the affinity of the antibody after vaccination. The conventional virus neutralisation test (cVNT) is the current gold standard and a robust serological test to detect neutralising antibodies against SARS‐CoV‐2. 47 The VNT was performed using different dilutions of sera in vaccinated mice and rabbits after the second and third injections, respectively. The results showed that the elevated antibodies have high affinity to neutralise the virus in cell culture. The immunisation of Rhesus macaques with the two‐dose BIV1‐CovIran vaccine led to efficient protection against 104 TCID50 of SARS‐CoV‐2 intratracheal challenge compared to controls.
Overall, after vaccination of challenged macaques, the lymphocyte cell increased and predominant subset of lymphocyte was CD8+ cell in peripheral blood, thereby increasing cytotoxic cellular immune responses. Evaluation of cytokines in mice showed that alum adjuvant with different doses of vaccine increased the pro‐inflammatory cytokine TNF‐α compared to the vaccine alone. In fact, the synergistic effect of the alum + vaccine stimulates the immune system. The amount of IFN‐γ also shows that a dose of 3 µg + alum has the ability to stimulate the immune system more than other groups. The synergistic effect of the alum + vaccine on immune stimulation has been well demonstrated in IL‐17 assays. IL‐4 assay shows that alum adjuvant alone or with vaccine did not has the ability to stimulate Th2 cells and cause allergic reactions, and the amount used as an adjuvant in this vaccine has a predominant effect on immune‐stimulating to its allergenicity.
Evaluation of cytokines in serums of monkey groups revealed that after vaccination, inflammatory cytokines (TNF‐α, IL‐6 and IFN‐γ) of vaccinated macaques increased in serum. But after challenge, TNF‐α and IL‐6 decreased in vaccinated group, while these main inflammatory cytokines rose in control group. This mean that vaccinated Rhesus could control excess inflammation after exposure to SARS‐CoV‐2 virus. On the other hand, the ratio of serum IL‐6/IL‐10 in the control group did not change whereas, in vaccinated groups, IL‐10 rose after challenge to limit inflammatory cytokines. Virus challenge increased serum IL‐4 in all groups but IFN‐γ did not change in control group. These changes of IL‐4 and IFN‐γ in the serum after challenge indicate the vaccine’s tendency to amplify of cellular immunity. Also, the ratio of IL‐4/IFN‐γ in the serum, after challenge, show synergic effect on down regulation of inflammation caused by virus especially in 3 µg vaccinated group.
Cytokine levels in lung of vaccinated macaques showed reduced IL‐6, an acute pre‐inflammatory cytokine which induces production of inflammatory mediators such as CRP and induction of neutrophils and also alteration in T lymphocyte subset, in comparison with control group macaques. As a result, from the clinical point of view, viral challenge causes the rise of inflammation in lungs and this vaccine decrease this inflammation. According to the western blot results, there was no change in IL‐4 level in both two groups of vaccine receivers. The vaccine with viral challenge can boost the production of IL‐4 and extension of acquired immune which is caused by Th2 cells and resist the increasing of Th1 and macrophage function.
Levels of IL‐10, an anti‐inflammatory cytokine, increased at the end of immune response. As with TGF‐β, the immune system shifts back to low function which is necessary for prevention of severe immune system response and incidence of over activation of immune system diseases. In contrast, virus‐induced inflammation may trigger the immune system but, with low amount of IL‐10, does not terminate immune system activity. Consequently, this endless immune system activity leads to pathologic effect of SARS‐COV‐2 virus.
The IFN‐γ cytokine, known as an amplifier and stimulator cytokine in immune system, leads to activation of numerous inherent immune cells such as macrophages and acquired immunity like T lymphocytes. The origin of this IFN‐γ can be the macrophage, TCD8+ and Th1 and even type 2 pneumocytes of lung. Macrophages and other immune cells activated by IFN‐γ perform their functions of containing augmentation of phagocytosis and increase the killer function of TCD8+.
One concern about COVID‐19 vaccines is the antibody‐dependent enhancement (ADE) phenomenon, whereby the vaccine might make the subsequent SARS‐CoV‐2 infection more severe. 39 , 48 The ADE phenomenon has been reported in studies of Middle East respiratory syndrome‐CoV and SARS‐CoV‐2 vaccines in animal challenge models. 49 However, this effect was not observed in an immunisation‐challenge model of Rhesus macaques using the same vaccines in the preclinical study or in the reports from preclinical studies of other inactivated COVID‐19 vaccine candidates. 40 , 50 Studies have shown that previous infection with SARS‐CoV‐2 could protect against re‐challenge in Rhesus macaques. The ADE phenomenon may happen due to an imbalance between Th1 and Th2 responses. 51 , 52
Another concern associated with whole‐inactivated virus‐based vaccines, particularly those with the alum adjuvant that can induce T helper 2 cell‐based responses, is the probability of vaccine‐associated enhanced respiratory disease (VAERD). VAERD was reported in young children in the 1960s when the whole‐inactivated virus vaccine with alum adjuvant was tested for measles and respiratory syncytial virus. 53 , 54 However, most of the inactivated vaccines under development against COVID‐19 have used alum adjuvant, and no evidence of VAERD has been observed yet. On the contrary, alum may reduce immunopathology compared with un‐adjuvanted coronavirus vaccines. 17 , 55
5. CONCLUSIONS
Taken together, data obtained from a preclinical study of BIV1‐CovIran candidate vaccine shows adequate safety and efficiency and thus, it may be a promising and feasible prophylactic vaccine to confer protection against the SARS‐CoV‐2 infection. Also, alum would serve as an effective adjuvant in the formulation of SARS‐CoV‐2 and other candidate vaccines.
CONFLICT OF INTERESTS
Mohammad Taqavian and Mohammadreza Hosseinpour are employees of Shifa Pharmed, with no stock options or incentives. Hamidreza Jamshidi and Hasan Jalili are the chairman and managing director of the vaccine research and development unit in Shifa Pharmed, respectively. Dr. Asghar Abdoli is the founder of Amirabad Virology Lab and the only shareholder of this laboratory; Dr Abdoli is also the scientific director of Amirabad Virology Lab. Further, Dr. Abdoli is a faculty member of the Pasteur Institute of Iran and a project consultant on the PastoCoAd vaccine project, which was initiated after the BIV1‐CovIran vaccine project.
All other authors declare no competing interests.
AUTHOR CONTRIBUTIONS
Asghar Abdoli, Mohammad Taqavian and Reza Aalizadeh helped immensely with the protocol design and report generation. Asghar Abdoli, Ebrahim Azimi and Nabbi Emamipour carried out purification of the inactivated viruses. Asghar Abdoli and Ali Teimoori contributed in virus characterisation and virus neutralizing tests. Asghar Abdoli, Reza Aalizadeh and Vahid Siavashi led the immunogenicity experiments. Hossein Aminianfar and Marzieh Eghtedardoost carried out pathological and toxicological assays. Zahra Kianmehr and Asghar Abdoli contributed to the data analysis. Asghar Abdoli, Zahra Kianmehr, Marzieh Eghtedardoost, Ali Teimoori and Hossein Aminianfar participated in manuscript preparation. Hamidreza Jamshidi and Hasan Jalili were the study coordinator and article reviewer.
Supporting information
Supplementary Material 1
Supplementary Material 2
Supplementary Material 3
ACKNOWLEDGEMENTS
We appreciate all the kind assistance of Dr. Mehrdad Jalilian to give us feedback to improve this manuscript and the site research staffs. This work is supported financially by Amirabad Virology Lab.
Abdoli A, Aalizadeh R, Aminianfar H, et al. Safety and potency of BIV1‐CovIran inactivated vaccine candidate for SARS‐CoV‐2: a preclinical study. Rev Med Virol. 2022;32(3):e2305. 10.1002/rmv.2305
[Correction added on 8 February 2022, after first online publication: the article category and Conflict of Interests were incorrect and have been changed in this version.]
Contributor Information
Reza Aalizadeh, Email: aalizadehreza19@gmail.com, Email: Hjalili@ut.ac.ir.
Hasan Jalili, Email: aalizadehreza19@gmail.com, Email: Hjalili@ut.ac.ir.
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the manuscript and supplementary material of this article.
REFERENCES
- 1. Plotkin S. History of vaccination. Proc Natl Acad Sci. 2014;111(34):12283‐12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jeyanathan M, Afkhami S, Smaill F, Miller MS, Lichty BD, Xing Z. Immunological considerations for COVID‐19 vaccine strategies. Nat Rev Immunol. 2020;20(10):615‐632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zakarya K, Kutumbetov L, Orynbayev M, et al. Safety and immunogenicity of a QazCovid‐in® inactivated whole‐virion vaccine against COVID‐19 in healthy adults: a randomized, single‐blind, placebo‐controlled phase 1 clinical trial with a 6 months follow‐up and an open‐label phase 2 clinical trial in Kazakhstan. Lancet Infect Dis. 2021;21(7):950‐961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Crooke SN, Ovsyannikova IG, Kennedy RB, Poland GA. Immunoinformatic identification of B cell and T cell epitopes in the SARS‐CoV‐2 proteome. Sci Rep. 2020;10(1):1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nunnally BK, Turula VE, Sitrin RD. Vaccine Analysis: Strategies, Principles, and Control. Verlag Berlin Heidelberg: Springer; 2015. doi: 10.1007/978-3-662-45024-6 [DOI] [Google Scholar]
- 6. Thomas F, Magill TP Vaccination of human subjects with virus of human influenza. Exp Biol Med. 1936;33(4):604–606. doi: 10.3181/00379727-33-8467p [DOI] [Google Scholar]
- 7. Salk JE, Krech U, Youngner J, Bennett BL, Lewis L, Bazeley P. Formaldehyde treatment and safety testing of experimental poliomyelitis vaccines. Am J Public Health Nations Health. 1954;44(5):563‐570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Provost P, Hughes J, Miller W, Giesa P, Banker F, Emini E. An inactivated hepatitis A viral vaccine of cell culture origin. J Med Virol. 1986;19(1):23‐31. [DOI] [PubMed] [Google Scholar]
- 9. Brouwer PJ, Caniels TG, van der Straten K, et al. Potent neutralizing antibodies from COVID‐19 patients define multiple targets of vulnerability. Science. 2020;369(6504):643‐650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Long Q‐X, Liu B‐Z, Deng H‐J, et al. Antibody responses to SARS‐CoV‐2 in patients with COVID‐19. Nat Med. 2020;26(6):845‐848. [DOI] [PubMed] [Google Scholar]
- 11. Liu W, Liu L, Kou G, et al. Evaluation of nucleocapsid and spike protein‐based enzyme‐linked immunosorbent assays for detecting antibodies against SARS‐CoV‐2. J Clin Microbiol. 2020;58(6):e00461‐e00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lecomte J, Cainelli‐Gebara V, Mercier G, et al. Protection from mouse hepatitis virus type 3‐induced acute disease by an anti‐nucleoprotein monoclonal antibody. Arch. Virol. 1987;97(1‐2):123‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wu L‐P, Wang N‐C, Chang Y‐H, et al. Duration of antibody responses after severe acute respiratory syndrome. Emerg Infect Dis. 2007;13(10):1562‐1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Marrack P, McKee AS, Munks MW. Towards an understanding of the adjuvant action of aluminium. Nat Rev Immunol. 2009;9(4):287‐293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Buonaguro FM, Ascierto PA, Morse GD, et al. Covid‐19: time for a paradigm change. Rev Med Virol. 2020;30(5):e2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fani M, Teimoori A, Ghafari S. Comparison of the COVID‐2019 (SARS‐CoV‐2) pathogenesis with SARS‐CoV and MERS‐CoV infections. Future Virol. 2020;15(5):317‐323. doi: 10.2217/fvl-2020-0050 [DOI] [Google Scholar]
- 17. Hotez PJ, Corry DB, Bottazzi ME. COVID‐19 vaccine design: the Janus face of immune enhancement. Nat Rev Immunol. 2020;20(6):347‐348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Laboratory Biosafety Guidance Related to Coronavirus Disease 2019 (COVID‐19): Interim Guidance. World Health Organization; 2020. [Google Scholar]
- 19. Abdoli A, Soleimanjahi H, Kheiri MT, Jamali A, Jamaati AJCJ. Determining influenza virus shedding at different time points in Madin‐Darby canine kidney cell line. Cell J. 2013;15(2):130‐135. [PMC free article] [PubMed] [Google Scholar]
- 20. Miller J, Ulrich R. On the analysis of psychometric functions: the Spearman‐Kärber method. Percept Psychophys. 2001;63(8):1399‐1420. [DOI] [PubMed] [Google Scholar]
- 21.Recommendations for the Production and Control of Poliomyelitis Vaccine (Oral), World Health Organization.
- 22. Abdoli A, Soleimanjahi H, Tavassoti Kheiri M, et al. An H1‐H3 chimeric influenza virosome confers complete protection against lethal challenge with PR8 (H1N1) and X47 (H3N2) viruses in mice. Pathog Dis. 2014;72(3):197‐207. [DOI] [PubMed] [Google Scholar]
- 23. Abdoli A, Soleimanjahi H, Kheiri MT, et al. Reconstruction of H3N2 influenza virus based virosome in‐vitro. Iran J Microbiol. 2013;5(2):166. [PMC free article] [PubMed] [Google Scholar]
- 24. Sakoda Y, Okamatsu M, Isoda N, et al. Purification of human and avian influenza viruses using cellulose sulfate ester (cellufine sulfate) in the process of vaccine production. Microbiol Immunol. 2012;56(7):490‐495. [DOI] [PubMed] [Google Scholar]
- 25. Kanlaya R, Thongboonkerd V. Cellufine sulfate column chromatography as a simple, rapid, and effective method to purify dengue virus. J Virol Methods. 2016;234:174‐177. [DOI] [PubMed] [Google Scholar]
- 26. Kianmehr Z, Soleimanjahi H, Ardestani SK, Fotouhi F, Abdoli AJMm, immunology. Influence of Brucella abortus lipopolysaccharide as an adjuvant on the immunogenicity of HPV‐16 L1VLP vaccine in mice. Med Microbiol Immunol. 2015;204(2):205‐213. [DOI] [PubMed] [Google Scholar]
- 27. The British Pharmacopoeia 2017 [Print Edition]. The Stationery Office; 2016. [Google Scholar]
- 28. Forster R. Study designs for the nonclinical safety testing of new vaccine products. J Pharmacol Toxicol methods. 2012;66(1):1‐7. [DOI] [PubMed] [Google Scholar]
- 29. Green MD, Al‐Humadi NH. Preclinical toxicology of vaccines. In: Said Faqi A, ed. A Comprehensive Guide to Toxicology in Nonclinical Drug Development. Elsevier; 2017:709‐735. [Google Scholar]
- 30. Sellers RS, Nelson K, Bennet B, et al. Scientific and Regulatory Policy Committee points to consider*: approaches to the conduct and interpretation of vaccine safety studies for clinical and anatomic pathologists. Toxicol Pathol. 2020;48(2):257‐276. [DOI] [PubMed] [Google Scholar]
- 31. Chandrashekar A, Liu J, Martinot AJ, et al. SARS‐CoV‐2 infection protects against rechallenge in rhesus macaques. Science. 2020;369(6505):812‐817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Woolsey C, Borisevich V, Prasad AN, et al. Establishment of an African Green Monkey Model for COVID‐19. Nat Immunol. 2021;22:86‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Meyer B, Reimerink J, Torriani G, et al. Validation and clinical evaluation of a SARS‐CoV‐2 surrogate virus neutralisation test (sVNT). Emerg Microbes Infect. 2020;9(1):2394‐2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rijkers G, Murk J‐L, Wintermans B, et al. Differences in antibody kinetics and functionality between severe and mild severe acute respiratory syndrome coronavirus 2 infections. J Infect Dis. 2020;222(8):1265‐1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Van Tol S, Mögling R, Li W, et al. Accurate serology for SARS‐CoV‐2 and common human coronaviruses using a multiplex approach. Emerg Microbes Infect. 2020;9(1):1965‐1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sardar R, Satish D, Birla S, Gupta D. Integrative analyses of SARS‐CoV‐2 genomes from different geographical locations reveal unique features potentially consequential to host‐virus interaction, pathogenesis and clues for novel therapies. Heliyon. 2020;6(9):e04658. doi: 10.1016/j.heliyon.2020.e04658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sopper S, Stahl‐Hennig C, Demuth M, Johnston I, Dörries R, Ter Meulen V. Lymphocyte subsets and expression of differentiation markers in blood and lymphoid organs of rhesus monkeys. Cytometry. 1997;29(4):351‐362. [PubMed] [Google Scholar]
- 38. Pious N, Ingole S. Race for COVID‐19 vaccine. Trends Biomater Artif Organs. 2020;34(S3):62‐65. [Google Scholar]
- 39. Stern PL. Key steps in vaccine development. Ann Allergy Asthma Immunol. 2020;125(1), 17‐27. [DOI] [PubMed] [Google Scholar]
- 40. Gao Q, Bao L, Mao H, et al. Development of an inactivated vaccine candidate for SARS‐CoV‐2. Science. 2020;369(6499):77‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Xia S, Duan K, Zhang Y, et al. Effect of an inactivated vaccine against SARS‐CoV‐2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. JAMA. 2020;324(10):951‐960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lin J, Zhang J‐S, Su N, et al. Safety and immunogenicity from a phase I trial of inactivated severe acute respiratory syndrome coronavirus vaccine. Antivir Ther. 2007;12(7):1107‐1113. [PubMed] [Google Scholar]
- 43. Brunner R, Jensen‐Jarolim E, Pali‐Schöll I. The ABC of clinical and experimental adjuvants—a brief overview. Immunol Lett. 2010;128(1):29‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dubensky TW, Jr & Reed SG. Seminars in immunology. [DOI] [PubMed]
- 45. Morefield GL, Sokolovska A, Jiang D, HogenEsch H, Robinson JP, Hem SLJV. Role of aluminum‐containing adjuvants in antigen internalization by dendritic cells in vitro. Vaccine. 2005;23(13):1588‐1595. [DOI] [PubMed] [Google Scholar]
- 46. Yu J, Tostanoski LH, Peter L, et al. DNA vaccine protection against SARS‐CoV‐2 in rhesus macaques. Science. 2020;369(6505):806‐811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tan CW, Chia WN, Qin X, et al. A SARS‐CoV‐2 surrogate virus neutralization test based on antibody‐mediated blockage of ACE2–spike protein–protein interaction. Nat Biotechnol. 2020;38(9):1073‐1078. [DOI] [PubMed] [Google Scholar]
- 48. Sahin U, Muik A, Derhovanessian E, et al. Concurrent human antibody and TH1 type T‐cell responses elicited by a COVID‐19 RNA vaccine. Medrxiv. 2020. [Google Scholar]
- 49. Graham BS. Rapid COVID‐19 vaccine development. Science. 2020;368(6494):945‐946. [DOI] [PubMed] [Google Scholar]
- 50. Corbett KS, Flynn B, Foulds KE, et al. Evaluation of the mRNA‐1273 vaccine against SARS‐CoV‐2 in nonhuman primates. N. Engl J Med. 2020;383(16):1544‐1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Banoun H. COVID19: Cross‐Immunity with Other Coronaviruses, Immunopathological Phenomena. SSRN; 2020. [Google Scholar]
- 52. Zellweger RM, Wartel TA, Marks F, Song M, Kim JH. Vaccination against SARS‐CoV‐2 and disease enhancement–knowns and unknowns. Expet Rev Vaccine. 2020;19(8):691‐698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fulginiti VA. Altered Reactivity to Measles Virus. JAMA. 1967;202(12):1075. doi: 10.1001/jama.1967.03130250057008 [DOI] [PubMed] [Google Scholar]
- 54. Kim HW, Canchola JG, Brandt CD, et al. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89(4):422‐434. [DOI] [PubMed] [Google Scholar]
- 55. Chen W‐H, Tao X, Agrawal AS, et al. Yeast‐expressed SARS‐CoV recombinant receptor‐binding domain (RBD219‐N1) formulated with aluminum hydroxide induces protective immunity and reduces immune enhancement. Vaccine. 2020;38(47):7533‐7541. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1
Supplementary Material 2
Supplementary Material 3
Data Availability Statement
The data that supports the findings of this study are available in the manuscript and supplementary material of this article.


