Abstract
The COVID‐19 pandemic has added another layer of complexity to the fears of patients with neuroendocrine tumors (NETs). Little is known regarding the psychological impact of the COVID‐19 outbreak on patients with gastroenteropancreatic or bronchopulmonary (BP) NETs. We longitudinally surveyed the mental symptoms and concerns of NET patients during the plateau phase of the first (W1) and second epidemic waves (W2) in Italy. Seven specific constructs (depression, anxiety, stress, health‐related quality of life, NET‐related quality of life, patient–physician relationship, psychological distress) were investigated using validated screening instruments, including DASS‐21, EORTC QLQ‐C30, EORTC QLQ GI.NET21, PDRQ9 and IES‐R. We enrolled 197 patients (98 males) with a median age of 62 years. The majority of the patients had G1/G2 neoplasms. Some 38% of the patients were on active treatment. At W1, the prevalence of depression, anxiety and stress was 32%, 36% and 26% respectively. The frequency of depression and anxiety increased to 38% and 41% at W2, whereas no modifications were recorded in the frequency of stress. Poor educational status was associated with higher levels of anxiety at both W1 (odds ratio [OR] = 1.33 ± 0.22; p = .07) and W2 (OR = 1.45 ± 0.26; p = .03). Notably, post‐traumatic stress symptoms were observed in the 58% of the patients, and both single marital status (OR = 0.16, 95% confidence interval [CI] = 0.06–0.48; p = .0009) and low levels of formal education (OR = 0.47, 95% CI = 0.23–0.99; p = .05) predicted their occurrence. No significant deteriorations of health‐related quality of life domains were observed from W1 to W2. High patient care satisfaction was documented despite the changes in health systems resource allocation. NET patients have an increased risk of developing post‐traumatic stress symptoms as result of the COVID‐19 pandemic. Specific screening measures and psychological interventions should be implemented in NET clinics to prevent, recognize and treat mental distress in this vulnerable population.
Keywords: anxiety, carcinoid, depression, HRQoL, post‐traumatic stress disorder
NET patients have an increased risk of developing post‐traumatic stress symptoms as result of the COVID‐19 pandemic. Specific screening measures and psychological interventions should be implemented in NET clinics to prevent, recognize and treat mental distress in this vulnerable population.

1. INTRODUCTION
In early December 2019, the first cluster of pneumonia cases of unknown origin was identified in Wuhan, the capital city of the Hubei province in China. 1 The causative agent was subsequently identified as a novel enveloped RNA betacoronavirus that was named severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). 2 Some 3 months after its first description, the SARS‐CoV‐2‐associated disease (COVID‐19) spread globally, and the World Health Organization (WHO) declared pandemic status on March 11, 2020. 3
The COVID‐19 pandemic poses unprecedented medical, economic and social challenges. The need for social distancing, the isolation induced by lockdowns and quarantine orders enforced by national governments, the fear of infection and death from the virus, and the financial problems caused by the epidemic (i.e., job loss, income cuts, etc.) have been described as major threats for mental health. 4 In this context, multiple studies have already shown rising levels of psychological distress and mental illness in the general population as result of the pandemic surge. 5 , 6 , 7
Patients with cancer are at higher risk of morbidity and mortality from COVID‐19 compared to the general population, probably as consequence of advanced age, coexisting chronic comorbidities, and cancer‐related and drug‐related immunosuppression. 8 Moreover, the COVID‐19 pandemic has transformed every aspect of cancer care, including deferring screening procedures and diagnosis, postponing elective surgeries and follow‐up visits, and adopting less‐intensive care regimens. Delays and changes in cancer diagnosis, treatment and follow‐up, in combination with concerns about the viral threat per se, have the potential to impair patients' mental and emotional well‐being, thus negatively impacting on their quality of life.
Neuroendocrine tumors (NETs) are a heterogeneous group of malignancies arising from the diffuse neuroendocrine system. 9 Although NETs may develop in almost any organs, they predominate in the gastroenteropancreatic (GEP) tract and bronchopulmonary (BP) system. Well‐differentiated NETs are characterized by a relatively indolent growth, and survival outcomes in the metastatic setting often span years. The rarity of the disease and the requirement of expertise available only in specialized centers, the long survival durations and the need of multiple therapy lines throughout the treatment journey, and the possible occurrence of clinical syndromes related to the ectopic secretion of peptide hormones or biogenic amines all render NET patients particularly vulnerable to the psychological distress induced by the COVID‐19 pandemic. In the present study, we aimed to investigate the impact of the COVID‐19 crisis on the mental health and quality of life of a heterogeneous, real‐world cohort of 197 patients with well‐differentiated GEP‐NET or BP‐NET.
2. MATERIALS AND METHODS
2.1. Study setting and design
The present study was carried out in two tertiary hospitals in Italy (Policlinico di Bari, Bari; National Cancer Institute Foundation “G. Pascale”, Naples). Both centers have a specific expertise in managing patients with NETs, and represent the two main institutions specialized in treatment of NETs in South Italy. As of the last day of data collection (November 14, 2020), 1,144,552 confirmed COVID‐19 infections and 44,683 deaths were recorded in Italy. To manage the pandemic, the Italian government instituted a full lockdown from March 11, 2020 to May 4, 2020, as well as a partial lockdown from October 13, 2020. During this period, we longitudinally surveyed the demographics, mental symptoms and concerns of NET patients twice, namely during the plateau phase of the first (W1) and second epidemic waves (W2) in Italy (Figure 1). In both occurrences, information was collected over 2 weeks via phone interviews by medical oncology fellows or research assistants. The study was approved by the Ethics Committee of both participating institutions. Enrolled patients provided their written informed consent to participate in the study.
FIGURE 1.
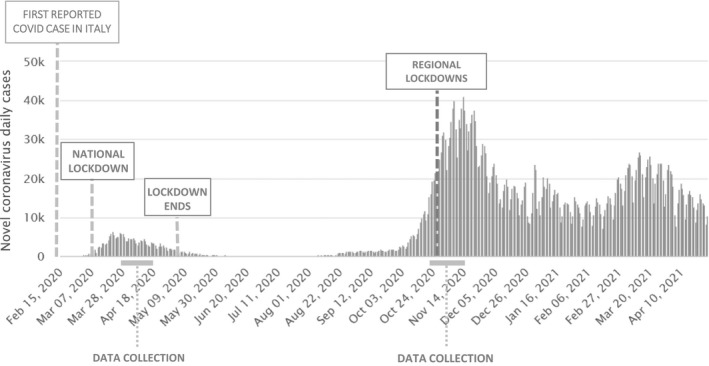
Timeline of events and data collection during the first and second waves of COVID‐19 in Italy
2.2. Patients
We searched a prospective database of patients with GEP or BP NETs managed at our institutions. Within this group, we identified adult patients (age ≥ 18 years) with advanced, inoperable disease or who underwent R0/R1 surgical resection up to 5 years prior to enrollment. Patients with stage IV disease could receive any type of treatments. Patients with an Eastern Cooperative Oncology Group performance status ≥ 2, subjects on active therapy with psychotropic agents and subjects with a history of infection by SARS‐CoV‐2 before enrollment were excluded from the study. Patients with mixed adenoneuroendocrine tumors were also excluded.
The following information was collected by review of patient medical records: demographics, marital status, level of education, date of initial diagnosis, location of primary tumor, stage at diagnosis and at study entry according to the American Joint Committee on Cancer classification, 10 , 11 , 12 presence of a functional hormonal syndrome, presence of a prior diagnosis of psychic illness needing active, chronic treatment with psychotropic agents. The tumor grade by WHO criteria 13 , 14 was obtained by review of surgical pathology reports.
2.3. Questionnaire instruments
Seven specific constructs (depression, anxiety, stress, quality of life, NET‐related quality of life, patient–physician relationship, psychological distress) were investigated using validated screening instruments, including the Depression anxiety stress scale‐21 (DASS‐21), 15 the European Organization for Research and Treatment of Cancer (EORTC) quality of life questionnaire (EORTC QLQ)‐C30, 16 the EORTC QLQ gastrointestinal NET 21 (GI.NET21), 17 the patient doctor relationship questionnaire 9 (PDRQ9) 18 and the Impact of event scale‐revised (IES‐R). 19 For each instrument, the overall score and the scores of the relative subscales were calculated as detailed in the Supporting information (Methods S1). All patients were asked to answer questions from the Italian version of DASS‐21, EORTC QLQ‐C30, EORTC QLQ‐GI.NET21 and PDRQ9 during the first and second waves of the pandemic outbreak in Italy. The IES‐R instrument was administered only during the second wave, given its ability in capturing features suggestive of post‐traumatic stress disorder (PTSD), a condition usually arising several months after a traumatic event.
2.4. Statistical analysis
Descriptive statistics were used for patient demographics and the results of questionnaire instruments. The association between ordinal classes obtained from individual questionnaire scores (i.e., normal vs non‐normal or normal vs mild depression vs moderate depression vs severe depression vs extremely severe depression) and patient clinicopathological features was evaluated by Fisher's test or one‐way ANOVA, as appropriate. Factors showing p ≤ .2 at univariate analysis were introduced in a multivariable logistic regression model or an ordinal regression model, as appropriate, in which variables were selected using backward stepwise elimination with p ≤ .05 being considered statistically significant. The assumption of proportionality was verified by likelihood ratio test. When this assumption was violated, the generalized ordered logit model was used. Exact 95% confidence intervals (CIs) were calculated for each proportion of interest. All tests were two‐sided, and statistical significance was declared at p ≤ .05. Statistical analysis was conducted using MedCalc, version 12.7 (MedCalc Software bvba) and STATA, version 16 (StataCorp. 2019: StataCorp LLC).
A potential confounding factor in an analysis of the psychological impact of COVID‐19 pandemic is the occurrence of the infection in patients themselves or their first‐degree relatives during the study. To mitigate this bias, we carried out separate analyses for patients with and without personal or family history of confirmed SARS‐CoV‐2 infection.
3. RESULTS
3.1. Demographics and tumor characteristics
Demographic variables and clinicopathological characteristics of the 197 patients included in the study are provided in Table 1. The number of male and female patients was similar, and the median age at NET diagnosis was 62 years (range 19–84 years). All patients were Caucasian. The majority of subjects (129/197; 65%) were married and approximately one quarter of the cohort received prior therapy with psychotropic agents, particularly benzodiazepines. The level of education was heterogeneous, with 52% of patients (104/197) harboring at least a high school degree. The majority of patients had pancreatic (29%) or small bowel (25%) primaries, and G1/G2 tumors were diagnosed in the 96% of cases (190/197). Seven patients harbored G3 NETs. The diagnosis of NET occurred within 2 years from enrollment in two‐thirds of patients (130/197). Presence of clinical syndromes associated with hormone secretion was documented in 31 patients (16%). At study entry, 75 patients (38%) were on treatment with anti‐cancer agents, 16 patients were on active surveillance for panNETs < 2 cm and 106 patients were on follow‐up after surgery. Nine patients were diagnosed with COVID‐19 when on study, and one of them died. Two non‐COVID‐19‐related deaths were also recorded before W2.
TABLE 1.
Patient demographics and clinical characteristics
| Characteristics |
Number of patients (n = 197) |
|---|---|
| Age (years) | |
| Median | 62 |
| Range | 19–84 |
| Gender | |
| Male | 98 (50%) |
| Female | 99 (50%) |
| Marital status | |
| Married | 129 (65%) |
| Single | 36 (18%) |
| Widow/widower | 32 (17%) |
| Level of instruction | |
| Bachelor's degree | 50 (25%) |
| High school | 54 (27%) |
| Middle school | 61 (31%) |
| Elementary school | 32 (17%) |
| Prior therapy for anxiety | |
| Yes | 51 (25%) |
| No | 146 (75%) |
| Date of diagnosis | |
| < 1 year | 28 (14%) |
| 1–2 years | 102 (52%) |
| > 3 years | 67 (34%) |
| Primary site | |
| Pancreas | 58 (29%) |
| Small intestine | 48 (25%) |
| Stomach | 37 (19%) |
| Colon–rectum | 26 (13%) |
| Appendix | 16 (8%) |
| Duodenum | 6 (3%) |
| Lung | 6 (3%) |
| Grade | |
| G1 | 83 (42%) |
| G2 | 107 (54%) |
| G3 | 5 (3%) |
| Unknown | 2 (1%) |
| Stage at diagnosis | |
| I | 62 (32%) |
| II | 43 (22%) |
| III | 29 (15%) |
| IV | 50 (25%) |
| Unknown | 13 (6%) |
| Hormone secretion | |
| Yes | 31 (16%) |
| No | 166 (84%) |
| Management | |
| Active surveillance | 122 (62%) |
| Active therapy | 75 (38%) |
3.2. Depression, anxiety and stress
At W1, the prevalence of depression, anxiety and stress by DASS‐21 questionnaire was 32%, 36% and 26%, respectively (Figure 2). The frequency of depression and anxiety increased to 38% and 41% at W2, in the absence of modifications in the frequency of stress. Although the levels of depression and anxiety (mild, moderate, severe and extremely severe 15 ) appeared to be similarly distributed at W1, moderate depression and anxiety tended to prevail at W2. No substantial modifications in the rate of depression, anxiety and stress at W2 were observed after removing patients who were diagnosed with COVID‐19 (see Supporting information, Figure S1). At W1, patients with hormonal syndromes showed a significantly higher frequency of depression (p = .001) and anxiety (p = .04). Moreover, subjects with education lower than secondary level displayed higher rates of depression (p = .02), whereas the prevalence of stress was significantly higher among females (p = .01). At W2, depression was documented more frequently in patients older than 65 years (p = .01), with poor education (p = .01) and in those who were previously treated with psychotropic agents (p = .007). Low‐level education (p = .009) and advanced age (p = .03) were also significantly associated with the occurrence of anxiety (Table 2). When patients with a personal or family history of COVID‐19 were removed from analysis, low tumor grade showed a significant association with depression at W2 (p = .04). After adjusting for variables that showed a p ≤ .2 in univariate analysis at W1, the presence of functioning tumors and the female sex remained associated with anxiety (odds ratio [OR] = 2.21, 95% CI = 1–4.8; p = .04) and stress (OR = 0.44, 95% CI = 0.22–0.84; p = .01), respectively. At W2, advanced age and poor education remained significantly associated with depression (OR = 2.31, 95% CI = 1.24–4.31; p = .009) and anxiety (OR = 1.47, 95% CI = 1.1–1.96; p = .009), respectively. By ordinal logistic regression analysis, female patients tended to show more severe forms of stress at W1 (OR = 0.45 ± 0.14; p = .01), whereas educational status was associated with the levels of anxiety at both W1 (OR = 1.33 ± 0.22; p = .07) and W2 (OR = 1.45 ± 0.26; p = .03).
FIGURE 2.
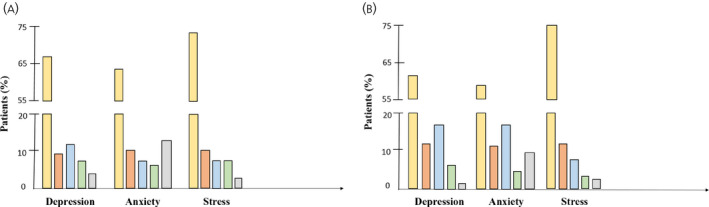
Prevalence of depression, anxiety and stress in the overall cohort. The frequency of depression and anxiety, but not stress, increased from (A) the first (W1) to (B) the second (W2) epidemic wave
TABLE 2.
Univariate analysis of potential predictors of depression, anxiety and stress
| Characteristics | First wave | Second wave | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Depression rate | p | Anxiety rate | p | Stress rate | p | Depression rate | p | Anxiety rate | p | Stress rate | p | |
| Sex | ||||||||||||
| Male | 27.5% | .18 | 31.6% | .25 | 18.4% | .01 | 33.3% | .2 | 40.6% | .93 | 19.8% | .14 |
| Female | 36.4% | 39.4% | 34.3% | 42.3% | 41.2% | 28.9% | ||||||
| Age at diagnosis (years) | ||||||||||||
| ≤ 65 | 30.9% | .67 | 34.7% | .74 | 27.6% | .61 | 30.8% | .01 | 35% | .03 | 22.3% | .39 |
| > 65 | 33.8% | 37% | 24.3% | 49.3% | 50.7% | 27.8% | ||||||
| Marital status | ||||||||||||
| Married | 33.1% | .66 | 35.2% | .88 | 25% | .54 | 38.9% | .68 | 38.9% | .43 | 23% | .55 |
| Single/widow | 30% | 36.2% | 29% | 35.8% | 44.8% | 26.9% | ||||||
| Level of instruction | ||||||||||||
| Bachelor degree | 40% | .02 | 38% | .09 | 42.9% | .27 | 37.5% | .01 | 35.4% | .009 | 29.2% | .35 |
| High school | 25.9% | 27.8% | 24.1% | 24.5% | 31.5% | 17% | ||||||
| Middle school | 21.3% | 31.1% | 19.7% | 37.7% | 39.3% | 22.9% | ||||||
| Elementary | 50% | 53.1% | 37.5% | 61.3% | 67.8% | 32.3% | ||||||
| Prior therapy for anxiety | ||||||||||||
| Yes | 27.4% | .42 | 33.3% | .7 | 29.1% | .59 | 51.6% | .007 | 45.1% | .48 | 21.6% | .59 |
| No | 33.6% | 36.3% | 25.3% | 31.3% | 39.4% | 25.3% | ||||||
| Stage | ||||||||||||
| I | 30.7% | .75 | 30.6% | .47 | 25.8% | .88 | 38.7% | .3 | 35.5% | .63 | 21% | .84 |
| II | 27.9% | 37.2% | 25.6% | 28.6% | 45.2% | 23.8% | ||||||
| III | 31% | 31% | 33.3% | 41.4% | 41.4% | 27.6% | ||||||
| IV | 38% | 44% | 28% | 48% | 46.8% | 27.7% | ||||||
| Hormonal syndrome | ||||||||||||
| Yes | 52.4% | .001 | 51.6% | .04 | 29% | .72 | 50% | .13 | 53.3% | .13 | 33.3% | .21 |
| No | 26.5% | 32.5% | 25.9% | 35.6% | 38.6% | 22.7% | ||||||
| Active treatment | ||||||||||||
| Yes | 32.5% | .97 | 36.8% | .73 | 25% | .77 | 41.7% | .38 | 45.8% | .23 | 23.6% | .9 |
| No | 32.2% | 34.5% | 26.9% | 35.3% | 37% | 24.4% | ||||||
| Time from diagnosis | ||||||||||||
| ≤ 12 months | 28.6% | .71 | 42.9% | .36 | 25% | .84 | 32.1% | .53 | 42.9% | .68 | 21.4% | .73 |
| > 12 months | 32.1% | 33.9% | 26.8% | 38.4% | 38.8% | 24.4% | ||||||
| Grading | ||||||||||||
| G1 | 34.9% | .5 | 38.5% | .35 | 31.3% | .21 | 44.6% | .08 | 42.2% | .65 | 27.7% | .38 |
| G2–G3 | 30.4% | 32.1% | 23.2% | 32.4% | 38.9% | 22.2% | ||||||
| Primary site | ||||||||||||
| Pancreas | 25.9% | .59 | 27.6% | .53 | 20.7% | .67 | 33.3% | .65 | 35.1% | .34 | 21.1% | .56 |
| Stomach/small bowel | 35.2% | 38.5% | 29.1% | 41.1% | 46.7% | 26.7% | ||||||
| Colon | 35.7% | 42.9% | 31% | 39% | 41.5% | 26.8% | ||||||
| Lung | 16.7% | 16.7% | 16.7% | 20% | 0% | 0% | ||||||
Bold values are statistically significant (p< 0.05)
3.3. Health‐related quality of life (HRQoL)
Mean and median HRQoL scores are detailed in Table 3. Overall, the global health status of study participants did not change between W1 and W2. Intriguingly, a significant improvement of the physical (p = .03) and emotional functioning domains (p = .001) was observed over time. Moreover, both nausea/vomiting (p = .0002) and appetite (p = .02) improved significantly between W1 and W2. Treatment‐related symptoms (p = .005) and disease‐related worries (p = .0006) were reported less commonly at W2 compared to W1, and an improvement of sexual function was also noted between W1 and W2 (p = .02). We then analyzed separately HRQoL changes in patients under surveillance (n = 122) and in those receiving active treatment (n = 75). No significant modifications of HRQoL domains were documented in actively treated patients, whereas an improvement of the physical functioning domain (p = .04), emotional functioning domain (p = .002), nausea/vomiting (p = .004), appetite (p = .009) and disease‐related worries (p = .001) was observed in patients on follow‐up. No changes were seen after excluding patients with a personal or family history of COVID‐19 from analysis.
TABLE 3.
Health‐related quality of life (HRQoL) scores in the first and second epidemic waves
| HRQoL domain | First wave | Second wave | p a | ||||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Median | Q1‐Q3 | Mean (SD) | Median | Q1‐Q3 | ||
| Global health status | 67.5 (20.3) | 66 | 50–83 | 67.2 (21.5) | 66 | 50–83 | .67 |
| Physical functioning | 81.5 (18.4) | 87 | 67–100 | 82.9 (18.3) | 87 | 67–100 | .03 |
| Role functioning | 79.5 (21.6) | 84 | 67–100 | 81.4 (20) | 84 | 67–100 | .13 |
| Emotional functioning | 68.7 (22.7) | 67 | 59–84 | 72 (21.4) | 67 | 67–92 | .001 |
| Cognitive functioning | 84.3 (18.5) | 84 | 67–100 | 84 (18.1) | 84 | 67–100 | .44 |
| Social functioning | 79.1 (23.3) | 84 | 67–100 | 78.3 (24) | 84 | 67–100 | .32 |
| Fatigue | 23.7 (20) | 20 | 0–33 | 22.6 (19.5) | 20 | 0–33 | .47 |
| Nausea/vomiting | 12 (18.6) | 0 | 0–20.2 | 8.2 (15) | 0 | 0–16 | .0002 |
| Pain | 18.6 (20) | 16 | 0–33 | 20.6 (24) | 16 | 0–33 | .33 |
| Dyspnea | 18 (22) | 0 | 0–33 | 16.2 (21) | 0 | 0–33 | .17 |
| Insomnia | 24.2 (24.6) | 33 | 0–33 | 21.9 (25.6) | 33 | 0–33 | .12 |
| Appetite loss | 16 (22) | 0 | 0–33 | 12.8 (20) | 0 | 0–33 | .02 |
| Constipation | 13.2 (20.2) | 0 | 0–33 | 12.1 (18.6) | 0 | 0–33 | .4 |
| Diarrhea | 12.6 (20.4) | 0 | 0–33 | 12.2 (20) | 0 | 0–33 | .9 |
| Financial difficulties | 20.2 (24.8) | 0 | 0–33 | 20.9 (25.4) | 0 | 0–33 | .54 |
| Endocrine scale | 11.1 (17.3) | 0 | 0–20 | 12 (16.3) | 0 | 0–20 | .37 |
| Gastrointestinal scale | 16.7 (16.3) | 13 | 0–26 | 16.9 (15.6) | 13 | 0–26 | .49 |
| Treatment scale | 17.3 (20.3) | 13 | 0–26 | 11.2 (16.7) | 0 | 0–16 | .005 |
| Disease‐related worries scale | 38.5 (25.7) | 33 | 20–53 | 33.8 (25.2) | 33 | 13–46 | .0006 |
| Social functioning scale | 27.3 (22.4) | 20 | 10–43 | 25.7 (21.3) | 20 | 10–43 | .19 |
| Muscle/bone pain symptom | 17.6 (25) | 0 | 0–33 | 17.1 (25.1) | 0 | 0–33 | .65 |
| Sexual function | 24.4 (30) | 33 | 0–33 | 13.6 (24.8) | 0 | 0–33 | .02 |
| Information/communication function | 53.9 (31.4) | 66 | 33–66 | 53.9 (32) | 66 | 33–66 | .44 |
| Weight loss | 10.9 (20.8) | 0 | 0–33 | 10.6 (19.4) | 0 | 0–33 | .76 |
| Weight gain | 10.2 (21.4) | 0 | 0–0 | 8 (18.1) | 0 | 0–0 | .05 |
Abbreviation: Q, quartile.
Wilcoxon matched‐pairs signed rank test.
Bold values are statistically significant (p< 0.05)
A 10‐point change in each EORTC‐QLQ‐C30 or EORTC‐QLQ‐GINET.21 domain score is frequently considered a minimal clinically important difference. 20 , 21 Figure 3 illustrates the clinically important changes that occurred in each HRQoL domain from W1 to W2. To identify those patients most likely to undergo HRQoL deterioration during the COVID‐19 pandemic, we evaluated the association between score modifications and selected clinical‐pathological features. By univariate analysis, advanced age, poor education and hormonal secretion were associated with a substantial deterioration of multiple HRQoL domains (Table 4). By multivariable analysis, advanced age remained significantly associated with a worsening in the physical functioning, cognitive functioning, fatigue, constipation and financial difficulties domains.
FIGURE 3.
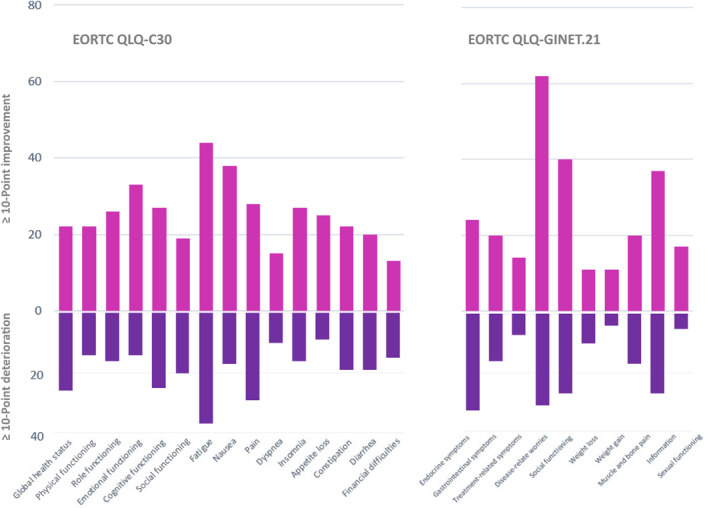
Effects of COVID‐19 pandemic on clinically significant health‐related quality of life changes between the first (W1) and second (W2) epidemic waves. EORTC, European Organization for Research and Treatment of Cancer; QLQ, quality of life questionnaire
TABLE 4.
Predictors of health‐related quality of life (HRQoL) deterioration during the COVID pandemic
| HRQoL domain deterioration | Univariate analysis | Multivariable analysis |
|---|---|---|
| Global health status | Prior therapy for anxiety: p = .04 | — |
| Poor instruction: p = .0002 | ||
| Physical functioning | Age > 65 years: p = .002 | Age > 65 years: p = .005 (OR = 6.66; 95% CI = 1.77–24.97) |
| Hormone secretion: p = .05 | ||
| Role functioning | — | — |
| Emotional functioning | — | — |
| Cognitive functioning | Age > 65 years: p = .01 | Age > 65 years: p = .01 (OR = 2.95; 95% CI = 1.25–6.97) |
| Social functioning | — | — |
| Fatigue | Age > 65 years: p = .0009 | Age > 65 years: p = .0007 (OR = 3.9; 95% CI = 1.77–8.63) |
| Active therapy: p = .04 | ||
| Poor instruction: p < .0001 | ||
| Nausea/vomiting | — | — |
| Pain | Age > 65 years: p = .03 | — |
| Poor instruction: p = .02 | ||
| Dyspnea | Single marital status: p= .02 | Single marital status: p = .003 (OR = 9.08; 95% CI = 2.13–38.80) |
| Poor instruction: p = .02 | ||
| Insomnia | — | — |
| Appetite loss | — | — |
| Constipation | Age > 65 years: p = .01 | Age > 65 years: p = .03 (OR = 2.96; 95% CI = 1.08–8.11) |
| Poor instruction: p = .001 | ||
| Diarrhea | Hormone secretion: p = .008 | Hormone secretion: p = .01 (OR = 5; 95% CI = 1.39–18) |
| Poor instruction: p = .01 | Poor instruction: p = .03 (OR = 0.15; 95% CI = 0.03–0.82) | |
| Financial difficulties | Age > 65 years: p = .01 | Age > 65 years: p = .02 (OR = 8.31; 95% CI = 2.15–32.15) |
| Married marital status: p = .005 | Married marital status: p = .04 (OR = 5.03; 95% CI = 1.09–23.30) | |
| Endocrine scale | Age > 65 years: p = .007 | — |
| Prior therapy for anxiety: p = .02 | ||
| Poor instruction: p < .0001 | ||
| Diagnosis > 1 year: p = .04 | ||
| Gastrointestinal scale | — | — |
| Treatment scale | — | — |
| Disease‐related worries scale | Hormone secretion: p = .05 | Hormone secretion: p = .002 (OR = 4.01; 95% CI = 1.68–9.6) |
| Social functioning scale | — | — |
| Muscle/bone pain | — | — |
| Sexual function | — | — |
| Information/communication | — | — |
| Weight loss | — | — |
| Weight gain | — | — |
Abbreviations: CI, confidence interval; OR, odds ratio.
3.4. Patient–physician relationship
A drastic reduction in the number of outpatient visits for NET patients has been recorded in our country during the COVID‐19 pandemic. 22 We therefore investigated possible changes in the patient–physician relationship between W1 and W2. The mean (± SD) score of the PDRQ9 questionnaire was 4.36/5 (±0.76) at W1 and 4.35/5 (±0.56) at W2.
3.5. Psychological distress
In the evaluable population (n = 195; two patients died before W2), the mean (± SD) total score of the IES‐R was 34.7 (±17). A score ≥ 33, suggestive of a higher risk of PTSD diagnosis, 19 was documented in 114 (58.4%) patients. The mean scoreds of the intrusion, avoidance and hyperarousal subscales were 1.8, 1.4 and 1.6, respectively. After excluding patients with a personal or family history of COVID‐19, 110/186 (59.1%) patients displayed a total score ≥ 33. Among patients with personal or family experience of the COVID‐19 disease, a score ≥ 33 was observed in 3/9 cases (33%). In the global population, IES‐R scores consistent with PTSD diagnosis were seen more frequently in patients that were single/widow (p = .0007) or in those with poor education (p = .002). By multivariable analysis, both the single marital status (OR = 0.16, 95% CI = 0.06–0.48; p = .0009) and the low level of education (OR = 0.47, 95% CI = 0.23–0.99; p = .05) remained significantly associated with IES‐R scores ≥ 33.
4. DISCUSSION
To our knowledge, this is the first study to evaluate the psychological impact of the COVID‐19 outbreak in patients with NET. Yet accurate information on mental health and QoL during the COVID‐19 pandemic is critically important: psychological interventions might be implemented in NET clinics if needed, COVID‐19‐influenced HRQoL levels can be assessed for future interpretation of QoL measures of ongoing clinical trials, and recommendations on health policy measures specifically concerning the NET patient population can be drawn upon our findings to improve the quality of care.
Several studies have already documented high rates of neuropsychiatric symptoms in patients with NETs. In particular, depression, anxiety and difficulty in impulse control have been described in 20%–50%, 35% and 75% of NET patients, respectively. 23 , 24 , 25 , 26 , 27 , 28 Moreover, NET patients have been shown to score considerably worse than healthy subjects in terms of HRQoL. 29
The COVID‐19 pandemic has added another layer of complexity to the fears of NET patients. Inability to travel, difficult access to hospitals and NET clinics, delayed imaging studies, and deferred surgeries or interventional procedures 22 , 30 are only a few factors potentially contributing to an enhanced psychological distress in NET patients. We longitudinally surveyed a bi‐institutional cohort of 197 patients with NET under active treatment or surveillance. Given the fluctuant nature of mental symptoms and the rapidly evolving pandemic scenario, patients were interviewed during the plateau phase of both the first and second waves of the epidemic in Italy. At W1, the frequency of depression, anxiety and stress was 32%, 36% and 26%, respectively, which is in line with prior reports preceding the onset of the COVID‐19 pandemic. Notably, the rate of depression (38%) and anxiety (41%) increased substantially at W2, possibly as a consequence of chronic exposure of patients to fears related to COVID‐19. By multivariable analysis, advanced age and poor education were found to be significantly associated with depression and anxiety at W2. This is in line with prior studies focusing on the mental impact of COVID‐19 in patients with cancer. 31 , 32 Tailored psycho‐oncological interventions should be offered, if possible, particularly to older patients with a low level of formal education, aiming to recognize, prevent or treat mental distress during the COVID‐19 pandemic.
According to a study carried out in the pre‐COVID‐19 era, 33 the prevalence of PTSD among patients with NET is approximately 15%, and patients with emotional distress caused by their cancer appear to be at higher risk for this condition. In the present study, we found a frequency of PTSD of approximately 60%, with single marital status and low level of education significantly predicting the occurrence of the disorder. Post‐traumatic stress symptoms are well characterized psychological effects of quarantine, particularly in subjects with poor education levels. 34 , 35 Nevertheless, a lower rate of PTSD (9%–36%) has been described in response to the COVID‐19 pandemic in patients with non‐neuroendocrine cancers, 36 , 37 , 38 thus suggesting that NET patients might be particularly vulnerable to this condition. Systematic screening of PTSD occurrence is advised for all NET patients and the bio‐psychological basis of such an elevated PTSD frequency should be investigated further.
HRQoL is increasingly recognized as a crucial endpoint in clinical trials for cancer patients, and contrasting data have been reported so far regarding the effects of the COVID‐19 pandemic on the QoL of patients with cancer. 39 , 40 , 41 , 42 In the present study, we longitudinally assessed intraindividual changes between W1 and W2 in patients with NETs. By contrast to our expectations, only a minority of patients underwent a clinically significant HRQoL deterioration throughout the pandemic, thus suggesting that NET patients were able to cope with the traumatic events associated with the epidemic outbreak. Several reasons might explain this phenomenon, at least theoretically. First, patients who are already accustomed to restrictions in everyday life might cope better with the additional restrictions imposed in response to the COVID‐19 pandemic. Second, the increase of intra‐familial proximity (especially for family members living in the same household) might potentially alleviate the physical and emotional distress suffered by NET patients. Not surprisingly, healthy subjects have reported loneliness as a result of the COVID‐19 pandemic more frequently than cancer patients. 43 Third, because NET patients have already re‐prioritized their life upon receiving a diagnosis of a rare, poorly understood form of cancer, they might be particularly resilient when exposed to a new, obscure, potentially life‐threatening situation. In our cohort, old patients appeared to be at higher risk of developing HRQoL deterioration, and particular attention should be paid to the worsening of physical and cognitive functioning areas in this frail subject category.
The relationship between patients and doctors is an essential component of patient care. Evidence demonstrates that HRQoL is positively associated with all aspects of care among cancer patients in general, 44 and also that care satisfaction is strictly related to better HRQoL and psychosocial function of NET patients in particular. 45 , 46 , 47 In the present study, the confidence in NET specialists was very high, and no longitudinal changes were noted despite the persistence of the pandemic threat. Although we acknowledge that the level of patient satisfaction could be overestimated in our study because the responses to questionnaires were not anonymized, it is also possible that the degree of confidence in physicians might be the consequence of patient management in two large‐volume institutions highly specialized in the treatment of NETs. A potential impact of elevated patient satisfaction on psychometric measures and HRQoL therefore cannot be excluded in our cohort.
The present study has several limitations. First, the study was conducted in two tertiary centers in South Italy, and our findings might thus have limited geographic generalizability. Nevertheless, although psychosocial regional differences should be always taken into account, we consider that the experiences and perceptions faced by study participants are similar to those experienced by NET patients internationally, or at least where lockdown measures were adopted. Second, fears and hopes related to the COVID‐19 pandemic have changed rapidly in recent months, and will likely continue to fluctuate over the next few months. Despite its longitudinal design, the present study only allows for an understanding of patient perceptions during the exact time frame of data collection. In this context, we are unable, for example, to evaluate the impact of anti‐SARS‐CoV‐2 vaccine development and mass vaccination campaigns on psychometric evaluations and HRQoL measures. Lastly, despite the sample size of this study being relatively large, a very heterogeneous population of patients was included. Although subanalysis exploring defined subclasses of patients have been carried out, reliable conclusions are hindered in several cases by small numbers.
Several lessons can be learnt from the current pandemic crisis with respect to minimizing the psychological impact of future pandemic outbreaks on NET patients. Although reallocation of health care resources can be necessary during a pandemic, pathways dedicated to patients with cancer (and in particular to those with rare cancers) should always remain active. Technology has undoubtedly facilitated uninterrupted cancer patient care during the COVID‐19 pandemic but, although being key in connecting patients with their physicians, telemedicine can also increase the disparity between low‐income and high‐income or old and young patients, providing suboptimal support to patient categories at high risk of psychological distress. Dedicated pathways should therefore be provided to high‐risk patients, and psychological consultations should be part of these pathways. Simplified access to anti‐cancer agents (ideally with door‐to‐door delivery of oral drugs), as well as rationalization of clinical trial procedures (i.e., shipment of investigational oral drugs to patients' homes, possibility of performing lab work in local facilities, and the use of telehealth services for follow‐up visits) are other important aspects that might reduce the psychological burden of future pandemics.
In conclusion, despite heightened vulnerability in terms of PTSD occurrence, NET patients show an elevated psychological resilience in response to the COVID‐19 pandemic. The high level of care satisfaction might contribute to explain the absence of significant HRQoL deterioration and the relatively small increase in depressive symptoms and anxiety from W1 to W2. We advise a systematic screening of post‐traumatic stress symptoms for all NET patients until the end of the pandemic. Specific psychological interventions should be developed to treat this vulnerable population.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
AUTHOR CONTRIBUTIONS
Eleonora Lauricella: Conceptualization; Data curation; Investigation; Methodology; Project administration; Writing – original draft. Mauro Cives: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Supervision; Writing – original draft. Alessandra Bracigliano: Data curation; Writing – review & editing. Ottavia Clemente: Data curation; Writing – review & editing. Valentina Felici: Conceptualization; Data curation; Methodology; Writing – review & editing. Rossella Lippolis: Conceptualization; Methodology; Writing – review & editing. Brunella Amoruso: Data curation; Writing – review & editing. Eleonora Pelle': Data curation; Writing – review & editing. Barbara Mandriani: Data curation; Writing – review & editing. Chiara Esposto: Data curation; Writing – review & editing. Cira Forte: Data curation; Writing – review & editing. Francesco Perri: Data curation; Writing – review & editing. Camillo Porta: Supervision; Writing – review & editing. Salvatore Tafuto: Conceptualization; Methodology; Writing – review & editing.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/jne.13041.
Supporting information
Figure S1
Methods S1
ACKNOWLEDGMENTS
This work was supported by the Associazione Italiana per la Ricerca sul Cancro (MFAG #23583) and Associazione per la Ricerca Biomolecolare Onlus, Acquaviva, Italy (2020).
Lauricella E, Cives M, Bracigliano A, et al. The psychological impact of COVID‐19 pandemic on patients with neuroendocrine tumors: Between resilience and vulnerability. J Neuroendocrinol. 2021;33:e13041. 10.1111/jne.13041
Eleonora Lauricella and Mauro Cives contributed equally to this work.
DATA AVAILABILITY STATEMENT
Data are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lu R, Zhao X, Li J, et al. Genomic characterization and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565‐574. 10.1016/S0140-6736(20)30251-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization . Coronavirus disease (COVID‐19) outbreak. https://www.who.int.Home/Diseases/Coronavirus‐19. Accessed 11 March 2020.
- 4. Wright L, Steptoe A, Fancourt D. Are we all in this together? Longitudinal assessment of cumulative adversities by socioeconomic position in the first 3 weeks of lockdown in the UK. J Epidemiol Community Health. 2020;74(9):683‐688. 10.1136/jech-2020-214475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fancourt D, Steptoe A, Bu F. Trajectories of anxiety and depressive symptoms during enforced isolation due to COVID‐19 in England: a longitudinal observational study. Lancet Psychiatry. 2021;8(2):141‐149. 10.1016/S2215-0366(20)30482-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pierce M, Hope H, Ford T, et al. Mental health before and during the COVID‐19 pandemic: a longitudinal probability sample survey of the UK population. Lancet Psychiatry. 2020;7(10):883‐892. 10.1016/S2215-0366(20)30308-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shanahan L, Steinhoff A, Bechtiger L, et al. Emotional distress in young adults during the COVID‐19 pandemic: evidence of risk and resilience from a longitudinal cohort study. Psychol Med. 2020;1‐10. 10.1017/S003329172000241X. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liang W, Guan W, Chen R, et al. Cancer patients in SARS‐CoV‐2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335‐337. 10.1016/S1470-2045(20)30096-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cives M, Strosberg JR. Gastroenteropancreatic neuroendocrine tumors. CA Cancer J Clin. 2018;68(6):471‐487. 10.3322/caac.21493 [DOI] [PubMed] [Google Scholar]
- 10. Bergsland EK, Woltering EA, Rindi G. Neuroendocrine tumors of the pancreas. In: Amin MB, eds. AJCC Cancer Staging Manual. 8th ed. AJCC; 2017:407. Corrected at 4th printing, 2018. [Google Scholar]
- 11. Rami‐Porta R, Asamura H, Travis WD, Rusch VW. Lung. In: Amin MB, eds. AJCC Cancer Staging Manual. 8th ed. AJCC; 2017:431. [DOI] [PubMed] [Google Scholar]
- 12. Woltering EA, Bergsland EK, Beyer DT, et al. Neuroendocrine tumors of the jejunum and ileum. In: Amin MB, eds. AJCC Cancer Staging Manual. 8th ed. AJCC; 2017:375. Corrected at 4th printing, 2018. [Google Scholar]
- 13. Klimstra DS, Kloppell G, La Rosa S, Rindi G. Classification of neuroendocrine neoplasms of the digestive system. In: WHO Classification of Tumours Editorial Board (Ed), WHO Classification of Tumours: Digestive System Tumours, 5th ed: International Agency for Research on Cancer; 2019:16. [Google Scholar]
- 14. Travis WD, Brambilla E, Burke AP, et al. (eds). WHO Classification of Tumours of the Lung, Pleura, Thymus, and Heart, 4th ed: IARC; 2015. [DOI] [PubMed] [Google Scholar]
- 15. Lovibond PF, Lovibond SH. The structure of negative emotional states: comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behav Res Ther. 1995;33(3):335‐343. 10.1016/0005-7967(94)00075-u [DOI] [PubMed] [Google Scholar]
- 16. Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ‐C30: a quality‐of‐life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365‐376. 10.1093/jnci/85.5.365 [DOI] [PubMed] [Google Scholar]
- 17. Yadegarfar G, Friend L, Jones L, et al. Validation of the EORTC QLQ‐GINET21 questionnaire for assessing quality of life of patients with gastrointestinal neuroendocrine tumours. Br J Cancer. 2013;108:301‐310. 10.1038/bjc.2012.560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Van der Feltz‐Cornelis CM, Van Oppen P, Van Marwijk HW, De Beurs E, Van Dyck R. A patient‐doctor relationship questionnaire (PDRQ‐9) in primary care: development and psychometric evaluation. Gen Hosp Psychiatry. 2004;26(2):115‐120. 10.1016/j.genhosppsych.2003.08.010 [DOI] [PubMed] [Google Scholar]
- 19. Weiss DS, Marmar CR. The impact of events scale–revised. In: Wilson JP, Keane TM, eds. Assessing Psychological Trauma and PTSD: The Guilford Press; 1997:399‐411. [Google Scholar]
- 20. Strosberg J, Wolin E, Chasen B, et al. Health‐related quality of life in patients with progressive midgut neuroendocrine tumors treated with 177 Lu‐Dotatate in the phase III NETTER‐1 trial. J Clin Oncol. 2018;36(25):2578‐2584. 10.1200/JCO.2018.78.5865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Khan S, Krenning EP, van Essen M, et al. Quality of life in 265 patients with gastroenteropancreatic or bronchial neuroendocrine tumors treated with [177Lu‐DOTA0, Tyr3] octreotate. J Nucl Med. 2011;52:1361‐1368. 10.2967/jnumed.111.087932 [DOI] [PubMed] [Google Scholar]
- 22. Panzuto F, Maccauro M, Campana D, et al. Impact of the SARS‐CoV2 pandemic dissemination on the management of neuroendocrine neoplasia in Italy: a report from the Italian Association for Neuroendocrine Tumors (Itanet). J Endocrinol Invest. 2021;44(5):989‐994. 10.1007/s40618-020-01393-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lewis AR, Wang X, Magdalani L, et al. Health‐related quality of life, anxiety, depression and impulsivity in patients with advanced gastroenteropancreatic neuroendocrine tumours. World J Gastroenterol. 2018;24(6):671‐679. 10.3748/wjg.v24.i6.671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Major LF, Brown GL, Wilson WP. Carcinoid and psychiatric symptoms. South Med J. 1973;66(7):787‐790. 10.1097/00007611-197307000-00013 [DOI] [PubMed] [Google Scholar]
- 25. Soliday EG, Garofalo J, Smith S, et al. Depression and antidepressant use in gastrointestinal carcinoid cancer patients. J Appl Biobehav Res. 2004;9(2):80‐90. 10.1111/j.1751-9861.2004.tb00093.x [DOI] [Google Scholar]
- 26. Beesley VL, Burge M, Dumbrava M, Callum J, Neale RE, Wyld DK. Perceptions of care and patient‐reported outcomes in people living with neuroendocrine tumours. Support Care Cancer. 2018;26(9):3153‐3161. 10.1007/s00520-018-4166-5 [DOI] [PubMed] [Google Scholar]
- 27. Russo S, Boon JC, Kema IP, et al. Patients with carcinoid syndrome exhibit symptoms of aggressive impulse dysregulation. Psychosom Med. 2004;66(3):422‐425. 10.1097/01.psy.0000126204.97565.91 [DOI] [PubMed] [Google Scholar]
- 28. Pezzilli R, Campana D, Morselli‐Labate AM, Fabbri MC, Brocchi E, Tomassetti P. Patient‐reported outcomes in subjects with neuroendocrine tumors of the pancreas. World J Gastroenterol. 2009;15(40):5067‐5073. 10.3748/wjg.15.5067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Larsson G, Sjödén PO, Oberg K, Eriksson B, von Essen L. Health‐related quality of life, anxiety and depression in patients with midgut carcinoid tumours. Acta Oncol. 2001;40(7):825‐831. 10.1080/02841860152703445 [DOI] [PubMed] [Google Scholar]
- 30. Weickert MO, Robbins T, Kyrou I, et al. Impact of the COVID‐19 pandemic on neuroendocrine tumour services in England. Endocrine. 2021;71(1):14‐19. 10.1007/s12020-020-02564-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Peng S, Lai X, Du Y, Li Y, Tian K, Gan Y. Prevalence and associated factors for depressive symptomatology in Chinese adults during COVID‐19 epidemic. Front Psychol. 2020;11:616723. 10.3389/fpsyg.2020.616723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yang S, Yao H, Song Y, et al. The status of anxiety state among cancer patients and their relatives during coronavirus disease 2019 (COVID‐19) in Hubei, China. Ann Palliat Med. 2021;10(4):4601‐4611. 10.21037/apm-21-745 [DOI] [PubMed] [Google Scholar]
- 33. Ezratty C, Kessel E, Kim MK, Lin JJ. Cancer beliefs associated with posttraumatic stress disorder in neuroendocrine tumor survivors. J Gastrointest Cancer. 2021;52(1):369‐373. 10.1007/s12029-021-00592-3 [DOI] [PubMed] [Google Scholar]
- 34. Brooks SK, Webster RK, Smith LE, et al. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet. 2020;395(10227):912‐920. 10.1016/S0140-6736(20)30460-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Taylor MR, Agho KE, Stevens GJ, Raphael B. Factors influencing psychological distress during a disease epidemic: data from Australia's first outbreak of equine influenza. BMC Public Health. 2008;8:347. 10.1186/1471-2458-8-347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang Y, Duan Z, Ma Z, et al. Epidemiology of mental health problems among patients with cancer during COVID‐19 pandemic. Transl Psychiatry. 2020;10(1):263. 10.1038/s41398-020-00950-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cui Q, Cai Z, Li J, et al. The psychological pressures of breast cancer patients during the COVID‐19 outbreak in China‐A comparison with frontline female nurses. Front Psychiatry. 2020;11:559701. 10.3389/fpsyt.2020.559701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Romito F, Dellino M, Loseto G, et al. Psychological distress in outpatients with lymphoma during the COVID‐19 pandemic. Front Oncol. 2020;10:1270. 10.3389/fonc.2020.01270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ciążyńska M, Pabianek M, Szczepaniak K, et al. Quality of life of cancer patients during coronavirus disease (COVID‐19) pandemic. Psychooncology. 2020;29(9):1377‐1379. 10.1002/pon.5434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gallo O, Bruno C, Locatello LG, et al. The impact of the COVID‐19 pandemic on the quality of life of head and neck cancer survivors. Support Care Cancer. 2021;1‐8. 10.1007/s00520-021-06198-6. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jeppesen SS, Bentsen KK, Jørgensen TL, et al. Quality of life in patients with cancer during the COVID‐19 pandemic ‐ a Danish cross‐sectional study (COPICADS). Acta Oncol. 2021;60(1):4‐12. 10.1080/0284186X.2020.1830169 [DOI] [PubMed] [Google Scholar]
- 42. Koinig KA, Arnold C, Lehmann J, et al. The cancer patient's perspective of COVID‐19‐induced distress—A cross‐sectional study and a longitudinal comparison of HRQOL assessed before and during the pandemic. Cancer Med. 2021;10(12):3928‐3937. 10.1002/cam4.3950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Van de Poll‐Franse LV, de Rooij BH, Horevoorts NJE, et al. Perceived care and well‐being of patients with cancer and matched norm participants in the COVID‐19 crisis: results of a survey of participants in the Dutch PROFILES Registry. JAMA Oncol. 2021;7(2):279‐284. 10.1001/jamaoncol.2020.6093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Brédart A, Razavi D, Robertson C, et al. Assessment of quality of care in an oncology institute using information on patients' satisfaction. Oncology. 2001;61(2):120‐128. 10.1159/000055362 [DOI] [PubMed] [Google Scholar]
- 45. Von Essen L, Larsson G, Oberg K, Sjödén PO. ‘Satisfaction with care’: associations with health‐related quality of life and psychosocial function among Swedish patients with endocrine gastrointestinal tumours. Eur J Cancer Care. 2002;11(2):91‐99. 10.1046/j.1365-2354.2002.00293.x [DOI] [PubMed] [Google Scholar]
- 46. Larsson G, Haglund K, Von Essen L. Distress, quality of life and strategies to ‘keep a good mood’ in patients with carcinoid tumours: patient and staff perceptions. Eur J Cancer Care. 2003;12(1):46‐57. 10.1046/j.1365-2354.2003.00322.x [DOI] [PubMed] [Google Scholar]
- 47. Fröjd C, Lampic C, Larsson G, von Essen L. Is satisfaction with doctors' care related to health‐related quality of life, anxiety and depression among patients with carcinoid tumours? A longitudinal report. Scand J Caring Sci. 2009;23(1):107‐116. 10.1111/j.1471-6712.2008.00596.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Methods S1
Data Availability Statement
Data are available from the corresponding author upon reasonable request.


