Dear Editor
In December 2020 the FDA authorized Pfizer/BioNTech (BNT162b2) and Moderna (mRNA‐1273) COVID‐19 vaccines. From December 2020 through June 2021, according to most recent data, over than 2.4 billion doses of COVID‐19 vaccine have been administrated globally (458 million fully vaccinated, that is 5.9% of global population) over the world. Numerous adverse reactions after messenger RNA (mRNA)‐based COVID‐19 vaccines have been reported. Both of them (Pfizer and Moderna) can be associated with cutaneous adverse effects. Several cutaneous reactions to Moderna (83%) or Pfizer (17%) COVID‐19 vaccines were reported by McMahon et al. for 414 unique patients. 1 The most common cutaneous reaction was delayed large local reaction, followed by local injection site reactions, urticaria, morbilliform eruptions, erythromelalgia, and pernio/chilblains. We report two cases of “COVID arm”: a localized erythematous reaction at the injection site after the first administration of Moderna COVID‐19 vaccine. 2 None of them had COVID‐19 infection symptoms prior to their COVID‐19 vaccinations and the onset of their skin rash. In Table S1 are reported patients' clinical data and management.
Patient 1 was a 60‐years‐old man who came at our department presenting a monolateral erythematous rash on his left arm (Figure 1A). The patient reported the appearance of the rash about 30 days earlier, 1 week after administration of the dose one of Moderna COVID 19 vaccine. No associated symptoms and no systemic involvement were reported. On dermatological physical examination it was appreciated a diffuse, pinkish‐colored erythematous patch along the entire surface of the left arm. Diascopy was negative.
FIGURE 1.
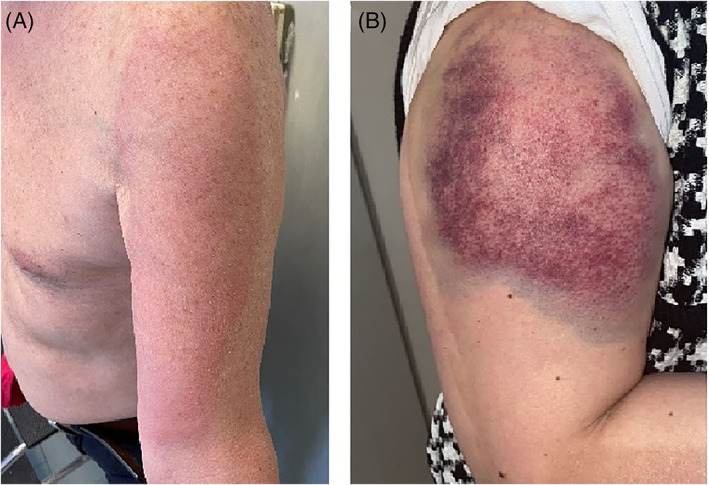
(A) Erythematous rash involving the entire surface of the left arm. (B) Erythematous purpuric rash in the right deltoid region
Patient 2 was a 62‐years‐old woman who developed a monolateral giant erythematous rash in the right deltoid region (Figure 1B). During the collection of the anamnesis, it emerged that the patient received the first dose of Moderna COVID 19 vaccine 7 days before the skin eruption. Soreness and burning pain were reported in the site of skin eruption, with no systemic involvement. On dermatological physical examination it was appreciated a purpuric rash with violet edge of dimensions of about 10 cm × 7 cm. It could not have just resulted from bleeding following the vaccination because it appeared 1 week after the dose administration, in addition, the laboratory examination showed elevated inflammatory markers. Diascopy was negative.
ANA, ANCA and complement, serum electrophoresis and blood count were performed in both patients, showing no alterations. For both of them a corticosteroid topic treatment was prescribed with emollients for 10 days. During the follow up visit, after 2 weeks, patient 1 showed an almost complete resolution of the rash, so it was started a gradual suspension of corticosteroid topic treatment. In patient 2 it was observed a partial improvement, with centripetal resolution of the skin eruption so corticosteroid topic therapy was continued for another week. “COVID arm” is an adverse event that may occur at the site of vaccine injection 7–10 days after the first administration of the Moderna COVID 19 vaccine, with the appearance of an erythematous rash associated or not with algic symptoms. 2 Currently in the literature there are no reported cases of “Covid arm” after the first dose of other vaccines used in the vaccination campaign against SARS‐Cov2. Nevertheless the underlying pathophysiological mechanism remains still unknown. 3 It is possible that this kind of m‐RNA vaccines cause endothelial damage to microcirculation, responsible for blood extravasation and purpuric lesions. 4 In several studies it has been reported that an activation of T‐lymphocytes after m‐RNA vaccine is the main cause of endothelial damage. 5 In conclusion, about our experience, “Covid arm” is a benign adverse event that resolves in a few weeks and does not represent a contraindication to the second dose of the vaccine; however, further studies are necessary to confirm our hypothesis (Informed consent: The patient gave the consent for photo acquisition and publication).
CONFLICT OF INTEREST
Gabriella Fabbrocini acted as a speaker or consultant for Abbvie, Amgen, Eli Lilly, Janssen, Leo‐Pharma, Almyrall, Novartis, and UCB. None of the contributing authors has any conflict of interest, including specific financial interests of relationships and affiliation relevant to the subject matter or discussed materials in the manuscript.
Supporting information
Table S1 Clinical Features And Management.
ACKNOWLEDGMENTS
Open Access Funding provided by Universita degli Studi di Napoli Federico II within the CRUI‐CARE Agreement. WOA Institution: Universita degli Studi di Napoli Federico II Blended DEAL: CARE
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions. Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
REFERENCES
- 1. McMahon DE, Amerson E, Rosenbach M, et al. Cutaneous reactions reported after Moderna and Pfizer COVID‐19 vaccination: a registry‐based study of 414 cases. J Am Acad Dermatol. 2021;85(1):46‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wei N, Fishman M, Wattenberg D, Gordon M, Lebwohl M. "COVID arm": a reaction to the Moderna vaccine. JAAD Case Rep. 2021;10:92‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Siegrist CA. Mechanisms underlying adverse reactions to vaccines. J Comp Pathol. 2007;137(1):46‐50. [DOI] [PubMed] [Google Scholar]
- 4. Lam M, Egail M, Bedlow AJ, Tso S. Ribonucleic acid COVID‐19 vaccine‐associated cutaneous adverse drug events: a case series of two patients. Clin Exp Dermatol. 2021;46:1131‐1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Blumenthal KG, Freeman EE, Saff RR, et al. Delayed large local reactions to mRNA‐1273 vaccine against SARS‐CoV‐2. N Engl J Med. 2021;384(13):1273‐1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Clinical Features And Management.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions. Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.


